Acromegaly
Excess Growth Hormone Secretion
Excess Growth Hormone–Releasing Hormone Secretion
Role of the Hypothalamus in the Etiology of Acromegaly
Integrated Treatment Approach to the Management of Acromegaly
Acromegaly is a disease of spectacular growth and metabolic disorders that has fascinated physicians for centuries. The natural history of the disorder, if left untreated, results in gross acral and facial disfigurement, musculoskeletal disability, cardiac failure, respiratory dysfunction, diabetes, and accelerated mortality.1–3 If the disease occurs before epiphyseal closure, gigantism results.1 After the first modern description of the disease in 1886 by Marie,4 it was subsequently recognized that the disorder is associated with a growth hormone (GH)-secreting adenohypophyseal adenoma, resulting in both a central mass lesion and the protean peripheral effects of sustained tissue exposure to high GH levels.1,5–7
Pathogenesis
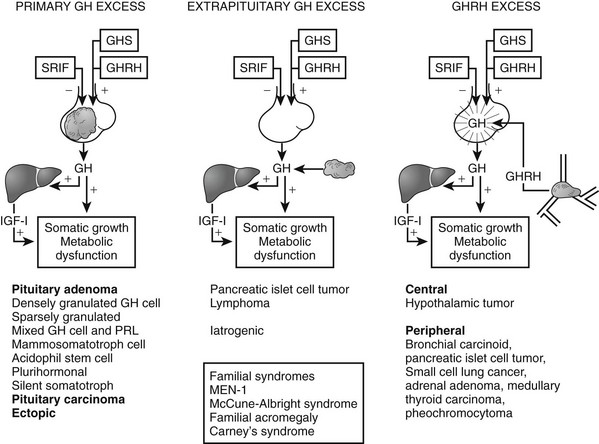
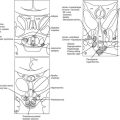
The pathogenetic events that underlie the etiology of pituitary acromegaly include excessive pituitary somatotroph cell proliferation and unrestrained GH hypersecretion (Fig. 7-1). GH is secreted by somatotroph cells, the largest differentiated compartment of the anterior pituitary. GH secretion is under dual hypothalamic inhibitory control: somatotropin release–inhibiting factor (SRIF) inhibits secretion, and GH-releasing hormone (GHRH) stimulates both GH synthesis and secretion.8,9 Ghrelin, a gut-derived peptide, binds to the GHS receptor and acts primarily at the hypothalamus to induce GH. Insulin-like growth factor 1 (IGF-1), the peripheral target molecule for GH action, participates in negative GH feedback inhibition by acting both at the hypothalamus to induce SRIF and directly at the pituitary to inhibit GH gene transcription.10–12 Peripheral sex and adrenal steroids also regulate GH secretion.8,13 GH itself binds to peripheral GH receptors that elicit signaling by JAK/STAT (Janus kinase/signal transducer and activator of transcription) intracellular phosphorylation cascades.14 GH acts directly to attenuate insulin action and induce lipolysis.15 The growth-promoting actions of GH are indirectly mediated by IGF-1, which is synthesized in the liver, kidney, pituitary, gastrointestinal tract, muscle, and cartilage.12,16,17 GH actions mediated by IGF-1 include protein synthesis; amino acid transportation; muscle, cartilage, and bone growth; DNA and RNA synthesis; and cell proliferation.18,19 Local production of IGF-1 may be under autocrine and paracrine regulation,3,20,21 acting in concert with circulating IGF-1 and GH to elicit a final tissue impact.
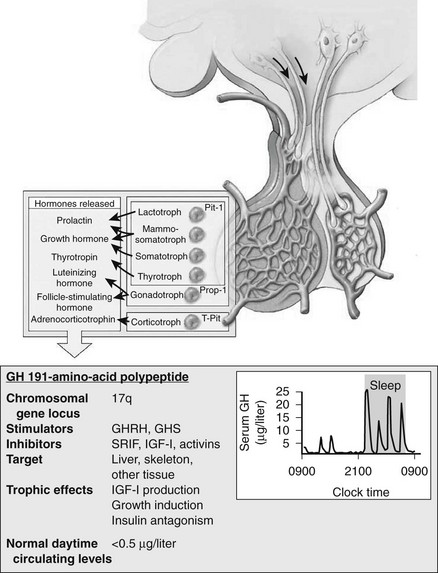
FIGURE 7-1 Synthesis and secretion of growth hormone by the anterior pituitary gland. (Modified from Melmed S: Acromegaly. New Engl J Med, 355:2558–2573, 2006.)
Excess Growth Hormone Secretion
Tumors may arise from clonal expansion of one or more of the anterior pituitary differentiated cell types22,23 and thereby result in specific hormone hypersecretory syndromes. The most common cause of acromegaly is a somatotroph (GH-secreting) adenoma of the anterior pituitary, which accounts for 30% of all hormone-secreting pituitary adenomas1 (Fig. 7-2). GH-secreting adenomas arise from differentiated cells secreting GH gene products1,23,24 (Table 7-1). These cells include somatotrophs, mixed mammosomatotrophs (secreting both GH and prolactin [PRL]), or more primitive acidophilic stem cells. Regardless of their cellular origin, transformation and subsequent replication of these cells result in adenoma formation as well as unrestrained GH secretion.1 Most patients harbor densely granulated GH-cell adenomas, which are commonly encountered in older patients with indolent disease progression. Sparsely granulated GH-cell adenomas occur in younger patients with more aggressive disease onset and higher GH levels.1,2 Mammosomatotroph cell tumors, or discrete mammotroph and somatotroph tumors, reflect the common stem cell origin of the somatotroph cell lineage.2,24–26 Although acidophil stem cell adenomas secrete GH, their predominant product is PRL, thus accounting for the high incidence of hyperprolactinemic symptoms (galactorrhea, amenorrhea, infertility) initially seen in these patients.27 Patients with McCune-Albright syndrome also may have acromegaly, although the presence of a discrete GH-cell adenoma has been inconsistently reported in these cases.28 Rarely, acromegaly may occur in patients with a partially empty sella.29 The rim of pituitary tissue surrounding the empty sella may harbor a small endocrinologically active GH-secreting adenoma not visible on magnetic resonance imaging (MRI) (i.e., <2 mm in diameter). Because embryonic pituitary tissue originates from the nasopharyngeal Rathke’s pouch, ectopic pituitary adenomas may arise in remnant nasopharyngeal tissue along the line of primitive adenohypophysial migration. These adenomas may not be detected on pituitary MRI fields, and more extensive skull base imaging may be required. Very rarely, ectopic GH production by pancreatic,30 lung,31 ovarian,32 or lymphocytic neoplasms may result in acromegaly.1
Table 7-1
Clinical and Pathologic Characteristics of Growth Hormone–Secreting Pituitary Tumors
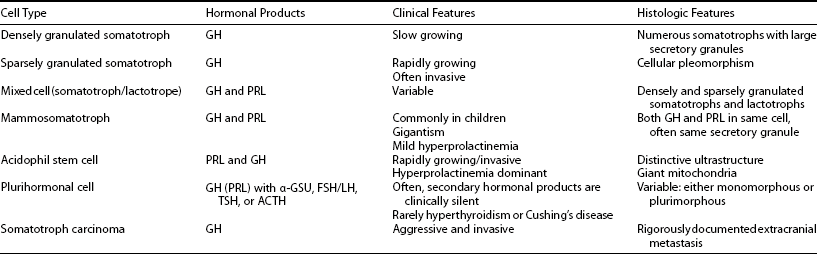
Adapted from Melmed S: Pathogenesis of pituitary tumors. Endocrinol Metab Clin North Am 21:553–574, 1992.
EXCESS GROWTH HORMONE–RELEASING HORMONE SECRETION
Excessive circulating levels of GHRH may overstimulate the pituitary and cause somatotroph hyperplasia, GH hypersecretion, and acromegaly.33 Central overproduction of GHRH may occur in patients harboring hypothalamic hamartomas or gangliocytomas.33 These rare tumors are usually diagnosed by pathologic examination of a surgically resected sellar mass causing GH hypersecretion and acromegaly.34 Ectopic GHRH production by carcinoid tumors, although rare, accounts for most cases of acromegaly.35 The clinical association of acromegaly with carcinoid disease had long been recognized, and the pathogenesis of ectopic GHRH production is now elucidated. Subclinical GHRH immunoreactivity has been demonstrated in about 40% of lung, abdominal, and bony carcinoid tissue specimens.
Pituitary somatotroph hyperplasia plus acromegaly associated with ectopic GHRH production has been reported in more than 100 patients, and the original isolation of GHRH was accomplished from a pancreatic carcinoid tumor.36 Because the peripheral features of hypersomatotropism are quite similar in all forms of pituitary and nonpituitary acromegaly, diagnosis of the etiology of the disease may be clinically challenging.1
Acromegaloidism is a very rare syndrome characterized by acromegalic features with no discernible pituitary tumor and normal serum GH and IGF-1 concentrations. It has been presumed that this disorder is due to excess secretion of a putative, as yet unidentified, growth factor.37
Role of The Hypothalamus in The Etiology of Acromegaly
Hypothalamic GHRH and SRIF selectively regulate GH gene expression and secretion.8 These hypothalamic peptide hormones are expressed both within the anterior pituitary gland itself and within GH-secreting pituitary tumors.38,39 GHRH, in addition to its hormonal regulation of GH production, induces somatotroph DNA synthesis.40 Mice bearing an overexpressing GHRH transgene are subject to somatotroph hyperplasia and ultimately to pituitary adenomas.41,42 In patients with carcinoid tumors and ectopic GHRH production, somatotroph hyperplasia and occasionally adenomas also may develop, which suggests that disordered endocrine or paracrine GHRH or SRIF action may be permissive for pituitary tumor growth.43 GHRH signaling defects also have been identified in acromegaly. Constitutive activation of the GHRH receptor G-protein signaling unit facilitates ligand-independent induction of GH gene expression. This gsp mutation results in guanosine triphosphatase (GTPase) inactivation, with subsequent elevated cyclic adenosine monophosphate (cAMP) levels and GH hypersecretion.44,45 Excessive CREB (cAMP response element binding protein) serine phosphorylation also may account for activation of the CREB–Pit-1 (pituitary-specific transcription factor 1) signaling unit in a subset of GH-cell adenomas.46
Pituitary tumor–derived paracrine GHRH or SRIF or both also may regulate tumor growth or function, although constitutively activating hormone receptor structural mutations have not been identified clinically. A truncated alternatively spliced GHRH receptor transcript has been described, but its functional significance is unclear.47 In light of compelling evidence favoring intrinsic genetic defects occurring in GH-secreting pituitary tumors, as discussed later, it is apparent that hypothalamic influences may be permissive of tumor growth rather than being proximally involved in the initiation of somatotroph tumorigenesis.43,48
Intrinsic Pituitary Lesions
Virtually all GH-cell adenomas arise as discrete clonal expansions of a transformed cell22 (Table 7-2). This monoclonal origin implies that intrinsic genetic alterations account for tumorigenic initiating events and supports abundant earlier clinical observations that resection of small well-circumscribed adenomas usually results in surgical cure of GH-secreting adenomas.24,43,49 Because adenohypophyseal tissue surrounding the pituitary adenoma is histologically normal, it is unlikely that multiple independent cellular growth events (e.g., generalized hyperplasia) precede adenoma formation. Increasing evidence points to complex molecular cascades accounting for the cellular progression, resulting in pituitary-cell transformation and, ultimately, tumor formation. Multistep development of pituitary acromegaly involves a spectrum of genetic alterations associated with dysregulation of cell proliferation, differentiation, and GH production.43 Activation of oncogene function or inactivation of tumor-suppressor genes or both may account for these changes43,50,51 (see Table 7-2).
Table 7-2
Evidence for an Intrinsic Pituitary Defect in the Pathogenesis of Acromegaly
GH-secreting adenomas are monoclonal
Absence of somatotroph hyperplasia in normal pituitary tissue surrounding pituitary adenomas
Successful surgical cure of well-circumscribed GH cell adenomas is achieved in >75% of patients
Adenoma transformation is rarely associated with generalized somatotroph hyperplasia
Unrestrained GH hypersecretion occurs independent of physiologic hypothalamic feedback control
Normalization of GH pulsatility often occurs after complete adenoma resection
Adapted from Drange MR, Melmed S: IGFs in the evaluation of acromegaly. In Rosenfeld RG, Roberts CT (eds): Contemporary endocrinology. The IGF system: molecular biology, physiology, and clinical applications. Totowa, NJ, 1999, Humana, pp 699–720.
Candidate Genes in The Etiology of Acromegaly
Several transgenic animal models have shown that disruption of tumor-suppressor genes (including RB [retinoblastoma] and p27) results in a high incidence of pituitary tumor formation in afflicted mice.52–54 Because a variety of chromosomal loss of heterogeneity (LOH) patterns are observed in human adenomas, loss of tumor-suppressor gene activity was similarly postulated for human tumors (Table 7-3).
Table 7-3
Tumor-Suppressor Genes and Oncogenes Associated With GH-Cell Adenomas
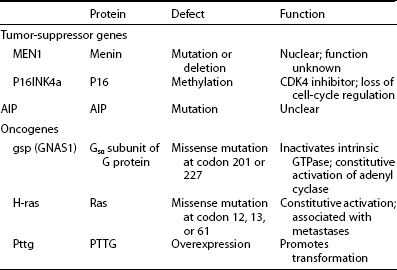
GH, Growth hormone; GTPase, guanosine triphosphatase; PTTG, pituitary tumor-transforming gene.
