CHAPTER 133 Acoustic Neuroma
History
In 1777, the first reported pathologic case of an acoustic neuroma (vestibular schwannoma) was made in an autopsy report by Eduard Sandidort.1,2 There are several reports from the early 19th century of patients with signs and symptoms consistent with an acoustic neuroma that were validated on postmortem studies. Lasource in 1810 and Bell in 1830 described the progression of symptoms along with postmortem descriptions of what were probably acoustic neuromas.3–5 Throughout most of the 18th century, attempts at resection of acoustic neuromas were associated with significantly high mortality. The high mortality and high morbidity were certainly associated with technique. Postoperative infections, as with other surgical procedures of the time, were common. Advancements in the understanding of aseptic technique and improved anesthetic skill enhanced patient tolerance of surgery and significantly decreased postoperative morbidity.
Some believe that the first successful removal of an acoustic neuroma was performed in 1894 by Sir Charles Balance.6 However, the case report described a tumor with a broad attachment to the dura on the posterior surface of the petrous portion of the temporal bone. In addition, there was no mention of diminished hearing. The absence of decreased hearing would be uncommon in the case of an acoustic neuroma. Cushing believed that these findings were more consistent with a meningioma.4,5 Others, including Cushing, have attributed the first successful removal of an acoustic neuroma to Thomas Annandale. The patient was a young pregnant woman with right-sided hearing loss who survived surgery and subsequently gave birth.
Survival in early attempts to remove acoustic neuromas, as mentioned, was the exception rather than the rule. Results from early series demonstrated a remarkably high mortality rate, with Borchardt reporting an operative mortality of 72%; Eiselberg, 74%; and Krause, 84%.5,7 These disappointing results were at least in part due to technique. Finger enucleation of the tumor would often avulse the anterior inferior cerebellar artery (AICA) and lead to significant bleeding and brainstem infarction.
Cushing was the most influential in ushering in a new era of acoustic surgery with significantly improved survival. In the early 20th century, Cushing refined surgical technique to reduce mortality significantly from higher than 50% to approximately 11%.4,6 Dandy built on Cushing’s work to further lower operative mortality and focused on gross total resection to limit recurrence.8 The disappointing results of early attempts at tumor resection lead Panse to suggest a translabyrinthine approach to avoid the severe complications seen with early retrosigmoid attempts.5 The translabyrinthine approach would be reintroduced by House in the 1960s as an alternative to the retrosigmoid approach.9
The importance of operative experience was evident in the 1960s. Compilations of surgeons infrequently performing the surgery demonstrated a 31% mortality rate.10 However, even with experienced surgeons such as Olivecrona, who operated on 415 acoustic neuromas between 1931 and 1960, there was a reported overall mortality of 19.7%.11 The mortality rate for large tumors in Olivecrona’s series was approximately 5 times what it was for tumors that were “hazelnut size,” thus establishing a differential between tumor size and outcome that persists today.12 In 1961, House would propose the middle fossa approach for acoustic neuroma surgery as part of his Triological thesis.13 The approach had been made popular for treating vestibular neuronitis and other temporal fossa pathology.
Natural History
Schuknecht found an incidence of occult acoustic neuromas of 0.57%, or 570 per 100,000 temporal bones.6,14 The National Institutes of Health Consensus Statement estimated an incidence of approximately 1 in 100,000 in 1991. However, advances in imaging, including routine use of gadolinium enhancement with magnetic resonance imaging (MRI), would probably place the incidence somewhere between the two. The increased sensitivity of current imaging modalities allows physicians to detect tumors at smaller sizes and also brings up the dilemma of what to do with small tumors in asymptomatic or mildly symptomatic patients.
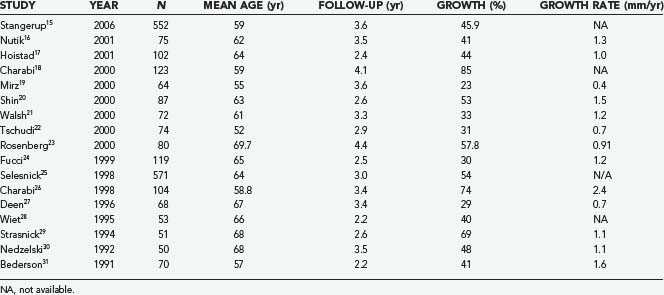
The expected rate of growth plays an important role in determining whether observation will suffice or a surgical intervention is indicated. Including only published series with at least 50 patients, the proportion of tumors that demonstrate growth ranges from 30% to 85% (Table 133-1).15–31 The growth rate also varies from 0.4 to 2.4 mm/yr. Stangerup and colleagues, in a large series of 522 patients with a mean observation time of 3.6 years, found that tumors demonstrating growth did so in the first 5 years after diagnosis. Interestingly, they also found that intrameatal and extrameatal tumors had a statistically different rate of growth, with 17% and 29% of these tumors demonstrating growth within 4 years of diagnosis, respectively.15 These findings suggest that both the time since diagnosis and the location of the tumor with respect to the internal auditory canal (IAC) play a role in the natural history of acoustic tumors. Acoustic neuromas in patients with neurofibromatosis type 2 (NF2) frequently exhibit a distinct growth pattern and are thus often treated as a separate entity. Acoustic neuromas in these patients occur at a younger age and may have a more unpredictable growth rate with more rapid growth the younger the patient at the time of diagnosis.32–35 Slattery and associates found the average growth rate measured in the tumor’s greatest diameter to be 1.3 mm/yr in patients with NF2. Patients who had a family history of NF2 did not experience more rapid growth. This growth rate falls within what is seen in patients without NF2 who harbor acoustic neuromas with gradual, consistent growth.36 NF2 patients often present a challenge because of the increased incidence of bilateral tumors and the younger age at which tumors develop. NF2 tumors present a more complex approach because attempts at maintaining serviceable hearing need to take into account future growth. Obviously, the loss of bilateral serviceable hearing is a more debilitating neurological deficit than unilateral hearing loss.
TABLE 133-1 Percentage of Acoustic Neuromas Demonstrating Growth in Series with at Least 50 Patients
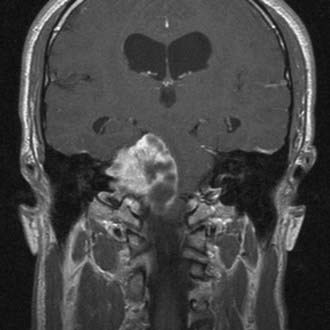
Cystic acoustic tumors are believed by some to have a more aggressive course with more rapid neurological deterioration (Fig. 133-1).37 There is debate within the literature about whether these tumors are associated with greater surgical morbidity, with some authors finding a higher risk for facial nerve palsy with operative treatment of cystic acoustic neuromas.38 However, others report that when corrected for other factors such as size, the surgical morbidity is comparable.39
Histopathologic Characteristics
The terminology “acoustic neuroma” is a misnomer because the cellular makeup of the tumor is actually more consistent with a schwannoma and it most commonly arises from the vestibular component of the vestibulocochlear nerve. In recent surgical studies, the most common origin has been from the inferior vestibular nerve, followed by the superior vestibular nerve.40,41
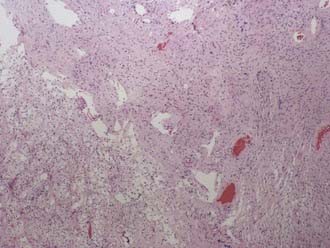
Classically, the microscopic appearance consists of two main histologic patterns: Antoni A and Antoni B (Fig. 133-2). Antoni A areas contain spindle-shaped cells with rod-shaped nuclei and dense reticulin arranged in compact intertwining fascicles. There may be palisading of nuclei forming what is known as a Verocay body. Antoni B areas have stellate or spindled-shaped cells with smaller and more hyperchromatic nuclei, less reticulin, prominent cytoplasmic processes, and a loose myxoid stroma.42 Most acoustic tumors are composed of predominantly Antoni A areas, but a higher component of Antoni B areas may be seen in more cystic tumors. Foamy histiocytes may be observed and are responsible for the bright yellow color associated with the tumor. On occasion, psammoma bodies may be found within an acoustic neuroma because the IAC is lined by dura. Immunohistochemical studies, although not routinely necessary, can be helpful in distinguishing a meningioma from a schwannoma in select cases. Vimentin and EMA are positive in a meningioma, whereas schwannomas express nuclear S-100 and vimentin positivity. Although S-100 can be positive in meningiomas, it is generally focal and cytoplasmic.43
The association of schwannomas with NF2 has led to increased interest in the genetics behind the tumor. Loss of heterozygosity on 22q and deficiency of the protein merlin have been linked to NF2 and have spurred interest in the molecular processes underlying acoustic neuromas. Merlin has been identified as a putative tumor suppressor, and increased understanding of related molecular mechanisms has recently identified potential targets for the development of pharmacotherapy, such as ErbB and Nrg.44
Clinical Findings
Cushing was the first to eloquently describe the progression of symptoms from early sensorineural hearing loss to the later symptoms associated with brainstem compression. Today, as fewer and fewer tumors are escaping detection, patients are less frequently initially seen with overt brainstem compression or hydrocephalus unless the tumor is almost entirely contained within the CPA. Although there is significant variability in the initial clinical findings, the most common initial symptom is asymmetric hearing loss. Asymmetric sensorineural hearing loss is seen in approximately 85% of patients with known acoustic neuromas and is the initial complaint in 65% of patients.45 Tinnitus involving the affected side occurs in a significant majority of patients, and persistent tinnitus should raise concern for an acoustic neuroma. Patients may have vestibular symptoms and complain of progressive imbalance or dizziness. Neurological examination may demonstrate evidence of vestibular dysfunction such as gait ataxia, nystagmus, and a positive Romberg sign. Headaches, facial paresthesia, and facial weakness or fasciculation are currently uncommon initial manifestations of acoustic neuromas.46 Classically, audiologic testing has played an important role in the evaluation of patients with sensorineural hearing loss.
