Adrenocorticotropic Hormone
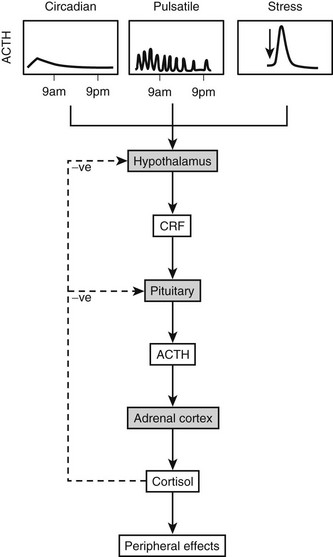
The hypothalamic-pituitary-adrenal (HPA) axis (Fig. 3-1) is well recognized for its role in the homeostatic mechanisms regulating the stress response. The hypothalamic secretion of corticotropin-releasing hormone (CRH) stimulates ACTH synthesis and release from the anterior pituitary, which in turn regulates the synthesis of glucocorticoids in the adrenal cortex. The impact of the host of factors and mechanisms known to regulate ACTH and related peptides is considered in the context of biological activity both at the adrenal and in other tissues.
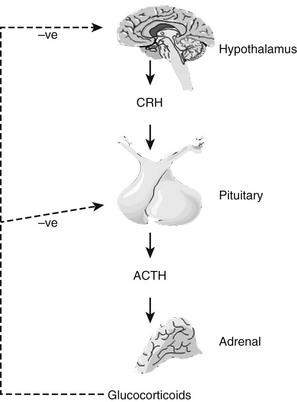
FIGURE 3-1 Schematic representation of the hypothalamic-pituitary-adrenal axis, showing sites of glucocorticoid negative feedback. ACTH, Adrenocorticotropic hormone; CRH, corticotropin-releasing hormone.
History
1930—Discovery by Smith that ACTH is a factor produced by the pituitary that maintains the weight of the adrenal cortex1
1954—Primary structure of ACTH2
1964—Isolation of β-lipotropic pituitary hormone (β-lipotropin)3
1975—Peptide with opioid activity isolated from the pituitary and named β-endorphin4
1978—Proof that POMC is the common precursor5
1979—Nucleotide sequence of POMC6
1981—Isolation and sequencing of corticotropin-releasing hormone (CRH)7
1992—Cloning of the ACTH receptor8,9
1998—Inherited mutations in POMC and PC1 associated with early-onset obesity, adrenal insufficiency, and red hair pigmentation10,11
2005—Differential control of hypothalamic POMC transcription12
2006—Mechanism for glucocorticoid regulation of POMC identified13
2007—Identification of the differential expression of an “HPA axis” equivalent in the skin14
Pro-Opiomelanocortin Gene
Humans have a single POMC gene located on the short arm of chromosome 2 at 2p23 (the mouse and pig have two copies of the gene). The structure of the gene is well conserved and has been characterized in humans15–17 as well as in other species.18 The POMC gene consists of three exons interspersed with two large introns (Fig. 3-2). The first exon, which consists of 87 base pairs (bp), contains no coding sequence, and its RNA transcript is thought to act as a leader sequence that binds the ribosome at the start of translation. Exon 2 (152 bp) contains the initiation sequence, a signal sequence that translocates the nascent peptide into the endoplasmic reticulum, and then the N-terminal part of the coding sequence for the POMC peptide. The third exon (835 bp) encodes most of the mature protein, including ACTH,15–17 the termination codon, and the signal for addition of the poly A tail.
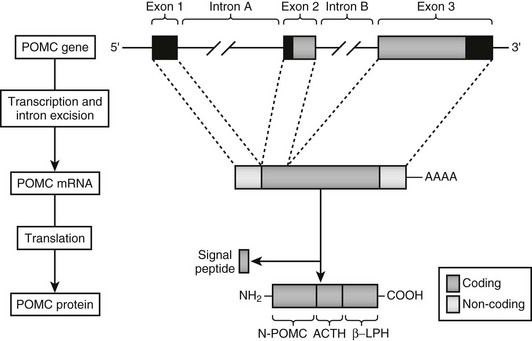
FIGURE 3-2 Genomic structure of human pro-opiomelanocortin (POMC) with the major spliced product and preprohormone. ACTH, Adrenocorticotropic hormone; βLPH, β-lipotropin.
POMC Transcripts
The POMC gene has three RNA transcripts of 1200 (T1), 800 (T2), and 1380 (T3) nucleotides, respectively (Fig. 3-3).
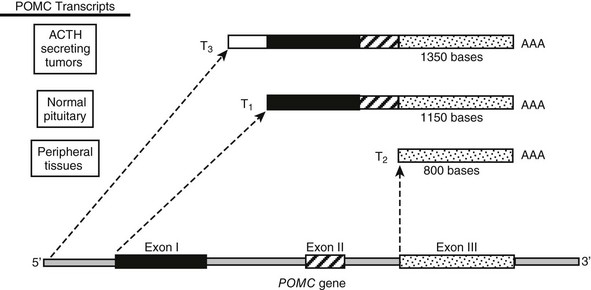
FIGURE 3-3 Tissue-specific transcriptional variants of the human pro-opiomelanocortin (POMC) gene. T1 has high-level expression of POMC in pituitary tissue. T2 has low-level expression in numerous extrapituitary tissues. T3 is present in some extrapituitary tumors causing the ectopic adrenocorticotropic hormone (ACTH) syndrome. AAA, Poly A tail.
The Pituitary Transcript T1: T1 is the mRNA transcript found in corticotrope cells of the anterior pituitary in man and has a size of 1200 nucleotides as detected by Northern blotting.19 In the hypothalamus, the POMC mRNA transcript seems to be identical to the pituitary transcript except for a longer poly A tail.20
The Upstream Transcript T3: The T3 transcript is 1380 nucleotides in size and is presumed to be under the regulation of promoter elements that lie upstream of the pituitary promoter. This transcript produces the same peptide product as T1, because the only relevant translation initiation site is in exon 1. This longer POMC mRNA transcript has been found in normal tissues (e.g., placenta21,22) and has also been associated with abnormal expression of POMC such as that seen in small cell lung carcinoma (SCLC).23
The Downstream Transcript T2: The downstream T2 transcript is an 800-nucleotide RNA transcript that has been shown in humans and rats to arise from transcription initiation at the 5′ end of exon 3.24,25 This finding suggests that regulatory sequences may occur starting from the 3′ end of intron 2.18 This transcript could not give rise to a mature POMC molecule and would lack a signal peptide, so its physiologic role is unclear.26 The smaller POMC transcript is found primarily in a variety of peripheral tissues, which indicates possible differential regulation in these tissues.
Regulatory Sites for Transcription
The POMC promoter has a number of common elements found in other genes that may contribute to regulation of POMC gene transcription (Fig. 3-4). Owing to the lack of availability of a human corticotrope cell line, the mouse-equivalent cell line (AtT20) has been used to assess POMC transcriptional regulation along with transgenic mouse models,27 although some studies have utilized primary human pituitary cells from surgical procedures.28
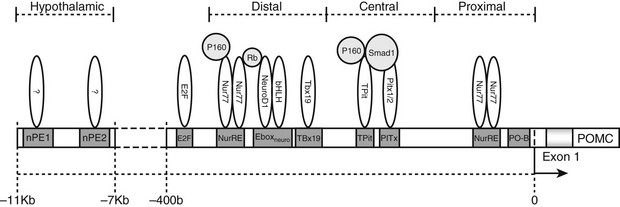
FIGURE 3-4 The promoter region of the pro-opiomelanocortin (POMC) gene. Response elements are shown along with bound transcription factors in association with known coregulators (light grey). bHLH, Basic-helix-loop-helix transcription factors; E2F, transcription factors involved in cell cycle regulation and synthesis of DNA; Ebox, specific DNA sequence that binds heterodimers of Neuro D1 and other bHLH proteins; Neuro D1, neurogenic differentiation 1 transcription factor; nPE, neural pro-opiomelanocortin enhancer; Nur77, also known as nerve growth factor IB (NGFIB), a member of a family of transcription factors involved in cell cycle mediation, inflammation, and apoptosis; NurRE, Nur factor response element; P160, family of coactivators; Pitx, a homeobox family member which is involved in organ development; PO-B, a transcription factor originally described as POMC specific. Rb, Retinoblastoma protein, a tumor suppressor protein; Smad1, a transcriptional modulator that mediates multiple signaling pathways; Tbx19, transcription factors present only in pituitary POMC-expressing cells and involved in the regulation of developmental processes; Tpit, transcription factors involved in regulation of development.
The correct spatial, temporal, and hormonal regulation of POMC transcription in the pituitary is conferred by two promoter regions immediately 5′ of exon 1. These regions are between −314 and −276 bp and between −67 and −27 bp in the human POMC gene.27,29,30
Hypothalamic and CNS-specific expression of POMC has been shown to require DNA control elements distal to those required for POMC expression in the pituitary. A 13 kb region immediately 5′ of the POMC gene has been demonstrated to control CNS and hypothalamic expression in transgenic mice.31 A 4-kb distal region of the POMC gene situated −13 to −9 kb in mouse and −11 to −7 kb in human from POMC exon 1 has been shown to contain two neuronal-specific enhancer regions capable of directing POMC expression in the arcuate nucleus of the hypothalamus.12 These two neuronal POMC enhancer regions (nPE1 and nPE2) have been shown by deletion studies to be individually capable of driving POMC transcription in the arcuate nucleus and have also been demonstrated to be inactive in the pituitary, implying that there is a modular independence between the promoter regions used to control pituitary and hypothalamic POMC transcription.12
The expression of the short POMC mRNA transcript (T2) seems to be regulated by “GC box” promoter sequences located in the 3′ end of intron 2.19
Expression of The POMC Gene
In humans, expression of POMC is most abundant in the corticotrope cells of the anterior pituitary, and in healthy subjects, these cells are the only ones that express the gene at high levels.32,33 POMC is one of the top 10 most abundant transcripts in the pituitary gland.34 The main POMC mRNA expressed in the pituitary is the T1 transcript with a size of 1200 nt. POMC mRNA is also detected in the intermediate lobe of the pituitary, which is present during fetal life in humans and is found in other species such as the mouse and rat.35–37
Pituitary expression is conferred by the 5′ flanking region of the gene25,27,38–40 (see Fig. 3-4), but there does not appear to be a specific element sufficient to direct high-level transcription, as for example, in the prolactin gene, where the pituitary-specific transcription factor Pit-1 binds to multiple sites to direct transcription. Rather, in the case of the POMC gene, there appears to be a requirement for integrity of the promoter.
The region just 5′ to the start site close to the TATA box confers basal expression and contains the binding sites for PO-B (at −27 nucleotides) and NUR 77 (−67 nucleotides). Further upstream, in the central region of the pituitary promoter, there is a response element which binds the homeobox protein Pitx141 and the related Pitx2.42 During development, Pitx1/2 play an important role in corticotrope development and in the development of the anterior pituitary in general.42–45 Close to the response element which binds Pitx1, there is a binding site for the T box factor, Tpit, which acts in synergy with Pitx1 and is required for expression of the POMC gene and for terminal differentiation of the pituitary corticotrope lineage.46 Tpit functions as an activator of transcription by recruiting SRC/p160 co-activators to its cognate DNA target in the POMC promoter.47
Evidence for the importance of the role of Tpit comes from Tpit-deficient mice, which represent a model of isolated ACTH deficiency, and from humans with Tpit gene mutations which are associated at high frequency with early-onset isolated ACTH deficiency (IAD).48,49 One cause of neonatal death has been identified as congenital IAD associated with mutations of the Tpit gene.50 Different Tpit mutations have been associated with IAD, but all these mutations are likely to manifest functionally through disruption of DNA-protein and protein-protein interactions. In one case, a mutation of the Tpit gene (M86R) has been shown to inhibit the binding of other DNA-bound proteins, ultimately leading to loss of recruitment of the p160 coactivator SRC-2.51
Other evidence for the importance of Tpit relates to bone morphogenic proteins (BMP) 4 and 2, which are signaling molecules associated with early organogenesis and cell differentiation of the pituitary. BMP4 stimulation leads to the recruitment of activated phospho-Smad1 by the POMC promoter through “tethered” interactions with Pitx1 and Tpit, thus reducing their transcriptional activity and resulting in repression of POMC transcription.52
The distal region of the pituitary promoter cannot confer activity independently of the central region but does contain a binding site (Eboxneuro) for NeuroD/1A acting as a heterodimer with other basic helix-loop-helix (bHLH) factors and synergizing with Pitx.40,53 The Eboxneuro element of the distal pituitary promoter, and a nearby Nur response element (NurRE), are required to modulate expression of POMC throughout pituitary development.54 A member of the T-box gene family, Tbx19, a pituitary development–specific transcription factor, has been shown to have a putative binding site at −310 nt, close to the Eboxneuro site. However, this protein may also exert POMC transcriptional effects synergistically with other pituitary-specific transcription factors.55
Expression in Other Tissues
POMC is also expressed, but at a much lower level, in other tissues such as the arcuate nucleus of the hypothalamus, skin, testis, ovary, placenta, duodenum, liver, kidney, adrenal medulla, lung, thymus, heart, and lymphocytes.14,19,22,25,56–59 Extrapituitary POMC mRNA is frequently expressed as the 1200 nt T1 transcript (similar to that in the pituitary), for example in the hypothalamus, skin, and placenta. However, POMC mRNA from extracranial tissues can also have a preponderance of the shorter 800 nt T3 transcript.58,60,61 As indicated earlier, this shorter T2 transcript could not give rise to mature POMC and would lack a signal sequence, so its physiologic role is unclear. It may be that low-level expression of longer POMC transcripts (T1 and T3) account for POMC transcription and expression even when there is high expression of the short transcript, for example as demonstrated in the testes.20,60,62
POMC expression and regulation in the skin is now well established.14,63,64 Current evidence would suggest that POMC transcription in the skin is regulated by the region immediately 5′ of exon 1 in keratinocytes64 and melanocytes.65
Expression in Pituitary Tumors
In corticotrope adenomas that give rise to pituitary-dependent Cushing’s disease, POMC is the most highly expressed gene,66 similar to that in the normal pituitary.34,67 The loss of retinoblastoma tumor-suppressor protein (Rb) expression has been linked to pituitary corticotrope tumor progression.68 It has been shown that Rb is a transcriptional activator of POMC by bridging between NeuroD and Nurr77 and potentiating interactions between Nurr77 and the p160 co-activator SRC-2.69,70
Expression in Nonpituitary Tumors
Tumors giving rise to the ectopic ACTH syndrome produce an mRNA transcript of 1200 bp, similar to that found in the pituitary, and approximately 20% of tumors express a larger transcript of 1400 to 1500 bp.67 This larger transcript seems to be under the regulation of a promoter region located at −392 and −432 bp relative to the conventional start site.21,71–73 Analysis of this domain in the human small cell lung carcinoma cell line DMS-79 showed that it binds the E2F family of trans-acting factors.74
The expression of this promoter in the ectopic ACTH syndrome suggests loss of the tight tissue-specific expression. This promoter is embedded within a CpG island which has been shown to be unmethylated in a number of tumors giving rise to the ectopic ACTH syndrome and in the POMC-expressing small cell lung carcinoma cell line, DMS-79.75 In contrast, the CpG island was methylated in normal nonexpressing tissues.27
Recently it has been shown that hypomethylation of the POMC promoter in thymic carcinoid tumors correlates with POMC overexpression and the ectopic ACTH syndrome. The region of the POMC promoter that underwent change in methylation status was shown to correspond to the E2F binding region of the POMC promoter.76
Regulation Of POMC Gene Expression
Regulation of the POMC Gene in the Pituitary
Numerous factors are known to regulate POMC gene expression in the pituitary, but perhaps the most important are CRH and glucocorticoids (Fig. 3-5). Expression of the POMC gene appears to be predominantly controlled at the level of gene transcription.77
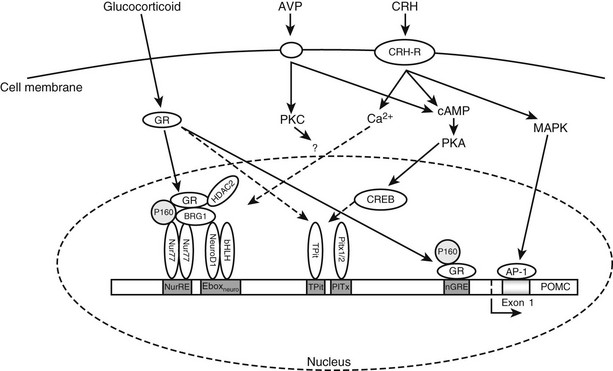
FIGURE 3-5 Intracellular signaling pathways regulating transcription of the pro-opiomelanocortin (POMC) gene. Through its receptor, corticotropin-releasing hormone (CRH) induces cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA) and thereby phosphorylation of cAMP response element–binding protein (CREB). CRH also activates mitogen-activated protein kinase (MAPK) pathways, which ultimately induce activator protein-1 (AP-1) binding to an exon 1 response element. Arginine-vasopressin (AVP) activates cAMP and/or protein kinase C (PKC) pathways, which may also feed into this pathway. Glucocorticoids acting through the glucocorticoid receptor (GR) can repress transcription through two cooperative binding sites.