Adapted from Drange M, Melmed S: Etiopatogenia de la acromegalia. In Webb S (ed): Libro De La Acromegalia. Barcelona, Spain, 1998, Accion Medica.
Several chromosomal lesions occur in pituitary tumor tissue derived from patients with sporadic nonfamilial acromegaly. LOH involving chromosomes 11q13, 13, and 9 occurs in up to 20% of sporadic50,55,56 pituitary tumors. Despite the multiple endocrine neoplasia type 1 (MEN1) gene location on chromosome 11, non-MEN1 patients with sporadic pituitary tumors and 11q LOH harbor intact coding and intronic sequences, with appropriately expressed MEN1 messenger RNA (mRNA).56 Lesions in chromosomes 13 and 9 also are more prevalent in invasive or larger adenomas.51 Chromosome 13q LOH occurs in proximity to the RB locus and was found in 13 aggressive pituitary tumors, whereas small circumscribed tumors exhibit intact RB alleles.57 These results suggest the presence of putative tumor-suppressor genes located on chromosomes 11 and 13 that may be involved in controlling the propensity for pituitary tumor proliferation. Despite these heterogeneous chromosomal LOH patterns, consistent loss of tumor-suppressor gene activity has not been identified for acromegaly. Although tumor invasiveness or size correlates with an increased propensity for chromosomal LOH,51 identification of a specific molecular lesion leading to loss of antiproliferative activity in GH-secreting tumors remains elusive43 (see Table 7-2).
Activating Mutations
GTPase acts to inactivate stimulatory G (Gs) proteins that induce adenyl cyclase and intracellular cAMP accumulation.58 Missense mutations replacing residue 201 (Arg → Cys or His) or 227 (Gln → Arg or Leu), termed gsp, result in persistently elevated ligand-independent Gs activity and constitutively elevated cAMP and GH hypersecretion.44 Gsp mutations occur in a subset of GH adenomas, with a prevalence ranging from 30% to 40% in whites58–63 to only 10% in Japanese64 patients with acromegaly. Clinical or biochemical correlations have not been associated with gsp mutations.44,65 Thus, although these mutational events suggest a compelling mechanism for explaining GH-cell hypersecretion, their clinical significance has not been apparent (see Table 7-3), because the natural course of the disease does not differ in gsp+ve or gsp−ve patients.
Rarely, ras mutations have been observed in highly invasive pituitary tumors or their extrapituitary metastases.66–68 Development of true GH-cell carcinoma with documented extracranial metastases, however, is exceedingly rare67 (see Table 7-3). A pituitary tumor-transforming gene (PTTG) was isolated from rat GH-secreting pituitary tumor cells69 and is functionally homologous to yeast securin, which regulates sister chromatid separation during mitosis.70 PTTG overexpression results in cell transformation in vitro and experimental pituitary tumor formation in vivo. PTTG mRNA is abundant in GH-producing tumors, with more than 10-fold increases evident in larger tumors.71 The strong transforming potential of PTTG indicates a role in early induction of GH-cell transformation, possibly by regulating the pituitary cell cycle70 (see Table 7-3).
Familial Syndromes
Acromegaly may occur as a component of MEN syndromes, including the Carney complex or MEN1. The Carney complex consists of myxomas, spotty skin pigmentation, and testicular, adrenal, and pituitary tumors.72–75 About 20% of patients with this autosomal-dominant syndrome associated with chromosome 2p16 harbor GH-secreting pituitary tumors.50,72
The MEN1 gene is located on chromosome 11q13, and LOH of chromosome 11q13 occurs in pancreatic, parathyroid, and pituitary tumors of patients with MEN1.56,76 Inactivation of the MEN1 tumor-suppressor gene likely accounts for the syndrome, in accordance with Knudson’s “two hit” theory whereby both inherited allelic germline mutations and a somatic deletion are required for inactivation of both specific alleles and subsequent tumor formation.77 MEN1, an autosomal-dominant syndrome, consists of hyperplastic or adenomatous parathyroid glands, endocrine pancreas, and anterior pituitary.76 Pituitary adenomas develop in almost half these patients, with GH-cell adenomas reported in about 10% of afflicted subjects.
Isolated familial acromegaly or gigantism not associated with MEN has rarely been reported.50,78,79 Chromosome 11q13 LOH with no discernible MEN 1 mutation was detected in the pituitary adenomas of two brothers with gigantism.80 Low prevalence germline mutations of the aryl hydrocarbon receptor–interacting protein (AIP, located on 11q 13.3) gene were reported as predisposing to a subset of patients with familial acromegaly and gigantism205; 15% of families with isolated familial acromegaly exhibit AIP mutations, and tumors are encountered earlier in subjects harboring a mutation.206,207 AIP has also been proposed as a tumor-suppressor gene for pituitary adenomas.209 The clinical significance of these findings to the broad population of sporadic GH-secreting tumors is at present unclear.208
Epidemiology of Acromegaly
Acromegaly is a rare disease, and accurate assessment of its prevalence in the community has been difficult to ascertain. In Newcastle, England, an annual incidence of 2.8 new patients per million adult population was reported, with an approximate point prevalence of 38 cases per million adult population.81 A higher incidence was reported in Sweden, where the average prevalence of the disease was reported to be 69 cases per million.2,82 If these data are projected to the population of the United States, 750 to 900 new cases would be expected annually, and GH-secreting pituitary adenomas would be present but undiagnosed in another 10,000 to 20,000 persons. The mean age at diagnosis is 40 to 45 years, and its insidious onset may cause the disease to not be diagnosed until 10 to 12 years after symptom onset.82–84 This long delay in diagnosis is often due to the subtle and slow onset of common symptoms, including headache, joint pains, jaw malocclusion, or mild type 2 diabetes. Furthermore, this relatively long time delay allows prolonged exposure of peripheral tissues to unacceptably elevated GH and IGF-1 levels.
Diagnosis
Persistent GH hypersecretion is the hallmark of acromegaly. Excess GH stimulates hepatic production of IGF-1, which is responsible for most of the clinical manifestations of acromegaly.85–87 The diagnosis is often delayed for up to 12 years because of slow clinical progression over many years. Heightened clinical awareness may result in earlier diagnosis.211 Although serum GH and IGF-1 concentrations are both increased in virtually all patients with acromegaly, serum IGF-1 levels may be discordant with GH increases. When a patient is suspected to have acromegaly, biochemical testing is required to confirm the clinical diagnosis, and imaging techniques are used to localize the cause of excess GH secretion (Table 7-4).
Documenting Growth-Hormone Hypersecretion
The diagnosis of acromegaly is confirmed by measurement of serum GH after a glucose load and by assessing levels of GH-dependent circulating molecules such as IGF-1 and IGF-binding protein 3 (IGFBP-3).29 IGF-1 levels reflect the integrated bioeffects of GH hypersecretion, and age- and gender-matched elevated IGF-1 levels are pathognomonic of acromegaly.88
Measurement of the serum IGF-1 concentration is the most precise screening test for acromegaly. Unlike those of GH, serum IGF-1 concentrations do not fluctuate hourly according to food intake, exercise, or sleep, but rather reflect integrated GH secretion during the preceding day or longer. Serum IGF-1 concentrations are elevated in virtually all patients with acromegaly, thus providing excellent discrimination from subjects without acromegaly.2,85 In normal subjects, serum IGF-1 concentrations are highest during puberty and decline gradually thereafter; values are significantly lower in adults older than 60 years than in younger subjects. Females have higher levels than do males, and pregnancy may also be associated with elevated IGF-1 levels. Thus, an inappropriately controlled “normal” IGF-1 value in an elderly male patient may in fact be truly elevated and indicative of acromegaly. Serum GH should be measured in patients with equivocal or elevated age- and sex-adjusted serum IGF-1 values.
Although all patients with acromegaly have increased GH secretion, it may be difficult to distinguish elevated random GH levels from normal. As GH levels fluctuate widely throughout the day and night, measuring random GH levels rarely provides useful information for diagnosis of the disorder.2 Short-term fasting, exercise, stress, and sleep are associated with elevated GH, and the availability of ultrasensitive GH assays has indicated that this pulsatile GH rhythm may occur at levels below the detectable sensitivity of previously available assays. Serum GH concentrations fluctuate widely, from less than 0.5 ng/mL (with ultrasensitive assays) during most of the day to as high as 20 or 30 ng/mL at night or after vigorous exercise. Random serum GH concentrations may be elevated in patients with uncontrolled diabetes mellitus, liver disease, and malnutrition, so dynamic tests have been proposed to confirm pituitary GH hypersecretion. The mean GH concentration obtained from 6-hourly samplings will generally provide an integrated summation of net GH secretion, and averaged pooled levels greater than 5 ng/mL are usually encountered in acromegaly.2
The diagnostic hallmark of excess GH hypersecretion was failure to suppress GH levels to 0.4 ng/mL or less (using a chemiluminescent immunoradiometric assay) during a 2-hour period after a 75-g oral glucose load.29 Several factors determine the measurement of serum GH values, including age, gender, BMI, and the type of assay employed. Spontaneous GH secretion is attenuated by 50% to 70% in subjects aged 65 years or more, and IGF-1 levels also decline progressively with age.200 GH levels also correlate inversely with BMI, and lean subjects exhibit higher GH values, as do female subjects.201 Accordingly, criteria for the diagnosis of acromegaly requires demonstrating a mean 24-hour GH level of >2.5 µg/L, a nadir GH of >1 ng/mL after a glucose load, and/or an elevated age- and gender-watched serum IGF-1 level.202
When GH levels were measured by different assays in 46 patients with controlled18 or uncontrolled28 disease,203 values obtained with Diagnostic Products Corporation’s IMMULITE assay were ∼2.3 fold higher than those determined by the Nichols assay. Using the IMMULITE assay, postglucose values of <1 µg/L were associated with disease control, while with the Nichols assay, the proposed cutoff value was 0.5 µg/L. Thus, interpretation of biochemical control should also be determined by knowledge of the specific GH assay employed, as well as the appropriateness of assay controls.204
Invariably, patients who fail to suppress GH after glucose exhibit elevated total IGF-1 levels, with a strong log-linear association between the 24-hour mean GH output and IGF-1 levels.2,89 About 10% or fewer patients may have apparently “normal” GH or IGF-1 levels or both at the time of diagnosis. Repeating the assays in a reputable laboratory may often resolve an apparent clinical/biochemical discordance. Alternatively, reinterpretation of a glucose suppression test or use of a rigorous GH assay may confirm the diagnosis.
Because IGFBP-3 secretion is GH dependent, concentrations may be elevated in patients with acromegaly, thus suggesting that IGFBP-3 measurement may prove useful in diagnosis.90 However, in contrast to the tight correlation of integrated mean 24-hour serum GH with total and free IGF-1 levels, IGFBP-3 levels do not correlate as tightly with disease activity.91,92 Thirty-two percent of subjects with active acromegaly had normal IGFBP-3 levels, and in patients who failed to suppress GH, no consistent elevation of IGFBP-3 was observed.91 Thus, the utility of IGFBP-3 measurements for acromegaly diagnosis or follow-up is limited.
Localizing The Source of Excess Growth Hormone
Nonpituitary Acromegaly
Rare nonpituitary causes of acromegaly include a hypothalamic tumor secreting GHRH,33,93 a nonendocrine tumor secreting GHRH,36,94 or ectopic GH secretion by a nonendocrine tumor.1,31,32 MRI of the head and pituitary should identify some of these tumors. If pituitary MRI findings are normal, abdominal and chest imaging should be performed, followed by catheterization studies in an attempt to demonstrate an arteriovenous GHRH gradient over the suspected tumor bed. In patients with ectopic GHRH secretion, serum GHRH and GH concentrations are both elevated, and pituitary MRI reveals a normal-sized or enlarged hyperplastic gland.95
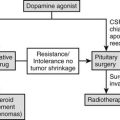
An algorithm for the diagnostic evaluation of patients suspected of having acromegaly is shown in Fig. 7-3. A normal age- and gender-controlled serum IGF-1 concentration is strong evidence excluding the diagnosis of acromegaly. If the serum IGF-1 concentration is high (or equivocal), serum GH should be measured within 2 hours after oral glucose administration. If pituitary MRI fails to reveal the presence of a discrete adenoma in the presence of clear-cut biochemical evidence of hypersomatotropism, studies to identify the rarely encountered GHRH- or GH-secreting tumors should be undertaken.
