Chapter 65 Acute Coronary Syndromes
Table 65-1 Pharmacologic Characteristics of Fibrinolytic Drugs Used in the Management of Patients With ST-Segment Elevation Myocardial Infarction
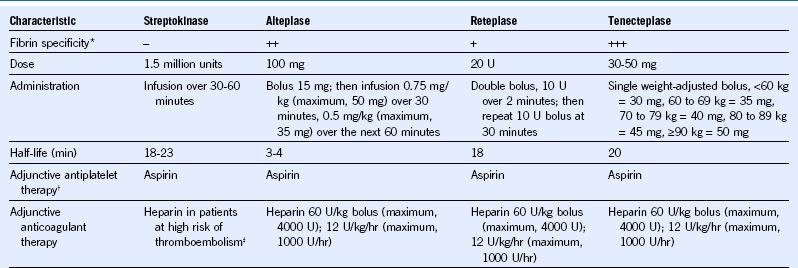
* Less fibrin specificity is associated with more systemic fibrinogen depletion.
Case 1: Bleeding After Fibrinolytic Therapy
Comment
Fibrinolytic trials report a 1% to 6% incidence of major bleeding and a 10% incidence of moderate bleeding during the first 30 days. The most common sources of major bleeding are the gastrointestinal tract and procedure-related bleeding. The principles of management of major bleeding in STEMI patients treated with fibrinolytic therapy are summarized in Table 65-2. Steps include (1) stop antithrombotic therapies; (2) use local measures when possible to control bleeding; (3) draw blood to measure fibrinogen, prothrombin time (PT), aPTT, and possibly anti-Xa levels (to measure the anticoagulant effect of low-molecular-weight heparin) and to crossmatch blood; and (4) administer therapies to mitigate or reverse the effects of fibrinolytic, antiplatelet, and anticoagulant drugs.
Table 65-2 Management of Major Bleeding in Patients With ST-Segment Elevation Myocardial Infarction Treated With Fibrinolytic Therapy
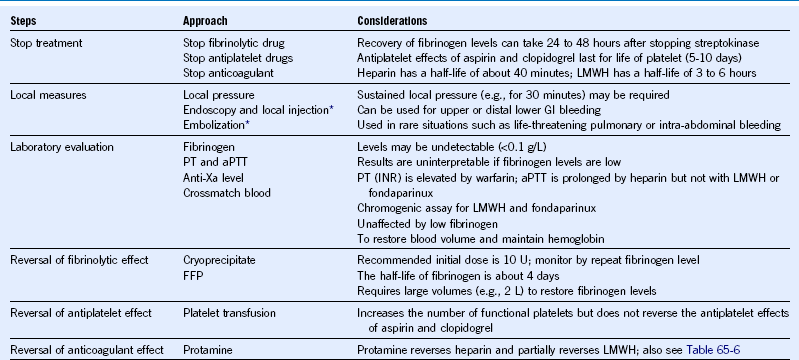
aPTT, Activated partial thromboplastin time; FFP, fresh-frozen plasma; GI, gastrointestinal; INR, international normalized ratio; LMWH, low-molecular-weight heparin; PT, prothrombin time
Table 65-3 Pharmacologic Characteristics of Oral Antiplatelet Drugs Commonly Used in the Management of Acute Coronary Syndromes
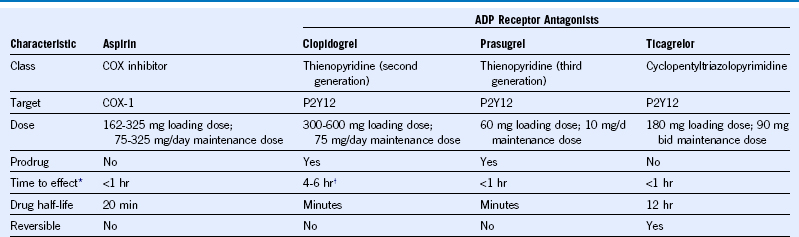
ADP, Adenosine diphosphate; bid, twice daily; COX, cyclooxygenase.