Corticotropin-Releasing Hormone Stimulation of the POMC Gene: CRH binds transmembrane receptors on corticotrope cells and stimulates cyclic adenosine monophosphate (cAMP) production and activation of protein kinase A (PKA)78 (see Fig. 3-5). CRH effects on POMC transcription do not require de novo protein synthesis.79 There is no cAMP response element (CRE) in the promoter region of the POMC immediately 5′ of exon 1, but two DNA elements have been identified that appear capable of conferring CRH responsiveness to the gene. One element at −171 to −160 nt upstream from the transcription start site binds a protein termed the CRH response element–binding protein.80,81 The second element reported to be CRH responsive is found in the noncoding exon 1 of the rat POMC gene.81,82 This element in exon 1 (+41/+47) shares close homology with a consensus activator protein-1 (AP-1) transcription factor–binding site and binds recombinant AP-1 protein and cAMP response element–binding protein (CREB) in a sequence-specific manner.82,83
CRH causes activation of mitogen-activated protein kinase (MAPK) and induction of the DNA-binding activity of AP-1 in the mouse pituitary corticotrope cell line, AtT20.78,84 In addition, the POMC exon 1 element confers both phorbol ester and CRH responsiveness to a heterologous promoter.84 Therefore, there is considerable evidence for a physiologic role of the MAPK/AP-1 cascade in mediating some actions of CRH.81,85,86
In AtT-20 corticotropes, CRH, and cAMP induce Nur77 expression, and POMC transcription is activated through the NurRE site by protein kinase A (PKA) and calcium-dependent and calcium-independent mechanisms.78 The NGFI-B (Nur77) subfamily of orphan nuclear receptors (NRs), which also includes Nurr1 and NOR1, bind the NurRE as either homo- or heterodimers formed between subfamily members. Nur factors behave as endpoint effectors of the PKA signaling pathway acting through dimers and AF-1-dependent recruitment of co-activators, such as TIF2.79
Tpit/PitxRE also mediates CRH-induced activation of POMC gene expression in a calcium-dependent manner. Clearly Tpit/PitxRE is an important element by which both CRH and Gcs regulate the POMC gene expression.87
Glucocorticoid Inhibition of the POMC Gene: Glucocorticoids are known to decrease ACTH levels, mainly as a result of inhibition of POMC transcription, as highlighted in the GR knockout mouse, where there is an increase in POMC expression in corticotropes,88 although they also act at the level of translation89 and antagonize actions of CRH90 (see Fig. 3-5). Considerable evidence indicates that glucocorticoids suppress transcription of the POMC gene.91–95
Glucocorticoids enter the cell, where they bind glucocorticoid receptors complexed to heat shock proteins in the cytoplasm. This results in the translocation of the ligand-bound receptor to the nucleus, where it recruits co-regulator proteins and acts as a transcription factor, binding (usually as a dimer with another glucocorticoid receptor) to the promoter region of a gene in order to regulate gene expression. This process is modulated by the presence of tissue-specific co-regulators.96
In the pituitary corticotrope, the glucocorticoid receptor mediates inhibition of the POMC gene. In the rat POMC gene, there are four sites through which glucocorticoid action is mediated, although only those at −63 and between −480 and −320 are needed in vivo.97 This latter glucocorticoid-regulated element is required to interact for the full glucocorticoid repression of pituitary POMC expression to be manifested. The −63 negative glucocorticoid-regulated element overlaps the putative COUP (chicken albumin upstream promoter) box98 and the proximal Nur response element.99 It has been suggested that the inhibitory effect of glucocorticoids on POMC transcription may occur by displacement of a stimulatory factor such as Nurr 77.99,100 In the mouse corticotrope cell line, AtT20, the Nurr77-mediated actions of CRH are antagonized by glucocorticoids. The mechanism of glucocorticoid transcriptional repression through this region of the POMC promoter involves the co-repressor HDAC2 and the Swi/Snf chromatin-remodeling protein Brg1 in the modulation of the recruitment of GR to the Nur77-bound NurRE site.13,101 The loss of nuclear expression of Brg1 or HDAC2 has been associated with 50% of glucocorticoid-resistant human and dog corticotrope adenomas.13
The importance of co-regulators in glucocorticoid receptor-mediated regulation of POMC transcription is demonstrated by the inhibition of pituitary POMC transcript levels in mice with a deletion of SRC-1, a glucocorticoid receptor co-activator.102 However, this inhibition of POMC may be through SRC-1 interaction with other transcription factors that affect POMC transcription.
Stimulation of the POMC Gene by Arginine Vasopressin: A number of other hypothalamic factors act on the pituitary corticotrope to influence POMC expression; however, their modes of action are less well defined. In particular, arginine vasopressin (AVP) augments the effect of CRH and can act independently, though rather weakly, to stimulate POMC expression.32,103–105 AVP acts on corticotropes via V1b receptors, resulting in the activation of the protein kinase C pathway and leading to a “cross-talk” interaction with the cAMP/protein kinase A pathway activated by binding of CRH to CRH1 receptors.106
Leukemia Inhibitory Factor Stimulation of the POMC Gene: A number of lines of evidence point to intrapituitary factors as important modulators of corticotrope function. One such factor is the proinflammatory cytokine, leukemia inhibitory factor (LIF). This factor has been shown to stimulate the POMC gene through STAT-3 at a response element that overlaps with the −166-nucleotide CRH response element, although this site does not directly bind STAT transcription factors.85,107,108 However, a functional STAT1-3 binding site was identified in the distal region of the POMC promoter,108 and this region has been shown to mediate LIF-CRH synergy through a mechanism involving synergy with the NurRE.109
Recently an interaction between LIF and glucocorticoids has been demonstrated that reduces the repressive properties of GR on POMC expression. This may occur by the loss of a co-repressor from GR tethered to Nurr77 at the NurRE.110 Other modulators of LIF effects on POMC transcription include CCAAT/enhancer–binding protein β (C/EBPβ) and glial cell–derived neurotrophic factor (GDNF)-inducible factor (GIF).111
Regulation of the POMC Gene in Other Tissues
POMC expression can occur in a wide range of nonpituitary tissues, as described earlier, although in many of these extrapituitary tissues, the shorter POMC transcript predominates, leading to extremely low levels of protein.19 However, there are several tissues where there is significant POMC expression, and subtle differences occur in regulation compared to the pituitary.
Brain: POMC is expressed mainly in the arcuate nucleus of the hypothalamus, although it is also expressed at lower levels in the hippocampus and cortex.112,113 In the arcuate nucleus, POMC plays a major role in regulating food intake and energy balance.114 Its importance is highlighted by children with mutations in the POMC gene who are obese.115 POMC is regulated by leptin, insulin, and glucose to generate an anorexigenic effect116,117 (Fig. 3-6). Fasting in rats causes a decrease in both POMC and ACTH peptides.118
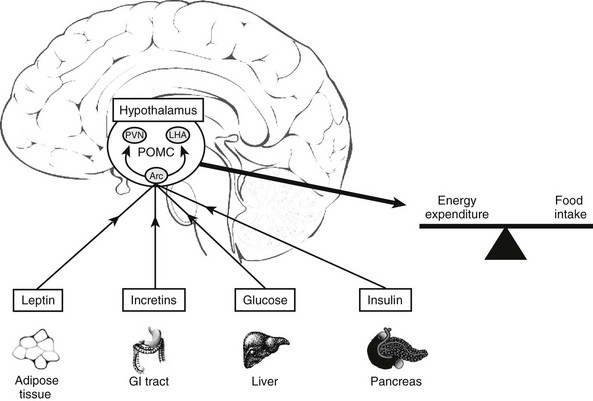
FIGURE 3-6 Factors that influence POMC expression in the arcuate nucleus (Arc) of the hypothalamus. PVN, Paraventricular nucleus; LHA, lateral hypothalamus.
Surprisingly, POMC expression is up-regulated by glucocorticoids in the hypothalamus. Adrenalectomized rats have a marked decrease in POMC mRNA, an effect that is completely reversed by glucocorticoid treatment.119,120
Skin: POMC is expressed in several components of the skin, including melanocytes, keratinocytes,121,122 and dermal microvascular endothelial cells123 (Fig. 3-7). The expression of POMC in the skin is regulated by both glucocorticoids and CRH but also by ultraviolet radiation.
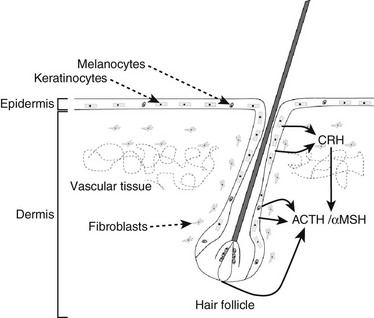
FIGURE 3-7 Components of the hypothalamic-pituitary axis present in the skin. Corticotropin-releasing hormone (CRH) released from skin and hair follicle cells can induce the secretion of adrenocorticotropic hormone (ACTH) and alpha melanocyte-stimulating hormone (αMSH) elsewhere in the skin.
The effect of glucocorticoids on POMC expression has been shown in mouse skin, where a down-regulation has been demonstrated which is coupled to hair follicle cycling.14,124 CRH expressed in skin cells up-regulates local POMC expression,14,64 and this is inhibited by glucocorticoids.125 POMC expression in the skin is increased by ultraviolet light,65,126 which also induces CRH production in human melanocytes, with subsequent stimulation of the CRH signaling pathway resulting in POMC expression.65 UV induction of POMC expression in mouse skin can be directly controlled by p53, and the mouse POMC promoter is stimulated by p53 in response to UV,127 although p53 is not the main or sole regulator of POMC expression.128
Regulation of the POMC Gene in Tumors
Corticotropin-Releasing Hormone Regulation of the POMC Gene in Tumors: In general, CRH stimulates POMC expression only in pituitary corticotrope tumors, but a few exceptions occur in ectopic tumors.132–134 However, it is likely that the increased ACTH stimulation of glucocorticoids will result in inhibition of the CRH gene. Therefore CRH may not be relevant in POMC-expressing tumors.
Glucocorticoid Regulation of the POMC Gene in Tumors: In pituitary corticotrope cells, expression of the POMC gene is repressed by glucocorticoids, and in pituitary corticotrope tumors, glucocorticoids are able to repress ACTH secretion. In contrast, in extrapituitary tumors, ACTH is characteristically resistant to glucocorticoids.135 This concept is the basis of the high-dose dexamethasone suppression test used to distinguish pituitary from ectopic sources of ACTH in Cushing’s syndrome. Most extrapituitary tumors are resistant to glucocorticoid inhibition of POMC expression, and this suggests that the mechanism of this glucocorticoid resistance is of importance.
A panel of human small cell lung carcinoma cell lines have been established as models of the ectopic ACTH syndrome.33,136 These cell lines express the POMC gene, and the glucocorticoid receptor has been found to be present, although at low levels.18,33,137 Significantly, all cell lines studied are resistant to glucocorticoid suppression. To determine whether glucocorticoid signaling was functional, a synthetic, glucocorticoid-responsive gene linked to a reporter gene was transfected into the cells. In contrast to the brisk induction of expression seen in control pituitary cells, none of the human small cell lung carcinoma cells responded to either natural or synthetic glucocorticoids.137 Thus resistance of the POMC gene to glucocorticoids is part of a global resistance of these malignant cells to glucocorticoid action. Expression of high concentrations of wild-type glucocorticoid receptor in the cells was found to be sufficient to restore glucocorticoid signaling.137 In two of the cell lines, mutations in the endogenous glucocorticoid receptor appeared to be the cause of the resistance,136,138 and in another cell line, altered co-regulator expression and recruitment by the glucocorticoid receptor was the cause of resistance.139
Because glucocorticoids can inhibit proliferation in some cell types and induce differentiation in others, it is possible that evasion of glucocorticoid signaling confers a survival advantage to the malignant cells. Indeed, when high levels of a wild-type glucocorticoid receptor were expressed in one of the resistant cell lines, it led to cell death by apoptosis,139a this suggests that the glucocorticoid resistance does confer a survival advantage, and because it also disinhibits POMC expression and ACTH secretion, these become biomarkers of a malignant phenotype.