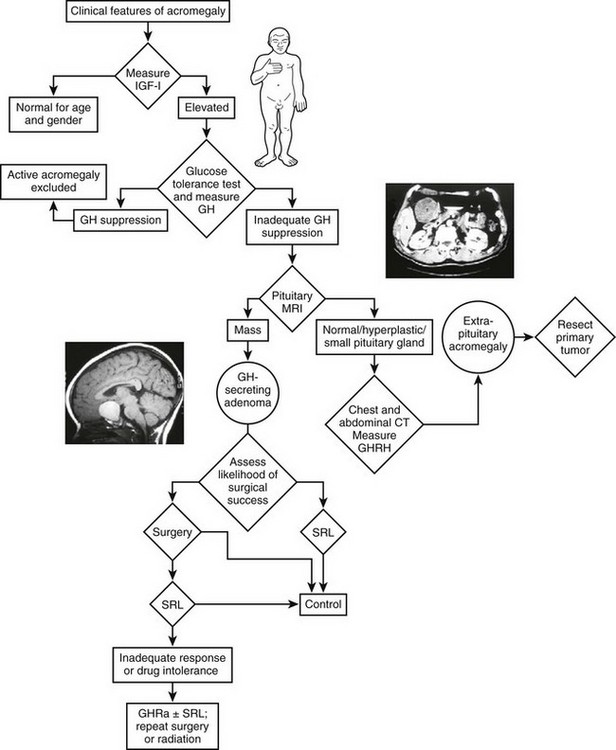
FIGURE 7-3 Diagnosis and management of acromegaly. GH, Growth hormone; GHRH, growth hormone–releasing hormone; IGF-1, insulin-like growth factor 1; MRI, magnetic resonance imaging; OGTT, oral glucose tolerance test; GHRA, GH receptor antagonist; SRL, somatostatin-receptor ligand. (Modified from Melmed S: Acromegaly, New Engl J Med 355:2558–2573, 2006.)
Clinical Manifestations
The systemic clinical features of acromegaly occur as a consequence of the deleterious impact of elevated serum concentrations of both GH and IGF-1 on peripheral tissues (Table 7-5). The somatic impact of elevated GH includes growth stimulation of a variety of tissues, such as skin, connective tissue, cartilage, bone, and many epithelial tissues, including mucosal surfaces. The metabolic effects of excess GH include nitrogen retention, insulin antagonism, and enhanced lipolysis.6
Table 7-5
Risk of Long-Term Exposure to Elevated Growth Hormone Levels
From Melmed S, Dowling RH, Frohman L, et al: Acromegaly: consensus for cure, Am J Med 97:468, 1994.
The onset of acromegaly is insidious, and disease progression is usually slow. At diagnosis, about 75% of patients are shown to harbor macroadenomas (tumor diameter of ≥10 mm), and some tumors extend to the parasellar or suprasellar regions.15 Headaches are the initial symptom in approximately 60% of patients, and 10% have visual symptoms.
Acral Overgrowth
Acral and soft-tissue overgrowth is invariably a feature of acromegaly. Characteristic findings include an enlarged, protruding jaw (macrognathia) with associated mandibular overbite and enlarged, swollen hands and feet, resulting in increasing shoe and glove size and the need to enlarge rings. Facial features are coarse, with enlargement of the nose and frontal bones, as well as the jaw; the upper incisors are consequently spread apart. Despite the prominence of these findings, the rate of change is so slow that few patients seek care because their appearance has changed (e.g., only 13% of 256 patients in one series5).
Rheumatologic Features
Musculoskeletal symptoms are leading causes of morbidity and serious functional disability in patients with acromegaly.96,97 In several studies encompassing large series, at least half of all patients exhibited minor arthralgias, and severe, debilitating arthritic features ultimately developed in more than one third of patients.97,98 The pathogenesis of joint disease in acromegaly generally begins with a noninflammatory osteoarthritic disorder and culminates in severe secondary joint and cartilage degeneration.97 Excess GH and IGF-1 exposure leads to uneven cartilage proliferation that results in a mechanically unstable joint surface. Joint spaces then narrow as weight-bearing surfaces erode cartilage and cause excess intraarticular new fibrocartilage deposits. Subchondral cysts and osteophytes then develop in an irreversible self-perpetuating process. Severe physical deformity and functional disability result from these inexorable pathologic and mechanical stresses. Although symptomatic and functional relief of arthritic disorders is observed in most patients after reducing GH levels, structural changes are unfortunately not reversible.98,99
Joint arthralgias are a common initial feature of the disease, and back pain and kyphosis are common.5 Synovial tissue and cartilage enlarge and cause hypertrophic arthropathy of the knees, ankles, hips, spine, and other joints.97 Back pain also may occur because of osteoporosis caused either by GH excess itself or concurrent gonadal insufficiency from the enlarging pituitary tumor. Spine and hip bone density may be increased in women with acromegaly, but not if estrogen deficiency is present.100 When excess GH secretion begins before epiphyseal fusion, linear growth increases and causes pituitary gigantism.
Skin and Soft Tissues
The skin thickens, and multiple recurrent skin tags may appear.3,101 Hyperhidrosis at rest is common (present in 50% of patients)2,3,5 and often malodorous. Hair growth increases, and some women have hirsutism.2,5,102 Other manifestations of soft-tissue overgrowth include macroglossia, deepening of the voice, and paresthesias of the hands (carpal tunnel syndrome) from nerve entrapment.2,5–7,15,98 Other patients have a symmetric sensorimotor peripheral (rarely hypertrophic) neuropathy unrelated to entrapment.96
Thyroid
Thyroid enlargement may be diffuse or multinodular. In a study of 37 patients with acromegaly, 92% had an enlarged thyroid gland when assessed with ultrasound; mean thyroid size was increased more than five times normal.103 Thyroid function is, however, usually normal.
Cardiovascular
Impaired cardiovascular function in acromegaly is an important determinant of morbidity and mortality,3,104 with exacerbated cardiovascular risk factors.198 The deleterious direct impact of excess GH and IGF-1 on the heart, as well as the effect of hypertension, which is present in 30% of patients, contributes to the disorder.5,105,106 Cardiac enlargement is disproportionate to the increased size of internal body organs,107–109 and the severity of cardiomyopathy correlates significantly with the duration of exposure to hypersomatotropism.104,108,110 Mean left ventricular mass may be significantly increased to more than 200 g, as opposed to a normal mean weight of 140 g, and end-systolic and diastolic volumes are attenuated. Concentric ventricular hypertrophy is associated with interstitial fibrosis, lymphocytic infiltration, and necrosis.108 Resting diastolic blood pressure and left and right ventricular peak filling rates are elevated. Post-exercise systolic and diastolic blood pressure may also be elevated, and the left ventricular ejection fraction is attenuated.111 Because physiologic doses of replacement GH also may actually improve cardiac function in patients with adult GH deficiency, a fine equilibrium may exist for the respective impacts of GH excess and GH deficiency on maintaining healthy myocardial function.108
Sleep Apnea
Peripheral airway obstruction caused by macroglossia, mandible deformation, mucosal hypertrophy, and inspirational laryngeal collapse has long been recognized as causing airway obstruction,5 snoring,3 and sleep apnea. Sleep apnea afflicts most patients with acromegaly and has been documented in up to 80% of cases.3 Macroglossia and enlargement of the soft tissues of the pharynx and larynx lead to obstructive sleep apnea in about 50% of patients; others have central sleep apnea, possibly resulting from altered central respiratory control.112 Sleep apnea may be an important cause of mortality in these patients. A central form of sleep apnea was recognized in acromegaly,112–114 which appears to correlate more closely with elevated GH and IGF-1 levels and may reflect central respiratory suppression caused by the dysregulated hypothalamic-GH axis. Clearly, the strong association of sleep apnea with hypertension, coronary artery disease, and cardiac arrest also reflects the clinical phenotype of patients with acromegaly. Attenuation of GH levels, especially with octreotide, improves or abrogates sleep apnea.112,115 Octreotide treatment is associated with improved indices for apnea, hypopnea, and oximetry.195 After 6 months of treatment of 14 apneic acromegalic patients with octreotide, a 40% decrease in the number of apneic events per hour was seen, as well as a decrease in total apneic time from 28% to 15%. Maximum O2 saturation rose from 76% to 84%, accompanied by a decline in daytime sleepiness, as well as improvement in central and obstructive apneic parameters.112
Diabetes
GH is a potent antagonist of insulin action, and glucose intolerance is encountered in up to 60% of patients. About 25% of patients may require insulin, and thus diabetes is an important systemic complication of hypersomatotropism. Diabetes is a major determinant of mortality, and only 30% of patients with diabetes at the time acromegaly is diagnosed appear to survive 20 years.83,116
Gonadal Function
Women with acromegaly may have amenorrhea, with or without galactorrhea,3,15,117 and some have hot flashes and vaginal atrophy. Men may have impotence, loss of libido, decreased facial hair growth, and testicular atrophy.3,7,117 Hypogonadism is caused either by hyperprolactinemia (present in about 30% of patients)102,117 or by impairment of gonadotropin secretion as the expanding pituitary tumor compresses normal pituitary gonadotroph cells. Asymptomatic, reversible prostatic enlargement also is common, even in men with hypogonadism.118,119
Neoplasms
Acromegaly is associated with an enhanced risk for development of colonic polyps,120–122 and prospective studies have reported premalignant adenomatous colonic polyps in up to 30% of patients, a prevalence not different from that in the general U.S. population.122–124 In patients with acromegaly, colon length is increased, and apoptotic activity is decreased significantly.121,209 Patients with acromegaly are more likely to have multiple adenomatous polyps, as well as polyps proximal to the splenic flexure, underscoring the need for full-length colonoscopy.121,125 No difference in the duration or degree of acromegaly is evident in patients with or without adenomatous polyps. A multicenter retrospective study of 1362 patients with acromegaly found a lower cancer rate than in the general population (standardized incidence ratio of 0.76) but an increased colon cancer mortality rate.126,127 The enhanced mortality correlated with persistently elevated serum GH concentrations but was not observed in patients with posttreatment serum GH levels less than 2.5 ng/mL.128,129 Overall, a recent meta-analysis suggests a moderate doubling of colon cancer risk in acromegaly.210 Colonoscopy is therefore recommended at diagnosis for all patients and periodically thereafter. Uncontrolled GH levels may act permissively to enhance morbidity and mortality from colon cancer. These findings underscore the requirement for tight GH control in these patients.
Laboratory Findings
Patients with acromegaly exhibit increased serum GH and IGF-1 concentrations and may have hyperglycemia, with frank diabetes occurring in 25% of patients. Some patients have hypertriglyceridemia. Hypercalciuria and hyperphosphatemia (not >5.5 mg/dL) occur in approximately 70% of patients as a result of direct stimulation of renal tubular phosphate reabsorption by IGF-1.102
Hyperprolactinemia occurs in about 30% of patients and is due to cosecretion of PRL and GH by the tumor or to stalk interference with hypothalamic-pituitary portal delivery of dopamine. Secretion of other pituitary hormones, especially gonadotropins, also may be decreased. Elevated plasma fibrinogen concentrations revert to normal with therapy, which suggests that effective treatment of acromegaly may prevent cardiovascular morbidity.130
Mortality
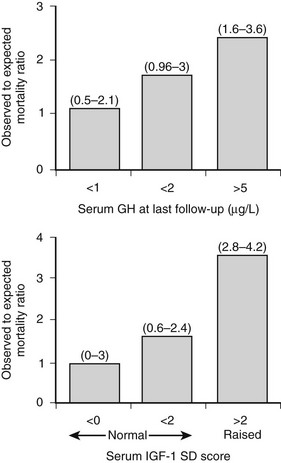
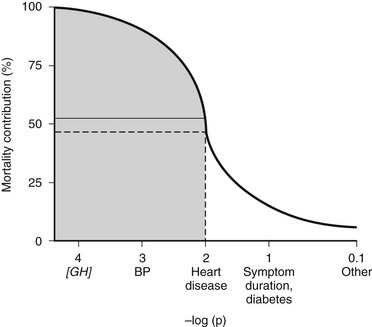
The overall mortality rate in acromegaly is about two to four times that of the general population.126,129,131,132 Up to 50% of patients die before age 50 years, and up to 89% die before age 60 years.2,133 In a series of 151 patients, survival was reduced an average of 10 years in comparison to age-matched controls.83 Analyzing 18 studies of mortality outcomes in acromegaly,126,131 there appears to be a 1.9-fold increase over expected mortality rates (range 1.16 to 3.3) for patients having undergone heterogenous treatments. Although standardized mortality outcomes have not been reported for untreated acromegaly, several mortality determinants are evident. These include the last measured GH or IGF-1 level, the prior use of radiotherapy, duration of the disease, and the presence of hypertension. Most significant predictors of survival include a postglucose GH level of <1 µg/L, a random serum GH of <2 to 2.5 µg/L, or a normal serum IGF-1 level (Fig. 7-4). Clearly attaining tight biochemical control should therefore be a goal of therapy. Aggressive management of comorbidities including hypertension, heart failure, sleep apnea, and diabetes likely also contribute to improved mortality rates83,132,197 (Table 7-6). Several retrospective studies now indicate that survival in acromegaly may be normalized to a control age-matched rate by controlling GH levels128,129; in particular, life-table analysis showed that GH levels less than 2.5 ng/mL were associated with survival rates equal to those of the general population.126,132,134 A recent postoperative follow-up of 53 patients for a mean of 12.7 years indicated that a normal IGF-1 level and GH nadir cutoff of less than 0.25 µg/L are associated with improved blood pressure control and glucose tolerance (Fig. 7-5).84 Thus, tight control of GH through aggressive multimodal therapy appears to reduce mortality risk to that expected for nonacromegalic subjects.84,128,129 In particular, the role of radiotherapy in adversely skewing mortality outcomes will have to be excluded from these evaluations.196
Table 7-6
Outcome Determinants of Acromegaly
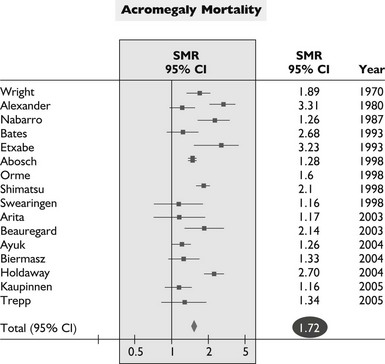
Data from Dekkers OM, Biermasz NR, Pereira AM, et al: Mortality in acromegaly: a metaanalysis, J Clin Endo Metab 93(1):61–67, 2008.