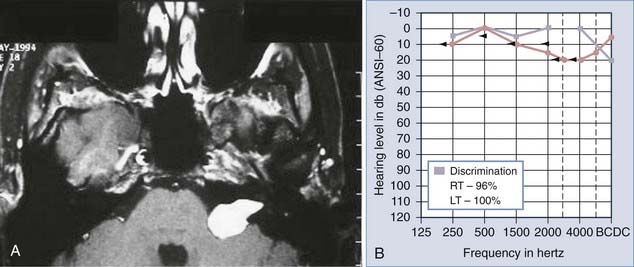
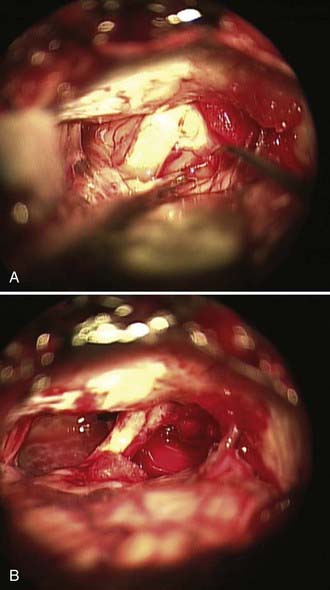
Auditory brainstem response (ABR) testing is the most sensitive audiologic examination for identifying an acoustic neuroma. ABR testing consists of recordings of neural activity within the cochlear nerve and auditory pathways after administration of a stimulus to the patient’s ear. Most large series have reported a sensitivity of 92% to 98% and specificity of 80% to 90% for ABRs.46–48 However, a more recent study found a sensitivity of 71% and specificity of 74% in the evaluation of asymmetric hearing loss. The sensitivity of ABRs was found to be lowest in patients harboring small acoustic neuromas.49 Increased awareness among clinicians has led to earlier diagnosis and changed the goals of surgery from prevention of brainstem compression to the potential for hearing preservation. We have used preoperative ABRs to evaluate the likelihood of hearing preservation. We have never saved hearing in a patient with a latency greater than 2 msec in comparison to the normal ear (Fig. 133-3A and B).
Preoperative Imaging Studies
The diagnostic evaluation of patients in whom there is a suspicion of an acoustic neuroma has advanced significantly with the advent of ABR testing, computed tomography (CT), and MRI. MRI and in particular MRI with paramagnetic contrast material have greatly increased the sensitivity of imaging in the detection of acoustic neuromas and in the distinction between other CPA pathology (Fig. 133-4). The sensitivity and specificity have increased so much that the utility of ABRs as a screening measure for asymmetric sensorineural hearing loss has been questioned.49 The use of specific “IAC” protocols to evaluate the CPA and bilateral IACs provides high-resolution, thin-cut sequences in a timely manner. Acoustic neuromas are the most common tumor seen within the CPA and account for 70% to 80% of tumors, followed by meningiomas and then epidermoids.50 Acoustic tumors usually demonstrate isointensity to brain parenchyma on T1-weighted sequences and hyperintensity on T2-weighted sequences.51,52 Acoustic neuromas demonstrate avid contrast enhancement after the intravenous injection of gadolinium. Tumors can have variable enhancement patterns: homogeneous (50% to 60%), heterogeneous (30% to 40%), or cystic (5% to 15%).37,51,52 The MRI appearance of the tumor varies somewhat depending on the size of the tumor and its histologic composition. Homogeneous small tumors are composed predominantly of the Antoni A type, whereas larger, more heterogeneous tumors have mixed Antoni A and Antoni B types or only the Antoni B type.52 In addition to aiding in the diagnosis of an acoustic neuroma, MRI also provides additional anatomic information useful in planning treatment. Information regarding the size, intracanalicular component, and CPA component is provided and can aid in planning treatment. Three-dimensional, fast spin-echo, heavily T2-weighted sequences such as FIESTA (fast imaging employing steady-state acquisition) and CISS (constructive interference in steady state) can also provide additional information regarding other CPA cranial nerves, including the facial nerve (Fig. 133-5). However, imaging never replaces the need for intraoperative observation and neurophysiologic monitoring because the facial nerve is often intimate with the tumor capsule.
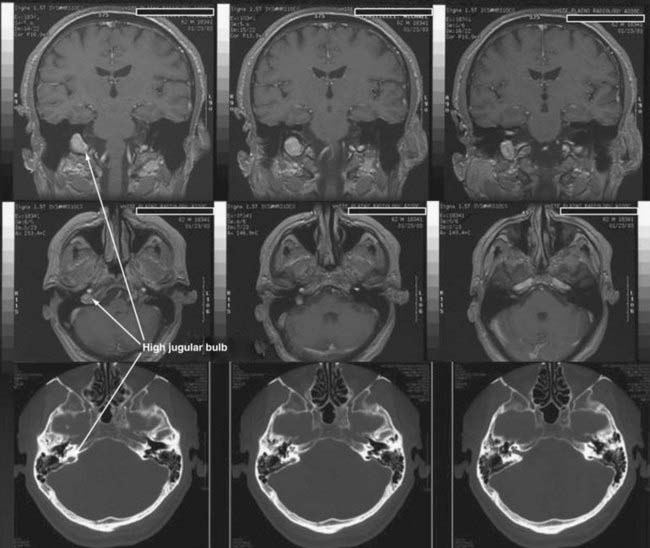
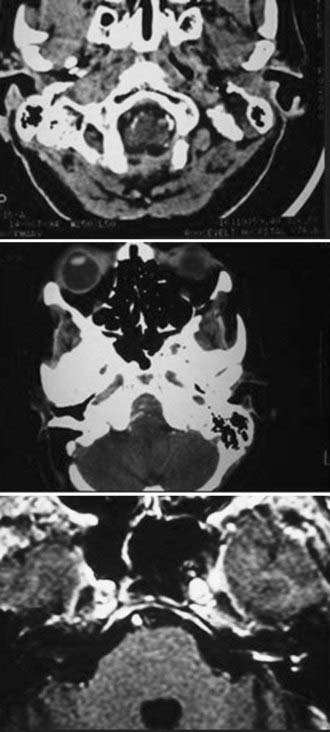
CT performed with bone algorithms can provide details on the bony anatomy not evident on MRI and still plays an important role in the preoperative evaluation of patients and in particular in surgical planning. The presence of a high-riding jugular bulb and its proximity to the IAC is important to be cognizant of when planning a retrosigmoid approach because it can limit lateral exposure of the tumor, particularly when performed with the patient in a supine position (Fig. 133-6). CT also provides useful information about the extent of mastoid and adjacent bone pneumatization, which is important in preventing postoperative cerebrospinal fluid (CSF) leaks (Fig. 133-7).
Preoperative Management
Discussion of all options available to a patient with a newly diagnosed acoustic neuroma is crucial. Patients can ultimately decide to be managed by observation until growth is observed or new clinical findings occur, or they may choose surgery or radiation therapy. Observation is an acceptable option for many patients with small or medium-sized tumors, particularly in elderly patients.53 Patient age, hearing status, and size of the tumor all play an important role in choosing to observe an acoustic tumor rather than intervene. The natural history of acoustic tumors has been described and is represented in Table 133-1. Measurable tumor growth demonstrated on MRI is a good predictor of future tumor growth and should encourage active management.
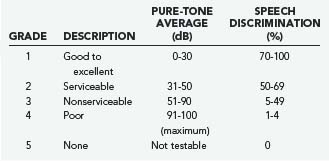
In general, initial follow-up MRI within 1 year is indicated to establish a growth trajectory. Annual scans for 3 to 5 years followed by every 2 years until 10 years and every 5 years thereafter is reasonable, but there is not sufficient evidence to suggest what the standard of care should be. Patients with useful hearing at the time of diagnosis are likely to lose hearing with a policy of observation inasmuch as more than half of patients lose hearing during the observation period.53 The specifics of the case will frequently determine the approach taken, and it is fruitless to generalize. Pure-tone audiometry and speech discrimination testing are usually performed, and hearing can be classified by using the scale proposed by Gardner and Robertson (Table 133-2).54,55 This provides an objective measure that can be followed and is useful in management decisions.
Surgical Approaches
There is much debate in the literature regarding the benefits of the three main approaches to an acoustic neuroma: the retrosigmoid, middle fossa, and translabyrinthine approaches. Although there are potential benefits and limitations with each approach, it is important to not be dogmatic about which surgical approach produces superior outcomes. The best approach in a particular case reflects the goals of the surgery, functional status of the patient, and the experience of the operative team with the proposed surgical approach. A learning curve is present with resection of acoustic neuromas, and the results therefore represent a moving target.56,57 Studies are thus limited by the particular surgeon’s preference and personalized results with each approach.
The translabyrinthine approach is not appropriate when hearing preservation is a goal. Some have suggested that the middle fossa approach is preferable in patients with a small, laterally located intracanalicular tumor and hearing preservation as a goal (see Fig. 133-7).58–60 However, excellent results in terms of hearing preservation can also be achieved via a retrosigmoid approach. Samii and coauthors recently reported a series of 200 patients in whom they achieved 51% functional hearing preservation and excellent or good facial function in 81% with a retrosigmoid approach.61 This represents an improvement on their previously reported 1000 patients, thus demonstrating that progress can be made even in the most experienced hands.62 Evaluation of the volume-outcome relationship at 265 hospitals found significantly improved results at hospitals with higher volumes and with surgeons with higher operative caseloads.63 There is clearly a steep learning curve that must be considered when evaluating the effectiveness of different surgical approaches.56,57,64,65 Therefore, conclusions about the appropriate surgical approach are thus limited by the personal experience of the surgeon and the particular aspects of the case (Table 133-3).
| CLASS | TUMOR EXTENSION |
|---|---|
| T1 | Intrameatal tumor |
| T2 | Intrameatal and extrameatal tumor |
| T3A | Tumor filling the cerebellopontine cistern |
| T3B | Tumor reaching the brainstem |
| T4A | Tumor compressing the brainstem |
| T4B | Tumor severely displacing the brainstem and compressing the fourth ventricle |
From Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): facial nerve preservation and restitution of function. Neurosurgery. 1997;40:684-695.
Retrosigmoid
The standard retrosigmoid approach is the most familiar to neurosurgeons and has many advantages. One major advantage is the flexibility of the approach with regard to tumor size and extracanalicular extension. One can address large extrameatal tumors causing compression of the brainstem to small intracanalicular tumors through a similar approach. There is an excellent view of the cranial nerves, and smaller tumors allow the surgeon to attempt hearing preservation (Fig. 133-8).
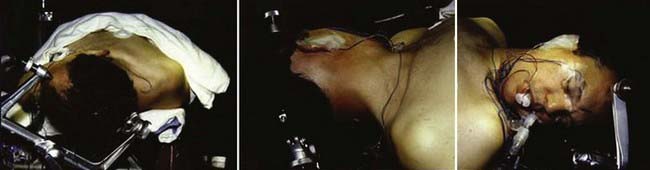
The semisitting, supine, supine-oblique, park bench, and lateral oblique positions have been used for suboccipital removal of acoustic neuromas.66 Most frequently, a supine position with shoulder bolsters is used at our institution (Fig. 133-9). It is important to make certain that the patient is secured to the operative table with multiple belts because there is frequent rotation of the table to improve exposure during surgery. Care should be taken to ensure that the patient has adequate padding at all potential pressure points. Three-point Mayfield skeletal fixation is used. The patient’s head is then turned to the contralateral side. If the extent of lateral rotation of the head is limited secondary to cervical spondylosis, a lateral position can be used. The key to adequate exposure of the posterior fossa and CPA is achieving a balance between the amount of flexion and rotation. With too little rotation, exposure of the CPA will be compromised, whereas too extensive rotation runs the risk of venous occlusion. We routinely give 0.5 to 1.0 g/kg of mannitol, 1.5 g of cefuroxime, and 10 mg of dexamethasone before the incision.
