4
Abdomen
Arterial supply and venous drainage
Arterial supply to the gastrointestinal tract
Nervous system in the posterior abdominal region
Sympathetic trunks and splanchnic nerves
ADDITIONAL LEARNING RESOURCES FOR CHAPTER 4, ABDOMEN, ON STUDENT CONSULT (www.studentconsult.com)
 Image Library—illustrations of abdominal anatomy, Chapter 4
Image Library—illustrations of abdominal anatomy, Chapter 4
 Self-Assessment—National Board style multiple-choice questions, Chapter 4
Self-Assessment—National Board style multiple-choice questions, Chapter 4
 Short Questions—these are questions requiring short responses, Chapter 4
Short Questions—these are questions requiring short responses, Chapter 4
 Interactive Surface Anatomy—interactive surface animations, Chapter 4
Interactive Surface Anatomy—interactive surface animations, Chapter 4
Traumatic rupture of the diaphragm
Chronic thrombosis of the inferior vena cava
Liver biopsy in patients with suspected liver cirrhosis
Complications of an abdominoperineal resection
Carcinoma of the head of the pancreas
Endoleak after endovascular repair of abdominal aortic aneurysm
Regional anatomy
The abdomen is the part of the trunk inferior to the thorax (Fig. 4.1). Its musculomembranous walls surround a large cavity (the abdominal cavity), which is bounded superiorly by the diaphragm and inferiorly by the pelvic inlet.
Fig. 4.1 Boundaries of the abdominal cavity.
The abdominal cavity may extend superiorly as high as the fourth intercostal space, and is continuous inferiorly with the pelvic cavity. It contains the peritoneal cavity and the abdominal viscera.
SURFACE TOPOGRAPHY
Topographical divisions of the abdomen are used to describe the location of abdominal organs and the pain associated with abdominal problems. The two schemes most often used are:
Four-quadrant pattern
A horizontal transumbilical plane passing through the umbilicus and the intervertebral disc between vertebrae LIII and LIV and intersecting with the vertical median plane divides the abdomen into four quadrants—the right upper, left upper, right lower, and left lower quadrants (Fig. 4.2).
Fig. 4.2 Four-quadrant topographical pattern.
Nine-region pattern
The nine-region pattern is based on two horizontal and two vertical planes (Fig. 4.3).
 The superior horizontal plane (the subcostal plane) is immediately inferior to the costal margins, which places it at the lower border of the costal cartilage of rib X and passes posteriorly through the body of vertebra LIII. (Note, however, that sometimes the transpyloric plane, halfway between the jugular notch and the symphysis pubis or halfway between the umbilicus and the inferior end of the body of the sternum, passing posteriorly through the lower border of vertebrae LI and intersecting with the costal margin at the ends of the ninth costal cartilages, is used instead.)
The superior horizontal plane (the subcostal plane) is immediately inferior to the costal margins, which places it at the lower border of the costal cartilage of rib X and passes posteriorly through the body of vertebra LIII. (Note, however, that sometimes the transpyloric plane, halfway between the jugular notch and the symphysis pubis or halfway between the umbilicus and the inferior end of the body of the sternum, passing posteriorly through the lower border of vertebrae LI and intersecting with the costal margin at the ends of the ninth costal cartilages, is used instead.)
Fig. 4.3 Nine-region organizational pattern.
These four planes establish the topographical divisions in the nine-region organization. The following designations are used for each region: superiorly the right hypochondrium, the epigastric region, and the left hypochondrium; inferiorly the right groin (inguinal region), pubic region, and left groin (inguinal region); and in the middle the right flank (lateral region), the umbilical region, and the left flank (lateral region) (Fig. 4.3).
Surface anatomy
Using abdominal quadrants to locate major viscera
The abdomen can be divided into quadrants by a vertical median plane and a horizontal transumbilical plane (Fig. 4.4):
 The liver and gallbladder are in the right upper quadrant.
The liver and gallbladder are in the right upper quadrant.
 The stomach and spleen are in the left upper quadrant.
The stomach and spleen are in the left upper quadrant.
 The cecum and appendix are in the right lower quadrant.
The cecum and appendix are in the right lower quadrant.
 The end of the descending colon and sigmoid colon are in the left lower quadrant.
The end of the descending colon and sigmoid colon are in the left lower quadrant.
Fig. 4.4 Abdominal quadrants and the positions of major viscera. Anterior view of a man.
Most of the liver is under the right dome of the diaphragm and is deep to the lower thoracic wall. The inferior margin of the liver can be palpated descending below the right costal margin when a patient is asked to inhale deeply. On deep inspiration, the edge of the liver can be felt “slipping” under the palpating fingers placed under the costal margin.
A common surface projection of the appendix is McBurney’s point, which is one third of the way up a line from the right anterior superior iliac spine to the umbilicus.
Surface anatomy
Defining surface regions to which pain from the gut is referred
The abdomen can be divided into nine regions by a midclavicular sagittal plane on each side and by the subcostal and intertubercular planes, which pass through the body transversely (Fig. 4.5).
Fig. 4.5 The nine regions of the abdomen. Anterior view of a woman.
Pain from the abdominal part of the foregut is referred to the epigastric region, pain from the midgut is referred to the umbilical region, and pain from the hindgut is referred to the pubic region.
ABDOMINAL WALL
The abdominal wall covers a large area. It is bounded superiorly by the xiphoid process and costal margins, posteriorly by the vertebral column, and inferiorly by the upper parts of the pelvic bones.
Its layers consist of skin, superficial fascia (subcutaneous tissue), muscles and their associated deep fascias, extraperitoneal fascia, and parietal peritoneum (Fig. 4.6).
Fig. 4.6 Layers of the abdominal wall.
Superficial fascia
The superficial fascia of the abdominal wall (subcutaneous tissue of abdomen) is a layer of fatty connective tissue. It is usually a single layer similar to, and continuous with, the superficial fascia throughout other regions of the body. However, in the lower region of the anterior part of the abdominal wall, below the umbilicus, it forms two layers: a superficial fatty layer and a deeper membranous layer.
Superficial layer
The superficial fatty layer of superficial fascia (Camper’s fascia) contains fat and varies in thickness (Figs. 4.7, 4.8). It is continuous over the inguinal ligament with the superficial fascia of the thigh and with a similar layer in the perineum.
Fig. 4.7 Superficial fascia.
Fig. 4.8 Continuity of membranous layer of superficial fascia into other areas.
In men, this superficial layer continues over the penis and, after losing its fat and fusing with the deeper layer of superficial fascia, continues into the scrotum where it forms a specialized fascial layer containing smooth muscle fibers (the dartos fascia). In women, this superficial layer retains some fat and is a component of the labia majora.
Deeper layer
The deeper membranous layer of superficial fascia (Scarpa’s fascia) is thin and membranous, and contains little or no fat (Fig. 4.7). Inferiorly, it continues into the thigh, but just below the inguinal ligament, it fuses with the deep fascia of the thigh (the fascia lata; Fig. 4.8). In the midline, it is firmly attached to the linea alba and the pubic symphysis. It continues into the anterior part of the perineum where it is firmly attached to the ischiopubic rami and to the posterior margin of the perineal membrane. Here, it is referred to as the superficial perineal fascia (Colles’ fascia).
In men, the deeper membranous layer of superficial fascia blends with the superficial layer as they both pass over the penis, forming the superficial fascia of the penis, before they continue into the scrotum where they form the dartos fascia (Figs. 4.7, 4.8). Also in men, extensions of the deeper membranous layer of superficial fascia attached to the pubic symphysis pass inferiorly onto the dorsum and sides of the penis to form the fundiform ligament of the penis. In women, the membranous layer of the superficial fascia continues into the labia majora and the anterior part of the perineum.
Anterolateral muscles
There are five muscles in the anterolateral group of abdominal wall muscles (Table 4.1):
Table 4.1 Abdominal wall muscles
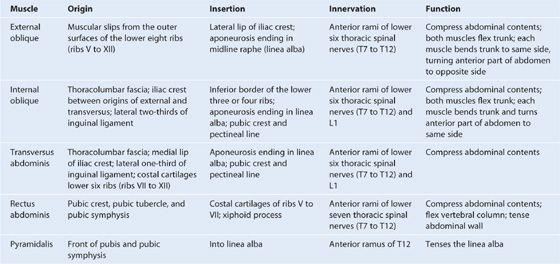
Each of these five muscles has specific actions, but together the muscles are critical:
 for the maintenance of many normal physiological functions,
for the maintenance of many normal physiological functions,
 to keep the abdominal viscera within the abdominal cavity,
to keep the abdominal viscera within the abdominal cavity,
 to protect the viscera from injury, and
to protect the viscera from injury, and
 to help maintain the position of the viscera in the erect posture against the action of gravity.
to help maintain the position of the viscera in the erect posture against the action of gravity.
Contraction of these muscles assists in both quiet and forced expiration by pushing the viscera upward (which helps push the relaxed diaphragm farther into the thoracic cavity) and in coughing and vomiting.
All these muscles are also involved in any action that increases intra-abdominal pressure, including parturition (childbirth), micturition (urination), and defecation (expulsion of feces from the rectum).
Flat muscles
External oblique
The most superficial of the three flat muscles in the anterolateral group of abdominal wall muscles is the external oblique, which is immediately deep to the superficial fascia (Table 4.1, Fig. 4.9). Its laterally placed muscle fibers pass in an inferomedial direction, while its large aponeurotic component covers the anterior part of the abdominal wall to the midline. Approaching the midline, the aponeuroses are entwined, forming the linea alba, which extends from the xiphoid process to the pubic symphysis.
Fig. 4.9 External oblique muscle and its aponeurosis.
The lower border of the external oblique aponeurosis forms the inguinal ligament on each side (Fig. 4.9). This thickened reinforced free edge of the external oblique aponeurosis passes between the anterior superior iliac spine laterally and the pubic tubercle medially (Fig. 4.10). It folds under itself forming a trough, which plays an important role in the formation of the inguinal canal.
Fig. 4.10 Ligaments formed from the external oblique aponeurosis.
Several other ligaments are also formed from extensions of the fibers at the medial end of the inguinal ligament:
 The lacunar ligament is a crescent-shaped extension of fibers at the medial end of the inguinal ligament that pass backward to attach to the pecten pubis on the superior ramus of the pubic bone (Figs. 4.10, 4.11).
The lacunar ligament is a crescent-shaped extension of fibers at the medial end of the inguinal ligament that pass backward to attach to the pecten pubis on the superior ramus of the pubic bone (Figs. 4.10, 4.11).
Fig. 4.11 Ligaments of the inguinal region.
Internal oblique
Deep to the external oblique muscle is the internal oblique muscle, which is the second of the three flat muscles (Table 4.1, Fig. 4.12). This muscle is smaller and thinner than the external oblique, with most of its muscle fibers passing in a superomedial direction. Its lateral muscular components end anteriorly as an aponeurosis that blends into the linea alba at the midline.
Fig. 4.12 Internal oblique muscle and its aponeurosis.
Transversus abdominis
Deep to the internal oblique muscle is the transversus abdominis muscle (Table 4.1, Fig. 4.13), so named because of the direction of most of its muscle fibers. It ends in an anterior aponeurosis, which blends with the linea alba at the midline.
Fig. 4.13 Transversus abdominis muscle and its aponeurosis.
Transversalis fascia
Each of the three flat muscles is covered on its anterior and posterior surfaces by a layer of deep (or investing) fascia. In general, these layers are unremarkable except for the layer deep to the transversus abdominis muscle (the transversalis fascia), which is better developed.
The transversalis fascia is a continuous layer of deep fascia that lines the abdominal cavity and continues into the pelvic cavity. It crosses the midline anteriorly, associating with the transversalis fascia of the opposite side, and is continuous with the fascia on the inferior surface of the diaphragm. It is continuous posteriorly with the deep fascia covering the muscles of the posterior abdominal wall and attaches to the thoracolumbar fascia.
After attaching to the crest of the ilium, the transversalis fascia blends with the fascia covering the muscles associated with the upper regions of the pelvic bones and with similar fascia covering the muscles of the pelvic cavity. At this point, it is referred to as the parietal pelvic (or endopelvic) fascia.
There is therefore a continuous layer of deep fascia surrounding the abdominal cavity that is thick in some areas, thin in others, attached or free, and participates in the formation of specialized structures.
Vertical muscles
The two vertical muscles in the anterolateral group of abdominal wall muscles are the large rectus abdominis and the small pyramidalis (Table 4.1, Fig. 4.14).
Fig. 4.14 Rectus abdominis and pyramidalis muscles.
Rectus abdominis
The rectus abdominis is a long, flat muscle and extends the length of the anterior abdominal wall. It is a paired muscle, separated in the midline by the linea alba, and it widens and thins as it ascends from the pubic symphysis to the costal margin. Along its course, it is intersected by three or four transverse fibrous bands or tendinous intersections (Fig. 4.14). These are easily visible on individuals with a well-developed rectus abdominis.
Pyramidalis
The second vertical muscle is the pyramidalis. This small, triangular muscle, which may be absent, is anterior to the rectus abdominis, has its base on the pubis, and its apex is attached superiorly and medially to the linea alba (Fig. 4.14).
Rectus sheath
The rectus abdominis and pyramidalis muscles are enclosed in an aponeurotic tendinous sheath (the rectus sheath) formed by a unique layering of the aponeuroses of the external and internal oblique, and transversus abdominis muscles (Fig. 4.15).
The rectus sheath completely encloses the upper three-quarters of the rectus abdominis and covers the anterior surface of the lower one-quarter of the muscle. As no sheath covers the posterior surface of the lower quarter of the rectus abdominis muscle, the muscle at this point is in direct contact with the transversalis fascia.
The formation of the rectus sheath surrounding the upper three-quarters of the rectus abdominis muscle has the following pattern:
 The posterior wall of the rectus sheath consists of the other half of the aponeurosis of the internal oblique and the aponeurosis of the transversus abdominis.
The posterior wall of the rectus sheath consists of the other half of the aponeurosis of the internal oblique and the aponeurosis of the transversus abdominis.
At a point midway between the umbilicus and the pubic symphysis, corresponding to the beginning of the lower one-fourth of the rectus abdominis muscle, all of the aponeuroses move anterior to the rectus muscle. There is no posterior wall of the rectus sheath and the anterior wall of the sheath consists of the aponeuroses of the external oblique, the internal oblique, and the transversus abdominis muscles. From this point inferiorly, the rectus abdominis muscle is in direct contact with the transversalis fascia. Marking this point of transition is an arch of fibers (the arcuate line; see Fig. 4.14).
Extraperitoneal fascia
Deep to the transversalis fascia is a layer of connective tissue, the extraperitoneal fascia, which separates the transversalis fascia from the peritoneum (Fig. 4.16). Containing varying amounts of fat, this layer not only lines the abdominal cavity but is also continuous with a similar layer lining the pelvic cavity. It is abundant on the posterior abdominal wall, especially around the kidneys, continues over organs covered by peritoneal reflections, and, as the vasculature is located in this layer, extends into mesenteries with the blood vessels. Viscera in the extraperitoneal fascia are referred to as retroperitoneal.
Fig. 4.16 Transverse section showing the layers of the abdominal wall.
Clinical app
Preperitoneal vs. retroperitoneal
In the description of specific surgical procedures, the terminology used to describe the extraperitoneal fascia is further modified. The fascia toward the anterior side of the body is described as preperitoneal (or, less commonly, properitoneal, and the fascia toward the posterior side of the body has been described as retroperitoneal (Fig. 4.17). Examples of the use of these terms would be the continuity of fat in the inguinal canal with the preperitoneal fat and a transabdominal preperitoneal laparoscopic repair of an inguinal hernia.
Peritoneum
Deep to the extraperitoneal fascia is the peritoneum (Fig. 4.16). This thin serous membrane lines the walls of the abdominal cavity and, at various points, reflects onto the abdominal viscera, providing either a complete or a partial covering. The peritoneum lining the walls is the parietal peritoneum; the peritoneum covering the viscera is the visceral peritoneum.
The continuous lining of the abdominal walls by the parietal peritoneum forms a sac. This sac is closed in men, but has two openings in women where the uterine tubes provide a passage to the outside. The closed sac in men and the semiclosed sac in women is called the peritoneal cavity.
Innervation
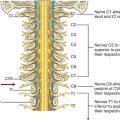
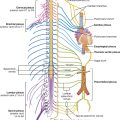
The skin, muscles, and parietal peritoneum of the anterolateral abdominal wall are supplied by T7 to T12 and L1 spinal nerves. The anterior rami of these spinal nerves pass around the body, from posterior to anterior, in an inferomedial direction (Fig. 4.18). As they proceed, they give off a lateral cutaneous branch and end as an anterior cutaneous branch.
Fig. 4.18 Innervation of the anterolateral abdominal wall.
The intercostal nerves (T7 to T11) leave their intercostal spaces, passing deep to the costal cartilages, and continue onto the anterolateral abdominal wall between the internal oblique and transversus abdominis muscles (Fig. 4.19). Reaching the lateral edge of the rectus sheath, they enter the rectus sheath and pass posterior to the lateral aspect of the rectus abdominis muscle. Approaching the midline, an anterior cutaneous branch passes through the rectus abdominis muscle and the anterior wall of the rectus sheath to supply the skin.
Fig. 4.19 Path taken by the nerves innervating the anterolateral abdominal wall.
Spinal nerve T12 (the subcostal nerve) follows a similar course as the intercostals. Branches of L1 (the iliohypogastric nerve and ilio-inguinal nerve), which originate from the lumbar plexus, follow similar courses initially, but deviate from this pattern near their final destination.
Along their course, nerves T7 to T12 and L1 supply branches to the anterolateral abdominal wall muscles and the underlying parietal peritoneum. All terminate by supplying skin (Fig. 4.20):
 Nerves T7 to T9 supply the skin from the xiphoid process to just above the umbilicus.
Nerves T7 to T9 supply the skin from the xiphoid process to just above the umbilicus.
 T10 supplies the skin around the umbilicus.
T10 supplies the skin around the umbilicus.
 T11, T12, and L1 supply the skin from just below the umbilicus to, and including, the pubic region.
T11, T12, and L1 supply the skin from just below the umbilicus to, and including, the pubic region.
Fig. 4.20 Dermatomes of the anterolateral abdominal wall.
Arterial supply and venous drainage
Numerous blood vessels supply the anterolateral abdominal wall (Fig. 4.21). Superficially:
Fig. 4.21 Arterial supply to the anterolateral abdominal wall.
At a deeper level:
 The lateral part of the wall is supplied by branches of the tenth and eleventh intercostal arteries and the subcostal artery.
The lateral part of the wall is supplied by branches of the tenth and eleventh intercostal arteries and the subcostal artery.
The superior and inferior epigastric arteries both enter the rectus sheath. They are posterior to the rectus abdominis muscle throughout their course, and anastomose with each other (Fig. 4.22).
Fig. 4.22 Superior and inferior epigastric arteries.
Veins of similar names follow the arteries and are responsible for venous drainage.
Lymphatic drainage
Lymphatic drainage of the anterolateral abdominal wall follows the basic principles of lymphatic drainage:
GROIN
The groin (inguinal region) is the area of junction between the anterior abdominal wall and the thigh. In this area, the abdominal wall is weakened from changes that occur during development and a peritoneal sac or diverticulum, with or without abdominal contents, can therefore protrude through it, creating an inguinal hernia. This type of hernia can occur in both sexes, but it is more common in males.
The inherent weakness in the anterior abdominal wall in the groin is caused by changes that occur during the development of the gonads. Before the descent of the testis and ovaries from their initial position high in the posterior abdominal wall, a peritoneal outpouching (the processus vaginalis) forms (Fig. 4.23), protruding through the various layers of the anterior abdominal wall and acquiring coverings from each:
 The transversalis fascia forms its deepest covering.
The transversalis fascia forms its deepest covering.
 Its most superficial covering is the aponeurosis of the external oblique.
Its most superficial covering is the aponeurosis of the external oblique.
Fig. 4.23 Descent of the testes from week 7 (postfertilization) to birth.
The final event in this development is the descent of the testes into the scrotum or of the ovaries into the pelvic cavity. This process depends on the development of the gubernaculum (Fig. 4.23), which extends from the inferior border of the developing gonad to labioscrotal swellings in the developing perineum.
The processus vaginalis is immediately anterior to the gubernaculum within the inguinal canal.
In men, as the testes descend, the testes and their accompanying vessels, ducts, and nerves pass through the inguinal canal and are therefore surrounded by the same fascial layers of the abdominal wall. Testicular descent completes the formation of the spermatic cord in men.
In women, the ovaries descend into the pelvic cavity and become associated with the developing uterus. Therefore, the only remaining structure passing through the inguinal canal is the round ligament of the uterus, which is a remnant of the gubernaculum.