Management
Treatment goals for acromegaly embody principles that apply to treating hormonal hypersecretory tumors (Table 7-7). Treatment should be both safe and efficacious. GH and IGF-1 levels should be normalized, especially because elevated GH levels have been associated with mortality in these patients. Thus, tight GH control is an important therapeutic end point.84 Tumor mass effects, especially central compression of visual tracts, should be alleviated. Importantly, the integrity of pituitary function should be preserved, and if hypopituitarism develops, patients require lifelong pituitary replacement. Clinical features of the disease that lead to the characteristic morbidity and ultimately to mortality should be ameliorated. Several treatment options are available for acromegaly. Transsphenoidal surgical resection of the adenoma; pharmacologic therapy with somatostatin analogs, dopamine agonists, and a GH-receptor antagonist; and various modes of radiation therapy are used to treat GH-secreting adenomas. The challenge of tight GH control in acromegaly can now be met with greater stringency by using single or multimodal forms of therapy. Effective disease control should thus include sustained hormone suppression, a contained adenoma mass, improved systemic morbidity, and ultimately, normalized mortality (see Table 7-7).
Table 7-7
Treatment Goals for Acromegaly
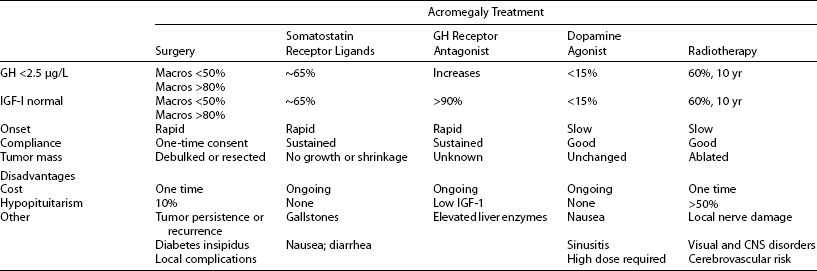
Data from Ben-Shlomo A, Melmed S: Acromegaly, Endocrinol Metab Clin N Am 37(1):102–122, 2008.
Surgery
Surgery for the treatment of GH-secreting pituitary tumors was pioneered in the early part of the century by Dr. Harvey Cushing, who demonstrated successful resection of such tumors by a transsphenoidal approach. This technique is standard, and only very rarely do large masses that extend far beyond the sella turcica require a transfrontal approach. The most important determinant of a successful surgical outcome is the experience of the surgeon. Recently, endoscopic approaches to pituitary adenoma resection have proved to be efficacious.135,136 Surgery rapidly alleviates acromegaly symptoms, removes the tumor mass, relieves optic-tract pressure effects, and relieves headache. Tumor debulking, even if partial, also may be helpful in enhancing the effectiveness of subsequent therapy. Surgical control also is inversely correlated with initial tumor size and GH levels.137,138 Not all patients are appropriate surgical candidates, usually because of coexisting cardiovascular and pulmonary disease, which may be a contraindication to anesthesia. If the tumor is sufficiently large or invasive and portends intraoperative damage to vital structures, the benefits of the procedure should be weighed against surgical risks.
Results of surgical resection of GH-secreting adenomas have only recently included more stringent biochemical criteria. Short-term remission (GH 5 µg/L) was achieved in 76% of 254 patients, with most of 129 patients remaining in long-term remission.129 Although the biochemical parameter of “basal” GH less than 5 µg/L used in this study does not reflect normalization of GH hypersecretion, even with this less stringent criterion, the postsurgical mortality outcome was equivalent to that of age- and sex-matched controls, in contrast to the 2.4- to 4.8-fold enhanced mortality observed in patients with persistent disease (GH > 5 µg/L). Using more stringent criteria (normalized IGF-1 levels or GH suppression to ≥2 µg/L after glucose loading), more than 90% of patients with microadenomas successfully achieved control.128,139,140 Unfortunately, most tumors encountered at diagnosis are large macroadenomas, and fewer than 50% of patients with macroadenomas are biochemically controlled.128,138,139 In these invasive tumors, surgical resection is invariably followed by persistent GH and or IGF-1 hypersecretion. Visible residual tumor mass is often contiguous with or involves the cavernous sinus, internal carotid arteries, or suprasellar regions. Mortality risk in patients cured at surgery does not differ from that of controls, whereas in patients with persistent disease, even after adjuvant irradiation or medical therapy, mortality remains significantly increased (almost twofold). Thus, the level of GH attained postsurgically is the most important determinant of mortality outcome.83 However, regardless of the treatment mode, normalization of GH restores mortality risk to that of age-matched population controls, and postoperative disease persistence is associated with a 3.5-fold relative mortality risk.126,128,132 Whether or not surgical debulking improves subsequent disease control by SRIF analogs has recently been studied.181–183 Surgical resection of at least 75% of the tumor mass enhanced the responsiveness of residual adenoma tissue to postoperative SRIF analog treatment.
Transnasal endoscopy135,136 appears to offer a more facile tumor access and lower complication rates. Long-term outcomes are awaited to assess the efficacy of this approach.
Side Effects
The most important adverse surgical event is failure to resect invasive tumor totally and, consequently, persistent hormonal hypersecretion. Postoperative complications occur in approximately 10% of patients, and their incidence is largely dependent on the experience of the operating surgeon.138,141 Complications include permanent diabetes insipidus, cerebrospinal fluid leaks requiring repair, meningitis, severe sinusitis, and hypopituitarism.82,133,139–141 Perioperative morbidity and residual pituitary failure remain of concern in patients with invasive tumors, especially when operated on by less-experienced surgeons.
In summary, surgical success is based largely on skill and experience and on tumor size or invasiveness. Surgery is useful for prompt reduction of GH levels, and tumor debulking may enhance the effectiveness of medical therapy. After apparent successful resection, however, up to 8% of tumors recur within 10 years. GH levels should be measured in the immediate postoperative period; evidence of GH hypersecretion at this time portends either disease persistence or long-term recurrence. Overall, by immunoradiometric GH assay, postglucose GH values less than 1.0 µg/L are found in 50% of patients after surgery, and in 39% of patients with normalized IGF-1 levels, GH levels still fail to be suppressed.142
Pituitary Irradiation
Techniques for pituitary radiotherapy include external radiation with either a cyclotron or a cobalt-60 source, and the radiotherapy is administered as a total dose of 4500 to 5000 rad. Higher doses are associated with a high incidence of side effects, whereas lower doses, although safer, appear to be less clinically effective. The total dose is given as 25 daily 180- to 200-rad fractions administered over a 6-week period.143 Maximal tumor irradiation with minimal damage to nontumorous surrounding tissue has been achieved by advances in stereotactic MRI-directed tumor localization, focused-beam direction, field-size simulation, head immobilization, and isocentral rotational techniques.144
Proton-beam therapy also decreases GH secretion but is not widely available. Stereotactic ablation of GH-secreting adenomas by gamma knife radiosurgery is a promising new technique for which long-term results are not yet available. In 16 postsurgical patients monitored for up to 2.6 years, GH levels of less than 5 ng/mL and normalized IGF-1 concentrations were observed within 16 months of stereotactic radiosurgery.145 However, the short follow-up precludes an assessment of complication outcome.
Tumor growth is invariably arrested after fractionated radiotherapy, but GH decline is slow, decreasing by approximately 20% per year. Within 18 years, 90% of patients have random serum GH concentrations lower than 5 ng/mL.143 The degree and rapidity of GH attenuation are highly dependent on pretreatment GH levels.143 However, few patients achieve the currently accepted rigorous goal of therapy, that is, a glucose-suppressed serum GH concentration less than 1 ng/mL. In one series, only 5 of 30 patients monitored for 10 or more years achieved this goal. After radiotherapy in 38 patients, 20 of whom had preradiotherapy IGF-1 data available, GH levels decreased by about 60% 3.5 years after irradiation and by about 80% 7 years after radiotherapy. However, plasma levels of IGF-1 remained almost unchanged and did not decrease to less than 80% of the initial value, even 7 years after radiotherapy. Only two patients ultimately exhibited normalized IGF levels.146 The failure of irradiation to normalize IGF-1 levels effectively in the long term implies persistent albeit low levels of GH hypersecretion in these patients. Subsequent studies have demonstrated improved efficacy, and longer-term outcomes are awaited.146
Side Effects
Pituitary failure develops in 50% of patients undergoing deep x-ray therapy by 10 years and require thyroid, gonadal, or adrenal steroid replacement or a combination of these.1,29 Rarely, optic-tract damage results in visual deficits. Ten years after radiotherapy, patients have a small but significant risk of a secondary brain malignancy, including glioma, in up to 1.7% of patients (relative risk of 16 versus expected).147–149 Radiation also may rarely induce brain parenchymal changes149–151 and brain dysfunction manifested as depression, decreased memory, decreased general quality of life, loss of vision, and cranial-nerve palsies.141,152 Short-term controlled results and side-effect profiles for gamma knife radiosurgery indicate fewer local side effects, with no visual deficits reported in 30 patients for up to 4 years.145 After maximal surgical debulking, a retrospective analysis of 83 patients who underwent single-session stereotactic radiotherapy (gamma knife), delivering high-dose radiation to the targeted mass184 showed that 50% of patients were biochemically controlled at 5 years. The 5-year cumulative risk of hypopituitarism in this selected group was less than 5%, suggesting a lower risk of pituitary damage than that observed for fractionated radiotherapy.185 In fact, no serious side effects were observed after 507 patient years of follow-up. In contrast, visual deficits were observed in 5.5% of patients treated for pituitary tumors with gamma knife radiation, likely reflecting high single retinal spot exposure.186
Thus, radiotherapy is effective in acromegaly, although its benefits are dose and time dependent, and GH reduction is delayed by 10 to 15 years. Even with the most accurate techniques, GH levels less than 2.0 ng/mL and normalization of IGF-1 levels are infrequently achieved.146,153 Therefore, radiation therapy may be useful for patients with growing pituitary tumors whose condition is not controlled by surgery or who are resistant to medical therapy.
Pharmacologic Management
Octreotide is a synthetic octapeptide analog of native, naturally occurring somatostatin. This 8-amino-acid analog (molecular weight of 1019) binds selectively to the SSTR2 somatostatin-receptor subtype.154 After subcutaneous injection, octreotide is rapidly absorbed, and peak drug concentrations (5.5 ng/mL) are achieved within 24 minutes of a 100-µg injection. The plasma distribution is about 12 minutes, and the elimination half-life is 1.5 hours, as compared with 2 minutes for natural SRIF.154,155 The drug inhibits pituitary GH secretion, also directly suppresses hepatic IGF-1 production,156 controls tumor growth, and relieves soft-tissue symptoms.
Octreotide inhibits GH, glucagon, and insulin release, but the analog exhibits greater selectivity in suppressing GH and glucagon than does somatostatin.154 In normal subjects, octreotide attenuates GH stimulation evoked by arginine,156 exercise, and insulin-induced hypoglycemia.157,158 The drug also may abrogate postprandial release of gastrointestinal and pancreatic peptides.158 Because native somatostatin suppresses thyroid-stimulating hormone (TSH) secretion, it is not surprising that octreotide also blocks the TRH-induced release of TSH.156,158,159 In acromegaly, octreotide reduces GH levels (by >50%) in more than 95% of all patients.
In the long term, about 70% of patients will have integrated GH levels suppressed to less than 5 ng/mL, and about 55% of patients have GH levels suppressed to less than 2 ng/mL. Seventy percent or more of patients will have their IGF-1 levels normalized after long-term treatment with octreotide (Fig. 7-6). Hypopituitarism does not develop during somatostatin receptor–ligand (SRL) treatment because SRIF analogs bind selectively to the somatostatin-receptor subtype that regulates GH secretion.160 In addition to suppressing GH and IGF-1 levels, headache, fatigue, perspiration, joint pains, carpal tunnel syndrome, and paresthesias improve in most patients treated over the long term.
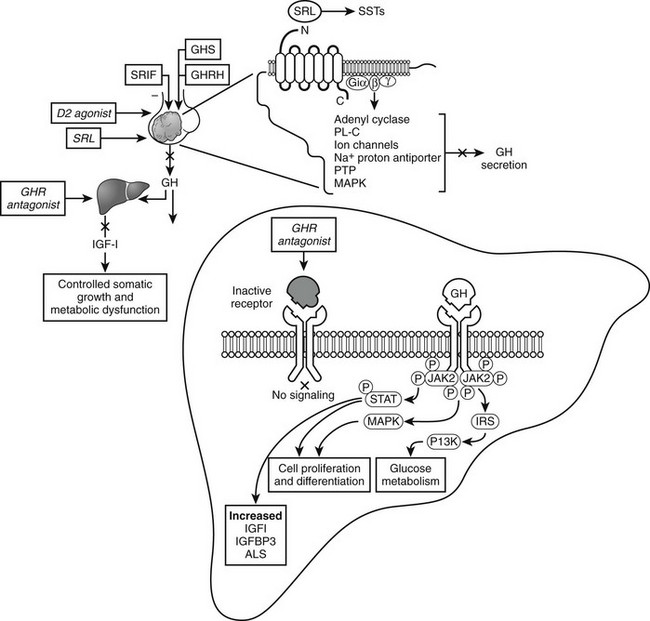
FIGURE 7-6 Acromegaly pharmacotherapy. Somatostatin-receptor ligands (SRLs) signal via G-protein coupled receptors (SST1-SST5) to suppress growth hormone (GH) secretion and action and control tumor growth. Growth hormone–receptor (GHR) antagonist blocks peripheral GH signaling to suppress insulin-like growth factor 1 (IGF-1). (Modified from Melmed S: Acromegaly, New Engl J Med 355:2558–2573, 2006.)