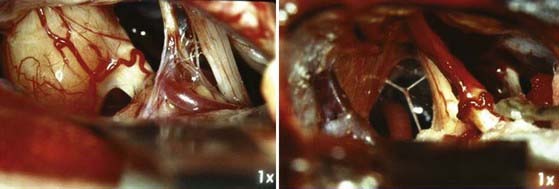
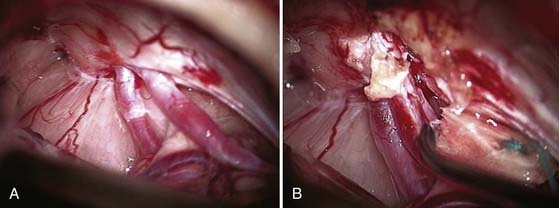
The operative microscope is brought into the field and the remainder of the surgery performed with microsurgical technique. The tumor is identified and the visualized cranial nerves are protected with a cottonoid. The arachnoid is dissected from the posterior aspect of the tumor. The posterior middle third of the tumor is the most commonly used entry zone for internal debulking. The facial nerve is usually found anteriorly in the middle third of the capsule, with the posterior part of the tumor being the least likely location of the nerve.67 A facial nerve stimulator is used to stimulate the dorsum of the tumor and confirm the absence of the facial nerve before initiating internal debulking. Although less common, both the facial and cochlear nerves can be found posterior to the tumor, thus emphasizing the importance of using a nerve stimulator before initiating the debulking (Fig. 133-10). The tumor is removed with ultrasound aspiration, microdissectors, and microcup forceps. Bipolar electrocautery is used sparingly, and we prefer to use Surgicel or Gelfoam for hemostasis to limit the potential for inadvertent cranial nerve injury. Once internal debulking of the tumor is adequate, the tumor capsule is gently moved into the field while maintaining the arachnoid plane (Fig. 133-11A-D). A facial nerve stimulator is again used to verify that there is no involvement of the capsule with the facial nerve. Whenever possible, early identification of the cranial nerves in the CPA extending to the porus acusticus is recommended.
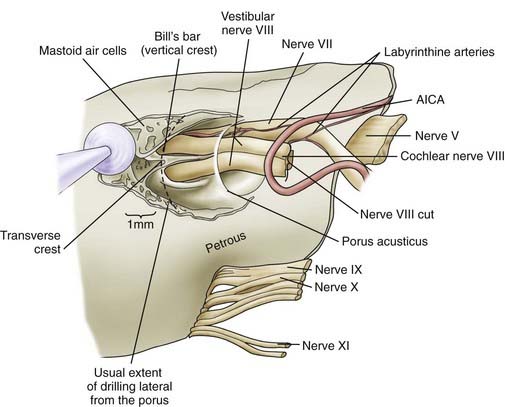
We routinely use a team approach, and our neurotologist generally drills the bone to open the posterior wall of the IAC. We place Gelfoam in the subarachnoid space to decrease the amount of bone dust accumulation during drilling of the IAC and thereby prevent severe postoperative headaches (Fig. 133-12).68 Once the dura on the posterior surface of the petrous ridge is removed, a diamond bur is used to uncover the IAC from its superior to its inferior surface. Particular care is taken to not enter the inner ear because the posterior semicircular canal and vestibule are in close proximity to the drilling. If a semicircular canal is breeched, it should be immediately packed and sealed with bone wax because hearing can still be preserved.69,70 The dura covering the canal is removed to expose the nerves as they enter the canal. The different contents of the IAC are identified, with the facial nerve being superior/anterior and separated from the superior/posterior superior vestibular nerve by Bill’s bar. The cochlear nerve is inferior/anterior and the inferior vestibular is inferior/posterior, with separation from the superior contents of the canal by the transverse crest (Fig. 133-13). The tumor is separated from the cochlear and facial nerves by progressing from either a lateral-to-medial direction or a medial-to-lateral direction, depending on the ease of separation of the planes. Both the facial nerve and cochlear nerve are monitored closely during this step to ensure that physiologic function is maintained during the dissection. The tumor is removed completely, after which the facial nerve is stimulated near the brainstem to assess its integrity.
Once the tumor has been removed, close attention is paid to hemostasis with various hemostatic agents. Any air cells within the canal are identified and carefully sealed with bone wax, muscle, or a combination of the two. This prevents a source of CSF leak via the eustachian tube. Endoscopy at this stage is helpful to ensure that all lateral tumor has been removed and all air cells have been sealed.68 The posterior fossa is irrigated throughout the procedure, particularly during drilling to limit the amount of bone dust and debris. Bone dust can contribute to both postoperative headaches and decreased arachnoid granulation drainage of CSF, which can lead to hydrocephalus. Before closing the dura, the CPA is inspected and irrigated as necessary. The dura is closed in watertight fashion with interrupted suture, and DuraSeal is applied over the suture line. The craniectomy is again inspected for evidence of air cells, and any additional air cells identified are waxed. A titanium mesh cranioplasty with methyl methacrylate is performed, or alternatively in the case of a craniotomy, the bone flap is replaced.71
Middle Cranial Fossa
The middle cranial fossa approach initially introduced by House for acoustic neuroma surgery has been described by many for hearing preservation surgery for small laterally located intracanalicular tumors.58,72–76 Comparison of this approach with the retrosigmoid approach remains a topic of debate, with excellent outcomes achieved with each approach in skilled hands. This approach is not ideally suited for patients with significant extracanalicular extension because of poor visualization of the CPA and brainstem, although extended middle fossa approaches have been used.
A linear vertical incision, curved incision, or a horseshoe incision is made through the skin and should be concealed behind the hairline whenever possible. A periosteal elevator is used to elevate the mucoperiosteal layer. A craniotomy approximately 4 cm in diameter is performed. The bone flap is dissected free from the dura and passed off for storage until the end of the procedure. If the inferior aspect of the craniotomy is not flush with the floor of the middle cranial fossa, a rongeur is used to carry the craniotomy inferiorly until it is flush. The microscope is brought in for elevation of the temporal dura off the floor of the middle cranial fossa. A House-Urban retractor is used for gentle medial retraction of the temporal lobe.77 Dissection proceeds in a posterior-to-anterior direction to avoid inadvertent injury to the greater superficial petrosal nerve, geniculate ganglion, or dehiscent petrous carotid. Anteriorly, the dissection is complete when there is adequate visualization of the foramen spinosum and middle meningeal artery. The posterior petrous ridge is identified.78
The temporal floor is evaluated for landmarks to identify the IAC. One method is to start drilling on the most medial aspect of the straight line parallel to the external auditory canal and perpendicular to the inner table. Another method is to draw a 100-degree angle between the greater superficial petrosal nerve and the arcuate eminence. The apex of the angle is bisected and drilling performed over the medial aspect of the petrous ridge.78,79 Drilling is performed with a diamond bur in a medial-to-lateral direction toward the fundus. Close attention is paid to avoiding injury to the underlying cochlea anteriorly and the labyrinth posteriorly. The geniculate ganglion and labyrinthine segment of the facial nerve are exposed.77 A direct nerve monitor can be applied to the anterior-inferior extradural region for nearly real-time monitoring of cochlear nerve function.
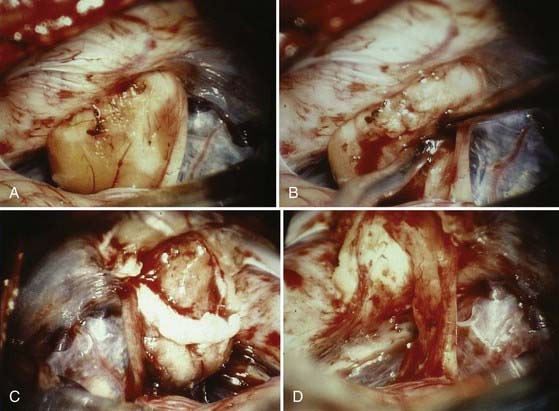
The dura of the IAC is incised along the posterior aspect and reflected anteriorly. The facial nerve is dissected away from the tumor starting at Bill’s bar. The tumor is then debulked internally and removed in a medial-to-lateral direction. The AICA is identified and dissected from the tumor to avoid hemorrhage. This is particularly challenging with larger tumors because they more often have complex involvement with the AICA (Fig. 133-14A and B).67 The cochlear nerve is dissected from the tumor, and the superior vestibular nerve can be sectioned if it is the origin of the tumor. Any change in ABRs should alert the surgeon that there could be too much traction on the cochlear nerve or stretching of the labyrinthine artery.80 Hemostasis is verified before closure, and an abdominal free fat graft is placed over the IAC. The bone around the IAC is inspected for air cells and bone wax applied as necessary. The bone flap is replaced and skin closure completed.
Translabyrinthine
The patient is placed in a supine position with head directed away from the side of the tumor. One of the benefits of this approach is that initial positioning is easier than with the other approaches. There is no requirement for a Mayfield head holder, and less attention to head postioning is required than for the retrosigmoid approach. A patch of skin is shaved from the postauricular crease to approximately 4 cm behind the auricle. The area is then prepared with povidone-iodine (Betadine) and draped in the usual sterile fashion. A C-shaped incision is made that extends from 1 cm above the ear and 2 cm behind the postauricular crease to 1 cm posteroinferior to the mastoid tip. The curve in the incision helps prevent foreshortening and keeps the incision posterior to the sigmoid sinus and the sinodural angle.81 The incision is initially carried only through the skin, and a skin flap is elevated. The incision is brought down through the muscle and periosteal layer with electrocautery while tracing the shape of the auricle with a 1-cm offset from the skin incision. A large superficial temporal fascial graft is harvested. The periosteum is elevated to the external auditory canal and retracted with dural hooks. This approach provides access to portions of the posterior digastric, which can be harvested for later use when a graft is often required for packing the middle ear.77
A complete mastoidectomy is performed with skeletonization of the tegmen and the bone overlying the sigmoid sinus. In patients with large tumors or an anteriorly placed sigmoid sinus, additional bone can be removed over the posterior cranial fossa posterior to the sigmoid sinus.82,83 The cutting bur is best used from the external auditory canal posteriorly because it provides superior control. The compact bone of the labyrinth is identified and the lateral semicircular canal used as a landmark for anatomic positioning of the facial nerve. Once the position of the facial nerve is known, a labyrinthectomy is completed by drilling down the superior petrosal sinus and slowly deepening and widening this area to include the labyrinth until the IAC and jugular bulb are identified. The lateral semicircular canal is opened until the ampulla is reached anteriorly. The posterior and superior semicircular canals are exposed to their entrance in the vestibule.82
The facial nerve is identified throughout its mastoid course and a diamond bur used to unroof it. The facial nerve is completely skeletonized from the mastoid segment around the genu to the tympanic segment. The vestibule is opened widely but with caution because a high-riding jugular bulb can be located more superficially than expected. Saleh and colleagues found that a high-riding jugular bulb limits exposure of the IAC in 24% of cases.84 The jugular bulb is particularly challenging to skeletonize because of hemorrhagic complications and the potential for air emboli. A high-riding jugular bulb can be managed by complete skeletonization of the sigmoid sinus and descending segment of the facial nerve. The jugular bulb is then progressively lowered to gain adequate exposure of the IAC.85,86 This does carry a potential risk for hemorrhage, venous infarction, and damage to contents of the pars nervosa.87 The bony opening is completed with skeletonization of the sigmoid sinus, jugular bulb, inferior IAC, and superior auditory canal. It is important to extend far enough laterally to allow identification of Bill’s bar and therefore the anterior superior facial nerve. One of the major proposed benefits of this surgical approach is early identification of the facial nerve. Once the bone between the middle fossa dura and superior IAC is removed and the facial nerve identified, the posterior fossa dura is incised. The junction of the posterior tumor with the cerebellum can often be identified. After the facial nerve has been reliably identified, the cochlear, superior, and vestibular nerves are removed from their canal.
