66 Abdominal Aortic Aneurysm
• The word aneurysm is derived from a Greek word meaning “widening.” It has further been defined as permanent and irreversible localized dilation of a vessel.1
• Abdominal aortic aneurysm (AAA) refers to pathologic widening of the aorta, and AAAs continue to be a significant cause of morbidity and mortality; early diagnosis is the key to reducing mortality.
• The risk for rupture relates linearly to the maximum cross-sectional diameter of the aneurysm, and 4.5 to 5 cm is the generally accepted size when intervention should occur.
• Elective repair of nonruptured AAAs lowers mortality significantly and warrants a high level of vigilance by health care providers to diagnose this condition in its potentially curative stages.
• Many risk factors promote the development of AAAs, but the most important are smoking, male gender, and family history.
• The two current operative approaches for repair of AAAs are open surgery and endovascular repair; the better approach for individual patients depends on several clinical variables and specific patient characteristics.
Epidemiology
Finding a unifying definition for abdominal aortic aneurysm (AAA) that encompasses the various classification schemes related to their size, shape, and location is an arduous task. Simplistically, most AAAs occur infrarenally and are defined as an aortic diameter greater than 3 cm, mostly measured in the anteroposterior dimension.1–3 Although normal aortic diameter differs between the sexes, it is not substantial enough to warrant changing the size limit of 3 cm that is currently used to characterize a small AAA.3 Finally, it is important to note that dilation of an aneurysm encompasses the three layers of the vascular wall; otherwise, the dilation is a pseudoaneurysm.1 For the commonly accepted descriptions and locations of most aneurysms, please see Box 66.1.
Box 66.1 Aneurysm Classification—Description versus Location
Description
Fusiform aneurysms are circumferential with respect to the artery.
Saccular aneurysms affect only part of the circumference.
Inflammatory aneurysms demonstrate extensive perianeurysmal and retroperitoneal fibrosis, as well as dense adhesions to neighboring organs, which frequently causes ureterohydronephrosis.
Location
Juxtarenal aneurysms arise distal but in very close proximity to the renal arteries.
Pararenal aneurysms originate from one or both renal arteries.
Suprarenal aneurysms affect the superior mesenteric and celiac arteries.
Type IV thoracoabdominal aneurysms are suprarenal aneurysms that extend upward to the crus of the diaphragm.
At present, AAA is the thirteenth leading cause of death in the United States. Once an AAA ruptures, death is a certainty in more than 65% of cases,1 with some studies reporting mortality rates as high as 90%, thus highlighting the necessity for rapid surgical repair.4 Low rates of postmortem examination and the high likelihood that some of these deaths have been erroneously attributed to cardiac causes severely hamper gathering true estimates of the prevalence of and mortality associated with ruptured AAAs.2 Elective repair of an AAA lowers the mortality to less than 5%, so there is an obvious benefit to diagnosing and treating these aneurysms before they rupture.4,5 Given that this disease affects 4% to 7% of adults 65 years or older, the onus increasingly falls on physicians to accomplish this task as the population ages.4
Pathophysiology
There is abundant research on identification of risk factors that potentiate the development and growth of AAAs (Box 66.2). Besides male gender, age, and hypertension, every study on the subject of AAAs singles out long-term tobacco smoking as the most important risk factor.2 AAAs develop in tobacco smokers more than four times more frequently than in lifelong nonsmokers.1 Lederle et al.6 compared the relative risk for different diseases in long-term cigarette smokers and found that risk for the development of AAAs is fivefold higher than that for cerebrovascular disease and threefold higher than that for coronary artery disease.1 Smoking not only raises the risk for the development of AAAs but also increases the annual growth rate of existent AAAs, with some studies reporting a rate of 2.83 mm/yr in smokers versus 2.53 mm/yr in nonsmokers.1,7 However, the exact means by which smoking enhances aneurysm formation remains a mystery.
