Chapter 18 Acute Coronary Syndromes
The term acute coronary syndrome covers a broad spectrum of clinical situations, from unstable angina to ST-segment elevation myocardial infarction (STEMI). These are, with rare exceptions (Table 18-1), a consequence of acute thrombus formation related to a disrupted coronary atherosclerotic plaque. Over the past decade, tremendous progress has been made in our understanding of the pathophysiology, classification, patient risk stratification, and management of acute coronary syndromes. However, they remain an important cause of morbidity and mortality; they were the most common cause of adult hospital admissions in the United States in 2001.
Table 18-1 Causes of Regional Myocardial Ischemia Other Than Atherosclerotic Disease
| Spontaneous coronary artery dissection |
| Coronary emboli (thrombus, vegetations, atrial myxoma, valve leaflet calcification) |
| Coronary artery spasm |
| Coronary arteritis |
| Aortic dissection involving the aortic root |
| Transplant vasculopathy |
PATHOPHYSIOLOGY
The coronary atherosclerotic plaque is the hallmark lesion of coronary artery disease. It is located in the intima of the artery and consists of a central lipid core surrounded by a fibrous capsule which separates the core from the vessel lumen (Fig. 18-1A). Atherosclerotic plaques most likely originate from preexisting intimal lesions (intimal masses or thickenings and intimal xanthoma or fatty streaks) that are present from childhood. The majority of these intimal lesions regress or remain stable. However, in the presence of atherogenic risk factors (smoking, hypertension, hyperglycemia, dyslipidemia)—which result in endothelial cell dysfunction and inflammation—these intimal lesions can lead to the formation of atherosclerotic plaques. Endothelial cell dysfunction and inflammation are key features of this process.1,2
Thrombus Formation on Vulnerable Atherosclerotic Plaques
The underlying pathophysiologic process of acute coronary syndromes involves thrombus formation on an atherosclerotic plaque (Fig. 18-1B).3 Three separate mechanisms appear to result in thrombus formation: (1) plaque rupture; (2) plaque erosion; (3) thrombosis associated with a calcified nodule (Table 18-2).
Table 18-2 Plaque Thrombosis: Mechanisms and Plaque Characteristics
Rights were not granted to include this table in electronic media. Please refer to the printed book.
(Figures from Naghavi M, Libby P, Falk E, et al: From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 108:1664, 2003.)
Following plaque disruption the subendothelial connective tissue, the tissue-factor-rich lipid core, or both are exposed to blood. This results in platelet activation and aggregation and in stimulation of the coagulation cascade, eventually leading to thrombus formation. Thrombus may be limited and remain within the plaque, in which case it is clinically silent. Alternatively, thrombus formation may progress and become exposed to blood flow within the artery lumen (mural thrombus). Platelet-thrombin emboli may pass distally and cause microvascular obstruction and result clinically in a non-ST-elevation acute coronary syndrome. Further growth of the thrombus eventually leads to intraluminal obstruction with resultant macrovascular ischemia. When thrombus causes total vessel occlusion, an ST-elevation acute coronary syndrome results.
The morphologies of plaques that are at high risk for thrombus formation through these mechanisms are quite distinct.4 Furthermore, the majority of these lesions are non-flow-limiting stenoses of less than 70% diameter. Therefore, the risk for future acute coronary ischemic events appears to be dependent largely on the presence of these morphologically distinct plaques, rather than on the presence of severely stenotic lesions. The term vulnerable plaque is used to describe lesions that are prone to thrombus formation.
Vulnerable Patient
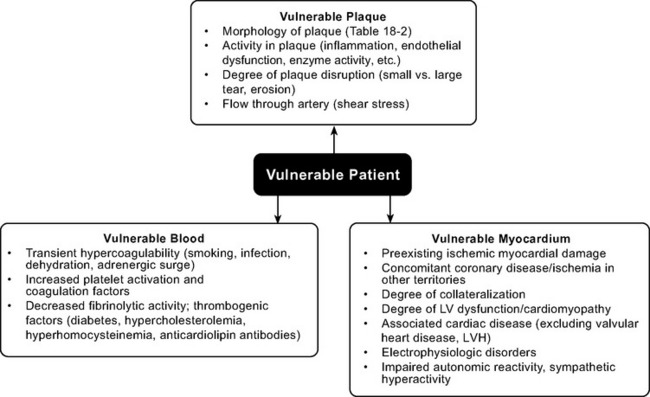
Whether disruption of a vulnerable plaque in an individual patient results in a clinical event is dependent not only on local plaque-related factors but also on the thrombogenicity of the patient’s blood and the state of the myocardium at the time of the event. The concept of the vulnerable plaque has been extended to include vulnerable blood, vulnerable myocardium, and the vulnerable patient (Fig. 18-2).5–7 Assessment and treatment strategies should address the vulnerable patient.