The development sequence is concluded in both sexes when the processus vaginalis obliterates. If this does not occur or is incomplete, a potential weakness exists in the anterior abdominal wall and an inguinal hernia may develop. In males, only proximal regions of the tunica vaginalis obliterate. The distal end expands to enclose most of the testes in the scrotum. In other words, the cavity of the tunica vaginalis in men forms as an extension of the developing peritoneal cavity that becomes separated off during development.
Inguinal canal
The inguinal canal is a slitlike passage that extends in a downward and medial direction, just above and parallel to the lower half of the inguinal ligament. It begins at the deep inguinal ring and continues for approximately 4 cm, ending at the superficial inguinal ring (Fig. 4.24). The contents of the canal are the genital branch of the genitofemoral nerve, the spermatic cord in men, and the round ligament of the uterus in women. Additionally, in both sexes, the ilio-inguinal nerve passes through part of the canal, exiting through the superficial inguinal ring with the other contents.
Fig. 4.24 Inguinal canal.
Deep inguinal ring
The deep (internal) inguinal ring is the beginning of the inguinal canal and is at a point midway between the anterior superior iliac spine and the pubic symphysis (Fig. 4.25). It is just above the inguinal ligament and immediately lateral to the inferior epigastric vessels. Although sometimes referred to as a defect or opening in the transversalis fascia, it is actually the beginning of the tubular evagination of transversalis fascia that forms one of the coverings (the internal spermatic fascia) of the spermatic cord in men or the round ligament of the uterus in women.
Fig. 4.25 Deep inguinal ring and the transversalis fascia.
Superficial inguinal ring
The superficial (external) inguinal ring is the end of the inguinal canal and is superior to the pubic tubercle (Fig. 4.26). It is a triangular opening in the aponeurosis of the external oblique, with its apex pointing superolaterally and its base formed by the pubic crest. The two remaining sides of the triangle (the medial crus and the lateral crus) are attached to the pubic symphysis and the pubic tubercle, respectively. At the apex of the triangle the two crura are held together by crossing (intercrural) fibers, which prevent further widening of the superficial ring.
Fig. 4.26 Superficial inguinal ring and the aponeurosis of the external oblique.
As with the deep inguinal ring, the superficial inguinal ring is actually the beginning of the tubular evagination of the aponeurosis of the external oblique onto the structures traversing the inguinal canal and emerging from the superficial inguinal ring. This continuation of tissue over the spermatic cord is the external spermatic fascia.
Anterior wall
The anterior wall of the inguinal canal is formed along its entire length by the aponeurosis of the external oblique muscle (Fig. 4.26). It is also reinforced laterally by the lower fibers of the internal oblique that originate from the lateral two-thirds of the inguinal ligament (Fig. 4.27). This adds an additional covering over the deep inguinal ring, which is a potential point of weakness in the anterior abdominal wall. Furthermore, as the internal oblique muscle covers the deep inguinal ring, it also contributes a layer (the cremasteric fascia containing the cremasteric muscle) to the coverings of the structures traversing the inguinal canal.
Fig. 4.27 Internal oblique muscle and the inguinal canal.
Posterior wall
The posterior wall of the inguinal canal is formed along its entire length by the transversalis fascia (see Fig. 4.25). It is reinforced along its medial one-third by the conjoint tendon (inguinal falx; Fig. 4.27). This tendon is the combined insertion of the transversus abdominis and internal oblique muscles into the pubic crest and pectineal line.
As with the internal oblique muscle’s reinforcement of the area of the deep inguinal ring, the position of the conjoint tendon posterior to the superficial inguinal ring provides additional support to a potential point of weakness in the anterior abdominal wall.
Roof
The roof (superior wall) of the inguinal canal is formed by the arching fibers of the transversus abdominis and internal oblique muscles (Figs. 4.27, 4.28). They pass from their lateral points of origin from the inguinal ligament to their common medial attachment as the conjoint tendon.
Fig. 4.28 Transversus abdominis muscle and the inguinal canal.
Floor
The floor (inferior wall) of the inguinal canal is formed by the medial one-half of the inguinal ligament. This rolled-under, free margin of the lowest part of the aponeurosis of the external oblique forms a gutter or trough on which the contents of the inguinal canal are positioned. The lacunar ligament reinforces most of the medial part of the gutter.
Contents
The contents of the inguinal canal are:
 the round ligament of the uterus, and
the round ligament of the uterus, and
 genital branch of the genitofemoral nerve in women.
genital branch of the genitofemoral nerve in women.
These structures enter the inguinal canal through the deep inguinal ring and exit it through the superficial inguinal ring.
Additionally, the ilio-inguinal nerve (L1) passes through part of the inguinal canal. This nerve is a branch of the lumbar plexus, and enters the abdominal wall posteriorly by piercing the internal surface of the transversus abdominis muscle and continues through the layers of the anterior abdominal wall by piercing the internal oblique muscle. As it continues to pass inferomedially, it enters the inguinal canal. It continues down the canal to exit through the superficial inguinal ring.
Spermatic cord
The spermatic cord begins to form proximally at the deep inguinal ring and consists of structures passing between the abdominopelvic cavities and the testes, and the three fascial coverings that enclose these structures (Fig. 4.29).
Fig. 4.29 Spermatic cord.
The structures in the spermatic cord include:
 the artery to ductus deferens (from the inferior vesical artery),
the artery to ductus deferens (from the inferior vesical artery),
 the testicular artery (from the abdominal aorta),
the testicular artery (from the abdominal aorta),
 the pampiniform plexus of veins (testicular veins),
the pampiniform plexus of veins (testicular veins),
 the cremasteric artery and vein (small vessels associated with the cremasteric fascia),
the cremasteric artery and vein (small vessels associated with the cremasteric fascia),
 the genital branch of the genitofemoral nerve (innervation to the cremasteric muscle),
the genital branch of the genitofemoral nerve (innervation to the cremasteric muscle),
 sympathetic and visceral afferent nerve fibers,
sympathetic and visceral afferent nerve fibers,
 remnants of the processus vaginalis.
remnants of the processus vaginalis.
These structures enter the deep inguinal ring, proceed down the inguinal canal, and exit from the superficial inguinal ring, having acquired the three fascial coverings during their journey. This collection of structures and fascias continues into the scrotum, where the structures connect with the testes and the fascias surround the testes.
The fascias enclosing the contents of the spermatic cord include (Fig. 4.29):
 the internal spermatic fascia, which is the deepest layer, arises from the transversalis fascia, and is attached to the margins of the deep inguinal ring;
the internal spermatic fascia, which is the deepest layer, arises from the transversalis fascia, and is attached to the margins of the deep inguinal ring;
 the external spermatic fascia, which is the most superficial covering of the spermatic cord, arises from the aponeurosis of the external oblique muscle, and is attached to the margins of the superficial inguinal ring (Fig. 4.29).
the external spermatic fascia, which is the most superficial covering of the spermatic cord, arises from the aponeurosis of the external oblique muscle, and is attached to the margins of the superficial inguinal ring (Fig. 4.29).
Round ligament of the uterus
The round ligament of the uterus is a cordlike structure that passes from the uterus to the deep inguinal ring where it enters the inguinal canal. It passes down the inguinal canal and exits through the superficial inguinal ring. At this point, it has changed from a cordlike structure to a few strands of tissue, which attach to the connective tissue associated with the labia majora. As it traverses the inguinal canal, it acquires the same coverings as the spermatic cord in men.
The round ligament of the uterus is the long distal part of the original gubernaculum in the fetus that extends from the ovary to the labioscrotal swellings. From its attachment to the uterus, the round ligament of the uterus continues to the ovary as the ligament of the ovary that develops from the short proximal end of the gubernaculum.
Clinical app
Surgical incisions
Traditionally, incisions have been placed at and around the region of surgical interest. The size of these incisions was usually large to allow good access and optimal visualization of the abdominal cavity.
Currently, the most commonly used large abdominal incision is a central craniocaudad incision from the xiphoid process to the pubic symphysis, which provides wide access to the whole of the abdominal contents and allows an exploratory procedure to be performed (laparotomy).
With the advent of small cameras and the development of minimal access surgery, tiny incisions can be made in the anterior abdominal wall and cameras inserted. The peritoneal cavity is “inflated” with carbon dioxide to increase the space in which the procedure is performed. Further instruments may be inserted through small portholes, and procedures such as cholecystectomy (removal of the gallbladder) and appendectomy (removal of the appendix) can be carried out, allowing the patient to return home sooner.
Surface anatomy
How to find the superficial inguinal ring
The superficial inguinal ring is superior to the pubic crest and tubercle and to the medial end of the inguinal ligament (Fig. 4.30):
 In women, the pubic tubercle can be palpated and the ring is superior and lateral to it.
In women, the pubic tubercle can be palpated and the ring is superior and lateral to it.
Because the superficial inguinal ring is the site where inguinal hernias appear, particularly in men, the ring and related parts of the inguinal canal are often evaluated during physical examination.
Clinical app
Cremasteric reflex
In men, the cremaster muscle and cremasteric fascia form the middle or second covering of the spermatic cord. This muscle and its associated fascia are supplied by the genital branch of the genitofemoral nerve (L1/L2). Contraction of this muscle can be stimulated by a reflex arc. Gentle touch at and around the skin of the medial aspect of the superior part of the thigh stimulates the sensory fibers in the ilio-inguinal nerve. These sensory fibers enter the spinal cord at level L1. At this level, the sensory fibers stimulate the motor fibers carried in the genital branch of the genitofemoral nerve and the testis elevates on the stimulated side.
Clinical app
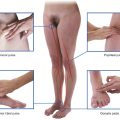
Masses around the groin
The most common masses in the groin are hernias. A hernia is the protrusion of a viscus, in part or in whole, through a normal or abnormal opening. The viscus usually carries a covering of parietal peritoneum, which forms the lining of the hernial sac.
The key to groin examination is determining the position of the inguinal ligament. The inguinal ligament passes between the anterior superior iliac spine laterally and the pubic tubercle medially. Inguinal hernias are above the inguinal ligament and are usually more apparent on standing. A visual assessment of the lump is necessary, bearing in mind the anatomical landmarks of the inguinal ligament.
In men, it is wise to examine the scrotum to check for a lump. If an abnormal mass is present, an inability to feel its upper edge suggests that it may originate from the inguinal canal and might be a hernia. By placing the hand over the lump and asking the patient to cough, the lump bulges outward.
An attempt should be made to reduce the swelling by applying gentle, firm pressure over the lump. If the lump is reducible, the hand should be withdrawn and careful observation will reveal recurrence of the mass.
The position of an abnormal mass in the groin relative to the pubic tubercle is very important, as are the presence of increased temperature and pain, which may represent early signs of strangulation or infection.
As a general rule:
 An inguinal hernia appears through the superficial inguinal ring above the pubic tubercle and crest.
An inguinal hernia appears through the superficial inguinal ring above the pubic tubercle and crest.
 A femoral hernia appears through the femoral canal below and lateral to the pubic tubercle.
A femoral hernia appears through the femoral canal below and lateral to the pubic tubercle.
Clinical app
Inguinal hernias
An inguinal hernia is the protrusion or passage of a peritoneal sac, with or without abdominal contents, through a weakened part of the abdominal wall in the groin. It occurs because the peritoneal sac enters the inguinal canal either:
 indirectly, through the deep inguinal ring, or
indirectly, through the deep inguinal ring, or
Clinical app
Indirect inguinal hernias
The indirect inguinal hernia is the most common of the two types of inguinal hernias and is much more common in men than in women (Fig. 4.31). It usually occurs because some part, or all, of the embryonic processus vaginalis remains open or patent. It is therefore referred to as being congenital in origin.
Fig. 4.31 Indirect inguinal hernia.
The protruding peritoneal sac enters the inguinal canal by passing through the deep inguinal ring, just lateral to the inferior epigastric vessels. The extent of its excursion down the inguinal canal depends on the amount of processus vaginalis that remains patent. If the entire processus vaginalis remains patent, the peritoneal sac may traverse the length of the canal, exit the superficial inguinal ring, and continue into the scrotum in men or the labia majus in women. In this case, the protruding peritoneal sac acquires the same coverings as those associated with the spermatic cord in men or the round ligament of the uterus in women.
Clinical app
Direct inguinal hernias
A peritoneal sac that enters the medial end of the inguinal canal directly through a weakened posterior wall is a direct inguinal hernia (Fig. 4.32). It is usually described as acquired because it develops when abdominal musculature has been weakened and is commonly seen in mature men.
Fig. 4.32 Direct inguinal hernia.
This type of inguinal hernia does not traverse the entire length of the inguinal canal, but may exit through the superficial inguinal ring. When this occurs, the peritoneal sac acquires a layer of external spermatic fascia and can extend, like an indirect hernia, into the scrotum. Also, unlike indirect inguinal hernias that originate lateral to the inferior epigastric artery, direct inguinal hernias originate medial to the artery (Fig. 4.32).
Femoral hernias
A femoral hernia passes through the femoral canal and into the medial aspect of the anterior thigh. The femoral canal lies at the medial edge of the femoral sheath, which contains the femoral artery, femoral vein, and lymphatics. The neck of the femoral canal is extremely narrow and is prone to trapping bowel within the sac, making this type of hernia irreducible and susceptible to bowel strangulation. Femoral hernias are usually acquired, and most commonly occur in middle-aged and elderly populations. In addition, because women generally have wider pelvises than men, they tend to occur more commonly in women.
Clinical app
Umbilical hernias
Umbilical hernias are rare. Occasionally, they are congenital and result from failure of the small bowel to return to the abdominal cavity from the umbilical cord during development. After birth, umbilical hernias may result from incomplete closure of the umbilicus (navel). Overall, most of these hernias close in the first year of life, and surgical repair is not generally attempted until later.
Para-umbilical hernias may occur in adults at and around the umbilicus and often have small necks, so requiring surgical treatment.
Clinical app
Incisional hernias
Incisional hernias occur through a defect in a scar of a previous abdominal operation. Usually, the necks of these hernias are wide and do not therefore strangulate the viscera they contain.
Clinical app
Sportsmen’s groin/sportsmen’s hernia
The groin can loosely be defined as the area where the leg meets the trunk near the midline. Here the abdominal muscles of the trunk blend in with the adductor muscles of the thigh, the medial end of the inguinal ligament attaches to the pubic tubercle, the pubic symphysis attaches the two pubic bones together, and the superficial (external) inguinal ring occurs. It also is in and around this region where there is considerable translation of force during most athletic and sporting activities. Pain in the groin or pubic region can be due to numerous causes, which include inflammatory changes at the pubic symphysis, insertional problems of the rectus abdominus/adductor longus, and hernias.
Clinical app
Other hernias
A spigelian hernia passes upward through the arcuate line into the lateral border at the lower part of the posterior rectus sheath. It may appear as a tender mass on one side of the lower anterior abdominal wall.
Abdominopelvic cavity hernias can also develop in association with the pelvic walls, and sites include the obturator canal, the greater sciatic foramen, above and below the piriformis muscle.
Clinical app
Potential problem of hernias
One of the potential problems with hernias is that bowel and fat may become stuck within the hernial sac. This can cause appreciable pain and bowel obstruction, necessitating urgent surgery. Another potential risk is strangulation of the hernia, in which the blood supply to the bowel is cut off at the neck of the hernial sac, rendering the bowel ischemic and susceptible to perforation.
ABDOMINAL VISCERA
Peritoneum
A thin membrane (the peritoneum) lines the walls of the abdominal cavity and covers much of the viscera. The parietal peritoneum lines the walls of the cavity and the visceral peritoneum covers the viscera. Between the parietal and visceral layers of peritoneum is a potential space (the peritoneal cavity). Abdominal viscera either are suspended in the peritoneal cavity by folds of peritoneum (mesenteries) or are outside the peritoneal cavity. Organs suspended in the cavity are referred to as intraperitoneal (Fig. 4.33); organs outside the peritoneal cavity, with only one surface or part of one surface covered by peritoneum, are retroperitoneal.
Fig. 4.33 A. Intraperitoneal. B. Retroperitoneal.
Peritoneal cavity
The peritoneal cavity is subdivided into the greater sac and the omental bursa (lesser sac; Fig. 4.34).
 The omental bursa is a smaller subdivision of the peritoneal cavity posterior to the stomach and liver and is continuous with the greater sac through an opening, the omental (epiploic) foramen (Fig. 4.35).
The omental bursa is a smaller subdivision of the peritoneal cavity posterior to the stomach and liver and is continuous with the greater sac through an opening, the omental (epiploic) foramen (Fig. 4.35).
Fig. 4.34 Greater and lesser sacs of the peritoneal cavity.
Surrounding the omental (epiploic) foramen are numerous structures covered with peritoneum (Fig. 4.35). They include the portal vein, hepatic artery proper, and bile duct anteriorly; the inferior vena cava posteriorly; the caudate lobe of the liver superiorly; and the first part of the duodenum inferiorly.
Clinical app
The peritoneum
The peritoneum has a large surface area, which facilitates the spread of disease through the peritoneal cavity and over the bowel and visceral surfaces. Conversely, this large surface area can be used for administering certain types of treatment and a number of procedures.
Clinical app
Innervation of peritoneum
The parietal peritoneum associated with the abdominal wall is innervated by somatic afferents carried in branches of the associated spinal nerves and is therefore sensitive to well-localized pain. The visceral peritoneum is innervated by visceral afferents that accompany autonomic nerves (sympathetic and parasympathetic) back to the central nervous system. Activation of these fibers can lead to referred pain and poorly localized sensations of discomfort, and to reflex visceral motor activity.
Clinical app
Ventriculoperitoneal shunts
Patients with obstructive hydrocephalus (an excessive accumulation of cerebrospinal fluid within the cerebral ventricular system) require continuous drainage of this fluid. This is achieved by placing a catheter through the skull into the cerebral ventricles and placing the extracranial part of the tube beneath the scalp and skin of the chest wall and then passing it through the abdominal wall into the peritoneal cavity. Cerebrospinal fluid drains through the tube into the peritoneal cavity where it is absorbed.
Dialysis and peritoneal dialysis
People who develop renal failure require dialysis to live. There are two methods.
In the first method (hemodialysis), blood is taken from the circulation, dialyzed through a complex artificial membrane, and returned to the body. A high rate of blood flow is required to remove excess body fluid, exchange electrolytes, and remove noxious metabolites. To accomplish this, either an arteriovenous fistula is established surgically and is cannulated each time the patient returns for dialysis, or a large-bore cannula is placed into the right atrium, through which blood can be aspirated and returned.
In the second method of dialysis, the peritoneum is used as the dialysis membrane. The large surface area of the peritoneal cavity is an ideal dialysis membrane for fluid and electrolyte exchange. To accomplish dialysis, a small tube is inserted through the abdominal wall and dialysis fluid is injected into the peritoneal cavity. Electrolytes and molecules are exchanged across the peritoneum between the fluid and blood. Once dialysis is completed, the fluid is drained.
Clinical app
Peritoneal spread of disease
The large surface area of the peritoneal cavity allows infection and malignant disease to spread easily throughout the abdomen. If malignant cells enter the peritoneal cavity by direct invasion (e.g., from colon or ovarian cancer), spread may be rapid. Similarly, a surgeon excising a malignant tumor and releasing malignant cells into the peritoneal cavity may cause an appreciable worsening of the patient’s prognosis.
Clinical app
Perforated bowel
A perforated bowel (e.g., caused by a perforated duodenal ulcer) often leads to the release of gas into the peritoneal cavity. This peritoneal gas can be easily visualized on a chest radiograph, with the patient standing, where gas can be demonstrated in extremely small amounts beneath the diaphragm. A patient with severe abdominal pain and subdiaphragmatic gas needs a laparotomy.
Omenta, mesenteries, and ligaments
Throughout the peritoneal cavity, numerous peritoneal folds connect organs to each other or to the abdominal wall. These folds (omenta, mesenteries, and ligaments) develop from the original dorsal and ventral mesenteries, which suspend the developing gastrointestinal tract in the embryonic coelomic cavity. Some contain vessels and nerves supplying the viscera, whereas others help maintain the proper positioning of the viscera.
Omenta
The omenta consist of two layers of peritoneum, which pass from the stomach and the first part of the duodenum to other viscera. There are two:
 the greater omentum derived from the dorsal mesentery, and
the greater omentum derived from the dorsal mesentery, and
 the lesser omentum derived from the ventral mesentery.
the lesser omentum derived from the ventral mesentery.
The greater omentum is a large, apron-like, peritoneal fold that attaches to the greater curvature of the stomach and the first part of the duodenum (Fig. 4.36). It drapes inferiorly over the transverse colon and the coils of the jejunum and ileum (see Fig. 4.34). Turning posteriorly, it ascends to associate with, and become adherent to, the peritoneum on the superior surface of the transverse colon and the anterior layer of the transverse mesocolon before arriving at the posterior abdominal wall.
Fig. 4.36 Greater omentum.
Usually a thin membrane, the greater omentum always contains an accumulation of fat, which may become substantial in some individuals. Additionally, there are two arteries and accompanying veins, the right and left gastro-omental vessels, between this double-layered peritoneal apron just inferior to the greater curvature of the stomach.