The facial nerve is most frequently found along the anterior margin of the tumor, but it can on occasion be splayed over the top of the tumor (Fig. 133-15).82 A facial nerve stimulator should be used to avoid inadvertent injury. The anterior aspect of the tumor is carefully dissected from the facial nerve down to the brainstem while maintaining a distinct plane.81 The medial dissection is the most challenging portion of the resection because of the associated risk of damage to both the facial nerve and the pons.
Radiosurgery and Fractionated Stereotactic Radiotherapy
Radiosurgery has played an increasingly important role in the management of acoustic neuromas. Leksell’s initial series from the Karolinska Institute from 1969 to 1974 achieved good short-term tumor control in eight of the nine patients who were treated, but post-treatment hearing loss was reported in the majority of patients.88 As seen in the initial open surgical series, refinement of technique would eventually lead to improvement in outcomes and a reduction in associated complications. The early series from Karolinska used high doses of 25 to 35 Gy. Kondziolka and associates published a series from 1987 to 1992 that included 162 consecutive patients with a mean transverse tumor diameter of 2.2 cm and an average dose to the tumor margin of 16 Gy. Their follow-up in the series included patients between 5 and 10 years after radiosurgery. Tumor control, defined as failure of progression requiring surgery, was excellent at 98%. Facial function was preserved at 5 years in 79% of patients. Normal trigeminal nerve function was also maintained over the same period in 73% of patients, whereas 51% did not have an alteration in their hearing ability.89 The initial results were not satisfying because of the approximately 30% of patients who experienced at least temporary facial nerve dysfunction and the morbidity associated with trigeminal neuropathy. The treatment-associated morbidity is probably influenced by the dose delivered and the volume of tumor to be treated. The development of more complex radiosurgical planning has led to more conformal radiation shapes; however, the conformity and dose gradient do not appear to have a clear effect on outcomes.90
As is the case in the open surgical treatment of acoustic neuromas, the goals of therapy with radiosurgery are not just good local tumor control but also preservation of facial nerve function and functional hearing. Adjustments have been made in both the mean dose delivered to the tumor margin and treatment planning to maintain tumor control and limit cranial neuropathy. Gamma Knife systems deliver doses to the periphery that are generally 50% of the peak dose delivered to the center of the tumor. Further reductions in the marginal dose have resulted in a decrease in side effects when doses lower than 13 Gy were used.91 The reduction in dose lowered the rate of facial neuropathy from 29% to 5% and lowered the morbidity of trigeminal neuropathy to 2%.74 It was not until the marginal dose was decreased to 10 Gy or less that there was a reduction in tumor control. A marginal dose of approximately 12 to 13 Gy probably limits the side effects while maintaining therapeutic effect. Recently, several series using lower marginal doses from 12 to 13 Gy have produced excellent results in terms of tumor control, facial nerve preservation, trigeminal preservation, and hearing preservation.92,93
In addition, the conversion from CT-based treatment plans to MRI-based planning has probably also contributed because doses can be delivered with greater precision. As is frequently the case in areas of medicine with rapidly advancing technology, the results will remain a moving target as advancements make improved results a reality.94,95
Fractionated stereotactic radiotherapy (FSRT) has the perceived benefit of fractionation, which allows lower treatment doses to sensitive neural structures while maintaining tumor control. There is a wide range seen in treatment paradigms, with varying dosage regimens from 18 to 21 Gy in 3 fractions to 60 Gy in 30 fractions.96,97 Andrews and coworkers compared the results of radiosurgery with FSRT in a study involving 125 patients. Both radiosurgery and FSRT yielded excellent results, but FSRT has been described by some to have a higher rate of hearing preservation if the patient had serviceable hearing preoperatively.98 However, fractionation requires that patients make frequent trips for treatment and may be more difficult for patients who do not live close to a treatment center.
Special Considerations—Facial Nerve Preservation
The operating microscope has made facial nerve preservation the expectation in acoustic neuroma surgery. Postoperative facial nerve function is the most important, followed by complete tumor removal and finally hearing preservation. For some patients, hearing preservation may be more important than complete tumor removal. A patient with a disfiguring facial paresis does not care whether there was anatomic preservation at the time of surgery. The most commonly used grading system for facial nerve function is the one described by House and Brackmann (Table 133-4). The relationship between postoperative facial nerve function and the size of the tumor has been well described.99–102 The facial nerve outcome with cystic tumors in some series is less favorable than that with solid tumors when tumor size is held the same.37,38,103 There are good and improving results in terms of functional preservation in both surgical and radiosurgical series.
| GRADE | DESCRIPTION |
|---|---|
| 1 | Normal in all areas |
| 2 | Mild dysfunction (slight weakness on close inspection, complete eye closure) |
| 3 | Moderate dysfunction (obvious but not disfiguring, forehead with slight to moderate movement, complete eye closure with maximal effort) |
| 4 | Moderate to severe dysfunction (obvious weakness with disfiguring asymmetry, no forehead movement, incomplete eye closure) |
| 5 | Severe dysfunction (barely perceptible motion) |
| 6 | Complete paralysis |
From House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146-147.
Surgery
The significant morbidity associated with a postoperative facial palsy has been evaluated by numerous authors.104 The postoperative likelihood of a facial paresis is directly related to the size of the tumor, with facial nerve injury being more likely with larger tumors (Fig. 133-16). The difference appears to be more dramatic when one looks at immediate postoperative facial weakness. In one series, the percentage of patients with House-Brackmann grade 3 or higher in the immediate postoperative period was 62.5% in those with tumors larger than 4 cm, whereas only 35.3% of patients had a House-Brackmann grade 3 or higher if the tumor size was less than 2.5 cm.105 However, 75% of the patients at 6 months had normal or nearly normal facial nerve function, thus demonstrating that although there is an effect related to tumor size, excellent results are still possible even with the largest tumors.105
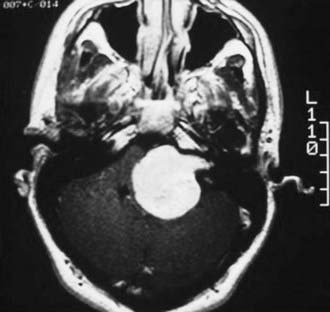
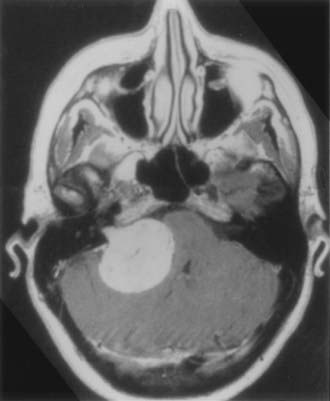
FIGURE 133-16 Magnetic resonance imaging with intravenous contrast enhancement demonstrating a 4.5-cm acoustic neuroma.
There is debate in the literature regarding whether a significant cystic component is a negative prognostic factor for facial nerve preservation. Numerous authors have reported that patients with cystic tumors have a greater likelihood of postoperative facial paresis than do those with more solid tumors.37,38,103 Samii and Matthies found that the anatomic preservation rate of the facial nerve decreased from 93% to 88% in patients with cystic tumors.106 Others have found that when series are corrected for size of the tumor because larger tumors are more likely to have a cystic component, there is no real difference in postoperative facial paresis.39 Cystic tumors are believed to be more difficult than their homogeneous solid counterparts to predict relationships with the facial nerve. However, varying definitions of cystic make generalizations based on the existing data difficult.