Adrenocorticotropic Hormone and Related Peptides
Structure and Processing of POMC and Related Peptides
Many bioactive peptides are synthesized from large precursor molecules, and a number of techniques have been used to elucidate the structures of these peptides. Studies have used pulse chase analysis whereby labeled amino acids are incubated with cells to detect the labeled precursors and the peptides derived from them. Subsequently, sequence analysis and cDNA cloning have been important approaches to determine peptide structures. Discovery of the structure and biosynthesis of POMC and ACTH-related peptides and the differences between species is reviewed extensively by Eipper and Mains.140
POMC
In 1973, high-molecular-weight forms of ACTH were identified in human plasma,141 mouse pituitary cells,142,143 and human tumors,144 which led to predictions of the presence of a precursors of ACTH.
Expression of the POMC gene leads to synthesis of the preprohormone POMC. This protein undergoes proteolytic cleavage at dibasic amino acid residues, which generates a series of small molecules, including ACTH140 (Fig. 3-8). Processing of POMC to its constituent peptides varies in a tissue-specific fashion, in that both the nature of the processing and the degree of processing varies in different tissues. This results in different groups of peptides being secreted from different tissues, although the exact ratios of the constituent peptides and precursors are still not fully understood.
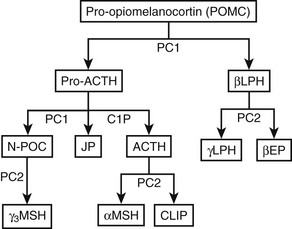
FIGURE 3-8 Processing of pro-opiomelanocortin (POMC). POMC is cleaved into pro-adrenocorticotropic hormone (pro-ACTH) and β-lipotropin (βLPH). Further processing of pro-ACTH yields ACTH, joining peptide, and N-pro-opiomelanocortin (N-POC), all of which are found in human plasma. Cleavage to smaller fragments occurs in a tissue- and species-specific manner. Shaded boxes represent peptides found in the human circulation. CLIP, Corticotropin-like intermediate lobe peptide; EP, endorphin; JP, joining peptide; LPH, lipotropin; MSH, melanocyte-stimulating hormone; PC, prohormone convertase.
ACTH
The ACTH peptide consists of 39 amino acids, is a single polypeptide chain, and has a molecular weight of 4.5 kD145 (see Fig. 3-8). The N-terminal 12 amino acids are highly conserved between species, thus reflecting the importance of this region for biological activity. In comparison with the human sequence, ACTH in other mammals has only one or two substitutions, which are in the region of amino acids 24 to 39. In birds, amphibians, and fish, although the N-terminal sequence is conserved, the ACTH sequence is more variable, particularly between amino acids 24 and 39. The melanocyte-stimulating hormone (MSH) sequence His-Phe-Arg-Trp is found at ACTH 6–9 and is termed α-MSH. Identical sequences are present in β-lipotropin (as β-MSH) and N-pro-opiomelanocortin (N-POC) (as γ-MSH). Given that these three forms of MSH bind different melanocortin receptors, it is thought that the surrounding amino acids influence their specific activity.
α-Melanocyte-Stimulating Hormone
α-MSH consists of ACTH 1–13 and is derived from ACTH 1–39 by proteolysis at the C-terminal, which is followed by C-terminal amidation and N-terminal acetylation. α-MSH is produced predominantly by melanotrope cells in the intermediate lobe of the pituitary, particularly in species such as the rat and mouse. The adult human pituitary does not have a distinct intermediate lobe, and therefore this is not a source of α-MSH in humans. In addition, α-MSH is not thought to be produced in the anterior lobe. Therefore, it is not clear whether α-MSH circulates in humans under normal circumstances.146,147
N-Pro-opiomelanocortin
Also called N-pro-opiomelanocortin, N-POC (see Fig. 3-8) comes from the N-terminal sequence of POMC, and in humans, it is a 76–amino acid peptide with an MSH sequence in the mid-region.148 The peptide has a tryptophan residue at the N terminus and two disulfide bridges linking cysteines 2 to 24 and 8 to 20, which are thought to be important for the sorting signal that directs POMC to the regulated pathway.149 N-POC can also undergo N-glycosylation at Asn65 and O-glycosylation at Thr45.
Joining Peptide
Joining peptide is found between N-POC and ACTH and is a 30–amino acid peptide, amidated at the C terminus. It was isolated from human pituitaries in 1981148 and has been shown to circulate in humans in the form of homodimers.150
β-Lipotropin
β-Lipotropin lies at the C terminus of POMC and can be cleaved to γ-lipotropin (which contains the β-MSH sequence at its C terminus) and β-endorphin (see Fig. 3-8). In the human anterior pituitary, cleavage appears to be limited, inasmuch as the main form of this peptide in the human circulation is β-lipotropin, with very little β-endorphin.151,152
The Processing Pathway and Processing Enzymes
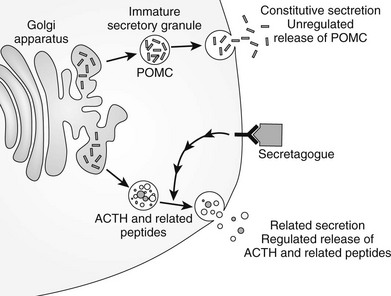
After translation of POMC mRNA into peptide, a series of processing stages are needed for release of the constituent peptides.112,114,130,153 The N-terminal signal sequence involved in movement of the peptide into the endoplasmic reticulum is no longer required and is removed at an early phase of posttranslational modification. Subsequently, POMC undergoes glycosylation and phosphorylation in the Golgi apparatus before transport to secretory vesicles, where it undergoes cleavage into its constituent peptides. The ACTH-related peptides are stored in dense core secretory granules and released from the cell on stimulation (e.g., by CRH), as in the stress response (Fig. 3-9). The prohormone, POMC, is found in the human circulation154 and could be released from the constitutive pathway perhaps as an “overflow” mechanism.
N-Glycosylation and Phosphorylation
These events occur in the Golgi apparatus before cleavage of the peptides. γ-MSH has the sequence Asn-X-Ser, which can be glycosylated on the Asn residue, and in mouse POMC, N-glycosylation of the CLIP sequence can occur. Some evidence indicates phosphorylation of serine 31 in ACTH, although the significance of this finding is unclear.155
Processing Enzymes: POMC is cleaved to its constituent peptides by limited proteolysis at pairs of basic amino acids, primarily Lys-Arg and Arg-Arg. The mammalian convertases responsible for this endoproteolytic cleavage are precursor converting enzymes from the subtilisin/Kex2 serine proteases, which include furin, a protease known to cleave peptides in the constitutive pathway of secretion.156 Prohormone convertase 1, or PC1 (also called PC3),157 cleaves POMC preferentially at pairs of basic residues and produces ACTH, β-lipotropin, N-POC, and joining peptide in the anterior pituitary. Although cleavage can begin in the Golgi apparatus, it continues in secretory vesicles. PC1 can be regulated in a manner similar to POMC in that PC1 mRNA is up-regulated by CRH and decreased by glucocorticoids in mouse AtT20 cells.158
ACTH 1–39 can be further cleaved by PC2 to produce ACTH 1–17 and CLIP. PC2 cleaves at different pairs of basic residues to PC1 to produce the smaller peptides159 (see Fig. 3-8). PC2 is not expressed in the human anterior pituitary but is found in the neurointermediate lobe, hypothalamus, and skin. This selective expression explains the tissue-specific presence of the MSH peptides and β-endorphin. PC2 can only cleave peptides within secretory vesicles, and therefore the MSH peptides are only produced intracellularly.
Cleavage by PC2 generates ACTH 1–17, which then has amino acids removed from the C terminus by carboxypeptidase E to produce ACTH 1–13. Subsequently, α-amidation is catalyzed by peptidylglycine α-amidating monoxygenase, an enzyme that has multiple molecular forms, and acetylation occurs by the action of specific acetyltransferases.112 These posttranslational modifications yield α-MSH (see Fig. 3-8).
Given that POMC can be released intact from cells, then cleavage by extracellular peptidases is theoretically possible in some circumstances. This phenomenon has been recently demonstrated by the extracellular peptidases angiotensin-converting enzyme and neprilysin derived from dermal microvascular endothelial cells.160
Processing in Different Tissues
POMC processing varies depending on the species and the tissue. Although POMC is expressed primarily in the pituitary, POMC mRNA has been detected in many extrapituitary tissues.130 However, such detection does not provide evidence that the peptides are synthesized or secreted there. POMC derived from the pituitary and placenta has been shown to be released into maternal blood.129,154 Whether the POMC peptides produced in other extrapituitary tissues reach the circulation is debatable, and it is more likely that they act in a paracrine role.
Anterior Pituitary
In the human anterior pituitary, POMC is cleaved to give pro-ACTH, which is then cleaved to ACTH, N-POC, and joining peptide (see Fig. 3-8). Interestingly, the ACTH precursors, POMC and pro-ACTH, are found in the human circulation with ACTH, N-POC, joining peptide, and β-lipotropin.154 That very little β-endorphin appears to be present indicates that processing of β-lipotropin is minimal (Fig. 3-10). However, reports can be confounded by the fact that in some β-endorphin assays, the antibodies also detect β-lipotropin. In the rat and sheep anterior pituitary, some ACTH is also processed to des-acetyl-α-MSH and α-MSH.112
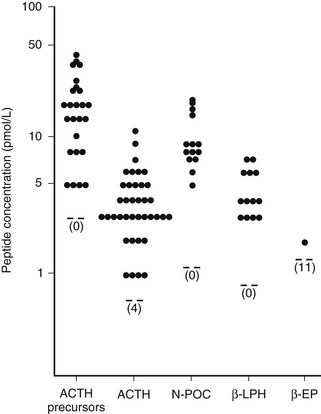
FIGURE 3-10 Concentrations of adrenocorticotropic hormone (ACTH) precursors and derived peptides in the circulation of normal subjects. EP, Endorphin; LPH, lipotropin; N-POC, N-pro-opiomelanocortin. (From Gibson S, Crosby SR, Stewart MF et al: Differential release of pro-opiomelanocortin-derived peptides from the human pituitary: Evidence from a panel of two-site immunoradiometric assays. J Clin Endocrinol Metab 78:835–841, 1994.)
Studies in the mouse pituitary tumor cell line, AtT20, suggest that cleavage is sequential, starting with the C terminus of ACTH.140 However, the same pair of basic amino acids is found between ACTH and β-lipotropin, joining peptide and ACTH, ACTH 1–16 and ACTH 17–39, and γ-lipotropin and β-endorphin. Therefore, the adjacent amino acids and peptide folding must influence the sequential processing.
Hypothalamus
POMC is produced primarily in the neurons of the hypothalamic arcuate nucleus where the peptides are central to regulation of food intake and energy balance114 (see Fig. 3-6). POMC is also expressed in the median eminence and ventromedial border of the third ventricle and much smaller amounts in the tractus solitarius. Processing is different from the anterior pituitary, in that smaller peptides characteristic of the neurointermediate lobe are produced.161 However, most of the studies are limited to the rat hypothalamus. In these extracts, high-performance liquid chromatographic separation of peptides suggests that ACTH is processed to CLIP and that des-acetyl-α-MSH is detected rather than α-MSH, thus indicating that N-terminal acetylation is limited. β-Endorphin 1–31 is found in the rat hypothalamus, again suggesting more extensive processing.114,112,162 PC1 and PC2 expression in the hypothalamus are increased by leptin and further regulated by the transcription factor Nescient Helix-Loop-Helix (Nhlh)-2, suggesting possible physiologic control of POMC peptide function at the level of peptide processing.163
POMC has also been detected in cerebrospinal fluid (CSF), although whether it originates from the pituitary or hypothalamus is uncertain. Evidence from changes in precursors and ACTH in rat CSF in relation to food intake and obesity suggest that they originate from the hypothalamus.118 In human CSF, the POMC precursor peptide has been shown to occur at high concentrations and predominates over ACTH when molar ratios are compared.164 However, several of the POMC peptides can be detected in CSF.165,166
Other Tissues
POMC peptides have been detected in the thyroid, pancreas, gastrointestinal tract, placenta, testis, ovary, adrenal gland, and immune system.112 However, in comparison to the pituitary, other tissues produce only low levels of POMC peptides.
POMC peptides are also produced in the skin (see Fig. 3-7). The first peptide to be detected, α-MSH, was found, by immunostaining, to predominate in human melanocytes, but ACTH has also been detected in human keratinocytes.167 A role for POMC peptides in hair pigmentation is also suggested by two patients with inherited mutations in POMC that prevented synthesis of the ACTH/α-MSH region; both patients had red hair pigmentation.11 The presence of PC1 and PC2 has been demonstrated in melanocytes and keratinocytes, along with functional POMC processing.63,168 The possibility of extracellular peptide processing of POMC by other peptidases has been demonstrated in dermal cells.160
Processing of POMC in the placenta results in significant amounts of POMC secretion along with ACTH, β-LPH, α-MSH, and β-endorphin.22,129
Pituitary Tumors
In patients with pituitary-dependent Cushing’s syndrome, the processing of precursors to ACTH appears to be relatively normal as judged by the molar ratios of these peptides in plasma.152 However, the molar ratio of precursors to ACTH is much higher for corticotrope macroadenomas, thus suggesting that processing is impaired.169 In pituitary corticotrope adenomas not associated with Cushing’s syndrome, defective PC1 expression may result in an increase of secreted unprocessed POMC.170
Extrapituitary Tumors
Data on tumor extracts suggest that most extrapituitary tumors causing the ectopic ACTH syndrome do not process the prohormone efficiently. In an early study, analysis of tumor tissue from patients without clinical features of hormone excess identified a high-molecular-weight form of ACTH, and this purified material could be cleaved to mature ACTH (4.5 kD) by the action of trypsin. The ACTH immunoreactivity was found to have no biological activity and was assumed to be due to ACTH precursors.171
Evidence that processing is impaired in tumors from patients with the ectopic ACTH syndrome also comes from the elevated levels of ACTH precursors in plasma and the high ratio of precursors to ACTH.152,172 Identification of ACTH precursors predominating in the circulation of patients with clinically apparent Cushing’s syndrome suggests that these precursors may have some activity at the ACTH receptor or are processed at the adrenal. Most of these patients had clinically obvious small cell carcinoma of the lung. However, patients with highly differentiated, slowly growing tumors, typically bronchial carcinoids, have lower but nevertheless elevated levels of ACTH precursors.173 CLIP has also been detected in four tumor extracts from patients with carcinoid tumors,174 thus suggesting that some tumors may process POMC in the manner of the neurointermediate lobe. It is not yet clear whether the same tumors give rise to increased precursors and smaller fragments or whether processing varies in different tumors.