In addition to tobacco smoking, there are other risk factors. Atherosclerosis is so highly associated with the development and expansion of AAAs that the American Heart Association (AHA) guidelines on the treatment of AAAs mandate that blood pressure and fasting serum lipid values be strictly monitored and controlled in patients with AAAs.3 The AHA also recommends that smoking cessation interventions be provided to patients with a personal or family history of aneurysms.3 Despite the importance of atherosclerosis, additional factors are probably present because not every patient with atherosclerosis has an AAA.1
Many authorities cite a possible causal link between the development of AAAs and chronic obstructive pulmonary disease (COPD). Researchers blame tobacco smoking–induced elastin degradation for this proposed association.3 A review of the literature notes that patients with COPD undergoing long-term treatment with corticosteroids have a much higher AAA expansion rate than do COPD patients not taking steroids.3 Hence, steroid use and coexisting disease are more likely to be responsible for the high prevalence of AAAs in patients with COPD.3 Finally, from a genetic standpoint, several screening studies suggest that male first-degree relatives of people with COPD are most at risk. Female first-degree relatives appear to be at similar risk, but the data are less certain.3 Familial aneurysms differ from nonfamilial aneurysms mainly in that they may develop at an earlier age.3 Existing genetic polymorphisms probably account for the familial clustering of AAAs, but this clustering could also result from exposure to common environmental factors, such as tobacco smoke.1
Histologically, AAAs develop as a result of a complex interplay of immunologically mediated proteases and antiproteases that cause destruction of elastin and collagen in the arterial wall. Recent research has focused on the role of matrix metalloproteinases (MMPs) in the degradation of elastin and collagen, the main proteins involved in AAA growth and rupture, respectively. In early aneurysm formation, elastic fibers are lost, fragmented, or attenuated, which contributes to development of the actual wall of the aneurysm.1,8 Once the concentration of medial elastin is severely decreased, the collagen-rich adventitial tissue constitutes the artery’s last mode of resistance, and once completely degraded, rupture inevitably ensues.1,8 The natural history of all arterial aneurysms encompasses gradual or sporadic growth in their diameter (or both) and accretion of mural thrombus.3 These features contribute to rupture, thromboembolic ischemic events, and compression or erosion of adjacent structures, three of the most common complications of AAAs.3
Presenting Signs and Symptoms
AAA continues to be such a difficult diagnosis because of vague and variable symptoms (Table 66.1), which carry large differential diagnoses. Vague symptoms such as back or abdominal pain often herald this diagnosis in its early (virtually asymptomatic) and potentially curative stages. The symptoms may masquerade as those of renal colic, diverticulitis, or gastrointestinal hemorrhage, thereby leading to a fatal misdiagnosis.3 Commonly, patients with nonruptured AAAs are seen only after suffering complications such as various thromboembolic events or after thorough evaluation for chronic vague abdominal and back pain.3
Table 66.1 Variations in Clinical Findings in Patients with Abdominal Aortic Aneurysms
| CLASSIC TRIAD | MOST COMMON FINDINGS | INFLAMMATORY ANEURYSM CLINICAL FINDINGS |
|---|---|---|
| Abdominal pain | Younger patients tend to be symptomatic earlier | Triad of chronic abdominal pain, weight loss, and elevated erythrocyte sedimentation rate |
| Pulsatile abdominal mass | Steady, aggravating back pain and hypogastric pain are the most common symptoms | These patients usually have concurrent peripheral vascular disease and coronary artery disease |
| Shock | Pain is usually unaffected by position or movement | These patients are more symptomatic at initial evaluation |
| Only 50% of patients have this triad | Pain can radiate to the scrotum, groin, buttocks, or legs | These patients tend to have more of a retroperitoneal inflammatory reaction |