CLASSIFICATION OF ACUTE CORONARY SYNDROMES
Establishing a Working Diagnosis of Acute Coronary Syndrome
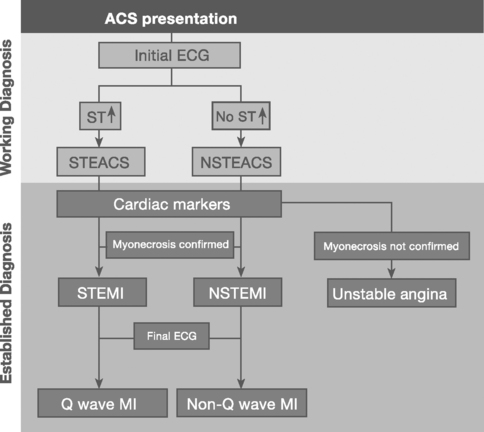
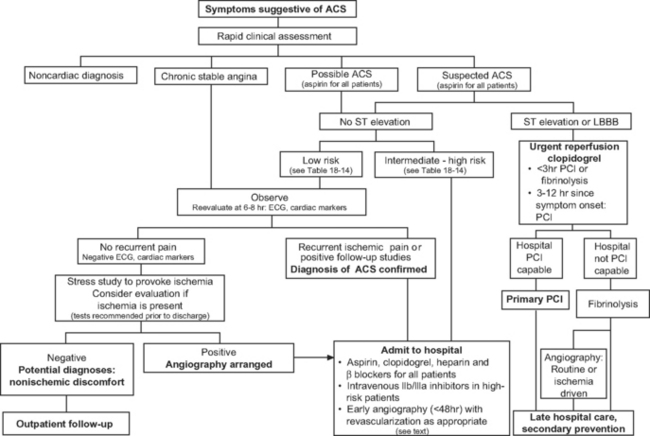
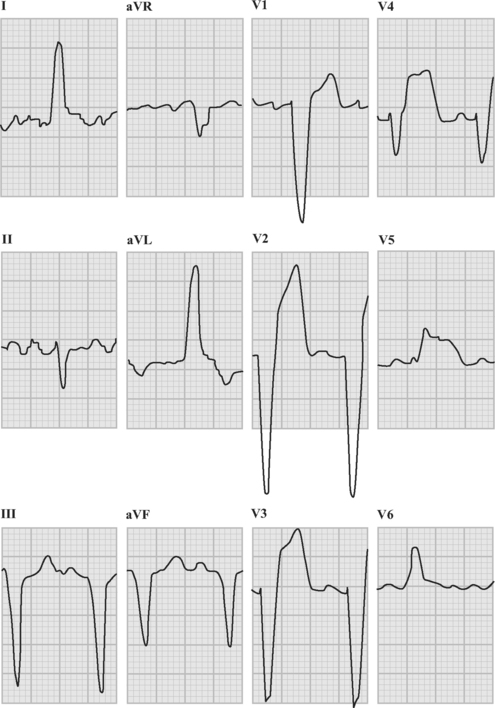
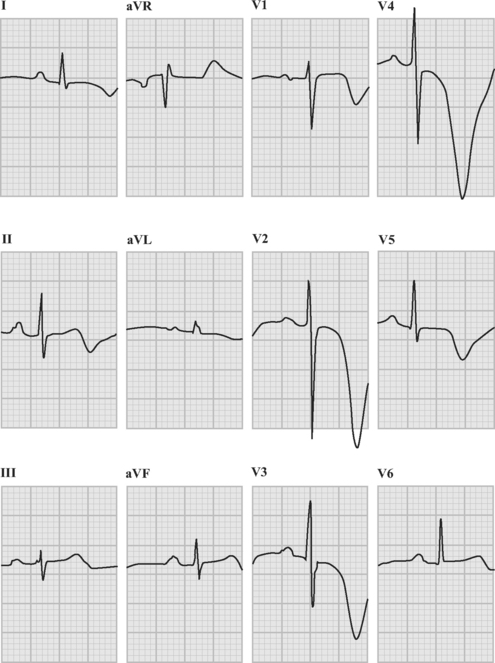
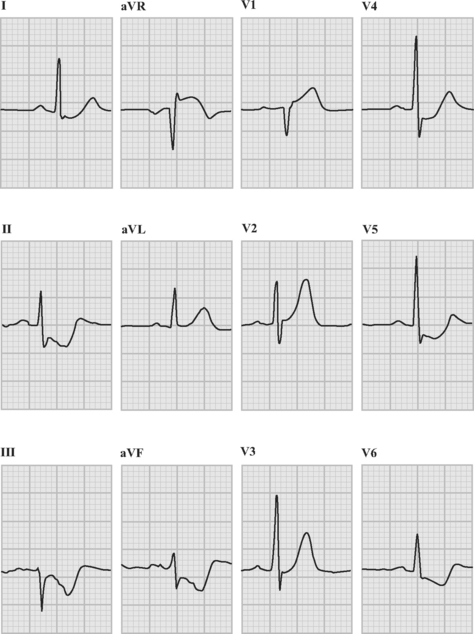
Patients with acute coronary syndromes are classified into two groups on the basis of ST-segment changes on their admission electrocardiograms (ECGs) (Fig. 18-3). This classification provides an initial working diagnosis so that appropriate therapy can be initiated.