The starting dose of subcutaneous octreotide is 50 µg given subcutaneously in 8-hourly doses, and after 2 weeks, the dose can be increased to 100 µg 3 times daily. Thereafter, dose titrations to a maximum of 1500 µg/day may be made, depending on the nadir 2-hour postinjection GH level. The efficacy of octreotide also can be improved by increasing the dose frequency, although not necessarily increasing the total daily drug dose, or by administering the drug in a continuous-infusion minipump. Interestingly, long-term (>3 years) use of the drug is associated with enhanced sensitivity and improved biochemical control. Tachyphylaxis does not occur, and down-regulation of receptor responses does not appear to be manifested clinically.159 About half of all patients will exhibit tumor shrinkage (30% average tumor volume change).
Responses to Long-Term Depot SRIF Analog Preparations: Long-term depot somatostatin preparations include octreotide LAR (long-acting release), and lanreotide Autogel.155,159,161,162 Octreotide LAR incorporates octreotide into microspheres of a biodegradable poly-d,l-lactide-co-glycolide glucose polymer, whereas lanreotide Autogel is a water-soluble preparation allowing deep subcutaneous slow release. After 3-monthly injections of depot preparations, sustained concentrations are maintained.
Fig. 7-7 depicts the pharmacokinetic response to octreotide LAR in acromegaly. After a single injection of octreotide LAR, drug levels peak at about 28 days after injection and decrease slowly thereafter. GH levels decline after the injection and by day 14 are suppressed to less than 2 µg/L. GH suppression (as determined by measurement of integrated secretion over a 4-hour period) is sustained through day 49 and starts increasing thereafter. From the pharmacokinetic curve, it is apparent that a single injection administered every 30 days will allow GH levels to be persistently suppressed throughout the month. Fig. 7-8 depicts the effects of a single, monthly injection of octreotide LAR in a group of patients with acromegaly whose average GH levels were suppressed for the duration of the study (≤54 months). GH suppression appears to be sustained as long as patients receive monthly injections of octreotide LAR. About 80% of a total of 110 patients had IGF-1 levels normalized and GH suppressed to less than 2.5 ng/mL within 36 months.155 Long-term studies indicate that >70% of patients receiving SRL therapy achieve GH levels of <2.5 µg/L and normal IGF-1 levels.187 Several published studies include subjects preselected for prior GH responsivity to SRLs, and a more rigorous assessment of the literature suggests an overall response rate of 40% to 50% in unselected patients.188 SRL therapy controls tumor growth in the majority of treated patients, and continued tumor expansion while receiving these drugs has rarely been reported. A critical analysis of 15 eligible studies showed that about one third of patients receiving primary SRL therapy experience a significant reduction in pituitary tumor mass.193,194 For those patients responding with tumor shrinkage, the dimensions of the mass decrease is ~50%. Nevertheless, several unresolved issues remain to be determined, including the effectiveness of SRL therapy in patients exhibiting optic chiasm compression, effects of SRL withdrawal on subsequent tumor mass reexpansion, and the effect of medically induced tumor shrinkage on surgical outcomes.
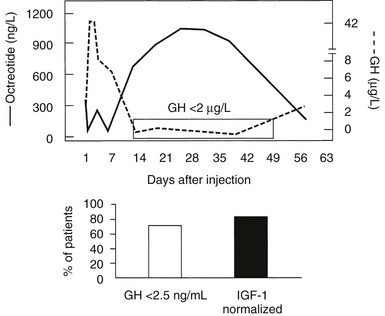
FIGURE 7-7 Pharmacokinetics of Octreotide LAR. GH, growth hormone; IGF-1, insulin-like growth factor 1; LAR, long-acting release.
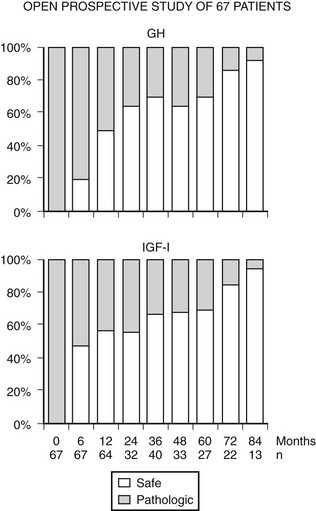
FIGURE 7-8 Nine years follow-up of selected patients treated with Octreotide LAR (long-acting release) shows that ~80% achieve GH and IGF-1 control. (Data from Cozzi R, Montini M, Attanasio R et al: Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage, J Clin Endocrinol Metab 91:1397–1403, 2006.)
Side Effects: Although somatostatin-receptor ligands are relatively safe in the long term, several important adverse events are reported. Asymptomatic echogenic gallbladder lesions develop in about 25% of patients.29 These lesions include both sludge and gallstones, which are usually diagnosed within the first 2 years of treatment, with few if any new echogenic events encountered thereafter. The prevalence of gallstones during octreotide therapy appears to vary geographically. In China, gallstones ultimately develop in most patients taking octreotide163; conversely, patients in southern Europe exhibit a far lower incidence. Clearly, dietary and/or other environmental factors play a role in their pathogenesis. Transient gastrointestinal symptoms, including anorexia, nausea, vomiting, flatulence, and loose stools, may occur, especially during the first 2 weeks of therapy; these symptoms may be ameliorated by injecting the medication between meals or at night. Rarely, fat malabsorption and bradycardia have been reported.
In summary, long-acting somatostatin analogs are effective and safe in managing GH hypersecretion, especially in patients in whom surgical resection has failed to achieve a stringent biochemical remission. Indications for SRL therapy of acromegaly include: postoperative persistence of GH secretion from residual tumor tissue; during the latency period of GH nonresponsiveness after radiation; primary therapy of patients who decline surgery or are medically unfit for surgery or who exhibit a low probability of surgical cure. Somatostatin analogs also may be offered as primary therapy for patients who have undergone irradiation, in whom GH levels may remain unacceptably elevated.29,164 Most patients with macroadenomas have persistent postsurgical GH hypersecretion, and the use of SRIF analogs should be weighed against radiotherapy for these patients. Long-acting injectable depot analogs administered once every 14 to 30 days provide enhanced patient convenience and compliance while retaining drug sensitivity.84,165,166 Prior surgery appears not to alter the long-term efficacy of somatostatin analogs in attaining biochemical control. However, drug cost and patient compliance must be factored in when deciding on therapeutic options.
Dopamine Agonists
High doses of dopamine agonists have been used in the management of these patients, and bromocriptine is associated with GH normalization in fewer than 15% of patients.167 A large meta-analysis revealed that 20% of patients will achieve GH levels less than 5 ng/mL, which is not a maximal criterion for control. Only 10% or fewer of all patients will actually have IGF-1 levels normalized. However, bromocriptine does not carry with it a risk for hypopituitarism, and because it is an orally available medication, it is extremely convenient and cost effective for the patient.168 Cabergoline, a long-acting dopamine agonist, has been used in acromegaly, but the long-term results are not compelling.168–170
Adverse Events: Because high doses (>20 mg/day) are required to achieve even moderate efficacy, the incidence of adverse events is far higher than usually seen when treating patients with prolactinomas. Patients receiving high doses of dopamine agonists complain of gastrointestinal symptoms, including nausea, vomiting, and abdominal cramps. Rarely, arrhythmias have been reported. Nasal stuffiness and sleep disturbances are common complaints.167,171,172
Growth Hormone–Receptor Antagonist
Pegvisomant, a growth hormone–receptor antagonist (GHRA), directly inhibits GH action in the periphery.173 Unlike somatostatin and dopamine agonists that act centrally to inhibit GH secretion through somatotroph-cell somatostatin and dopamine receptors, pegvisomant interferes with the functional association of GH receptor subunits, suppressing peripheral IGF-1 generation in almost all patients with GH-secreting pituitary tumors treated for up to 36 months. Pegvisomant binds one GHR unit on site 1 but cannot bind the mutated site 2.173 The site 2 mutation in pegvisomant involves replacement of glycine by lysine at position 120 (G120), preventing functional GHR dimerization, blocking initiation of subsequent GH signal transduction.
Daily doses of pegvisomant (10, 15, or 20 mg), given for 12 weeks, normalized IGF-1 levels in 38%, 75%, and 82% of patients with acromegaly, respectively.174,175 A concomitant dose-dependent reduction in serum IGF-1 levels is accompanied by a dose-dependent regression of soft-tissue swelling, excessive perspiration, and fatigue, with no significant improvement in arthralgia or headache. Pegvisomant also improves insulin sensitivity and glucose tolerance in patients with acromegaly, reducing fasting serum insulin levels and fasting serum glucose levels, without observed decreased glycated hemoglobin.
Short-term (≤3 months of treatment) side effects encountered in pegvisomant-treated patients (versus placebo) included reversible injection-site reactions (11%), diarrhea, and nausea (14% versus 3%) in an 18-month study.174 GH levels are reversibly increased approximately twofold, mirroring the IGF-1 decrease. Despite detection of anti-GH antibodies in 17% of patients, no evidence of tachyphylaxis was found. Serum cholesterol elevation also was reported.
The availability of pegvisomant allows a new approach for monitoring biochemical control of acromegaly. Pegvisomant therapy effectively normalizes IGF-1 levels in >90% of patients and also enhances insulin sensitivity in patients exhibiting glucose intolerance (Fig. 7-9). Persistent pituitary tumor growth is observed infrequently (<2% of patients), and transiently elevated (3× upper limit of normal) liver transaminase levels are observed in up to 5% of patients, with rarely reported drug-induced hepatitis.189 Other side effects include lipohypertrophy190 and features of hypopituitarism if IGF-1 levels are attenuated to below normal ranges.
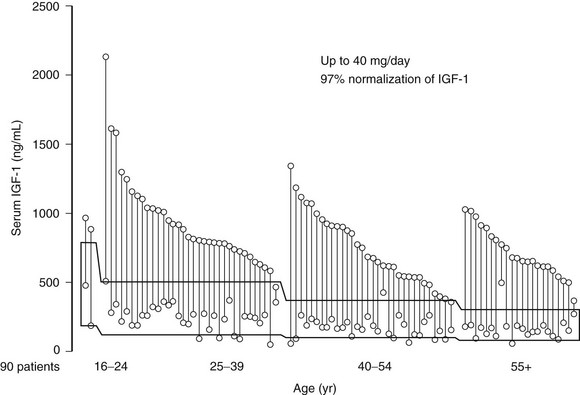
FIGURE 7-9 Insulin-like growth factor 1 levels at baseline and after 12 months of pegvisomant therapy. (Data from van der Lely AJ, Hutson RK, Trainer PJ et al: Long-term treatment of acromegaly with pegvisomant, a growth hormone–receptor antagonist, Lancet 358:1754–1759, 2001.)
GH levels are elevated in patients receiving pegvisomant therapy, so IGF-1 levels must be used to monitor efficacy, emphasizing the need to standardize commercial IGF-1 assays and develop age- and gender-specific reference ranges. Possible overtreatment with pegvisomant may create clinical features of GH deficiency, as pegvisomant may suppress IGF-1 levels below the lower limit of normal. It seems reasonable to titrate IGF-1 levels to midnormal ranges in these patients. Pegvisomant normalizes IGF-1 levels in somatostatin-resistant patients,176 and promising results have been reported for combined weekly or twice-weekly pegvisomant injections and somatostatin analog cotreatment.191,192 Pegvisomant is approved for patients who are intolerant, only partially responsive, or unresponsive to conventional treatment and effectively normalizes IGF-1 levels in patients (Fig. 7-10).
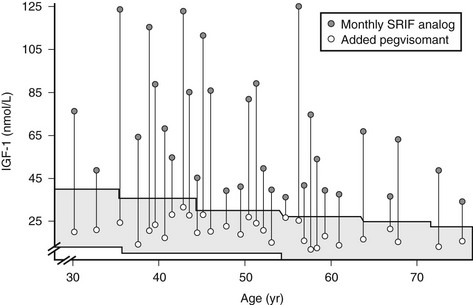
FIGURE 7-10 Combined somatostatin release–inhibiting factor (SRIF) analog and pegvisomant treatment controls insulin-like growth factor 1 (IGF-1) levels in patients resistant to SRIF analog monotherapy. (Data from Neggers SJ, van Aken MO, Janssen JA, et al: Long-term efficacy and safety of combined treatment of somatostatin analogs and pegvisomant in acromegaly, J Clin Endocrinol Metab 92[12]:4598–4601, 2007.)