Nonruptured aneurysms are sometimes found incidentally. An astute clinician can find them on physical examination. Palpation of AAAs is safe and has not been reported to precipitate rupture.3 Abdominal palpation is moderately sensitive for the detection of AAAs that meet the size criteria necessary for surgical intervention, but even large aneurysms may be difficult to palpate, especially in obese patients. However, in the case of AAAs that are small or have already ruptured, physical examination alone is not nearly precise enough.3
When aneurysms rupture, the extent of shock varies according to the location and size of the rupture and the amount of delay before the patient is examined.1 AAAs that rupture anterolaterally dramatically violate the peritoneal cavity and are most often associated with sudden death. Patients with ruptured AAAs who reach a physician tend to have ruptures involving the posterolateral wall in the retroperitoneal space. Some of these patients are fortunate enough that their bodies temporarily tamponade a small tear so that they suffer relatively small initial blood loss.1 A few of these patients with contained ruptures may even exhibit flank ecchymosis, a physical finding known as the Grey Turner sign.3 However, AAAs are not contained for long, and even these patients suffer the deleterious effects of a larger rupture if they fail to seek medical attention early or if the physician fails to recognize the signs of impending rupture.1
Differential Diagnosis and Medical Decision Making
As a result of the variable manifestations of AAAs just mentioned, the differential diagnosis is broad. Table 66.2 lists the differential diagnostic entities for AAA, along with priority actions for each. The correct diagnosis of AAA, regardless of whether it is ruptured, hinges on maintaining a high level of suspicion and pursuing an appropriate work-up.
Table 66.2 Differential Diagnosis of Abdominal Aortic Aneurysm and Priority Actions
| ALTERNATIVE DIAGNOSIS | PRIORITY ACTIONS AND COMMENTS |
|---|---|
| Myocardial infarction | Pursue a thorough cardiac work-up, and if the diagnosis is in doubt, be sure to consult both cardiothoracic surgery and cardiology specialists before initiating thrombolytic or anticoagulant therapy. |
| Ischemic bowel | Assuming that the patient has no clinical signs of rupture and is hemodynamically stable, obtain a CT scan to allow a definitive diagnosis. |
| Acute appendicitis | If patient is hemodynamically stable, obtain both an immediate surgical consultation and a CT scan. |
| Gastrointestinal bleeding | This diagnosis is not usually subtle and is fairly easy to make at the bedside with a rectal examination or nasogastric lavage; be wary of an aortoenteric fistula. |
| Pancreatitis | Though a relatively common diagnosis and one that usually requires medical treatment only, pancreatitis is still a life-threatening condition. Be sure to obtain a serum lipase measurement to screen for this diagnosis. |
| Bowel obstruction | If bowel obstruction is a concern, an acute abdominal radiographic series will aid in quick diagnosis. |
| Peptic ulcer disease, perforated ulcer | Upright bedside chest radiography to evaluate for free air should be a routine part of the work-up for an AAA to quickly rule out or rule in peptic ulcer disease and perforated ulcer. |
| Cholelithiasis | On the basis of the patient’s clinical symptoms, it may be prudent to order a hepatic function test at initial evaluation. |
| Diverticulitis | If the abdominal examination raises enough concern for diverticulitis, immediate surgical consultation is mandatory; if the patient is hemodynamically stable enough for CT, the diagnosis is made easily. |
| Gastritis | Though part of the differential diagnosis for AAA, gastritis is more of a diagnosis of exclusion after life-threatening possibilities have been evaluated or treated. |
| Cauda equine, epidural abscess, vertebral osteomyelitis | Consider obtaining an erythrocyte sedimentation rate measurement, magnetic resonance imaging, and neurosurgical consultation. |
| Urinary tract infection (females), pyelonephritis, nephrolithiasis | Urinalysis and CT are useful in differentiating these possibilities. |
| Musculoskeletal pain | Often a diagnosis of exclusion. |
AAA, abdominal aortic aneurysm; CT, computed tomography.