ST-Segment Elevation Acute Coronary Syndrome
Sustained ST-segment elevation on an ECG, in the context of an acute coronary syndrome, is usually indicative of an occluded coronary artery. Included within this subset are those patients presenting with a presumed new left bundle branch block (LBBB) pattern on the initial ECG. These patients require urgent pharmacologic or catheter-based reperfusion.8
Non-ST-Segment Elevation Acute Coronary Syndrome
In the absence of sustained ST elevation, patients with acute coronary syndromes represent a heterogeneous population, spanning transient ST-segment elevation, ST-segment depression, T-wave inversion, and the absence of ECG changes. This group of patients does not require urgent reperfusion therapies and, in fact, pharmacologic reperfusion is harmful. The ECG provides important prognostic information upon which antithrombotic treatment and early revascularization can be initiated—either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG).9
Establishing a Final Diagnosis of Myocardial Infarction
Myocardial infarction is defined as myocyte necrosis in the setting of an acute coronary syndrome, PCI, or CABG (Table 18-3). The diagnosis of myocardial infarction in a patient with an acute coronary syndrome is based on: (1) biochemical markers; (2) evolving ECG changes; (3) clinical features.
The European Society of Cardiology and American College of Cardiology: Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur Heart J 21:1502-1513, 2000.
CK-MB, MB fraction of creatine kinase; PCI, percutaneous coronary intervention.
Biochemical evidence of myocardial infarction involves a typical pattern of elevation followed by a gradual fall of the troponins (either I or T) or of the creatine kinase MB fraction (CK-MB) (see Table 18-6 and Fig. 18-11 in Laboratory Tests, later in the text). When using troponin T, a level above 0.03 μg/l is considered elevated; the cut-off for troponin I depends on the assay used. CK-MB increases more rapidly than the troponins and provides early evidence of myocardial infarction, but CK-MB is a less sensitive and less specific marker of myocyte damage.
ASSESSMENT AND DIAGNOSIS OF ACUTE CORONARY SYNDROMES
A targeted history, a clinical examination, and investigations are needed to confirm a diagnosis of acute coronary syndrome, to exclude other causes of the presenting symptoms (Table 18-4), to risk-stratify the patient, and to initiate appropriate therapy without delay. The assessment and risk stratification of a patient should be a dynamic process throughout his or her hospitalization (Fig. 18-4).
Table 18-4 Differential Diagnosis of Symptoms Associated With Acute Coronary Syndromes
| Aortic dissection |
| Pulmonary embolus |
| Peptic ulcer |
| Tension pneumothorax |
| Esophageal rupture with mediastinitis |
| Pericarditis |
| Gastroesophageal reflux and spasm |
| Musculoskeletal/chest wall pain |
| Pneumonia/pleurisy |
| Biliary or pancreatic pain |
| Cervical disk or neuropathic pain |
History
Chest pain or discomfort is the principal symptom of myocardial ischemia in the majority of patients with acute coronary syndromes. Most often the discomfort is central or retrosternal and is described as crushing, squeezing, heavy, or tight and may radiate into the back between the shoulder blades, neck, jaw, left shoulder, or arm. Accompanying symptoms may include shortness of breath, weakness, sweating, nausea, and vomiting. Some patients, notably diabetic patients and the elderly, may have no discomfort but present with breathlessness, syncope, palpitations, or nonspecific symptoms of general lack of wellness.
Clinical Examination
Physical signs of left ventricular failure may be present, such as S3 and S4 heart sounds, crackles and wheezes on chest auscultation, and evidence of systemic hypoperfusion. A mid or late systolic murmur may indicate the presence of mitral regurgitation. Cardiogenic shock occurs in about 4% of patients with ST-elevation acute coronary syndrome (see Chapter 19).
Electrocardiogram
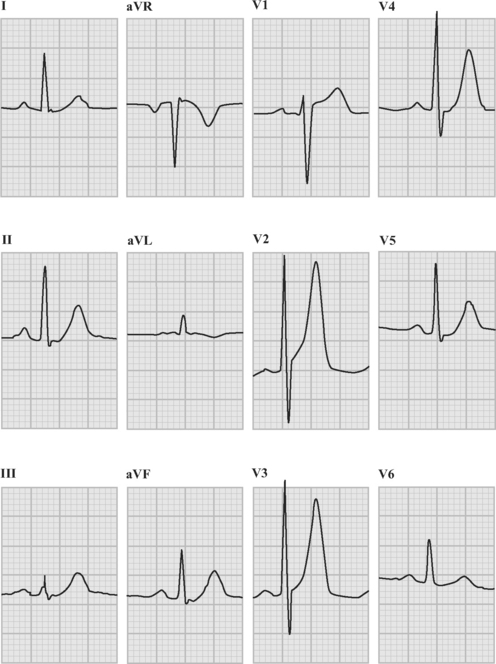
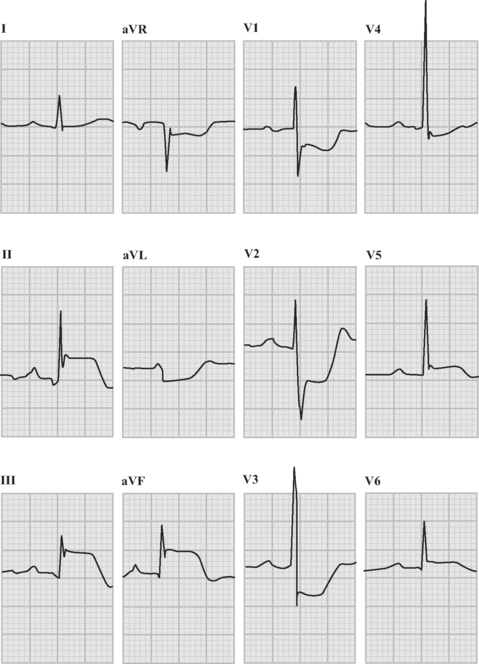
An ECG should be obtained and reviewed immediately on presentation. If the initial ECG is normal or only mildly abnormal (Fig. 18-5) and there is a high clinical suspicion of myocardial infarction, serial ECGs should be performed at 5- to 10-minute intervals or, ideally, continuous ST-segment monitoring should be instituted. ECGs should be obtained every 6 to 8 hours in all other patients until an established diagnosis has been made.