The other two-layered peritoneal omentum is the lesser omentum (Fig. 4.37). It extends from the lesser curvature of the stomach and the first part of the duodenum to the inferior surface of the liver (Fig. 4.37; also see Fig. 4.34).
Fig. 4.37 Lesser omentum.
A thin membrane continuous with the peritoneal coverings of the anterior and posterior surfaces of the stomach and the first part of the duodenum, the lesser omentum is divided into:
 a medial hepatogastric ligament, which passes between the stomach and liver, and
a medial hepatogastric ligament, which passes between the stomach and liver, and
 a lateral hepatoduodenal ligament, which passes between the duodenum and liver.
a lateral hepatoduodenal ligament, which passes between the duodenum and liver.
The hepatoduodenal ligament ends laterally as a free margin and serves as the anterior border of the omental foramen (see Fig. 4.35). Enclosed in this free edge are the hepatic artery proper, the bile duct, and the portal vein. Additionally, the right and left gastric vessels are between the layers of the lesser omentum near the lesser curvature of the stomach.
Clinical app
The greater omentum
When a laparotomy is performed and the peritoneal cavity is opened, the first structure usually encountered is the greater omentum. This fatty double-layered vascular membrane hangs like an apron from the greater curvature of the stomach, drapes over the transverse colon, and lies freely suspended within the abdominal cavity. It is often referred to as the “policeman of the abdomen” because of its apparent ability to migrate to any inflamed area and wrap itself around the organ to wall off inflammation.
The greater omentum is also an important site for metastatic tumor spread. Direct omental spread by a transcoelomic route is common for carcinoma of the ovary.
Mesenteries
Mesenteries are peritoneal folds that attach viscera to the posterior abdominal wall. They allow some movement and provide a conduit for vessels, nerves, and lymphatics to reach the viscera and include:
 the mesentery—associated with parts of the small intestine,
the mesentery—associated with parts of the small intestine,
 the transverse mesocolon—associated with the transverse colon, and
the transverse mesocolon—associated with the transverse colon, and
 the sigmoid mesocolon—associated with the sigmoid colon.
the sigmoid mesocolon—associated with the sigmoid colon.
All of these are derivatives of the dorsal mesentery.
The mesentery is a large, fan-shaped, double-layered fold of peritoneum that connects the jejunum and ileum to the posterior abdominal wall (Fig. 4.38). Its superior attachment is at the duodenojejunal junction, just to the left of the upper lumbar part of the vertebral column. It passes obliquely downward and to the right, ending at the ileocecal junction near the upper border of the right sacro-iliac joint. In the fat between the two peritoneal layers of the mesentery are the arteries, veins, nerves, and lymphatics that supply the jejunum and ileum.
Fig. 4.38 Peritoneal reflections, forming mesenteries, outlined on the posterior abdominal wall.
The transverse mesocolon is a fold of peritoneum that connects the transverse colon to the posterior abdominal wall (Fig. 4.38, also see Fig. 4.34). Its two layers of peritoneum leave the posterior abdominal wall across the anterior surface of the head and body of the pancreas and pass outward to surround the transverse colon. Between its layers are the arteries, veins, nerves, and lymphatics related to the transverse colon. The anterior layer of the transverse mesocolon is adherent to the posterior layer of the greater omentum.
The sigmoid mesocolon is an inverted, V-shaped peritoneal fold that attaches the sigmoid colon to the abdominal wall (Fig. 4.38). The apex of the V is near the division of the left common iliac artery into its internal and external branches, with the left limb of the descending V along the medial border of the left psoas major muscle and the right limb descending into the pelvis to end at the level of vertebra SIII. The sigmoid and superior rectal vessels, along with the nerves and lymphatics associated with the sigmoid colon, pass through this peritoneal fold.
Ligaments
Peritoneal ligaments consist of two layers of peritoneum that connect two organs to each other or attach an organ to the body wall, and may form part of an omentum. They are usually named after the structures being connected. For example, the splenorenal ligament connects the left kidney to the spleen and the gastrophrenic ligament connects the stomach to the diaphragm.
Organs
Abdominal esophagus
The abdominal esophagus represents the short distal part of the esophagus located in the abdominal cavity. Emerging through the right crus of the diaphragm, usually at the level of vertebra TX, it passes from the esophageal hiatus to the cardial orifice of the stomach just left of the midline (Fig. 4.39).
Fig. 4.39 Abdominal esophagus.
Associated with the esophagus, as it enters the abdominal cavity, are the anterior and posterior vagal trunks:
The arterial supply to the abdominal esophagus (Fig. 4.40) includes:
 esophageal branches from the left gastric artery (from the celiac trunk), and
esophageal branches from the left gastric artery (from the celiac trunk), and
 esophageal branches from the left inferior phrenic artery (from the abdominal aorta).
esophageal branches from the left inferior phrenic artery (from the abdominal aorta).
Fig. 4.40 Arterial supply to the abdominal esophagus and stomach.
Stomach
The stomach is the most dilated part of the gastrointestinal tract and has a J-like shape (Figs. 4.41, 4.42). Positioned between the abdominal esophagus and the small intestine, the stomach is in the epigastric, umbilical, and left hypochondrium regions of the abdomen.
Fig. 4.41 Stomach.
Imaging app
Visualizing the stomach
Fig. 4.42 Double contrast radiograph of the stomach and duodenum.
The stomach is divided into four regions (Fig. 4.41):
 the cardia, which surrounds the opening of the esophagus into the stomach;
the cardia, which surrounds the opening of the esophagus into the stomach;
 the fundus of stomach, which is the area above the level of the cardial orifice;
the fundus of stomach, which is the area above the level of the cardial orifice;
 the body of stomach, which is the largest region of the stomach; and
the body of stomach, which is the largest region of the stomach; and
 the pyloric part, which is divided into the pyloric antrum and pyloric canal and is the distal end of the stomach (Fig. 4.41).
the pyloric part, which is divided into the pyloric antrum and pyloric canal and is the distal end of the stomach (Fig. 4.41).
The most distal portion of the pyloric part of the stomach is the pylorus (Fig. 4.41). It is marked on the surface of the organ by the pyloric constriction and contains a thickened ring of gastric circular muscle, the pyloric sphincter, which surrounds the distal opening of the stomach, the pyloric orifice. The pyloric orifice is just to the right of midline in a plane that passes through the lower border of vertebra LI (the transpyloric plane).
Other features of the stomach include:
 the lesser curvature, which is a point of attachment for the lesser omentum;
the lesser curvature, which is a point of attachment for the lesser omentum;
 the cardial notch, which is the superior angle created when the esophagus enters the stomach; and
the cardial notch, which is the superior angle created when the esophagus enters the stomach; and
 the angular incisure, which is a bend on the lesser curvature.
the angular incisure, which is a bend on the lesser curvature.
The arterial supply to the stomach (Fig. 4.40) includes:
 the left gastric artery from the celiac trunk,
the left gastric artery from the celiac trunk,
 the right gastric artery from the hepatic artery proper,
the right gastric artery from the hepatic artery proper,
 the right gastro-omental artery from the gastroduodenal artery,
the right gastro-omental artery from the gastroduodenal artery,
 the left gastro-omental artery from the splenic artery, and
the left gastro-omental artery from the splenic artery, and
 the posterior gastric artery from the splenic artery (variant and not always present).
the posterior gastric artery from the splenic artery (variant and not always present).
Small intestine
The small intestine is the longest part of the gastrointestinal tract and extends from the pyloric orifice of the stomach to the ileocecal fold. This hollow tube, which is approximately 6 to 7 m long with a narrowing diameter from beginning to end, consists of the duodenum, the jejunum, and the ileum.
Duodenum
The first part of the small intestine is the duodenum. This C-shaped structure, adjacent to the head of the pancreas, is 20 to 25 cm long and is above the level of the umbilicus; its lumen is the widest of the small intestine (Fig. 4.43). It is retroperitoneal except for its beginning, which is connected to the liver by the hepatoduodenal ligament, a part of the lesser omentum.
Fig. 4.43 Duodenum.
The duodenum is divided into four parts (Fig. 4.43).
 The inferior part (third part) of the duodenum is the longest section, crossing the inferior vena cava, the aorta, and the vertebral column (Figs. 4.42, 4.43). It is crossed anteriorly by the superior mesenteric artery and vein.
The inferior part (third part) of the duodenum is the longest section, crossing the inferior vena cava, the aorta, and the vertebral column (Figs. 4.42, 4.43). It is crossed anteriorly by the superior mesenteric artery and vein.
This duodenojejunal flexure is surrounded by a fold of peritoneum containing muscle fibers called the suspensory muscle (ligament) of duodenum (ligament of Treitz).
The arterial supply to the duodenum (Fig. 4.44) includes:
 branches from the gastroduodenal artery,
branches from the gastroduodenal artery,
 the supraduodenal artery from the gastroduodenal artery,
the supraduodenal artery from the gastroduodenal artery,
 the first jejunal branch from the superior mesenteric artery.
the first jejunal branch from the superior mesenteric artery.
Fig. 4.44 Arterial supply to the duodenum.
Jejunum
The jejunum and ileum make up the last two sections of the small intestine (Fig. 4.45). The jejunum represents the proximal two-fifths. It is mostly in the left upper quadrant of the abdomen and is larger in diameter and has a thicker wall than the ileum. Additionally, the inner mucosal lining of the jejunum is characterized by numerous prominent folds that circle the lumen (plicae circulares). The less prominent arterial arcades and longer vasa recta (straight arteries) compared with those of the ileum are a unique characteristic of the jejunum (Fig. 4.46).
Fig. 4.46 Differences in the arterial supply to the small intestine. A. Jejunum. B. Ileum.
The arterial supply to the jejunum includes jejunal arteries from the superior mesenteric artery (Fig. 4.47).
Fig. 4.47 Arterial supply to the ileum and jejunum.
Ileum
The ileum makes up the distal three fifths of the small intestine and is mostly in the right lower quadrant. Compared with the jejunum, the ileum has thinner walls, fewer and less prominent mucosal folds (plicae circulares), shorter vasa recta, more mesenteric fat, and more arterial arcades (Fig. 4.46).
The ileum opens into the large intestine where the cecum and ascending colon join together. Two flaps projecting into the lumen of the large intestine (the ileocecal fold) surround the opening (Fig. 4.48). The flaps of the ileocecal fold come together at their end forming ridges. Musculature from the ileum continues into each flap, forming a sphincter. Possible functions of the ileocecal fold include preventing reflux from the cecum to the ileum, and regulating the passage of contents from the ileum to the cecum.
Fig. 4.48 Illustration showing ileocecal junction and the ileocecal fold.
The arterial supply to the ileum (Fig. 4.47) includes:
 ileal arteries from the superior mesenteric artery, and
ileal arteries from the superior mesenteric artery, and
 an ileal branch from the ileocolic artery (from the superior mesenteric artery).
an ileal branch from the ileocolic artery (from the superior mesenteric artery).
Epithelial transition between the abdominal esophagus and stomach
The gastroesophageal junction is demarcated by a transition from one epithelial type to another epithelial type. In some people, the histological junction does not lie at the physiological gastroesophageal junction, but is in the lower one third of the esophagus. This may predispose these people to esophageal ulceration, and is also associated with an increased risk of adenocarcinoma.
Clinical app
Surgery for obesity
Surgical procedures for obesity are divided into malabsorption and restrictive procedures.
In these procedures, the stomach is surgically anastomosed to the distal jejunum/ileum so that the stomach contents bypass most of the small intestine. There are certain complications of malabsorption procedures, which include anemia, osteoporosis, and diarrhea.
These procedures involve placing bands around the stomach in order to reduce the amount of food that can be ingested and also produce an earlier feeling of satiety.
Clinical app
Duodenal ulceration
Duodenal ulcers usually occur in the superior part of the duodenum:
 Anterior duodenal ulcers erode into the peritoneal cavity, causing peritonitis. This intense inflammatory reaction and the local ileus promote adhesion of the greater omentum, which attempts to seal off the perforation. The stomach and duodenum usually contain considerable amounts of gas, which enters the peritoneal cavity and can be observed on a chest radiograph of an erect patient as subdiaphragmatic gas.
Anterior duodenal ulcers erode into the peritoneal cavity, causing peritonitis. This intense inflammatory reaction and the local ileus promote adhesion of the greater omentum, which attempts to seal off the perforation. The stomach and duodenum usually contain considerable amounts of gas, which enters the peritoneal cavity and can be observed on a chest radiograph of an erect patient as subdiaphragmatic gas.
Clinical app
Examination of the bowel lumen
Barium sulfate solutions may be swallowed by the patient and can be visualized using an X-ray fluoroscopy unit. The lumen can be examined for masses (e.g., polyps and tumors) and peristaltic waves can be assessed. Patients may also be given carbon dioxide–releasing granules to fill the stomach so that the barium thinly coats the mucosa, resulting in images displaying fine mucosal detail.
Clinical app
Meckel’s diverticulum
A Meckel’s diverticulum is the remnant of the proximal part of the yolk stalk (vitelline duct), which extends into the umbilical cord in the embryo and lies on the antimesenteric border of the ileum. Although it is an uncommon finding (occurring in approximately 2% of the population), it is always important to consider the diagnosis of Meckel’s diverticulum because it does produce symptoms in a small number of patients.
Clinical app
Carcinoma of the stomach
Carcinoma of the stomach is a common gastrointestinal malignancy. Chronic gastric inflammation (gastritis), pernicious anemia, and polyps predispose to the development of this aggressive cancer, which is usually not diagnosed until late in the course of the disease.
The diagnosis may be made using barium and conventional radiology or endoscopy, which allows a biopsy to be obtained at the same time. Ultrasound scanning is used to check the liver for metastatic spread, and, if negative, computed tomography is carried out to assess for surgical resectability. If carcinoma of the stomach is diagnosed early, a curative surgical resection is possible.
Imaging app
Endoscopic examination of the abdominal gastrointestinal tract
Endoscopy is a minimally invasive diagnostic medical procedure that can be used to assess the interior surfaces of an organ by inserting a tube into the body. The instrument is typically made of a flexible plastic material through which a light source and eye piece are attached at one end.
In gastrointestinal and abdominal medicine, an endoscope is used to assess the esophagus, stomach, duodenum, and proximal small bowel (Fig. 4.49, A-E). The tube is swallowed by the patient under light sedation and is extremely well tolerated.
Assessment of the colon is performed by passage of the tube through the anus and into the rectum. The whole of the colon can be readily assessed (Fig. 4.49F-J).
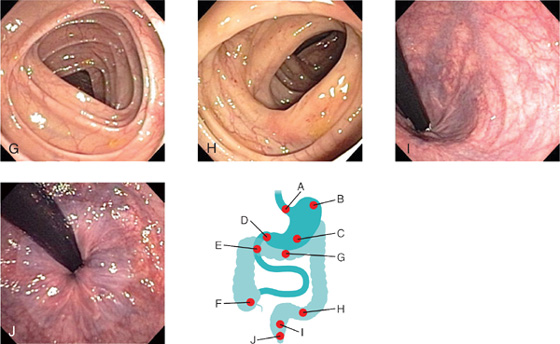
Large intestine
The large intestine extends from the distal end of the ileum to the anus, a distance of approximately 1.5 m in adults. It absorbs fluids and salts from the gut contents, thus forming feces, and consists of the cecum, appendix, colon, rectum, and anal canal (Figs. 4.50, 4.51).
Fig. 4.50 Large intestine.
Imaging app
Visualizing the large intestine
Fig. 4.51 Radiograph, using barium, showing the large intestine.
Beginning in the right groin as the cecum, with its associated appendix, the large intestine continues upward as the ascending colon through the right flank and into the right hypochondrium (Fig. 4.52). Just below the liver, it bends to the left, forming the right colic flexure (hepatic flexure), and crosses the abdomen as the transverse colon to the left hypochondrium. At this position, just below the spleen, the large intestine bends downward, forming the left colic flexure (splenic flexure), and continues as the descending colon through the left flank and into the left groin.
Fig. 4.52 Position of the large intestine in the nine-region organizational pattern.
It enters the upper part of the pelvic cavity as the sigmoid colon, continues on the posterior wall of the pelvic cavity as the rectum, and terminates as the anal canal.
The general characteristics of most of the large intestine (Fig. 4.53) are:
 its large internal diameter compared to that of the small intestine;
its large internal diameter compared to that of the small intestine;
 peritoneal-covered accumulations of fat (the omental appendices) are associated with the colon;
peritoneal-covered accumulations of fat (the omental appendices) are associated with the colon;
 the sacculations of the colon (the haustra of colon).
the sacculations of the colon (the haustra of colon).
Fig. 4.53 Cecum and appendix.
Cecum and appendix
The cecum is the first part of the large intestine (Fig. 4.53). It is inferior to the ileocecal opening and in the right iliac fossa. It is an intraperitoneal structure because of its mobility not because of its suspension by a mesentery.
The cecum is continuous with the ascending colon at the entrance of the ileum and is usually in contact with the anterior abdominal wall. It may cross the pelvic brim to lie in the true pelvis. The appendix is attached to the posteromedial wall of the cecum, just inferior to the end of the ileum (Fig. 4.53).
The appendix is a narrow, hollow, blind-ended tube connected to the cecum. It has large aggregations of lymphoid tissue in its walls and is suspended from the terminal ileum by the mesoappendix (Fig. 4.54), which contains the appendicular vessels. Its point of attachment to the cecum is consistent with the highly visible free taenia leading directly to the base of the appendix, but the location of the rest of the appendix varies considerably (Fig. 4.55). It may be:
 suspended over the pelvic brim in a pelvic or descending position;
suspended over the pelvic brim in a pelvic or descending position;
 below the cecum in a subcecal location; or
below the cecum in a subcecal location; or
Fig. 4.54 Mesoappendix.
Fig. 4.55 Positions of the appendix.
The arterial supply to the cecum and appendix (Fig. 4.57) includes:
 the anterior cecal artery from the ileocolic artery (from the superior mesenteric artery),
the anterior cecal artery from the ileocolic artery (from the superior mesenteric artery),
 the posterior cecal artery from the ileocolic artery (from the superior mesenteric artery), and
the posterior cecal artery from the ileocolic artery (from the superior mesenteric artery), and
 the appendicular artery from the ileocolic artery (from the superior mesenteric artery).
the appendicular artery from the ileocolic artery (from the superior mesenteric artery).
Clinical app
Appendicitis
Acute appendicitis is an abdominal emergency. It usually occurs when the appendix is obstructed by either a fecalith or enlargement of the lymphoid nodules. Within the obstructed appendix, bacteria proliferate and invade the appendix wall, which becomes damaged by pressure necrosis. In some instances, this may resolve spontaneously; in other cases, inflammatory change continues and perforation ensues, which may lead to localized or generalized peritonitis.
Most patients with acute appendicitis have localized tenderness in the right groin. Initially, the pain begins as a central/periumbilical, which tends to come and go. As the disease progresses, the pain shifts to the lower right groin and is focal.
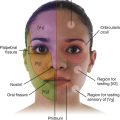
When the appendix first becomes inflamed, the visceral sensory fibers are stimulated (Fig. 4.56). These fibers enter the spinal cord at spinal cord level T10. The pain is referred to the dermatome of T10 in the periumbilical region. The pain is diffuse, not focal; every time a peristaltic wave passes through the ileocecal region, the pain recurs. This intermittent type of pain is referred to as colic.
Fig. 4.56 Mechanism for referred pain from an inflamed appendix to the T10 dermatome.
In later stages of the disease, the appendix contacts and irritates the parietal peritoneum in the right iliac fossa, which is innervated by somatic sensory nerves. This produces a constant focal pain, which predominates over the colicky pain that the patient felt some hours previously and the patient no longer perceives the referred pain.
Colon
The colon extends superiorly from the cecum and consists of the ascending, transverse, descending, and sigmoid colon (Fig. 4.58). Its ascending and descending segments are (secondarily) retroperitoneal and its transverse and sigmoid segments are intraperitoneal.
Fig. 4.58 Colon.
At the junction of the ascending and transverse colon is the right colic flexure, which is just inferior to the right lobe of the liver (Fig. 4.59). A similar, but more acute bend (the left colic flexure) occurs at the junction of the transverse and descending colon (Fig. 4.59). This bend is just inferior to the spleen, higher and more posterior than the right colic flexure, and is attached to the diaphragm by the phrenicocolic ligament.
Fig. 4.59 Right and left colic flexures.
Immediately lateral to the ascending and descending colons are the right and left paracolic gutters (Fig. 4.58). These depressions are formed between the lateral margins of the ascending and descending colon and the posterolateral abdominal wall and are gutters through which material can pass from one region of the peritoneal cavity to another. Because major vessels and lymphatics are on the medial or posteromedial sides of the ascending and descending colon, a relatively blood-free mobilization of the ascending and descending colon is possible by cutting the peritoneum along these lateral paracolic gutters.