There are numerous reports in the literature comparing facial nerve preservation rates with the various approaches. It is again important to not be dogmatic because the published results probably reflect some of a group’s surgical bias rather than solely approach-related factors. Numerous surgical series have demonstrated excellent facial nerve results with each approach, thus making the experience of the surgical team with an approach the most important factor. There has been debate about facial nerve outcomes, particularly with the middle cranial fossa approach. It was generally believed that a middle cranial fossa approach would produce worse facial nerve results because the facial nerve lies between the surgeon and the tumor. Therefore, it was argued that prolonged traction on the facial nerve would produce inferior results. Conversely, proponents of the translabyrinthine approach argued that early identification of the facial nerve before tumor dissection would provide results superior to those of all other approaches. However, several series have demonstrated no real difference in functional facial preservation depending on the approach.62,77,100,105,107–112 It is important to note that because tumor size influences facial nerve preservation results, comparison of different series must eliminate tumor size as a confounding variable. There is higher risk for facial nerve injury with both surgical approaches as tumor size increases, but it is comparatively higher with the middle fossa approach than with the suboccipital or translabyrinthine approaches.110,111 This finding produces a bias in many of the series assessing facial nerve outcome with the middle cranial fossa approach. Many centers will recommend a middle cranial fossa approach for tumors that are small or intracanalicular or with only a small amount of CPA extension when hearing preservation is a goal. Nonetheless, some authors have reported success with the enlarged middle cranial fossa approach, with one series reporting 92% good facial nerve results (House-Brackmann grade 1 or 2), including tumors with up to 2.9-cm extension into the CPA.92
Radiosurgery and Fractionated Stereotactic Radiotherapy
Radiosurgery has seen significant improvements in facial nerve preservation from early studies. Early studies had an unacceptably high rate of facial neuropathy (33%) with the early treatment doses and planning.113 Early focus was on increasing the number of isocenters to create more conformal treatment plans as a means of reducing facial neuropathy. Investigators have examined numerous variables, with tumor volume and treatment dose emerging as important predictors of post-treatment facial neuropathy. Friedman and colleagues found that with each cubic centimeter increase in tumor volume, the odds of facial weakness increased by 17%.114 The treatment dose also plays an important role in complications involving the facial nerve. Work over the past decade has focused on determining the optimum treatment dose that does not sacrifice tumor control and yet maintains a low rate of post-treatment facial paresis. Investigators have found that with each 250-cGy increase in dose there is an eightfold increase in the odds of facial weakness.114 Recent literature suggests that doses between 12 and 13 Gy probably maintain tumor control while decreasing post-treatment facial weakness.115,116
Special Considerations—Hearing Preservation
Surgery
There is much debate in the literature regarding the best surgical approach to maintain serviceable hearing, and the conclusion of this debate probably lies in its nuances (see Table 133-2). The first important factor, as mentioned earlier, is the comfort of the surgeon with the particular approach. Tumor size and extension, along with the preoperative hearing level, are important predictors of the likelihood of hearing preservation.61,117,118 Good results of hearing preservation have been reported with both the suboccipital approach and middle fossa approach to acoustic neuromas. The middle fossa approach is best used for small laterally placed tumors in the IAC. Hearing preservation results ranging from 52% to 100% have been described in the literature.58,72–76 However, the correlation of tumor size is present in the middle fossa approach as well. Satar and coworkers found that in tumors with extrameatal extension from 10 to 18 mm into the CPA, the rate of hearing preservation was 34%.111
Comparable results have also been reported with the retrosigmoid approach, with functional hearing being preserved in 14% to 83% and the best results achieved with tumors less than 1 cm and without significant CPA extension.75,118–121 Staeker and associates compared the middle fossa and suboccipital approaches for small intracanalicular tumors in 15 patients matched for audiologic criteria and tumor size. They found a significant difference in both the postoperative change in the average pure-tone threshold and word recognition score and superior results with the middle fossa approach. The hearing preservation rates for the middle fossa approach and suboccipital approach were 57% and 47%, respectively.58 Kumon and colleagues found that for tumors between 1 and 2 cm, hearing preservation rates for the middle fossa approach and retrosigmoid approach were 0% and 47%, respectively. Also of importance is that only 50% of patients undergoing the middle fossa approach for these larger tumors had excellent or good facial nerve function postoperatively versus 87% in the retrosigmoid group.110 Samii and coworkers were able to achieve a 29% hearing preservation rate in patients with Hannover grade 4A and 27% hearing preservation in patients with grade 4B lesions (see Table 133-3).61 Attempts at hearing preservation are important as artificial auditory augmentation systems develop, and it is our belief that attempts at hearing preservation should be made in all patients with serviceable hearing. In experienced hands, the middle fossa approach can achieve good hearing preservation results with small intracanalicular tumors, but as tumor size increases, the results experience a more significant drop-off than with the retrosigmoid approach. Hearing preservation is possible even for larger tumors with the retrosigmoid approach in experienced hands. However, as noted earlier, we have never preserved hearing in a patient with a latency of greater than 2 msec in comparison to the normal ear. If hearing is preserved, long-term follow-up has shown that it remains relatively stable.122
Radiosurgery and Fractionated Stereotactic Radiotherapy
As previously described, earlier series of acoustic neuromas treated with radiosurgery or fractionated radiotherapy had unsatisfactory results of hearing preservation. However, as planning systems were upgraded, MRI improved localization, and the dose was lowered to 12 to 13 Gy, the hearing preservation results improved significantly. Flickinger and coauthors reported a hearing preservation rate of 78.6% as defined by maintenance of serviceable hearing.116 Paek and associates reported maintenance of serviceable hearing in 52% of patients treated with a prescription dose of 12 Gy with a median tumor volume of 3 cm3.123 Iwai and coauthors reported maintenance of serviceable hearing in 56%.93 These results compare favorably with the results seen with both the middle fossa and retrosigmoid approaches. The etiology of the hearing loss in radiosurgery and fractionated radiotherapy are not clearly defined. There is a dose effect, as previously mentioned, with improved hearing preservation as the dose is reduced. The key is balancing good hearing preservation rates without sacrificing tumor control.
Management of Complications
Facial Nerve Injury
The Acoustic Neuroma Association reported that facial nerve injury is the number one concern of patients undergoing surgery in the CPA.105,124,125 Facial nerve injury can occur because of direct trauma, stretching, vascular injury, or thermal effects.105 Recent retrospective studies have evaluated the role of vasospasm in postoperative facial paresis and found positive results with the use of nimodipine and hydroxyethyl starch.126 The introduction of facial nerve evoked responses from intracranial stimulation allowed surgeons to continuously assess the functional viability of the facial nerve during CPA dissection.127 Injury to the facial nerve is occasionally identified intraoperatively because of identified physiologic transection or loss of facial nerve evoked responses. In the event of anatomic transection of the nerve, immediate neurorrhaphy should be attempted to minimize the long-term postoperative facial deficit. Sampath and colleagues found that of eight patients with anatomic transection, none improved to normal facial function but five would improve to a House-Brackmann grade 3 or 4.105 Facial nerve reanastomosis involves identification of the proximal part of the nerve at the brainstem, rerouting the distal facial nerve stump, and anastomosis with 9-0 suture.128–130 An interposed sural nerve graft may be used if apposition of the ends is not possible. On occasion, the proximal stump is not available, and a 12th-to-7th cranial nerve jump graft can be created by using a sural nerve or great auricular nerve.131 The downside to this technique is that patients frequently require prolonged training for facial expression and a gold weight is needed for ocular protection.132 An alternative approach that is more technically challenging but has potentially better orbicularis oculi innervation is a straight end-to-side hypoglossal-facial anastomosis without interruption of the hypoglossal nerve.133–135
In a patient with postoperative facial palsy and incomplete eye closure, we favor early gold weight placement along with immediate ocular precautions to prevent exposure keratitis and corneal ulceration. Gold weights placed in the eyelid for loading to assist in closure of the eye is an effective management strategy for patients with facial palsy preventing complete closure of the eye.136 There is often a waiting period to see the extent of facial nerve recovery before planning alternative measures for reanimation. Gold weights have been shown to provide good ocular protection during that period along with low complication rates on removal if facial function recovers.137 Other facial reanimation techniques, including a temporal fascia sling, are useful in patients after they have demonstrated failure to recover facial function and complete eye closure.138
Cochlear Nerve Injury
Intraoperative cochlear nerve monitoring is an important tool in hearing preservation acoustic neuroma surgery. There are two primary methods of intraoperative cochlear monitoring: ABRs and direct cochlear nerve monitoring. ABRs give intraoperative feedback of cochlear nerve function. However, ABR testing is limited by the low amplitude of the signals and the 2- to 3-minute lag time during recording. ABR testing does not provide immediate feedback, thus leaving the surgeon to question what maneuver was directly responsible for the change. Direct cochlear nerve monitoring has the advantage of higher amplitudes and more rapid feedback.139 This technique is most compatible with the suboccipital approach and requires placement of an electrode directly on the cochlear nerve. Access to the cochlear nerve at the brainstem may be difficult with larger tumors compressing the brainstem. Direct cochlear nerve monitoring provides more feedback and can improve hearing preservation results. Danner and coworkers found a hearing preservation rate of 41% with the use of ABRs and 71% with direct cochlear nerve monitoring for tumors less than 1 cm in one study.140 Prospective evaluation would provide additional support to this study.
Recent studies have identified vasospasm as a potential source of postoperative loss of hearing. In a prospective study, Strauss and colleagues found that implementing nimodipine and hydroxyethyl starch for an average of 9 days significantly increased the rate of hearing preservation in comparison to controls, thus suggesting a vasoactive role in hearing loss.141
Cerebrospinal Fluid Leaks
CSF leaks are the next most common complication of surgery for acoustic neuromas, after injury to the seventh or eighth cranial nerve, and it has a reported incidence of 2% to 30%.142–147 CSF leaks are associated with increased length of hospital stay, increased rates of postoperative meningitis, and in some cases, surgical intervention.142,148–150 There are multiple locations that may serve as the site of CSF egress, including the nose, incision, or ear in the order of most common occurrence. CSF leaks can occur whenever there is a communication between the pneumatized cells of the temporal bone and the subarachnoid space. CSF rhinorrhea occurs when CSF enters the mastoid air cell system and finds an outlet via the eustachian tube into the nasopharynx. CSF rhinorrhea after the retrosigmoid approach is usually secondary to entrance of CSF through temporal bone air cells along the craniotomy site or surgically exposed air cells in the perilabyrinthine region.151 Close scrutiny of any exposed air cells during both opening and closing is crucial in the prevention of CSF rhinorrhea. Any exposed air cells can be sealed with bone wax, muscle, or hydroxyapatite cement.152 CSF rhinorrhea can occur after the translabyrinthine approach by egress of CSF through the posterior fossa dural defect; it gains access first to the mastoid and then to the eustachian tube via the aditus ad antrum, facial recess cells, sinus tympani cells exposed during skeletonization of the facial nerve, or retrofacial air cells. In addition, CSF can enter the middle ear through the fundus of the IAC if the stapes is accidentally dislocated.142 Incisional leaks occur secondary to CSF dissection through the closure layers and may occur in early or delayed fashion.
We have rarely found it necessary to obliterate the eustachian tube surgically because the techniques just described usually cease the CSF leak. However, with a refractory CSF leak in a patient without hearing, a blind sac closure of the external auditory canal and packing of the eustachian tube can be performed. If the patient has hearing and a refractory CSF leak, a middle cranial fossa approach with segmental division, removal, and cauterization of the ipsilateral eustachian tube can be performed.145 CSF otorrhea after resection of acoustic neuromas occurs only when CSF has access to the external auditory canal through a hole in the bony-cartilaginous junction made inadvertently secondary to retraction or through a defect in the tympanic membrane.
The effect of the surgical approach and tumor size on the rate of CSF leakage remains a matter of debate. Some series have suggested that rates of CSF leakage vary depending on the surgical approach used.149,153,154 However, other series have demonstrated no significant difference in CSF leakage rates when the surgical approach or tumor size is taken into account.148,155
Vascular Complications
The potentially dire vascular complications that could arise from acoustic neuroma surgery have long been known. Cushing referred to the cerebellopontine angle as the “bloody angle,” with reference to the fence corner at the Battle of Gettysburg.5 The incidence of serious vascular complications has decreased significantly with the advent of microsurgical technique.
