CHAPTER 36 Adult Hydrocephalus
The Role of Endoscopic Third Ventriculostomy
The history of the neurosurgical use of third ventriculostomy is fascinating and filled with ebbs and flows of popularity. This history has been elegantly detailed by both Hellwig and colleagues1 and Enchev and Oi.2 It is not the intention of this chapter to reiterate these previous accounts; instead, we urge readers to refer to these references.
Comparison of Adult and Pediatric Hydrocephalus
Epidemiology
A close examination of the current literature on pediatric and adult hydrocephalus highlights the paucity of scientific data, especially in the adult hydrocephalus arena. There are approximately 10 articles dealing with pediatric hydrocephalus for every publication on adult hydrocephalus. Therefore, much more is known about the incidence, prevalence, epidemiologic factors, inpatient hospitalization, health care costs, and mortality associated with the treatment of hydrocephalus in children. Simon and associates estimated that there are approximately 38,000 to 39,000 admissions for hydrocephalus each year in the United States, with approximately 400,000 hospital days and $2 billion per year in charges for admissions alone for the care of pediatric hydrocephalus.3 The authors found that pediatric hydrocephalus accounts for more than three times the inpatient utilization but just 4% of the National Institutes of Health (NIH) funding for a comparable chronic disease such as cystic fibrosis. Studies such as this help quantify potential impediments to the progress of pediatric hydrocephalus research in the hope of bringing this disparity to the attention of the NIH.
Tisell and coworkers conducted a cross-sectional study in Sweden to evaluate the incidence of adult hydrocephalus from 1996 through 1998.4 During that time, they found 891 new adult patients in whom hydrocephalus was diagnosed and treated. This represents an incidence of 3.6 per 100,000. They also found that the incidence was increasing over the 3 years of study. In a North American setting, Patwardhan and Nanda used the Nationwide Inpatient Sample database to determine that 8305 new cases of pediatric and adult hydrocephalus were treated in 2000 in the United States.5 This study also estimated an inpatient mortality of 2.7% for hydrocephalus and an overall cost to the health care system of $1.1 billion in 2000. Bondurant and Jimenez used a similar database to estimate the overall prevalence of hydrocephalus in the United States to be 125,000 in 1988, with 36,000 cerebrospinal fluid (CSF) shunt operations being performed each year.6 Neither of these studies, however, addressed adult hydrocephalus specifically because the pediatric cohort was intermingled with the adult cases.
One common theme between the pediatric and adult hydrocephalus populations is that they both include a heterogeneous group of hydrocephalus subtypes; however, the causes and incidences of these subtypes differ vastly between children and adults. Unpublished data from the Hydrocephalus Clinical Research Network, which is a five-center network dedicated to studying pediatric hydrocephalus, indicate that the five most common causes of pediatric hydrocephalus are intraventricular hemorrhage (IVH) resulting from premature birth (22%), myelomeningocele (14%), posterior fossa tumor (9%), aqueductal stenosis (8%), and congenital communicating hydrocephalus (8%). These prospective data represent only new diagnoses of hydrocephalus and will be published in detail in the near future. In contrast, Tisell and associates found that the most common causes of adult hydrocephalus were normal-pressure hydrocephalus (NPH) (47%), acquired communicating high-pressure hydrocephalus from bleeding such as after subarachnoid hemorrhage (15%), adult-onset aqueductal stenosis (10%), other noncommunicating hydrocephalus (e.g., tectal glioma) (9%), and acquired communicating hydrocephalus from trauma (5%).4 However, these incidences are not stagnant. Technologic advances to improve the care of pediatric patients with hydrocephalus have begun to translate into diminished mortality. Thus, many hydrocephalic children reach adulthood, which makes treatment of the adult cohort perhaps more complex.3
Use of Endoscopic Third Ventriculostomy
Another major difference between pediatric and adult hydrocephalus is that ETV is much more commonly used and therefore much more frquently described in the literature in the pediatric context. For instance, Drake reviewed the cases of 368 patients who had undergone ETV in Canada during a 15-year period.7 Analysis of this cohort demonstrated that younger age was associated with ETV failure whereas other factors, including etiology, surgeon experience, previous surgery, and center volume, were not. No similar studies have been conducted in the adult cohort, although there is a burgeoning literature on the role of ETV in patients with NPH. This specific issue is explored later in this chapter.
