Action of physical and chemical agents on microorganisms
Geoffrey W. Hanlon and Norman A. Hodges
Chapter contents
D value, or decimal reduction time
Antimicrobial effects of moist and dry heat
Resistance of microorganisms to moist and dry heat
Factors affecting heat resistance and its measurement
Effect of ionizing radiations on materials
Factors affecting the radiation resistance of microorganisms
Factors affecting resistance to UV light
Antimicrobial effects of chemical agents
Key points
Introduction
The subject of this chapter is of importance because pharmaceutical scientists have a responsibility for:
• the production of sterile medicaments having no living microorganisms, e.g. injections and eye drops
Thus, the major pharmaceutical interest in microorganisms is that of killing them, or at least preventing their growth. Consequently it is necessary to have both an understanding of the physical processes, e.g. heating and irradiation that are used to kill microorganisms and a knowledge of the more diverse subject of antimicrobial chemicals.
This background knowledge must include an understanding of the kinetics of cell inactivation, the calculation of parameters by which microbial destruction and growth inhibition are measured, and an appreciation of the factors that influence the efficiency of the physical and chemical processes used. These aspects, together with a synopsis of the major groups of antimicrobial chemicals, are the subject of this chapter.
Kinetics of cell inactivation
The death of a population of cells exposed to heat or radiation is often found to follow or approximate to first-order kinetics. In this sense, it is similar to bacterial growth during the logarithmic phase of the cycle, the graphs representing these processes being similar but of opposite slope. Assuming first-order kinetics (the exceptions will be considered later), an initial population of No cells per mL will, after a time t minutes, be reduced to Nt cells per mL, according to the following equations in which k is the inactivation rate constant:
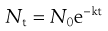 (15.1)
(15.1)
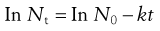 (15.2)
(15.2)
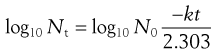 (15.3)
(15.3)
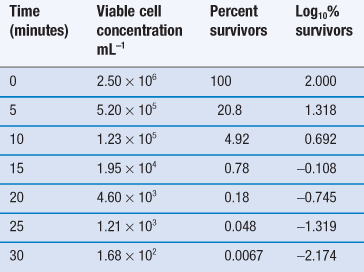
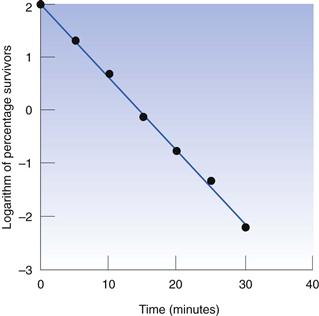
Thus, the data in Table 15.1 may be used to produce a plot of logarithm of cell concentration against exposure time (Fig. 15.1), where the intercept is log No and the slope is −k/2.303. This may be plotted with the logarithm of the percentage of survivors as the ordinate; thus the largest numerical value on this axis is 2.0 (100%). An important feature of Figure 15.1 is the fact that there is no lower endpoint to the ordinate scale – it continues indefinitely. If the initial population was 1000 cells mL−1 the logarithmic value would be 3.0; at 100 cells mL−1 the value would be 2.0; at 10 cells mL−1 1.0, and at 1 cell mL−1 zero. The next incremental point on the logarithmic scale would be −1, which corresponds to 0.1 cells mL−1. It is clearly nonsense to talk of a fraction of a viable cell per mL but this value corresponds to one whole cell in 10 mL of liquid. The next point, −2.0, corresponds to one cell in 100 mL, and so on. Sterility is the complete absence of life, i.e. zero cells mL−1, which has a log value of −∞. Guaranteed sterility would therefore require an infinite exposure time.
D value, or decimal reduction time
It is characteristic of first-order kinetics that the same percentage change in concentration occurs in successive time intervals. Thus in Figure 15.1 it can be seen that the viable population falls to 10% of its initial value after 7.5 minutes; in the next 7.5-minute period the population again falls to 10% of its value at the start of that period. This time period for a 90% reduction in count is related to the slope of the line and is one of the more useful parameters by which the death rate may be indicated. It is known as the decimal reduction time, or D value, and usually has a subscript showing the temperature at which it was measured, e.g. D121 or D134. It is quite possible to indicate the rate of destruction by the inactivation rate constant calculated from the slope of the line but the significance of this value cannot be as readily appreciated during conversation as that of a D value, and so the former is rarely used.
Z values
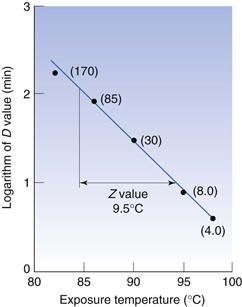
When designing steam sterilization processes, it is necessary to know both the D value, which is a measure of the effectiveness of heat at any given temperature, and the extent to which a particular increase in temperature will reduce the D value, i.e. it is necessary to have a measure of the effect of temperature change on death rate. One such measure is the Z value, which is defined as the number of degrees of temperature change required to achieve a 10-fold change in D value, e.g. if the D value for Bacillus stearothermophilus1 spores at 110 °C is 20 minutes and they have a Z value of 9 °C, this means that at 119°C the D value would be 2.0 minutes and at 128 °C the D value would be 0.20 minutes. The relationship between D and Z values is shown in Figure 15.2. The Z value is one of several parameters that relate change in temperature to change in death rate, and is probably the most commonly used and readily understood.
The activation energy obtained from an Arrhenius plot (see Chapter 7) or a temperature coefficient, a Q10 value (change in rate for a 10 °C change in temperature, Chapter 14), do the same but are rarely used.
Alternative survivor plots
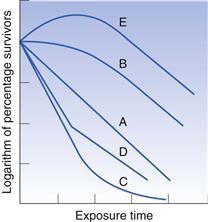
It was stated earlier that bacterial death often approximates to first-order kinetics, although exceptions do arise; some of the more common are illustrated in Figure 15.3. The plot labelled A is that conforming to first-order kinetics, which has already been described. A shoulder on the curve, as in case B, is not uncommon and various explanations have been offered. Cell aggregation or clumping may be responsible for such a shoulder, because it would be necessary to apply sufficient heat to kill all the cells in the clump, not merely the most sensitive, before a fall is observed in the number of colonies appearing on the agar. Under normal circumstances one single colony could arise both from one cell alone or, say, from 100 cells aggregated together. In the latter case, if sufficient heat was applied to kill the 99 most sensitive cells in the clump, the colony count would be unaltered. Clumping is not the only explanation, because substantial shoulders may arise using suspensions where the vast majority of cells exist individually.