The final segment of the colon (the sigmoid colon) begins above the pelvic inlet and extends to the level of vertebra SIII, where it is continuous with the rectum (Fig. 4.58). This S-shaped structure is quite mobile except at its beginning, where it continues from the descending colon, and at its end, where it continues as the rectum. Between these points, it is suspended by the sigmoid mesocolon.
The arterial supply to the ascending colon includes (Fig. 4.60):
 the colic branch from the ileocolic artery (from the superior mesenteric artery),
the colic branch from the ileocolic artery (from the superior mesenteric artery),
 the anterior cecal artery from the ileocolic artery (from the superior mesenteric artery),
the anterior cecal artery from the ileocolic artery (from the superior mesenteric artery),
 the posterior cecal artery from the ileocolic artery (from the superior mesenteric artery), and
the posterior cecal artery from the ileocolic artery (from the superior mesenteric artery), and
 the right colic artery from the superior mesenteric artery.
the right colic artery from the superior mesenteric artery.
Fig. 4.60 Arterial supply to the colon.
The arterial supply to the transverse colon includes (Fig. 4.60):
 the right colic artery from the superior mesenteric artery,
the right colic artery from the superior mesenteric artery,
 the middle colic artery from the superior mesenteric artery, and
the middle colic artery from the superior mesenteric artery, and
 the left colic artery from the inferior mesenteric artery.
the left colic artery from the inferior mesenteric artery.
The arterial supply to the descending colon includes the left colic artery from the inferior mesenteric artery (Fig. 4.60).
The arterial supply to the sigmoid colon includes sigmoidal arteries from the inferior mesenteric artery (Fig. 4.60).
Rectum and anal canal
Extending from the sigmoid colon is the rectum (Fig. 4.61). The rectosigmoid junction is usually described as being at the level of vertebra SIII or at the end of the sigmoid mesocolon because the rectum is a retroperitoneal structure.
Fig. 4.61 Rectum and anal canal.
The anal canal is the continuation of the large intestine inferior to the rectum.
The arterial supply to the rectum and anal canal includes (Fig. 4.62):
 the superior rectal artery from the inferior mesenteric artery,
the superior rectal artery from the inferior mesenteric artery,
 the middle rectal artery from the internal iliac artery, and
the middle rectal artery from the internal iliac artery, and
 the inferior rectal artery from the internal pudendal artery (from the internal iliac artery).
the inferior rectal artery from the internal pudendal artery (from the internal iliac artery).
Fig. 4.62 Arterial supply to the rectum and anal canal. Posterior view.
Congenital disorders of the gastrointestinal tract
The normal positions of the abdominal viscera result from a complex series of rotations that the gut tube undergoes and from the growth of the abdominal cavity to accommodate changes in the size of the developing organs.
Malrotation is incomplete rotation and fixation of the midgut after it has passed from the umbilical sac and returned to the abdominal coelom. The proximal attachment of the small bowel mesentery begins at the suspensory muscle of duodenum (ligament of Treitz), which determines the position of the duodenojejunal junction. The mesentery of the small bowel ends at the level of the ileocecal junction in the right lower quadrant. This long line of fixation of the mesentery prevents accidental twists of the gut.
If the duodenojejunal flexure or the cecum does not end up in its usual site, the origin of the small bowel mesentery shortens, which permits twisting of the small bowel around the axis of the superior mesenteric artery. Twisting of the bowel, in general, is termed volvulus. Volvulus of the small bowel may lead to a reduction of blood flow and infarction.
Clinical app
Bowel obstruction
A bowel obstruction can be either mechanical or functional:
Clinical app
Diverticular disease
Diverticular disease is the development of multiple colonic diverticula, predominantly throughout the sigmoid colon, though the whole colon may be affected (Fig. 4.63). The sigmoid colon has the smallest diameter of any portion of the colon and is therefore the site where intraluminal pressure is potentially the highest.
Patients tend to develop symptoms and signs when the neck of the diverticulum becomes obstructed by feces and becomes infected. Inflammation may spread along the wall, causing abdominal pain.
Because of the anatomical position of the sigmoid colon there are a number of complications that may occur. The diverticula can perforate to form an abscess in the pelvis. The inflammation may produce an inflammatory mass, obstructing the left ureter. Inflammation may also spread to the bladder, producing a fistula between the sigmoid colon and the bladder.
Liver
The liver is the largest visceral organ in the body and is primarily in the right hypochondrium and epigastric region, extending into the left hypochondrium (or in the right upper quadrant, extending into the left upper quadrant) (see Fig. 4.4).
Surfaces of the liver include:
 a diaphragmatic surface in the anterior, superior, and posterior directions, and
a diaphragmatic surface in the anterior, superior, and posterior directions, and
 a visceral surface in the inferior direction (Fig. 4.64).
a visceral surface in the inferior direction (Fig. 4.64).
Fig. 4.64 Surfaces of the liver and recesses associated with the liver.
Diaphragmatic surface
The diaphragmatic surface of the liver, which is smooth and domed, lies against the inferior surface of the diaphragm (Fig. 4.65). Associated with it are the subphrenic and hepatorenal recesses (Fig. 4.64):
 The subphrenic recess separates the diaphragmatic surface of the liver from the diaphragm and is divided into right and left areas by the falciform ligament, a structure derived from the ventral mesentery in the embryo.
The subphrenic recess separates the diaphragmatic surface of the liver from the diaphragm and is divided into right and left areas by the falciform ligament, a structure derived from the ventral mesentery in the embryo.
Fig. 4.65 Diaphragmatic surface of the liver.
The subphrenic and hepatorenal recesses are continuous anteriorly.
Visceral surface
The visceral surface of the liver is covered with visceral peritoneum except in the fossa for the gallbladder and at the porta hepatis (gateway to the liver; Fig. 4.66). Structures related to it include the following (Fig. 4.66):
 right anterior part of the stomach,
right anterior part of the stomach,
 superior part of the duodenum,
superior part of the duodenum,
Fig. 4.66 Posterior view of the bare area of the liver and associated ligaments.
The porta hepatis serves as the point of entry into the liver for the hepatic arteries and the portal vein, and the exit point for the hepatic ducts (Fig. 4.66).
Associated ligaments
The liver is attached to the anterior abdominal wall by the falciform ligament and, except for a small area of the liver against the diaphragm (the bare area), the liver is almost completely surrounded by visceral peritoneum (Fig. 4.66). Additional folds of peritoneum connect the liver to the stomach (hepatogastric ligament), the duodenum (hepatoduodenal ligament), and the diaphragm (right and left triangular ligaments and anterior and posterior coronary ligaments).
The bare area of the liver is a part of the liver on the diaphragmatic surface where there is no intervening peritoneum between the liver and the diaphragm (Fig. 4.66):
Lobes
The liver is divided into right and left lobes by fossae for the gallbladder and the inferior vena cava (Figs. 4.66, 4.67, 4.68). The right lobe of liver is the largest lobe, whereas the left lobe of liver is smaller. The quadrate and caudate lobes are described as arising from the right lobe of liver, but functionally are distinct.
Fig. 4.67 Visceral surface of the liver.
Imaging app
Visualizing the liver
Fig. 4.68 Abdominal computed tomogram, with contrast, in the axial plane, showing the visceral surface of the liver.
The arterial supply to the liver includes (Fig. 4.69):
Fig. 4.69 Arterial supply to the liver and gallbladder.
Clinical app
Ostomies
It is occasionally necessary to surgically externalize bowel to the anterior abdominal wall. Externalization of bowel plays an important role in patient management.
Gastrostomy is performed when the stomach is attached to the anterior abdominal wall and a tube is placed through the skin into the stomach. Typically this is performed to feed the patient when it is impossible to take food and fluid orally (e.g., complex head and neck cancer).
In jejunostomy, the jejunum is brought to the anterior abdominal wall and fixed. The jejunostomy is used as a site where a feeding tube is placed through the anterior abdominal wall into the proximal efferent small bowel.
Clinical app
Segmental anatomy of the liver
For many years the segmental anatomy of the liver was of little importance. However, since the development of liver resection surgery, the size, shape, and segmental anatomy of the liver has become clinically important, especially with regard to liver resection for metastatic disease.
The liver is divided by the principal plane, which divides the organ into halves of approximately equal size. This imaginary line is defined by a parasagittal line that passes through the gallbladder fossa to the inferior vena cava. It is in this plane that the middle hepatic vein is found. Importantly, the principal plane divides the left half of the liver from the right half. The lobes of the liver are unequal in size and bear only little relevance to operative anatomy.
The traditional eight segment anatomy of the liver relates to the hepatic arterial, portal, and biliary drainage of these segments (Fig. 4.70).
The caudate lobe is defined as segment I, the remaining segments are numbered in a clockwise fashion up to segment VIII. The features are extremely consistent among individuals.
From a surgical perspective a right hepatectomy would involve division of the liver in the principal plane in which segments V, VI, VII, and VIII would be removed, leaving segments I, II, III, and IV.
Gallbladder
The gallbladder is a pear-shaped sac lying on the visceral surface of the right lobe of the liver in a fossa between the right and quadrate lobes (Fig. 4.66). It has:
 a rounded end (fundus of gallbladder), which may project from the inferior border of the liver;
a rounded end (fundus of gallbladder), which may project from the inferior border of the liver;
 a narrow part (neck of gallbladder) with mucosal folds forming the spiral fold.
a narrow part (neck of gallbladder) with mucosal folds forming the spiral fold.
The arterial supply to the gallbladder (see Fig. 4.69) is the cystic artery from the right hepatic artery (a branch of the hepatic artery proper).
The gallbladder receives, concentrates, and stores bile from the liver.
Pancreas
The pancreas lies mostly posterior to the stomach (Figs. 4.71, 4.73). It extends across the posterior abdominal wall from the duodenum, on the right, to the spleen, on the left.
Fig. 4.71 Pancreas.
The pancreas is (secondarily) retroperitoneal except for a small part of its tail and consists of a head, uncinate process, neck, body, and tail (Fig. 4.71).
 The head of pancreas lies within the C-shaped concavity of the duodenum.
The head of pancreas lies within the C-shaped concavity of the duodenum.
 The body of pancreas is elongate and extends from the neck to the tail of the pancreas.
The body of pancreas is elongate and extends from the neck to the tail of the pancreas.
 The tail of pancreas passes between layers of the splenorenal ligament.
The tail of pancreas passes between layers of the splenorenal ligament.
The pancreatic duct begins in the tail of the pancreas (Fig. 4.72). It passes to the right through the body of the pancreas and, after entering the head of the pancreas, turns inferiorly. In the lower part of the head of pancreas, the pancreatic duct joins the bile duct. The joining of these two structures forms the hepatopancreatic ampulla (ampulla of Vater), which enters the descending (second) part of the duodenum at the major duodenal papilla. Surrounding the ampulla is the sphincter of ampulla (sphincter of Oddi), which is a collection of smooth muscle.
Fig. 4.72 Pancreatic duct system.
Visualizing the pancreas
Fig. 4.73 Abdominal computed tomogram, with contrast, in the axial plane.
The accessory pancreatic duct empties into the duodenum just above the major duodenal papilla at the minor duodenal papilla (Fig. 4.72). If the accessory duct is followed from the minor papilla into the head of the pancreas, a branch point is discovered:
The main and accessory pancreatic ducts usually communicate with each other. The presence of these two ducts reflects the embryological origin of the pancreas from dorsal and ventral buds from the foregut.
The arterial supply to the pancreas (Fig. 4.74) includes the:
 gastroduodenal artery from the common hepatic artery (a branch of the celiac trunk),
gastroduodenal artery from the common hepatic artery (a branch of the celiac trunk),
 anterior superior pancreaticoduodenal artery from the gastroduodenal artery,
anterior superior pancreaticoduodenal artery from the gastroduodenal artery,
 posterior superior pancreaticoduodenal artery from the gastroduodenal artery,
posterior superior pancreaticoduodenal artery from the gastroduodenal artery,
 dorsal pancreatic artery from the inferior pancreatic artery (a branch of the splenic artery),
dorsal pancreatic artery from the inferior pancreatic artery (a branch of the splenic artery),
 great pancreatic artery from the inferior pancreatic artery (a branch of the splenic artery),
great pancreatic artery from the inferior pancreatic artery (a branch of the splenic artery),
 dorsal pancreatic and greater pancreatic arteries (branches of the splenic artery),
dorsal pancreatic and greater pancreatic arteries (branches of the splenic artery),
Fig. 4.74 Arterial supply to the pancreas. Posterior view.
Clinical app
Annular pancreas
The pancreas develops from ventral and dorsal buds from the foregut. The dorsal bud forms most of the head, neck, and body of pancreas. The ventral bud, which consists of right and left portions that normally fuse, rotates posteriorly around the bile duct to form part of the head and the uncinate process. If the two components of the ventral bud fail to fuse, they may encircle the duodenum. The duodenum is therefore constricted and may even undergo atresia, and be absent at birth because of developmental problems. After birth, the child may fail to thrive and vomit due to poor gastric emptying.
Sometimes an annular pancreas is diagnosed in utero by ultrasound scanning. The obstruction of the duodenum may prevent the fetus from swallowing enough amniotic fluid, which may increase the overall volume of amniotic fluid in the amniotic sac surrounding the fetus (polyhydramnios).
Pancreatic cancer
Pancreatic cancer accounts for a significant number of deaths and is often referred to as the “silent killer.” Malignant tumors of the pancreas may occur anywhere within the pancreas, but are most frequent within the head and the neck. There are a number of nonspecific findings in patients with pancreatic cancer, including upper abdominal pain, loss of appetite, and weight loss. Depending on the exact site of the cancer, obstruction of the common bile duct may occur, which can produce obstructive jaundice. Although surgery is indicated in patients where there is a possibility of cure, most detected cancers have typically spread locally invading the portal vein, and superior mesenteric vessels, and may extend into the porta hepatis. Lymph node spread also is common and these factors would preclude curative surgery.
Given the position of the pancreas, a surgical resection is a complex procedure involving resection of the region of pancreatic tumor usually with part of the duodenum necessitating a complex bypass procedure.
Duct system for bile
The duct system for the passage of bile extends from the liver, connects with the gallbladder, and empties into the descending part of the duodenum (Fig. 4.75). The coalescence of ducts begins in the liver parenchyma and continues until the right and left hepatic ducts are formed. These drain the respective lobes of the liver.
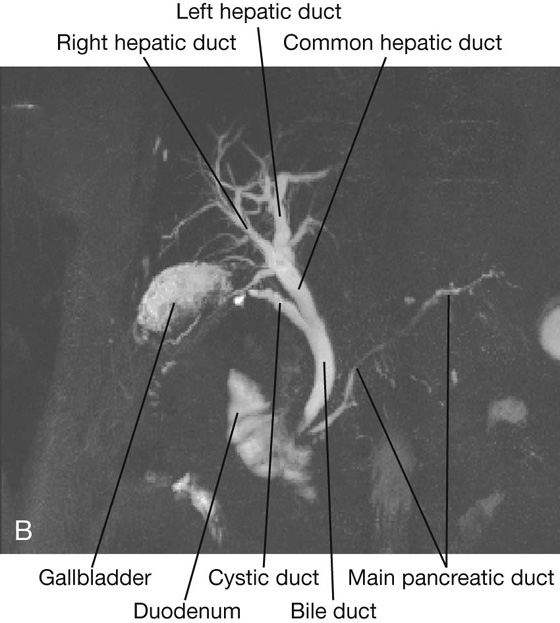
The two hepatic ducts combine to form the common hepatic duct, which runs, near the liver, with the hepatic artery proper and portal vein in the free margin of the lesser omentum.
As the common hepatic duct continues to descend, it is joined by the cystic duct from the gallbladder. This completes the formation of the bile duct. At this point, the bile duct lies to the right of the hepatic artery proper and usually to the right of, and anterior to, the portal vein in the free margin of the lesser omentum (see Fig. 4.35). The omental foramen is posterior to these structures at this point.
The bile duct continues to descend, passing posteriorly to the superior part of the duodenum before joining with the pancreatic duct to enter the descending part of the duodenum at the major duodenal papilla (Fig. 4.75).
Clinical app
Gallstones
Gallstones are present in approximately 10% of people over the age of 40 and are more common in women. They consist of a variety of components, but are predominantly a mixture of cholesterol and bile pigment. They may undergo calcification, which can be demonstrated on plain radiographs. From time to time, gallstones lodge in the neck of the gallbladder. The gallbladder cannot empty normally and contractions of the gallbladder wall produce severe pain. If this persists, a cholecystectomy (removal of the gallbladder) may be necessary.
Sometimes the gallbladder may become inflamed (cholecystitis). If the inflammation involves the related parietal peritoneum of the diaphragm, pain may not only occur in the right upper quadrant of the abdomen but may also be referred to the shoulder on the right side (phrenic nerve, C3-C5, innervation of diaphragm).
From time to time, small gallstones pass into the bile duct and are trapped in the region of the sphincter of the ampulla, which obstructs the flow of bile into the duodenum. This, in turn, produces jaundice.
Jaundice
Jaundice is a yellow discoloration of the skin caused by excess bile pigment (bilirubin) within the plasma. The yellow color is best appreciated by looking at the normally white sclerae of the eyes, which turn yellow.
Any obstruction to the biliary tree can produce jaundice, but the two most common causes are gallstones within the bile duct and an obstructing tumor at the head of the pancreas.
Spleen
The spleen develops as part of the vascular system in the part of the dorsal mesentery that suspends the developing stomach from the body wall. In the adult, the spleen lies against the diaphragm, in the area of rib IX to rib X (Fig. 4.76). It is therefore in the left upper quadrant, or left hypochondrium, of the abdomen.
Fig. 4.76 Location of the spleen in a lateral view.
The spleen is connected to the:
 left kidney by the splenorenal ligament (Fig. 4.77), which contains the splenic vessels.
left kidney by the splenorenal ligament (Fig. 4.77), which contains the splenic vessels.
Fig. 4.77 Splenic ligaments and related vasculature.
Both these ligaments are parts of the greater omentum.
The spleen is surrounded by visceral peritoneum except in the area of the hilum on the medial surface of the spleen (Fig. 4.78). The splenic hilum is the entry point for the splenic vessels and occasionally the tail of the pancreas reaches this area.
Fig. 4.78 Surfaces and hilum of the spleen.
The arterial supply to the spleen (Fig. 4.79) is the splenic artery from the celiac trunk.
Fig. 4.79 Arterial supply to the spleen.
Clinical app
From a clinical point of view, there are two main categories of spleen disorders: rupture and enlargement.
This tends to occur when there is localized trauma to the left upper quadrant. It may be associated with left lower rib fractures. Because the spleen has such an extremely thin capsule it is susceptible to injury even when there is no damage to surrounding structures, and because the spleen is highly vascular, when ruptured, it bleeds profusely into the peritoneal cavity.
The spleen is an organ of the reticuloendothelial system. Diseases that affect the reticuloendothelial system (e.g., leukemia, lymphoma, and certain infections) may produce generalized lymphadenopathy and enlargement of the spleen (splenomegaly).
Arterial supply to the gastrointestinal tract
The abdominal aorta begins at the aortic hiatus of the diaphragm, anterior to the lower border of vertebra TXII (Fig. 4.80). It descends through the abdomen, anterior to the vertebral bodies, and by the time it ends at the level of vertebra LIV it is slightly to the left of midline. The terminal branches of the abdominal aorta are the two common iliac arteries.
Fig. 4.80 Anterior branches of the abdominal aorta.
Anterior branches of the abdominal aorta
The abdominal aorta has anterior, lateral, and posterior branches as it passes through the abdominal cavity. The three anterior branches supply the gastrointestinal viscera: the celiac trunk and the superior mesenteric and inferior mesenteric arteries (Fig. 4.80).
The primitive gut tube can be divided into foregut, midgut, and hindgut regions. The boundaries of these regions are directly related to the areas of distribution of the three anterior branches of the abdominal aorta (Fig. 4.81).
 The foregut begins with the abdominal esophagus and ends just inferior to the major duodenal papilla, midway along the descending part of the duodenum. It includes the abdominal esophagus, stomach, duodenum (superior to the major papilla), liver, pancreas, and gallbladder. The spleen also develops in relation to the foregut region. The foregut is supplied by the celiac trunk (Fig. 4.81).
The foregut begins with the abdominal esophagus and ends just inferior to the major duodenal papilla, midway along the descending part of the duodenum. It includes the abdominal esophagus, stomach, duodenum (superior to the major papilla), liver, pancreas, and gallbladder. The spleen also develops in relation to the foregut region. The foregut is supplied by the celiac trunk (Fig. 4.81).
 The midgut begins just inferior to the major duodenal papilla, in the descending part of the duodenum, and ends at the junction between the proximal two thirds and distal one third of the transverse colon. It includes the duodenum (inferior to the major duodenal papilla), jejunum, ileum, cecum, appendix, ascending colon, and the right two thirds of the transverse colon. The midgut is supplied by the superior mesenteric artery (Fig. 4.81).