Patient Selection and Outcomes
The overall success rate of ETV lies somewhere between 50% and 90% at 1 year,7–10 primarily because hydrocephalus has such a heterogeneous compilation of causes, which vary with the age of the population being studied. For example, ETV in children with hydrocephalus secondary to tectal glioma has a success rate of 88%,9 but ETV performed in the context of IVH associated with prematurity succeeds just 0% to 33% of the time.11,12 Therefore, careful patient selection is absolutely essential in maximizing the chance of success and minimizing the complications inherent in ETV.
Young age (<6 months) has been shown to be associated with a lower success rate independent of etiology; however, this age effect either plateaus or diminishes after the patient reaches the age of 2 years, perhaps because of cranial maturation.7,13 The literature indicates that in patients older than 2 years, the success rate of ETV is high for noncommunicating hydrocephalus caused by such conditions as aqueductal stenosis, tectal glioma, and posterior fossa tumors.7,9 In contrast, there appears to be a lower success rate in patients with hydrocephalus caused by IVH associated with prematurity or in those with postinfectious hydrocephalus, thus leading to some controversy regarding its use in these populations.11,12,14 Who are the best candidates for ETV in the adult setting? What are the factors that matter in making the decisions?
Because the surgery is associated with a slightly higher complication rate and is longer and more involved than placement of a CSF shunt, patients must be relatively healthy. Primarily, they should be devoid of a coagulopathy. The patient must also be willing and able to return for follow-up care. It is folly to think that ETV is a “cure” because ETV can and will fail over time.15 It is significant to note that these failures can rarely result in sudden death.16
Patient selection for ETV is inescapably linked to outcome, and therefore one cannot be discussed without the other. The most important factor in selecting adult patients for ETV is the subtype of hydrocephalus itself. Treatment with ETV appears to offer the most benefit for late-onset idiopathic aqueductal stenosis (LIAS), secondary noncommunicating hydrocephalus, NPH, and conversion of a shunt in a patient who was treated for hydrocephalus as a child.17
Late-Onset Idiopathic Aqueductal Stenosis
LIAS, also known as delayed or compensated aqueductal stenosis, is a triventricular hydrocephalus with little or no flow through the aqueduct of Sylvius and a normal to small fourth ventricle. This form of hydrocephalus occurs in adult patients in the absence of space-occupying lesions or previous central nervous system insults (e.g., previous meningitis). LIAS is the cause of approximately 3% to 10% of all adult cases of hydrocephalus.18,19 In a meta-analysis of 190 patients, Tisell found that the clinical symptoms included headaches (70%), cognitive impairment (55%), urinary incontinence (40%), gait disturbance (28%), diplopia (15%), and endocrine dysfunction (12%).19 Fukuhara and Luciano evaluated 31 patients and added papilledema, swallowing difficulty, Parinaud’s syndrome, and seizures to this list.18 Interestingly, patients tended to dichotomize into either a younger cohort (mean age of 33 years) who had primarily headache and signs and symptoms of raised intracranial pressure or an older cohort (mean age of 63 years) with larger ventricles and NPH-like symptoms (cognitive impairment, urinary incontinence, and gait disturbance).18
Patients with LIAS respond to ETV if properly selected on the basis of signs and symptoms, radiologic findings of triventricular hydrocephalus, and minimal subarachnoid spaces. Success typically results in complete or nearly complete resolution of preexisting signs and symptoms and no need for further CSF diversion procedures. The preponderance of the literature suggests that the success rate of ETV in these patients is 50% to 86.5%, with most authors reporting a success rate of approximately 80% with 6 to 22 months of follow-up.10,17,18
Secondary Noncommunicating Hydrocephalus
Secondary noncommunicating adult-onset hydrocephalus is defined as obstructive hydrocephalus resulting from a lesion impeding the CSF pathway.17 The first choice for such lesions is removal or partial resection to reestablish CSF flow; however, in many cases this is not possible or warranted. In such cases, ETV may provide an alternative.