Tailing of survivor curves, as in plot C, is often observed if the initial cell concentration is high. This has been attributed to the presence of mutants that are exceptionally resistant to the lethal agent. If the proportion of mutants was 1 in 106 cells and the initial concentration only 105 cells mL−1 the mutant would not be detected, but an initial population of 109 cells mL−1 would permit easy detection if the inactivation plot were continued down to low levels of survivors. Again there are alternative explanations, one of the most common being that the cells dying during the early exposure period release chemicals which help to protect those that are still alive.
A sharp break in the line, as in D, usually indicates that there are two distinct populations of cells present which have markedly different resistances. Contamination of a cell suspension or culture is a possible explanation, or it may be that a mutant has arisen naturally and the cultural conditions are such that it has a selective advantage and its numbers have increased until it is a substantial proportion of the population.
Plot E is uncommon and is usually only seen as a result of ‘heat activation’ of bacterial spores. This is a situation in which a significant proportion of a population of spores (usually a thermophile) remains dormant and fails to germinate and produce colonies under ‘normal’ conditions. If the suspension receives a heat stimulus or shock which is insufficient to kill the spores, some or all of those that would otherwise remain dormant become activated, germinate and thus produce a rise in the colony count.
First-order kinetics are less commonly observed when microorganisms are being killed by chemicals than when heat or radiation are the lethal agents. This is because the chemical must interact with a target molecule within the cell, and the concentration of both the chemical and the intracellular target might influence death rate; this results in second-order kinetics. In practice, however, the antimicrobial chemical is often present in such a high concentration that the proportion of it that is ‘used up’ by interaction with the cell is negligible; this means its concentration is effectively constant and pseudo first-order kinetics result.
Antimicrobial effects of moist and dry heat
Moist heat (steam) and dry heat (hot air) both have the potential to kill microorganisms but their efficiencies and their mechanisms of action differ. In autoclaves, dry saturated steam, i.e. 100% water vapour with no liquid water present, is used at temperatures between 121 and 135 °C, at which it rapidly kills microorganisms. An advantage of using steam is that it possesses a large latent heat of vaporization, which it transfers to any object upon which it condenses. It is essential to use dry saturated steam if maximal autoclaving efficiency is to be achieved. If the steam is wet, i.e. contains liquid water, penetration of vapour-phase steam into dressings may be retarded. If the steam is superheated, i.e. its temperature has been raised while the pressure remains constant, or the pressure fell while the temperature remains constant, it contains less moisture and latent heat than dry saturated steam at the same temperature. In this case the effect is similar to using a steam–air mixture at that temperature. The process by which steam kills cells is hydrolysis of essential proteins (enzymes) and nucleic acids. In contrast, dry heat causes cell death by oxidative processes, although again it is the proteins and nucleic acids that are the vulnerable targets. Dry heat is much less effective at killing microorganisms than steam at the same temperature. Exposures of not less than 2 hours at 160 °C (or an equivalent temperature/time combination) are recommended in the PhEur for sterilization by dry heat methods. The state of hydration of a cell is thus an important factor determining its resistance to heat.
Resistance of microorganisms to moist and dry heat
Numerous factors influence the observed heat resistance of microbial cells and it is difficult to make comparisons between populations unless these factors are controlled. Not surprisingly, marked differences in resistance exist between different genera, species and strains, and between the spore and vegetative cell forms of the same organism. The resistance may be influenced, sometimes extensively, by: the age of the cell, i.e. lag, exponential or stationary phase; its chemical composition, which in turn is influenced by the medium in which the cell is grown; and by the composition and pH of the fluid in which the cell is heated. It is difficult to obtain strictly comparable heat resistance data for grossly dissimilar organisms, but the values quoted in Table 15.2 indicate the relative order of heat resistance of the various microbial groups. Tabulation of D values at a designated temperature is perhaps the most convenient way of comparing resistance but this is only suitable for first-order kinetics. Alternative methods of comparison include the time to achieve a particular percentage kill or the time required to achieve no survivors; the latter is, of course, dependent upon the initial population level.
Table 15.2
A ‘league table’ of heat resistances of different microorganisms and infectious agents
| Organism or agent | Heat resistance (values are for fully hydrated organisms unless otherwise stated) |
| Prions | The most heat-resistant infectious agent. May survive steam sterilization at 134–138 °C for 1 hour |
| Bacterial spores (endospores) | Little or no inactivation at <80 °C. Some species survive boiling for several hours |
| Fungal spores | Ascospores of Byssochlamys species may survive 88 °C for 60 minutes but most fungal spores are less resistant |
| Actinomycete spores | Spores of Nocardia sebivorans reported to survive for 10 minutes at 90 °C but the majority of species are less resistant |
| Mycobacterium tuberculosis | May survive for 30 minutes at 100 °C in the dry state but when hydrated is killed by pasteurization (63 °C for 30 minutes or 72 °C for 15 seconds) |
| Yeasts | Ascospores and vegetative cells show little difference in resistance. Survival for 20 minutes at 60 °C is typical |
| Most non-sporing bacteria of pharmaceutical or medical importance | D60 of 1–5 minutes is typical of staphylococci and many Gram-negative enteric organisms. Enterococci may be more resistant, and pneumococci may survive for 30 minutes at 110 °C when dry |
| Fungi and actinomycetes | Vegetative mycelia exhibit similar resistance to that of non-sporing bacteria described above |
| Viruses | Rarely survive for >30 minutes at 55–60 °C except perhaps in blood or tissues, but papovaviruses and hepatitis viruses are more resistant |
| Protozoa and algae | Most are no more resistant than mammalian cells and survive only a few hours at 40–45 °C. However, cysts of Acanthamoeba species are more resistant |
The most heat-resistant infectious agents (as distinct from microbial cells) are prions, which are proteins rather than living cells and are the cause of spongiform encephalopathies, e.g. Creutzfeldt–Jakob disease (CJD) and bovine spongiform encephalopathy (BSE or ‘mad cow disease’). Prion proteins are so resistant to heat inactivation that an autoclave cycle of 134–138 °C for 18 minutes has been recommended for the decontamination of prion-contaminated materials, and the efficacy of even this extreme heat treatment has been questioned. The World Health Organization recommends that prion-contaminated surgical instruments are autoclaved at 121 °C for one hour in the presence of 1M sodium hydroxide.
Bacterial endospores are invariably found to be the most heat-resistant cell type, and those of certain species may survive boiling water for many hours. The term ‘endospore’ refers to the spores produced by Bacillus and Clostridium species and is not to be confused with the spores produced by other bacteria, such as actinomycetes, which do not develop within the vegetative cell. The majority of Bacillus and Clostridium species normally form spores which survive in water for 15–30 minutes at 80 °C without significant damage or loss of viability. Because endospores are more resistant than other cells, they have been the subject of a considerable amount of research in the food and pharmaceutical industries and much of the earlier work has been reviewed by Russell (1999).
