Chapter 52 Adhesion Prevention
INTRODUCTION
The relevance of pelvic and peritoneal adhesions to the practice of reproductive endocrinology and infertility clearly relates to their association with infertility and chronic pelvic pain. In addition, postoperative adhesions are also responsible for significant complications, most notably adhesive bowel obstruction, and increase the difficulty of subsequent surgical procedures, which enhances the potential for intraoperative complications. Interestingly, the first case report of fatal intestinal obstruction caused by intra-abdominal adhesions was a woman who developed adhesions after removal of an ovarian tumor.1 Although the peritoneal cavity is of interest to the reproductive surgeon, postoperative adhesions also occur in other spaces, such as the pleural and pericardial cavities.
HISTORICAL PERSPECTIVE
Ancient Egyptians, known for their detailed descriptions of human anatomy, described pelvic adhesions several thousands years ago. Pleural adhesions were first described in the Babylonian Talmud in 440 A.D.2 Although adhesions caused by peritonitis have been recognized since the early 1700s, it was not until the widespread use of anesthesia in the mid-1800s when invasive abdominal procedures became more prevalent that the extent of the problems caused by intra-abdominal adhesions was realized.
By the 1880s, the first published reports describing the use of adjuvants for adhesion prevention began to appear in the surgical literature. Over the next 100 years, a plethora of scientific reports and anecdotal accounts described the use of everything from amniotic fluid, bovine cecum, gold-beater’s skin, shark peritoneum, fish bladder, vitreous of calf’s eyes, various gums, lubricants, fluids, gels, polymers, physical barriers, and a host of mechanical separation methods to prevent adhesions. Unfortunately, the results of most of these studies were equivocal, with no more than a small percentage of success. Even in this age of surgical sophistication and new operating room technology, our age-old nemesis, the intra-abdominal adhesion, remains a significant, long-term, and recurrent postoperative problem.3
DEFINITION, TYPE, AND EXTENT OF DISEASE
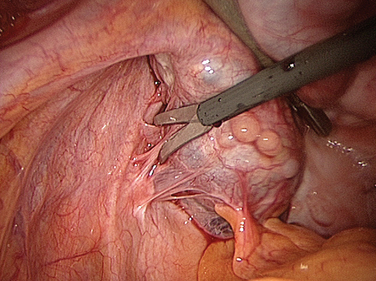
Intra-abdominal adhesions are strands or membranes of fibrous tissue that can be attached to the various intra-abdominal organs, sometimes connecting them together (Fig. 52-1). The term adhesion in the medical field refers to the abnormal joining of anatomic structures at sites where no such anatomic attachment should exist. Adhesions usually develop in conjunction with surgery at sites of adhesiolysis and other operative sites, but also occur at “distant” sites where no surgical procedure was done.
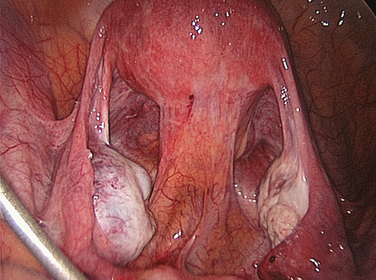
Figure 52-1 Pelvic adhesions involving the uterus and bowel. This patient had a previous myomectomy.
To accurately describe the extent of peritoneal adhesions during clinical investigations, various scoring systems have been developed. Systematic assessment of adhesions is mandatory to decrease interobserver variation and to provide quantitative data corresponding to their extent and clinical significance.4 Most of the available scoring systems incorporate adhesion location, vascularity, and type (thickness). However, the current scoring systems suffer from the lack of validation for outcomes such as fertility, pain, and bowel obstruction, which makes the interpretation of the results of research related to adhesion development and prevention difficult. Thus, the question is often asked that if a study demonstrates a significant change in an adhesion score, does that reflect true clinical relevance in the extent of adhesive disease?
PREVALENCE OF DISEASE
Despite the application of microsurgical technique and use of surgical adjuvants by experienced surgeons, the development of intraperitoneal postoperative adhesions is common. Currently, no serum marker or scanning technique is consistently able to identify adhesions, and a repeat operative procedure is required for evaluation.5
In a postmortem study of victims of motor vehicle accidents, intra-abdominal adhesions were encountered in 67% of individuals who had a history of abdominal operation.6 The prevalence among patients who had undergone major operations and multiple procedures was 81% and 93%, respectively. Most studies have shown that after an intra-abdominal operation, most patients developed adhesions. One study found that after merely one previous abdominal operation, 93% of patients had adhesions.7 On the other hand, intra-abdominal adhesions among patients who had never experienced a laparotomy were found in only 10.4%.
Within 1 year of laparotomy, 1% of patients develop adhesion-related intestinal obstruction;7 11% to 12% of them will suffer from recurrence later.8 In reports based on data from laparoscopies9 and autopsies,6 60% and 69% of women, respectively, had pelvic adhesions after previous abdominopelvic operations. Variation in the incidence of postoperative adhesions may be explained by differences in the extent of surgery, differences in the incidence or severity of prior surgery or other etiologic events, and in what each investigator considered to be a “significant” adhesion. It is important to emphasize that although some investigators believe all adhesions may not be clinically significant, others would emphasize that you cannot tell which adhesions will cause pain or contribute to bowel obstruction.
ECONOMIC IMPACT OF ADHESIONS
The economic impact of adhesions as a complication of surgery is enormous. In the United States, 446,000 procedures were performed annually to remove abdominopelvic adhesions.10 This included 347,000 operations to release peritoneal adhesions and approximately 100,000 procedures to liberate intestinal adhesions. The cost of morbidity associated with adhesions is large.11 Few studies have been conducted to evaluate the financial impact of adhesion-related problems on the medical budget, and many of the studies could have underestimated the actual financial impact of adhesive disease by not considering the costs of performing diagnostic tests, consultations with other services such as gastroenterology, the increase in operative time required to free adhesions, and the complications due to inadvertent injury to other vital structures while freeing adhesions. In addition, a significant number of planned laparoscopic surgeries are converted to laparotomy because of failure to safely enter the abdomen and achieve adequate pneumoperitoneum. Finally, there are costs associated with long-term morbidity such as adhesion-related infertility and repeated admissions for bowel obstruction. The psychological impact of adhesion-related conditions that lead to loss of work might add to the economic burden.
In the United States, an analysis of all hospitalizations for adhesions was performed using the 1988 National Hospital Discharge Survey. Of a total 281,982 hospitalizations, 51,100 were adhesion-related admissions. In total, there were more than 948,000 hospital-days of care, accounting for a cost of approximately $1.18 billion.10 In 1994, the same authors updated their database and found the annual overall cost to be approximately $1.3 billion. They also reported that lysis of adhesions was responsible for 1% of hospitalization in the United States.12
In a study that evaluated hospital discharge for adhesion-related bowel obstruction between 1990 and 1996, the total number of patients increased from 115,067 in 1990 with a total length of hospitalization of 962,642 days to 139,716 patients with a total length of hospitalization of 885,396 days in 1996.13 The total costs increased steadily from $924 million in 1990 to $1.4 billion in 1996. Among those who were treated medically, there were 88,601 hospitalizations in 1990 and 110,817 in 1996, with a parallel increase of cost from $261 million in 1990 to $386 million in 1996.
In the United Kingdom, Menzies and coworkers14 reviewed 110 hospital admissions resulting from adhesion-related small-bowel obstruction over a 2-year period. Of 110 admissions, surgical treatment was performed in 37% of patients and conservative management in the remaining patients. The total costs per admission were $7,521.28 (£4,677.41) and $2582.69 (£1,606.15) for the surgical and conservative treatment groups, respectively.