Pineal region tumors, tectal gliomas, and posterior fossa tumors are the most common types of mass lesions causing secondary noncommunicating hydrocephalus in adults. Rare entities such as thalamic masses, cerebellar infarction, and neurocysticercosis have also been described.20,21 Dusick and colleagues reviewed a series of 108 adult patients who underwent ETV for the treatment of hydrocephalus and found that a mass lesion was an indication for the procedure in 47 (43.5%).22 Their overall success rate for this indication was 76.6%, although the 8-month median follow-up was short. In their study, pineal region masses were the most prevalent indication for ETV, and the success rate in these cases was 71%. These results are compatible in both incidence and success rates with other similar studies.23–25 Even higher success rates of 81% to 88% are seen for ETV performed to treat patients with tectal gliomas, although 18% of these patients may require a second ETV for ostomy blockage.9,26 Tectal gliomas are uncommon and, in general, are the indication for only about 4% of all ETVs—both pediatric and adult.26 Arachnoid cysts, especially those found in the suprasellar, prepontine, third ventricle, or posterior fossa region, can often cause obstructive hydrocephalus. Dusick and associates found that ETV was successful in treating this cause of hydrocephalus in 86% of patients.22
Frequently, ETV performed for tectal and pineal region masses is paired with endoscopic biopsy of the lesion through the third ventricle. Despite a high success rate of the ETV procedure itself, diagnostic rates for the masses are generally lower (69% to 90%). Complication rates associated with the biopsies are typically documented at approximately 18%.8,23 Common complications of these types of biopsies include intracerebellar and intraventricular hemorrhage, upgaze palsy, ventriculitis, and disturbances in sodium balance.23
Normal-Pressure Hydrocephalus
NPH is a very controversial subject in the neurosurgical literature in both its diagnosis and its treatment. It is characterized by gradual blockage of the drainage of CSF, which causes a slow buildup of fluid and a less dramatic increase in fluid pressure. Patients with NPH are, in general, elderly patients with a triad of clinical signs and symptoms, including gait abnormalities such as a “magnetic” or “festinating” gait, bladder incontinence, and cognitive impairments or dementia. Findings on magnetic resonance imaging (MRI) include ventriculomegaly, focal enlargements of the subarachnoid space, and an open aqueduct.17 Most authors use an open aqueduct as a necessary criterion for the diagnosis of NPH. A normal-sized fourth ventricle is seen in 35% of patients with an open aqueduct, whereas it is slightly enlarged in 37% of patients and grossly enlarged in 27%.27
The diagnosis of NPH can be supported by formal neuropsychological testing, gait testing, clinical improvement after a lumbar CSF tap test28 or an external CSF lumbar drainage test,17 or a high-pressure steady-state plateau found on a lumbar infusion test.28 There is as yet no consensus on which of these tests either defines the diagnosis or predicts good outcomes after CSF diversion.
The use of ETV is relatively new and controversial for the management of NPH. Detractors of its use in this context note that CSF shunts have a high success rate in patients with NPH17,29,30 and ETV does not lower intracranial pressure enough to maximally improve clinical outcomes. Proponents emphasize that use of ETV will enable these patients to be free of hardware and point out that CSF shunts cause frequent complications in these patients, including infection, overdrainage symptoms, a need for revision, and subdural hematomas.29
Reported success rates of ETV in patients with NPH have varied considerably from 21% in a study of 14 patients30 to 87% in a study of 15 patients with 27 months of follow-up.29 However, in a recent study by Gangemi and coworkers, 110 patients with NPH were treated by ETV at four centers and monitored for a minimum of 2 years; the success rate in these patients was 69%.27 A successful procedure was defined as resulting in a significant improvement in clinical symptoms. Another 22% of these patients had no change clinically and 9% deteriorated. Potential predictors of success included a shorter duration of symptoms and milder symptomatology, but neither patient age nor size of the fourth ventricle predicted response.
Secondary Endoscopic Third Ventriculostomy for Revision of Pediatric-Onset Hydrocephalus
As noted earlier, the most common causes of pediatric hydrocephalus are IVH secondary to prematurity, myelomeningocele, posterior fossa tumors, aqueductal stenosis, and congenital communicating hydrocephalus (authors’ unpublished data). Many patients with aqueductal stenosis, previous posterior fossa tumors, or IVH secondary to prematurity underwent placement of a shunt as children because their diagnosis preceded the recent resurgence of ETV or because they required treatment before the age of 1 year, for which ETV has been demonstrated to have a poor success rate.7 If these patients are seen with a shunt malfunction as adults, ETV with shunt removal is a viable option in lieu of shunt revision. Most studies show a 70% success rate in these patients at a median follow-up of 36 months.15,25 It is important to consider that success rates will always vary with each study’s selection criteria (the underlying cause for performance of ETV). This may explain why other studies show similar initial success rates that diminish rapidly to 25% at 2 years.31 Another consideration when assessing adult patients for possible ETV who have previously placed shunts is that a much higher complication rate (31%) has been demonstrated for ETV performed after shunt malfunction than for ETV performed as a primary procedure (8%).32
Special consideration should be made for patients with myelomeningocele who have an implanted shunt. ETV is rarely used as a primary procedure for treating hydrocephalus in these children because it can be technically challenging and has poor rates of success (11% to 37%).11,33 However, when ETV is undertaken in the setting of shunt malfunction in older children or adults with myelomeningocele, the success rate increases to 63% to 93%.11,33 Therefore, ETV is a viable option in adult myelomeningocele patients with shunt failure even if they were not candidates for the procedure as children.