ECG in ST-Elevation Acute Coronary Syndromes
The following criteria are used to define ST-segment elevation:
The following criteria are used to define new Q waves:
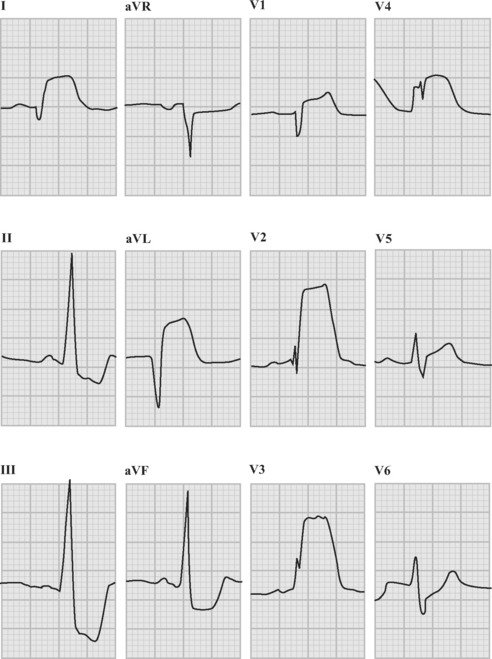
The absence of ST elevation or a new LBBB pattern does not exclude the presence of complete epicardial coronary artery occlusion, but the benefit of pharmacologic reperfusion therapy (fibrinolysis) has not been demonstrated in these patients. This situation arises in the setting of a posterior myocardial infarction due to circumflex artery occlusion, in which there is marked ST-segment depression in leads V1 through V4 associated with tall R waves and upright T waves in the right precordial leads (V1 through V3). These changes are shown in Figure 18-7. In this example there is also inferior ST-segment elevation.
ECG in Non-ST-Elevation Acute Coronary Syndromes
The following ECG findings may be seen in patients with non-ST-elevation acute coronary syndromes:
The ECG provides important prognostic information in patients with non-ST-elevation acute coronary syndromes. Patients with ST-segment depression have a poorer prognosis when compared to patients with T wave abnormalities only and those with normal ECGs. Furthermore, the magnitude of ST-segment depression and the number of leads in which it occurs provides clear independent prognostic information.
Laboratory Tests
Certain laboratory tests should be performed at baseline in all patients with suspected acute coronary syndromes (Table 18-5).10
Table 18-5 Laboratory Examination in Acute Coronary Syndromes
| Cardiac troponin |
| Electrolytes (including potassium and magnesium) |
| Urea and creatinine |
| Glucose |
| Complete blood count |
| Lipid profile |
| Coagulation (PT, aPTT) |
| Consider hsCRP and BNP |
aPTT, activated partial thromboplastin time; BNP, B-type natriuretic peptide; hsCRP, high-sensitivity C-reactive protein; PT, prothrombin time.
Cardiac Markers: Troponin, CK-MB, and Myoglobin
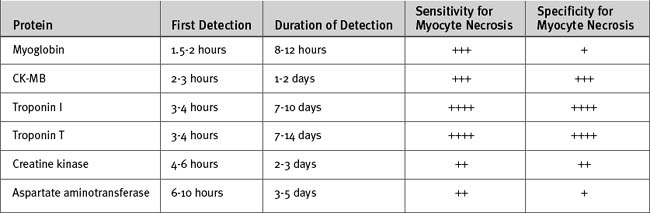
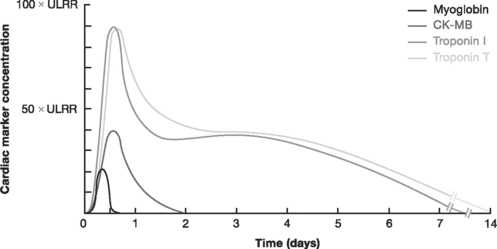
The cardiac markers (Fig. 18-11 and Table 18-6) are proteins released into the bloodstream by necrotic myocardium and leaky cell myocyte membranes. Besides being central to the diagnosis of myocardial infarction (see earlier discussion), the troponins, T and I, provide other important information, including the following:
In patients with ST-elevation acute coronary syndromes, initiation of reperfusion therapy based on the initial ECG should take priority over cardiac marker analysis. Confirmation of myocardial infarction can be determined subsequently by the results of marker levels. Blood samples for troponin analysis should be obtained within 10 minutes of presentation and should be repeated at 6 to 8 hours. Troponin levels obtained 8 hours after the onset of symptoms detect most myocardial infarctions, whereas troponin levels obtained 12 hours after the onset of symptoms detect all myocardial infarctions.