In Sweden, Holmdhal and Riseberg15 conducted a cost analysis study on adhesion-related admission among all general surgeons. Collection of data was performed after the authors analyzed the questionnaire sent to all department heads of Swedish surgical units. There were a total of 6200 patients requiring hospitalizations, accounting for 3.5% of all laparotomies. They found that the total cost for adhesion-related hospitalization was $6.1 million US annually or close to $1 million US per million Swedish inhabitants (the population of Sweden is 8.5 million).
Because of the difference in medical costs in various countries, meaningful comparisons are difficult. However, all studies suggest a huge financial impact of adhesion-related conditions to the healthcare system. Recently, Wilson16 calculated that a low-cost product with a 25% efficacy in preventing surgical adhesions could potentially generate a cost saving of £55 million over a 10-year period in the UK. In a prior study, they concluded that demonstrating the clinical effectiveness of adhesion reduction products in a randomized, controlled setting is unlikely to be feasible due to the large number of patients required. They suggested that products costing £200 (around $300 US) or more are unlikely to pay back their direct costs.17
COMPLICATIONS OF ADHESIONS
Postoperative adhesions develop after virtually every transperitoneal operation, ranging from minimal scarring present on serosal surface to dense agglutination of nearly all structures. However, postoperative adhesions are much more common than symptoms would suggest; therefore, most adhesions probably do not lead to acute symptoms or clinical sequelae.
In certain situations, adhesions may be of benefit. They may serve as adjuncts to the body’s natural defenses against intra-abdominal insults and can be lifesaving in the postoperative period by localizing leakage from suture lines, isolating an inflammatory collection, or preventing the spread of infection. Other potential benefits include neovascularization of ischemic structures such as anastomoses. However, adhesions are associated with significant morbidity such as infertility, pain, and bowel obstruction.18
Reproductive Problems
Adhesions play a significant role in the etiology of several reproductive disorders, including infertility, ectopic pregnancy, and recurrent pregnancy loss (intrauterine adhesions). From 15% to 20% of female infertility is caused by adhesions.19 Adhesions causing infertility or ectopic pregnancy may originate from endometriosis, infection such as pelvic inflammatory disease, appendicitis, or tuberculosis, as well as inflammatory bowel disease and surgery. More details on adhesions and infertility, ectopic pregnancy, and recurrent pregnancy loss are provided in other chapters of this text (see Chapters 34, 41, 47 and 48).
Intestinal Obstruction
Adhesive intestinal obstruction is the most serious complication associated with peritoneal adhesions (Fig. 52-2). In addition to the significant pain, the condition can be life-threatening and in some cases fatal. In the early 20th century, most of the cases of intestinal obstruction were due to strangulated external hernias. As abdominal and gynecologic surgeries began to be performed more routinely, the number of intestinal obstructions caused by postsurgical adhesions increased and now has surpassed those produced secondary to hernias. This statistic, however, only holds true for the western world. In poorer regions of the world, the percentage of obstruction from hernias is still greater than that caused by adhesions.20 In advanced countries, adhesions account for intestinal obstructions in 49% to 74% of cases.21 Intestinal surgeries involving the left side of the colon and rectum, appendectomies, and gynecologic procedures are the three most common surgical procedures accounting for adhesion-related intestinal obstruction.7
Both the extent and indication (e.g., cancer) of a gynecologic operation correlate significantly with the risk of postoperative intestinal obstruction. A Japanese group looked at the type of adhesions that caused intestinal obstruction and found that in 29% of cases, obstructive adhesions were small bowel to small bowel; in 48% the adhesions were small bowel to other abdominopelvic structures.22
Gynecologic malignancy increases the incidence of intestinal obstruction because more extensive operations are often required, and blockage by a tumor mass can occur. A report on 283 gynecologic patients treated for mechanical small-bowel obstruction showed that 175 (61.8%) were due to a primary or recurrent gynecologic malignancy, almost exclusively arising from the ovary.23 However, the second most common cause of obstruction was postoperative adhesions (41 patients, 14.5%), most of which followed an operation performed for a gynecologic cancer.23 Intestinal obstruction occurring in the immediate postoperative period and those that follow treatment for early-stage ovarian cancer usually are related to adhesions, whereas delayed obstruction, especially that which occurs in the presence of advanced disease, is usually tumor-related, particularly if radiotherapy is administered.24
Although small-bowel obstruction on a gynecology service occurs most frequently in women with ovarian cancer, it can occur after other extensive pelvic operations, particularly ones associated with significant pelvic infection, such as surgery for tubo-ovarian abscess. It has been suggested that the two structures most commonly involved in adhesions associated with gynecologic surgery are the omentum and the distal small intestine.18 Short, obese women have a particular tendency to develop omental and small-bowel postoperative adhesions, perhaps in part related to longer, more difficult operative procedures.6 Because colonic adhesions are less common and the restricted mesentery can preclude twisting and kinking of the lumen, the colon is involved in adhesive obstruction only 2% to 10% as frequently as the small bowel.6 Among gynecology patients colonic obstruction usually is related to recurrent or persistent pelvic tumor causing extrinsic compression of the rectosigmoid colon.23,24
Adhesive intestinal obstruction after previous surgery can be early or late. According to the general surgery literature, 17% to 29% of intestinal obstruction cases occur within 1 month,25,26 whereas the rest of the cases develop later, from 1 month to several decades after the surgery. After definitive operative management of an intestinal obstruction, the incidence of recurrence is approximately 14%. The factors contributing to occurrence or recurrence of intestinal obstruction are poorly understood.26,27
Fevang and colleagues studied a series of patients that included 500 patients who had a median follow-up of 10 years and a maximum follow-up time of 40 years after adhesive small-bowel obstruction.28 The cumulative recurrence rate for patients operated once for adhesive intestinal obstruction was 18% after 10 years and 29% at 30 years. For patients admitted several times for adhesive intestinal obstruction, the relative risk of recurrent adhesive intestinal obstruction increased with increasing number of prior episodes. The cumulative recurrence rate reached 81% for patients with four or more admissions. Other factors influencing the recurrence rate were the method of treatment of the last previous adhesive intestinal obstruction episode (conservative versus surgical) and the number of abdominal operations before the initial adhesive intestinal obstruction operation. Most recurrent adhesive small-bowel obstruction episodes occurred within 5 years after the previous one, but a considerable risk is still present 10 to 20 years after an adhesive small-bowel obstruction episode.28
The Conundrum of Chronic Pain Syndromes and Adhesions
The issue of adhesion-related pain, including chronic pelvic and abdominal pain, dysmenorrhea, and dyspareunia, has been the subject for intense debate.29 There are three important questions that need to be addressed: Do adhesions cause pain? If yes, does the extent of adhesions correlate with the nature and severity of pain? Does adhesiolysis relieve adhesion-related pain?
Adhesions May or May Not Cause Pain
The question as to whether or not adhesions cause pain does not have an entirely satisfactory answer. In favor of this relationship are the findings that adhesions contain nerve fibers30 and are innervated with substance P-containing sensory neurons, suggesting that adhesions themselves are capable of generating pain stimuli and perhaps finally suggesting a mechanism to explain the pain associated with adhesions.31 However, some have criticized reports such as this because of the potential for “tenting” of normal peritoneum during adhesion excision, such that the nerves may have been in normal peritoneum. Additionally, the apparent absence of pain in some patients with massive adhesions in contrast to claims of intense pain by patients with only minimal adhesive disease has led many clinicians to doubt that adhesions alone are “enough” pathology to “cause” pain.