Preoperative Imaging
Some authors also rely on the sagittal kinematic, or CINE, phase-contrast sequence to identify absence of flow through the cerebral aqueduct for selection of patients for ETV.34 Although we are not fervently against this sequence, we do not place much emphasis on the kinematic sequence because we have found it to overestimate the level of CSF flow through the aqueduct. In these cases, ETV candidates who might otherwise have benefited from this procedure might not be offered the treatment.
Radiologic Outcomes
In general, the success of ETV is measured by resolution of the clinical signs and symptoms and absence of the need for a repeat procedure—either ETV or CSF shunting. The diminution in ventricular size after ETV is usually considered a much smaller factor for determining success, if it is used at all; however, there are growing concerns that persistently enlarged ventricles, even without signs of raised intracranial pressure, may have chronic cognitive consequences.35,36 In a study that included both children and adults who underwent ETV, Fukuhara and associates demonstrated that only 25% of patients’ ventricles diminished in size the next day, with 52% being diminished at 6 months and 58% at 1 year.34 Other authors have found a significant correlation between diminution of ventricular size and clinical improvement.37 Despite these improvements in ventriculomegaly, however, in most cases the ventricles remain larger than normalized correlates for age and sex, which is contrary to the findings from patients with shunts.38
Operative Technique for Endoscopic Third Ventriculostomy
Anatomic Considerations
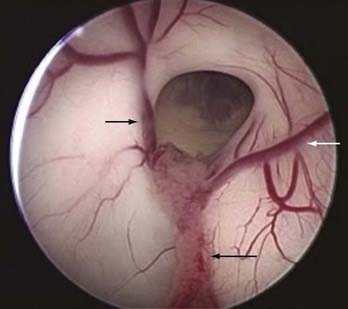
A knowledge of ventricular anatomy is paramount and should be reviewed with the assistants before the operation. The venous anatomy of the lateral ventricle gives visual confirmation of which ventricle the endoscope has entered. The thalamostriate vein runs along the lateral wall and converges with the anterior septal vein before converging and entering the foramen of Monro as the internal cerebral vein. The choroid plexus together with the superior choroidal vein runs along the floor of the lateral ventricle (Fig. 36-1).
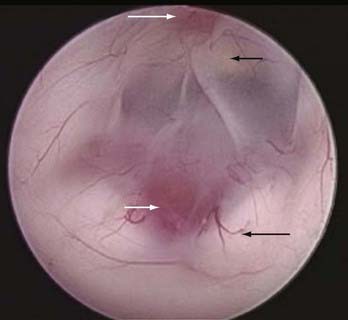
The floor of the third ventricle is generally thin and translucent in a hydrocephalic patient. After the third ventricle is entered, the paired mamillary bodies should be evident midway along the floor, with the basilar complex generally just anterior to them in the midline (Fig. 36-2). The infundibular recess is usually evident as a pinkish orange spot on the anterior midline floor. Slightly posterior to this recess is a white rectangular transverse band of the dorsum sellae. The ideal spot for fenestration is midway between the basilar complex and the dorsum sellae in the midline. The oculomotor and abducens (deeper) nerves can at times be visualized laterally along the floor. Some patients have a thicker and somewhat opaque floor. In this scenario the infundibular recess, clivus, and basilar pulsations can usually be seen and the fenestrations then directed over the bony aspect of the clivus in the midline for maximal safety.