Biological Activity Of ACTH-Related Peptides
The major role of ACTH is to stimulate steroidogenesis in the adrenal cortex, which results in the synthesis and release of cortisol in humans and corticosterone in rodents. In pathologic conditions, it is evident that ACTH can increase the production of adrenal androgens and aldosterone; however, under physiologic situations these pathways are regulated by other factors. Long-term overexpression of ACTH can cause adrenal cell proliferation,175,176 although peptides from the N-terminal of POMC have also been implicated in this process. ACTH is thought to have a role in adrenal cortical development,177,178 particularly since ACTH replacement in POMC knockout mice causes normal adrenal development.179
In situations with prolonged ACTH excess such as Nelson’s syndrome, Addison’s disease, and ectopic ACTH syndrome, skin pigmentation can occur and is thought to be due to ACTH binding through its MSH sequence to melanocortin receptors in the skin, although whether the skin pigmentation results from cleavage of ACTH to MSH peptides is unclear. ACTH receptors are also present on human mononuclear leukocytes and have been identified on other rat and mouse immune cells, which suggests that ACTH may have a role in immune function.130
ACTH Receptors and Signaling
ACTH binds to the melanocortin-2 receptor (MC2R),180 which has been identified in human adrenal glands,181,182 although in rat and ovine adrenocortical cells, there is a suggestion that low-affinity ACTH binding sites are also present. Binding of ACTH to human receptors requires calcium181 and occurs with a Kd of approximately 2.0 nmol/L. However, ACTH at 0.01 nmol/L causes maximal steroidogenesis, and therefore only a small number of the predicted 3500 sites per cell need to be occupied to achieve this activity. ACTH 1–16 is the minimal peptide required for binding to the human MC2R and signaling; the presence of a broad binding pocket in human MC2R, utilizing both conserved and unique amino acid residues, may be the reason why α-MSH was not able to bind hMC2R.183
The ACTH receptor is a member of the melanocortin receptor family, which all have similar seven-membrane-spanning domains and are G-protein coupled.182 On binding to its receptor, ACTH stimulates cAMP production,181 and cAMP in turn stimulates a cAMP-dependent protein kinase that activates the steroidogenic pathway. Calcium is also involved in ACTH stimulation of cAMP in human adrenal cells.
ACTH Effects on the Adrenal
ACTH acts at a number of levels to increase cortisol production. On binding to its receptor, it stimulates lipoprotein uptake, activates hydrolysis of cholesterol, and increases transport of cholesterol to mitochondria. Importantly, ACTH also regulates cholesterol side-chain cleavage, which is the rate-limiting step in steroidogenesis and results in the production of pregnenolone. This activity takes place in the inner membrane of the mitochondria and is catalyzed by cytochrome P450 side-chain cleavage enzyme.184
α-Melanocyte-Stimulating Peptide
In most mammals, α-MSH is produced in the melanotrope cells of the neurointermediate lobe, but because these cells are absent from the human pituitary, it is unlikely that this peptide has a role as a secreted peptide in humans. In mice, α-MSH acting at the MC-1 receptor causes changes in coat color, and in frogs, it affects skin pigmentation. It is also thought that locally produced α-MSH peptides stimulate melanogenesis in human skin.167 Recently α-MSH has been shown to have immunoregulatory functions in the human hair follicle185 as well as cytoprotective activity against UVB-induced apoptosis and DNA damage in the skin.65,186
α-MSH-related peptides produced in the arcuate nucleus of the hypothalamus and acting at the MC-4 receptor in the paraventricular nucleus of the hypothalamus are important in the regulation of food intake and energy balance and are the principal mediators of the effects of leptin.161 The role of POMC peptides is evidenced by a number of inherited deletions in the POMC gene which are associated with obesity.11,115,187 Recently, antagonists of α-MSH function have been shown to increase food intake in mice when injected peripherally, thus demonstrating the importance of α-MSH in the regulation of appetite and energy balance.188
The antiinflammatory and immunomodulatory properties of α-MSH have resulted in the hypothesis that α-MSH and its cognate receptors might present potential antiinflammatory treatment options.189,190
N-Pro-opiomelanocortin and Joining Peptide
N-POC has been reported to potentiate ACTH-induced steroidogenesis in human and rat adrenocortical cells, and it is thought that the γ3-MSH region, from mid- to C-terminal of N-POC, is responsible for this activity. It has also been shown that N-POC 1–48 and not the γ3-MSH region stimulates adrenal growth after unilateral adrenalectomy in the rat.191 N-POC 1–28 has also been shown to decrease adrenal steroidogenesis in opposition to ACTH.192 Since N-POC circulates intact, it has been proposed that cleavage of N-POC occurs at the adrenal gland, and a serine protease capable of cleaving N-POC has been identified in the outer adrenal cortex.193 In addition, N-POC stimulates the release of aldosterone from human adrenal tumor cells.194,195
The role of joining peptide is unclear. It has been suggested that it is the adrenal androgen-stimulating hormone, but subsequently, several reports have shown that joining peptide lacks the ability to increase adrenal androgens.196
β-Lipotropin and β-Endorphin
Data are conflicting regarding whether β-endorphin circulates in human plasma.151,154 It may have a more important role when released locally in the brain because, when administered, it has opiate-like analgesic activity associated with the met-enkephalin sequence at its N terminus, and mice lacking β-endorphin exhibit absence of stress-induced analgesia.197 β-Endorphin has also been shown to affect sexual behavior and learning. In the skin, β-endorphin modifies human hair follicle physiology via its ability to up-regulate melanogenesis, dendricity, and proliferation in melanocytes.198
ACTH Precursors
It has proved difficult to get a clear indication of ACTH precursor bioactivity because of problems in obtaining pure preparations of the peptides and the limitations of available bioassays. POMC itself is thought to have relatively little biological activity,171 whereas pro-ACTH was shown to be equipotent with ACTH in a rat adrenal cell bioassay or 8% to 33% as potent in a cytochemical ACTH bioassay.199 Nothing is currently known about the binding of POMC and pro-ACTH to the ACTH receptor (MC2-R) and the other MSH receptors (MC3-R, MC5-R).200 However, at the MC4-R, the receptor for α-MSH in the brain, β-MSH and ACTH bind MC4-R with similar affinity to α-MSH, and POMC itself functions with low potency.201 Also, at the MC1-R which is considered to be the receptor for α-MSH in the skin, again ACTH and α-MSH have similar affinity, and POMC can function at low potency.63 The possible biological effect of POMC was examined in human pigment cells, where functional activity was shown but only at concentrations in excess of 10−7 M, considerably higher than the concentrations of POMC released from the cells (∼1 × 10−10 M).63 However, it is possible that POMC is degraded extracellularly to ACTH-like peptides.
Because ACTH precursors are present in the circulation at concentrations greater than those of ACTH,154 it would be valuable to examine the agonist/antagonist activity of the precursors at the other human melanocortin receptors. In patients with hyperpigmentation related to post-adrenalectomy Cushing’s disease, concentrations of both ACTH and ACTH precursors correlated with pigmentation scores.202 The interaction of ACTH precursor peptides with the recently cloned receptor MC4-R found exclusively in the brain may also be of interest, inasmuch as concentrations of ACTH precursors in CSF are 100-fold those of ACTH (414 versus 3.2 pmol/L).164
Some information regarding in vivo POMC bioactivity can be gained from clinical studies. If patients with the ectopic ACTH syndrome produce ACTH precursors in preference to ACTH,152 it must either have biological activity when present at very high levels or be cleaved to ACTH at the level of the adrenal as previously suggested.191
Factors Regulating Secretion of ACTH And Related Peptides
Glucocorticoids exert a classic feedback inhibitory effect on the production of CRH and ACTH (Fig. 3-11). Therefore, ACTH stimulation of cortisol release from the adrenal directly determines the concentration of cortisol feeding back to inhibit the ACTH release from the pituitary. However, there is also regeneration of cortisol from cortisone by the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD1) in tissues such as the liver and adipose,203–205 and this will influence circulating cortisol concentrations, particularly in conditions such as obesity.
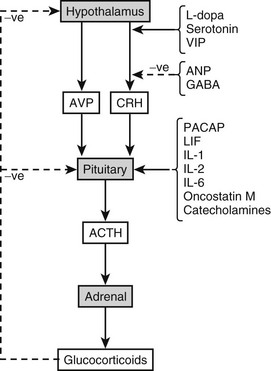
FIGURE 3-11 Factors regulating pituitary secretion of adrenocorticotropic hormone (ACTH)-related peptides. ANP, Atrial natriuretic peptide; AVP, arginine-vasopressin; CRH, corticotropin-releasing hormone; GABA, γ-aminobutyric acid; LIF, leukemia inhibitory factor; PACAP, pituitary adenylate cyclase-activating polypeptide; VIP, vasoactive intestinal polypeptide.
Fast Glucocorticoid Feedback: Fast feedback occurs over minutes and is linked to the rate of increase in glucocorticoid concentration. It was first identified in humans in 1979.206 An acute reduction in ACTH release takes place, but fast feedback has no impact on gene expression or peptide synthesis. It appears that the targets of glucocorticoid action are hypothalamic CRH secretion and direct action on the pituitary corticotrope to reduce ACTH release. Given the timeframe of the effect, this fast feedback glucocorticoid inhibition of ACTH is most likely to act on release of secretory granules. Experiments in rats, using antagonists of the glucocorticoid receptor and mineralocorticoid receptor, have shown that glucocorticoids may mediate fast feedback via the mineralocorticoid receptor.207,208
Intermediate Glucocorticoid Feedback: Intermediate feedback occurs over a few hours (typically maximal at 2 hours in vivo) and again appears to be due to acute inhibition of ACTH and CRH release, with no discernible effect on gene transcription or peptide synthesis. Annexin 1 is a key mediator of the inhibitory effects of glucocorticoids over this time frame, and because it is produced by the folliculo-stellate cells in the pituitary, it is probably a paracrine mediator of glucocorticoid action.209
Slow Glucocorticoid Feedback: The slow component is dependent on the concentration and time of exposure and occurs over days. POMC gene transcription and POMC peptide synthesis are reduced, but changes in CRH expression are uncertain, and glucocorticoids may also inhibit hypothalamic AVP levels. To effect inhibition of the POMC gene, glucocorticoids enter the cell, where they bind to the glucocorticoid receptor (GR) in the cytoplasm, which translocates to the nucleus, where it inhibits POMC gene transcription. A recent study in the rat, using a GR antagonist, has shown that GR modulates slow but not fast feedback of regulation of basal corticosterone secretion.210
Differential Glucocorticoid Regulation in Other Tissues: Glucocorticoids also act on POMC gene expression in the hypothalamus, where they influence food intake and energy balance. However, the evidence is controversial, since adrenalectomy (and therefore loss of glucocorticoids) has been shown to increase POMC mRNA in rat hypothalamus211 and to decrease POMC mRNA in the hypothalamus.119 Nevertheless, in rodents, adrenalectomy reverses many forms of obesity, suggesting removal of glucocorticoids increases POMC; alternatively, this could be explained by adrenalectomy affecting a number of other related pathways.211a
Corticotropin-Releasing Hormone
CRH is an important physiologic activator of ACTH. This is evidenced by mice with inactivating mutations in the CRH gene which die at birth with dysplastic lungs, preventable by prenatal maternal glucocorticoids.212 Thus CRH activation of ACTH is necessary to provide sufficient glucocorticoids for lung development. Both CRH and arginine vasopressin (AVP) are considered important for activation of pituitary ACTH, but CRH is a more potent secretagogue in rat and horse, whereas AVP is thought to be more potent in sheep. In all cases, there is a marked synergism, with CRH functioning permissively, while AVP is the main dynamic signal.
CRH stimulates ACTH secretion from dispersed pituitary cells in a sustained manner, initially causing the release of preformed peptide but simultaneously stimulating peptide synthesis (see Fig. 3-9). In humans, a biphasic response to exogenous CRH reflects these two mechanisms of action.213
The effects of CRH on the levels of ACTH precursors in the human circulation have been examined only during petrosal sinus sampling, which is used as a diagnostic test in patients with suspected Cushing’s syndrome. In this test, CRH is given intravenously, and ACTH peptides are measured in the petrosal sinuses draining the pituitary. In this situation, the increase in ACTH is much greater than the increase in precursors, which suggests that CRH is stimulating release of processed ACTH from secretory granules.154
Hypothalamic CRH is subject to regulation by multiple afferent signals and will in turn influence ACTH release. These signals include upregulation by catecholamines via β- and α1-adrenoceptors, serotonin (5-HT) acting via the 5-HT1A, 5-HT2A, and 5-HT2C receptors,214 acetylcholine acting through both muscarinic and nicotinic receptors, and the cytokines interleukin 1 (IL-1) and IL-6 possibly acting by generation of prostaglandins. In addition, CRH expression may be inhibited by glucocorticoids, catecholamines via α2-receptors, and γ-aminobutyric acid (GABA) released by neuronal input from the hippocampus and amygdala.
Vasopressin
Vasopressin (AVP) is synthesized in the same cells of the paraventricular nucleus of the hypothalamus as CRH (see Fig. 3-11). The two peptides are released concurrently from the median eminence into the hypophysial portal system. In addition, AVP reaches portal blood from the supraoptic nucleus. AVP exerts weak, direct stimulation on ACTH release but powerfully synergizes with CRH. In vivo evidence indicates a role for AVP in stress-induced ACTH secretion.215,216 In contrast to CRH, which acts via protein kinase A, AVP acts by stimulation of protein kinase C. AVP also increases the cAMP response to CRH in isolated pituicytes, which suggests multiple sites of interaction between the two signaling cascades.