The magnitude of the problem is great. As many as 20% of patients with acute pelvic inflammatory disease will have chronic pain, much of which is felt to be associated with adhesions.32 It is possible that adhesions that restrict the free movement of pelvic organs would be implicated as a cause of chronic pelvic pain.33 Approximately 20% to 50% of patients with chronic pain have pelvic adhesions.33,34
Adhesion Extent Does Not Correlate with Nature and Severity of Pain
Although several studies have suggested a significant association between adhesions and chronic pelvic pain, and the location of pain to reflect the site of adhesions, most of these studies failed to show a significant correlation between the extent of adhesions and the severity of pain.20 Similar observations have been made for the relationship between endometriosis and pain.
Adhesiolysis Does Not Always Relieve Adhesion-Related Pain
Lysis of adhesions has been proposed as the therapeutic modality of choice for patients suffering from pelvic pain in association with adhesions, and some investigators report the resolution of chronic pain in individuals after lysis of adhesions, whereas others have not noted this effect consistently or have noticed only a very short period of benefit.35,36 Obviously, the “gold standard” study to determine the utility of adhesiolysis for pain relief would be a double-blinded, randomized trial with a control arm, which would control for adhesion reformation and de novo adhesion formation, and follow-up occurring past the point of the expected placebo effect of adhesiolysis surgery. Not surprisingly, such a study would be difficult to execute because of the need to recruit a large number of patients, to perform a second-look procedure to ascertain whether adhesion reformation occurred in those patients having pain relapse after adhesiolysis, and to debate the method to use to quantify patient pain.20
Probably the closest to this ideal study are reports by Swank and colleagues,37,38 who conducted both laparotomy and laparoscopy. Neither of these studies was able to demonstrate a significant benefit of adhesiolysis to the patient population as a whole, although trends were identified. Although each of these studies was randomized, the length of follow-up was limited, and neither included second-look laparoscopy so as to allow correction for new or persisting adhesions.
Other Problems Complicating Adhesions
There are several other clinical implications of adhesions, including interference with intraperitoneal therapeutics and difficult repeat surgery. In an effort to improve both the response rates and the overall survival of patients with ovarian carcinoma, investigators have explored the safety and efficacy of antineoplastic agents delivered intraperitoneally for this malignancy. A major impediment to this innovative therapy is inadequate intraperitoneal distribution of drugs whenever extensive adhesions are present.39 Other adhesion-related complications of gynecologic surgery that do not affect fertility have not been well-studied. Voiding dysfunction, ureteral obstruction, and nonspecific gastrointestinal complaints may also be related to postoperative adhesion development.
PATHOPHYSIOLOGY OF ADHESION FORMATION
Peritoneal Cavity Repair
The abdominal cavity is lined by the peritoneum, which consists of a single layer of mesothelial cells, supported by a basement membrane and an underlying sheet of connective tissue. When it covers the abdominal wall it is called the parietal peritoneum and when it covers viscera it is called the visceral peritoneum.
Peritoneal trauma results in mesothelial damage and is accompanied by inflammation. Mesothelial cells balloon and detach from the basal membrane, thereby creating denuded areas.40 Peritoneal injury due to surgery, irradiation, infection, or irritation initiates an inflammatory reaction that increases peritoneal fluid, including proteins and cells. This fibrinous exudate leads to formation of fibrin41 by activation of the coagulation cascade.42 Within this fibrinous exudate, polymorphonuclear cells, macrophages, fibroblasts, and mesothelial cells migrate, proliferate, or differentiate (Table 52-1).
Table 52-1 The Temporal Relationship of Different Cell Types and Products in the Peritoneal Cavity (Days) after Surgical Injury
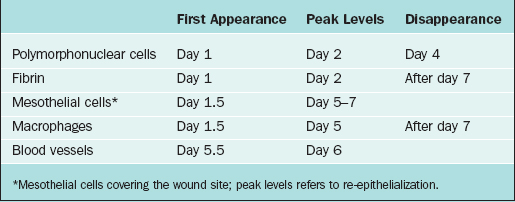
Macrophages increase in number and change functions from mainly phagocytosis into secretion of a variety of substances that cause differentiation of progenitor cells into mesothelial cells on the injured surface. Mesothelial cells form islands throughout the injured area, proliferate, and cover the denuded area in short periods of time, usually estimated to be within 5 to 7 days of injury.43 It is important to realize that this process occurs, not just at the edge of the denuded peritoneum, but throughout the surface of the injured area. All these cells, as well as fibroblasts that migrate from underlying tissues, release a variety of substances such as plasminogen system components, arachidonic acid metabolites, reactive oxygen species, cytokines, and growth factors such as interleukins, tumor necrosis factor-α, and transforming growth factors α and β. These factors modulate the process of peritoneal healing and adhesion development at different stages.44
Abnormal Repair
Fibroblasts will invade the fibrin matrix with extracellular matrix deposition, leading to peritoneal adhesions. In addition to fibroblast invasion and extracellular matrix deposition, the formation of new blood vessels has been universally claimed to be important in adhesion development as a means of resupplying oxygen and nutrients and removing metabolic waste.44
During peritoneal healing, cell–cell interactions between mesothelial cells, macrophages, and fibroblasts contribute to the healing of the peritoneum. Adhesion fibroblasts have developed a specific phenotype. Compared with normal peritoneal fibroblasts, adhesion fibroblasts have increased basal levels of collagen I, fibronectin, and other adhesion substances and decreased levels of tPA.45 Readers with greater interest in this area of investigation are referred to a more detailed review.46
To conclude, the balance between fibrin deposition and degradation in the initial days after surgery is critical in determining normal peritoneal healing or adhesion development. If fibrin is completely degraded, remesothelialization leading to normal peritoneal healing without adhesions will occur. In contrast, if fibrin is not completely degraded, it will serve as a scaffold for fibroblast ingrowth with subsequent extracellular matrix deposition and angiogenesis. After abdominal surgery and infection, however, the equilibrium between coagulation and fibrinolysis is disturbed in favor of the coagulation system.47–50
Risk Factors for Developing Adhesions
As mentioned earlier, peritoneal repair and adhesion development is the net result of a balance between fibrin deposition as an outcome of the inflammatory process associated with peritoneal injury and fibrinolysis. Fibrinolysis plays a central role in the resolution of the inflammatory exudate, thereby minimizing the risk of adhesion development. This process has primarily been thought to be initiated by mesothelial cells in the region of tissue injury because fibrinolytic activity has been documented within normal mesothelium.51 However, tPA occurs in fibroblasts from human peritoneum and adhesions as well.52,53 Adequate blood supply is critical for normal fibrinolysis to occur. Peritoneal injury associated with ischemia interferes with fibrinolysis and leads to organization rather than resolution of the fibrin–cellular matrix.43 In the absence of ischemia, even large denuded areas of peritoneum usually will heal normally without developing adhesions.54,55
Agents that compromise blood flow within the area of tissue injury increase adhesion development. Thermal injury,56 infection,57 foreign body reaction (i.e., suture),57,58 radiation-induced endarteritis,59 and any other impediment to fibrin degradation increase intraperitoneal adhesions. The effect of electrocautery devoid of significant thermal injury or incomplete hemostasis on fibrinolysis and ultimately on adhesion development has not been adequately studied. Thus, the necessity to control small bleeding vessels and the optimal method to do such (cautery or suture) has yet to be defined. Only conflicting reports concerning these issues exist in the infertility literature.56,60 Fibrinolysis in the abdominal cavity is even more depressed in the presence of infection.61