Third Ventriculostomy
The fenestration through the floor of the ventricle is made bluntly with a blunt trocar, closed forceps, or a laser wire. Sharp fenestration and cautery are not recommended because both these techniques increase risk to the basilar artery complex, a disastrous scenario. Some authors maintain that fenestrations may create tension along the walls of the third ventricle and increase the risk for postoperative hypothalamic dysfunction,32 but we have not found this to be problematic. Others use a small Doppler probe to locate the basilar complex definitively before fenestration, especially in patients with opaque third ventricular floors.39
The fenestration is then dilated to 4 to 6 mm in diameter by using a double-balloon catheter, Fogarty balloon, spreaders, or forceps. Next, the endoscope is perched atop the ostomy to inspect the subarachnoid spaces for membranes such as the membrane of Liliequist. These, too, must be carefully fenestrated or the operation will be at risk for failure. A rule of thumb is that if the endoscope can fit within the fenestration, it is large enough. After the fenestration, the floor of the third ventricle should pulsate and flap with the respiration and heart rates. These pulsations have been shown to be a strong predictor of ETV success.40
Closure and Postoperative Issues
Many centers encourage the use of subcutaneous bur hole reservoirs after ETV. These can be tapped in emergency or potentially infectious scenarios and can also be used postoperatively to measure intracranial pressure.39,41 However, ventricular reservoirs may encourage patency of the cortical tract and thus promote CSF leakage; their use may also disallow the possibility of rendering the patient hardware free.
As stated previously, ventriculomegaly in patients who have undergone ETV decreases at a much slower rate and to a much less extent than in patients with implanted shunts.38 Therefore, we perform postoperative imaging (CT) at 6 to 8 weeks rather than on postoperative day 1 as is our practice in patients with shunts. Typical in-hospital stays for patients after ETV vary between 1 and 3 days, with most patients going home on postoperative day 2; this is longer than their counterparts with shunts.
Complications
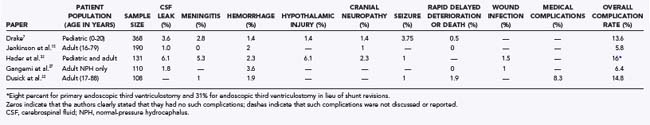
Preoperative informed consent and postoperative patient monitoring require a detailed understanding of both the types and frequencies of complications associated with ETV. Despite allowing the patient to be hardware free and therefore invulnerable to shunt infection, ETV is certainly not without risk, especially in the short term. The risk for ETV failure was described earlier and ranges from 10% to 50% in most studies. This variation is a result of the wide range of causes of hydrocephalus. The overall complication rates for this procedure vary from 5.8% to 16% (Table 36-1). These rates vary primarily based on indications for or cause of the hydrocephalus, patient comorbidity, surgeon experience, and vigilance in reporting.
Major complications that should be disclosed to the patient before surgery include CSF leakage (1% to 6%), meningitis (1% to 5%), cranial neuropathies (1% to 2%), seizures (1%), and medical complications (2% to 9%). Overall hemorrhage rates vary from 1% to 3.6% and include intraventricular bleeding requiring either abandonment of the procedure or reoperation (hemorrhage blocking the ostomy), postoperative hematoma, or rare catastrophic basilar injury. Hypothalamic injuries are generally underreported, especially pathologic weight gain, and include diabetes insipidus, amenorrhea, and precocious puberty.32
Hydrocephalic patients treated with ETV must be considered treated and not cured; therefore, they require follow-up just like their counterparts with shunts. This is especially important because rapid delayed deterioration has been reported in upward of 1 in 200 ETV patients.7 Sixteen such cases have been reported, with a staggering mortality rate of 81%. All the survivors except 1 have been disabled as a result of the rapid deterioration.42
Repeated Endoscopic Third Ventriculostomy After Primary Failure
Such patients with ETV failure should undergo MRI with high-resolution T2-weighted sagittal sequencing. This sequence will allow the clinician to evaluate ventricle size, the presence or absence of an interruption in the floor of the third ventricle (ostomy), and whether a flow void exists through the opening. If the opening is present with or without a flow void, serial lumbar punctures to encourage flow through the ostomy can be considered.43 If there is no resolution after two or three attempts, CSF shunting is mandated. In a patient in whom neither an opening nor a flow void is seen on MRI, re-exploration or repeated ETV is warranted. During re-exploration, if an opening is visualized and is of adequate size, conversion to a CSF shunt is suggested. In the event that the ostomy is occluded either by debris or by scar tissue, refenestration is warranted. Gangemi and coauthors reported that in 10 of 110 patients who worsened clinically after primary ETV, 4 were found to have occluded ostomies on MRI and agreed to undergo a repeat ETV procedure.27 All 4 improved clinically.