Mould spores and those of yeasts and actinomycetes usually exhibit a degree of moist heat resistance intermediate between endospores and vegetative cell forms; D-values of the order of 30 minutes at 50 °C would be typical of such organisms, although some species may be substantially more resistant. Bacterial and yeast vegetative cells and mould mycelia all vary significantly in heat resistance: mycobacteria, which possess a high proportion of lipid in their cell wall, tend to be more resistant than others. Protozoa and algae are, by comparison, susceptible to heat and when in the vegetative (uncysted) state they, like mammalian cells, rapidly die at temperatures much in excess of 40 °C. Information on the heat resistance of viruses is limited but the available data suggest that they may vary significantly between types. The majority of viruses are no more heat resistant than vegetative bacteria, but hepatitis viruses, particularly hepatitis B, is less susceptible and exposures of 80 °C for 10 minutes or more are required for effective decontamination.
Resistance to dry heat by different groups of infectious agents and microorganisms usually follows a pattern similar to that in aqueous environments. Again, prions head the ‘league table’ by exhibiting extreme heat resistance and endospores are substantially more resilient than other cell types, with those of B. stearothermophilus and B. subtilis usually more resistant than other species. Exposures of 2 hours at 160 °C are required by the European Pharmacopoeia (2004) to achieve an acceptable level of sterility assurance for materials sterilized by dry heat.
Cells of pneumococci have been reported to survive dry heat at 110 °C for 30 minutes but this represents exceptional resistance for vegetative cells, most of which may be expected to die after a few minutes heating at 100 °C or less.
Valid comparisons of dry heat resistance among dissimilar organisms are even less common than those for aqueous environments because there is the additional problem of distinguishing the effects of drying from those of heat. For many cells, desiccation is itself a potentially lethal process, even at room temperature, so that experiments in which the moisture content of the cells is uncontrolled may produce results that are misleading or difficult to interpret. This is particularly so when the cells are heated under conditions where their moisture content is changing and they become progressively drier during the experiment.
Factors affecting heat resistance and its measurement
The major factors affecting heat resistance are listed in the previous section and will be considered in some detail here. The subject has been extensively studied and again, many of the experimental data and consequently many of the examples quoted in this section come from the field of spore research.
The measurement of heat resistance in fully hydrated cells, i.e. those suspended in aqueous solutions or exposed to dry saturated steam, does not normally represent a problem when conducted at temperatures less than 100 °C, but errors may occasionally arise when spore heat resistance is measured at higher temperatures. In these circumstances, it is necessary to heat suspensions sealed in glass ampoules immersed in glycerol or oil baths or to expose the spores to steam in a modified autoclave. Monitoring and control of heat-up and cool-down times become important, and failure to pay adequate attention to these aspects may lead to apparent differences in resistance, which may be due simply to factors such as variations in the thickness of glass in two batches of ampoules.
Species and strain differences
Variations in heat resistance between the species within a genus are very common, although it is difficult to identify from the published reports the precise magnitude of these differences because different species may require different growth media and incubation conditions which, together with other factors, might influence the results. For example, one report described a 700-fold variation in spore heat resistance within 13 Bacillus species, but to produce the spore crops for testing, the authors necessarily had to use eight culture media, three incubation temperatures and six procedures for cleaning the spores. Differences between strains of a single species are, not surprisingly, more limited; D90 values ranging from 4.5 to 120 minutes have been reported for five strains of Clostridium perfringens spores.
Cell form
Whether or not the heated cells exist in the vegetative or the spore form may in some cases be related to the age of the culture or the cell population being heated. In cultures of Bacillus and Clostridium species, the proportion of spores usually increases as the incubation period is extended and the culture ages. This may be due to more and more of the vegetative cells producing spores, in which case the spore count increases. Alternatively, the spore count may remain unchanged but the vegetative cell count falls as a result of the action of lytic enzymes produced by the cells themselves. Among the common mesophilic Bacillus species, spore formation is largely complete 6–10 hours after the end of exponential growth under optimal cultural conditions. The degree of heat resistance and the concentration of spores would not be expected to rise much after this time. Conducting heat resistance studies on a mixture of spores and vegetative cells is undesirable because the likely result is a rapid initial fall in count due to killing of the vegetative cells, and a subsequent slower rate due to death of spores. If necessary, the vegetative cells can usually be removed by addition of the enzymes lysozyme and trypsin.
The degree of heat resistance shown by vegetative cells may also be influenced by the stage of growth from which the cells were taken. It is normally found that stationary-phase cells are more heat resistant than those taken from the logarithmic phase of growth, although several exceptions have been reported.
Culture conditions
The conditions under which the cells are grown is another factor that can markedly affect heat resistance. Insufficient attention has been paid to this potential source of variation in a substantial part of the research conducted. Not infrequently, insufficient details of the cultivation procedures are described in the scientific reports, or materials of variable composition, e.g. tap water or soil extracts, were used in media without regard to the possible differences that might have arisen between successive batches or populations of cells.
Factors such as growth temperature, medium pH and buffering capacity, oxygen availability and concentrations of culture medium components may all affect resistance.
Thermophilic organisms are generally more heat resistant than mesophils, which in turn tend to be more resistant than psychrophils. If a ‘league table’ of spore heat resistance were to be constructed, it is probable that B. stearothermophilus, B. coagulans and Cl. thermosaccharolyticum would head the list; all three have growth optima of 50–60 °C. Variable results have arisen when single species have been grown at a variety of temperatures. Escherichia coli and Streptococcus faecalis have both been the subject of conflicting reports on the influence of growth temperature on heat resistance, whereas spores of B. cereus produced at temperatures between 20 and 41 °C showed maximal resistance at 30 °C.
The effects of medium pH, buffering capacity, oxygen availability and the concentrations of culture medium components are often complex and interrelated. An unsuitable pH, inadequate buffer or insufficient aeration may all limit the extent of growth, with the result that the cells that do grow each have available to them a higher concentration of nutrients than would be the case if a higher cell density had been achieved. The levels of intracellular storage materials and metal ions may therefore differ and so influence resistance to heat and other lethal agents. Cells existing in, or recently isolated from, their ‘natural’ environment, e.g. water, soil, dust or pharmaceutical raw materials, have often been reported to have a greater heat resistance than their progeny that have been repeatedly subcultured in the laboratory and then tested under similar conditions.
pH and composition of heating menstruum
It is frequently found that cells survive heating more readily when they are at neutrality (or their optimum pH for growth if this differs from neutrality). The combination of heat and an unfavourable pH may be additive or even synergistic in killing effects; thus B. stearothermophilus spores survive better at 110 °C in dilute pH 7.0 phosphate buffer than at 85 °C in pH 4.0 acetate buffer. Differences in heat resistance may also result merely from the presence of the buffer, regardless of the pH it confers. Usually an apparent increase in resistance occurs when cells are heated in buffer rather than in water alone. A similar increase is often found to occur on the addition of other dissolved or suspended solids, particularly those of a colloidal or proteinaceous nature, e.g. milk, nutrient broth and serum.