Intraoperative tissue damage, infections, tissue ischemia, and intra-abdominal presence of foreign material, blood, or bile62,63 all have been shown to be potent causes of peritoneal adhesions. Foreign materials, such as glove powder,64 fluff from surgical packs (gauze lint),65 sutures,58,66 and material extruded from the digestive tract, cause a peritoneal inflammatory reaction, hence increasing the chance of adhesions.67
PREVENTION OF ADHESION DEVELOPMENT
Although gynecologic surgery has been a major source of intraperitoneal adhesions and associated morbidity over the past century, most related complications, including adhesions, have been traditionally managed by general surgeons and related surgical subspecialties.68 However, with the establishment of the gynecologic subspecialties of reproductive endocrinology and infertility and gynecologic oncology, gynecologic surgeons developed a directed interest in adhesion development, prevention, and management. This interest is stimulated particularly for reproductive endocrinologists by the relationship between adhesions and impaired fertility4 and for gynecologic oncologists by the increased aptitude to perform all aspects of intestinal surgery,69 as well as the ability to deal with adhesion-related intestinal complications. As residency training programs in obstetrics and gynecology universally provide exposure to both of these subspecialties, all obstetricians and gynecologists should be familiar with the principles of adhesion development and their management.70
Surgical Techniques
Hemostasis and Tissue Handling
Achieving meticulous hemostasis and minimizing tissue handling without doubt constitute the two most important surgical principles in minimizing adhesions. Although the presence of blood at the operative site increases postoperative adhesion development, it is important that hemostasis be achieved in a manner that devitalizes as little surrounding tissue as possible. When possible, the size of pedicles should be minimized and use of electrosurgery should be restricted to the actual bleeding site. With regard to tissue handling, manipulation of structures is required to achieve exposure and perform the procedure. However, tissue damage can often be minimized by the use of atraumatic graspers, moist (not dry) pads and, when feasible, grasping of tissue structures to be excised.4
Value of Precise Tissue Approximation
The value of tissue approximation, including peritoneal closure, is still a subject of intense debate without clearcut recommendations to be generalized. Traditionally, closure of the peritoneum was thought to possibly allow for (1) restoration of anatomy and approximation of tissues for healing; (2) reestablishment of the peritoneal barrier to reduce the risk of infection; (3) reduction of the risk of wound herniation or dehiscence; and (4) minimization of adhesion development.71
The Cochrane database examined the issue of peritoneal closure versus nonclosure in cesarean section. They concluded that there was “no significant difference in short-term morbidity from nonclosure of the peritoneum in cesarean section.”72 It is, however, important to appreciate that the conclusions drawn from cesarean section may not be applicable to general gynecologic surgery due to the obvious differences in the nature of the two surgery types. Although suturing the peritoneum appears to have a more anatomic result than leaving it to heal by secondary intention, the presence of ischemic tissue by sutures causes a predisposition to adhesion development.55 In animal models,73,74 laparotomy closure without peritoneal suturing healed with a lower incidence of adhesions to the wound compared with animals with peritoneal suturing. Postoperative adhesions at the site of closure of the pelvic peritoneum were responsible for bowel obstruction in 85% of cases, with adhesions to the anterior abdominal wall occurring in another 15%.75 Tulandi76 suggested that the currently available evidence suggests that peritoneal suturing is not only unnecessary, but could also be associated with a greater risk of small-bowel obstruction.
Another important issue involving whether to precisely approximate tissues together or not is the ovarian cortex closure, such as in surgeries involving the removal of ovarian cysts and masses. In animal studies, suture closure of the ovarian cortex was associated with greater, not lesser, adhesion development.77
Use of Laparoscopy
It had been suggested, anecdotally, that procedures performed by laparoscopy, as opposed to laparotomy, might be less likely to be followed by the postoperative development of pelvic adhesions. Potential explanations included reductions in tissue drying, tissue manipulation, introduction of foreign materials, and abrasion of peritoneal sutures, as well as lack of packing of bowel.78,79 However, in a multicenter study evaluating adhesion reformation at a second-look procedure after laparoscopic adhesiolysis, adhesion reformation was identified in 66 of 68 subjects (97%).80 Laparoscopic adhesiolysis was able to significantly reduce the extent of pelvic adhesions to approximately half of what was present initially. De novo adhesion development occurred in only 8 (12%) of these 68 women, and in 11 (23%) of 47 available sites in those affected. This suggests that de novo adhesion development (not adhesion reformation) may occur less frequently after laparoscopic surgery, but confirmation of this hypothesis will require properly controlled studies.
A recent study made the observation that the increased use of laparoscopy for abdominal procedures between 1988 and 1994 did not appear to be associated with a concomitant reduction in the hospitalization rate for adhesive intestinal obstruction, suggesting that although minimally invasive techniques may offer advantages such as decreased morbidity, whether such procedures actually reduce adhesion development remains unclear at this time.12
Laparoscopic adhesiolysis for adhesive intestinal obstruction has also come to the forefront81 and obviously offers similar advantages over laparotomy. However, there are no long-term results; thus, the question concerning decreased recurrence after laparoscopy compared with laparotomy requires further investigation.
CO2 Pneumoperitoneum-Enhanced Adhesion Development
Recently, the effects of carbon dioxide (CO2) pneumoperitoneum have become increasingly scrutinized.82–90 It has been hypothesized that CO2 pneumoperitoneum induces adverse effects, including hypercarbia, acidosis,88 hypothermia, and desiccation,89 as well as altered peritoneal fluid90 and morphology of the mesothelial cells.91 Adhesions have been found to increase with the duration of the pneumoperitoneum and with increased insufflation pressure in rabbits82 and mice.83 Potentially, such adhesions may be due to drying or cooling of tissues from insufflation gas flow; however, this remains controversial. Cooling has been suggested both to cause89 and reduce adhesions,84 while humidification of insufflation gas has been implicated in adhesion reduction85 or having no effect. The pneumoperitoneum-enhanced adhesion development has been suggested to be mediated by mesothelial hypoxia because similar effects were observed with helium pneumoperitoneum, and because the addition of 2% to 4% oxygen to both CO2 and helium pneumoperitoneum decreased adhesion development.83,86
Use of Energy Source
In general, there has been no evidence that use of a specific energy source per se (e.g., CO2 laser, bipolar electrocautery, unipolar electrocautery, harmonic scalpel) results in a greater reduction of adhesions or improvement in pregnancy outcome, compared with other surgical modalities.92–94 However, individual surgeons, based on their own experience, equipment availability, and preference, may find use of a particular modality to be most advantageous for the performance of these procedures.95–98
Technique for Treatment of Existing Adhesions to Prevent Adhesion Reformation
There is no single best method of dividing adhesions, in spite of dogma to the contrary. In some situations, particularly when the adhesions are flimsy and are easily separable, gentle blunt dissection may be the safest method. In other situations, when adhesions are dense and especially when important adjacent structures such as the urinary bladder are involved, blunt dissection or pulling on the small intestine results in tears of the bowel or adherent viscus. This happens primarily because the tensile strength of the adhesions exceeds that required to maintain bowel or other visceral seromuscular layer intact. Consequently, when adhesions are dense, it is generally safer to use a sharp method of dissection (Fig. 52-3).