Amini A, Schmidt RH. Endoscopic third ventriculostomy in a series of 36 adult patients. Neurosurg Focus. 2005;19(6):E9.
Bergsneider M, Miller C, Vespa PM, et al. Surgical management of adult hydrocephalus. Neurosurgery. 2008;62(suppl 2):643-659.
Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg. 1995;23:254-258.
Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317-324.
Drake JM. Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery. 2007;60:881-886.
Drake J, Chumas P, Kestle J, et al. Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg. 2006;105:118-126.
Enchev Y, Oi S. Historical trends of neuroendoscopic surgical techniques in the treatment of hydrocephalus. Neurosurg Rev. 2008;31:249-262.
Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol. 2001;55:132-136.
Gangemi M, Maiuri F, Naddeo M, et al. Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus: an Italian multicenter study. Neurosurgery. 2008;63:62-67.
Hader WJ, Drake J, Cochrane D, et al. Death after late failure of third ventriculostomy in children. Report of three cases. J Neurosurg. 2002;97:211-215.
Hader WJ, Walker RL, Myles ST, et al. Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery. 2008;63:ONS168-174.
Hellwig D, Grotenhuis JA, Tirakotai W, et al. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev. 2005;28:1-34.
Jenkinson MD, Hayhurst C, Al-Jumaily M, et al. The role of endoscopic third ventriculostomy in adult patients with hydrocephalus. J Neurosurg. 2009;110:861-866.
Longatti PL, Fiorindi A, Martinuzzi A. Failure of endoscopic third ventriculostomy in the treatment of idiopathic normal pressure hydrocephalus. Minim Invasive Neurosurg. 2004;47:342-345.
Patwardhan RV, Nanda A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139-144.
Simon TD, Riva-Cambrin J, Srivastava R, et al. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131-137.
St. George E, Natarajan K, Sgouros S. Changes in ventricular volume in hydrocephalic children following successful endoscopic third ventriculostomy. Childs Nerv Syst. 2004;20:834-838.
Tisell M, Hoglund M, Wikkelso C. National and regional incidence of surgery for adult hydrocephalus in Sweden. Acta Neurol Scand. 2005;112:72-75.
Warf BC. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg. 2005;102:1-15.
1 Hellwig D, Grotenhuis JA, Tirakotai W, et al. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev. 2005;28:1-34.
2 Enchev Y, Oi S. Historical trends of neuroendoscopic surgical techniques in the treatment of hydrocephalus. Neurosurg Rev. 2008;31:249-262.
3 Simon TD, Riva-Cambrin J, Srivastava R, et al. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131-137.
4 Tisell M, Hoglund M, Wikkelso C. National and regional incidence of surgery for adult hydrocephalus in Sweden. Acta Neurol Scand. 2005;112:72-75.
5 Patwardhan RV, Nanda A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139-144.
6 Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg. 1995;23:254-258.
7 Drake JM. Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery. 2007;60:881-886.
8 Beems T, Grotenhuis JA. Long-term complications and definition of failure of neuroendoscopic procedures. Childs Nerv Syst. 2004;20:868-877.
9 Jallo GI, Kothbauer KF, Abbott IR. Endoscopic third ventriculostomy. Neurosurg Focus. 2005;19(6):E11.
10 Tisell M, Almstrom O, Stephensen H, et al. How effective is endoscopic third ventriculostomy in treating adult hydrocephalus caused by primary aqueductal stenosis? Neurosurgery. 2000;46:104-110.
11 Brockmeyer D, Abtin K, Carey L, et al. Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg. 1998;28:236-240.
12 Peretta P, Ragazzi P, Carlino CF, et al. The role of Ommaya reservoir and endoscopic third ventriculostomy in the management of post-hemorrhagic hydrocephalus of prematurity. Childs Nerv Syst. 2007;23:765-771.
13 Oertel JM, Baldauf J, Schroeder HW, et al. Endoscopic options in children: experience with 134 procedures. J Neurosurg Pediatr. 2009;3:81-89.
14 Warf BC. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg. 2005;102:1-15.
15 Jenkinson MD, Hayhurst C, Al-Jumaily M, et al. The role of endoscopic third ventriculostomy in adult patients with hydrocephalus. J Neurosurg. 2009;110:861-866.
16 Hader WJ, Drake J, Cochrane D, et al. Death after late failure of third ventriculostomy in children. Report of three cases. J Neurosurg. 2002;97:211-215.