Before the 1970s, standard plain radiographs were the only means by which to monitor the expansion rate of aneurysms. However, plain films are useful for this purpose only when the aneurysm has mural calcifications, which can be easily seen on radiographs. Additionally, the film may show obscuration of the psoas margin by a soft tissue mass and, possibly, extension of mural calcification into a periaortic soft tissue mass, thus hinting at a possible ruptured aneurysm.3 It is not the current standard of care to use plain radiographs for surveillance of AAAs, but quite a few such lesions are initially discovered as incidental findings on plain abdominal films obtained for other purposes.3
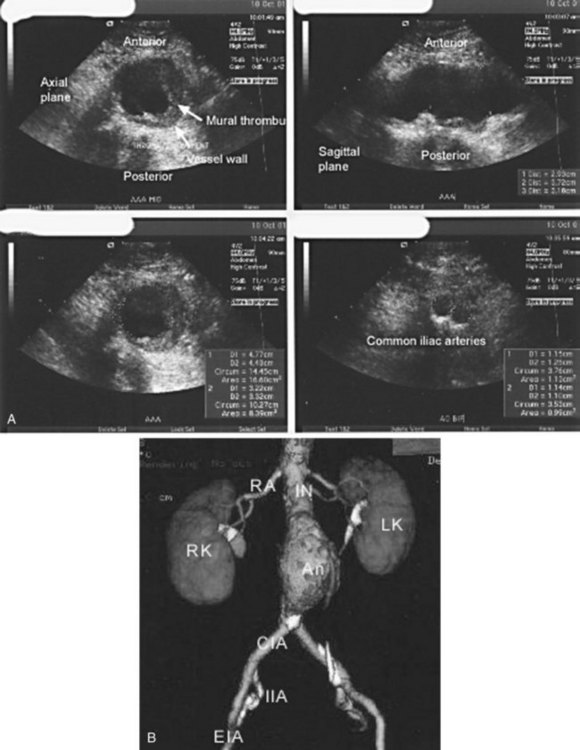
Ultrasonography is an excellent choice for diagnosing AAAs for several reasons (Fig. 66.1, A). First, it is an easy, inexpensive, and accurate diagnostic modality that can evaluate the aorta in the transverse, longitudinal, and anteroposterior dimensions. For these reasons, it is ideal for initial evaluation, surveillance, and population screening with a sensitivity ranging from 92% to 99% and a diagnostic specificity of nearly 100%.3 In addition, ultrasonography provides the emergency physician with a rapid, accurate mode of bedside assessment of a hypotensive patient with a suspected AAA who is too unstable to leave the emergency department (ED) for computed tomography (CT), magnetic resonance imaging (MRI), or magnetic resonance angiography (MRA). However, ultrasonography does have its limitations. Despite its efficacy in ascertaining the size of infrarenal aortic aneurysms, it is unreliable for imaging pararenal, juxtarenal, and suprarenal aneurysms and for imaging the common and internal iliac arteries for aneurysms.3 Spiral CT scans of the abdomen and pelvis with three-dimensional reconstruction are far superior to ultrasonography for this purpose and are most physicians’ first choice for diagnostic purposes (Fig. 66.1, B).3 Before the advent of CT, transcatheter arteriography was the “gold standard” for the preoperative assessment of AAAs despite its inability to determine the exact size of an aneurysm in the presence of a mural thrombus.3 CT has several advantages over this technique; it is cheaper, less invasive, carries a lower radiation dose, and provides information about the aorta and surrounding structures simultaneously. MRI and MRA can provide the same information as CT and can do so without the use of nephrotoxic agents. MRI and MRA are viable, albeit more costly and time-consuming options for patients with contraindications to iodinated contrast dye who are stable enough to leave the ED for an extended period.