Vascular complications can be broken down into ischemic and hemorrhagic types. Ischemic complications can arise from inadvertent coagulation of brainstem arteries that are adherent to the tumor. It is important to be aware that brainstem arteries can take a recurrent course initially, course out toward the IAC, and then loop back to supply the brainstem.156 Occlusion of the sigmoid or transverse sinus can lead to venous infarction.157 One study found the incidence of sigmoid sinus injury to be 4.7% in patients undergoing suboccipital and translabyrinthine approaches.157 The mechanism of the injury can be secondary to retraction, drilling, compression from bone wax, or excessive drying from a prolonged procedure. Approximately half of the resulting venous infarctions will have a hemorrhagic component.158 CPA hemorrhages are most commonly related to venous infarction and represent a complication that frequently requires surgical intervention.62
The AICA is increasingly involved with the tumor as the size of the tumor increases (see Fig. 133-14). Studies have shown that the AICA is involved in approximately 37% of tumors ranging from 2.5 to 4 cm and in 92% of tumors greater than 4 cm.67 The labyrinthine branch of the AICA is involved in 40% of tumors less than 2.5 cm, 58% of tumors 2.5 to 4 cm, and 100% of tumors greater than 4 cm.67 The posterior inferior cerebellar artery, superior cerebellar artery, and vertebrobasilar arteries can also be involved with tumor, particularly in larger lesions. Early identification and cautious dissection are important to avoid potentially devastating vascular injury.
Hydrocephalus
The association of acoustic tumors with both communicating and noncommunicating hydrocephalus is well described, with the incidence ranging from 3.7% to 43%.159 The mass effect from large tumors on the fourth ventricle can lead to obstructive hydrocephalus. In most cases, resection of the tumor will remove the source of the mass effect with improvement in the hydrocephalus. However, these patients must be monitored closely with subsequent clinical and radiographic examinations. Communicating hydrocephalus can also be seen with smaller tumors less than 2 cm, thus suggesting alternative mechanisms such as elevated CSF protein levels. Patients with CSF protein levels greater than 151 mg/dL were 6 times more likely to have hydrocephalus than were patients with CSF protein levels less than 80 mg/dL in multiple logistic regression analysis in one study.159 Hydrocephalus can complicate radiotherapy, with symptomatic communicating hydrocephalus developing in 11% of patients after the administration of FSRT in one study.160 These findings suggest that increased necrotic debris secondary to radiation could play a role in the development of communicating hydrocephalus and further support at least a partial role of increased CSF protein.
Headaches
Postoperative headaches are seen in the immediate postoperative period but resolve in most patients. However, a significant percentage of patients will have persistent headaches that can affect quality of life. There is variability in the exact percentage of patients experiencing postoperative headaches ranging from 11% to 50%.104,161–163 Surveys addressing quality of life after acoustic tumor resection have demonstrated a statistically significant decrease in self-assessed quality of life associated with persistent headaches.104 The cause of the headaches, as well as differences in prevalence among the different surgical approaches, has been explored. The retrosigmoid approach appears to be associated with a higher incidence of persistent postoperative headaches. Headaches are increased in patients who undergo a retrosigmoid craniectomy versus a retrosigmoid craniotomy, thus suggesting a role of adherence of the cervical musculature to the dura in generation of the headaches.164 Consistent with this concept are several reports describing a decrease in the incidence of headaches in patients who underwent retrosigmoid craniectomy with cranioplasty versus those who underwent craniectomy without cranioplasty.165,166 The amount of bone dust from drilling is also probably related to the postoperative headaches. When there is bone dust in the CPA from intradural drilling, the incidence of headaches is increased regardless of whether a cranioplasty is completed after craniectomy (see Fig. 133-13).68,167 The exact etiology remains debatable and is probably multifactorial. We routinely perform extensive irrigation when widespread drilling has been performed and cranioplasty to take both potential causes into account. Another potential cause of postoperative headaches is hydrocephalus from impaired CSF absorption.
Anderson DE, Leonetti J, Wind JJ, et al. Resection of large vestibular schwannomas: facial nerve preservation in the context of surgical approach and patient-assessed outcome. J Neurosurg. 2005;102:643-649.
Barker FG2nd, Carter BS, Ojemann RG, et al. Surgical excision of acoustic neuroma: patient outcome and provider caseload. Laryngoscope. 2003;113:1332-1343.
Becker SS, Jackler RK, Pitts LH. Cerebrospinal fluid leak after acoustic neuroma surgery: a comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol. 2003;24:107-112.
Betchen SA, Walsh J, Post KD. Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg. 2005;102:6-9.
Betchen SA, Walsh J, Post KD. Self-assessed quality of life after acoustic neuroma surgery. J Neurosurg. 2003;99:818-823.
Brackmann DE, Barrs DM. Assessing recovery of facial function following acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1984;92:88-93.
Catalano PJ, Jacobowitz O, Post KD. Prevention of headache after retrosigmoid removal of acoustic tumors. Am J Otol. 1996;17:904-908.
Colletti V, Fiorino F. Middle fossa versus retrosigmoid-transmeatal approach in vestibular schwannoma surgery: a prospective study. Otol Neurotol. 2003;24:927-934.
Day JD, Chen DA, Arriaga M. Translabyrinthine approach for acoustic neuroma. Neurosurgery. 2004;54:391-395.
Hammerschlag PE. Facial reanimation with jump interpositional graft hypoglossal facial anastomosis and hypoglossal facial anastomosis: evolution in management of facial paralysis. Laryngoscope. 1999;109(suppl 90):1-23.
Hasegawa T, Kida Y, Kobayashi T, et al. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005;102:10-16.
Irving RM, Jackler RK, Pitts LH. Hearing preservation in patients undergoing vestibular schwannoma surgery: comparison of middle fossa and retrosigmoid approaches. J Neurosurg. 1998;88:840-845.
Kondziolka D, Lundsford L, McLaughlin M, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426-1433.
Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope. 2000;110:497-508.
Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): facial nerve preservation and restitution of function. Neurosurgery. 1997;40:684-695.
Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11-21.
Sampath P, Holliday MJ, Brem H, et al. Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: etiology and prevention. J Neurosurg. 1997;87:60-66.
Sampath P, Rini D, Long DM. Microanatomical variations in the cerebellopontine angle associated with vestibular schwannomas (acoustic neuromas): a retrospective study of 1006 consecutive cases. J Neurosurg. 2000;92:70-78.
Sennaroglu L, Slattery WH3rd. Petrous anatomy for middle fossa approach. Laryngoscope. 2003;113:332-342.
Strauss C, Bischoff B, Neu M, et al. Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J Neurosurg. 2001;95:771-777.
1 Sandidort E. Observationes anatomico-pathologicae. Lugduni Batavorum. 1777:116-120.
2 House WF, Luetje CM. Acoustic Tumors. Baltimore: University Park Press; 1979.
3 Bell C. The Nervous System of the Human Body, Embracing the Papers Delivered to the Royal Society On the Subject of Nerves. London: Longman, Rees, Orme, Brown, and Green; 1830. 112-114
4 Cushing H. Tumors of the Nervous Acousticus and the Syndrome of the Cerebellopontine Angle. Philadelphia: Saunders; 1917.
5 Ramsden RT. The bloody angle: 100 years of acoustic neuroma surgery. J R Soc Med. 1995;89:464-468.
6 Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope. 2000;110:497-508.
7 Krause F. Zur Freilegung der hinteren Felsenbeinfliche und des Kleinhirns. Beitr Klin Chir. 1903;37:728-764.
8 Dandy WE. An operation for the total removal of cerebellopontine (acoustic) tumors. Surg Gynecol Obstet. 1925;41:129-148.
9 House WF. Transtemporal bone microsurgical removal of acoustic neuromas. Evolution of transtemporal bone removal of acoustic tumors. Arch Otolaryngol. 1964;80:731-742.
10 Block JM, Nathanson M. A review of acoustic neuromas at the Mount Sinai Hospital. J Mt Sinai Hosp N Y. 1963;30:217-227.
11 Olivecrona H. The removal of acoustic neurinomas. J Neurosurg. 1967;26:100-103.
12 House WF. A history of acoustic tumor surgery:1800-1960. In: House WR, Luetje CM, Doyle KJ, editors. Acoustic Tumors: Diagnosis and Management. San Diego, CA: Singular; 1997:1-19.
13 House WF. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. Laryngoscope. 1961;71:1363-1385.
14 Schuknecht HF, editor. Pathology of Vestibular Schwannoma (Acoustic Neuroma). Birmingham, AL: Aesculapius. 1977:193-197.
15 Stangerup SE, Caye-Thomasen P, Tos M, et al. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27:547-552.
16 Nutik SL, Babb MJ. Determinants of tumor size and growth in vestibular schwannomas. J Neurosurg. 2001;94:922-926.
17 Hoistad DL, Melnik G, Mamikoglu B, et al. Update on conservative management of acoustic neuroma. Otol Neurotol. 2001;22:682-685.
18 Charabi S, Tos M, Thomsen J, et al. Vestibular schwannoma growth—long-term results. Acta Otolaryngol Suppl. 2000;543:7-10.
19 Mirz F, Pedersen CB, Fiirgaard B, et al. Incidence and growth pattern of vestibular schwannomas in a Danish county, 1977-98. Acta Otolaryngol Suppl. 2000;543:30-33.
20 Shin YJ, Fraysse B, Cognard C, et al. Effectiveness of conservative management of acoustic neuromas. Am J Otol. 2000;21:857-862.
21 Walsh RM, Bath AP, Bance ML, et al. The role of conservative management of vestibular schwannomas. Clin Otolaryngol Allied Sci. 2000;25:28-39.
22 Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000;21:722-728.
23 Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope. 2000;110:497-508.
24 Fucci MJ, Buchman CA, Brackmann DE, et al. Acoustic tumor growth: implication for treatment choices. Am J Otol. 1999:495-499.
25 Selesnick SH, Johnson G. Radiologic surveillance of acoustic neuromas. Am J Otol. 1998;19:846-849.
26 Charabi S, Thomsen J, Tos M, et al. Acoustic neuroma/vestibular schwannoma growth: past, present, and future. Acta Otolaryngol. 1998;118:327-332.
27 Deen HG, Ebersold MJ, Harner SG, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery. 1996;39:260-264.
28 Wiet RJ, Zappia JJ, Hecht CS, et al. Conservative management of patients with small acoustic tumors. Laryngoscope. 1995;105:795-800.
29 Strasnick B, Glasscock ME3rd, Haynes D, et al. The natural history of untreated acoustic neuromas. Laryngoscope. 1994;104:1115-1119.
30 Nedzelski JM, Schessel DA, Pfleiderer A, et al. Conservative management of acoustic neuromas. Otolaryngol Clin North Am. 1992;25:691-705.
31 Bederson JB, von Ammon K, Wichmann WW, et al. Conservative treatment of patients with acoustic tumors. Neurosurgery. 1991;28:646-650. discussion 650-651
32 Bance M, Ramsden RT. Management of neurofibromatosis type 2. Ear Nose Throat J. 1999;78:91-94. 96
33 Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26:93-97.
34 Baser ME, Makariou EV, Parry DM. Predictors of vestibular schwannoma growth in patients with neurofibromatosis type 2. J Neurosurg. 2002;96:217-222.
35 Mautner VF, Baser ME, Thakkar SD, et al. Vestibular schwannoma growth in patients with neurofibromatosis type 2: a longitudinal study. J Neurosurg. 2002;96:223-228.