17 Bergsneider M, Miller C, Vespa PM, et al. Surgical management of adult hydrocephalus. Neurosurgery. 2008;62(suppl 2):643-659.
18 Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol. 2001;55:132-136.
19 Tisell M. How should primary aqueductal stenosis in adults be treated?—A review. Acta Neurol Scand. 2005;111:145-153.
20 Baldauf J, Oertel J, Gaab MR, et al. Endoscopic third ventriculostomy for occlusive hydrocephalus caused by cerebellar infarction. Neurosurgery. 2006;59:539-544.
21 Bergsneider M, Holly LT, Lee JH, et al. Endoscopic management of cysticercal cysts within the lateral and third ventricles. Neurosurg Focus. 1999;6(4):E7.
22 Dusick JR, McArthur DL, Bergsneider M. Success and complication rates of endoscopic third ventriculostomy for adult hydrocephalus: a series of 108 patients. Surg Neurol. 2008;69:5-15.
23 Depreitere B, Dasi N, Rutka J, et al. Endoscopic biopsy for intraventricular tumors in children. J Neurosurg. 2007;106:340-346.
24 Gangemi M, Mascari C, Maiuri F, et al. Long-term outcome of endoscopic third ventriculostomy in obstructive hydrocephalus. Minim Invasive Neurosurg. 2007;50:265-269.
25 O’Brien DF, Hayhurst C, Pizer B, et al. Outcomes in patients undergoing single-trajectory endoscopic third ventriculostomy and endoscopic biopsy for midline tumors presenting with obstructive hydrocephalus. J Neurosurg. 2006;105:219-226.
26 Javadpour M, Mallucci C. The role of neuroendoscopy in the management of tectal gliomas. Childs Nerv Syst. 2004;20:852-857.
27 Gangemi M, Maiuri F, Naddeo M, et al. Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus: an Italian multicenter study. Neurosurgery. 2008;63:62-67.
28 Kahlon B, Sundbarg G, Rehncrona S. Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2002;73:721-726.
29 Meier U. Shunt-Operation versus endoskopische Ventrikulostomie beim Normaldruckhydrozephalus: Diagnostik und Outcome. Zentralbl Neurochir. 2003;64:19-23.
30 Longatti PL, Fiorindi A, Martinuzzi A. Failure of endoscopic third ventriculostomy in the treatment of idiopathic normal pressure hydrocephalus. Minim Invasive Neurosurg. 2004;47:342-345.
31 Woodworth G, McGirt MJ, Thomas G, et al. Prior CSF shunting increases the risk of endoscopic third ventriculostomy failure in the treatment of obstructive hydrocephalus in adults. Neurol Res. 2007;29:27-31.
32 Hader WJ, Walker RL, Myles ST, et al. Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery. 2008;63:ONS168-174.
33 Teo C, Jones R. Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg. 1996;25:57-63.
34 Fukuhara T, Vorster SJ, Luciano MG. Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery. 2000;46:1100-1109.
35 Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317-324.
36 Golomb J, de Leon MJ, George AE, et al. Hippocampal atrophy correlates with severe cognitive impairment in elderly patients with suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1994;57:590-593.
37 Santamarta D, Martin-Vallejo J, Diaz-Alvarez A, et al. Changes in ventricular size after endoscopic third ventriculostomy. Acta Neurochir (Wien). 2008;150:119-127.
38 St George E, Natarajan K, Sgouros S. Changes in ventricular volume in hydrocephalic children following successful endoscopic third ventriculostomy. Childs Nerv Syst. 2004;20:834-838.
39 Amini A, Schmidt RH. Endoscopic third ventriculostomy in a series of 36 adult patients. Neurosurg Focus. 2005;19(6):E9.
40 Greenfield JP, Hoffman C, Kuo E, et al. Intraoperative assessment of endoscopic third ventriculostomy success. J Neurosurg Pediatr. 2008;2:298-303.
41 Aquilina K, Edwards RJ, Pople IK. Routine placement of a ventricular reservoir at endoscopic third ventriculostomy. Neurosurgery. 2003;53:91-96.
42 Drake J, Chumas P, Kestle J, et al. Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg. 2006;105:118-126.
43 Cinalli G, Spennato P, Ruggiero C, et al. Intracranial pressure monitoring and lumbar puncture after endoscopic third ventriculostomy in children. Neurosurgery. 2006;58:126-136.