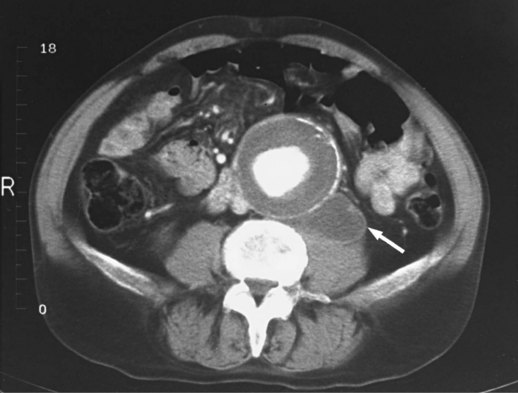
Serial CT scans can be used preoperatively to give the vascular team charged with repairing the AAA several pieces of important information, especially if endovascular repair is being considered. CT can adequately visualize the proximal neck (the transition between the normal and aneurysmal aorta) and map out any dangerous venous anomalies that would make access difficult.1 CT can also measure the thickness of a mural thrombus and display the presence of blood within a thrombus.1 Blood within the thrombus is known as the crescent sign and has been highly touted by some as a reliable sign of impending rupture.1,3,9 Another important marker of aneurysm rupture is extravasation of contrast material. Finally, CT can demonstrate a contained rupture by showing clear evidence of draping of the posterior aspect of the aorta over the adjacent vertebral body; sometimes concomitant vertebral body erosion may be seen (Fig. 66.2).9
Currently, many researchers are actively investigating markers of rupture other than size. Much research revolves around the use of MMPs. These zinc- and calcium-dependent enzymes are produced by smooth muscle and inflammatory cells, and several of these proteinases may participate in AAA formation. McMillan and Pearce10 found that the amount of circulating MMP-9 is significantly higher in patients with AAAs. Lindholt et al.11 then noted an impressive association with the size and expansion rate of these aneurysms, which prompted many to theorize that serum levels of MMP may soon become a standard part of physicians’ diagnostic arsenal.1
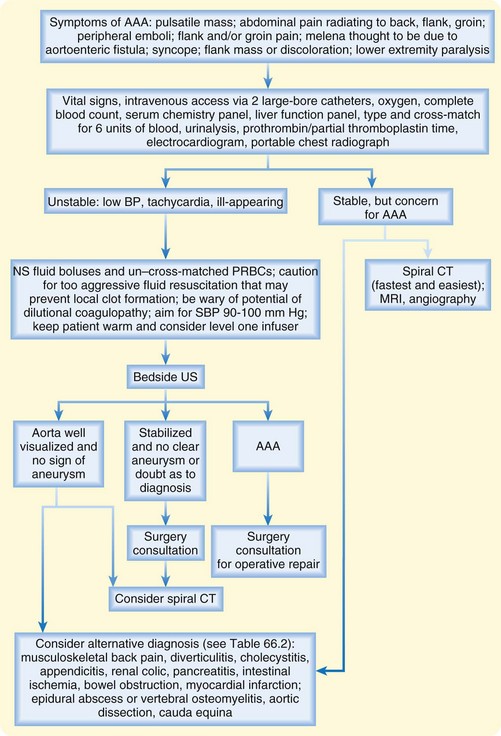
An approach to the diagnosis and management of AAAs is shown in Figure 66.3.
Tips and Tricks
At baseline, women have slightly smaller normal aortic diameters than men do (1.9 versus 2.3 cm); however, this difference in normal aortic diameter between the sexes is not substantial enough to warrant changing the upper limit of 3 cm that is used to characterize a small abdominal aortic aneurysm (AAA).
Patients who undergo emergency surgery for AAAs and are found to have intact, symptomatic aneurysms still have a mortality of 20% to 25% versus 5% for those undergoing elective repair.
In stable patients with abdominal or back pain in whom AAA is high on the differential diagnosis, the emergency physician should choose the type of computed tomography (CT) scan that allows diagnosis of the most likely cause; rapid abdominal CT without oral contrast enhancement may be most beneficial.
Treatment
Any patient in whom a ruptured AAA or a symptomatic intact AAA is diagnosed needs emergency surgical intervention. Volume resuscitation and cross-matching of large amounts of blood should be undertaken immediately (see Fig. 66.3). Vascular surgery consultation should be sought as soon as the diagnosis is made.