The midgut begins just inferior to the major duodenal papilla, in the descending part of the duodenum, and ends at the junction between the proximal two thirds and distal one third of the transverse colon. It includes the duodenum (inferior to the major duodenal papilla), jejunum, ileum, cecum, appendix, ascending colon, and the right two thirds of the transverse colon. The midgut is supplied by the superior mesenteric artery (Fig. 4.81).
 The hindgut begins just before the left colic flexure (the junction between the proximal two thirds and distal one third of the transverse colon) and ends midway through the anal canal. It includes the left one third of the transverse colon, descending colon, sigmoid colon, rectum, and upper part of the anal canal. The hindgut is supplied by the inferior mesenteric artery (Fig. 4.81).
The hindgut begins just before the left colic flexure (the junction between the proximal two thirds and distal one third of the transverse colon) and ends midway through the anal canal. It includes the left one third of the transverse colon, descending colon, sigmoid colon, rectum, and upper part of the anal canal. The hindgut is supplied by the inferior mesenteric artery (Fig. 4.81).
Celiac trunk
The celiac trunk is the anterior branch of the abdominal aorta supplying the foregut. It arises from the abdominal aorta immediately below the aortic hiatus of the diaphragm (Fig. 4.82), anterior to the upper part of vertebra LI. It immediately divides into the left gastric, splenic, and common hepatic arteries.
Fig. 4.82 Distribution of the celiac trunk.
The left gastric artery is the smallest branch of the celiac trunk. It ascends to the cardioesophageal junction and sends esophageal branches upward to the abdominal part of the esophagus (Fig. 4.82). Some of these branches continue through the esophageal hiatus of the diaphragm and anastomose with esophageal branches from the thoracic aorta. The left gastric artery itself turns to the right and descends along the lesser curvature of the stomach in the lesser omentum. It supplies both surfaces of the stomach in this area and anastomoses with the right gastric artery.
The splenic artery, the largest branch of the celiac trunk, takes a tortuous course to the left along the superior border of the pancreas (Fig. 4.82). It travels in the splenorenal ligament and divides into numerous branches, which enter the hilum of the spleen.
As the splenic artery passes along the superior border of the pancreas, it gives off numerous small branches to supply the neck, body, and tail of the pancreas (Fig. 4.83).
Fig. 4.83 Branches of the gastroduodenal artery.
Approaching the spleen, the splenic artery gives off short gastric arteries, which pass through the gastrosplenic ligament to supply the fundus of the stomach. It also gives off the left gastro-omental artery, which runs to the right along the greater curvature of the stomach, and anastomoses with the right gastro-omental artery.
The common hepatic artery is a medium-sized branch of the celiac trunk that runs to the right and divides into its two terminal branches, the hepatic artery proper and the gastroduodenal artery (Figs. 4.82, 4.83).
The hepatic artery proper ascends toward the liver in the free edge of the lesser omentum. It runs to the left of the bile duct and anterior to the portal vein, and divides into the right and left hepatic arteries near the porta hepatis (Fig. 4.84). As the right hepatic artery nears the liver, it gives off the cystic artery to the gallbladder.
Fig. 4.84 Distribution of the common hepatic artery.
The gastroduodenal artery may give off the supraduodenal artery and does give off the posterior superior pancreaticoduodenal artery near the upper border of the superior part of the duodenum. After these branches the gastroduodenal artery continues descending posterior to the superior part of the duodenum. Reaching the lower border of the superior part of the duodenum, the gastroduodenal artery divides into its terminal branches, the right gastro-omental artery and the anterior superior pancreaticoduodenal artery (Fig. 4.83).
The right gastro-omental artery passes to the left, along the greater curvature of the stomach, eventually anastomosing with the left gastro-omental artery from the splenic artery. The right gastro-omental artery sends branches to both surfaces of the stomach and additional branches descend into the greater omentum.
The anterior superior pancreaticoduodenal artery descends and, along with the posterior superior pancreaticoduodenal artery, supplies the head of the pancreas and the duodenum (Fig. 4.83). These vessels eventually anastomose with the anterior and posterior branches of the inferior pancreaticoduodenal artery.
Superior mesenteric artery
The superior mesenteric artery is the anterior branch of the abdominal aorta supplying the midgut. It arises from the abdominal aorta immediately below the celiac artery (Fig. 4.85), anterior to the lower part of vertebra LI.
Fig. 4.85 Initial branching and relationships of the superior mesenteric artery.
The superior mesenteric artery is crossed anteriorly by the splenic vein and the neck of pancreas. Posterior to the artery are the left renal vein, the uncinate process of the pancreas, and the inferior part of the duodenum (Fig. 4.85). After giving off its first branch (the inferior pancreaticoduodenal artery), the superior mesenteric artery gives off jejunal and ileal arteries on its left (Figs. 4.85, 4.86). Branching from the right side of the main trunk of the superior mesenteric artery are three vessels—the middle colic, right colic, and ileocolic arteries—which supply the terminal ileum, cecum, ascending colon, and two thirds of the transverse colon (Fig. 4.86).
Fig. 4.86 Distribution of the superior mesenteric artery.
Inferior pancreaticoduodenal artery
The inferior pancreaticoduodenal artery is the first branch of the superior mesenteric artery (Fig. 4.85). It divides immediately into anterior and posterior branches, which ascend on the corresponding sides of the head of the pancreas. Superiorly, these arteries anastomose with anterior and posterior superior pancreaticoduodenal arteries (Figs. 4.83, 4.85). This arterial network supplies the head and uncinate process of the pancreas and the duodenum.
Distal to the inferior pancreaticoduodenal artery, the superior mesenteric artery gives off numerous branches. Arising on the left is a large number of jejunal and ileal arteries supplying the jejunum and most of the ileum (Fig. 4.86). These branches leave the main trunk of the artery, pass between two layers of the mesentery, and form anastomosing arches or arcades as they pass outward to supply the small intestine. The number of arterial arcades increases distally along the gut.
There may be single and then double arcades in the area of the jejunum, with a continued increase in the number of arcades moving into and through the area of the ileum (Fig. 4.86). Extending from the terminal arcade are vasa recta (straight arteries), which provide the final direct vascular supply to the walls of the small intestine. The vasa recta supplying the jejunum are usually long and close together, forming narrow windows visible in the mesentery. The vasa recta supplying the ileum are generally short and far apart, forming low broad windows.
The middle colic artery is the first of the three branches from the right side of the main trunk of the superior mesenteric artery (Fig. 4.86). Arising as the superior mesenteric artery emerges from beneath the pancreas, the middle colic artery enters the transverse mesocolon and divides into right and left branches. The right branch anastomoses with the right colic artery while the left branch anastomoses with the left colic artery, which is a branch of the inferior mesenteric artery.
Continuing distally along the main trunk of the superior mesenteric artery, the right colic artery is the second of the three branches from the right side of the main trunk of the superior mesenteric artery (Fig. 4.86). It is an inconsistent branch, and passes to the right in a retroperitoneal position to supply the ascending colon. Nearing the colon, it divides into a descending branch, which anastomoses with the ileocolic artery, and an ascending branch, which anastomoses with the middle colic artery.
The final branch arising from the right side of the superior mesenteric artery is the ileocolic artery (Fig. 4.86). This passes downward and to the right toward the right iliac fossa where it divides into superior and inferior branches:
 The inferior branch continues toward the ileocolic junction dividing into colic, cecal, appendicular, and ileal branches (Fig. 4.86).
The inferior branch continues toward the ileocolic junction dividing into colic, cecal, appendicular, and ileal branches (Fig. 4.86).
The specific pattern of distribution and origin of these branches is variable:
 The appendicular branch enters the free margin of and supplies the mesoappendix and the appendix.
The appendicular branch enters the free margin of and supplies the mesoappendix and the appendix.
Inferior mesenteric artery
The inferior mesenteric artery is the anterior branch of the abdominal aorta that supplies the hindgut. It is the smallest of the three anterior branches of the abdominal aorta and arises anterior to the body of vertebra LIII. Initially, the inferior mesenteric artery descends anteriorly to the aorta and then passes to the left as it continues inferiorly (Fig. 4.87). Its branches include the left colic artery, several sigmoid arteries, and the superior rectal artery.
Fig. 4.87 Distribution of the inferior mesenteric artery.
The left colic artery is the first branch of the inferior mesenteric artery (Fig. 4.87). It ascends retroperitoneally, dividing into ascending and descending branches:
The sigmoid arteries consist of two to four branches, which descend to the left, in the sigmoid mesocolon, to supply the lowest part of the descending colon and the sigmoid colon (Fig. 4.87). These branches anastomose superiorly with branches from the left colic artery and inferiorly with branches from the superior rectal artery.
The terminal branch of the inferior mesenteric artery is the superior rectal artery (Fig. 4.87). This vessel descends into the pelvic cavity in the sigmoid mesocolon, crossing the left common iliac vessels. Opposite vertebra SIII, the superior rectal artery divides. The two terminal branches descend on each side of the rectum, dividing into smaller branches in the wall of the rectum. These smaller branches continue inferiorly to the level of the internal anal sphincter, anastomosing along the way with branches from the middle rectal arteries (from the internal iliac artery) and the inferior rectal arteries (from the internal pudendal artery).
Clinical app
Vascular supply to the gastrointestinal system
Arteriosclerosis may occur throughout the abdominal aorta and at the openings of the celiac trunk and the superior mesenteric and inferior mesenteric arteries. Not infrequently, the inferior mesenteric artery becomes occluded. Interestingly, many of these patients do not suffer any complications because anastomoses between the right, middle, and left colic arteries gradually enlarge, forming a continuous marginal artery. The distal large bowel therefore becomes supplied by this enlarged marginal artery (marginal artery of Drummond), which replaces the blood supply of the inferior mesenteric artery.
Venous drainage
Venous drainage of the spleen, pancreas, gallbladder, and the abdominal part of the gastrointestinal tract, except for the inferior part of the rectum, is through the portal system of veins, which deliver blood from these structures to the liver. Once blood passes through the hepatic sinusoids, it passes through progressively larger veins until it enters the hepatic veins, which return the venous blood to the inferior vena cava just inferior to the diaphragm.
Portal vein
The portal vein is the final common pathway for the transport of venous blood from the spleen, pancreas, gallbladder, and the abdominal part of the gastrointestinal tract. It is formed by the union of the splenic vein and the superior mesenteric vein posterior to the neck of the pancreas at the level of vertebra LII (Figs. 4.88, 4.89).
Fig. 4.88 Portal vein.
Fig. 4.89 Venous drainage of the abdominal portion of the gastrointestinal tract.
Ascending toward the liver, the portal vein passes posterior to the superior part of the duodenum and enters the right margin of the lesser omentum. As it passes through this part of the lesser omentum, it is anterior to the omental foramen and posterior to both the bile duct, which is slightly to its right, and the hepatic artery proper, which is slightly to its left (see Fig. 4.84).
On approaching the liver, the portal vein divides into right and left branches, which enter the liver parenchyma. Tributaries to the portal vein include:
 right and left gastric veins draining the lesser curvature of the stomach and abdominal esophagus,
right and left gastric veins draining the lesser curvature of the stomach and abdominal esophagus,
 cystic veins from the gallbladder, and
cystic veins from the gallbladder, and
 para-umbilical veins, which are normally small veins associated with the obliterated umbilical vein, and connect to veins on the anterior abdominal wall (Fig. 4.90).
para-umbilical veins, which are normally small veins associated with the obliterated umbilical vein, and connect to veins on the anterior abdominal wall (Fig. 4.90).
Splenic vein
The splenic vein forms from numerous smaller vessels leaving the hilum of the spleen (Fig. 4.89). It passes to the right, passing through the splenorenal ligament with the splenic artery and the tail of pancreas. Continuing to the right, the large, straight splenic vein is in contact with the body of the pancreas as it crosses the posterior abdominal wall. Posterior to the neck of the pancreas, the splenic vein joins the superior mesenteric vein to form the portal vein.
Tributaries to the splenic vein include:
 short gastric veins from the fundus and left part of the greater curvature of the stomach,
short gastric veins from the fundus and left part of the greater curvature of the stomach,
 the left gastro-omental vein from the greater curvature of the stomach,
the left gastro-omental vein from the greater curvature of the stomach,
 pancreatic veins draining the body and tail of pancreas, and
pancreatic veins draining the body and tail of pancreas, and
 usually the inferior mesenteric vein.
usually the inferior mesenteric vein.
Superior mesenteric vein
The superior mesenteric vein drains blood from the small intestine, cecum, ascending colon, and transverse colon (Fig. 4.89). It begins in the right iliac fossa as veins draining the terminal ileum, cecum, and appendix join, and ascends in the mesentery to the right of the superior mesenteric artery.
Posterior to the neck of the pancreas, the superior mesenteric vein joins the splenic vein to form the portal vein (Fig. 4.88).
As a corresponding vein accompanies each branch of the superior mesenteric artery, tributaries to the superior mesenteric vein include jejunal, ileal, ileocolic, right colic, and middle colic veins. Additional tributaries include:
 the right gastro-omental vein, draining the right part of the greater curvature of the stomach; and
the right gastro-omental vein, draining the right part of the greater curvature of the stomach; and
Inferior mesenteric vein
The inferior mesenteric vein drains blood from the rectum, sigmoid colon, descending colon, and splenic flexure (Figs. 4.89, 4.90). It begins as the superior rectal vein and ascends, receiving tributaries from the sigmoid veins and the left colic vein. All these veins accompany arteries of the same name. Continuing to ascend, the inferior mesenteric vein passes posterior to the body of the pancreas and usually joins the splenic vein. Occasionally, it ends at the junction of the splenic and superior mesenteric veins or joins the superior mesenteric vein.
Hepatic cirrhosis
Cirrhosis is a complex disorder of the liver, the diagnosis of which is confirmed histologically. When a diagnosis is suspected, a liver biopsy is necessary.
Cirrhosis is characterized by widespread hepatic fibrosis interspersed with areas of nodular regeneration and abnormal reconstruction of pre-existing lobular architecture. The presence of cirrhosis implies previous or continuing liver cell damage. The poorly functioning liver cells (hepatocytes) are unable to break down blood and blood products, leading to an increase in the serum bilirubin level, which manifests as jaundice.
As the cirrhosis progresses, the intrahepatic vasculature is distorted, which in turn leads to increased pressure in the portal vein and its draining tributaries (portal hypertension). Portal hypertension produces increased pressure in the splenic venules leading to splenic enlargement. At the sites of portosystemic anastomosis, large dilated varicose veins develop. These veins are susceptible to bleeding and may produce marked blood loss, which in some instances can be fatal.
Clinical app
Portosystemic anastomosis
The hepatic portal system drains blood from the visceral organs of the abdomen to the liver. In normal individuals, 100% of the portal venous blood flow can be recovered from the hepatic veins, whereas in patients with elevated portal vein pressure (e.g., portal hypertension from cirrhosis), there is significantly less blood flow to the liver. The rest of the blood enters collateral channels, which drain into the systemic circulation at specific points (Fig. 4.90). The largest of these collaterals occur at:
When the pressure in the portal vein is elevated, venous enlargement (varices) tends to occur at and around the sites of portosystemic anastomoses and these enlarged veins are called:
 hemorrhoids at the anorectal junction,
hemorrhoids at the anorectal junction,
 esophageal varices at the gastroesophageal junction, and
esophageal varices at the gastroesophageal junction, and
 caput medusae at the umbilicus.
caput medusae at the umbilicus.
Fig. 4.90 Portosystemic anastomoses.
Lymphatics
Lymphatic drainage of the abdominal part of the gastrointestinal tract, as low as the inferior part of the rectum, as well as the spleen, pancreas, gallbladder, and liver, is through vessels and nodes that eventually end in large collections of pre-aortic lymph nodes at the origins of the three anterior branches of the abdominal aorta, which supply these structures. These collections are therefore referred to as the celiac, superior mesenteric, and inferior mesenteric groups of pre-aortic lymph nodes. Lymph from viscera supplied by (Fig. 4.91):
 the celiac trunk (i.e., structures that are part of the abdominal foregut) drains to pre-aortic nodes near the origin of the celiac trunk (Fig. 4.91)—these celiac nodes also receive lymph from the superior mesenteric and inferior mesenteric groups of pre-aortic nodes, and lymph from the celiac nodes enters the cisterna chyli;
the celiac trunk (i.e., structures that are part of the abdominal foregut) drains to pre-aortic nodes near the origin of the celiac trunk (Fig. 4.91)—these celiac nodes also receive lymph from the superior mesenteric and inferior mesenteric groups of pre-aortic nodes, and lymph from the celiac nodes enters the cisterna chyli;
 the superior mesenteric artery (i.e., structures that are part of the abdominal midgut) drains to pre-aortic nodes near the origin of the superior mesenteric artery (Fig. 4.91)—these superior mesenteric nodes also receive lymph from the inferior mesenteric groups of pre-aortic nodes, and lymph from the superior mesenteric nodes drains to the celiac nodes; and
the superior mesenteric artery (i.e., structures that are part of the abdominal midgut) drains to pre-aortic nodes near the origin of the superior mesenteric artery (Fig. 4.91)—these superior mesenteric nodes also receive lymph from the inferior mesenteric groups of pre-aortic nodes, and lymph from the superior mesenteric nodes drains to the celiac nodes; and
 the inferior mesenteric artery (i.e., structures that are part of the abdominal hindgut) drains to pre-aortic nodes near the origin of the inferior mesenteric artery (Fig. 4.91), and lymph from the inferior mesenteric nodes drains to the superior mesenteric nodes.
the inferior mesenteric artery (i.e., structures that are part of the abdominal hindgut) drains to pre-aortic nodes near the origin of the inferior mesenteric artery (Fig. 4.91), and lymph from the inferior mesenteric nodes drains to the superior mesenteric nodes.
Fig. 4.91 Lymphatic drainage of the abdominal portion of the gastrointestinal tract.
Innervation
Abdominal viscera are innervated by both extrinsic and intrinsic components of the nervous system:
Abdominal viscera receiving extrinsic innervation include the abdominal part of the gastrointestinal tract, the spleen, the pancreas, the gallbladder, and the liver. These viscera send sensory information back to the central nervous system through visceral afferent fibers and receive motor impulses from the central nervous system through visceral efferent fibers.
The visceral efferent fibers are part of the sympathetic and parasympathetic parts of the autonomic division of the peripheral nervous system.
Structural components serving as conduits for these afferent and efferent fibers include posterior and anterior roots of the spinal cord, respectively; spinal nerves; anterior rami; white and gray rami communicantes; the sympathetic trunks; splanchnic nerves carrying sympathetic fibers (thoracic, lumbar, and sacral); parasympathetic fibers (pelvic); the prevertebral plexus and related ganglia; and the vagus nerves [X].
The enteric nervous system consists of motor and sensory neurons in two interconnected plexuses in the walls of the gastrointestinal tract. These neurons control the coordinated contraction and relaxation of intestinal smooth muscle and regulate gastric secretion and blood flow.
Sympathetic trunks
The sympathetic trunks are two parallel nerve cords extending on either side of the vertebral column from the base of the skull to the coccyx (Fig. 4.92). As they pass through the neck, they lie posterior to the carotid sheath. In the upper thorax, they are anterior to the necks of the ribs, whereas in the lower thorax they are on the lateral aspect of the vertebral bodies. In the abdomen, they are anterolateral to the lumbar vertebral bodies and, continuing into the pelvis, they are anterior to the sacrum. The two sympathetic trunks come together anterior to the coccyx to form the ganglion impar.
Fig. 4.92 Sympathetic trunks.
Throughout the extent of the sympathetic trunks, small raised areas are visible. These collections of neuronal cell bodies outside the CNS are the paravertebral sympathetic ganglia (Fig. 4.92). There are usually:
 three ganglia in the cervical region,
three ganglia in the cervical region,
 eleven or twelve ganglia in the thoracic region,
eleven or twelve ganglia in the thoracic region,
 four ganglia in the lumbar region,
four ganglia in the lumbar region,
 four or five ganglia in the sacral region, and
four or five ganglia in the sacral region, and
 the ganglion impar anterior to the coccyx.
the ganglion impar anterior to the coccyx.
The ganglia and trunks are connected to adjacent spinal nerves by gray rami communicantes throughout the length of the sympathetic trunk and by white rami communicantes in the thoracic and upper lumbar parts of the trunk (T1 to L2). Neuronal fibers found in the sympathetic trunks include preganglionic and postganglionic sympathetic fibers and visceral afferent fibers.
Splanchnic nerves
The splanchnic nerves are important components in the innervation of the abdominal viscera. They pass from the sympathetic trunk or sympathetic ganglia associated with the trunk to the prevertebral plexus and ganglia anterior to the abdominal aorta.
There are two different types of splanchnic nerves, depending on the type of visceral efferent fiber they are carrying:
Thoracic splanchnic nerves
Three thoracic splanchnic nerves pass from sympathetic ganglia along the sympathetic trunk in the thorax to the prevertebral plexus and ganglia associated with the abdominal aorta in the abdomen (Fig. 4.93):
Fig. 4.93 Splanchnic nerves.