Because dissolved solids can have such a marked effect on heat resistance, great care must be taken in attempting to use experimental data from simple solutions to predict the likely heat treatment required to kill the same cells in a complex formulated medicine or food material. An extreme case of protection of cells from a lethal agent is the occlusion of cells within crystals. When spores of B. subtilis var. niger were occluded within crystals of calcium carbonate, their resistances to inactivation were approximately 900 times and nine times higher than for unoccluded spores when subjected to steam and dry heat, respectively; an exposure period of 2.5 hours at 121 °C (moist heat) was required to eliminate survivors within the crystals. It is to minimize the risk of such situations arising that the Rules and Guidance for Pharmaceutical Manufacturers and Distributors places such emphasis on hygiene and cleanliness in the manufacture of medicines.
The solute concentrations normally encountered in dilute buffer solutions used as suspending media for heat resistance experiments cause no significant reduction in the vapour pressure of the solution relative to that of pure water, i.e. they do not reduce the water activity, Aw, of the solution (which has a value of 1.0 for water). If high solute concentrations are used, or the cells are heated in a ‘semi-dry’ state, the Aw is significantly lower and the resistance is increased, e.g. a 1000-fold increase in D value has been reported for B. megaterium spores when the water activity was reduced from 1.0 to between 0.2 and 0.4.
Recovery of heat-treated cells
The recovery conditions available to cells after exposure to heat may influence the proportion of cells that produce colonies. A heat-damaged cell may require an incubation time longer than normal to achieve a colony of any given size, and the optimum incubation temperature may be several degrees lower. The composition of the medium may also affect the colony count, with nutritionally rich media giving a greater percentage survival than a ‘standard’ medium, whereas little or no difference can be detected between the two when unheated cells are used. Adsorbents such as charcoal and starch have been found to have beneficial effects in this context.
Ionizing radiations
Ionizing radiations can be divided into electromagnetic and particulate (corpuscular) types and are of sufficient energy to cause ejection of an electron from an atom or molecule in their path. Electromagnetic radiations include γ-rays and X-rays, whereas particulate radiation includes α and β particles, positrons and neutrons.
Particulate radiation
The nuclear disintegration of radioactive elements results in the production of charged particles. α particles are heavy and positively charged, being equivalent to the nuclei of helium atoms. They travel relatively slowly in air and although they cause a great deal of ionization along their paths, they have very little penetrating power, their range being just a few centimetres in air. α particles cannot penetrate skin but may cause damage when emitted by radionuclides inserted into the body. β particles are negatively charged and have the same mass as an electron. In air the penetrating power of these particles is a few metres but they will be stopped by a thin sheet of aluminium. β particles resulting from radioactive decay are therefore not sufficiently penetrative for use in sterilization processes, but the production of accelerated electrons from man-made machines (cathode rays) results in particles of great energy with enhanced penetrating power.
Electromagnetic radiation
γ radiation results when the nucleus still has too much energy even after the emission of α or β particles. This energy is dissipated in the form of very short wavelength radiation which, as it has no mass or charge, travels with the speed of light, penetrating even sheets of lead. Although travelling in a wave form, γ radiation behaves as if composed of discrete packets of energy called quanta (photons). A 60Co source emits γ-rays with photons of 1.17 and 1.33 MeV and the source has a half-life of 5.2 years. X-rays are generated when a heavy metal target is bombarded with fast electrons. They have similar properties to γ-rays despite originating from a shift in electron energy rather than from the nucleus.
Units of radioactivity
The unit of activity is the becquerel (Bq), which is equal to one nuclear transformation per second. This replaces the term curie (Ci); 3.7 × 1010 becquerels = 1 curie. The unit of absorbed dose according to the SI system is the gray (Gy), which is equal to one joule per kilogram. However, the old term ‘rad’ is still used occasionally and is equivalent to 100 ergs per gram of irradiated material.
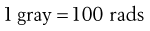
The energy of radiation is measured in electron volts (eV) or millions of electron volts (MeV). An electron volt is the energy acquired by an electron falling through a potential difference of 1 volt.
Effect of ionizing radiations on materials
Ionizing radiations are absorbed by materials in a variety of ways, depending upon the energy of the incident photons:
The ionization caused by the primary radiation results in the formation of free radicals, excited atoms, etc., along a discrete track through the material. However, if secondary electrons contain sufficient energy they may cause excitation and ionization of adjacent atoms, thereby effectively widening the track. Accelerated electrons used in electron beam sterilizers are essentially equivalent to the secondary electrons arising from γ irradiation – they cause direct ionization of molecules within materials. Temperature rise during irradiation is very small and even high-energy radiation resulting in pair production is only accompanied by an increase of approximately 2 °C, but nevertheless, the chemical changes that occur in irradiated materials are very widespread. Of particular significance here are the deleterious changes that may occur in packaging materials at normal dosage levels. Such effects may include changes in tensile strength, colour, odour and gas formation of polymers. Materials most affected include acetal, FEP, PTFE and PVA. Total absorbed energy determines the extent of physical and chemical reactions that occur, and so damage is cumulative. For sterilization purposes, exposure times can be long but the process is predictable and delivers a reproducible level of lethality.
The lethal effect of irradiation on microorganisms can occur in two ways:
Some of the possible reactions are as follows:
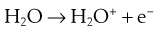
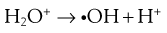
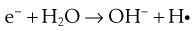

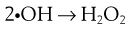
The presence of oxygen has a significant effect on the destructive properties of ionizing radiation owing to the formation of hydroperoxyl radicals.
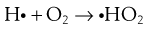
Peroxides and free radicals can act as both oxidizing and reducing agents according to the conditions.
Factors affecting the radiation resistance of microorganisms
Across the spectrum of microorganisms, viruses are the forms most resistant to the effects of radiation, followed by bacterial endospores, then Gram-positive cells and finally Gram-negative cells. Resistance to radiation is genetically determined and a particularly resistant bacterium called Deinococcus radiodurans can withstand a radiation dose up to 5 000 Gy compared to E. coli which is killed by 800 Gy. For comparison, a dose of 5 Gy is lethal to humans. Fortunately, this microorganism does not have any clinical significance. It is worth noting that microbial products such as endotoxins will not be inactivated by normal doses of ionizing radiations. Consequently it is important to ensure that initial bioburden levels are low.