The single most compelling reason to repair AAAs is to prevent fatal rupture. Besides rupture, other rare complications also mandate emergency repair, such as distal embolization and fistulous connections between the aorta and adjacent structures.2–4 Once an AAA is identified, the obvious next step is to identify its size via ultrasonography, CT, MRI, or MRA to discern whether immediate intervention or periodic surveillance is warranted. One prospective but nonrandomized study demonstrated that observation alone is safe until an aneurysm undergoes a growth spurt or attains a threshold diameter of 5.0 cm.3,12 Aneurysms with a diameter of this size or larger weaken the aortic wall, which is then at higher risk for rupture.3 Estimates place the risk for rupture at 1% to 3% per year for AAAs measuring up to 5 cm, 11% per year for 5- to 7-cm aneurysms, and nearly 20% per year for aneurysms larger than 7 cm.9 Several studies have confirmed the linear association between AAA diameter and annual expansion rate. An observed rate of growth that surpasses these estimates is indicative of a “growth spurt” that may necessitate early elective aneurysm repair. Katzen et al.4 defined this “growth spurt” as an increase of 1 cm/yr. In addition, if the patient is a woman with smaller native vessels, one must remember that the relative size representing aneurysmal disease may be less than the conventional range of 5 to 5.5 cm.3
Prospective randomized trials comparing early intervention with expectant observation for infrarenal AAAs measuring 4.0 to 5.4 cm in diameter were conducted in the United Kingdom and by the U.S. Department of Veterans Affairs during the past decade.13,14 Elective surgical treatment was delayed in patients in the nonoperative cohort in each trial until their aneurysms exceeded 5.4 cm on serial imaging studies.3 On the basis of data regarding gender differences in the United Kingdom trial, a guidelines subcommittee of the American Association for Vascular Surgery and the Society for Vascular Surgery now recommends a diameter of 4.5 to 5.0 cm as the appropriate threshold for elective repair of asymptomatic infrarenal aortic aneurysms in women.3,15 The general consensus regarding suprarenal, pararenal, or type IV thoracoabdominal aortic aneurysms is that because of the higher risk for surgical complications with these aneurysms, elective intervention should be considered at a slightly larger diameter than with infrarenal aortic aneurysms.3
A caveat in successful watchful waiting is patient cooperation. Valentine et al.16 studied 101 patients with aneurysms less than 5.0 cm in diameter and did not find ruptures in patients who adhered to their follow-up program but did find a 10% rupture rate in patients who complied poorly. If continued surveillance is planned, lifestyle and dietary modification should be encouraged. As mentioned earlier, patients who smoke should be strongly encouraged to stop smoking and use any and all available smoking cessation aids, and they should undergo rigorous monitoring and treatment of blood pressure and cholesterol levels, as currently recommended for patients with atherosclerosis.3
![]() Red Flags
Red Flags
The rate of abdominal aortic aneurysms (AAAs) in tobacco smokers is more than four times that in lifelong nonsmokers.
Estimates place the risk for rupture at 1% to 3% per year for AAAs 4 to 5 cm in diameter, at 6% to 11% per year for those 5 to 7 cm, and nearly 20% per year for those larger than 7 cm.
Patients usually complain of pain that is steady and aggravating in nature, lasts for hours to days, and is unaffected by movement or position.
The triad of chronic abdominal pain, weight loss, and elevated erythrocyte sedimentation rate may signal the presence of an inflammatory aneurysm.
One complication of AAA is rupture into the bowel, commonly involving the duodenum, and exsanguination is the usual fatal result, but slow leaks may be manifested as melena and masquerade as peptic ulcer disease.
Patients who stabilize with fluid resuscitation should be monitored closely because they are at high risk for rapid deterioration.