Similarly, the role of adhesion incision versus excision has not been well studied. Although excision of adhesion bands may in many cases be intuitive so that the resulting raw surfaces will no longer be in apposition, it is perhaps better to leave raw surface area as small as possible, and therefore avoid denudation of visceral surfaces. In all situations, immediate recognition of enterotomy is important because if the operation is terminated without closing the defect, peritonitis will occur in the immediate postoperative period.99
Adhesion Development versus Adhesion Reformation
Three groups have demonstrated fundamental differences between adhesion development and adhesion reformation in animal models. Holtz and associates100,101 described reduction in adhesion development with 32% dextran 70, but a similar inhibition of adhesion reformation could not be achieved with higher doses of dextran. Similarly, Elkins and coworkers102,103 observed a greater extent of adhesion reformation than adhesion formation after dextran treatment. Finally, Diamond and associates104,105 compared adhesion formation and reformation models and noted a greater extent of adhesions in the latter. Consistent with these observations, Diamond and Nezhat have proposed a classification of postsurgical adhesion development that differentiates de novo adhesion formation (type I) from adhesion reformation (type II) and subcategorizes each of these groups as to whether there was a lack of or presence of treatment of pathology106 at sites during the initial procedure. Importantly, a recent meta-analysis has validated this classification system by demonstrating increasing risk of adhesions in association with advanced stages.107 Such a system provides a method to assess efficacy of surgical techniques, new instrumentation, and antiadhesion adjuvant therapy.
Second-Look Laparoscopy
Nonetheless, these outcomes do not prove clinical benefits regarding pregnancy. Although Tulandi and colleagues were unable to identify a benefit of second-look laparoscopy 1 year after reproductive surgery, this study was limited by the lack of randomization and the differences in the initial surgeons.108 In contrast, when looking at adhesions as the endpoints, early second-look laparoscopy has been shown to reduce the presence of adhesions at the time of a “third-look” procedure.109 Additionally, reduced rates of ectopic pregnancy have been reported in women having second-look laparoscopies.110
If second-look laparoscopy is to be performed for assessment and possible management of postoperative adhesions, Swolin111 recommended that it be done early (6 to 8 weeks) to improve the possibility of lysis of postoperative adhesions. Subsequently, Raj and Hulka112 examined second-look laparoscopies performed up to 2 years after the initial procedure and demonstrated that bleeding was more common if the procedure was performed more than 12 weeks after surgery or sooner than 2 weeks after surgery. In the former case, bleeding was attributed to increased density and vascularity of the adhesions; in the latter, bleeding was attributed to granulation tissue.
Adjuvant Therapy
In addition to adopting microsurgical principles and optimal surgical techniques to reduce adhesion development, several other approaches have been suggested to help prevent and reduce the severity of adhesion development. Such approaches include the application of intraoperative devices and agents as well as the use of adjuvant medications to prevent adhesions. Table 52-2 includes a list of characteristics possessed by the ideal antiadhesive adjuvant.
Table 52-2 Characteristics of an Ideal Antiadhesive Adjuvant
| Highly efficacious over range of conditions for which it will be utilized |
| High safety profile |
| No interference with tissue healing |
| Easy to handle |
| Easy to deliver |
| Stays where placed |
| Remains in abdominal cavity for sufficient time |
| Will not promote infection |
| Will not interfere with surgical procedure |
| Treats large area |
| Utilizable at open and endoscopic procedures |
| Biosorbable |
| Inexpensive |
When reviewing the potential applications of adhesion barriers, it is important to distinguish the prevention of adhesions at the injury site versus other sites. It is not clear whether applying physical barriers to reduce adhesions at site of application can provide protection in areas other than the site of application.113 It has been shown that adhesions can readily develop at uninjured peritoneal sites distant from the midline incision and that a midline laparotomy initiates a generalized peritoneal inflammatory response.114 Hence, a single preventive measure, such as a physical barrier alone applied to one area, may not completely eliminate adhesion development throughout the abdominal cavity. In fact, the antiadhesion material barrier trials have used, as their endpoint, adhesion development at the site of barrier placement rather than at distant sites.
Potential Mechanisms for Antiadhesion Barriers
There are several different classes of antiadhesion barriers (Table 52-3). Approaches that have been suggested to minimize adhesion development involve one or more of the following mechanisms of action:
| Fibrinolytic agents |
| Anticoagulants |
| Anti-inflammatory agents |
| Antibiotics |
| Mechanical separation |
Table 52-4 gives some examples of various adjuvants that are generally used to prevent surgical adhesions.115,116 Several substances and materials have been studied and used over the years. These commercial adhesion-reducing substances are relatively expensive, with their cost ranging between $100 and $300 per unit. Although much has been written about use of these agents to prevent adhesion development, too little is known about the economic impact of adhesion-reducing agents on the healthcare system.
Table 52-4 Examples of Antiadhesion Barriers
Human trials have not investigated the use of surgical adjuvants in preventing adhesions after radical pelvic surgery; therefore, it remains only speculative that this potential exists. Adhesions may simply represent the normal healing process after peritoneal injury. Indeed, with the degree of tissue destruction associated with radical operations, adhesion development may be physiologic rather than pathologic. Fortunately, animal studies and second-look laparoscopies, once largely limited to the infertility arena, are now being used in gynecologic oncology research and operative procedures by other surgical specialties.18
Activation of the fibrinolytic system is considered beneficial in the prevention of intra-abdominal adhesions. In the late 19th century, agents with potential fibrinolytic capacity, including liquor thiosinamine with sodium salicylate117 and oral phosphorus,2 were advocated. Streptokinase and streptodornase were the first agents with proven fibrinolytic properties that were effective in preventing adhesion development in rabbits and rats.118,119 The value of activation of the fibrinolytic system remains unproven in humans.
Plasminogen Activator
Tissue plasminogen activator, the main plasminogen activator, has often been studied for prevention of adhesions. Although tPA proved to be effective, the risk of hemorrhage has been the main obstacle to its routine use. Anticoagulants such as heparin are also effective in adhesion prevention, although the use of local heparin in abdominal surgery remains controversial because of the risk of bleeding.120–124
Mechanical Separtion of Injured Surfaces
In the past decade several mechanical barriers have been developed. Membranes of oxidized regenerated cellulose,125,126 modified hyalouronic acid and carboxymethylcellulose, or expanded polytetrafluoroethylene127 were found to prevent the development of adhesions. All three coat the trauma site for the time (>7 days) required for re-epithelialization. Johns has reviewed the literature for the available evidence-based prevention of postoperative adhesions.128 He concluded that level 1 evidence supports the efficacy of three barrier methods for the prevention of postoperative adhesions: Interceed, Seprafilm, and Gore-Tex Surgical Membrane. Adhesions are not eliminated by these barriers, and the main debate is the clinical significance with the level of adhesions prevented. Furthermore, most of the efficacy is at the site of application.
Hyaluronan
Hyaluronan-based agents have been shown to prevent adhesions after surgery. The potential of these agents to reduce intra-abdominal adhesion in abdominal sepsis is a new promising concept. Hyaluronan-based antiadhesive agents, including modified hyaluronan–carboxymethylcellulose bioresorbable membrane and 0.4% hyaluronan solution, have been shown to be effective and safe for clinical application. Only the membrane, however, has been approved by the U.S. Food and Drug Administration (FDA) for use in benign diseases under noninfectious conditions.129
Methods of Unknown Benefit
Hydrofloatation, the use of large-volume isotonic solutions such as normal saline and Ringer’s lactate, has not been directly tested in randomized studies for preventing postsurgical adhesions. However, lack of benefit of crystalloids (and in fact statistically significant worsening) was demonstrated by a recent meta-analysis.107 Failure of effectiveness of crystalloids is due, at least in part, from the rapid absorption time. Most crystalloids are absorbed at approximately 30 to 60mL/hour.