36 Slattery WH3rd, Fisher LM, Iqbal Z, et al. Vestibular schwannoma growth rates in neurofibromatosis type 2 natural history consortium subjects. Otol Neurotol. 2004;25:811-817.
37 Charabi S, Klinken L, Tos M, et al. Histopathology and growth pattern of cystic acoustic neuromas. Laryngoscope. 1994;104:1348-1352.
38 Charabi S, Tos M, Borgesen SE, et al. Cystic acoustic neuromas. Results of translabyrinthine surgery. Arch Otolaryngol Head Neck Surg. 1994;120:1333-1338.
39 Jones SEM, Baguley DM, Moffat DA. Are facial nerve outcomes worse following surgery for cystic vestibular schwannoma? Skull Base. 2007;17:281-284.
40 Jacob A, Robinson LLJr, Bortman JS, et al. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope. 2007;117:2087-2092.
41 Khrais T, Romano G, Sanna M. Nerve origin of vestibular schwannoma: a prospective study. J Laryngol Otol. 2008;122:128-131.
42 Ellison D, Love S, Chimelli L, et al. Peripheral nerve sheath neoplasms. In Neuropathology: A Reference Text of CNS pathology, 2nd ed, Edinburgh: Mosby; 2004:695-699.
43 Gruskin P, Carberry JN, Chandrasekhar SS. Pathology of acoustic tumors. In: House WR, C.M. L, Doyle KJ, editors. Acoustic Tumors Diagnosis and Management. San Diego, CA: Singular; 1997:27-80.
44 Doherty JK, Ongkeko W, Crawley B, et al. ErbB and Nrg: potential molecular targets for vestibular schwannoma pharmacotherapy. Otol Neurotol. 2007;29:50-57.
45 Chandrasekhar SS, Brackmann DE, Devgan KK. Utility of auditory brainstem audiometry in diagnosis of acoustic neuromas. Am J Otol. 1995;16:63-67.
46 Selesnick SH, Jackler RK, Pitts LH. The changing presentation of acoustic tumors in the MRI era. Laryngoscope. 1993;103:431-435.
47 Tucci DL. Audiologic testing. In: House WR, Luetje CM, Doyle KJ, editors. Acoustic Tumors: Diagnosis and Management. San Diego, CA: Singular; 1997:93-103.
48 Welling DB, Glasscock ME, Woods CI, et al. Acoustic neuroma: a cost-effective approach. Arch Otolaryngol. 1990;103:364-370.
49 Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope. 2004;114:1686-1692.
50 Moffat DA, Ballagh RH. Rare tumours of the cerebellopontine angle. Clin Oncol (R Coll Radiol). 1995;7:28-41.
51 Bonneville F, Savatovsky J, Chiras J. Imaging of cerebellopontine angle lesions: an update. Part 1: enhancing extra-axial lesions. Eur Radiol. 2007;17:2472-2482.
52 Gomez-Brouchet A, Delisle MB, Cognard C. Vestibular schwannomas: correlations between magnetic resonance imaging and histopathologic appearance. Otol Neurotol. 2001;22:79-86.
53 Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115:450-454.
54 Martuza RL, Parker SW, Nadol JBJr, et al. Diagnosis of cerebellopontine angle tumors. Clin Neurosurg. 1985;32:177-213.
55 Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55-66.
56 Buchman CA, Chen DA, Flannagan P, et al. The learning curve for acoustic tumor surgery. Laryngoscope. 1996;106:1406-1411.
57 Elsmore AJ, Mendoza ND. The operative learning curve for vestibular schwannoma excision via the retrosigmoid approach. Br J Neurosurg. 2002;16:448-455.
58 Staeker H, Nadol JB, Ojeman R, et al. Hearing preservation in acoustic neuroma surgery: middle fossa versus retrosigmoid approach. Am J Otol. 2000;21:399-404.
59 Slattery WH3rd, Brackmann DE, Hitselberger W. Middle fossa approach for hearing preservation with acoustic neuromas. [published erratum appears in Am J Otol 1997:18:796]. Am J Otol. 1997;18;:596-601.
60 Arts HA, Telian SA, El-Kashlan H, et al. Hearing preservation and facial nerve outcomes in vestibular schwannoma surgery: results using middle cranial fossa approach. Otol Neurotol. 2006;27:234-241.
61 Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105:527-535.
62 Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): facial nerve preservation and restitution of function. Neurosurgery. 1997;40:684-695.
63 Barker FG2nd, Carter BS, Ojemann RG, et al. Surgical excision of acoustic neuroma: patient outcome and provider caseload. Laryngoscope. 2003;113:1332-1343.
64 Moffat DA, Hardy DG, Grey PL, et al. The operative learning curve and its effect on facial nerve outcome in vestibular schwannoma surgery. Am J Otol. 1996;17:643-647.
65 Welling DB, Slater PW, Thomas RD, et al. The learning curve in vestibular schwannoma surgery. Am J Otol. 1999;20:644-648.
66 Ojeman RG, Martuza RL. Acoustic neuroma. In: Youman JR, editor. Neurological Surgery. Philadelphia: Saunders; 1990:3316-3350.
67 Sampath P, Rini D, Long DM. Microanatomical variations in the cerebellopontine angle associated with vestibular schwannomas (acoustic neuromas): a retrospective study of 1006 consecutive cases. J Neurosurg. 2000;92:70-78.
68 Catalano PJ, Jacobowitz O, Post KD. Prevention of headache after retrosigmoid removal of acoustic tumors. Am J Otol. 1996;17:904-908.
69 Ojeman RG, Martuza RL. Suboccipital transmeatal approach to vestibular schwannoma. In: Schmidek HH, Roberts DW, editors. Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results. 5th ed. Philadelphia: Saunders; 2006:920-931.
70 Tatagiba M, Samii M, Matthies C, et al. The significance for postoperative hearing of preserving the labyrinth in acoustic neurinoma surgery. J Neurosurg. 1992;77:677-684.
71 Glasscock ME3rd, Bohrer PS. Suboccipital approach. In: House WR, Luetje CM, Doyle KJ, editors. Acoustic Tumors: Diagnosis and Management. San Diego, CA: Singular; 1997:189-203.
72 Brackmann DE, Owens RM, Friedman RA, et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol. 2000;21:417-424.
73 Briggs RJ, Fabinyi G, Kaye AH. Current management of acoustic neuromas: review of surgical approaches and outcomes. J Clin Neurosci. 2000;7:521-526.
74 Dornhoffer JL, Helms J, Hoehman DH. Hearing preservation in acoustic tumor surgery: results and prognostic factors. Laryngoscope. 1995;105:184-187.
75 Irving RM, Jackler RK, Pitts LH. Hearing preservation in patients undergoing vestibular schwannoma surgery: comparison of middle fossa and retrosigmoid approaches. J Neurosurg. 1998;88:840-845.
76 Yates PD, Jackler RK, Satar B, et al. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas? Otol Neurotol. 2003;24:460-464.
77 Garcia-Ibanez E, Garcia-Ibanez JL. Middle fossa vestibular neurectomy: a report of 373 cases. Arch Otolaryngol. 1980;88:486-490.
78 Bennet M, Haynes DS. Surgical approaches and complications in the removal of vestibular schwannomas. Otolaryngol Clin North Am. 2007;40:589-609.
79 Sennaroglu L, Slattery WH3rd. Petrous anatomy for middle fossa approach. Laryngoscope. 2003;113:332-342.
80 Syms CA3rd, Brackmann DE. Middle fossa approach. In: House WR, Luetje CM, Doyle KJ, editors. Acoustic Tumors: Diagnosis and Management. San Diego, CA: Singular; 1997:177-188.
81 House WF. Translabyrinthine Approach. In: House WR, Luetje CM, Doyle KJ, editors. Acoustic Tumors Diagnosis and Management. San Diego, CA: Singular; 1997:151-170.
82 Poulsgaard L. Translabyrinthine approach to vestibular schwannomas. In: Schmidek HH, Roberts DW, editors. Schmidek and Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results. 5th ed. Philadelphia: Saunders; 2006:932-941.
83 Day JD, Chen DA, Arriaga M. Translabyrinthine approach for acoustic neuroma. Neurosurgery. 2004;54:391-395.
84 Saleh EA, Aristegui M, Taibah AK, et al. Management of the high jugular bulb in the translabyrinthine approach. Otolaryngol Head Neck Surg. 1994;110:397-399.
85 Moffat DA, Quaranta N, Chang P. Management of the high jugular bulb in translabyrinthine surgery. Laryngoscope. 2003;113:580-582.
86 Roche PH, Moriyama T, Thomassin JM, et al. High jugular bulb in the translabyrinthine approach to the cerebellopontine angle: anatomical considerations and surgical management. Acta Neurochir (Wien). 2006;148:415:-420.
87 Friedman RA, Brackmann DE, van Loveren HR, et al. Management of the contracted mastoid in the translabyrinthine removal of acoustic neuroma. Arch Otolaryngol Head Neck Surg. 1997;123:342-344.
88 Hirsch A, Norén G, Anderson H. Audiologic findings after stereotactic radiosurgery in nine cases of acoustic neurinomas. Acta Otolaryngol. 1979;99:155-160.
89 Kondziolka D, Lundsford L, McLaughlin M, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426-1433.
90 Beegle RD, Friedman WA, Bova FJ. Effect of treatment plan quality on outcomes after radiosurgery for vestibular schwannoma. J Neurosurg. 2007;107:913-916.
91 Foote KD, Friedman WA. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440-449.
92 Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68:845-851.
93 Iwai Y, Yamanaka K, Shiotani M, et al. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery. 2003;53:282-287.
94 Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68:845-851.
95 Hasegawa T, Kida Y, Kobayashi T, et al. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005;102:10-16.
96 Bush DA, McAllister CJ, Loredo LN, et al. Fractionated proton beam radiotherapy for acoustic neuroma. Neurosurgery. 2002;50:270-273.
97 Chang SD, Gibbs IC, Sakamoto GT, et al. Staged stereotactic irradiation for acoustic neuroma. Neurosurgery. 2005;56:1254-1261.
98 Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265-1278.
99 Ruth HR, Luetje CM, Whittaker CK. Acoustic tumors: preoperative measurement and correlation with postoperative facial nerve function. Otolaryngol Head Neck Surg. 1985;93:160-163.
100 Grey PL, Moffat DA, Palmer CR, et al. Factors which influence the facial nerve outcome in vestibular schwannoma surgery. Clin Otolaryngol Allied Sci. 1996;21:409-413.
101 Arriaga MA, Luxford WM, Atkins JSJr, et al. Predicting long-term facial nerve outcome after acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1993;108:220-224.