Case 2: Stent Thrombosis
Comment
Stent thrombosis is a potentially life-threatening complication of PCI, affecting 1% to 2% of patients during the first year and presenting in almost all cases as death or MI.1 Risk factors for stent thrombosis can be categorized as patient related, technical (procedure, stent, or lesion), or drug related. The single most important predictor of stent thrombosis is premature discontinuation of clopidogrel. High on-treatment platelet reactivity during clopidogrel therapy has also emerged as a predictor of stent thrombosis and is affected by clinical (e.g., age, diabetes, renal insufficiency) and genetic factors. Carriers of reduced function CYP2C19 alleles have low levels of the active metabolite of clopidogrel, diminished platelet inhibition, and an increased risk of stent thrombosis. Unlike clopidogrel, which undergoes two-step, cytochrome P450-dependen, metabolic conversion in the liver, prasugrel and ticagrelor are not affected by CYP polymorphisms and consistently produce a greater and more consistent level of platelet inhibition than standard clopidogrel doses. Higher doses of clopidogrel (e.g., 225 or 300 mg/day) in patients heterozygous for CYP2C19*2 alleles achieve levels of platelet inhibition similar to those seen with standard (75 mg) doses but have not been evaluated in clinical outcome studies.
Case 3: Thrombocytopenia After Stenting
Comment
Severe thrombocytopenia (platelet count <50 × 109/L) occurs in about 0.5% and less severe thrombocytopenia in 2% to 4% of patients treated with GP IIb/IIIa inhibitors.3 Thrombocytopenia caused by GP IIb/IIIa inhibitors is readily distinguished from other causes by its rapid onset, typically within 24 hours of exposure and sometimes within with first hour, and severity (count often <10 × 109/L). By contrast, heparin-induced thrombocytopenia is usually delayed until at least 4 days after starting heparin therapy (with the exception of patients with prior exposure to heparin in the past 3 months) and platelet counts rarely fall below 30 × 109/L. The very rapid onset of thrombocytopenia is believed to be caused by preformed antibodies that react with GP IIb/IIIa inhibitor–coated platelets. Spontaneous recovery of the platelet count usually occurs within days but can take several weeks. Patients with GP IIb/IIIa inhibitor–induced thrombocytopenia respond normally to platelet transfusions, which should be considered when the count is below 10 × 109/L to reduce the risk of spontaneous bleeding. There is no evidence that steroids or IV gamma globulin alter the natural history of GP IIb/IIIa inhibitor–induced thrombocytopenia. Repeated exposure to GP IIb/IIIa inhibitors should be avoided because there is a risk of recurrent thrombocytopenia, which may be more severe than the initial episode.
Table 65-5 Pharmacologic Characteristics of Parenteral Anticoagulants Commonly Used in the Management of Patients With Acute Coronary Syndromes
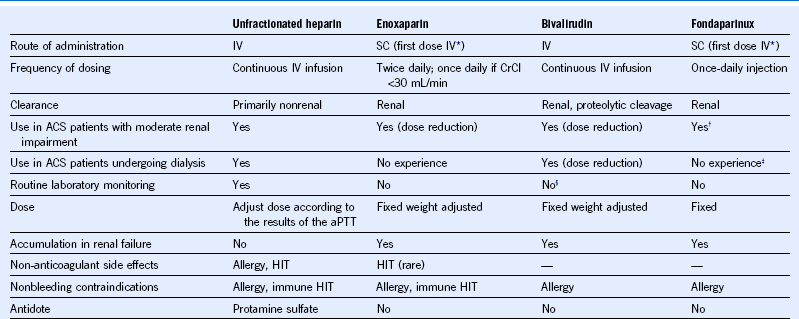
ACS, acute coronary syndromes; aPTT, activated partial thromboplastin time; CrCl, creatinine clearance; HIT, heparin-induced thrombocytopenia; IV, intravenous; SC, subcutaneous.
§ Monitoring and dose adjustment required in patients with creatinine clearance below 30 mL/min.