However, recent studies have suggested that these crystalloids remain in the peritoneal fluid longer.130 After the instillation of 300mL of Ringer’s lactate solution, 78mL was still present in the peritoneal cavity after 48 hours, compared with 30mL in patients in the control group, in whom no Ringer’s lactate was left.130 By 96 hours there was no difference between the two groups. However, this is still too short a time to have a beneficial effect on adhesion formation.
The disturbed equilibrium between fibrin synthesis and degradation leads to persistence of fibrinous adhesions. These will become ingrown with fibroblasts, and subsequent collagen deposition results in the development of permanent fibrous adhesions. Treatment with halofuginone, an inhibitor of collagen type I synthesis, decreases the development of experimentally induced surgical adhesions.131 Clinical trials have not been conducted yet.
Anti-inflammatory drugs, including corticosteroids and prostaglandin synthetase inhibitors, have been tested for their ability to prevent adhesions.132–134 Down-regulation of the inflammatory response in this way, however, gave conflicting results. Swolin135 successfully reduced adhesion development in patients by applying intraperitoneal steroids, but others have reported equivocal or even deleterious effects.134
Dextran
1 Bryant T. Clinical lectures on intestinal obstruction. Med Tim Gaz. 1872;1:363-365.
2 Wiseman DM. Adhesion prevention: Past the future. In: DiZerega G, DeCherney A, Diamond M, et al, editors. Peritoneal Surgery. New York: Springer; 2000:401-417.
3 Becker JM, Stucchi AF. Intra-abdominal adhesion prevention: Are we getting any closer? Ann Surg. 2004;240:202-204.
4 Hulka JF, Omran K, Berger GS. Classification of adnexal adhesions: A proposal and evaluation of its prognostic value. Fertil Steril. 1978;30:661-665.
5 Diamond MP. Surgical aspects of infertility. In: Sciarra JJ, editor. Gynecology and Obstetrics. Philadelphia: Harper and Row, 2004.
6 Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery: A postmortem study. Am J Surg. 1973;126:345-353.
7 Menzies D, Ellis H. Intestinal obstruction from adhesion: How big is the problem? Ann R Coll Surg Engl. 1990;72:60-63.
8 Ellis H. The clinical significance of adhesions: Focus on intestinal obstruction. Eur J Surg Suppl. 1997;577:5-9.
9 Stovall TG, Elder RF, Ling FW. Predictors of pelvic adhesions. J Reprod Med. 1989;34:345-348.
10 Ray NF, Larsen JW, Stillman RJ, et al. Economic impact of hospitalizations for lower abdominal adhesiolysis in the United States in 1988. Surg Gynecol Obstet. 1993;176:271-276.
11 Al-Jaroudi D, Tulandi T. Adhesion prevention in gynecologic surgery. Obstet Gynecol Surv. 2004;59:360-367.
12 Ray NF, Denton WG, Thamer M, et al. Abdominal adhesiolysis: Inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1-9.
13 Moscowitz I, Wexner S. Contributions of adhesions to the cost of health care. Health Care Financing Administration. MEDPAR Database 1990–1996. In: DiZerega G, DeCherney A, Diamond M, et al, editors. Peritoneal Surgery. New York: Springer-Verlag, 2000.
14 Menzies D, Parker M, Hoare R, et al. Small bowel obstruction due to postoperative adhesions: Treatment patterns and associated costs in 110 hospital admissions. Ann R Coll Surg Engl. 2001;83:40-46.
15 Holmdhal L, Riseberg B. Adhesions: Prevention and complications in general surgery. Eur J Surg. 1997;163:169-174.
16 Wilson M. Cost and economics of adhesions. Hosp Med. 2004;65:343-347.
17 Wilson MS, Menzies D, Knight AD, Crowe AM. Demonstrating the clinical and cost effectiveness of adhesion reduction strategies. Colorectal Dis. 2002;4:355-360.
18 Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: Clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170:1396-1403.
19 Hershlag A, Diamond MP, DeCherney AH. Adhesiolysis. Clin Obstet Gynecol. 1991;34:395-402.
20 Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7:567-576.
21 Welch JP. Adhesions. In: Welch JP, editor. Bowel Obstruction. Philadelphia: WB Saunders; 1990:154-165.
22 Maetani S, Tobe T, Kashiwara S. Neglected role of torsion and constriction in pathogenesis of simple adhesive bowel obstruction. Br J Surg. 1984;71:127-130.
23 Krebs HB, Goplerud DR. Mechanical intestinal obstruction in patients with gynecologic disease: A review of 368 patients. Am J Obstet Gynecol. 1987;157:577-583.
24 Helmkamp BF, Kimmel J. Conservative management of small bowel obstruction. Am J Obstet Gynecol. 1985;152:677-679.
25 Miller EM, Winfield JM. Acute intestinal obstruction secondary to postoperative adhesions. Surgery. 1959;78:952-957.
26 Krook SS. Obstruction of the small intestine due to adhesions and bands. Acta Chir Scand. 1947;95:130-136.
27 Brightwell NL, McFee AS, Aust JB. Bowel obstruction and the long tube stent. Arch Surg. 1977;112:505-511.
28 Fevang BT, Fevang J, Lie SA, et al. Long-term prognosis after operation for adhesive small bowel obstruction. Ann Surg. 2004;240:193-201.
29 Hammoud A, Gago LA, Diamond MP. Adhesions in patients with chronic pelvic pain: A role for adhesiolysis? Fertil Steril. 2004;82:1483-1491.
30 Kligman I, Drachenberg C, Papadimitriou J, Katz E. Immunohistochemical demonstration of nerve fibers in pelvic adhesions. Obstet Gynecol. 1993;82:566-568.
31 Sulaiman H, Gabella G, Davis MC, et al. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann Surg. 2001;234:256-261.
32 Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol. 1980;138:880-892.
33 Rapkin AJ. Adhesions and pelvic pain: A retrospective study. Obstet Gynecol. 1986;68:13-15.
34 Cunanan RG, Courey NG, Lippes J. Laparoscopic findings in patients with pelvic pain. Am J Obstet Gynecol. 1983;146:587-591.
35 Steege JF, Stout AL. Resolution of chronic pelvic pain after laparoscopic lysis of adhesions. Am J Obstet Gynecol. 1991;165:278-281.
36 Peters AAW, Trimbos-Kemper GCM, Admiraal C, Trimbos JB. A randomized clinical trial on the benfit of adhesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. BJOG. 1992;99:59-62.
37 Swank DJ, Van Erp WF, Repelaer Van Driel OJ, et al. A prospective analysis of predictive factors on the results of laparoscopic adhesiolysis in patients with chronic abdominal pain. Surg Laparosc Endosc Percutan Tech. 2003;13:88-94.
38 Swank DJ, Swank-Bordewijk SC, Hop WC, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: A blinded randomised controlled multi-centre trial. Lancet. 2003;361:1247-1251.
39 Markman M, Jones W, Lewis JL, et al. Impact of laparotomy finding of significant intra-abdominal adhesions on the surgically defined complete response rate to subsequent salvage intraperitoneal chemotherapy. J Cancer Res Clin Oncol. 1992;118:163-165.
40 Raftery AT. Regeneration of peritoneal and visceral peritoneum. A light microscopical study. Br J Surg. 1973;60:293-299.
41 Holmdahl L. The plasmin system, a marker of the propensity to develop adhesions. In: DiZerega G, DeCherney A, Diamond M, et al, editors. Peritoneal Surgery. New York: Springer; 2000:117-131.
42 DiZerega GS. Peritoneum, peritoneal healing and adhesion formation. In: DiZerega G, DeCherney A, Diamond M, et al, editors. Peritoneal Surgery. New York: Springer; 2000:3-38.