Other Regulatory Factors
L-Dopa and serotonin both increase ACTH secretion by means of neuronal release into the paraventricular nucleus of the hypothalamus.217–219 Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) both enhance ACTH secretion, but while PACAP directly stimulates pituitary ACTH, VIP promotes release of CRH. The role of these two peptides is probably most relevant in regulating HPA responses to inflammatory and cold stressors.220
In contrast, GABA inhibits ACTH when released from hippocampal afferents to the hypothalamus by inhibiting release of CRH and AVP.221 Atrial natriuretic peptide (ANP) has been shown to decrease ACTH secretion and inhibit CRH gene expression. In comparison, opiate receptor agonists inhibit ACTH release, probably by effects at the hypothalamic or hippocampal level, although it has been reported that met-enkephalin can directly inhibit ACTH release at the level of the corticotrope. Oxytocin inhibits CRH stimulated ACTH secretion in humans, but in rats, oxytocin stimulates ACTH, probably by binding to AVP receptors.
Cytokines and Growth Factors
Cytokines are pleiotropic polypeptides released from immune cells in response to inflammation, infection, and tissue injury.222,223 Proinflammatory cytokines stimulate the HPA axis in vivo,224 and while some act via CRH, several cytokines, including interleukin 2 (IL-2), interferons, and the gp130 cytokine family (IL-6, leukemia inhibitory factor [LIF], oncostatin M) act at the pituitary.
Interleukin 1: IL-1 α and β are endogenous pyrogenic proteins induced by bacterial endotoxin. The two forms bind to the same receptor, the IL-1 receptor type 1, and display identical biological activities. IL-1β is released by several cell types, including activated macrophages, monocytes, and cells within the hypothalamus, where it can stimulate its own expression.225
The specifics of the action of IL-1 on ACTH release are controversial. In the intact rat, infusion of human IL-1 increased circulating levels of ACTH, but IL-1 acted at the hypothalamus by stimulating CRH release.226 However, primary cultures of rat pituitary cells responded to IL-1β by increasing secretion of ACTH,227 showing a direct effect can occur. In another study using primary rat pituitary cultures, no effects of acute IL-1 administration on POMC gene transcription or ACTH peptide release were observed. Interestingly, chronic treatment of these cultures with either IL-1α or IL-1β exerted weak induction of ACTH release with no effect on POMC mRNA accumulation.228 An explanation for these divergent results may be that IL-1 modulates the actions of other ACTH secretagogues, including catecholamines.
Interleukin 2: Expression of IL-2 mRNA and IL-2 receptor mRNA was detected in human corticotrope adenoma cells and in mouse pituitary AtT20 cells.229
IL-2 enhances POMC gene expression in the pituitary and ACTH secretion in AtT20 cells and primary rat pituitary cultures. IL-2, when administered to human subjects during cancer therapy trials, was found to increase circulating β-endorphin and ACTH levels,230,231 thus demonstrating a role for IL-2 in activating the HPA axis in vivo.
Interleukin 6: IL-6 is synthesized and secreted by bovine pituitary folliculostellate cells, which do not express pituitary trophic hormones or their precursors in vitro.232 In addition, cultured primary rat pituitary cells release IL-6 relatively abundantly,233 and IL-6 is synthesized by both normal human and neoplastic anterior pituitary tissue.234–236
In vivo, IL-6 is a potent stimulus of the HPA axis in humans and probably acts at the hypothalamus to stimulate AVP release and subsequent ACTH induction.237 Because IL-6 is also present in the circulation, especially during inflammatory stress, the relative importance of locally derived versus systemically available IL-6 in pituitary function remains to be determined.238
Leukemia Inhibitory Factor: LIF regulates differentiation and development of pituitary corticotropes during ontogenesis and is involved in the HPA response to inflammation.239 The peptide is produced by human pituitary cells, and its receptors (LIF-Rs) are present in murine AtT20 pituicytes and human fetal corticotropes.240 Pituitary LIF-R mRNA is induced by lipopolysaccharide (LPS) in vivo, although the changes were less pronounced than those observed for LIF mRNA.241
LIF acts principally on the pituitary corticotrope, potently inducing POMC gene transcription and enhancing ACTH secretion.240,242,243 In addition, it potentiates the action of CRH to induce ACTH secretion in AtT20 cells242 and has been shown to reverse glucocorticoid-dependent repression of POMC expression.110
Studies of the HPA axis in LIF knockout mice revealed a defect in activation of the axis in response to stress. Circulating ACTH levels are attenuated after fasting in the knockout animals, and chronic replacement by LIF infusion restores HPA responses to levels seen in wild-type littermates.244 Interestingly, in mice with a double knockout of LIF and CRH, the POMC response to inflammation was robust and similar to wild-type animals. These animals had increased TNF-α, IL-1β, and IL-6, suggesting that increased central proinflammatory cytokines may compensate for the impaired HPA axis function caused by loss of CRH and LIF.239 A study in LIF-receptor knockout mice shows a decrease in POMC expression in the fetus, highlighting the importance of LIF signaling in HPA axis development.245
Integrated Control of ACTH Secretion
Three tiers of control subserve the regulation of ACTH secretion (see Fig. 3-11).
Tier I: Tier 1 consists of central signals from the brain and hypothalamus and includes the hypothalamic hormones, neurotransmitters, and brain peptides. These molecules traverse the portal venous system in classic endocrine fashion to impinge on their respective distal receptors located on the corticotrope cell surface. These highly differentiated receptors transduce their signals to the cell nucleus, thus determining biosynthesis and ultimate secretion of POMC peptides. The hypothalamic hormones also determine pituitary cell mitotic activity, and clinically, pathologic oversecretion of these hormones results in pituitary hyperplasia and adenoma formation.
Tier II: The second tier of pituitary control consists of an intrapituitary network of cytokines. These molecules provide highly specific unique signals to the pituicyte or an overlapping redundancy (e.g., interleukin regulation of ACTH). Furthermore, they may often synergize with hypothalamic hormones (e.g., LIF and CRH).
Tier III: The third tier of pituitary control comes from the peripheral target hormones such as glucocorticoids. Clinically, loss of negative feedback inhibition by glucocorticoid target hormones results in hypersecretion of ACTH, pituitary hyperplasia, and sometimes adenoma formation, as may be encountered in hypoadrenalism.
Differential Regulation of POMC and ACTH
The relative concentrations of POMC and ACTH in the circulation will depend not only on regulatory mechanisms influencing expression of the POMC gene but also on precursor processing and mechanisms of secretion from the corticotrope cells. Evidence from studies with the mouse corticotrope adenoma cell line AtT20 suggests that in the absence of stimulation, corticotrope cells release newly synthesized POMC.246 Therefore, the levels of POMC and ACTH in the circulation at any given time could well vary because of the differing regulatory mechanisms.
Mechanisms Regulating Secretion of ACTH and Related Peptides
The many factors which regulate secretion of ACTH and related peptides are integrated into the mechanisms which underpin the regulatory processes (see Fig. 3-11). There is a marked circadian rhythm for ACTH, and underlying this is a pulsatile release process. However, it is clear that stress responses can be superimposed on these, as can the feedback regulation of cortisol, which down-regulates ACTH secretion. The details of this feedback inhibition are described in the section on glucocorticoid regulation. A number of studies describe the factors involved in the stress response and feedback regulation, but much less is known about circadian control and pulsatile secretion.
Circadian Rhythmicity
The circadian rhythm for ACTH originates from a primary “clock” which is located in the suprachiasmatic nucleus. Neuronal afferents from this nucleus feed into the paraventricular nucleus of the hypothalamus and regulate CRH expression. ACTH secretion is pulsatile, and the circadian rhythm is generated by variation in the amplitude of the pulses rather than variation in pulse frequency. Therefore, the amplitude of ACTH pulses during peak secretion is fourfold higher than during the ACTH nadir. Peak levels of ACTH, and concordantly cortisol, are reached at 6 am, decline during the day to 4 pm, and then further decline to a nadir between 11 pm and 3 am (Fig. 3-12). The 6 am peak is reached after an abrupt increase in ACTH secretion. Although all the circulating POMC peptides show a diurnal variation and peak at the same time, their decline occurs at different rates, probably conferred by different circulatory half-lives and/or variation in extrapituitary processing.
Pulsatility
ACTH is secreted in a pulsatile manner which is reflected in the pulsatile release of cortisol. However, the analysis of this is very much dependent on the sampling techniques. In humans, studies have shown 12 or 40 pulses per 24 hours, depending on the sampling frequency.247,248 There are fewer pulses during the nadir of ACTH secretion, and there are more pulses, with greater peak amplitude and higher mean level, in males.249 The pulsatility may be a mechanism for overcoming desensitization of the ACTH receptor and may reflect pulsatile release of CRH.
The Stress Response
In response to stress, peripheral and central signals are integrated by the pituitary to modulate adrenal glucocorticoid production. Several lines of evidence suggest a unifying hypothesis linking hypothalamic releasing factors, activation of peripheral cytokine cascades, and intrapituitary cytokine expression with pituitary-mediated modulation of the systemic inflammatory response.247,250
An acute septic insult provokes a local inflammatory response, with coordinated and sequential activation of a series of proinflammatory cytokines238,251,252 and neural and bacterial toxin signals that activate the HPA axis.253 Initially, peripheral activation of local and distal tumor necrosis factor (TNF) expression is followed by IL-1, IL-6, and LIF.238 A number of the proinflammatory cytokines exert most if not all their activities at the hypothalamus, notably IL-6 acting on hypothalamic AVP237 and IL-1 and TNF acting on CRH.226 Hypothalamic LIF mRNA is also up-regulated by lipopolysaccharide (LPS) treatment, a well-recognized model of gram-negative septic shock.241
Other cytokines clearly act at the pituitary, notably LIF.242,254 The pituitary is also a site of de novo cytokine synthesis, and thus in addition to the circulating, peripherally derived cytokines, an intrapituitary network of cytokines is established in the acute phase of septic shock. IL-1β and LIF are up-regulated by LPS,241,255 and macrophage migration inhibitory factor is acutely released from pituicytes in vitro and in vivo in response to LPS.256 In addition, intrapituitary IL-6 is up-regulated by IL-1.238,257
The pituitary response to septic shock involves cytokines such as IL-6 and LIF, which limit the inflammatory response. These cytokines cause activation of the HPA axis and increased glucocorticoid production, thus limiting the extent of the inflammatory response and protecting against lethality. The increase in intrapituitary LIF stimulates POMC expression and strongly potentiates CRH action on the corticotrope.242,244 The key role of HPA activation in limiting the lethal effects of unrestrained activation of proinflammatory cytokine cascades is underscored by the poor performance of the CRH knockout mouse exposed to endotoxin.258 IL-1, TNF, and IL-6 have also been shown by some but not all studies to exert direct effects on pituitary ACTH secretion.227,259,260
A further level of action of the cytokines is to antagonize the negative feedback loop of adrenal glucocorticoids on hypothalamic CRH expression and pituitary POMC secretion.253 The pattern of acute proinflammatory cytokines induced by septic shock opposes effective glucocorticoid signaling,261,262 in part by activation of the NF-κB nuclear transcription factor, which inhibits glucocorticoid receptor action.263–265
Stress-associated disorders, such as melancholic depression, are characterized by persistent activation of the HPA axis. In this situation, there are multiple feedback loops which activate central CRH pathways, including down-regulation of the glucocorticoid receptor which prevents the normal negative feedback on the HPA axis, resulting in the vicious circle of continued activation of the HPA.266
Measurement of ACTH and Related Peptides
ACTH was one of the first peptides to be measured by radioimmunoassay and presented a significant challenge because of the difficulty in generating high-affinity antisera and in labeling ACTH. The development of sensitive immunoradiometric assays for ACTH has improved the reliability of ACTH measurement.173,267 The assays are based on a labeled monoclonal antibody that usually binds the N-terminal region of ACTH and a solid-phase antibody that recognizes a different sequence in ACTH. Because binding of both antibodies is required to generate a signal, the assay does not recognize α-MSH or CLIP. However, it is not always clear whether current ACTH assays recognize the ACTH precursors.
However, we must recognize that in some clinical situations, the use of an assay that is highly specific for ACTH 1–39 may be insufficient or misleading. In one patient with the ectopic ACTH syndrome, shown by chromatography to be producing high-molecular-weight ACTH precursors, the ACTH concentration was very low when measured by immunoradiometric assay.267 To ensure that patients with the ectopic ACTH syndrome are flagged by an ACTH assay, it is important that the ACTH precursors have a high degree of cross-reactivity in the ACTH assay or that a separate specific assay for ACTH precursors be available.
Detection of ACTH precursors in plasma was first demonstrated in normal subjects after stimulation with metyrapone,268 and it was later observed after insulin-induced hypoglycemia.269 However, complex chromatographic techniques were required to separate ACTH precursors from ACTH. Clearly, this approach cannot be used for large numbers of patient samples and would not provide a quantitative assessment of the concentrations of ACTH precursors in plasma.
Direct measurement of ACTH precursors was made possible by the development of a two-site immunoradiometric assay for the ACTH precursors POMC and pro-ACTH.270 The assay is based on a labeled monoclonal antibody that binds within the ACTH region of POMC and a solid-phase antibody that recognizes N-POC (see Fig. 3-8). Because binding of both antibodies is required to generate a signal, the assay does not detect ACTH. With this assay, the concentrations of ACTH precursors in normal subjects were found to be 5 to 40 pmol/L, which is equivalent to or greater than the concentrations of ACTH, N-POC, β-lipotropin, and β-endorphin.154 Measurement of ACTH precursors in patients with ectopic ACTH syndrome has indicated that the precursors are present at much higher concentrations than is ACTH.172,173 A similar approach has been used to measure POMC in aggressive ACTH-secreting tumors.271
Other POMC-Derived Peptides
The development of radioimmunoassays and/or immunoradiometric assays for N-POC, γ-MSH, α-MSH, β-lipotropin, and β-endorphin has proved extremely valuable in understanding the production and action of POMC peptides. The development of specific immunometric assays that distinguish circulating levels of β-lipotropin and β-endorphin has shown that β-lipotropin is the main form in human plasma and that relatively little β-endorphin is secreted.151 Nevertheless, questions relating to the relative molar ratios of the family of POMC peptides are still unanswered.
References
1. Smith, PE. Hypophysectomy and a replacement therapy in the rat. American Journal of Anatomy. 1930;45:205–273.
2. Bell, PH. Purification and structure of beta-corticotropin. J Am Chem Soc. 1954;76:5565–5567.
3. Li, CH. Lipotropin, a new active peptide from pituitary glands. Nature. 1964;201:924.
4. Li, CH. Isolation, characterization and opiate activity of beta-endorphin from human pituitary glands. Biochem Biophys Res Commun. 1976;72:1542–1547.
5. Eipper, BA, Mains, RE. Analysis of the common precursor to corticotropin and endorphin. J Biol Chem. 1978;253:5732–5744.