102 Sekhar LN, Gormley WB, Wright DC. The best treatment for vestibular schwannoma (acoustic neuroma): microsurgery or radiosurgery? Am J Otol. 1996;17:676-682.
103 Deguine O, Maillard A, Bonafe A, et al. Pre-operative and per-operative factors conditioning long-term facial nerve function in vestibular schwannoma surgery through translabyrinthine approach. J Laryngol Otol. 1998;112:441-445.
104 Betchen SA, Walsh J, Post KD. Self-assessed quality of life after acoustic neuroma surgery. J Neurosurg. 2003;99:818-823.
105 Sampath P, Holliday MJ, Brem H, et al. Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: etiology and prevention. J Neurosurg. 1997;87:60-66.
106 Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11-21.
107 Anderson DE, Leonetti J, Wind JJ, et al. Resection of large vestibular schwannomas: facial nerve preservation in the context of surgical approach and patient-assessed outcome. J Neurosurg. 2005;102:643-649.
108 Colletti V, Fiorino F. Middle fossa versus retrosigmoid-transmeatal approach in vestibular schwannoma surgery: a prospective study. Otol Neurotol. 2003;24:927-934.
109 Gjuric M, Wigand ME, Wolf SR. Enlarged middle fossa vestibular schwannoma surgery: experience with 735 cases. Otol Neurotol. 2001;22:223-230.
110 Kumon Y, Sakaki S, Kohno K, et al. Selection of surgical approaches for small acoustic neurinomas. Surg Neurol. 2000;53:52-59.
111 Satar B, Jackler RK, Oghalai J, et al. Risk-benefit analysis of using the middle fossa approach for acoustic neuromas with >10 mm cerebellopontine angle component. Laryngoscope. 2002;112:1500-1506.
112 Stripf T, Braun K, Gouveris H, et al. Influence of different approaches to the cerebellopontine angle on the function of the intermediate nerve. J Neurosurg. 2007;107:927-931.
113 Flickinger JC, Lunsford LD, Linskey ME, et al. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993;27:91-98.
114 Friedman WA, Bradshaw P, Myers A, et al. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg. 2006;105:657-661.
115 Beegle RD, Friedman WA, Bova FJ. Effect of treatment plan quality on outcomes after radiosurgery for vestibular schwannoma. J Neurosurg. 2007;107:913-916.
116 Flickinger JC, Kondziolka D, Niranjan A, et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60:225-230.
117 Meyer TA, Canty PA, Wilkinson EP, et al. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol. 2006;27:380-392.
118 Post KD, Eisenberg MB, Catalano PJ. Hearing preservation in vestibular schwannoma surgery: what factors influence outcome? J Neurosurg. 1995;83:191-196.
119 Koos WT, Day JD, Matula C, et al. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. 1998;88:506-512.
120 Nadol JBJr, Chiong CM, Ojemann RG, et al. Preservation of hearing and facial nerve function in resection of acoustic neuroma. Laryngoscope. 1992;102:1153-1158.
121 Cohen NL, Lewis WS, Ransohoff J. Hearing preservation in cerebellopontine angle tumor surgery: the NYU experience 1974-1991. Am J Otol. 1993;14:423-433.
122 Betchen SA, Walsh J, Post KD. Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg. 2005;102:6-9.
123 Paek SH, Chung HT, Jeong SS, et al. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer. 2005;104:580-590.
124 Leonetti JP, Brackmann DE, Prass RL. Improved preservation of facial nerve function in the infratemporal approach to the skull base. Otolaryngol Head Neck Surg. 1989;101:74-78.
125 Lye RH, Dutton J, Ramsden RT, et al. Facial nerve preservation during surgery for removal of acoustic nerve tumors. J Neurosurg. 1982;57:739-746.
126 Strauss C, Romstock J, Fahlbusch R, et al. Preservation of facial nerve function after postoperative vasoactive treatment in vestibular schwannoma surgery. Neurosurgery. 2006;59:577-584.
127 Delgado TE, Bucheit WA, Rosenholtz HR, et al. Intraoperative monitoring of facial muscle evoked responses obtained by intracranial stimulation of the facial nerve: a more accurate technique for facial nerve dissection. Neurosurgery. 1979;4:418-421.
128 Arriaga MA, Brackmann DE. Facial nerve repair techniques in cerebellopontine angle tumor surgery. Am J Otol. 1992;13:356-359.
129 Brackmann DE, Barrs DM. Assessing recovery of facial function following acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1984;92:88-93.
130 Brackmann DE, Hitselberger WE, Robinson JV. Facial nerve repair in cerebellopontine angle surgery. Ann Otol Rhinol Laryngol. 1978;87:772-777.
131 May M, Sobol SM, Mester SJ. Hypoglossal-facial nerve interpositional-jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg. 1991;104:818-825.
132 Hammerschlag PE. Facial reanimation with jump interpositional graft hypoglossal facial anastomosis and hypoglossal facial anastomosis: evolution in management of facial paralysis. Laryngoscope. 1999;109(suppl 90):1-23.
133 Atlas MD, Lowinger DS. A new technique for hypoglossal-facial nerve repair. Laryngoscope. 1997;107:984-991.
134 Darrouzet V, Guerin J, Bebear JP. New technique of side-to-end hypoglossal-facial nerve attachment with translocation of the infratemporal facial nerve. J Neurosurg. 1999;90:27-34.
135 Ferraresi S, Garozzo D, Migliorini V, et al. End-to-side intrapetrous hypoglossal-facial anastomosis for reanimation of the face. Technical note. J Neurosurg. 2006;104:457-460.
136 O’Connell JE, Robin PE. Eyelid gold weights in the management of facial palsy. J Laryngol Otol. 1991;105:471-474.
137 Snyder MC, Johnson PJ, Moore GF, et al. Early versus late gold weight implantation for rehabilitation of the paralyzed eyelid. Laryngoscope. 2001;111:2109-2113.
138 Gupta AK, Jain S. Temporalis muscle sling revisited: a technique to restore ocular sphincter function. Ann Plast Surg. 1994;33:496-499.
139 Roberson J, Senne A, Brackmann D, et al. Direct cochlear nerve action potentials as an aid to hearing preservation in middle fossa acoustic neuroma resection. Am J Otol. 1996;17:653-657.
140 Danner C, Mastrodimos B, Cueva RA. A comparison of direct eighth nerve monitoring and auditory brainstem response in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2004;25:826-832.
141 Strauss C, Bischoff B, Neu M, et al. Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J Neurosurg. 2001;95:771-777.
142 Becker SS, Jackler RK, Pitts LH. Cerebrospinal fluid leak after acoustic neuroma surgery: a comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol. 2003;24:107-112.
143 Fishman AJ, Hoffman RA, Roland JTJr, et al. Cerebrospinal fluid drainage in the management of CSF leak following acoustic neuroma surgery. Laryngoscope. 1996;106:1002-1004.
144 Fishman AJ, Marrinan MS, Golfinos JG, et al. Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Laryngoscope. 2004;114:501-505.
145 Friedman RA, Cullen RD, Ulis J, et al. Management of cerebrospinal fluid leaks after acoustic tumor removal. Neurosurgery. 2007;61(suppl 3):35-39.
146 Hoffman RA. Cerebrospinal fluid leak following acoustic neuroma removal. Laryngoscope. 1994;104:40-58.
147 Magliulo G, Sepe C, Varacalli S, et al. Cerebrospinal fluid leak management following cerebellopontine angle surgery. J Otolaryngol. 1998;27:258-262.
148 Bryce GE, Nedzelski JM, Rowed DW, et al. Cerebrospinal fluid leaks and meningitis in acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1991;104:81-87.
149 Glasscock ME3rd, Kveton JF, Jackson CG, et al. A systematic approach to the surgical management of acoustic neuroma. Laryngoscope. 1986;96:1088-1094.
150 Harner SG, Beatty CW, Ebersold MJ. Retrosigmoid removal of acoustic neuroma: experience 1978-1988. Otolaryngol Head Neck Surg. 1990;103:40-45.
151 Harner SG, Laws ERJr. Translabyrinthine repair for cerebrospinal fluid otorhinorrhea. J Neurosurg. 1982;57:258-261.
152 Catalano PJ, Post K, Sen C, et al. Prevention of cerebrospinal fluid rhinorrhea in neurotologic surgery. Am J Otol. 2000;21:265-269.
153 Shea MC, Robertson JT. Acoustic neuroma removal: a comparative study of translabyrinthine and suboccipital approaches. Am J Otol. 1979;1:94-99.
154 Wiet RJ, Kazan RP, Raslan W, et al. Complications in the approach to acoustic tumor surgery. Ann Otol Rhinol Laryngol. 1986;95:28-31.
155 Brennan JW, Rowed DW, Nedzelski JM, et al. Cerebrospinal fluid leak after acoustic neuroma surgery: influence of tumor size and surgical approach on incidence and response to treatment. J Neurosurg. 2001;94:217-223.
156 Rhoton ALJ. Microsurgical anatomy of the cerebellopontine angle. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:1063-1084.
157 Sade B, Mohr G, Dufour JJ. Vascular complications of vestibular schwannoma surgery: a comparison of the suboccipital retrosigmoid and translabyrinthine approaches. J Neurosurg. 2006;105:200-204.
158 de Bruijn SF, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry. 2001;70:105-108.
159 Fukuda M, Oishi M, Kawaguchi T, et al. Etiopathological factors related to hydrocephalus associated with vestibular schwannoma. Neurosurgery. 2007;61:1186-1192.
160 Sawamura Y, Shirato H, Sakamoto T, et al. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J Neurosurg. 2003;99:685-692.
161 Parving A, Tos M, Thomsen J, et al. Some aspects of life quality after surgery for acoustic neuroma. Arch Otolaryngol Head Neck Surg. 1992;118:1061-1064.
162 Andersson G, Ekvall L, Kinnefors A, et al. Evaluation of quality of life and symptoms after translabyrinthine acoustic neuroma surgery. Am J Otol. 1997;18:421-426.
163 Martin HC, Sethi J, Lang D, et al. Patient-assessed outcomes after excision of acoustic neuroma: postoperative symptoms and quality of life. J Neurosurg. 2001;94:211-216.
164 Koperer H, Deinsberger W, Jodicke A, et al. Postoperative headache after the lateral suboccipital approach: craniotomy versus craniectomy. Minim Invasive Neurosurg. 1999;42:175-178.
165 Harner SG, Beatty CW, Ebersold MJ. Impact of cranioplasty on headache after acoustic neuroma removal. Neurosurgery. 1995;36:1097-1099.
166 Wazen JJ, Sisti M, Lam SM. Cranioplasty in acoustic neuroma surgery. Laryngoscope. 2000;110:1294-1297.
167 Jackson CG, McGrew BM, Forest JA, et al. Comparison of postoperative headache after retrosigmoid approach: vestibular nerve section versus vestibular schwannoma resection. Am J Otol. 2000;21:412-416.