43 Montz FJ, Shimanuki T, DiZerega GS. Postsurgical mesothelial re-epithelization. In: DeCherney AH, Polan ML, editors. Reproductive Surgery. Chicago: Year Book; 1987:31-47.
44 Binda MM, Molinas CR, Koninck PR. Reactive oxygen species and adhesion formation. Clinical implications in adhesion prevention. Hum Reprod. 2003;18:2503-2507.
45 Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-β1 in human peritoneal fibroblasts. Fertil Steril. 2002;78:144-147.
46 Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: The adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11:307-314.
47 Saed GM, Diamond MP. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2003;79:164-168.
48 Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763-768.
49 Saed GM, Collins KL, Diamond MP. Transforming growth factors β1, β2 and β3 and their receptors are differentially expressed in human peritoneal fibroblasts in response to hypoxia. Am J Reprod Immunol. 2002;48:387-393.
50 Saed GM, Munkarah AR, Diamond MP. Cyclooxygenase-2 is expressed in human fibroblasts isolated from intraperitoneal adhesions but not from normal peritoneal tissues. Fertil Steril. 2003;79:1404-1408.
51 Myhre-Johnson O, Larsen SB, Astrup T. Fibrinolytic activity in serosal and synovial membranes. Arch Pathol. 1969;88:623-630.
52 Diamond MP, El-Hammady E, Wang R, et al. Regulation of expression of tissue plasminogen activator and plasminogen activator inhibitor-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Am J Obstet Gynecol. 2004;190:926-934.
53 Ivarsson ML, Diamond MP, Falk P, Holmdahl L. Plasminogen activator/plasminogen activator inhibitor-1 and cytokine modulation by the PROACT System. Fertil Steril. 2003;79:987-992.
54 Robbins GF, Brunschwig A, Foote FW. Deperitonealization: Clinical and experimental observations. Ann Surg. 1949;130:466-479.
55 Buckman RF, Buckman PD, Hufnagel HV, Gervin AS. A physiologic basis for the adhesion-free healing of deperitonealized surfaces. J Surg Res. 1976;21:67-76.
56 Mecke H, Schunke M, Schulz S, Semm K. Incidence of adhesions following thermal tissue damage. Res Exp Med. 1991;191:405-411.
57 O’Leary DP, Coakley JB. The influence of suturing and sepsis on the development of postoperative peritoneal adhesions. Ann R Coll Surg Eng. 1992;74:134-137.
58 Holtz G. Adhesion induction by suture of varying tissue reactivity and caliber. Int J Fertil. 1982;27:134-135.
59 Morgenstern L, Hart M, Lugo D, Friedman NB. Changing aspects of radiation enteropathy. Arch Surg. 1985;120:1225-1228.
60 Soderstrom RM. Preventing adhesions: Electrosurgery—advantages and disadvantages. In: diZerega GS, Malinak LR, Diamond MP, Linsky DB, editors. Treatment of Post-Surgical Adhesions. New York: Wiley-Liss; 1990:841-844.
61 Ivarsson M-L, Holmdahl L, Eriksson E, et al. Expression and kinetics of fibrinolytic components in plasma and peritoneum during abdominal surgery. Fibrin Proteo. 1998;12:61-67.
62 Ellis H. The cause and prevention of postoperative intraperitoneal adhesions. Surg Gynecol Obstet. 1971;133:497-511.
63 Almdahl SM, Burhol PG. Peritoneal adhesions: Causes and prevention. Dig Dis. 1990;8:37-44.
64 Ellis H. The hazards of surgical glove dusting powders. Surg Gynecol Obstet. 1990;171:521-527.
65 Down RHL, Whitehead R, Watts JMcK. Do surgical packs cause peritoneal adhesions? Aust NZ J Surg. 1979;49:379-382.
66 Elkins TE, Stovall TG, Warren J, et al. A histologic evaluation of peritoneal injury and repair: Implications for adhesion formation. Obstet Gynecol. 1987;70:225-228.
67 Saxén L, Myllärniemi H. Foreign material and postoperative adhesions. NEJM. 1968;279:200-202.
68 Perry JF, Smith GA, Yonehiro EG. Intestinal obstruction caused by adhesions. Ann Surg. 1955;142:810-816.
69 Barnhill D, Doering D, Remmenga S, et al. Intestinal surgery performed on gynecologic cancer patients. Gynecol Oncol. 1991;40:38-41.
70 Alvarez RD. Gastrointestinal complications in gynecologic surgery: A review for the general gynecologist. Obstet Gynecol. 1988;71:533-1540.
71 Duffy DM, diZerega GS. Is peritoneal closure necessary? Obstet Gynecol Surv. 1994;49:817-822.
72 Wilkinson C, Enkin M: Peritoneal non-closure at cesarean section. In Neilson J, Crowther C, Hodnett E, Hofmeryr G (eds). Pregnancy and Childbirth Module. Cochrane Database Syst Rev 1998; CD000163.
73 Conolly WB, Stephens FO. Factors influencing the incidence of intraperitoneal adhesions: An experimental study. Surgery. 1968;63:976-979.
74 Ellis H. The etiology of postoperative abdominal adhesions. Br J Surg. 1962;50:10-16.
75 Al-Took S, Platt R, Tulandi T. Adhesion-related small bowel obstruction after gynecologic operations. Am J Obstet Gynecol. 1999;180:313-315.
76 Tulandi T. Peritoneal closure and adhesions. Hum Reprod. 2002;17:249-250.
77 Meyer WR, Grainger DA, DeCherney AH, et al. Ovarian surgery on the rabbit: Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
78 Larsson B, Perbeck L. The possible advantage of keeping the uterine and intestinal serosa irrigated with saline to prevent intra-abdominal adhesions in operations for fertility. An experimental study in rats. Acta Chir Scand Suppl. 1986;530:15-18.
79 Zamir G, Bloom AI, Reissman P. Prevention of intestinal adhesions after laparotomy in a rat model—a randomized prospective study. Res Exp Med (Berl). 1998;197:349-353.
80 Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: Evaluation at early second look procedures. Fertil Steril. 1991;55:700-704.
81 Nagle A, Ujiki M, Denham W, et al. Laparoscopic adhesiolysis for small bowel obstruction. Am J Surg. 2004;187:464-470.
82 Ordonez JL, Dominguez J, Evrard V, Koninckx PR. The effect of training and duration of surgery on adhesion formation in the rabbit model. Hum Reprod. 1997;12:2654-2657.
83 Yesildaglar N, Ordonez JL, Laermans I, Koninckx PR. The mouse as a model to study adhesion formation following endoscopic surgery: A preliminary report. Hum Reprod. 1999;14:55-59.
84 Binda MM, Molinas CR, Mailova K, Koninckx PR. Effect of temperature upon adhesion formation in a laparoscopic mouse model. Hum Reprod. 2004;19:2626-2632.
85 Hazebroek EJ, Schreve MA, Visser P, et al. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A. 2000;12:355-364.
86 Molinas CR, Koninckx PR. Hypoxaemia induced by CO2 or helium pneumoperitoneum is a co-factor in adhesion formation in rabbits. Hum Reprod. 2000;15:1758-1763.
87 Molinas CR, Mynbaev O, Pauwels A, et al. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560-567.
88 West MA, Hackam DJ, Baker J, et al. Mechanism of decreased in vitro murine macrophage cytokine release after exposure to carbon dioxide: Relevance to laparoscopic surgery. Ann Surg. 1997;226:179-190.
89 Gray RI, Ott DE, Henderson AC, et al. Severe local hypothermia from laparoscopic gas evaporative jet cooling: A mechanism to explain clinical observations. JSLS. 1999;3:171-177.