6. Inoue, A, Kita, T, Nakamura, M, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature. 1979;278:423–427.
7. Vale, W, Spiess, J, Rivier, C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta endorphin. Science. 1981;213:1394–1397.
8. Mountjoy, K, Robbins, L, Mortrud, M, et al. The cloning of a family of genes the encode the melanocortin receptors. Science. 1992;257:1248–1251.
9. Lefkowitz, RJ, Roth, J, Pricer, W, et al. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970;65:745–752.
10. Jackson, RS, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306.
11. Krude, H, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157.
12. de Souza, FS, et al. Identification of neuronal enhancers of the proopiomelanocortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol Cell Biol. 2005;25:3076–3086.
13. Bilodeau, S, et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 2006;20:2871–2886.
14. Slominski, A, Wortsman, J, Tuckey, RC, et al. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149.
15. Cochet, M, Chang, ACY, Cohen, SN. Characterisation of the structural gene and putative 5′ regulatory sequences for human proopiomelanocortin. Nature. 1982;297:335–339.
16. Takahashi, H, Teranishi, Y, Nakanishi, S, et al. Isolation and structural organisation of the human corticotropin-beta-lipotropin precursor gene. FEBS Lett. 1981;135:97–102.
17. Whitfield, PL, Shire, J. The human proopiomelanocortin gene: organisation sequence and interspersion with repetitive DNA. DNA. 1982;1:133–143.
18. White, A, Clark, AJ, Stewart, MF. The synthesis of ACTH and related peptides by tumours. Baillieres Clin Endocrinol Metab. 1990;4:1–27.
19. Lacaze-Masmonteil, T, De Keyzer, Y, Luton, JP, et al. Characterization of proopiomelanocortin transcripts in human nonpituitary tissues. Proc Natl Acad Sci USA. 1987;84:7261–7265.
20. Jeannotte, L, Burbach, JP, Drouin, J. Unusual proopiomelanocortin ribonucleic acids in extrapituitary tissues: intronless transcripts in testes and long poly(A) tails in hypothalamus. Mol Endocrinol. 1987;1:749–757.
21. Nakai, Y, Nakao, K. Adrenocorticotropic hormone and related peptides in human tissue. In: Black PM, et al, eds. Secretory Tumours of the Pituitary Gland. New York: Raven; 1984:227–243.
22. Grigorakis, SI, Anastasiou, E, Dai, K, et al. Three mRNA transcripts of the proopiomelanocortin gene in human placenta at term. Eur J Endocrinol. 2000;142:533–536.
23. Chang, AC, Israel, A, Gazdar, A, et al. Initiation of pro-opiomelanocortin mRNA from a normally quiescent promoter in a human small cell lung cancer cell line. Gene. 1989;84:115–126.
24. Jeannotte, L, Trifiro, MA, Plante, RK, et al. Tissue-specific activity of the pro-opiomelanocortin gene promoter. Mol Cell Biol. 1987;7:4058–4064.
25. Jingami, H, Nakanishi, S, Numa, S. Tissue distribution of messenger RNAs coding for opioid peptide precursors and related RNA. Eur J Biochem. 1984;142:441–447.
26. Clark, AJ, Lavender, PM, Coates, P, et al. In vitro and in vivo analysis of the processing and fate of the peptide products of the short proopiomelanocortin mRNA. Mol Endocrinol. 1990;4:1737–1743.
27. Newell-Price, J. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing’s syndrome and beyond. J Endocrinol. 2003;177:365–372.
28. Kraus, J, Buchfelder, M, Hollt, V. Regulatory elements of the human proopiomelanocortin gene promoter. DNA Cell Biol. 1993;12:527–536.
29. Liu, B, Mortrud, M, Low, MJ. DNA elements with AT-rich core sequences direct pituitary cell-specific expression of the pro-opiomelanocortin gene in transgenic mice. Biochem J. 1995;312(Pt 3):827–832.
30. Liu, B, Hammer, GD, Rubinstein, M, et al. Identification of DNA elements cooperatively activating proopiomelanocortin gene expression in the pituitary glands of transgenic mice. Mol Cell Biol. 1992;12:3978–3990.
31. Young, JI, et al. Authentic cell-specific and developmentally regulated expression of pro-opiomelanocortin genomic fragments in hypothalamic and hindbrain neurons of transgenic mice. J Neurosci. 1998;18:6631–6640.
32. Lundblad, JR, Roberts, JL. Regulation of proopiomelanocortin gene expression in pituitary. Endocr Rev. 1988;9:135–158.
33. White, A, Clark, AJ. The cellular and molecular basis of the ectopic ACTH syndrome. Clin Endocrinol (Oxf). 1993;39:131–141.
34. Nishida, Y, Yoshioka, M, St-Amand, J. The top 10 most abundant transcripts are sufficient to characterize the organs functional specificity: evidences from the cortex, hypothalamus and pituitary gland. Gene. 2005;344:133–141.
35. Mauri, A, et al. Alpha-melanocyte-stimulating hormone during human perinatal life. J Clin Endocrinol Metab. 1993;77:113–117.
36. Rosa, PA, Policastro, P, Herbert, E. A cellular basis for the differences in regulation of synthesis and secretion of ACTH/endorphin peptides in anterior and intermediate lobes of the pituitary. J Exp Biol. 1980;89:215–237.
37. Schachter, BS, Johnson, LK, Baxter, JD, et al. Differential regulation by glucocorticoids of proopiomelanocortin mRNA levels in the anterior and intermediate lobes of the rat pituitary. Endocrinology. 1982;110:1442–1444.
38. Roberts, JL, Lundblad, JR, Eberwine, JH. Hormonal regulation of POMC gene expression in the pituitary. Ann N Y Acad Sci. 1988;512:275–285.
39. Hammer, GD, Fairchild-Huntress, V, Low, MJ. Pituitary specific and hormonally regulated gene expression directed by the rat proopiomelanocortin promoter in transgenic mice. Mol Endocrinol. 1990;4:1689–1697.
40. Therrien, M, Drouin, J. Cell-specific helix-loop-helix factor required for pituitary expression of the pro-opiomelanocortin gene. Mol Cell Biol. 1993;13:2342–2353.
41. Lamonerie, T, et al. Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev. 1996;10:1284–1295.
42. Suh, H, Gage, PJ, Drouin, J, et al. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337.
43. Drouin, J, Lamolet, B, Lamonerie, T, et al. The PTX family of homeodomain transcription factors during pituitary developments. Mol Cell Endocrinol. 1998;140:31–36.
44. Tremblay, JJ, Goodyer, CG, Drouin, J. Transcriptional properties of Ptx1 and Ptx2 isoforms. Neuroendocrinology. 2000;71:277–286.
45. Tremblay, JJ, Lanctot, C, Drouin, J. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol. 1998;12:428–441.
46. Lamolet, B, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859.
47. Maira, M, et al. The T-box factor Tpit recruits SRC/p160 co-activators and mediates hormone action. J Biol Chem. 2003;278:46523–46532.
48. Pulichino, AM, et al. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17:711–716.
49. Metherell, LA, et al. TPIT mutations are associated with early-onset, but not late-onset isolated ACTH deficiency. Eur J Endocrinol. 2004;151:463–465.
50. Vallette-Kasic, S, et al. Congenital isolated adrenocorticotropin deficiency: an underestimated cause of neonatal death, explained by TPIT gene mutations. J Clin Endocrinol Metab. 2005;90:1323–1331.
51. Vallette-Kasic, S, et al. The TPIT gene mutation M86R associated with isolated adrenocorticotropin deficiency interferes with protein: protein interactions. J Clin Endocrinol Metab. 2007;92:3991–3999.
52. Nudi, M, Ouimette, JF, Drouin, J. Bone morphogenic protein (Smad)-mediated repression of proopiomelanocortin transcription by interference with Pitx/Tpit activity. Mol Endocrinol. 2005;19:1329–1342.
53. Poulin, G, Turgeon, B, Drouin, J. NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol. 1997;17:6673–6682.
54. Lavoie, PL, Budry, L, Balsalobre, A, et al. Developmental dependence on NurRE and EboxNeuro for expression of pituitary proopiomelanocortin. Mol Endocrinol. 2008;22:1647–1657.
55. Liu, J, et al. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci U S A. 2001;98:8674–8679.
56. Buzzetti, R, McLoughlin, L, Lavender, PM, et al. Expression of pro-opiomelanocortin gene and quantification of adrenocorticotropic hormone-like immunoreactivity in human normal peripheral mononuclear cells and lymphoid and myeloid malignancies. J Clin Invest. 1989;83:733–737.
57. Chen, C-LC, Chang, AC, Krieger, DT, et al. Expression and regulation of pro-opiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology. 1986;118:2382–2389.
58. DeBold, CR, Menefee, JK, Nicholson, WE, et al. Proopiomelanocortin gene is expressed in many normal human tissues and in tumors not associated with ectopic adrenocorticotropin syndrome. Mol Endocrinol. 1988;2:862–870.
59. Millington, WR, Rosenthal, DW, Unal, CB, et al. Localization of pro-opiomelanocortin mRNA transcripts and peptide immunoreactivity in rat heart. Cardiovasc Res. 1999;43:107–116.
60. Gizang-Ginsberg, E, Wolgemuth, DJ. Localization of mRNAs in mouse testes by in situ hybridization: distribution of alpha-tubulin and developmental stage specificity of pro-opiomelanocortin transcripts. Dev Biol. 1985;111:293–305.
61. Andjelkov, N, et al. Detection of mRNA transcripts of truncated opiate precursor (POMC) in human cartilage. Cell Biochem Funct. 2006;24:229–235.
62. Ivell, R. The proopiomelanocortin gene is expressed as both full-length and 5′truncated transcripts in rodent Leydig cells. Reprod Fertil Dev. 1994;6:791–794.
63. Rousseau, K, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–1856.
64. Slominski, A, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248.
65. Zbytek, B, Wortsman, J, Slominski, A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–2547.
66. Morris, DG, et al. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol. 2005;153:143–151.
67. De Keyzer, Y, et al. Altered proopiomelanocortin gene expression in adrenocorticotropin-producing nonpituitary tumors. Comparative studies with corticotropic adenomas and normal pituitaries. J Clin Invest. 1985;76:1892–1898.
68. Hinton, DR, Hahn, JA, Weiss, MH, et al. Loss of Rb expression in an ACTH-secreting pituitary carcinoma. Cancer Lett. 1998 Apr 24;126(2):209–214.
69. Batsche, E, Moschopoulos, P, Desroches, J, et al. Retinoblastoma and the related pocket protein p107 act as coactivators of NeuroD1 to enhance gene transcription. J Biol Chem. 2005;280:16088–16095.
70. Batsche, E, Desroches, J, Bilodeau, S, et al. Rb enhances p160/SRC coactivator-dependent activity of nuclear receptors and hormone responsiveness. J Biol Chem. 2005;280:19746–19756.
71. De Keyzer, Y, Bertagna, X, Luton, JP, et al. Variable modes of proopiomelanocortin gene transcription in human tumors. Mol Endocrinol. 1989;3:215–223.
72. De Keyzer, Y, et al. Pro-opiomelanocortin gene expression in human phaeochromocytomas. J Mol Endocrinol. 1989;2:175–181.
73. Texier, PL, et al. Proopiomelanocortin gene expression in normal and tumoral human lung. J Clin Endocrinol Metab. 1991;73:414–420.
74. Picon, A, Bertagna, X, De Keyzer, Y. Analysis of the human proopiomelanocortin gene promoter in a small cell lung carcinoma cell line reveals an unusual role for E2F transcription factors. Oncogene. 1999;18:2627–2633.
75. Newell-Price, J, King, P, Clark, AJ. The CpG island promoter of the human proopiomelanocortin gene is methylated in nonexpressing normal tissue and tumors and represses expression. Mol Endocrinol. 2001;15:338–348.
76. Ye, L, et al. Hypomethylation in the promoter region of POMC gene correlates with ectopic overexpression in thymic carcinoids. J Endocrinol. 2005;185:337–343.
77. Birnberg, NC, Lissitsky, J-C, Hinman, M, et al. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci USA. 1983;80:6982–6986.
78. Kovalovsky, D, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638–1651.
79. Maira, M, Martens, C, Batsche, E, et al. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol. 2003;23:763–776.
80. Boutillier, AL, Sassone-Corsi, P, Loeffler, JP. The protooncogene c-fos is induced by corticotropin-releasing factor and stimulates proopiomelanocortin gene transcription in pituitary cells. Mol Endocrinol. 1991;5:1301–1310.
81. Jin, WD, et al. Characterization of a corticotropin-releasing hormone-responsive element in the rat proopiomelanocortin gene promoter and molecular cloning of its binding protein. Mol Endocrinol. 1994;8:1377–1388.
82. Boutillier, AL, Gaiddon, C, Lorang, D, et al. Transcriptional activation of the proopiomelanocortin gene by cyclic AMP-responsive element binding protein. Pituitary. 1998;1:33–43.
83. Autelitano, DJ. Stress-induced stimulation of pituitary POMC gene expression is associated with activation of transcription factor AP-1 in hypothalamus and pituitary. Brain Res Bull. 1998;45:75–82.
84. Boutillier, AL, et al. Corticotropin-releasing hormone stimulates proopiomelanocortin transcription by cFos-dependent and -independent pathways: characterization of an AP1 site in exon 1. Mol Endocrinol. 1995;9:745–755.
85. Bousquet, C, Ray, DW, Melmed, S. A common pro-opiomelanocortin-binding element mediates leukemia inhibitory factor and corticotropin-releasing hormone transcriptional synergy. J Biol Chem. 1997;272:10551–10557.
86. Becquet, D, Guillaumond, F, Bosler, O, et al. Long-term variations of AP-1 composition after CRH stimulation: consequence on POMC gene regulation. Mol Cell Endocrinol. 2001;175:93–100.
87. Murakami, I, Takeuchi, S, Kudo, T, et al. Corticotropin-releasing hormone or dexamethasone regulates rat proopiomelanocortin transcription through Tpit/Pitx-responsive element in its promoter. J Endocrinol. 2007;193:279–290.
88. Reichardt, HM, Schutz, G. Feedback control of glucocorticoid production is established during fetal development. Mol Med. 1996;2:735–744.
89. Svec, F. Interactions of glucocorticoids with the AtT-20 cell: effect on protein accumulation. Arch Biochem Biophys. 1985;240:184–190.
90. Itoi, K, Jiang, YQ, Iwasaki, Y, et al. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16:348–355.
91. Eberwine, JH, Jonassen, JA, Evinger, MJ, et al. Complex transcriptional regulation by glucocorticoids and corticotropin-releasing hormone of proopiomelanocortin gene expression in rat pituitary cultures. DNA. 1987;6:483–492.