Once an aneurysm has met the criteria for repair, the vascular surgeon can choose between open and endovascular repair when determining the operative approach. The most important factor determining the utility of either approach is patient selection. Generally, open surgical repair is appropriate for younger, low-risk patients, whereas endovascular repair is preferred for older, higher-risk patients4 (Box 66.3). Studies have shown lower 30-day mortality for endovascular repair (roughly 1.2%) than for open surgery (4.6%).4 Further study is required to determine whether either affords a long-term survival advantage. It is quite clear that both approaches decrease the risk for death from AAA rupture.4
Elective repair has resulted in a drastic reduction in mortality in comparison with emergency intervention because patients undergoing elective AAA repair are not suffering the catastrophic physiologic demands of sudden, rapid, high-volume loss at the time of repair. Additionally, they have had time to undergo a thorough preoperative evaluation. A number of studies have demonstrated that the mortality rate for open aortic aneurysm repair can be reduced to less than 2% in settings in which approximately 5% to 15% of patients undergo preliminary coronary artery intervention.17 Numerous studies have also been performed to elicit risk stratification methods to help the surgeon identify markers that herald higher morbidity and mortality risks in patients requiring intervention. Multiple studies have shown that the mortality rate with elective operations is so much lower than that with ruptured aneurysms that octogenarians should be offered surgical repair. Generally, AAA repair should be offered regardless of age.3,19,20 Study results do not agree regarding the extent to which race influences outcome. Some suggest that race plays no role, whereas others suggest that the mortality rate with elective AAA repair is higher in African Americans.3,21,22 Surprisingly, gender has proved to be influential. According to larger, population-based data sets in various states and countries, the mortality rate with both elective and ruptured aneurysm repair may be as much as 50% higher in women than in men.3
With regard to emergency repair of ruptured AAAs, mortality depends on the hemodynamic status of patients at the time of surgery. In contrast to the decreased mortality associated with elective repair, no improvement in the mortality of patients with operatively managed ruptured aneurysms has been reported during the past decades, and it remains 30% to 70%.1 Clinical variables that have been found to significantly influence the mortality rate after ruptured aneurysm repair are presented in Box 66.4.
Box 66.4
Variables That Significantly Increase the Mortality Rate in Patients After Repair of Ruptured Abdominal Aortic Aneurysms3
Hypotension that requires resuscitation
High Acute Physiological and Chronic Health Evaluation (APACHE) score
From Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-654.
Future Therapy
Given the delineation of the role that MMPs play in aneurysm development and rupture, a fair amount of research has been undertaken in an effort to find inhibitors of these proteases and the utility of such agents in the treatment of small asymptomatic AAAs. Tetracyclines are potentially effective treatment for this purpose. Protracted administration of doxycycline has been associated with reduced plasma MMP-9 levels, but the long-term effects of this medication on the rate of aneurysm growth have yet to be determined.1,3 Interestingly, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors have been found to decrease the expression of MMP in addition to their effects on cholesterol. Perhaps in time, statins will prove to be useful adjuncts to the prevention and treatment of AAAs.1,3 Finally, nonsteroidal antiinflammatory drugs and beta-blockers are being studied as possible medical treatments to prohibit the development of AAAs or inhibit their expansion rate.1,3
Follow-Up, Next Steps in Care, and Patient Education
Complications
As mentioned, AAAs are the thirteenth leading cause of death in the United States. Consequently, they are also associated with several other complications. First, as many as 13% of patients with aortic aneurysms have multiple aneurysms elsewhere,3 with some studies finding that more than 20% of patients with thoracic aortic aneurysms have concomitant AAAs.3 Thus, a patient in whom an aneurysm is discovered at any level should undergo a thorough examination of the entire aorta.3 A less common complication is aortocaval fistula. The overall prevalence of aortocaval fistula is 3% to 6% of all ruptured aortic aneurysms.1 The clinical features of patients with an acute aortocaval fistula usually consist of lower extremity swelling, engorged veins, and high-output cardiac failure.9 In fact, the development of high-output congestive heart failure with the perception of continuous abdominal noise is pathognomonic of an aortocaval fistula.1
Other, even more rare complications of AAA can develop. One is rupture into the bowel, usually involving the duodenum.9 Exsanguination is the fatal result, but slow leaks may be manifested as melena and masquerade as peptic ulcer disease.9 The incidence of this complication is very low, and it is found less than 0.1% of the time at autopsy. However, the incidence rate of aortoduodenal fistula following previous repair is 0.5% to 2.3%.9 Exceptionally large or inflammatory aortic aneurysms can occasionally be associated with early satiety or gastric outlet symptoms because of duodenal compression. Finally, infectious or mycotic aneurysms are worthy of mention. Such an aneurysm may arise by one of two means. It may occur secondary to infection of a preexisting aneurysm,3 or the aortic wall itself may become infected and give rise to the development of an aneurysm, usually saccular in nature.3 Primary aortic infections are most commonly caused by Staphylococcus and Salmonella.3,23 Tuberculosis has also been found to be responsible for infection of aortic pseudoaneurysms.3
1 Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589.