90 Ott DE. Laparoscopy and tribology: The effect of laparoscopic gas on peritoneal fluid. J Am Assoc Gynecol Laparosc. 2001;8:117-123.
91 Volz J, Koster S, Spacek Z, Paweletz N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13:611-614.
92 Martin DC, Diamond MP, Yussman MA. Laser laparoscopy for infertility surgery. In: Sanfillippo J, Levine R, editors. Operative Gynecologic Endoscopy. New York: Springer-Verlag; 1989:211-235.
93 Diamond MP. Assessment of results of laser surgery. In: Sutton CJG, editor. Bailliere’s Clinical Obstetrics and Gynecology: Laparoscopic Surgery. London: Bailliere Tindall; 1989:649-654.
94 Diamond MP. Assessment of results of laparoscopic laser surgery. In: Sutton CJG, editor. Lasers in Gynaecology. London: Chapman & Hall Medical; 1992:55-72.
95 Daly DC. Hysteroscopy and infertility. In: Sciarra JJ, editor. Gynecology and Obstetrics. Philadelphia: Harper & Row, 1986.
96 Martin DC, Diamond MP. Extended laparoscopic surgery: Comparison of laser and other techniques. Curr Probl Obstet Gynecol Fertil. 9, 1986.
97 Daniell JF. The role of lasers in infertility surgery. Fertil Steril. 1984;42:815-823.
98 Dixon JA. Lasers in surgery. Curr Probl Surg. 1984;21:1-65.
99 Paloyan D. Intestinal problems in gynecologic surgery. In: Schiarra JJ, editor. Gynecology and Obstetrics. Philadelphia: Harper and Row, 2004.
100 Holtz G, Baker E, Tsai C. Effect of 32% dextran 70 on peritoneal adhesion formation and reformation after lysis. Fertil Steril. 1980;33:660-662.
101 Holtz G, Baker ER. Inhibition of peritoneal adhesion reformation after lysis with 32% dextran 70. Fertil Steril. 1980;34:394-395.
102 Elkins TE, Bury RJ, Ritter JL, et al. Adhesion prevention by solutions of sodium carboxymethylcellulose in the rat: I. Fertil Steril. 1984;41:926-928.
103 Elkins TE, Ling FW, Ahokas RA, et al. Adhesion prevention by solutions of sodium carboxymethylcellulose in the rat: II. Fertil Steril. 1984;41:929-932.
104 Diamond MP, DeCherney AH, Linsky CB, et al. Assessment of carboxymethylcellulose and 32% dextran 70 for prevention of adhesions in a rabbit uterine horn model. Int J Fertil. 1988;33:278-282.
105 Diamond MP, DeCherney AH, Linsky CB, et al. Adhesion reformation in the rabbit uterine horn model: I. Reduction with carboxymethylcellulose. Int J Fertil. 1988;33:372-375.
106 Diamond MP, Nezhat F. Adhesions after resection of ovarian endometriomas. Fertil Steril. 1993;59:934-935.
107 Wiseman DM, Trout JR, Franklin RR, Diamond MP. Meta-analysis of the safety and efficacy of an adhesion barrier (Interceed TC7) in laparotomy. J Reprod Med. 1999;44:325-331.
108 Tulandi T, Falcone T, Kafka I. Second-look operative laparoscopy 1 year following reproductive surgery. Fertil Steril. 1989;52:421-424.
109 Ugur M, Turan C, Mungan T, et al. Laparoscopy for adhesion prevention following myomectomy. Int J Gynaecol Obstet. 1996;53:145-149.
110 Lavy G, Diamond MP, DeCherney AH. Ectopic pregnancy: Its relationship to tubal reconstructive surgery. Fertil Steril. 1987;47:543-556.
111 Swolin K. Electromicrosurgery and salpingostomy long-term results. Am J Obstet Gynecol. 1975;121:418-419.
112 Raj SC, Hulka JF. Second-look laparoscopy in infertility surgery: Therapeutic and prognostic value. Fertil Steril. 1982;38:325-329.
113 Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: A prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297-306.
114 Reed KL, Fruin AB, Bishop-Bartolomei KK, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. J Surg Res. 2002;108:165-172.
115 Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: Application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
116 Adhesion Study Group. Reduction of postoperative pelvic adhesions with intraperitoneal 32% dextran 70: A prospective, randomized clinical trial. Fertil Steril. 1983;40:612-619.
117 Boys F. The prophylaxis of peritoneal adhesions. A review of the literature. Surgery. 1942;11:118-168.
118 Wright LT, Smith DH, Rothman M, et al. Prevention of postoperative adhesions in rabbits with streptococcal metabolites. Proc Soc Exp Biol Med. 1950;75:602-604.
119 James DCO, Ellis H, Hugh TB. The effect of streptokinase on experimental intraperitoneal adhesion formation. J Pathol Bacteriol. 1965;90:279-287.
120 Lai HS, Chen Y, Chang KJ, Chen WJ. Tissue plasminogen activator reduces intraperitoneal adhesion after intestinal resection in rats. J Formos Med Assoc. 1998;97:323-327.
121 Buckenmaier CC3rd, Pusateri AE, Harris RA, Hetz SP. Comparison of antiadhesive treatments using an objective rat model. Am Surg. 1999;65:274-282.
122 Menzies D, Ellis H. The role of plasminogen activator in adhesion prevention. Surg Gynecol Obstet. 1991;172:362-366.
123 Dunn RC, Mohler M. Effect of varying days of tissue plasminogen activator therapy on the prevention of postsurgical adhesions in a rabbit model. J Surg Res. 1993;54:242-245.
124 Evans DM, McAree K, Guyton DP, et al. Dose dependency and wound healing aspects of the use of tissue plasminogen activator in the prevention of intra-abdominal adhesions. Am J Surg. 1993;165:229-232.
125 Farquhar C, Vandekerckhove P, Watson A, et al. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000. CD000475.
126 Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
127 Hellebrekers BW, Trimbos-Kemper GC, van Blitterswijk CA, et al. Effects of five different barrier materials on postsurgical adhesion formation in the rat. Hum Reprod. 2000;15:1358-1363.
128 Johns A. Evidence-based prevention of post-operative adhesions. Hum Reprod Update. 2001;7:577-579.
129 Reijnen MM, Bleichrodt PJ, van Goor RP. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg. 2003;90:533-541.
130 Muzii L, Bellati F, Manci N, et al. Ringer’s lactate solution remains in the peritoneal cavity after laparoscopy longer than expected. Fertil Steril. 2005;84:148-153.
131 Nagler A, Rivkind AI, Raphael J, et al. Halofuginone—an inhibitor of collagen type I synthesis—prevents postoperative formation of abdominal adhesions. Ann Surg. 1998;227:575-582.
132 Siegler AM, Kontopoulos V, Wang CF. Prevention of postoperative adhesions in rabbits with ibuprofen, a nonsteroidal anti-inflammatory agent. Fertil Steril. 1980;34:46-49.
133 Jansen RP. Failure of intraperitoneal adjuncts to improve the out-come of pelvic operations in young women. Am J Obstet Gynecol. 1985;153:363-371.
134 Larsson B. Prevention of postoperative formation, reformation of pelvic adhesions. In: Treutner KH, Schumpelick V, editors. Peritoneal Adhesions. Berlin: Springer; 1997:331-334.
135 Swolin K. The effect of a massive intraperitoneal dose of glucocorticoid on the formation of postoperative adhesions: Clinical studies using laparoscopy in patients operated on for extrauterine pregnancy. Acta Obstet Gynecol Scand. 1967;46:204-218.