92. Eberwine, JH, Roberts, JL. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem. 1984;259:2166–2170.
93. Fremeau, RT, Lundblad, JR, Pritchett, DB, et al. Regulation of pro-opiomelanocortin gene transcription in individual cell nuclei. Science. 1986;234:1265–1269.
94. Gagner, JP, Drouin, J. Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Mol Cell Endocrinol. 1985;40:25–32.
95. Israel, A, Cohen, SN. Hormonally mediated negative regulation of human pro-opiomelanocortin gene expression after transfection into mouse L-cells. Mol Cell Biol. 1985;5:2443–2453.
96. Stevens, A, et al. Dissociation of steroid receptor coactivator 1 and nuclear receptor corepressor recruitment to the human glucocorticoid receptor by modification of the ligand-receptor interface: the role of tyrosine 735. Mol Endocrinol. 2003;17:845–859.
97. Riegel, AT, et al. Proopiomelanocortin gene promoter elements required for constitutive and glucocorticoid-repressed transcription. Mol Endocrinol. 1991;5:1973–1982.
98. Therrien, M, Drouin, J. Pituitary pro-opiomelanocortin gene expression requires synergistic interactions of several regulatory elements. Mol Cell Biol. 1991;11:3492–3503.
99. Philips, A, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–5951.
100. Philips, A, et al. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol. 1997;17:5952–5959.
101. Martens, C, Bilodeau, S, Maira, M, et al. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol Endocrinol. 2005;19:885–897.
102. Winnay, JN, Xu, J, O’Malley, BW, et al. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147:1322–1332.
103. Abou-Samra, AB, Harwood, JP, Manganiello, VC, et al. Phorbol 12-myristate 13-acetate and vasopressin potentiate the effect of corticotropin-releasing factor on cyclic AMP production in rat anterior pituitary cells. Mechanisms of action. J Biol Chem. 1987;262:1129–1136.
104. Smoak, B, Deuster, P, Rabin, D, et al. Corticotropin releasing hormone is not the sole factor mediating exercise induced adrenocorticotropin release in humans. J Clin Endocrinol Metab. 1991;73:302–306.
105. Watts, AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130.
106. Carvallo, P, Aguilera, G. Protein kinase C mediates the effect of vasopressin in pituitary corticotrophs. Mol Endocrinol. 1989;3:1935–1943.
107. Bousquet, C, Melmed, S. Critical role for STAT3 in murine pituitary adrenocorticotropin hormone leukemia inhibitory factor signaling. J Biol Chem. 1999;274:10723–10730.
108. Mynard, V, Guignat, L, vin-Leclerc, J, et al. Different mechanisms for leukemia inhibitory factor-dependent activation of two proopiomelanocortin promoter regions. Endocrinology. 2002;143:3916–3924.
109. Mynard, V, et al. Synergistic signaling by corticotropin-releasing hormone and leukemia inhibitory factor bridged by phosphorylated 3′,5′-cyclic adenosine monophosphate response element binding protein at the Nur response element (NurRE)-signal transducers and activators of transcription (STAT) element of the proopiomelanocortin promoter. Mol Endocrinol. 2004;18:2997–3010.
110. Latchoumanin, O, et al. Reversal of glucocorticoids-dependent proopiomelanocortin gene inhibition by leukemia inhibitory factor. Endocrinology. 2007;148:422–432.
111. Abbud, RA, Kelleher, R, Melmed, S. Cell-specific pituitary gene expression profiles after treatment with leukemia inhibitory factor reveal novel modulators for proopiomelanocortin expression. Endocrinology. 2004;145:867–880.
112. Smith, AI, Funder, JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9:159–179.
113. Wikberg, JES, et al. New aspects on the melanocortins and their receptors. Pharmacological Research. 2000;42:393–420.
114. Pritchard, LE, White, A. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology. 2007;148:4201–4207.
115. Krude, H, Biebermann, H, Gruters, A. Mutations in the human proopiomelanocortin gene. Ann N Y Acad Sci. 2003;994:233–239.
116. Chaptini, L, Peikin, S. Neuroendocrine regulation of food intake. Curr Opin Gastroenterol. 2008;24:223–229.
117. Valassi, E, Scacchi, M, Cavagnini, F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168.
118. Pritchard, LE, et al. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology. 2003;144:760–766.
119. Wardlaw, SL, McCarthy, KC. Glucocorticoid regulation of hypothalamic proopiomelanocortin. Neuroendocrinol. 1998;67:51–57.
120. Pelletier, G. Regulation of proopiomelanocortin gene expression in rat brain and pituitary as studied by in situ hybridization. Ann N Y Acad Sci. 1993;680:246–259.
121. Schauer, E, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–2262.
122. Wintzen, M, Yaar, M, Burbach, JP, et al. Proopiomelanocortin gene product regulation in keratinocytes. J Invest Dermatol. 1996;106:673–678.
123. Scholzen, TE, et al. Expression of proopiomelanocortin peptides in human dermal microvascular endothelial cells: evidence for a regulation by ultraviolet light and interleukin-1. J Invest Dermatol. 2000;115:1021–1028.
124. Paus, R, et al. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann N Y Acad Sci. 1999 Oct;885:350–363.
125. Ito, N, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334.
126. Schiller, M, et al. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and alpha-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol. 2004;122:468–476.
127. Cui, R, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864.
128. Slominski, A, Tobin, DJ, Paus, R. Does p53 regulate skin pigmentation by controlling proopiomelanocortin gene transcription? Pigment Cell Res. 2007;20:307–308.
129. Raffin-Sanson, ML, et al. High precursor level in maternal blood results from the alternate mode of proopiomelanocortin processing in human placenta. Clin Endocrinol (Oxf). 1999;50:85–94.
130. Bicknell, AB. The tissue-specific processing of pro-opiomelanocortin. J Neuroendocrinol. 2008;20:692–699.
131. Margioris, AN, Grino, M, Protos, P, et al. Corticotropin-releasing hormone and oxytocin stimulate the release of placental proopiomelanocortin peptides. Journal of Clinical Endocrinology Metabolism. 1988;66:922–926.
132. Kubo, M, Nakagawa, K, Akikawa, K, et al. In vivo and in vitro ACTH response to ovine corticotropin-releasing factor in a bronchial carcinoid from a patient with ectopic ACTH syndrome. Endocrinol Jpn. 1985;32:577–581.
133. Malchoff, CD, et al. Ectopic ACTH syndrome caused by a bronchial carcinoid tumor responsive to dexamethasone, metyrapone, and corticotropin-releasing factor. Am J Med. 1988;84:760–764.
134. Suda, T, et al. Corticotropin-releasing hormone, proopiomelanocortin, and glucocorticoid receptor gene expression in adrenocorticotropin-producing tumors in vitro. J Clin Invest. 1993;92:2790–2795.
135. Liddle, GW, et al. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283–314.
136. Gaitan, D, et al. Glucocorticoid receptor structure and function in an adrenocorticotropin-secreting small cell lung cancer. Mol Endocrinol. 1995;9:1193–1201.
137. Ray, DW, Littlewood, AC, Clark, AJ, et al. Human small cell lung cancer cell lines expressing the proopiomelanocortin gene have aberrant glucocorticoid receptor function. J Clin Invest. 1994;93:1625–1630.
138. Ray, DW. Molecular mechanisms of glucocorticoid resistance. J Endocrinol. 1996;149:1–5.
139. Waters, CE, Stevens, A, White, A, et al. Analysis of co-factor function in a glucocorticoid-resistant small cell carcinoma cell line. J Endocrinol. 2004;183:375–383.
139a. Sommer, P, Le Rouzic, P, Gillingham, H, et al. Glucocorticoid receptor expression exerts an anti-survival effect on human small cell lung cancer cells. Oncogene. 2007;26:1–11.
140. Eipper, BA, Mains, RE. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980;1:1–27.
141. Yalow, RS, Berson, SA. Characteristics of “big ACTH” in human plasma and pituitary extracts. J Clin Endocrinol Metab. 1973;36:415–423.
142. Eipper, BA, Mains, RE. High molecular weight forms of adrenocorticotropic hormone in the mouse pituitary and in a mouse pituitary cell line. Biochemistry. 1975;14:3836.
143. Orth, DN, et al. ACTH and MSH production by a single cloned mouse pituitary tumor cell line. Endocrinology. 1973;92:385–393.
144. Lowry, PJ, Rees, L, Tomlin, S, et al. Chemical characterization of ectopic ACTH purified from a malignant thymic carcinoid tumour. J Clin Endocrinol Metab. 1976;43:831.
145. Schwyzer, R. ACTH: a short introductory review. Ann N Y Acad Sci. 1977;297:3–26.
146. Croughs, RJ, Thijssen, JH, Mol, JA. Absence of detectable immunoreactive alpha melanocyte stimulating hormone in plasma in various types of Cushing’s disease. J Endocrinol Invest. 1991;14:197–200.
147. Kortlandt, W, De Rotte, AA, Arts, CJM, et al. Characterization of alpha-MSH-like immunoreactivity in human plasma. Acta Endocrinological (Copenh). 1986;113:175–180.
148. Seidah, NG, Chretien, M. Complete amino acid sequence of a human pituitary glycopeptide: an important maturation product of proopiomelanocortin. Proc Natl Acad Sci USA. 1981;78:4236–4240.
149. Cool, DR, Fenger, M, Snell, CR, et al. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem. 1995;270:8723–8729.
150. Bertagna, X, Camus, F, Lenne, F, et al. Human joining peptide: a proopiomelanocortin product secreted as a homodimer. Mol Endocrinol. 1988;2:1108–1114.
151. Gibson, S, Crosby, SR, White, A. Discrimination between beta-endorphin and beta-lipotropin in human plasma using two-site immunoradiometric assays. Clin Endocrinol (Oxf). 1993;39:445–453.
152. Stewart, PM, et al. ACTH precursors characterize the ectopic ACTH syndrome. Clin Endocrinol (Oxf). 1994;40:199–204.
153. Wilson, H, White, A. Prohormones: their clinical relevance. Trends Endocrinol Metab. 1998;9:396–402.
154. Gibson, S, et al. Differential release of proopiomelanocortin-derived peptides from the human pituitary: evidence from a panel of two-site immunoradiometric assays. J Clin Endocrinol Metab. 1994;78:835–841.
155. Mountjoy, KG, Wong, J. Obesity, diabetes and functions for proopiomelanocortin-derived peptides. Mol Cell Endocrinol. 1996;128:171–177.
156. Steiner, DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39.
157. Thomas, L, Leduc, R, Thorne, BA, et al. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci USA. 1991;88:5297–5301.
158. Bloomquist, BT, Eipper, BA, Mains, RE. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991;5:2014–2024.
159. Benjannet, S, Rondeau, N, Day, R, et al. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA. 1991;88:3564–3568.
160. Scholzen, TE, Konig, S, Fastrich, M, et al. Terminating the stress: peripheral peptidolysis of proopiomelanocortin-derived regulatory hormones by the dermal microvascular endothelial cell extracellular peptidases neprilysin and angiotensin-converting enzyme. Endocrinology. 2007;148:2793–2805.
161. Pritchard, LE, Turnbull, AV, White, A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172:411–421.
162. Castro, MG, Morrison, E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57.
163. Nillni, EA. Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology. 2007;148:4191–4200.
164. Tsigos, C, Crosby, SR, Gibson, S, et al. Proopiomelanocortin is the predominant adrenocorticotropin-related peptide in human cerebrospinal fluid. J Clin Endocrinol Metab. 1993;76:620–624.
165. McLoughlin, L, Lowry, PJ, Ratter, SJ, et al. Characterisation of the proopiocortin family of peptides in human cerebrospinal fluid. Neuroendocrinology. 1981;32:209–212.
166. Nakao, K, Oki, S, Tanaka, I. Immunoreactive beta-endorphin and adrenocorticotropin in human cerebrospinal fluid. J Clin Invest. 1980;66:1383–1390.
167. Thody, AJ, Graham, A. Does alpha-MSH have a role in regulating skin pigmentation in humans? Pigment Cell Res. 1998;11:265–274.
168. Peters, EM, Tobin, DJ, Seidah, NG, et al. Pro-opiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol. 2000;114:430–437.
169. Gibson, S, et al. Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J Clin Endocrinol Metab. 1996;81:497–502.
170. Tateno, T, et al. Defective expression of prohormone convertase 1/3 in silent corticotroph adenoma. Endocr J. 2007;54:777–782.
171. Odell, WD. Ectopic ACTH syndrome: a misnomer. Endocrinol Metab Clin North Am. 1991;20:371–379.
172. Oliver, RL, Davis, JR, White, A. Characterisation of ACTH related peptides in ectopic Cushing’s syndrome. Pituitary. 2003;6:119–126.
173. White, A, Gibson, S. ACTH precursors: biological significance and clinical relevance. Clin Endocrinol (Oxf). 1998;48:251–255.
174. Vieau, D, Massias, JF, Girard, F, et al. Corticotrophin-like intermediary lobe peptide as a marker of alternate pro-opiomelanocortin processing in ACTH-producing non-pituitary tumours. Clin Endocrinol (Oxf). 1989;31:691–700.
175. Dallman, MF, Makara, GB, Roberts, JL, et al. Corticotrope response to removal of releasing factors and corticosteroids in vivo. Endocrinology. 1985;117:2190–2197.
176. Dallman, MF, et al. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173.
177. Karpac, J, Kern, A, Hochgeschwender, U. Pro-opiomelanocortin peptides and the adrenal gland. Mol Cell Endocrinol. 2007;265–266:29–33.
178. Kempna, P, Fluck, CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22:77–93.
179. Coll, AP, et al. The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology. 2004;145:4721–4727.
180. Clark, AJ. The melanocortin-2 receptor in normal adrenocortical function and familial adrenocorticotrophic hormone resistance. In: Cone RD, ed. The Melanocortin Receptors. Totowa NJ: Humana Press; 2000:361–384.
181. Catalano, RD, Stuve, L, Ramachandran, J. Characterization of corticotrophin receptors in human adrenocortical cells. J Clin Endocrinol Metab. 1986;62:300–304.
182. Cone, RD, et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317.
183. Chen, M, Aprahamian, CJ, Kesterson, RA, et al. Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry. 2007;46:11389–11397.
184. Miller, WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318.
185. Bohm, M, et al. Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of alpha-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology. 2005;146:4635–4646.