Because of its rapid rise and fall, CK-MB is preferred over troponin T or I (which may remain elevated for 2 weeks) for the diagnosis of reinfarction. Although troponins are the most specific cardiac markers, they are also elevated in conditions other than acute coronary syndromes (Table 18-7).
Table 18-7 Causes of Elevated Troponin Levels Other Than Acute Coronary Syndromes
| Iatrogenic |
| Cardiac surgery |
| PCI |
| Electrophysiologic radiofrequency ablation |
| Defibrillation |
| Cardiotoxic drugs (e.g., doxorubicin, 5-fluorouracil) |
| Myopericarditis |
| Rheumatic fever |
| Rheumatoid arthritis |
| Systemic vasculitis |
| Postviral |
| Infiltrative Diseases of the Myocardium |
| Amyloidosis |
| Sarcoidosis |
| Miscellaneous |
| Tachyarrhythmia |
| Hypertension |
| Congestive heart failure |
| Renal failure |
| Hypothyroidism |
| Pulmonary embolism with right ventricular infarction |
| Sepsis (including sepsis occurring with shock) |
| Transient ischemic attack, stroke, or subarachnoid hemorrhage |
| Pheochromocytoma |
| Rhabdomyolysis with myocyte necrosis |
| Chest wall trauma |
| False-positive: heterophile antibodies |
PCI, percutaneous coronary intervention.
Natriuretic Peptides
B-type natriuretic peptide (BNP) has been shown to be an independent prognostic factor in both ST-elevation and non-ST-elevation myocardial infarction. Like inflammatory markers, measurement of BNP is not currently recommended for routine clinical use in patients with acute coronary syndromes. A low level of BNP together with negative troponins may identify patients at very low risk.
Risk Stratification
There are a number of risk scores for patients with acute coronary syndromes. The TIMI (Thrombolysis In Myocardial Infarction trials group) risk score (Table 18-8) is widely used to predict 30-day mortality in patients with ST-elevation myocardial infarction.11 Risk assessment of patients with non-ST-elevation acute coronary syndromes (see Table 18-14 under subsequent heading Management of Acute Coronary Syndromes: Patients Without ST Elevation) is important for making prognoses and for initiating appropriate therapies.
MANAGEMENT OF ACUTE CORONARY SYNDROMES
Patients with ST Elevation
Urgent reperfusion of the ischemic myocardium through restoration of flow in the occluded epicardial coronary artery is the primary therapeutic goal in patients with ST-elevation acute coronary syndromes who present within 12 hours of symptom onset. The earlier reperfusion therapy is initiated after the onset of symptoms, the smaller the infarct size and the greater the survival benefit. When epicardial flow is restored within 30 minutes of occlusion, myocardial infarction can be aborted. If flow is achieved within 2 hours, considerable myocardial salvage can occur despite infarction—with beneficial effects on ventricular function and the likelihood of mortality. When reperfusion is achieved after 2 to 3 hours, myocardial salvage is progressively reduced, and recovery of ventricular function is dependent on established collateral flow. Beyond 6 hours, myocardial salvage is minimal or absent.12 Reperfusion can be achieved using either fibrinolysis or primary PCI.
Primary PCI Versus Fibrinolysis
Infarct artery patency rates at 90 minutes with PCI are superior to rates with fibrinolysis (90% versus 60%).13 In a recent metaanalysis of 23 trials comparing PCI to fibrinolysis, PCI was superior in reducing short-term mortality, reinfarction, and stroke.14 However, there is some evidence that very early (prehospital) administration of fibrinolysis results in the same early survival rate as primary PCI.15 Thus, for patients presenting less than 3 hours after symptom onset, fibrinolytic therapy may be the treatment of choice, especially if there is a delay in performing PCI, whereas for patients presenting between 3 and 12 hours after symptom onset, primary PCI is the reperfusion therapy of choice (Table 18-9). Contraindications to fibrinolysis are outlined in Table 18-10.