Oxygen has already been mentioned as having a significant influence on the antimicrobial effects of radiation, as increased levels of hydroperoxyl radicals lead to marked increases in kill. Vegetative cells such as E. coli and Pseudomonas aeruginosa are 3–4 times more sensitive in the presence of oxygen than in its absence. The presence of moisture will also influence sensitivity with dehydration causing an increase in resistance owing to an indirect effect on the formation and mobility of free radicals. Freezing increases radiation resistance due to the reduction of mobility of free radicals in the menstruum, preventing them from diffusing to sites of action at the cell membrane. Above the freezing point there is very little effect of temperature.
A variety of organic materials provide a protective environment for microorganisms, and comparison of radiation resistance is greatly complicated by different complexities of the media used. Sulphydryl groups, such as may be found in amino acids and proteins, have a protective effect on microorganisms owing to their interaction with free radicals. In contrast, compounds that combine with –SH groups, such as halogenated acetates, tend to increase sensitivity. Some naturally occurring materials, particularly foods, may have a profound protective effect on contaminant bacteria. This is of concern to the food-processing industry.
Ultraviolet radiation
Although UV radiation covers a range of wavelengths from approximately 15 to 330 nm, its range of maximum bactericidal activity is much narrower (220–280 nm), with an optimum of about 265 nm. Whereas ionizing radiations cause electrons to be ejected from atoms in their path, UV radiation does not possess sufficient energy for this and merely causes the electrons to become excited. It has much less penetrating power than ionizing radiations and tends to be used for the destruction of microorganisms in air, water and on surfaces.
The bactericidal effect of UV light is due to the formation of linkages between adjacent pyrimidine bases in the DNA molecule to form dimers. These are usually thymine dimers, although other types have been identified. The presence of thymine dimers alters the structural integrity of the DNA chain, thereby hindering chromosome replication. Certain cells can repair damaged DNA in a variety of ways, enhancing their radiation resistance.
Exposure of UV-damaged cells to visible light (photoreactivation) enables a light-dependent photoreactivating enzyme to split the thymine dimers into monomers. A second mechanism is not light dependent and is called dark recovery. In this case, the thymine dimers are removed by a specific endonuclease enzyme that nicks the damaged DNA strand either side of the dimer. DNA polymerase then replaces the missing nucleotides and the ends are joined by a ligase enzyme.
Factors affecting resistance to UV light
As already mentioned, UV light has very little penetrating power and anything that acts as a shield around the cells will afford a degree of protection. The formation of aggregates of cells will result in those cells at the centre of the aggregate surviving an otherwise lethal dose of radiation. Similarly, microorganisms suspended in water withstand considerably higher doses of radiation than in the dry state owing to lack of penetration of the radiation. Suspension of bacteria in broth containing organic matter such as proteins increases the resistance of the cells still further. The stage of growth of the culture will affect the sensitivity of the cells, with maximum sensitivity being shown during the logarithmic phase.
Other factors shown to influence radiation resistance include pH, temperature and humidity, although the effect of the last parameter is still somewhat confused.
Gases
The use of gases as antimicrobial agents has been documented for centuries, although it is only recently that their mechanisms of action and factors affecting activity have been elucidated. A wide variety of gaseous agents has been used for their antimicrobial properties and a few of the major ones will be dealt with here.
Ethylene oxide
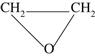
Ethylene oxide is a gas at room temperature (with a boiling point at 10.7 °C) that readily permeates a variety of materials (plastics, cardboard, cloth, etc.) but not crystals. Its odour is reported as being rather pleasant, although the levels at which it is detected in the atmosphere (700 ppm) greatly exceed the 5 ppm maximum safety limit for humans. Toxicity problems include burns and blistering if the material comes into contact with the skin, whereas inhalation results in lachrymation, headache, dizziness and vomiting. Great care must be taken to ensure the removal of residual ethylene oxide from treated products (e.g. rubber gloves) to avoid the risk of skin reactions. Explosive mixtures are formed when ethylene oxide is mixed with air at any concentration above 3% and this is especially dangerous if the gas mixture is confined. The addition of carbon dioxide or fluorinated hydrocarbons will eliminate this risk, and for sterilization purposes gas mixtures of 10% ethylene oxide/90% carbon dioxide are typically used.
Ethylene oxide is extremely effective at killing microorganisms and its activity is related to its action as an alkylating agent. Reactive hydrogen atoms on hydroxyl, carboxyl, sulphydryl and amino groups can all be replaced with hydroxyethyl groups, thereby interfering with a wide range of metabolic activities. Ethylene oxide inactivates the complete spectrum of microorganisms, including endospores and viruses. The difference in resistance between endospore-forming bacteria and vegetative cells is only of the order of 5–10 times, compared to several thousand-fold differences with other physical and chemical processes. In addition, no microorganism of genetically determined high resistance has been found. Spores of B. subtilis var. niger are among the most resistant to the effect of ethylene oxide. The moist heat-resistant spore former B. stearothermophilus and spores of Clostridium sporogenes are no more resistant than a number of vegetative organisms, such as Staph. aureus and Micrococcus luteus. Fungal spores exhibit the same order of resistance as vegetative cells.
Factors affecting the activity of ethylene oxide
The bactericidal activity of ethylene oxide is proportional to the partial pressure of gas in the reaction chamber, time of exposure, temperature of treatment and level and type of contamination. At room temperature, the time taken to reduce the initial concentration of cells by 90% can be very slow. For this reason elevated temperatures of 50–60 °C are recommended and these result in greatly increased rates of kill. Concentrations of ethylene oxide between 500 and 1000 mg L−1 are usually employed. Relative humidity has a most pronounced effect, as at very high humidities ethylene oxide may be hydrolysed to the much less active ethylene glycol. This is borne out by the observation that the gas is 10 times more active at 30% RH than at 97% RH. The optimum value for activity appears to be between 28% and 33% RH. Below 28% RH the alkylating action of ethylene oxide is inhibited due to lack of water. The degree of dehydration of cells greatly influences activity and it may not be possible to rehydrate very dry organisms simply by exposure to increased RH. The RH value chosen in practice is usually between 40% and 70%.
Microorganisms may be protected from the action of ethylene oxide by occlusion within crystalline material or when coated with organic matter or salts. B. subtilis var. niger spores dried from salt-water solutions are much more resistant to the gas than are suspensions dried from distilled water.
Biological indicators used to test the efficacy of ethylene oxide treatment employ spores of B. subtilis dried on to suitable carriers, such as pieces of aluminium foil.