186. Bohm, M, Luger, TA, Tobin, DJ, et al. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126:1966–1975.
187. Challis, BG, et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11:1997–2004.
188. Sutton, GM, Josephine, BM, Gu, X, et al. A derivative of the melanocortin receptor antagonist SHU9119 (PG932) increases food intake when administered peripherally. Peptides. 2008;29:104–111.
189. Maaser, C, Kannengiesser, K, Kucharzik, T. Role of the melanocortin system in inflammation. Ann N Y Acad Sci. 2006;1072:123–134.
190. Lasaga, M, Debeljuk, L, Durand, D, et al. Role of alpha-melanocyte stimulating hormone and melanocortin 4 receptor in brain inflammation. Peptides. 2008;29:1825–1835.
191. Lowry, PJ, Silas, L, McLean, C, et al. Pro-γ-melanocyte-stimulating hormone cleavage in adrenal gland undergoing compensatory growth. Nature. 1983;306:70–73.
192. Fassnacht, M, et al. N-terminal proopiomelanocortin acts as a mitogen in adrenocortical tumor cells and decreases adrenal steroidogenesis. J Clin Endocrinol Metab. 2003;88:2171–2179.
193. Bicknell, AB, et al. Characterization of a serine protease that cleaves pro-gamma-melanotropin at the adrenal to stimulate growth. Cell. 2001;105:903–912.
194. Al-Dujaili, EAS, Hope, J, Estivariz, FE, et al. Circulating human pituitary pro-y-melanotropin enhances the adrenal response to ACTH. Nature. 1981;291:156–159.
195. Rochemont, J, Hamelin, J. The missing fragment of the pro-sequence of human pro-opiomelanocortin: sequence and evidence for C-terminal amidation. Biochem Biophys Res Commun. 1981;102:710–716.
196. Robinson, P, et al. Isolation and characterization of three forms of joining peptide from adult human pituitaries: lack of adrenal androgen-stimulating activity. Endocrinology. 1991;129:859–867.
197. Rubinstein, M, et al. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci USA. 1996;93:3995–4000.
198. Kauser, S, Thody, AJ, Schallreuter, KU, et al. Beta-endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol. 2004;123:184–195.
199. Ratter, SJ, et al. Pro-opiocortin related peptides in human pituitary and ectopic ACTH secreting tumours. Clin Endocrinol (Oxf). 1983;18:211–218.
200. Hruby, VJ, Han, G. The molecular pharmacology of alpha-melanocyte stimulating hormone. In: Cone RD, ed. The melanocortin receptors. Totowa, New Jersey: Humana Press Inc; 2000:255–257.
201. Pritchard, LE, et al. Agouti-related protein (83–132) is a competitive antagonist at the human melanocortin-4 receptor: no evidence for differential interactions with pro-opiomelanocortin-derived ligands. J Endocrinol. 2004;180:183–191.
202. Ray, DW, et al. Elevated levels of adrenocorticotropin (ACTH) precursors in post-adrenalectomy Cushing’s disease and their regulation by glucocorticoids. J Clin Endocrinol Metab. 1995;80:2430–2436.
203. Morton, NM, Seckl, JR. 11Beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res. 2008;36:146–164.
204. Tomlinson, JW, Stewart, PM. Modulation of glucocorticoid action and the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2007;21:607–619.
205. Hughes, KA, Webster, SP, Walker, BR. 11-Beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitors in type 2 diabetes mellitus and obesity. Expert Opin Investig Drugs. 2008;17:481–496.
206. Daly, JR, Reader, SCJ, Alaghband-Zadeh, J, et al. Observations on feedback regulation of corticotropin (ACTH) in man. In: Jones MT, Gillham B, Dallman MF, Chattopadhyay S, eds. Interaction within the brain-pituitary-adrenocortical system. London: Academic; 1979:181–188.
207. de Kloet, ER, Vreugdenhil, E, Oitzl, MS, et al. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301.
208. Atkinson, HC, et al. Corticosteroids mediate fast feedback of the rat hypothalamic-pituitary-adrenal axis via the mineralocorticoid receptor. Am J Physiol Endocrinol Metab. 2008;294:E1011–E1022.
209. Tierney, T, Christian, HC, Morris, JF, et al. Evidence from studies on co-cultures of TtT/GF and AtT20 cells that Annexin 1 acts as a paracrine or juxtacrine mediator of the early inhibitory effects of glucocorticoids on ACTH release. J Neuroendocrinol. 2003;15:1134–1143.
210. Spiga, F, et al. Effect of the glucocorticoid receptor antagonist Org 34850 on fast and delayed feedback of corticosterone release. J Endocrinol. 2008;196:323–330.
211. Beaulieu, S, Gagne, B, Barden, N. Glucocorticoid regulation of proopiomelanocortin messenger ribonucleic acid content of rat hypothalamus. Mol Endocrinol. 1988;2:727–731.
211a. Drazen, DL, Wortman, MD, Schwartz, MW, et al. Adrenalectomy alters the sensitivity of the central nervous system melanocortin system. Diabetes. 2003;52(12):2928–2934.
212. Venihaki, M, Carrigan, A, Dikkes, P, et al. Circadian rise in maternal glucocorticoid prevents pulmonary dysplasia in fetal mice with adrenal insufficiency. Proc Natl Acad Sci USA. 2000;97:7336–7341.
213. DeBold, CR, et al. Effect of synthetic ovine corticotropin-releasing factor: prolonged duration of action and biphasic response of plasma adrenocorticotropin and cortisol. J Clin Endocrinol Metab. 1983;57:294–298.
214. Jorgensen, H, Knigge, U, Kjaer, A, et al. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J Neuroendocrinol. 2002;14:788–795.
215. Guillaume, V, Conte-Devolx, B, Magnan, E. Effect of chronic active immunization with antiarginine vasopressin on pituitary-adrenal function in sheep. Endocrinology. 1992;130:3007–3014.
216. Whitnall, MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629.
217. Elias, AN, Valenta, LJ, Szekeres, AV, et al. Regulatory role of gamma-aminobutyric acid in pituitary hormone secretion. Psychoneuroendocrinology. 1982;7:15–30.
218. Fish, HR, Chernow, B, O’Brian, JT. Endocrine and neurophysiologic responses of the pituitary to insulin-induced hypoglycemia: a review. Metabolism. 1986;35:763–780.
219. Hornby, PJ, Piekut, DT. Opiocortin and catecholamine input to CRF-immunoreactive neurons in rat forebrain. Peptides. 1989;10:1139–1146.
220. Nussdorfer, GG, Malendowicz, LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides. 1998;19:1443–1467.
221. Koenig, JI. Pituitary gland: neuropeptides, neurotransmitters and growth factors. Toxicol Pathol. 1989;17:256–265.
222. Johnson, KL, Rn, CR. The hypothalamic-pituitary-adrenal axis in critical illness. AACN Clin Issues. 2006;17:39–49.
223. Ray, D, Melmed, S. Pituitary cytokine and growth factor expression and action. Endocr Rev. 1997;18:206–228.
224. Turnbull, AV, Rivier, CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71.
225. Skurlova, M, Stofkova, A, Jurcovicova, J. Exogenous IL-1beta induces its own expression, but not that of IL-6 in the hypothalamus and activates HPA axis and prolactin release. Endocr Regul. 2006;40:125–128.
226. Sapolsky, R, Rivier, C, Yamamoto, G, et al. Interleukin 1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524.
227. Bernton, EW, Beach, JE, Holaday, JW, et al. Release of multiple hormones by a direct action of interleukin 1 on pituitary cells. Science. 1987;238:652–654.
228. Suda, T, Tozawa, F, Ushiyama, T. Effects of protein kinase C related adrenocorticotrophin secretogogues and interleukin 1 on proopiomelanocortin gene expression in rat anterior pituitary cells. Endocrinology. 1989;124:1444–1449.
229. Arzt, E, et al. Interleukin 2 and interleukin-receptor expression in human corticotrophic adenoma and mouse pituitary cell cultures. J Clin Invest. 1992;90:1944–1951.
230. Denicoff, KD, Durkin, TM, Lotze, MT. The neuroendocrine effects of interleukin 2 treatment. Clin Endocrinol Metab. 1989;69:402–410.
231. Lotze, MT, Frana, LW, Sharrow, SO, et al. In vivo administration of purified human interleukin alpha I half-life and immunologic effects of the JURKAT cell-line derived IL-2. J Immunol. 1985;134:157–166.
232. Vankelecom, H, Carmeliet, P, Van Damme, J, et al. Production of interleukin 6 by folliculostellate cells of the anterior pituitary gland in a histiotypic cell aggregate culture system. Neuroendocrinol. 1989;49:102–106.
233. Spangelo, BL, MacLeod, RM, Isakson, PC. Production of interleukin 6 by anterior pituitary cells in-vitro. Endocrinology. 1990;126:582.
234. Jones, TH, Justice, S, Price, A, et al. Interleukin-6-secreting human pituitary adenomas in vitro. J Clin Endocrinol Metab. 1991;73:207–209.
235. Jones, TH, et al. Production of bioactive and immunoreactive interleukin 6 (IL-6) and expression of IL-6 messenger ribonucleic acid by human pituitary adenomas. J Clin Endocrinol Metab. 1994;78:180–187.
236. Tsagarakis, S, Kontogeorgos, G, Giannou, P. Interleukin-6, a growth promoting cytokine, is present in human pituitary adenomas: an immunocytochemical study. Clin Endocrinology. 1992;37:163–167.
237. Mastorakos, G, Weber, JS, Magiakou, MA, et al. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 1994;79:934–939.
238. Fong, Y, Moldawer, LL, Marano, M. Endotoxemia elicits increased circulating beta2-IFN/IL-6 in man. J Immunol. 1989;142:2321–2324.
239. Kariagina, A, Romanenko, D, Ren, SG, et al. Hypothalamic-pituitary cytokine network. Endocrinology. 2004;145:104–112.
240. Akita, S, et al. Human and murine pituitary expression of leukemia inhibitory factor: novel intrapituitary regulation of adrenocorticotropin synthesis and secretion. J Clin Invest. 1995;95:1288–1298.
241. Wang, Z, Ren, SG, Melmed, S. Hypothalamic and pituitary leukemia inhibitory factor gene expression in vivo: a novel endotoxin-inducible neuro-endocrine interface. Endocrinology. 1996;137:2947–2953.
242. Ray, DW, Ren, SG, Melmed, S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J Clin Invest. 1996;97:1852–1859.
243. Stefana, B, Ray, DW, Melmed, S. Leukemia inhibitory factor induces differentiation of pituitary corticotroph function: an immuno-neuroendocrine phenotypic switch. Proc Natl Acad Sci USA. 1996;93:12502–12506.
244. Akita, S, Malkin, J, Melmed, S. Disrupted murine leukemia inhibitory fact (LIF) gene attenuates adrenocorticotrophic hormone (ACTH) secretion. Endocrinology. 1996;137:3140–3143.
245. Ware, CB, Kariagina, A, Zonis, S, et al. Leukemia inhibitory factor signaling is implicated in embryonic development of the HPA axis. FEBS Lett. 2005;579:4465–4469.
246. Kelly, RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32.
247. Lightman, SL, et al. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583:255–262.
248. Gudmundsson, A, Carnes, M. Pulsatile adrenocorticotropic hormone: an overview. Biol Psychiatry. 1997;41:342–365.
249. Horrocks, PM, et al. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf). 1990;32:127–134.
250. Smith, SM, Vale, WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395.
251. Hesse, DG, Tracey, KJ, Fong, Y. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988;166:147–153.
252. Van Deveter, SJH, et al. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrolytic and complement pathways. Blood. 1990;76:2500–2526.
253. Chrousos, GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362.
254. Ray, DW, Stefana, B, Zand, O, et al, Leukaemia inhibitory factor: a potent modulator of CRH action on pituitary corticotroph cells. Proceedings of the Annual Meeting of the Endocrine Society. 1996:532.
255. Takao, T, Culp, SG, De Souza, EB. Reciprocal modulation of interleukin 1 beta and interleukin-1 receptors by lipopolysaccharide (endotoxin) treatment in the mouse brain-endocrine-immune axis. Endocrinology. 1993;132:1497–1504.
256. Bernhagen, J, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–959.
257. Yamaguchi, M, Matsuzaki, N, Hirota, K, et al. Interleukin 6 possibly induced by interleukin-1 beta in the pituitary gland stimulates the release of gonadotrophins and prolactin. Acta Endocrinology (Copenh). 1990;122:201–20556.
258. Muglia, L, Jacobson, L, Dikkes, P, et al. Corticotrophin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432.
259. Milenkovic, L, Rettori, V, Snyder, GD, et al. Cachectin alters pituitary hormone release by a direct action in vitro. Proc Natl Acad Sci USA. 1989;86:2418–2422.
260. Spangelo, BL, Judd, AM, Isakson, PC, et al. Interleukin 6 stimulates anterior pituitary hormone release in vitro. Endocrinology. 1989;125:575–577.
261. Almawi, WY, et al. Abrogation of glucocorticoid-mediated inhibition of T-cell proliferation by the synergistic actions of IL-6 and IFN-gamma. J Immunol. 1991;146:3523–3527.
262. Kam, JC, Szeffer, SJ, Surs, W, et al. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993;151:3460–3466.
263. Caldenhoven, E, et al. Negative cross-talk between Re1A and the glucocorticoid receptor: a possible mechanism for the anti-inflammatory action of glucocorticoids. Mol Endocrinol. 1995;9:401–412.
264. Ray, A, Prefontaine, KE. Physical association and functional antagonism between the p65 subunit of transcription factor NK-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–756.
265. Scheinman, RI, Gualberto, A, Jewell, CM, et al. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953.
266. Makino, S, Hashimoto, K, Gold, PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73:147–158.
267. Raff, H, Findling, JW. A new immunoradiometric assay for corticotropin evaluated in normal subjects and patients with Cushing’s syndrome. Clin Chem. 1989;35:596–600.
268. Yalow, RS, Berson, SA. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971;44:439–445.
269. Hale, AC, Besser, GM, Rees, LH. Characterization of pro-opiomelanocortin-derived peptides in pituitary and ectopic adrenocorticotrophin-secreting tumours. J Endocrinol. 1986;108:49–56.
270. Crosby, SR, Stewart, MF, Ratcliffe, JG, et al. Direct measurement of the precursors of adrenocorticotropin in human plasma by two-site immunoradiometric assay. J Clin Endocrinol Metab. 1988;67:1272–1277.
271. Raffin-Sanson, ML, et al. High plasma proopiomelanocortin in aggressive adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab. 1996;81:4272–4277.