Integrated Treatment Approach to the Management of Acromegaly
Patients With Likelihood of Good Surgical Outcome
Once acromegaly is diagnosed, the likelihood of surgical cure is assessed. For small, well-circumscribed tumors, surgical excision by an experienced pituitary surgeon is the treatment of choice. Surgical cure rates are maximal for noninvasive, well-encapsulated smaller tumors. If a good surgical outcome is predicted, that is, a 60% chance or better that the disease will be controlled by tumor excision, surgery is indicated. After surgery, patients are monitored to ensure that GH responses to a glucose load are less than 1 ng/mL and that IGF-1 levels are normalized. If, however, after surgery, hormone levels are not controlled, indicative of disease persistence or recurrence, either short- or long-acting somatostatin analogs are indicated.29 Pegvisomant is indicated after failure of other therapy. Careful assessment of pituitary tumor size by using MRI is recommended every 6 to 12 months, depending on baseline tumor size and location. Patients should be followed up by measuring age- and gender-matched IGF-1 levels, aiming for the midnormal range levels. Pegvisomant treatment should be avoided in patients who have LFT abnormalities until further data are available, and LFTs should be evaluated monthly during the first 6 months of therapy. If the patient is still not biochemically controlled, a dopamine agonist is added, or reoperation or irradiation is considered.
Patients With A Poor Likelihood of Successful Surgical Outcome
Patients with large adenomas are likely to have a poor surgical outcome, and fewer than half of them will be biochemically controlled. Most patients in whom acromegaly is newly diagnosed have large macroadenomas, which portend a poor surgical outcome. These patients can be offered primary treatment with somatostatin analogs, as would patients in whom surgery is contraindicated or who decline surgery.176 In 67 consecutive patients treated prospectively with octreotide LAR and followed for up to 9 years,199 70% exhibited GH levels <2.5 µg/L and/or normal age-matched IGF-1 levels. Interestingly, in this study over 80% of patients experienced tumor-mass shrinkage, and in 8 patients, the tumor disappeared or was reduced to an empty sella by MRI visualization. Testosterone levels and eugonadism were also restored in 64% of hypogonadal patients. Similar results were observed in an 18-year follow-up of 36 patients.187 Use of preoperative octreotide to enhance subsequent surgical outcomes has been advocated. In a 3-month postoperative follow-up of 62 patients randomized to receive preoperative octreotide for 6 months, the drug appeared to offer improved postsurgical biochemical results for those patients harboring macroadenomas.183 Because the drug reduces tumor bulk by approximately 50%,161,176 subsequent surgery may therefore be facilitated. If biochemical control is not achieved, drug efficacy may be improved by dose increases and the addition of a dopamine agonist, and these patients should be offered a GHRA.29 If patients are resistant to medical treatment, second surgery or irradiation is indicated, depending on the size and location of the tumor remnant and the skill of the neurosurgeon.
General
Patients with acromegaly require management of multiple associated medical disorders. Colonoscopy should be performed at the time of diagnosis. The presence of more than three skin tags, a family history, age older than 50 years, or the presence of previous polyps requires more aggressive colonoscopic monitoring. Because cardiovascular morbidity is so high in patients with acromegaly, aggressive management of hypertension, left ventricular hypertrophy, cardiac failure, and arrhythmias should be pursued. Pulmonary function and sleep evaluation should be undertaken in all patients early in the course of the disorder, and debilitating arthritis requires aggressive rheumatologic management. Screening and therapy for insulin resistance and diabetes are important, and somatostatin analogs usually improve diabetes control dramatically. Insulin requirements may immediately decrease to 90% of pretreatment needs as GH is effectively suppressed. Headache is an extremely common symptom and usually improves with somatostatin analogs; if not, potent analgesics may be indicated. Maxillofacial disorders may require dental, maxillary, and facial cosmetic surgery. Fertility is commonly of concern to patients, and several recent reports of successful pregnancies in women treated with octreotide provide optimistic guidelines for pregnancy management.111,177 Nevertheless, octreotide is not approved by the Food and Drug Administration for use during pregnancy. Patients with acromegaly may be depressed and have low self-esteem and other psychosocial sensitivity.174,178 Thus, careful individual or group counseling may be indicated to assist patients with these issues. Finally, because all the available treatment modes for acromegaly are associated with therapy-specific complications, they should be carefully watched for and, if they occur, promptly treated.
The availability of long-acting depot preparations of somatostatin analogs179 and the GHRA have changed the approach to management, inasmuch as patient compliance and medication acceptance are expected to improve markedly. Slow-release somatostatin formulations require single injections once every 2 to 4 weeks and control acromegaly in about 70% of patients; their side-effect profile appears quite similar. Availability of new formulations of SRIF receptor ligands provide promising therapeutic avenues for patients manifesting GH hypersecretory syndromes.
References
1. Melmed, S. Acromegaly. New Engl J Med. 2006;355:2558–2573.
2. Barkan, AL. Acromegaly: diagnosis and therapy. Endocrinol Metab Clin North Am. 1989;18:277–310.
3. Molitch, ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am. 1992;21:597–614.
4. Marie, P. Sur deux cas d’acromegalie: hypertrophie singuliere non congenitale des extremities superieures et cephalique. Rev Med. 1886;6:297–333.
5. Nabarro, JDN. Acromegaly. Clin Endocrinol (Oxf). 1987;26:481–512.
6. Colao, A, Ferone, D, Marzullo, P, et al. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–152.
7. Jadresic, A, Banks, LM, Child, DF, et al. The acromegaly syndrome: relation between clinical features, growth hormone values and radiological characteristics of the pituitary tumours. Q J Med. 1982;51:189–204.
8. Frohman, LA, Jansson, JO. Growth hormone-releasing hormone. Endocr Rev. 1986;7:223–253.
9. Thorner, MO, Vance, ML. Growth hormone. J Clin Invest. 1988;82:745–747.
10. Yamashita, S, Melmed, S. Insulin-like growth factor I regulation of growth hormone gene transcription in primary rat pituitary cells. J Clin Invest. 1987;79:449–452.
11. Berelowitz, M, Szabo, M, Frohman, LA, et al. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212:1279–1281.
12. Melmed, S, Yamashita, S, Yamasaki, H, et al. IGF-I receptor signaling: lessons from the somatotroph. Recent Prog Horm Res. 1996;51:189–215.
13. Veldhuis, JD, Liem, AY, South, S, et al. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1995;80:3209–3222.
14. Carter-Su, C, Schwartz, J, Smit, LS. Molecular mechanism of growth hormone action. Annu Rev Physiol. 1996;58:187–207.
15. Fan, Y, Menon, RK, Cohen, P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944.
16. D’Ercole, AJ, Stiles, AD, Underwood, LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984;81:935–939.
17. LeRoith, D, Yakar, S. Metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab. 2007;3:302–310.
18. Jones, JI, Clemmons, DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34.
19. Van Wyk, JJ. The somatomedins: biological and physiologic control mechanisms. In: Growth Factors. Orlando, FL: Academic Press; 1984:81–125.
20. Isaksson, OG, Lindahl, A, Nilsson, A, et al. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev. 1987;8:426–438.
21. Spencer, GS, Hodgkinson, SC, Bass, JJ. Passive immunization against insulin-like growth factor-I does not inhibit growth hormone-stimulated growth of dwarf rats. Endocrinology. 1991;128:2103–2109.
22. Herman, V, Fagin, J, Gonsky, R, et al. Clonal origin of pituitary adenomas. J Clin Endocrinol Metab. 1990;71:1427–1433.
23. Melmed, S, Braunstein, GD, Horvath, E, et al. Pathophysiology of acromegaly. Endocr Rev. 1983;4:271–290.
24. Drange, MR, Melmed, S. Molecular pathogenesis of acromegaly. Pituitary. 1999;2:43–50.
25. Frawley, LS, Boockfor, FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12:337–355.
26. Asa, SL, Kovacs, K, Horvath, E, et al. Human fetal adenohypophysis: Electron microscopic and ultrastructural immunocytochemical analysis. Neuroendocrinology. 1988;48:423–431.
27. Horvath, E, Kovacs, K, Singer, W, et al. Acidophil stem cell adenoma of the human pituitary: clinicopathologic analysis of 15 cases. Cancer. 1981;47:761–771.
28. Weinstein, LS, Shenker, A, Gejman, PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695.
29. Melmed, S, Colao, A, Barkan, A, et al. Guidelines for acromegaly management. J Clin Endocrinol Metab. 2004;94:1504–1517.
30. Melmed, S, Ezrin, C, Kovacs, K, et al. Acromegaly due to secretion of growth hormone by an ectopic pancreatic islet-cell tumor. N Engl J Med. 1985;312:9–17.
31. Sparagana, M, Phillips, G, Hoffman, C, et al. Ectopic growth hormone syndrome associated with lung cancer. Metabolism. 1971;20:730–736.
32. Kaganowicz, A, Farkouh, NH, Frantz, AG, et al. Ectopic human growth hormone in ovaries and breast cancer. J Clin Endocrinol Metab. 1979;48:5–8.
33. Sano, T, Asa, SL, Kovacs, K. Growth hormone-releasing hormone-producing tumors: clinical, biochemical, and morphological manifestations. Endocr Rev. 1988;9:357–373.
34. Asa, SL, Scheithauer, BW, Bilbao, JM, et al. A case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factor. J Clin Endocrinol Metab. 1984;58:796–803.
35. Oberg, K, Norheim, I, Wide, L. Serum growth hormone in patients with carcinoid tumours: basal levels and response to glucose and thyrotrophin releasing hormone. Acta Endocrinol (Copenh). 1985;109:13–18.
36. Thorner, MO, Perryman, RL, Cronin, MJ, et al. Somatotroph hyperplasia: successful treatment of acromegaly by removal of a pancreatic islet tumor secreting a growth hormone-releasing factor. J Clin Invest. 1982;70:965–977.
37. Ashcraft, MW, Hartzband, PI, Van Herle, AJ, et al. A unique growth factor in patients with acromegaloidism. J Clin Endocrinol Metab. 1983;57:272–276.
38. Levy, A, Lightman, SL. Growth hormone-releasing hormone transcripts in human pituitary adenomas. J Clin Endocrinol Metab. 1992;74:1474–1476.
39. Levy, L, Bourdais, J, Mouhieddine, B, et al. Presence and characterization of the somatostatin precursor in normal human pituitaries and in growth hormone secreting adenomas. J Clin Endocrinol Metab. 1993;76:85–90.
40. Billestrup, N, Swanson, LW, Vale, W. Growth hormone–releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A. 1986;83:6854–6857.
41. Mayo, KE, Hammer, RE, Swanson, LW, et al. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone–releasing factor gene. Mol Endocrinol. 1988;2:606–612.
42. Kovacs, M, Kineman, RD, Schally, AV, et al. Effects of antagonists of growth hormone–releasing hormone (GHRH) on GH and insulin-like growth factor I levels in transgenic mice overexpressing the human GHRH gene, an animal model of acromegaly. Endocrinology. 1997;138:4536–4542.
43. Shimon, I, Melmed, S. Genetic basis of endocrine disease: pituitary tumor pathogenesis. J Clin Endocrinol Metab. 1997;82:1675–1681.
44. Spada, A, Arosio, M, Bochicchio, D, et al. Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase. J Clin Endocrinol Metab. 1990;71:1421–1426.
45. Vallar, L, Spada, A, Giannattasio, G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987;330:566–568.
46. Bertherat, J, Chanson, P, Montminy, M. The cyclic adenosine 3′,5′-monophosphate-responsive factor CREB is constitutively activated in human somatotroph adenomas. Mol Endocrinol. 1995;9:777–783.
47. Hashimoto, K, Koga, M, Motomura, T, et al. Identification of alternatively spliced messenger ribonucleic acid encoding truncated growth hormone–releasing hormone receptor in human pituitary adenomas. J Clin Endocrinol Metab. 1995;80:2933–2939.
48. Molitch, ME. Prolactinoma. In: Melmed S, ed. The Pituitary. Cambridge: Blackwell; 1995:443–477.
49. Melmed, S, Ho, K, Klibanski, A, et al. Clinical review 75: recent advances in pathogenesis, diagnosis, and management of acromegaly. J Clin Endocrinol Metab. 1995;80:3395–3402.
50. Gadelha, MR, Prezant, TR, Une, KN, et al. Loss of heterozygosity on chromosome 11q13 in two families with acromegaly/gigantism is independent of mutations of the multiple endocrine neoplasia type I gene. J Clin Endocrinol Metab. 1999;84:249–256.
51. Bates, AS, Farrell, WE, Bicknell, EJ, et al. Allelic deletion in pituitary adenomas reflects aggressive biological activity and has potential value as a prognostic marker. J Clin Endocrinol Metab. 1997;82:818–824.
52. Fero, ML, Rivkin, M, Tasch, M, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744.
53. Jacks, T, Fazeli, A, Schmitt, EM, et al. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300.
54. Nakayama, K, Ishida, N, Shirane, M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720.
55. Herman, V, Drazin, NZ, Gonsky, R, et al. Molecular screening of pituitary adenomas for gene mutations and rearrangements. J Clin Endocrinol Metab. 1993;77:50–55.
56. Prezant, TR, Levine, J, Melmed, S. Molecular characterization of the MEN-1 tumor-suppressor gene in sporadic pituitary tumors. J Clin Endocrinol Metab. 1998;83:1388–1391.
57. Pei, L, Melmed, S, Scheithauer, B, et al. Frequent loss of heterozygosity at the retinoblastoma susceptibility gene (RB) locus in aggressive pituitary tumors: evidence for a chromosome 13 tumor-suppressor gene other than RB. Cancer Res. 1995;55:1613–1616.