Lumbar and sacral splanchnic nerves
There are usually two to four lumbar splanchnic nerves, which pass from the lumbar part of the sympathetic trunk or associated ganglia and enter the prevertebral plexus (Fig. 4.93).
Similarly, the sacral splanchnic nerves pass from the sacral part of the sympathetic trunk or associated ganglia and enter the inferior hypogastric plexus, which is an extension of the prevertebral plexus into the pelvis.
Pelvic splanchnic nerves
The pelvic splanchnic nerves (parasympathetic root) are unique. They are the only splanchnic nerves that carry parasympathetic fibers. In other words, they do not originate from the sympathetic trunks. Rather, they originate directly from the anterior rami of S2 to S4. Preganglionic parasympathetic fibers originating in the sacral spinal cord pass from the S2 to S4 spinal nerves to the inferior hypogastric plexus (see Fig. 4.93). Once in this plexus, some of these fibers pass upward, enter the abdominal prevertebral plexus, and distribute with the arteries supplying the hindgut. This provides the pathway for innervation of the distal one third of the transverse colon, the descending colon, and the sigmoid colon by preganglionic parasympathetic fibers.
Abdominal prevertebral plexus and ganglia
The abdominal prevertebral plexus is a collection of nerve fibers that surrounds the abdominal aorta and is continuous onto its major branches. Scattered throughout the length of the abdominal prevertebral plexus are cell bodies of postganglionic sympathetic fibers. Some of these cell bodies are organized into distinct ganglia, whereas others are more random in their distribution. The ganglia are usually associated with specific branches of the abdominal aorta and named after these branches.
The three major divisions of the abdominal prevertebral plexus and associated ganglia are the celiac, aortic, and superior hypogastric plexuses (Fig. 4.94).
 The celiac plexus is the large accumulation of nerve fibers and ganglia associated with the roots of the celiac trunk and superior mesenteric artery immediately below the aortic hiatus of the diaphragm. Ganglia associated with the celiac plexus include two celiac ganglia, a single superior mesenteric ganglion, and two aorticorenal ganglia.
The celiac plexus is the large accumulation of nerve fibers and ganglia associated with the roots of the celiac trunk and superior mesenteric artery immediately below the aortic hiatus of the diaphragm. Ganglia associated with the celiac plexus include two celiac ganglia, a single superior mesenteric ganglion, and two aorticorenal ganglia.
Fig. 4.94 Abdominal prevertebral plexus and ganglia.
Each of these major plexuses gives origin to a number of secondary plexuses, which may also contain small ganglia. These plexuses are usually named after the vessels with which they are associated. For example, the celiac plexus is usually described as giving origin to the superior mesenteric plexus and the renal plexus, as well as other plexuses that extend out along the various branches of the celiac trunk. Similarly, the aortic plexus has secondary plexuses consisting of the inferior mesenteric plexus, the spermatic plexus, and the external iliac plexus.
Inferiorly, the superior hypogastric plexus divides into the hypogastric nerves, which descend into the pelvis and contribute to the formation of the inferior hypogastric or pelvic plexus (Fig. 4.94).
The abdominal prevertebral plexus receives:
 preganglionic parasympathetic and visceral afferent fibers from the vagus nerves [X],
preganglionic parasympathetic and visceral afferent fibers from the vagus nerves [X],
 preganglionic parasympathetic fibers from the pelvic splanchnic nerves.
preganglionic parasympathetic fibers from the pelvic splanchnic nerves.
Parasympathetic innervation
Parasympathetic innervation of the abdominal part of the gastrointestinal tract, and of the spleen, pancreas, gallbladder, and liver is from two sources—the vagus nerves [X] and the pelvic splanchnic nerves.
Vagus nerves
The vagus nerves [X] enter the abdomen associated with the esophagus as the esophagus passes through the diaphragm (Fig. 4.95) and provides parasympathetic innervation to the foregut and midgut.
Fig. 4.95 Parasympathetic innervation of the abdominal portion of the gastrointestinal tract.
After entering the abdomen as the anterior and posterior vagal trunks, they send branches to the abdominal prevertebral plexus. These branches contain preganglionic parasympathetic fibers and visceral afferent fibers, which are distributed with the other components of the prevertebral plexus along the branches of the abdominal aorta.
Pelvic splanchnic nerves
The pelvic splanchnic nerves, carrying preganglionic parasympathetic fibers from S2 to S4 spinal cord levels, enter the inferior hypogastric plexus in the pelvis. Some of these fibers move upward into the inferior mesenteric part of the prevertebral plexus in the abdomen (Fig. 4.95). Once there, these fibers are distributed with branches of the inferior mesenteric artery and provide parasympathetic innervation to the hindgut.
Enteric system
The enteric system is a division of the visceral part of the nervous system and is a local neuronal circuit in the wall of the gastrointestinal tract. It consists of motor and sensory neurons organized into two interconnected plexuses (the myenteric and submucosal plexuses) between the layers of the gastrointestinal wall, and the associated nerve fibers that pass between the plexuses and from the plexuses to the adjacent tissue (Fig. 4.96).
Fig. 4.96 The enteric system.
The enteric system regulates and coordinates numerous gastrointestinal tract activities, including gastric secretory activity, gastrointestinal blood flow, and the contraction and relaxation cycles of smooth muscle (peristalsis).
Although the enteric system is generally independent of the central nervous system, it does receive input from postganglionic sympathetic and preganglionic parasympathetic neurons that modifies its activities.
Example—sympathetic innervation of stomach
The pathway of sympathetic innervation of the stomach is as follows:
 The white ramus communicans, containing the preganglionic fiber, connects to the sympathetic trunk.
The white ramus communicans, containing the preganglionic fiber, connects to the sympathetic trunk.
 In the celiac ganglion, the preganglionic fiber synapses with a postganglionic neuron.
In the celiac ganglion, the preganglionic fiber synapses with a postganglionic neuron.
POSTERIOR ABDOMINAL REGION
The posterior abdominal region is posterior to the abdominal part of the gastrointestinal tract, the spleen, and the pancreas (Fig. 4.97). This area, bounded by bones and muscles making up the posterior abdominal wall, contains numerous structures that not only are directly involved in the activities of the abdominal contents but also use this area as a conduit between body regions. Examples include the abdominal aorta and its associated nerve plexuses, the inferior vena cava, the sympathetic trunks, and lymphatics. There are also structures originating in this area that are critical to the normal function of other regions of the body (i.e., the lumbar plexus of nerves), and there are organs that associate with this area during development and remain in it in the adult (i.e., the kidneys and suprarenal glands).
Fig. 4.97 Posterior abdominal region.
Posterior abdominal wall
Bones
Lumbar vertebrae and the sacrum
Projecting into the midline of the posterior abdominal area are the bodies of the five lumbar vertebrae (Fig. 4.98). The prominence of these structures in this region is due to the secondary curvature (a forward convexity) of the lumbar part of the vertebral column.
Fig. 4.98 Osteology of the posterior abdominal wall.
The lumbar vertebrae can be distinguished from cervical and thoracic vertebrae because of their size. They are much larger than any other vertebrae in any other region. The vertebral bodies are massive and progressively increase in size from vertebra LI to LV. The pedicles are short and stocky, the transverse processes are long and slender, and the spinous processes are large and stubby. The articular processes are large and oriented medially and laterally, which promotes flexion and extension in this part of the vertebral column.
Between each lumbar vertebra is an intervertebral disc, which completes this part of the midline boundary of the posterior abdominal wall.
The midline boundary of the posterior abdominal wall, inferior to the lumbar vertebrae, consists of the upper margin of the sacrum (Fig. 4.98). The sacrum is formed by the fusion of the five sacral vertebrae into a single, wedge-shaped bony structure that is broad superiorly and narrows inferiorly. Its concave anterior surface and its convex posterior surface contain anterior and posterior sacral foramina for the anterior and posterior rami of spinal nerves to pass through.
Pelvic bones
The ilia, which are components of each pelvic bone, attach laterally to the sacrum at the sacro-iliac joints (Fig. 4.98). The upper part of each ilium expands outward into a thin winglike area (the iliac fossa). The medial side of this region of each iliac bone, and the related muscles, are components of the posterior abdominal wall.
Ribs
Superiorly, ribs XI and XII complete the bony framework of the posterior abdominal wall (Fig. 4.98). These ribs are unique in that they do not articulate with the sternum, they have a single articular facet on their heads, and they do not have necks or tubercles.
Rib XI is posterior to the superior part of the left kidney, and rib XII is posterior to the superior part of both kidneys. Also, rib XII serves as a point of attachment for numerous muscles and ligaments.
Muscles
Muscles forming the medial, lateral, inferior, and superior boundaries of the posterior abdominal region fill in the bony framework of the posterior abdominal wall (Table 4.2). Medially are the psoas major and minor muscles, laterally is the quadratus lumborum muscle, inferiorly is the iliacus muscle, and superiorly is the diaphragm (Figs. 4.99, 4.100).
Table 4.2 Posterior abdominal wall muscles
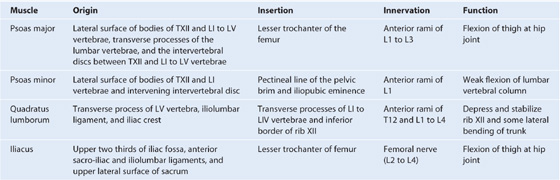
Fig. 4.99 Muscles of the posterior abdominal wall.
Fig. 4.100 Diaphragm.
Psoas major and minor
Medially, the psoas major muscles cover the anterolateral surface of the bodies of the lumbar vertebrae, filling in the space between the vertebral bodies and the transverse processes (Table 4.2, Fig. 4.99). Associated with the psoas major muscle is the psoas minor muscle, which is sometimes absent.
Quadratus lumborum
Laterally, the quadratus lumborum muscles fill the space between ribs XII and the iliac crest on both sides of the vertebral column (Table 4.2, Fig. 4.99). They are overlapped medially by the psoas major muscles; along their lateral borders are the transversus abdominis muscles.
Iliacus
Inferiorly, an iliacus muscle fills the iliac fossa on each side (Table 4.2, Fig. 4.99). From this expansive origin covering the iliac fossa, the muscle passes inferiorly, joins with the psoas major muscle, and passes into the thigh. These combined muscles are referred to as the iliopsoas muscle.
Diaphragm
Superiorly, the diaphragm forms the boundary of the posterior abdominal region. This musculotendinous sheet also separates the abdominal cavity from the thoracic cavity.
Structurally, the diaphragm consists of a central tendinous part into which the circumferentially arranged muscle fibers attach (Fig. 4.100). The diaphragm is anchored to the lumbar vertebrae by musculotendinous crura, which blend with the anterior longitudinal ligament of the vertebral column.
 The right crus is the longest and broadest of the crura and is attached to the bodies of vertebrae LI to LIII, and the intervening intervertebral discs (Fig. 4.101).
The right crus is the longest and broadest of the crura and is attached to the bodies of vertebrae LI to LIII, and the intervening intervertebral discs (Fig. 4.101).
Fig. 4.101 Crura of the diaphragm.
 Similarly, the left crus is attached to vertebrae LI and LII and the associated intervertebral disc (Fig. 4.101).
Similarly, the left crus is attached to vertebrae LI and LII and the associated intervertebral disc (Fig. 4.101).
The crura are connected across the midline by a tendinous arch (the median arcuate ligament), which passes anterior to the aorta (Fig. 4.101).
Lateral to the crura, a second tendinous arch is formed by the fascia covering the upper part of the psoas major muscle. This is the medial arcuate ligament, which is attached medially to the sides of vertebrae LI and LII and laterally to the transverse process of vertebra LI (Fig. 4.101).
A third tendinous arch, the lateral arcuate ligament, is formed by a thickening in the fascia that covers the quadratus lumborum. It is attached medially to the transverse process of vertebra LI and laterally to rib XII (Fig. 4.101).
Structures passing through or around the diaphragm
Numerous structures pass through or around the diaphragm (Fig. 4.100):
 The third large opening in the diaphragm is the caval opening through which the inferior vena cava passes from the abdominal cavity to the thoracic cavity (Fig. 4.100) at approximately vertebra TVIII in the central tendinous part of the diaphragm.
The third large opening in the diaphragm is the caval opening through which the inferior vena cava passes from the abdominal cavity to the thoracic cavity (Fig. 4.100) at approximately vertebra TVIII in the central tendinous part of the diaphragm.
 Accompanying the inferior vena cava through the caval opening is the right phrenic nerve.
Accompanying the inferior vena cava through the caval opening is the right phrenic nerve.
Additional structures pass through small openings either in or just outside the diaphragm as they pass from the thoracic cavity to the abdominal cavity (Fig. 4.100):
 The greater and lesser splanchnic nerves pass through the crura, on either side.
The greater and lesser splanchnic nerves pass through the crura, on either side.
 The hemiazygos vein passes through the left crus.
The hemiazygos vein passes through the left crus.
 Passing anterior to the diaphragm, just deep to the ribs, are the superior epigastric vessels.
Passing anterior to the diaphragm, just deep to the ribs, are the superior epigastric vessels.
The classic appearance of the right and left domes of the diaphragm is caused by the underlying abdominal contents pushing these lateral areas upward, and by the fibrous pericardium, which is attached centrally, causing a flattening of the diaphragm in this area (Fig. 4.102).
Imaging app
Visualizing the diaphragm
Fig. 4.102 Right and left domes of the diaphragm. Chest radiograph.
The domes are produced by:
Although the height of these domes varies during breathing, a reasonable estimate in normal expiration places the left dome at the fifth intercostal space and the right dome at rib V. This is important to remember when percussing the thorax.
During inspiration, the muscular part of the diaphragm contracts, causing the central tendon of the diaphragm to be drawn inferiorly. This results in some flattening of the domes, enlargement of the thoracic cavity, and a reduction in intrathoracic pressure. The physiological effect of these changes is that air enters the lungs and venous return to the heart is enhanced.
There is blood supply to the diaphragm on its superior and inferior surfaces:
 inferiorly, the inferior phrenic arteries, branches of the abdominal aorta, supply the diaphragm (see Fig. 4.100).
inferiorly, the inferior phrenic arteries, branches of the abdominal aorta, supply the diaphragm (see Fig. 4.100).
Venous drainage is through companion veins to these arteries.
Innervation of the diaphragm is primarily by the phrenic nerves. These nerves, from the C3 to C5 spinal cord levels, provide all motor innervation to the diaphragm and sensory fibers to the central part. They pass through the thoracic cavity, deep to the mediastinal pleura, in the fibrous pericardium, to the superior surface of the diaphragm. At this point, the right phrenic nerve accompanies the inferior vena cava through the diaphragm and the left phrenic nerve passes through the diaphragm by itself (see Fig. 4.100). Additional sensory fibers are supplied to the peripheral areas of the diaphragm by intercostal nerves.
Clinical app
Psoas muscle abscess
At first glance, it is difficult to appreciate why the psoas muscle sheath is of greater importance than any other muscle sheath. The psoas muscle and its sheath arise not only from the lumbar vertebrae but also from the intervertebral discs between each vertebra. This disc origin is of critical importance. In certain types of infection, the intervertebral disc is preferentially affected (e.g., tuberculosis and salmonella discitis). As the infection of the disc progresses, the infection spreads anterolaterally and passes into the psoas muscle sheath. The infection spreads inferiorly in the sheath and may appear below the inguinal ligament as a mass.
Clinical app
Diaphragmatic hernias
To understand why a hernia occurs through the diaphragm, it is necessary to consider the embryology of the diaphragm.
The diaphragm is formed from four structures—the septum transversum, the posterior esophageal mesentery, the pleuroperitoneal membrane, and the peripheral rim—which eventually fuse together, separating the abdominal cavity from the thoracic cavity. The septum transversum develops from a mesodermal origin in front of the embryo’s head and then moves to its more adult position during formation of the head fold.
Fusion of the various components of the diaphragm may fail, and hernias may occur through the failed points of fusion. The commonest sites are:
 between the xiphoid process and the costal margins on the right (Morgagni’s hernia), and
between the xiphoid process and the costal margins on the right (Morgagni’s hernia), and
Hernias may also occur through the central tendon and through a congenitally large esophageal hiatus.
Morgagni’s and Bochdalek’s hernias tend to appear at or around the time of birth or in early infancy. They allow abdominal bowel to enter the thoracic cavity, which may compress the lungs and reduce respiratory function. Most of these hernias require surgical closure of the diaphragmatic defect.
Clinical app
Hiatal hernia
At the level of the esophageal hiatus, the diaphragm may be lax, allowing the fundus of the stomach to herniate into the posterior mediastinum. This typically causes symptoms of acid reflux. Ulceration may occur and may produce bleeding and anemia.
Viscera
Kidneys
The bean-shaped kidneys are retroperitoneal in the posterior abdominal region (Fig. 4.103). They lie in the extraperitoneal connective tissue immediately lateral to the vertebral column. In the supine position, the kidneys extend from approximately vertebra TXII superiorly to vertebra LIII inferiorly, with the right kidney somewhat lower than the left because of its relationship with the liver. Although they are similar in size and shape, the left kidney is a longer and more slender organ than the right kidney, and nearer to the midline.
Fig. 4.103 Retroperitoneal position of the kidneys in the posterior abdominal region.
Relationships to other structures
The anterior surface of the right kidney is related to numerous structures, some of which are separated from the kidney by a layer of peritoneum and some of which are directly against the kidney (Fig. 4.104):
 A small part of the superior pole is covered by the right suprarenal gland.
A small part of the superior pole is covered by the right suprarenal gland.
 Medially, the descending part of the duodenum is retroperitoneal and contacts the kidney.
Medially, the descending part of the duodenum is retroperitoneal and contacts the kidney.
Fig. 4.104 Structures related to the anterior surface of each kidney.
The anterior surface of the left kidney is also related to numerous structures, some with an intervening layer of peritoneum and some directly against the kidney (Fig. 4.104):
 A small part of the superior pole, on its medial side, is covered by the left suprarenal gland.
A small part of the superior pole, on its medial side, is covered by the left suprarenal gland.
 The rest of the superior pole is covered by the intraperitoneal stomach and spleen.
The rest of the superior pole is covered by the intraperitoneal stomach and spleen.
 Moving inferiorly, the retroperitoneal pancreas covers the middle part of the kidney.
Moving inferiorly, the retroperitoneal pancreas covers the middle part of the kidney.
Posteriorly, the right and left kidneys are related to similar structures (Fig. 4.105). Superiorly is the diaphragm and inferior to this, moving in a medial to lateral direction, are psoas major, quadratus lumborum, and transversus abdominis muscles.
Fig. 4.105 Structures related to the posterior surface of each kidney.
The superior pole of the right kidney is anterior to rib XII, whereas the same region of the left kidney is anterior to ribs XI and XII. The pleural sacs, and specifically, the costodiaphragmatic recesses, therefore extend posterior to the kidneys.
Also passing posterior to the kidneys are the subcostal vessels and nerves and the iliohypogastric and ilio-inguinal nerves.
Renal fat and fascia
The kidneys are enclosed in and associated with a unique arrangement of fascia and fat. Immediately outside the renal capsule, there is an accumulation of extraperitoneal fat—the perinephric fat (perirenal fat), which completely surrounds the kidney (Fig. 4.106). Enclosing the perinephric fat is a membranous condensation of the extraperitoneal fascia (the renal fascia). The suprarenal glands are also enclosed in this fascial compartment, usually separated from the kidneys by a thin septum. The renal fascia must be incised in any surgical approach to this organ.
Fig. 4.106 Organization of fat and fascia surrounding the kidney.
At the lateral margins of each kidney, the anterior and posterior layers of the renal fascia fuse (Fig. 4.106). This fused layer may connect with the transversalis fascia on the lateral abdominal wall.
Above each suprarenal gland, the anterior and posterior layers of the renal fascia fuse and blend with the fascia that covers the diaphragm.
Medially, the anterior layer of the renal fascia continues over the vessels in the hilum and fuses with the connective tissue associated with the abdominal aorta and the inferior vena cava (Fig. 4.106). In some cases, the anterior layer may cross the midline to the opposite side and blend with its companion layer.
The posterior layer of the renal fascia passes medially between the kidney and the fascia covering the quadratus lumborum muscle to fuse with the fascia covering the psoas major muscle.
Inferiorly, the anterior and posterior layers of the renal fascia enclose the ureters.
In addition to perinephric fat and the renal fascia, a final layer of paranephric fat (pararenal fat) completes the fat and fascias associated with the kidney (Fig. 4.106). This fat accumulates posterior and posterolateral to each kidney.
Kidney structure
Each kidney has a smooth anterior and posterior surface covered by a fibrous capsule, which is easily removable except during disease.
On the medial margin of each kidney is the hilum of kidney, which is a deep vertical slit through which renal vessels, lymphatics, and nerves enter and leave the substance of the kidney (Fig. 4.107). Internally, the hilum is continuous with the renal sinus. Perinephric fat continues into the hilum and sinus and surrounds all structures.