2 Golledge J, Muller J, Daugherty A, et al. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613.
3 Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654.
4 Katzen BT, Dake MD, MacLean AA, et al. Endovascular repair of abdominal and thoracic aortic aneurysms. Circulation. 2005;112:1663–1675.
5 Noel AA, Gloviczki P, Cherry Jr, KJ., et al. Ruptured abdominal aortic aneurysms: the excessive mortality rate of conventional repair. J Vasc Surg. 2001;34:41–46.
6 Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg. 2003;38:329–334.
7 Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21.
8 Dobrin PB, Mrkvicka R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 1994;2:484–488.
9 Schwartz SA, Taljanovic MS, Smyth S, et al. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR Am J Roentgenol. 2007;188:W57–W62.
10 McMillan WD, Pearce WH. Increased plasma levels of metalloproteinase-9 are associated with abdominal aortic aneurysms. J Vasc Surg. 1999;29:122–127.
11 Lindholt JS, Jørgensen B, Shi GP, et al. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2003;25:546–551.
12 Brown PM, Pattenden R, Gutelius JR. The selective management of small abdominal aortic aneurysms: the Kingston study. J Vasc Surg. 1992;15:21–27.
13 Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999;230:289–297.
14 United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445–1452.
15 Brewster DC, Cronenwett JL, Hallett JW, Jr., et al. Guidelines for the treatment of abdominal aortic aneurysms: report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117.
16 Valentine RJ, Decaprio JD, Castillo JM, et al. Watchful waiting in cases of small abdominal aortic aneurysms: appropriate for all patients? J Vasc Surg. 2000;32:441–450.
17 McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804. erratum in: N Engl J Med 2005;95:19
18 Prance SE, Wilson YG, Cosgrove CM, et al. Abdominal aortic aneurysms: selecting patients for surgery. Eur J Vasc Endovasc Surg. 1999;17:129–132.
19 Kazmers A, Perkins AJ, Jacobs LA. Outcomes after abdominal aortic aneurysm repair in those > or = 80 years of age: recent Veterans Affairs experience. Ann Vasc Surg. 1998;12:106–112.
20 O’Hara PJ, Hertzer NR, Krajewski LP, et al. Ten-year experience with abdominal aortic aneurysm repair in octogenarians: early results and late outcome. J Vasc Surg. 1995;21:830–838.
21 Collins TC, Johnson M, Daley J, et al. Preoperative risk factors for 30-day mortality after elective surgery for vascular disease in Department of Veterans Affairs hospitals: is race important? J Vasc Surg. 2001;34:634–640.
22 Heller JA, Weinberg A, Arons R, et al. Two decades of abdominal aortic aneurysm repair: have we made any progress? J Vasc Surg. 2000;32:1091–1100.
23 Fiessinger JN, Paul JF. [Inflammatory and infectious aortitis.]. Rev Prat. 2002;52:1094–1099.