Table 18-9 Preferred Strategy of Reperfusion for ST Elevation Myocardial Infarction
| Primary PCI Preferred | Fibrinolysis Preferred |
|---|---|
| PCI-capable lab available (emergency department to balloon time <90 min, appropriate operator and team experience) | No PCI-capable lab available Duration of symptoms <3 hr (significant delay exists to get to lab) |
| Difficult arterial access Renal failure | |
| Duration of symptoms >3 hr Cardiogenic shock Significant heart failure (Killip class III/IV; see Table 19-1) Contraindications to fibrinolysis (see Table 18-10) Diagnosis of STEMI in doubt |
PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
Table 18-10 Contraindications to Fibrinolysis
| Absolute |
| Any prior intracerebral hemorrhage |
| Known structural cerebrovascular lesion |
| Known malignant intracranial or spinal neoplasm or arteriovenous malformation |
| Ischemic stroke within 6 months |
| Neurosurgery within 6 months |
| Suspected aortic dissection |
| Active bleeding or bleeding diathesis (excluding menses) |
| Significant closed-head or facial trauma within 3 months |
| Uncontrolled hypertension on presentation (SBP ≥180 mmHg or DBP ≥110 mmHg) |
| Recent internal bleeding within 6 weeks |
| Major surgery or major trauma within 2 weeks |
| Relative |
| Transient ischemic attack within 6 months Patients younger than 84 years of age with |
| Traumatic CPR within 2 weeks |
| Patients in whom a saphenous vein graft is |
| Noncompressible vascular puncture |
| Patients who have previously received |
| Pregnancy |
| Active peptic ulcer |
| Current use of anticoagulants with INR >2 |
| Advanced liver disease |
| Infective endocarditis |
| Active cavitating tuberculosis |
| Acute pancreatitis |
| Intracardiac thrombus |
CPR, cardiopulmonary resuscitation; DBP, diastolic blood pressure; INR, international normalized ratio; SBP, systolic blood pressure.
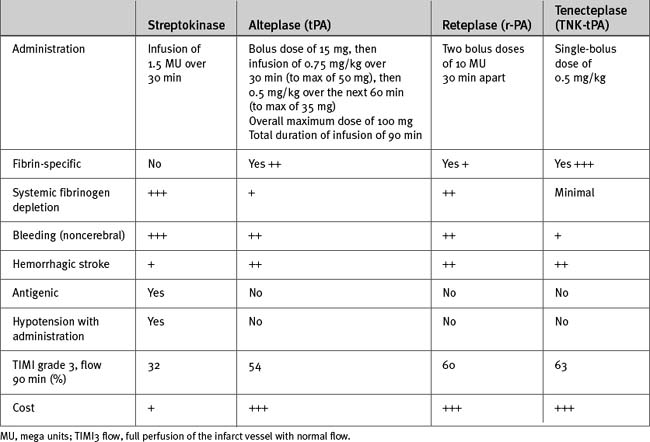
Choice of Fibrinolytic
In choosing a fibrinolytic (Table 18-11), a fibrin-specific agent is most effective in the following situations:
For all other patients with STEMI, streptokinase can be used.
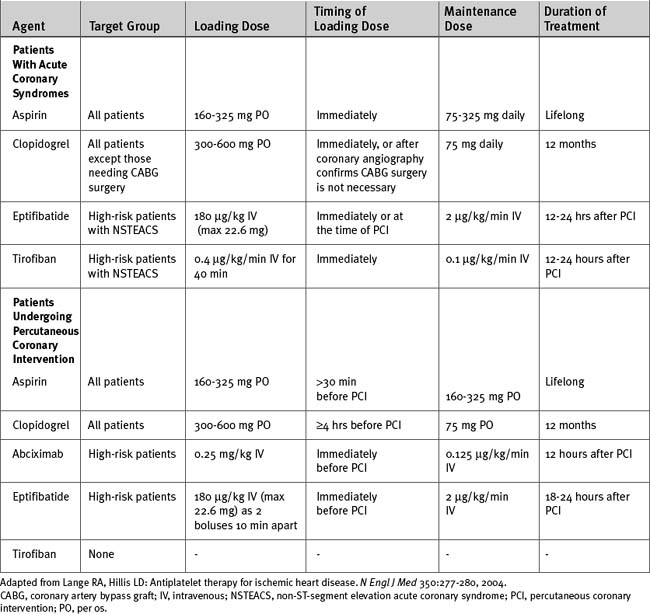
Antiplatelet Therapy
Aspirin at a dose of 150 to 325 mg should be administered to all patients. Clopidogrel should be considered at presentation for all patients with STEMI. For patients treated with fibrinolysis who are younger than 75 years of age, it is recommended that a loading dose of 300 mg of clopidogrel followed by 75 mg daily be given for 2 weeks. For patients older than 75 years, 75 mg of clopidogrel should be commenced at presentation. For patients undergoing primary PCI, clopidogrel can be given (with a loading dose of 300 to 600 mg) at presentation or after defining the coronary anatomy and continued for up to 12 months, depending on the type of stent used (Table 18-12).
Heparin Therapy in Fibrinolysis
Unfractionated or low molecular weight heparin should be commenced immediately after fibrinolytic therapy has started (Table 18-13). Low molecular weight heparin is the preferred therapy. The dose of unfractionated heparin has recently been adjusted downward because of concerns about the risks for intracranial hemorrhage when used in conjunction with fibrinolytic therapy.16 A bolus dose of 60 units/kg (maximum 4000 units) followed by an infusion of 12 units/kg/hr (maximum 1000 units/hr) is indicated. At 3 hours the heparin infusion rate should be adjusted according to the activated partial thromboplastin time, aiming for a value of 50 to 80 seconds. The heparin infusion should continue for 48 hours.