Fig. 4.107 Internal structure of the kidney.
Each kidney consists of an outer renal cortex and an inner renal medulla. The renal cortex is a continuous band of pale tissue that completely surrounds the renal medulla. Extensions of the renal cortex (the renal columns) project into the inner aspect of the kidney, dividing the renal medulla into discontinuous aggregations of triangular-shaped tissue (the renal pyramids).
The bases of the renal pyramids are directed outward, toward the renal cortex, whereas the apex of each renal pyramid projects inward, toward the renal sinus. The apical projection (renal papilla) is surrounded by a minor calyx.
The minor calices receive urine and represent the proximal parts of the tube that will eventually form the ureter (Fig. 4.107). In the renal sinus, several minor calices unite to form a major calyx, and two or three major calices unite to form the renal pelvis, which is the funnel-shaped superior end of the ureters.
Renal vasculature and lymphatics
A single large renal artery, a lateral branch of the abdominal aorta, supplies each kidney. These vessels usually arise just inferior to the origin of the superior mesenteric artery between vertebrae LI and LII (Fig. 4.108). The left renal artery usually arises a little higher than the right, and the right renal artery is longer and passes posterior to the inferior vena cava.
Fig. 4.108 Renal vasculature.
As each renal artery approaches the renal hilum, it divides into anterior and posterior branches, which supply the renal parenchyma. Accessory renal arteries are common. They originate from the lateral aspect of the abdominal aorta, either above or below the primary renal arteries, enter the hilum with the primary arteries or pass directly into the kidney at some other level, and are commonly called extrahilar arteries.
Multiple renal veins contribute to the formation of the left and right renal veins, both of which are anterior to the renal arteries (Fig. 4.108). Importantly, the longer left renal vein crosses the midline anterior to the abdominal aorta and posterior to the superior mesenteric artery and can be compressed by an aneurysm in either of these two vessels.
The lymphatic drainage of each kidney is to the lateral aortic (lumbar) nodes around the origin of the renal artery.
Ureters
The ureters are muscular tubes that transport urine from the kidneys to the bladder. They are continuous superiorly with the renal pelvis, which is a funnel-shaped structure in the renal sinus. The renal pelvis is formed from a condensation of two or three major calices, which in turn are formed by the condensation of several minor calices (see Fig. 4.108). The minor calices surround a renal papilla.
The renal pelvis narrows as it passes inferiorly through the hilum of the kidney and becomes continuous with the ureter at the ureteropelvic junction (Fig. 4.109). Inferior to this junction, the ureters descend retroperitoneally on the medial aspect of the psoas major muscle. At the pelvic brim, the ureters cross either the end of the common iliac or the beginning of the external iliac arteries, enter the pelvic cavity, and continue their journey to the bladder.
Fig. 4.109 Ureters.
At three points along their course the ureters are constricted (Fig. 4.109):
 the first point is at the ureteropelvic junction;
the first point is at the ureteropelvic junction;
 the second point is where the ureters cross the common iliac vessels at the pelvic brim;
the second point is where the ureters cross the common iliac vessels at the pelvic brim;
 the third point is where the ureters enter the wall of the bladder.
the third point is where the ureters enter the wall of the bladder.
Kidney stones can become lodged at these constrictions.
Ureteric vasculature and lymphatics
The ureters receive arterial branches from adjacent vessels as they pass toward the bladder (Fig. 4.109):
 The renal arteries supply the upper end.
The renal arteries supply the upper end.
In all cases, arteries reaching the ureters divide into ascending and descending branches, which form longitudinal anastomoses.
Lymphatic drainage of the ureters follows a pattern similar to that of the arterial supply. Lymph from:
 the upper part of each ureter drains to the lateral aortic (lumbar) nodes;
the upper part of each ureter drains to the lateral aortic (lumbar) nodes;
 The middle part of each ureter drains to lymph nodes associated with the common iliac vessels.
The middle part of each ureter drains to lymph nodes associated with the common iliac vessels.
Ureteric innervation
Ureteric innervation is from the renal, aortic, superior hypogastric, and inferior hypogastric plexuses through nerves that follow the blood vessels.
Visceral efferent fibers come from both sympathetic and parasympathetic sources, whereas visceral afferent fibers return to T11 to L2 spinal cord levels. Ureteric pain, which is usually related to distention of the ureter, is therefore referred to cutaneous areas supplied by T11 to L2 spinal cord levels. These areas would most likely include the posterior and lateral abdominal wall below the ribs and above the iliac crest, the pubic region, the scrotum in males, the labia majora in females, and the proximal anterior aspect of the thigh.
Clinical app
Urinary tract stones
Urinary tract stones (calculi) occur more frequently in men than in women, are most common in people aged between 20 and 60 years, and are usually associated with sedentary lifestyles. The stones are polycrystalline aggregates of calcium, phosphate, oxalate, urate, and other soluble salts within an organic matrix. The urine becomes saturated with these salts, and small variations in the pH cause the salts to precipitate.
Typically the patient has pain that radiates from the infrascapular region (loin) into the groin, and even into the scrotum or labia majora. Blood in the urine (hematuria) may also be noticed.
Clinical app
Urinary tract cancer
Most tumors that arise in the kidney are renal cell carcinomas. These tumors develop from the proximal tubular epithelium. Approximately 5% of tumors within the kidney are transitional cell tumors, which arise from the urothelium of the renal pelvis. Most patients typically have blood in the urine (hematuria), pain in the infrascapular region (loin), and a mass.
Renal cell tumors are unusual because not only do they grow outward from the kidney, invading the fat and fascia, but they also spread into the renal vein. This venous extension is rare for any other type of tumor, so when seen, renal cell carcinoma should be suspected. Transitional cell carcinoma arises from the urothelium. The urothelium is present from the calices to the urethra and behaves as a “single unit.” Therefore, when patients develop transitional carcinomas within the bladder, similar tumors may also be present within upper parts of the urinary tract.
Clinical app
Kidney transplant
Kidney transplantation began in the United States in the 1950s. Since the first transplant, the major problem for kidney transplantation has been tissue rejection. A number of years have passed since this initial procedure and there have been significant breakthroughs in transplant rejection medicine. Renal transplantation is now a common procedure undertaken in patients with end-stage renal failure.
An ideal place to situate the transplant kidney is in the left or the right iliac fossa (Fig. 4.110). A curvilinear incision is made paralleling the iliac crest and pubic symphysis. The external oblique muscle, internal oblique muscle, transverse abdominis muscle, and transversalis fascia are divided. The surgeon identifies the parietal peritoneum but does not enter the peritoneal cavity. The parietal peritoneum is medially retracted to reveal the external iliac artery, external iliac vein, and the bladder. In some instances the internal iliac artery of the recipient is mobilized and anastomosed directly as an end-to-end procedure onto the renal artery of the donor kidney. Similarly the internal iliac vein is anastomosed to the donor vein. The ureter is easily tunneled obliquely through the bladder wall with a straightforward anastomosis.
Fig. 4.110 Abdominal computed tomogram, in the axial plane, showing the transplanted kidney in the left iliac fossa.
The left and right iliac fossae are ideal locations for the transplant kidney, because a new space can be created without compromise to other structures. The extraperitoneal approach enables patients to make a swift recovery.
Imaging app
Investigation of the urinary tract
After an appropriate history and examination of the patient, including a digital rectal examination to assess the prostate in men, special investigations are required.
An IVU is one of the most important and commonly carried out radiological investigations (Fig. 4.111A). The patient is injected with iodinated contrast medium. Most contrast media contain three iodine atoms spaced around a benzene ring. The relatively high atomic number of iodine compared with the atomic number of carbon, hydrogen, and oxygen, attenuates the radiation beam. After intravenous injection, contrast media are excreted predominantly by glomerular filtration, although some are secreted by the renal tubules. This allows visualization of the collecting system as well as the ureters and bladder.
Ultrasound can be used to assess kidney size and the size of the calices, which may be dilated when obstructed. Although the ureters are poorly visualized using ultrasound, the bladder can be easily seen when full. Ultrasound measurements of bladder volume can be obtained before and after micturition.
Computed tomography can be used to assess the kidneys, ureters, bladder, and adjacent structures and is a powerful tool for staging primary urinary tract tumors (Fig. 4.111, B).
Nuclear medicine is an extremely useful tool for investigating the urinary tract, because radioisotope compounds can be used to estimate renal cell mass and function, and assess the parenchyma for renal scarring. These tests are often very useful in children when renal scarring and reflux disease is suspected.
Suprarenal glands
The suprarenal glands are associated with the superior pole of each kidney (Fig. 4.112). They consist of an outer cortex and an inner medulla. The right gland is shaped like a pyramid, whereas the left gland is semilunar in shape and the larger of the two.
Fig. 4.112 Arterial supply to the suprarenal glands.
Anterior to the right suprarenal gland is part of the right lobe of the liver and the inferior vena cava, whereas anterior to the left suprarenal gland is part of the stomach, pancreas, and, on occasion, the spleen. Parts of the diaphragm are posterior to both glands.
The suprarenal glands are surrounded by the perinephric fat and enclosed in the renal fascia, although a thin septum separates each gland from its associated kidney.
Suprarenal vasculature
The arterial supply to the suprarenal glands is extensive and arises from three primary sources (Fig. 4.112):
 As the bilateral inferior phrenic arteries pass upward from the abdominal aorta to the diaphragm, they give off multiple branches (superior suprarenal arteries) to the suprarenal glands.
As the bilateral inferior phrenic arteries pass upward from the abdominal aorta to the diaphragm, they give off multiple branches (superior suprarenal arteries) to the suprarenal glands.
In contrast to this multiple arterial supply is the venous drainage, which usually consists of a single vein leaving the hilum of each gland. On the right side, the right suprarenal vein is short and almost immediately enters the inferior vena cava; whereas on the left side, the left suprarenal vein passes inferiorly to enter the left renal vein.
Vasculature
Abdominal aorta
The abdominal aorta begins at the aortic hiatus of the diaphragm as a midline structure at approximately the lower level of vertebra TXII (Fig. 4.113). It passes downward on the anterior surface of the bodies of vertebrae LI to LIV, ending just to the left of midline at the lower level of vertebra LIV. At this point, it divides into the right and left common iliac arteries. This bifurcation can be visualized on the anterior abdominal wall as a point approximately 2.6 cm below the umbilicus or even with a line extending between the highest points of the iliac crest.
Fig. 4.113 Abdominal aorta.
As the abdominal aorta passes through the posterior abdominal region, the prevertebral plexus of nerves and ganglia covers its anterior surface. It is also related to numerous other structures:
 Several lumbar veins cross it posteriorly as they pass to the inferior vena cava.
Several lumbar veins cross it posteriorly as they pass to the inferior vena cava.
 On its left side is the left crus of the diaphragm.
On its left side is the left crus of the diaphragm.
Branches of the abdominal aorta (Table 4.3) can be classified as:
 visceral branches supplying organs,
visceral branches supplying organs,
 posterior branches supplying the diaphragm or body wall, or
posterior branches supplying the diaphragm or body wall, or
Table 4.3 Branches of the abdominal aorta

Visceral branches
The visceral branches are either unpaired or paired vessels.
The three unpaired visceral branches that arise from the anterior surface of the abdominal aorta (Table 4.3, Fig. 4.113) are:
 the celiac trunk, which supplies the abdominal foregut,
the celiac trunk, which supplies the abdominal foregut,
 the superior mesenteric artery, which supplies the abdominal midgut, and
the superior mesenteric artery, which supplies the abdominal midgut, and
 the inferior mesenteric artery, which supplies the abdominal hindgut.
the inferior mesenteric artery, which supplies the abdominal hindgut.
The paired visceral branches of the abdominal aorta (Table 4.3; also see Fig. 4.113) include:
Posterior branches
The posterior branches of the abdominal aorta are vessels supplying the diaphragm or body wall. They consist of the inferior phrenic arteries, the lumbar arteries, and the median sacral artery (Table 4.3; also see Fig. 4.113).
Inferior phrenic arteries
The inferior phrenic arteries arise immediately inferior to the aortic hiatus of the diaphragm either directly from the abdominal aorta, as a common trunk from the abdominal aorta, or from the base of the celiac trunk (see Fig. 4.113). Whatever their origin, they pass upward, provide some arterial supply to the suprarenal gland, and continue onto the inferior surface of the diaphragm.
Lumbar arteries
There are usually four pairs of lumbar arteries arising from the posterior surface of the abdominal aorta (see Fig. 4.113). They run laterally and posteriorly over the bodies of the lumbar vertebrae, continue laterally, passing posterior to the sympathetic trunks and between the transverse processes of adjacent lumbar vertebrae, and reach the abdominal wall. From this point onward, they demonstrate a branching pattern similar to a posterior intercostal artery, which includes providing segmental branches that supply the spinal cord.
Median sacral artery
The final posterior branch is the median sacral artery (see Fig. 4.113). This vessel arises from the posterior surface of the abdominal aorta just superior to the bifurcation and passes in an inferior direction, first over the anterior surface of the lower lumbar vertebrae and then over the anterior surface of the sacrum and coccyx.
Clinical app
Abdominal aortic stent graft
An abdominal aortic aneurysm is a dilatation of the aorta and generally tends to occur in the infrarenal region (the region at or below the renal arteries). As the aorta expands, the risk of rupture increases, and it is now generally accepted that when an aneurysm reaches 5.6 cm or greater an operation will significantly benefit the patient.
Treatment of aneurysms prior to rupture can involve inserting an endovascular graft (Fig. 4.114). The technique involves surgically dissecting the femoral artery below the inguinal ligament. A small incision is made in the femoral artery and the preloaded compressed graft with metal support struts is passed on a large catheter into the abdominal aorta through the femoral artery. Using X-ray for guidance the graft is opened so that it lines the inside of the aorta. Attachments are made to the graft that extend into the common iliac vessels. This bifurcated tube device effectively excludes the abdominal aortic aneurysm.
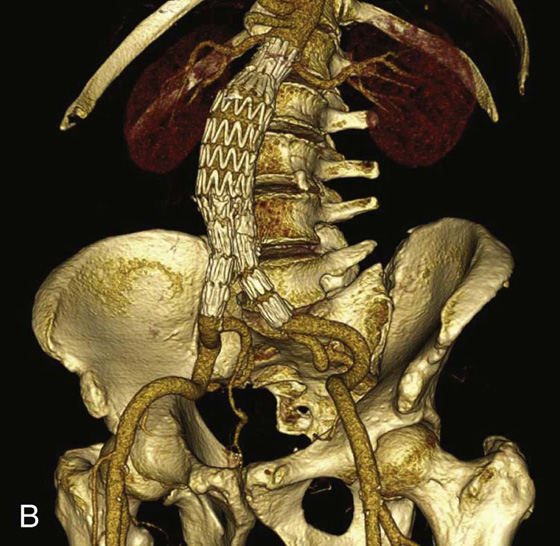
Inferior vena cava
The inferior vena cava returns blood from all structures below the diaphragm to the right atrium of the heart (Fig. 4.115). It is formed when the two common iliac veins come together at the level of vertebra LV, just to the right of the midline. It ascends through the posterior abdominal region anterior to the vertebral column immediately to the right of the abdominal aorta (Fig. 4.115), continues in a superior direction, and leaves the abdomen by piercing the central tendon of the diaphragm at the level of vertebra TVIII.
Fig. 4.115 Inferior vena cava.
During its course, the anterior surface of the inferior vena cava is crossed by the right common iliac artery, the root of the mesentery, the right testicular or ovarian artery, the inferior part of the duodenum, the head of the pancreas, the superior part of the duodenum, the bile duct, the portal vein, and the liver, which overlaps and on occasion completely surrounds the vena cava (Fig. 4.115).
Tributaries to the inferior vena cava include the:
 right testicular or ovarian vein,
right testicular or ovarian vein,
There are no tributaries from the abdominal part of the gastrointestinal tract, the spleen, the pancreas, or the gallbladder, because veins from these structures are components of the portal venous system, which first passes through the liver.
Of the venous tributaries mentioned above, the lumbar veins are unique in their connections and deserve special attention. Not all of the lumbar veins drain directly into the inferior vena cava:
 The third and fourth lumbar veins usually drain into the inferior vena cava.
The third and fourth lumbar veins usually drain into the inferior vena cava.
 The first and second lumbar veins may empty into the ascending lumbar veins.
The first and second lumbar veins may empty into the ascending lumbar veins.
The ascending lumbar veins are long, anastomosing venous channels that connect the common iliac, iliolumbar, and lumbar veins with the azygos and hemiazygos veins of the thorax (Fig. 4.116).
Fig. 4.116 Lumbar veins.
If the inferior vena cava becomes blocked, the ascending lumbar veins become important collateral channels between the lower and upper parts of the body.
Clinical app
Inferior vena cava filter
Deep vein thrombosis is a potentially fatal condition where a clot (thrombus) is formed in the deep venous system of the legs and the veins of the pelvis. Common predisposing factors include hospitalization, surgery, oral contraceptives, smoking, and air travel. Other factors include clotting abnormalities (e.g., protein S and protein C deficiency).
The diagnosis of deep vein thrombosis may be difficult to establish, with symptoms including leg swelling and pain and discomfort in the calf.
Occasionally the clot may dislodge and pass into the venous system through the right side of the heart and into the main pulmonary arteries. If the clots are of significant size they obstruct blood flow to the lung and may produce instantaneous death.
Other complications include destruction of the normal valvular system in the legs, which may lead to venous incompetency and chronic leg swelling with ulceration.
In certain situations it is not possible to optimize the patient with prophylactic treatment, and it may be necessary to insert a filter into the inferior vena cava that traps any large clots. It may be removed after the risk period has ended.
Surface anatomy
Visualizing the position of major blood vessels
Each of the vertebral levels in the abdomen is related to the origin of major blood vessels (Fig. 4.117):
 The celiac trunk originates from the aorta at the upper border of the LI vertebra.
The celiac trunk originates from the aorta at the upper border of the LI vertebra.
 The superior mesenteric artery originates at the lower border of the LI vertebra.
The superior mesenteric artery originates at the lower border of the LI vertebra.
 The renal arteries originate at approximately the LII vertebra.
The renal arteries originate at approximately the LII vertebra.
 The inferior mesenteric artery originates at the LIII vertebra.
The inferior mesenteric artery originates at the LIII vertebra.
 The aorta bifurcates into the right and left common iliac arteries at the level of the LIV vertebra.
The aorta bifurcates into the right and left common iliac arteries at the level of the LIV vertebra.
 The left and right common iliac veins join to form the inferior vena cava at the LV vertebral level.
The left and right common iliac veins join to form the inferior vena cava at the LV vertebral level.
Fig. 4.117 Major vessels projected onto the body’s surface. Anterior view of the abdominal region of a man.
Lymphatic system
Lymphatic drainage from most deep structures and regions of the body below the diaphragm converges mainly on collections of lymph nodes and vessels associated with the major blood vessels of the posterior abdominal region (Fig. 4.118). The lymph then predominantly drains into the thoracic duct.
Fig. 4.118 Abdominal lymphatics.
Pre-aortic and lateral aortic or lumbar nodes (para-aortic nodes)
Approaching the aortic bifurcation, the collections of lymphatics associated with the two common iliac arteries and veins merge, and multiple groups of lymphatic vessels and nodes associated with the abdominal aorta and inferior vena cava pass superiorly. These collections may be subdivided into pre-aortic nodes, which are anterior to the abdominal aorta, and right and left lateral aortic or lumbar nodes (para-aortic nodes), which are positioned on either side of the abdominal aorta (Table 4.4, Fig. 4.118).
Table 4.4 Lymphatic drainage
|
Lymphatic vessel |
Area drained |
|
Right jugular trunk |
Right side of head and neck |
|
Left jugular trunk |
Left side of head and neck |
|
Right subclavian trunk |
Right upper limb, superficial regions of thoracic and upper abdominal wall |
|
Left subclavian trunk |
Left upper limb, superficial regions of thoracic and upper abdominal wall |
|
Right bronchomediastinal trunk |
Right lung and bronchi, mediastinal structures, thoracic wall |
|
Left bronchomediastinal trunk |
Left lung and bronchi, mediastinal structures, thoracic wall |
|
Thoracic duct |
Lower limbs, abdominal walls and viscera, pelvic walls and viscera, thoracic wall |
As these collections of lymphatics pass through the posterior abdominal region, they continue to collect lymph from a variety of structures. The lateral aortic or lumbar lymph nodes (para-aortic nodes) receive lymphatics from the body wall, the kidneys, the suprarenal glands, and the testes or ovaries.
The pre-aortic nodes are organized around the three anterior branches of the abdominal aorta that supply the abdominal part of the gastrointestinal tract, as well as the spleen, pancreas, gallbladder, and liver. They are divided into celiac, superior mesenteric, and inferior mesenteric nodes, and receive lymph from the organs supplied by the similarly named arteries.