Table 65-6 Pharmacological Characteristics of Warfarin and New Oral Anticoagulants Evaluated in Phase 3 Trials for the Long-Term Management of Acute Coronary Syndromes
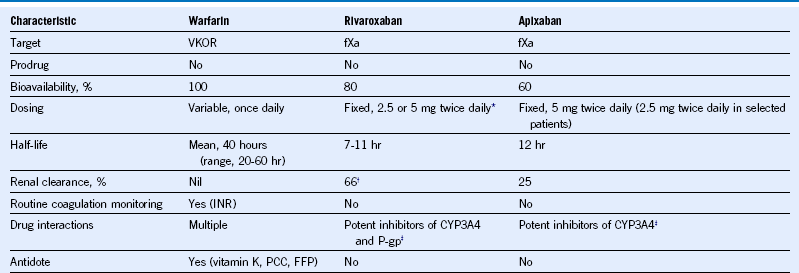
CYP-3A4, Cytochrome p-450 3A4; FFP, fresh-frozen plasma; fXa, activated factor X; INR, international normalized ratio; PCC, prothrombin complex concentrates; P-gp, P-glycoprotein; VKOR, vitamin K epoxide reductase.
* A once-daily regimen was tested in atrial fibrillation.
† Half of renally cleared rivaroxaban is cleared as unchanged drug and half as inactive metabolites.
Table 65-7 Strategies Aimed at Minimizing the Risk of Bleeding in Patients Treated With Triple Therapy (Dual Antiplatelet Therapy and an Oral Anticoagulant)
| Proposed Approach | Rationale |
|---|---|
| Aspirin maintenance dose ≤100 mg/day | Higher aspirin maintenance doses increase bleeding, and there is no evidence that they improve efficacy. |
| PPI with a preference for agents that interfere less with CYP 2C19 (e.g., pantoprazole) | Much of the excess bleeding is from the GI tract. The use of acid-suppressive agents that interfere less with CYP 2C19 minimizes the potential for a negative interaction with clopidogrel. |
| For warfarin, use a target INR of 2 to 2.5 | Some evidence that a restricted target INR range reduces the risk of bleeding. |
| Manage warfarin in a specialized anticoagulation clinic | Compared with usual care, specialist clinics achieve a higher time-in-therapeutic-range of the INR. |
| Minimize duration of triple therapy | The risk of bleeding is highest during the first 30 days but remains elevated with long-term treatment. |
| Avoid NSAIDs | NSAIDs are a common cause of upper GI bleeding. |
| Avoid prasugrel and ticagrelor | Prasugrel and ticagrelor cannot be recommended because they are more potent and cause more bleeding than clopidogrel. |
CYP, Cytochrome P450; GI, gastrointestinal; INR, international normalized ratio; NSAID, nonsteroidal antiinflammatory drug; PPI, proton pump inhibitor.
Case 4: Triple Therapy
Comment
Anticoagulation is indicated for the management of left ventricular thrombosis, and the combination of aspirin and clopidogrel is indicated for the management of patients with drug-eluting stents. The combination of anticoagulation and dual antiplatelet therapy is associated with a 2% to 3% incidence of major bleeding during the first 30 days and a 4% to 12% incidence during the first year. Strategies that may help to minimize the risk of bleeding in patients receiving triple antithrombotic are summarized in Table 65-7. The new oral anticoagulants dabigatran etexilate (110 mg twice a day) and apixaban (5 mg twice a day) may offer an advantage if they are used instead of warfarin in patients who require triple antithrombotic therapy because they cause less bleeding than warfarin in direct head-to-head comparisons.5,6 However, all anticoagulants, including the new oral agents, increase the risk of bleeding when added to dual antiplatelet therapy.
1 Marchini JF, Manica A, Croce K. Stent thrombosis: Understanding and managing a critical problem. Curr Treat Options Cardiovasc Med. 2012;14:91.
2 Holmes MV, Perel P, Shah T, et al. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704.
3 Aster RH, Curtis BR, Bougie DW, et al. Thrombocytopenia associated with the use of GPIIb/IIIa inhibitors: position paper of the ISTH working group on thrombocytopenia and GPIIb/IIIa inhibitors. J Thromb Haemost. 2006;4:678.
4 Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv. 2011;4:522.
5 Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139.
6 Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin Q in patients with atrial fibrillation. N Engl J Med. 2011;365:981.