Table 18-13 Dosing of Unfractionated and Low Molecular Weight Heparin in Acute Coronary Syndromes
| Unfractionated heparin | Bolus 60 IU/kg (maximum 4000 IU) IV, followed by infusion of 12 IU/kg/hr (modified to achieve an aPTT of 50-80 sec). |
| Enoxaparin | 1 mg/kg subcutaneously every 12 hr;* the first dose may be preceded by a 30 mg IV bolus† |
| Dalteparin | 120 IU/kg subcutaneously every 12 hr (max 10,000 IU twice daily) |
aPTT, activated partial thromboplastin time; IU, international units.
* Omit bolus dose if age >75 years.
† If creatinine clearance < 30 ml/min reduce dose to every 24 hr.
Angiography in Patients Who Have Received Fibrinolytic Therapy
Following fibrinolysis, elective angiography may be performed in all patients or limited to those with demonstrable inducible ischemia on subsequent stress testing. In one study, a strategy of routine angiography with or without PCI resulted in a lower incidence of adverse outcome.17 Angiography also provides important prognostic information and may facilitate early discharge.
Surgical Revascularization
The role of urgent CABG surgery in patients with STEMI is limited to a few select circumstances18,19:
The outcomes of CABG surgery in patients with STEMI are better if surgery is delayed for several days after the acute event.21 Thus, in patients who are suitable for CABG rather than PCI and do not have one of the criteria listed above, surgery should be delayed until myocardial stunning has resolved—that is, for at least 48 hours and preferably longer.19
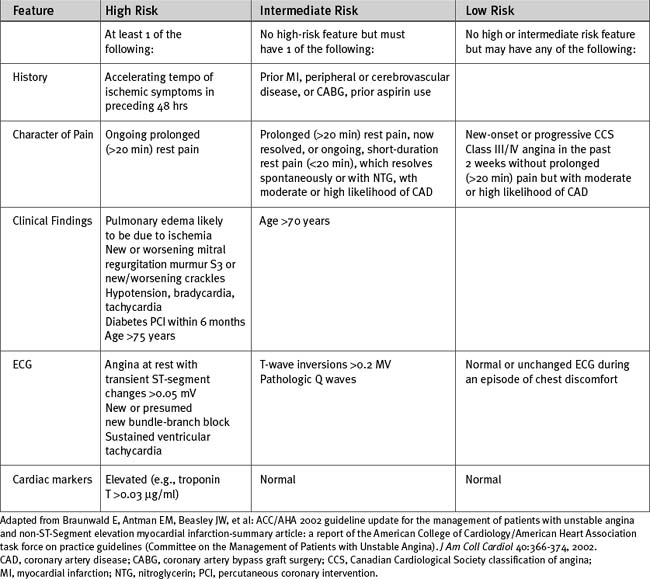
Patients Without ST Elevation
Early stratification into low-, intermediate-, or high-risk groups (Table 18-14) is vital to determine the appropriate therapy for patients with non-ST-elevation acute coronary syndromes.9
Low-Risk Patients
Low-risk patients should be monitored, and if they remain stable and their troponins are not elevated at 8 hours after first assessment, they should undergo investigation for inducible ischemia as described in Chapter 5. If there is no evidence of inducible ischemia at a moderate workload, they should be discharged, with outpatient follow-up. If inducible ischemia is demonstrated, angiography and revascularization are indicated.
Intermediate- and High-Risk Patients
Intermediate- and high-risk patients should receive aggressive antithrombotic therapy followed by early angiography and revascularization. In high-risk patients with elevated troponins, ST-segment depression, or diabetes, combination treatment with aspirin, clopidogrel, a glycoprotein IIb/IIIa inhibitor, and either unfractionated or low molecular weight heparin is appropriate (Tables 18-12 and 18-13). In all other intermediate- or high-risk patients, treatment with aspirin, clopidogrel, and either unfractionated or low molecular weight heparin is appropriate. Clopidogrel should be avoided in patients who have a high likelihood of requiring urgent CABG surgery. Switching among various antithrombin therapies should be avoided because it increases the risk for bleeding and adverse ischemic events. As an alternative to unfractionated or low molecular weight heparin, bivalirudin, a direct thrombin inhibitor, may be administered. Bivalirudin is associated with less bleeding than either unfractionated heparin or enoxaparin with a IIb/IIIa inhibitor.22
Strategies for Angiography and Revascularization
Based on the findings at angiography, patients may undergo PCI with coronary stenting or be referred for CABG surgery. The primary determinant of suitability for PCI is the nature of the coronary lesions (Table 18-15). The indications for CABG surgery are outlined in Chapter 9.