Formaldehyde
Formaldehyde (H.CHO) in its pure form is a gas at room temperature, with a boiling point of −19 °C but readily polymerizes at temperatures below 80 °C to form a white solid. The vapour, which is extremely irritating to the eyes, nose and throat, can be generated either from solid polymers such as paraformaldehyde or from a solution of 37% formaldehyde in water (formalin). Formalin usually contains about 10% methanol to prevent polymerization.
As with ethylene oxide, formaldehyde is a very reactive molecule and there is only a small differential in resistance between bacterial spores and vegetative cells. Its bactericidal powers are superior to those of ethylene oxide (concentrations of 3–10 mg L−1 are effective) but it has weak penetrating power and is really only a surface bactericide. It is also more readily inactivated by organic matter. Adsorbed gas is very difficult to remove and long airing times are required. Its mechanism of action is thought to involve the production of intramolecular crosslinks between proteins, together with interactions with RNA and DNA. It acts as a mutagenic agent and an alkylating agent, reacting with carbonyl, thiol and hydroxyl groups. In order to be effective, the gas must dissolve in a film of moisture surrounding the bacteria. For this reason, relative humidities in the order of 75% are required. Formaldehyde used in conjunction with low-temperature steam is a very effective sterilization medium.
Peracetic acid
The toxic nature of ethylene oxide and formaldehyde has prompted the search for further gaseous sterilants. Peracetic acid has been widely used as an aqueous solution but its use in the gaseous phase is more limited. It is a liquid at room temperature, requiring heat treatment to vaporize. Although it is highly active against bacteria (including mycobacteria and endospores), fungi and viruses, it is rather unstable and is damaging to certain materials such as metals and rubber.
Hydrogen peroxide
Hydrogen peroxide is similar to peracetic acid in that it is a solution at room temperature and must be heated to generate the gaseous phase. The main attraction of hydrogen peroxide as an antimicrobial agent is the fact that its decomposition products are oxygen and water. Most work on the antimicrobial properties of hydrogen peroxide has been carried out on aqueous solutions where it has been shown to have a good range of activity, including against bacterial spores. The biocidal efficacy of the vapour phase is less than that in solution and is influenced by environmental conditions.
Chlorine dioxide
Chlorine dioxide is a gas at room temperature but is primarily used in aqueous solution where it has good broad-spectrum activity. If it is to be employed in the gaseous phase then it must be generated at the point of use, and in this form it is highly effective and relatively safe.
Propylene oxide
Propylene oxide is a liquid (boiling point = 34 °C) at room temperature which requires heating to volatilize. It is inflammable between 2.1% and 21.5% by volume in air but this can be reduced by mixing with CO2. Its mechanism of action is similar to that of ethylene oxide and involves the esterification of carbonyl, hydroxyl, amino and sulphydryl groups present on protein molecules. It is, however, less effective than ethylene oxide in terms of its antimicrobial activity and its ability to penetrate materials. Whereas ethylene oxide breaks down to give ethylene glycol or ethylene chlorohydrin, both of which are toxic, propylene oxide breaks down to propylene glycol, which is much less so.
Methyl bromide
Methyl bromide boils at 3.46 °C and so is a gas at room temperature. It is used as a disinfectant and a fumigant at concentrations of 3.5 mg L−1 with a relative humidity between 30% and 60%. It has inferior antimicrobial properties compared to the previous compounds but has good penetrating power.
Gas plasmas
A plasma is formed by applying energy to a gas or vapour under vacuum. Natural examples are lightning and sunlight but plasmas can also be generated under low energy such as in fluorescent strip lights. Within a plasma, positive and negative ions, electrons and neutral molecules collide to produce free radicals. The destructive power of these entities has already been described and so plasmas can be used as biocidal agents in a variety of applications. This type of system can be produced at temperatures below 50 °C using vapours generated from hydrogen peroxide or peracetic acid. Dusseau et al (2004) have produced a useful review of the applications of gas plasmas in the sterilization of medical devices.
Antimicrobial effects of chemical agents
Chemical agents have been used since very early times to combat such effects of microbial proliferation as spoilage of foods and materials, infection of wounds and decay of bodies. Thus, long before the role of microorganisms in disease and decay was recognized, salt and sugar were used in food preservation, a variety of oils and resins were applied to wounds and employed for embalming, and sulphur was burned to fumigate sick rooms.
The classic research work of Pasteur, which established microorganisms as causative agents of disease and spoilage, paved the way for the development and rational use of chemical agents in their control. There are different definitions to describe the antimicrobial use of chemical agents in different settings. Those agents used to destroy microorganisms on inanimate objects are described as disinfectants, while those used to treat living tissues, as in wound irrigation, cleansing of burns or eye washes, are called antiseptics. The term preservative describes those antimicrobial agents used to protect medicines, pharmaceutical formulations, cosmetics, foods and general materials against microbial spoilage. Other definitions have been introduced to give more precise limits of meaning, namely, bactericide and fungicide for chemical agents that kill bacteria and fungi, respectively, and bacteriostat and fungistat for those that prevent the growth of a bacterial or fungal population. The validity of drawing a rigid demarcation line between those compounds that kill and those that inhibit growth without killing is doubtful. In many instances concentration and time of contact are the critical factors. Biocide is a general term for antimicrobial chemicals but it excludes antibiotics and other agents used for systemic treatment of infections.
The mechanisms by which biocides exert their effects have been intensively investigated and the principal sites of their attack upon microbial cells identified. These are the cell wall, the cytoplasmic membrane and the cytoplasm. Chemical agents may weaken the cell wall, thereby allowing the extrusion of cell contents, distortion of cell shape, filament formation or complete lysis. The cytoplasmic membrane, controlling as it does permeability and being a site of vital enzyme activity, is vulnerable to a wide range of substances that interfere with reactive groups or can disrupt its phospholipid layers. Chemical and electrical gradients exist across the cell membrane and these represent a proton-motive force which drives such essential processes as oxidative phosphorylation, adenosine triphosphate (ATP) synthesis and active transport; several agents act by reducing the proton-motive force. The cytoplasm, which is the site of genetic control and protein synthesis, presents a target for those chemical agents that disrupt ribosomes, react with nucleic acids or generally coagulate protoplasm.
Principal factors affecting activity
The factors most easily quantified are temperature and concentration. In general, an increase in temperature increases the rate of kill for a given concentration of agent and inoculum size. The commonly used nomenclature is Q10 (temperature coefficient), which is the change in activity of the agent per 10 °C rise in temperature (e.g. Q10 phenol = 4).