58. Spada, A, Lania, A, Ballare, E. G protein abnormalities in pituitary adenomas. Mol Cell Endocrinol. 1998;142:1–14.
59. Barlier, A, Gunz, G, Zamora, AJ, et al. Prognostic and therapeutic consequences of Gs alpha mutations in somatotroph adenomas. J Clin Endocrinol Metab. 1998;83:1604–1610.
60. Landis, CA, Masters, SB, Spada, A, et al. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696.
61. Clementi, E, Malgaretti, N, Meldolesi, J, et al. A new constitutively activating mutation of the Gs protein alpha subunit-gsp oncogene is found in human pituitary tumours. Oncogene. 1990;5:1059–1061.
62. Lyons, J, Landis, CA, Harsh, G, et al. Two G-protein oncogenes in human endocrine tumors. Science. 1990;249:655–659.
63. Boggild, MD, Jenkinson, S, Pistorello, M, et al. Molecular genetic studies of sporadic pituitary tumors. J Clin Endocrinol Metab. 1994;78:387–392.
64. Hosoi, E, Yokogoshi, Y, Horie, H, et al. Analysis of the Gs alpha gene in growth hormone-secreting pituitary adenomas by the polymerase chain reaction-direct sequencing method using paraffin-embedded tissues. Acta Endocrinol (Copenh). 1993;129:301–306.
65. Adams, EF, Brockmeier, S, Friedmann, E, et al. Clinical and biochemical characteristics of acromegalic patients harboring gsp-positive and gsp-negative pituitary tumors. Neurosurgery. 1993;33:198–203.
66. Cai, WY, Alexander, JM, Hedley-Whyte, ET, et al. Ras mutations in human prolactinomas and pituitary carcinomas. J Clin Endocrinol Metab. 1994;78:89–93.
67. Pei, L, Melmed, S, Scheithauer, B, et al. H-ras mutations in human pituitary carcinoma metastases. J Clin Endocrinol Metab. 1994;78:842–846.
68. Karga, HJ, Alexander, JM, Hedley-Whyte, ET, et al. Ras mutations in human pituitary tumors. J Clin Endocrinol Metab. 1992;74:914–919.
69. Pei, L, Melmed, S. Isolation and characterization of a pituitary tumor–transforming gene (PTTG). Mol Endocrinol. 1997;11:433–441.
70. Melmed, S. Mechanisms for pituitary tumorigenesis: The plastic pituitary. J Clin Invest. 2003;112:1603–1618.
71. Zhang, X, Horwitz, GA, Heaney, AP, et al. Pituitary tumor–transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999;84:761–767.
72. Stratakis, CA, Carney, JA, Lin, JP, et al. Carney complex, a familial multiple neoplasia and lentiginosis syndrome: analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705.
73. Stratakis, CA, Jenkins, RB, Pras, E, et al. Cytogenetic and microsatellite alterations in tumors from patients with the syndrome of myxomas, spotty skin pigmentation, and endocrine overactivity (Carney complex). J Clin Endocrinol Metab. 1996;81:3607–3614.
74. Carney, JA, Hruska, LS, Beauchamp, GD, et al. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc. 1986;61:165–172.
75. Carney, JA, Gordon, H, Carpenter, PC, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985;64:270–283.
76. Teh, BT, Kytola, S, Farnebo, F, et al. Mutation analysis of the MEN1 gene in multiple endocrine neoplasia type 1, familial acromegaly and familial isolated hyperparathyroidism. J Clin Endocrinol Metab. 1998;83:2621–2626.
77. Knudson, AGJ. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823.
78. Benlian, P, Giraud, S, Lahlou, N, et al. Familial acromegaly: a specific clinical entity: further evidence from the genetic study of a three-generation family. Eur J Endocrinol. 1995;133:451–456.
79. Ackermann, F, Krohn, K, Windgassen, M, et al. Acromegaly in a family without a mutation in the menin gene. Exp Clin Endocrinol Diabetes. 1999;107:93–96.
80. Gadelha, MR, Rhode, K, Kineman, RD, et al. Author’s response: isolated familial somatotropinomas: does the disease map to 11q13 or to 2p16? J Clin Endocrinol Metab. 2000;85:4921.
81. Alexander, L, Appleton, D, Hall, R, et al. Epidemiology of acromegaly in the Newcastle region. Clin Endocrinol (Oxf). 1980;12:71–79.
82. Bengtsson, BA, Eden, S, Ernest, I, et al. Epidemiology and long-term survival in acromegaly. Acta Med Scand. 1988;223:327–335.
83. Holdaway, IM, Rojasoorya, CR, Gamble, GD, et al. Long-term treatment outcome in acromegaly. Growth Horm IGF-1 Res. 2003;13:185–192.
84. Serri, O, Beauregard, C, Hardy, J. Long-term biochemical status and disease-related morbidity in 53 postoperative patients with acromegaly. J Clin Endocrinol Metab. 2004;89:658–661.
85. Clemmons, DR, Van Wyk, JJ, Ridgway, EC, et al. Evaluation of acromegaly by radioimmunoassay of somatomedin-C. N Engl J Med. 1979;301:1138–1142.
86. Rieu, M, Kuhn, JM, Bricaire, H, et al. Evaluation of treated acromegalic patients with normal growth hormone levels during oral glucose load. Acta Endocrinol. 1984;107:1–8.
87. Lee, PD, Durham, SK, Martinez, V, et al. Kinetics of insulin-like growth factor (IGF) and IGF-binding protein responses to a single dose of growth hormone. J Clin Endocrinol Metab. 1997;82:2266–2274.
88. Melmed, S. Confusion in clinical laboratory GH and IGF-1 reports. Pituitary. 1999;2:171–172.
89. Barkan, AL, Beitins, IZ, Kelch, RP. Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab. 1988;67:69–73.
90. Grinspoon, S, Clemmons, D, Swearingen, B, et al. Serum insulin-like growth factor–binding protein-3 levels in the diagnosis of acromegaly. J Clin Endocrinol Metab. 1995;80:927–932.
91. de Herder, WW, van der Lely, AJ, Janssen, JA, et al. IGFBP-3 is a poor parameter for assessment of clinical activity in acromegaly. Clin Endocrinol (Oxf). 1995;43:501–505.
92. van der Lely, AJ, de Herder, WW, Janssen, JA, et al. Acromegaly: the significance of serum total and free IGF-I and IGF-binding protein-3 in diagnosis. J Endocrinol. 1997;155(Suppl):9–16.
93. Shibasaki, T, Kiyosawa, Y, Masuda, A, et al. Distribution of growth hormone-releasing hormone-like immunoreactivity in human tissue extracts. J Clin Endocrinol Metab. 1984;59:263–268.
94. Guillemin, R, Brazeau, P, Bohlen, P, et al. Growth hormone–releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982;218:585–587.
95. Melmed, S, Ziel, FH, Braunstein, GD, et al. Medical management of acromegaly due to ectopic production of growth hormone-releasing hormone by a carcinoid tumor. J Clin Endocrinol Metab. 1988;67:395–399.
96. Lieberman, SA, Bjorkengren, AG, Hoffman, AR. Rheumatologic and skeletal changes in acromegaly. Endocrinol Metab Clin North Am. 1992;21:615–631.
97. Scarpa, R, De Brasi, D, Pivonello, R, et al. Acromegalic axial arthropathy: a clinical case-control study. J Clin Endrocrinol Metab. 2004;89:598–603.
98. Bluestone, R, Bywaters, EG, Hartog, M, et al. Acromegalic arthropathy. Ann Rheum Dis. 1971;30:243–258.
99. Dons, RF, Rosselet, P, Pastakia, B, et al. Arthropathy in acromegalic patients before and after treatment: a long-term follow-up study. Clin Endocrinol (Oxf). 1988;28:515–524.
100. Lesse, GP, Fraser, WD, Farquharson, R, et al. Gonadal status is an important determinant of bone density in acromegaly. Clin Endocrinol (Oxf). 1998;48:59–65.
101. Melmed, S. Acromegaly. In: Melmed S, ed. The Pituitary. Cambridge, MA: Blackwell, 2002.
102. Wass, J. Acromegaly and gigantism. In: Besser GM, Thorner MO, eds. Comprehensive Clinical Endocrinology. ed 3. St. Louis: Mosby; 2002:57–71.
103. Cheung, NW, Boyages, SC. The thyroid gland in acromegaly: An ultrasonographic study. Clin Endocrinol (Oxf). 1997;46:545–549.
104. Pereira, AM, van Thiel, SW, Lindner, JR, et al. Increased prevalence of regurgitant valvular heart disease in acromegaly. J Clin Encrinol Metab. 2004;89:71–75.
105. Chanson, P, Megnien, JL, del Pino, M, et al. Decreased regional blood flow in patients with acromegaly. Clin Endocrinol (Oxf). 1998;49:725–731.
106. Lieberman, SA, Hoffman, AR. Sequelae to acromegaly: reversibility with treatment of the primary disease. Horm Metab Res. 1990;22:313–318.
107. Colao, A, Cuocolo, A, Marzullo, P, et al. Effects of 1-year treatment with octreotide on cardiac performance in patients with acromegaly. J Clin Endocrinol Metab. 1999;84:17–23.
108. Lombardi, G, Colao, A, Marzullo, P, et al. Is growth hormone bad for your heart? Cardiovascular impact of GH deficiency and of acromegaly. J Endocrinol. 1997;155(Suppl):33–39.
109. Sacca, L, Cittadini, A, Fazio, S. Growth hormone and the heart. Endocr Rev. 1994;15:555–573.
110. Colao, A, Cuocolo, A, Marzullo, P, et al. Impact of patient’s age and disease duration on cardiac performance in acromegaly: A radionuclide angiography study. J Clin Endocrinol Metab. 1999;84:1518–1523.
111. Colao, A, Merola, B, Ferone, D, et al. Acromegaly. J Clin Endocrinol Metab. 1997;82:2777–2781.
112. Grunstein, RR, Ho, KK, Sullivan, CE. Effect of octreotide, a somatostatin analog, on sleep apnea in patients with acromegaly. Ann Intern Med. 1994;121:478–483.
113. Grunstein, RR, Ho, KY, Sullivan, CE. Sleep apnea in acromegaly. Ann Intern Med. 1991;115:527–532.
114. Grunstein, RR, Ho, KY, Berthon-Jones, M, et al. Central sleep apnea is associated with increased ventilatory response to carbon dioxide and hypersecretion of growth hormone in patients with acromegaly. Am J Respir Crit Care Med. 1994;150:496–502.
115. Chanson, P, Timsit, J, Benoit, O, et al. Rapid improvement in sleep apnea of acromegaly after short-term treatment with somatostatin analogue SMS 201-995 [letter]. Lancet. 1986;1:1270–1271.
116. Melmed, S. Unwanted effects of growth hormone excess in the adult. J Pediatr Endocrinol Metab. 1996;9:369–374.
117. Duncan, E, Wass, JAH. Investigation protocol: Acromegaly and its investigation. Clin Endocrinol (Oxf). 1999;50:285–293.
118. Colao, A, Marzullo, P, Spiezia, S, et al. Effect of growth hormone (GH) and insulin-like growth factor I on prostate diseases: an ultrasonographic and endocrine study in acromegaly, GH deficiency, and healthy subjects. J Clin Endocrinol Metab. 1999;84:1986–1991.
119. Colao, A, Marzullo, P, Ferone, D, et al. Prostatic hyperplasia: an unknown feature of acromegaly. J Clin Endocrinol Metab. 1998;83:775–779.
120. Melmed, S. Acromegaly and cancer: not a problem? J Clin Endocrinol Metab. 2001;86:2929–2934.
121. Renehan, AG, Pudhupalayan, B, Painter, JE, et al. The prevalence and characteristics of colorectal neoplasia in acromegaly. J Clin Endocrinol Metab. 2000;85:3417–3424.
122. Jenkins, PJ, Besser, M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab. 2001;86:2935–2941.
123. Rokkas, T, Pistiolas, D, Sechopoulos, P, et al. Risk of colorectal neoplasm in patients with acromegaly: a meta-analysis. World J Gastroenterol. 2008;14:3484–3489.
124. Leiberman, DA, Weiss, DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560.
125. Jenkins, PJ, Fairclough, PD, Richards, T, et al. Acromegaly, colonic polyps and carcinoma. Clin Endocrinol (Oxf). 1997;47:17–22.
126. Holdaway, IM, Rajasoorja, RC, Gamble, GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–674.
127. Orme, SM, McNally, RJ, Cartwright, RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study: United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–2734.
128. Swearingen, B, Barker, FG, Katznelson, L, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–3426.
129. Abosch, A, Tyrrell, JB, Lamborn, KR, et al. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998;83:3411–3418.
130. Landin-Wilhelmsen, K, Tengborn, L, Wilhelmsen, L, et al. Elevated fibrinogen levels decrease following treatment of acromegaly. Clin Endocrinol (Oxf). 1997;46:69–74.
131. Biermasz, NR, Dekker, FW, Pereira, AM, et al. Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J Clin Endocrinol Metab. 2004;89:2789–2796.
132. Ayuk, J, Clayton, RN, Holder, G, et al. Growth hormone and pituitary radiotherapy, but not serum insulin-like growth factor-I concentrations, predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab. 2004;89:1613–1617.