Table 18-15 Anatomic Risk Groups for Successful Percutaneous Coronary Intervention
| Procedural Success | Risk | Lesion Characteristics |
|---|---|---|
| High (>85%) | Low | Discrete lesion (length <10 mm) Concentric Readily accessible Nonangulated segment (<45 degrees) Smooth contour Little or no calcification Less than totally occlusive Not ostial in location No major side branch involvement Absence of thrombus |
| Intermediate (60%-85%) | Moderate | Tubular (length 10-20 mm) Eccentric Moderate tortuosity of proximal segment Moderately angulated segment Irregular contour Moderate or heavy calcification Total occlusions <3 months old Ostial in location Bifurcation lesions requiring double guide wires Some thrombus present |
| Low (<60%) | High | Diffuse (length >20 mm) Excessive tortuosity of proximal segment Extremely angulated segments >90 degrees Total occlusions >3 months old and/or bridging collaterals Inability to protect major side branches Degenerated vein grafts with friable lesions |
A concern in coronary stenting is the risk for in-stent restenosis, which occurs due to neointimal proliferation. With bare metal stents, in-stent restenosis occurs in 15% to 30% of stented lesions within 6 to 9 months,23 and a higher incidence occurs in certain situations, notably unstable angina, diabetes, renal failure, small vessel caliber, long lesion length, and bifurcation lesions. However, stents impregnated with antiproliferative medications such as sirolimus and paclitaxel (so-called drug-eluting stents) blunt this neointimal response and have been shown to reduce significantly the rate of in-stent restenosis (to about 5% within the same time frame).23 Although the longevity of drug-eluting stents remains to be confirmed, the proportion of patients treated with PCI continues to rise. Increasingly, patients with triple vessel and left main coronary disease who have lesions suitable for the procedure are being treated with PCI.
Adjunctive Therapy in Acute Coronary Syndromes
Oxygen
Supplemental oxygen should be administered to all patients who present with ischemic chest pain.
β Blockers
Intravenous β Blockers should be considered in a patient with ST-elevation acute coronary syndrome because they reduce the incidence of reinfarction and ventricular fibrillation. However, in certain high-risk groups (age >70, systolic BP<120, tachycardia >110, or Killip class III or IV; see Table 19-1), the use of intravenous β blockers on the day of admission is associated with an increased incidence of cardiogenic shock, which offsets the benefits. Thus, in such a patient it is prudent to withhold β blockers for 24 hours or until the patient’s hemodynamic state has stabilized.24 The benefits of β blockers in nonST-elevation acute coronary syndromes are less clearly defined, but they may reduce progression to myocardial infarction and are usually recommended.
Angiotensin-Converting Enzyme Inhibitors
Antihyperglycemic Therapy
Diabetics with acute coronary syndromes represent a high-risk group for adverse outcomes. Some small trials have shown a benefit from glucose/insulin/potassium therapy in acute coronary syndromes but a recent large randomized trial of patients with STEMI reported no benefit.25 Nevertheless, optimal long-term glycemic control remains an important goal.
COMPLICATIONS OF ACUTE CORONARY SYNDROMES
Patients with acute coronary syndromes are at risk for a range of acute complications, including arrhythmias and heart block (see Chapter 21), acute heart failure (see Chapter 19), and cardiac arrest (see Chapter 20) with subsequent hypoxic ischemic encephalopathy (see Chapter 37). Mechanical complications (mitral regurgitation, ventricular septal rupture, and free wall rupture) may develop in the first few days following myocardial infarction (see Chapter 9). Long-term complications include ventricular remodeling and chronic heart failure (see Chapter 19) and the formation of left ventricular aneurysms and pseudoaneurysms.
1 Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868-874.
2 Libby P. The vascular biology of atherosclerosis. In Zipes DP, Libby P, Bonow RO, Braunwald E, editors: Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, ed 7, Philadelphia: WB Saunders, 2005.
3 Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361-366.
4 Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-1275.
5 Casscells W, Naghavi M, Willerson JT. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003;107:2072-2075.
6 Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation. 2003;108:1772-1778.
7 Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664-1672.
8 Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110:588-636.
9 Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2002;40:366-374.
10 French JK, White HD. Clinical implications of the new definition of myocardial infarction. Heart. 2004;90:99-106.
11 Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031-2037.
12 Giugliano RP, Braunwald E, The TIMI Study Group. Selecting the best reperfusion strategy in ST-elevation myocardial infarction: it ’s all a matter of time. Circulation. 2003;108:2828-2830.
13 Grines CL. Should thrombolysis or primary angioplasty be the treatment of choice for acute myocardial infarction ? Primary angioplasty —the strategy of choice. N Engl J Med. 1996;335:1313-1316. discussion 1316-1317
14 Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
15 Bonnefoy E, Lapostolle F, Leizorovicz A, et al. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet. 2002;360:825-829.
16 White HD, Yusuf S. Issues regarding the use of heparin following streptokinase therapy. J Thromb Thrombol. 1995;2:5-10.
17 Fernandez-Aviles F, Alonso JJ, Castro-Beiras A, et al. Routine invasive strategy within 24 hours of thrombolysis versus ischaemia-guided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet. 2004;364:1045-1053.
18 Stone GW, Brodie BR, Griffin JJ, et al. Role of cardiac surgery in the hospital phase management of patients treated with primary angioplasty for acute myocardial infarction. Am J Cardiol. 2000;85:1292-1296.
19 Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:340-437.
20 Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
21 Braxton JH, Hammond GL, Letsou GV, et al. Optimal timing of coronary artery bypass graft surgery after acute myocardial infarction. Circulation. 1995;92:II66-II68.
22 Direct Thrombin Inhibitor Trialists’ Collaborative Group. Direct thrombin inhibitors in acute coronary syndromes: principal results of a meta-analysis based on individual patients’ data. Lancet. 2002;359:294-302.
23 Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354:483-495.
24 Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622-1632.
25 Mehta SR, Yusuf S, Diaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437-446.