The effect of change in concentration of a chemical agent upon the rate of kill can be expressed as:
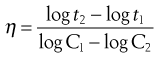 (15.4)
(15.4)
where C1 and C2 represent the concentrations of agent required to kill a standard inoculum in times t1 and t2. The concentration exponent η represents the slope of the line when log death time (t) is plotted against log concentration (C).
When values of η are greater than 1, changes of concentration will have a pronounced effect. Thus, in the case of phenol, when η = 6, halving the concentration will decrease its activity by a factor of 26 (i.e. 64-fold), whereas for a mercurial compound, η = 1, the same dilution would only reduce activity twofold (21). Further details and tabulations of both temperature coefficients and concentration exponents may be found in Denyer & Wallhaeusser (1990).
Range of chemical agents
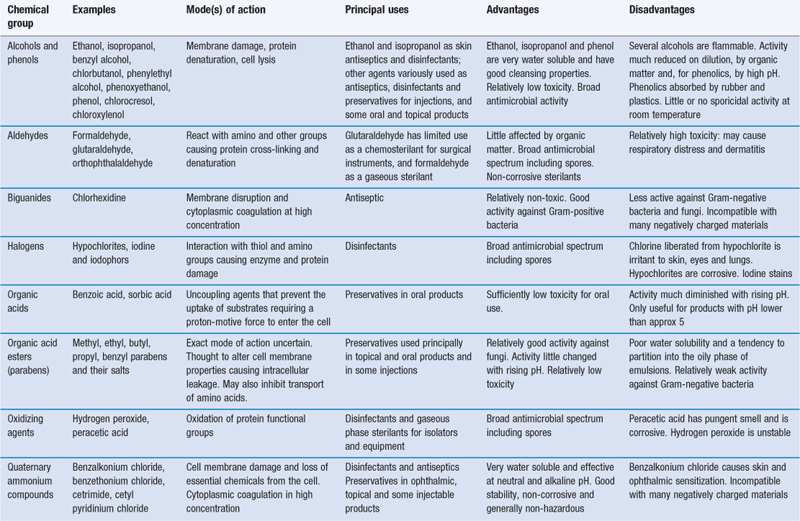
The broad categories of antibacterial chemical compounds have remained surprisingly constant over the years, with phenolics and hypochlorites comprising the major disinfectants and quaternary ammonium compounds widely used as antiseptics. The compounds capable of use as preservatives in preparations for oral, parenteral or ophthalmic administration are obviously strictly limited by toxicity requirements. As concerns over toxicity have intensified, the range of available preservatives has diminished: mercury-containing compounds, for example, are now very little used for the preservation of parenteral and ophthalmic products. The high cost of research and testing coupled with the poor prospects for an adequate financial return militate against the introduction of new agents. For this reason there is a tendency towards the use of existing preservatives in combination, with a view to achieving one or more of the following benefits: synergy, a broader antimicrobial spectrum or reduced human toxicity resulting from the use of lower concentrations. The subjects of preservative toxicity and their potentiation and synergy are reviewed by Denyer & Wallhaeusser (1990), and Moore & Payne (2004) have described in detail characteristics of the commonly used biocides. Table 15.3 summarizes the properties and uses of the major groups of biocides.
Phenolics
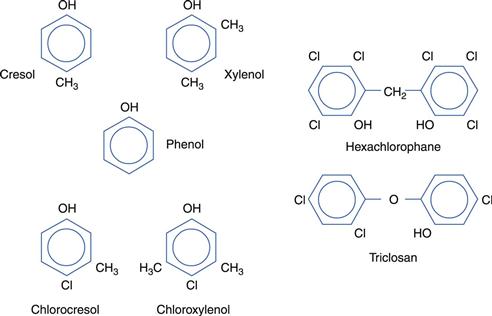
A limited selection of phenolic compounds is shown in Figure 15.4.
Various distillation fractions of coal tar yield phenolic compounds, including cresols, xylenols and phenol itself, all of which are toxic and caustic to skin and tissues. Disinfectant formulations traditionally described as ‘black fluids’ and ‘white fluids’ are prepared from higher-boiling coal tar fractions. The former make use of soaps to solubilize the tar fractions in the form of stable homogeneous solutions, whereas the latter are emulsions of the tar products and unstable on dilution.
Remarkable success has been achieved in modifying the phenol molecule by the introduction of chlorine and methyl groups, as in chlorocresol and chloroxylenol. This has the dual effect of eliminating toxic and corrosive properties while at the same time enhancing and prolonging antimicrobial activity. Thus, chlorocresol is used as a bactericide in injections and to preserve oil-in-water creams, whereas chloroxylenol is employed as a household and hospital antiseptic. Phenol may itself be rendered less caustic by dilution to 1% w/v or less for lotions and gargles, or by dissolving in glycerol for use as ear drops. Bisphenols, such as hexachlorophane and triclosan (Irgasan), share the low solubility and enhanced activity of the other phenol derivatives described but have a substantive effect which makes them particularly useful as skin antiseptics. Formulated as creams, cleansing lotions or soaps, they have proved valuable in reducing postoperative infections and cross-infection. Again toxicity concerns have emerged. Consequently, hexachlorophane, for example, is restricted in the UK both in respect of the concentrations that may be employed and the type of product in which it may be used.
Phenols are generally active against vegetative bacteria and fungi, are readily inactivated by dilution and organic matter and are most effective in acid conditions. Depending on concentration, phenols may cause cell lysis at low concentrations or general coagulation of cell contents at higher concentrations.
Alcohols, aldehydes, acids and esters
Ethanol has long been used, usually as ‘surgical spirit’ for rapid cleansing of preoperative areas of skin before injection. It is most effective at concentrations of 60–70%. It is rapidly lethal to bacterial vegetative cells and fungi but has no activity against bacterial endospores and little effect on viruses. The effect of aromatic substitution is to produce a range of compounds which are less volatile and less rapidly active and find general use as preservatives, e.g. phenylethanol for eye drops and contact lens solutions, benzyl alcohol in injections, Bronopol (2-bromo-2-nitropropane-1,3-diol) in shampoos and other toiletries. Phenoxyethanol, which has good activity against Ps. aeruginosa, has been used as an antiseptic. In general the alcohols act by disrupting the bacterial cytoplasmic membrane and can also interfere with the functioning of specific enzyme systems contained within the membrane.
Formaldehyde and glutaraldehyde are both powerful disinfectants, denaturing protein and destroying vegetative cells and spores. Formaldehyde is used in sterilization procedures both as a gas and as a solution in ethanol. Glutaraldehyde solutions are also used to sterilize surgical instruments.
The organic acids, sorbic and benzoic and their esters, because of their low toxicity, are well established as preservatives for food products and medicines (see Chapter 50). The exact mode of action of these agents on microorganisms is still uncertain but they have been shown to influence the pH gradient across the cell membrane. At higher concentrations, the parabens (esters of parahydroxybenzoic acid) induce leakage of intracellular constituents.