133. Krieger, MD, Couldwell, WT, Weiss, MH. Assessment of long-term remission of acromegaly following surgery. J Neurosurg. 2003;98:719–724.
134. Bates, AS, Van’t Hoff, W, Jones, JM, et al. Does treatment of acromegaly affect life expectancy? Metabolism. 1995;44(Suppl 1):1–5.
135. Laws, ER. Surgery for acromegaly: evolution of the techniques and outcomes. Rev Endocr Metab Disord. 2008;9:67–70.
136. Cappabianca, P, Cavallo, LM, Colao, A, et al. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97:293–298.
137. Biermasz, NR, van Dulken, H, Roelfsema, F. Ten-year follow-up results of transsphenoidal microsurgery in acromegaly. J Clin Encrinol Metab. 2000;85:4596–4602.
138. Ahmed, S, Elsheikh, M, Stratton, IM, et al. Outcome of transsphenoidal surgery for acromegaly and its relationship to surgical experience. Clin Endocrinol (Oxf). 1999;50:561–567.
139. Kreutzer, J, Vance, ML, Lopes, MB, et al. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86:4072–4077.
140. Shimon, RL, Cohen, ZR, Ram, Z, et al. Transsphenoidal surgery for acromegaly: endocrinological follow-up of 98 patients. Neurosurgery. 2001;48:1239–1243.
141. Ciric, I, Ragin, A, Baumgartner, C, et al. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236.
142. Freda, PU, Post, KD, Powell, JS, et al. Evaluation of disease status with sensitive measures of growth hormone secretion in 60 postoperative patients with acromegaly. J Clin Endocrinol Metab. 1998;83:3808–3816.
143. Eastman, RC, Gorden, P, Glatstein, E, et al. Radiation therapy of acromegaly. Endocrinol Metab Clin North Am. 1992;21:693–712.
144. Laws, JE, Vance, ML. Radiosurgery for pituitary tumors and craniopharyngiomas. Neurosurg Clin North Am. 1999;10:327–336.
145. Attanasio, R, Epaminonda, P, Motti, E, et al. Gamma-knife radiosurgery in acromegaly: a 4-year follow-up study. J Clin Endocrinol Metab. 2003;88:3105–3112.
146. Barkan, AL, Halasz, I, Dornfeld, KJ, et al. Pituitary irradiation is ineffective in normalizing plasma insulin-like growth factor I in patients with acromegaly. J Clin Endocrinol Metab. 1997;82:3187–3191.
147. Tsang, RW, Laperriere, NJ, Simpson, WJ, et al. Glioma arising after radiation therapy for pituitary adenoma: A report of four patients and estimation of risk [published erratum appears in Cancer 73:492, 1994]. Cancer. 1993;72:2227–2233.
148. Ahmed, M, Kanaan, I, Rifai, A, et al. An unusual treatment-related complication in a patient with growth hormone–secreting pituitary tumor. J Clin Endocrinol Metab. 1997;82:2816–2820.
149. Brada, M, Ford, D, Ashley, S, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. Br Med J. 1992;304:1343–1346.
150. Al-Mefty, O, Kersh, JE, Routh, A, et al. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73:502–512.
151. Alexander, MJ, DeSalles, AA, Tomiyasu, U. Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma: Case report. J Neurosurg. 1998;88:111–115.
152. Crossen, JR, Garwood, D, Glatstein, E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642.
153. Jaffe, CA. Reevaluation of conventional pituitary irradiation in the therapy of acromegaly. Pituitary. 1999;2:55–62.
154. Lamberts, SWJ, van der Lely, AJ, de Herder, WW, et al. Octreotide. N Engl J Med. 1996;334:246–254.
155. Cozzi, R, Attanasio, R, Montini, M, et al. Four-year treatment with octreotide long-acting repeatable in 110 acromegalic patients: predictive value of short-term results? J Clin Endocrinol Metab. 2003;88:3090–3098.
156. Murray, RD, Kim, K, Ren, S-G, et al. Central and peripheral actions of somatostatin on the growth hormone-insulin-like growth factor-I axis. J Clin Invest. 2004;114:349–356.
157. Lightman, SL, Fox, P, Dunne, MJ. The effect of SMS 201-995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl. 1986;119:84–95.
158. Battershill, PE, Clissold, SP. Octreotide: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs. 1989;38:658–702.
159. Gillis, JC, Noble, S, Goa, KL. Octreotide long-acting release (LAR): a review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs. 1997;53:618–699.
160. Shimon, I, Yan, X, Taylor, JE, et al. Somatostatin receptor (SSTR) subtype-selective analogues differentially suppress in vitro growth hormone and prolactin in human pituitary adenomas: novel potential therapy for functional pituitary tumors. J Clin Invest. 1997;100:2386–2392.
161. Colao, A, Ferone, D, Marzullo, P, et al. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab. 2001;86:2779–2786.
162. Attanasio, R, Baldelli, R, Pivonello, R, et al. Lanreotide 60 mg, a new long-acting formulation: effectiveness in the chronic treatment of acromegaly. J Clin Endocrinol Metab. 2003;88:5258–5265.
163. Shi, YF, Zhu, XF, Harris, AG, et al. Prospective study of the long-term effects of somatostatin analog (octreotide) on gallbladder function and gallstone formation in Chinese acromegalic patients. J Clin Endocrinol Metab. 1993;76:32–37.
164. Ayuk, J, Stewart, SE, Stewart, PM, et al. Long-term safety and efficacy of depot long-acting somatostatin analogs for the treatment of acromegaly. J Clin Endocrinol Metab. 2002;87:4142–4146.
165. Newman, CB, Melmed, S, Snyder, PJ, et al. Safety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients: A clinical research center study. J Clin Endocrinol Metab. 1995;80:2768–2775.
166. Caron, P, Cogne, M, Gusthiot-Joudet, B, et al. Intramuscular injections of slow-release lanreotide (BIM 23014) in acromegalic patients previously treated with continuous subcutaneous infusion of octreotide (SMS 201-995). Eur J Endocrinol. 1995;132:320–325.
167. Jaffe, CA, Barkan, AL. Treatment of acromegaly with dopamine agonists. Endocrinol Metab Clin North Am. 1992;21:713–735.
168. Jackson, SN, Fowler, J, Howlett, TA. Cabergoline treatment of acromegaly: a preliminary dose finding study. Clin Endocrinol (Oxf). 1997;46:745–749.
169. Abs, R, Verhelst, J, Maiter, D, et al. Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab. 1998;83:374–378.
170. Muratori, M, Arosio, M, Gambino, G, et al. Use of cabergoline in the long-term treatment of hyperprolactinemic and acromegalic patients. J Endocrinol Invest. 1997;20:537–546.
171. Colao, A, Ferone, D, Marzullo, P, et al. Effect of different dopaminergic agents in the treatment of acromegaly. J Clin Endocrinol Metab. 1997;82:518–523.
172. Vance, ML, Evans, WS, Thorner, MO. Drugs five years later: bromocriptine. Ann Intern Med. 1984;100:78–91.
173. Kopchick, JJ, Parkinson, C, Stevens, EC, et al. Growth hormone receptor antagonists: discovery, development and use in patients with acromegaly. Endocr Rev. 2002;23:623–646.
174. van der Lely, AJ, Hutson, RK, Trainer, PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759.
175. Trainer, PJ, Drake, WM, Katznelson, L, et al. Treatment of acromegaly with the growth hormone–receptor antagonist pegvisomant. N Engl J Med. 2000;342:1171–1177.
176. Bonert, VH, Zib, K, Scarlett, JA, et al. Growth hormone receptor antagonist therapy in acromegalic patients resistant to somatostatin analogs. J Clin Endocrinol Metab. 2000;85:2958–2961.
177. Herman-Bonert, V, Seliverstov, M, Melmed, S. Pregnancy in acromegaly: successful therapeutic outcome. J Clin Endocrinol Metab. 1998;83:727–731.
178. Furman, K, Ezzat, S. Psychological features of acromegaly. Psychother Psychosom. 1998;67:147–153.
179. van der Hoek, J, de Herder, WW, Feelders, RA, et al. A single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patients. J Clin Endocrinol Metab. 2004;89:638–645.
180. Bevan, JS, Atkin, SL, Atkinson, AB, et al. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor I, and tumor size. J Clin Endocrinol Metab. 2002;87:4554–4563.
181. Colao, A, Attanasio, R, Pivonello, R, et al. Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2006;91:85–92.
182. Petrossians, P, Borges-Martins, L, Espinoza, C, et al. Gross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogs. Eur J Endocrinol. 2005;152:61–66.
183. Carlsen, SM, Lund-Johansen, M, Schreiner, T, et al. Preoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trial. J Clin Endocrinol Metab. 2008;93:2984–2990.
184. Losa, M, Gioia, L, Picozzi, P, et al. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93:2546–2552.
185. Jenkins, PJ, Bates, P, Carson, MN, et al. Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor I in patients with acromegaly. J Clin Endocrinol Metab. 2006;91:1239–1245.
186. Jagannathan, J, Sheehan, JP, Pouratian, N, et al. Gamma Knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980–987.
187. Maiza, JC, Vezzosi, D, Matta, M, et al. Long-term (up to 18 years) effects on GH/IGF-1 hypersecretion and tumour size of primary somatostatin analogue (SSTa) therapy in patients with GH-secreting pituitary adenoma responsive to SSTa. Clin Endocrinol (Oxf). 2007;67:282–289.
188. Mercado, M, Borges, F, Bouterfa, H, et al. A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol (Oxf). 2007;66:859–868.
189. Schreiber, I, Buchfelder, M, Droste, M, et al. Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol. 2007;156:75–82.
190. Bonert, VS, Kennedy, L, Petersenn, S, et al. Lipodystrophy in patients with acromegaly receiving pegvisomant. J Clin Endocrinol Metab. 2008;93:3515–3518.
191. Feenstra, J, de Herder, WW, ten Have, SM, et al. Combined therapy with somatostatin analogues and weekly pegvisomant in active acromegaly. Lancet. 2005;365:1644–1646.
192. Jorgensen, JO, Feldt-Rasmussen, U, Frystyk, J, et al. Cotreatment of acromegaly with a somatostatin analog and a growth hormone receptor antagonist. J Clin Endocrinol Metab. 2005;90:5627–5631.
193. Melmed, S, Sternberg, R, Cook, D, et al. A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:4405–4410.
194. Bevan, JS. Clinical review: the antitumoral effects of somatostatin analog therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:1856–1863.
195. Herrmann, BL, Wessendorf, TE, Ajaj, W, et al. Effects of octreotide on sleep apnoea and tongue volume (magnetic resonance imaging) in patients with acromegaly. Eur J Endocrinol. 2004;151:309–315.
196. Ayuk, J, Clayton, RN, Holder, G, et al. Growth hormone and pituitary radiotherapy, but not serum insulin-like growth factor I concentrations, predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab. 2004;89:1613–1617.
197. Kauppinen-Makelin, R, Sane, T, Reunanen, A, et al. A nationwide survey of mortality in acromegaly. J Clin Endocrinol Metab. 2005;90:4081–4086.
198. Ronchi, CL, Varca, V, Beck-Peccoz, P, et al. Comparison between six-year therapy with long-acting somatostatin analogs and successful surgery in acromegaly: effects on cardiovascular risk factors. J Clin Endocrinol Metab. 2006;91:121–128.
199. Cozzi, R, Montini, M, Attanasio, R, et al. Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab. 2006;91:1397–1403.
200. Lamberts, SW, van den Beld, AW, van der Lely, AJ. The endocrinology of aging. Science. 1997;278:419–424.
201. Colao, A, Amato, G, Pedroncelli, AM, et al. Gender- and age-related differences in the endocrine parameters of acromegaly. J Endocrinol Invest. 2002;25:532–538.
202. Giustina, A, Barkan, A, Casanueva, FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526–552.
203. Arafat, AM, Mohlig, M, Weickert, MO, et al. Growth hormone response during oral glucose tolerance test: the impact of assay method on the estimation of reference values in patients with acromegaly and in healthy controls, and the role of gender, age, and body mass index. J Clin Endocrinol Metab. 2008;93:1254–1262.
204. Colao, A, Lombardi, G. Should we still use glucose-suppressed growth hormone levels for the evaluation of acromegaly? J Clin Endocrinol Metab. 2008;93:1181–1182.
205. Vierimaa, O, Georgitsi, M, Lehtonen, R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230.
206. Daly, AF, Vanbellinghen, JF, Khoo, SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab. 2007;92:1891–1896.
207. Georgitsi, M, Raitila, A, Karhu, A, et al. Molecular diagnosis of pituitary adenoma predisposition caused by aryl hydrocarbon receptor-interacting protein gene mutations. Proc Natl Acad Sci U S A. 2007;104:4101–4105.
208. Melmed, S. Update in pituitary disease. J Clin Endocrinol Metab. 2008;93:331–338.
209. Bogazzi, F, Russo, D, Locci, MT, et al. Apoptosis is reduced in the colonic mucosa of patients with acromegaly. Clin Endocrinol (Oxf). 2005;63:683–688.
210. Renehan, AG, O’Connell, J, O’Halloran, D, et al. Acromegaly and colorectal cancer: a comprehensive review of epidemiology, biological mechanisms, and clinical implications. Horm Metab Res. 2003;35:712–725.
211. Beauregard, C, Utz, AL, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93(6):2063–2071.