Quaternary ammonium compounds
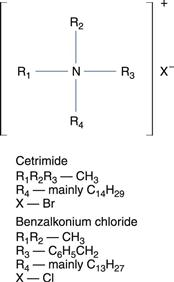
The chemical formula for quaternary ammonium compounds is shown in Figure 15.5.
These cationic surface-active compounds are, as their name implies, derivatives of an ammonium halide in which the hydrogen atoms are substituted by at least one lipophilic group, a long-chain alkyl or aryl-alkyl radical containing C8–C18 carbon atoms. In marked contrast to phenol and the cresols, these compounds are mild in use and active at such high dilutions as to be virtually nontoxic. Their surface-active properties make them powerful cleansing agents, a useful adjunct to their common use as skin antiseptics and preservatives in contact lens cleansing and soaking solutions. They are also safe for formulating into eye drops and injections, and are widely used in gynaecology and general surgery. Active as cations, ambient pH is important, as is interference caused by anions. Thus, alkaline conditions promote activity and it is important that all traces of soap, which is anion active, are removed from the skin prior to treatment with a quaternary ammonium compound. Foreign organic matter and grease also cause inactivation.
One effect of the detergent properties of these compounds is to interfere with cell permeability such that susceptible bacteria (mainly Gram-positive cells) leak their contents and eventually undergo lysis. Gram-negative bacteria are less susceptible and, to widen the spectrum to include these, mixtures of quaternary ammonium compounds with other antimicrobial agents such as phenoxyethanol or chlorhexidine are used.
Biguanides and amidines
Chlorhexidine (Fig. 15.6) is a widely used biocide which has activity against Gram-positive and Gram-negative bacteria, but little activity against endospores or viruses. It is widely used in general surgery, both alone and in combination with cetrimide, and can also be used as a preservative in eye drops. Polyhexamethylene biguanide (PHMB) is a polymeric biguanide used widely in the food, brewing and dairy industries. It has also found application as a disinfectant in contact lens cleaning solutions. The biguanides act on the cytoplasmic membrane, causing leakage of intracellular constituents.
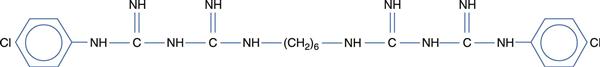
Fig. 15.6 Chemical structure of chlorhexidine.
The aromatic diamidines, propamidine and dibromopropamidine, are non-toxic antiseptics mainly active against Gram-positive bacteria and fungi. However, resistance to these agents can develop quickly during use.
Halogens and their compounds
Chlorine gas is a powerful disinfectant used in the municipal treatment of drinking water and in swimming baths. Solutions of chlorine in water may be made powerful enough for use as general household bleach, and disinfectant and dilute solutions are used for domestic hygiene. The high chemical reactivity of chlorine renders it lethal to bacteria, fungi and viruses, and to some extent spores. This activity is optimal at acid pH levels around 5.0. Unionized hypochlorous acid (HOC1) is an extremely potent and widely used bactericidal agent that acts as a non-selective oxidant, reacting readily with a variety of cellular targets. Salt solutions subjected to electrolysis in an electrochemical cell yield a mixture of biocidal species of which the predominant one is hypochlorous acid. This system is available commercially for use in endoscope washers.
Two traditional chlorine-containing pharmaceutical formulations, which are used much less frequently now, are Eusol (Edinburgh University solution of lime, also known as Chlorinated Lime and Boric Acid Solution BPC 1973) and Dakin’s Solution (Surgical Chlorinated Soda Solution BPC 1973), both of which are designed to provide slow release of chlorine.
An alternative method of obtaining more prolonged release of chlorine is by the use of organic chlorine compounds such as Chloramine T (sodium p-toluene-sulphonchloramide) and Halazone BPC 1973 (p-sulphondichloramide benzoic acid). These are used in pharmaceutical products much less frequently now but have retained some application in the disinfection of water such as in whirlpool spas and in fish farms.
Iodine, which, like chlorine, is a highly reactive element, denatures cell proteins and essential enzymes by its powerful oxidative effects. Traditionally it has been used in alcoholic solutions such as Tincture of Iodine (BP 1973) or complexed with potassium iodide to form an aqueous solution (Lugol’s Iodine BP 1973). The latter product, although highly effective as a bactericide, probably fell out of favour because of the tendency to stain both the clothes and skin.
The staining and irritant properties of iodine have resulted in the development of iodophores, mixtures of iodine with surface-active agents, which hold the iodine in micellar combination from which it is released slowly. Such a preparation is Betadine (polyvinylpyrrolidone-iodine formulated as 10% povidone iodine), used as a non-staining, non-irritant antiseptic.
Metals
Many metallic ions are toxic to essential enzyme systems, particularly those utilizing thiol (-SH) groups, but those used medically are restricted to mercury, silver and aluminium. The extreme toxicity of mercury has rendered its use obsolete apart from in organic combination. The organic compounds that still have a limited use in pharmacy are phenylmercuric nitrate (and acetate) as a bactericide in eye drops and injections, and thiomersal (sodium ethylmercurithiosalicylate) as a preservative in biological products and certain eye drops.
Silver, in the form of the nitrate, has been used to treat infections of the eyes, as have silver protein solutions. Aluminium foil has been used as a wound covering in the treatment of burns and venous ulcers. It has been shown to adsorb microorganisms and inhibit their growth.
The acridines
This group of compounds interferes specifically with nucleic acid function and has some ideal antiseptic properties. Thus, aminacrine hydrochloride is non-toxic, non-irritant, non-staining and active against Gram-positive and Gram-negative bacteria even in the presence of serum.
References
1. Denyer SP, Wallhaeusser KH. Antimicrobial preservatives and their properties. In: Denyer SP, Baird R, eds. Guide to Microbiological Control in Pharmaceuticals. Chichester: Ellis Horwood; 1990.
2. Dusseau J-Y, Duroselle P, Freney J. Gaseous sterilization. In: Fraise AP, Lambert PA, Maillard J-Y, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 4th edn Oxford: Blackwell Science; 2004.
2011. European Pharmacopoeia. 7th edn Strasbourg: Council of Europe; 2011.
4. Moore SL, Payne DN. Types of antimicrobial agents. In: Fraise AP, Lambert PA, Maillard J-Y, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 4th edn Oxford: Blackwell Science; 2004.
2007. 2007.
6. Russell AD. Destruction of bacterial spores by thermal methods. In: Russell AD, Hugo WB, Ayliffe GAJ, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 3rd edn Oxford: Blackwell Science; 1999.