Retroperitoneal lymph node surgery
From a clinical perspective, retroperitoneal lymph nodes are arranged in two groups. The pre-aortic lymph node group drains lymph from the embryological midline structures, such as the liver, bowel, and pancreas. The para-aortic lymph node group (the lateral aortic or lumbar nodes), on either side of the aorta, drain lymph from bilateral structures, such as the kidneys and adrenal glands. Organs embryologically derived from the posterior abdominal wall also drain lymph to these nodes. These organs include the ovaries and the testes (importantly, the testes do not drain lymph to the inguinal regions).
There are a number of causes for enlarged retroperitoneal lymph nodes. Massively enlarged lymph nodes are a feature of lymphoma, and smaller lymph node enlargement is observed in the presence of infection and metastatic malignant spread of disease (e.g., colon cancer).
The surgical approach to retroperitoneal lymph node resection involves a lateral paramedian incision in the midclavicular line. The three layers of the anterolateral abdominal wall (external oblique, internal oblique, and transversus abdominis) are opened and the transversalis fascia is divided. The next structure the surgeon sees is the parietal peritoneum. Instead of entering the parietal peritoneum, which is standard procedure for most intra-abdominal surgical operations, the surgeon gently pushes the parietal peritoneum toward the midline, which moves the intra-abdominal contents and allows a clear view of the retroperitoneal structures. On the left, the para-aortic lymph node group (lateral aortic or lumbar nodes) are easily demonstrated with a clear view of the abdominal aorta and kidney. On the right the inferior vena cava is demonstrated, which has to be retracted to access to the right para-aortic lymph node chain (lateral aortic or lumbar nodes).
The procedure of the retroperitoneal lymph node dissection is extremely well tolerated and lacks the problems of entering the peritoneal cavity (e.g., paralytic ileus). Unfortunately, the complication of a vertical incision in the midclavicular line is to divide the segmental nerve supply to the rectus abdominis muscle. This produces muscle atrophy and asymmetrical proportions to the anterior abdominal wall.
Nervous system in the posterior abdominal region
Several important components of the nervous system are in the posterior abdominal region. These include the sympathetic trunks and associated splanchnic nerves, the plexus of nerves and ganglia associated with the abdominal aorta, and the lumbar plexus of nerves.
Sympathetic trunks and splanchnic nerves
The sympathetic trunks pass through the posterior abdominal region anterolateral to the lumbar vertebral bodies, before continuing across the sacral promontory and into the pelvic cavity (Fig. 4.119). Along their course, small raised areas are visible. These represent collections of neuronal cell bodies—primarily postganglionic neuronal cell bodies—which are located outside the central nervous system. They are sympathetic paravertebral ganglia. There are usually four ganglia along the sympathetic trunks in the posterior abdominal (lumbar) region.
Fig. 4.119 Prevertebral plexus and ganglia in the posterior abdominal region.
Also associated with the sympathetic trunks in the posterior abdominal region are the lumbar splanchnic nerves (Fig. 4.119). These components of the nervous system pass from the sympathetic trunks to the plexus of nerves and ganglia associated with the abdominal aorta. Usually two to four lumbar splanchnic nerves carry preganglionic sympathetic fibers and visceral afferent fibers.
Abdominal prevertebral plexus and ganglia
The abdominal prevertebral plexus is a network of nerve fibers surrounding the abdominal aorta. It extends from the aortic hiatus of the diaphragm to the bifurcation of the aorta into the right and left common iliac arteries. Along its route, it is subdivided into smaller, named plexuses (Fig. 4.119):
 Continuing inferiorly, the plexus of nerve fibers extending from just below the superior mesenteric artery to the aortic bifurcation is the abdominal aortic plexus (Fig. 4.119).
Continuing inferiorly, the plexus of nerve fibers extending from just below the superior mesenteric artery to the aortic bifurcation is the abdominal aortic plexus (Fig. 4.119).
 At the bifurcation of the abdominal aorta, the abdominal prevertebral plexus continues inferiorly as the superior hypogastric plexus (Fig. 4.119).
At the bifurcation of the abdominal aorta, the abdominal prevertebral plexus continues inferiorly as the superior hypogastric plexus (Fig. 4.119).
Throughout its length, the abdominal prevertebral plexus is a conduit for:
 preganglionic sympathetic and visceral afferent fibers from the thoracic and lumbar splanchnic nerves (Fig. 4.120),
preganglionic sympathetic and visceral afferent fibers from the thoracic and lumbar splanchnic nerves (Fig. 4.120),
Fig. 4.120 Nerve fibers passing through the abdominal prevertebral plexus and ganglia.
 preganglionic parasympathetic and visceral afferent fibers from the vagus nerves [X] (Fig. 4.120), and
preganglionic parasympathetic and visceral afferent fibers from the vagus nerves [X] (Fig. 4.120), and
 preganglionic parasympathetic fibers from the pelvic splanchnic nerves.
preganglionic parasympathetic fibers from the pelvic splanchnic nerves.
Associated with the abdominal prevertebral plexus are clumps of nervous tissue (the prevertebral ganglia), which are collections of postganglionic sympathetic neuronal cell bodies in recognizable aggregations along the abdominal prevertebral plexus; they are usually named after the nearest branch of the abdominal aorta. They are therefore referred to as celiac, superior mesenteric, aorticorenal, and inferior mesenteric ganglia (Fig. 4.119). These structures, along with the abdominal prevertebral plexus, play a critical role in the innervation of the abdominal viscera.
Common sites for pain referred from the abdominal viscera are given in Table 4.5.
Table 4.5 Referred pain pathways (visceral afferents)
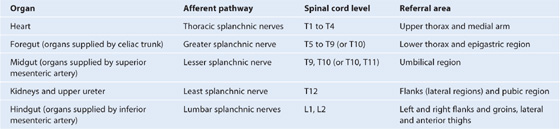
Lumbar plexus
The lumbar plexus is formed by the anterior rami of nerves L1 to L3, and most of the anterior ramus of L4 (Table 4.6, Fig. 4.121). It also receives a contribution from the T12 (subcostal) nerve.
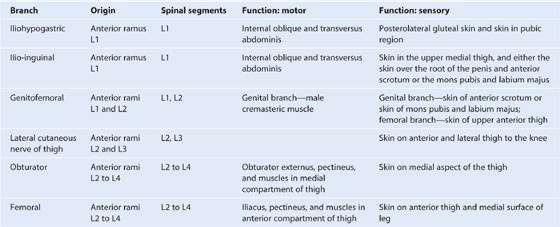
Fig. 4.121 Lumbar plexus.
Branches of the lumbar plexus include the iliohypogastric, ilio-inguinal, genitofemoral, lateral cutaneous nerve of thigh (lateral femoral cutaneous), femoral, and obturator nerves. The lumbar plexus forms in the substance of the psoas major muscle anterior to its attachment to the transverse processes of the lumbar vertebrae (Fig. 4.122). Therefore, relative to the psoas major muscle, the various branches emerge either:
Fig. 4.122 Lumbar plexus in the posterior abdominal region.
Iliohypogastric and ilio-inguinal nerves (L1)
The iliohypogastric and ilio-inguinal nerves arise as a single trunk from the anterior ramus of nerve L1 (Fig. 4.122). Either before or soon after emerging from the lateral border of the psoas major muscle, this single trunk divides into the iliohypogastric and the ilio-inguinal nerves.
The iliohypogastric nerve passes across the anterior surface of the quadratus lumborum muscle, posterior to the kidney. It pierces the transversus abdominis muscle and continues anteriorly around the body between the transversus abdominis and internal oblique muscles. Above the iliac crest, a lateral cutaneous branch pierces the internal and external oblique muscles to supply the posterolateral gluteal skin (Fig. 4.123).
Fig. 4.123 Cutaneous distribution of the nerves from the lumbar plexus.
The remaining part of the iliohypogastric nerve (the anterior cutaneous branch) continues in an anterior direction, piercing the internal oblique just medial to the anterior superior iliac spine as it continues in an obliquely downward and medial direction. Becoming cutaneous, just above the superficial inguinal ring, after piercing the aponeurosis of the external oblique, it distributes to the skin in the pubic region (Fig. 4.123). Throughout its course, it also supplies branches to the abdominal musculature.
The ilio-inguinal nerve is smaller than, and inferior to, the iliohypogastric nerve as it crosses the quadratus lumborum muscle (Fig. 4.122). Its course is more oblique than that of the iliohypogastric nerve, and it usually crosses part of the iliacus muscle on its way to the iliac crest. Near the anterior end of the iliac crest, it pierces the transversus abdominis muscle, and then pierces the internal oblique muscle and enters the inguinal canal.
The ilio-inguinal nerve emerges through the superficial inguinal ring, along with the spermatic cord, and provides cutaneous innervation to the upper medial thigh, the root of the penis, and the anterior surface of the scrotum in men, or the mons pubis and labium majus in women (Fig. 4.123). Throughout its course, it also supplies branches to the abdominal musculature.
Genitofemoral nerve (L1 and L2)
The genitofemoral nerve arises from the anterior rami of the nerves L1 and L2 (Fig. 4.121). It passes downward in the substance of the psoas major muscle until it emerges on the anterior surface of psoas major. It then descends on the surface of the muscle, in a retroperitoneal position, passing posterior to the ureter. It eventually divides into genital and femoral branches.
The genital branch continues downward and enters the inguinal canal through the deep inguinal ring. It continues through the canal and:
The femoral branch descends on the lateral side of the external iliac artery and passes posterior to the inguinal ligament, entering the femoral sheath lateral to the femoral artery. It pierces the anterior layer of the femoral sheath and the fascia lata to supply the skin of the upper anterior thigh (Fig. 4.123).
Lateral cutaneous nerve of thigh (L2 and L3)
The lateral cutaneous nerve of thigh arises from the anterior rami of nerves L2 and L3 (see Fig. 4.121). It emerges from the lateral border of the psoas major muscle, passing obliquely downward across the iliacus muscle toward the anterior superior iliac spine (see Fig. 4.122). It passes posterior to the inguinal ligament and enters the thigh.
The lateral cutaneous nerve of thigh supplies the skin on the anterior and lateral thigh to the level of the knee (Fig. 4.123).
Obturator nerve (L2 to L4)
The obturator nerve arises from the anterior rami of the nerves L2 to L4 (see Fig. 4.121). It descends in the psoas major muscle, emerging from its medial side near the pelvic brim (see Fig. 4.122).
The obturator nerve continues posterior to the common iliac vessels, passes across the lateral wall of the pelvic cavity, and enters the obturator canal, through which the obturator nerve gains access to the medial compartment of the thigh (see Fig. 4.122).
In the area of the obturator canal, the obturator nerve divides into anterior and posterior branches. On entering the medial compartment of the thigh, the two branches are separated by the obturator externus and adductor brevis muscles. Throughout their course through the medial compartment, these two branches supply:
 articular branches to the hip joint,
articular branches to the hip joint,
 cutaneous branches to the medial aspect of the thigh, and
cutaneous branches to the medial aspect of the thigh, and
 in association with the saphenous nerve, cutaneous branches to the medial aspect of the upper part of the leg, and articular branches to the knee joint (Fig. 4.123).
in association with the saphenous nerve, cutaneous branches to the medial aspect of the upper part of the leg, and articular branches to the knee joint (Fig. 4.123).
Femoral nerve (L2 to L4)
The femoral nerve arises from the anterior rami of nerves L2 to L4 (see Fig. 4.121). It descends through the substance of the psoas major muscle, emerging from the lower lateral border of the psoas major (see Fig. 4.122). Continuing its descent, the femoral nerve lies between the lateral border of the psoas major and the anterior surface of the iliacus muscle. It is deep to the iliacus fascia and lateral to the femoral artery as it passes posterior to the inguinal ligament and enters the anterior compartment of the thigh. Upon entering the thigh, it immediately divides into multiple branches.
Cutaneous branches of the femoral nerve include (Fig. 4.123):
 the saphenous nerve supplying the skin on the medial surface of the leg (Fig. 4.123).
the saphenous nerve supplying the skin on the medial surface of the leg (Fig. 4.123).
Muscular branches innervate the iliacus, pectineus, sartorius, rectus femoris, vastus medialis, vastus intermedius, and vastus lateralis muscles. Articular branches supply the hip and knee joints.

 Medical Clinical Case Studies
Medical Clinical Case Studies Clinical Cases
Clinical Cases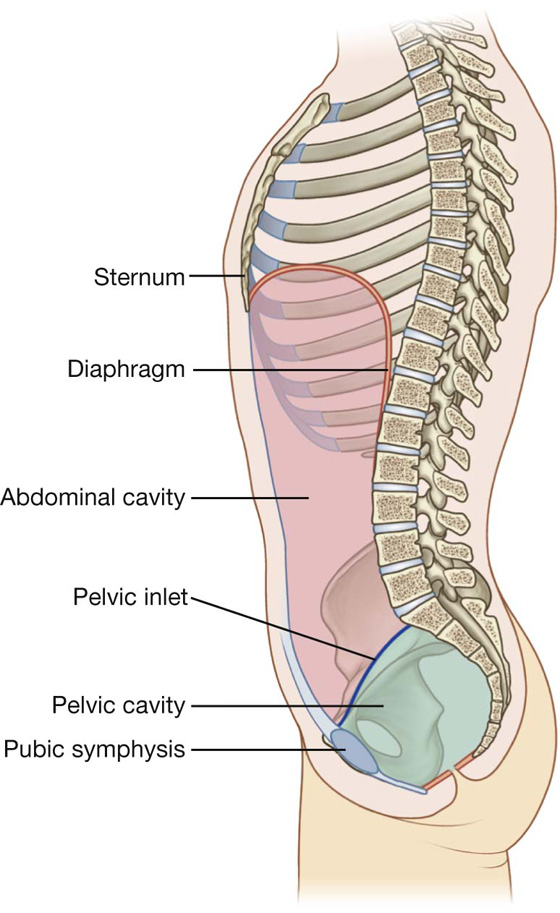
 a four-quadrant pattern, and
a four-quadrant pattern, and a nine-region pattern.
a nine-region pattern.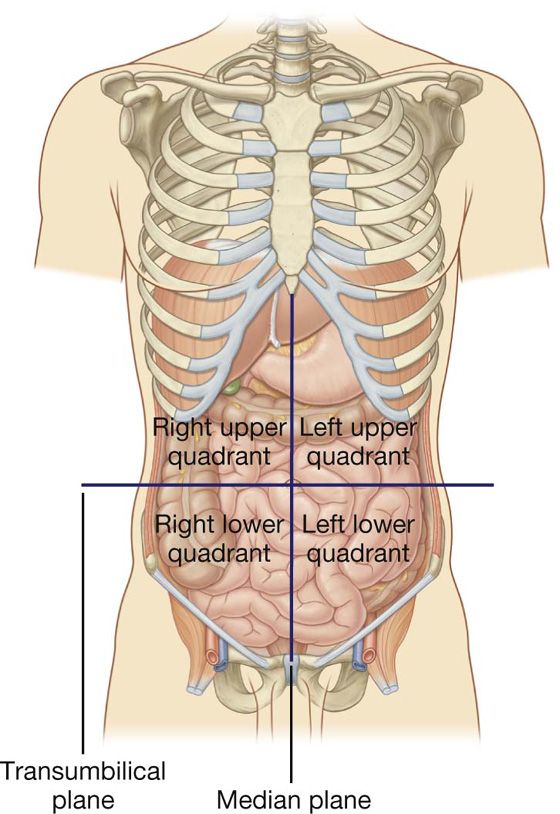
 The inferior horizontal plane (the intertubercular plane) connects the tubercles of the iliac crests, which are palpable structures 5 cm posterior to the anterior superior iliac spines, and passes through the upper part of the body of vertebra LV.
The inferior horizontal plane (the intertubercular plane) connects the tubercles of the iliac crests, which are palpable structures 5 cm posterior to the anterior superior iliac spines, and passes through the upper part of the body of vertebra LV. The vertical planes pass from the midpoint of the clavicles inferiorly to a point midway between the anterior superior iliac spine and pubic symphysis.
The vertical planes pass from the midpoint of the clavicles inferiorly to a point midway between the anterior superior iliac spine and pubic symphysis.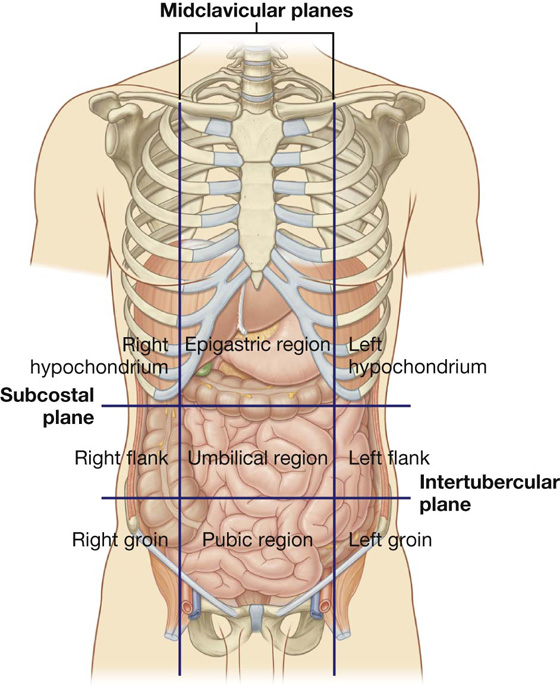
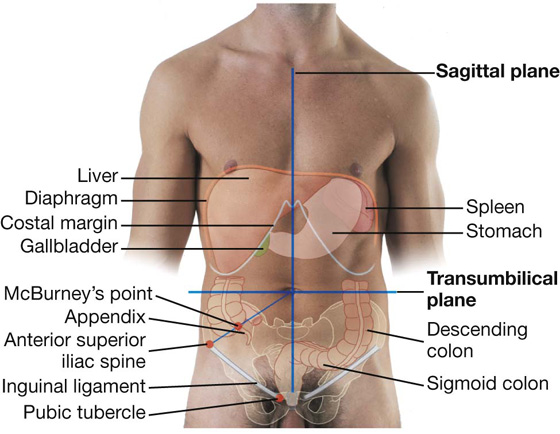
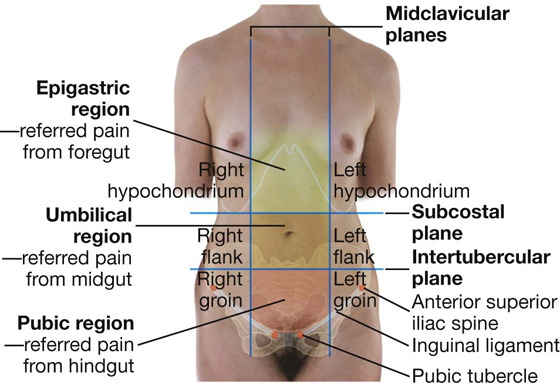
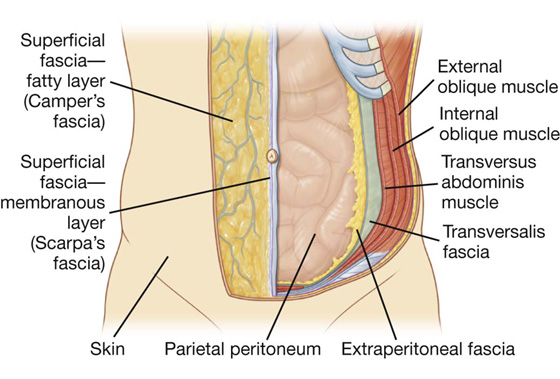
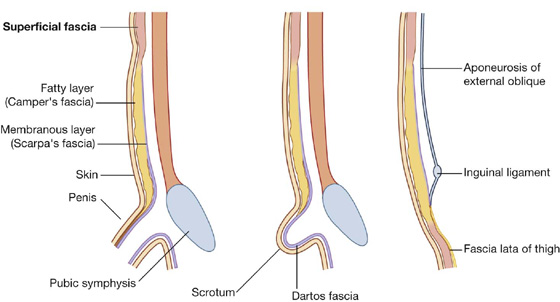
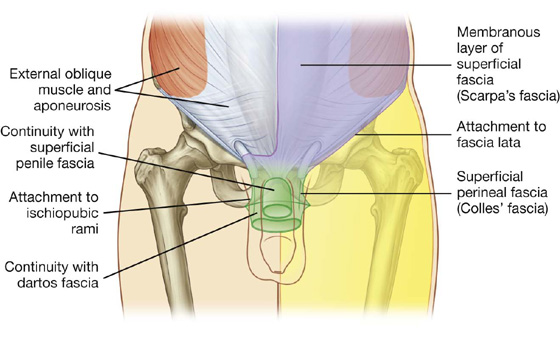
 three flat muscles whose fibers begin posterolaterally, pass anteriorly, and are replaced by an aponeurosis as the muscle continues toward the midline—the external oblique, internal oblique, and transversus abdominis muscles;
three flat muscles whose fibers begin posterolaterally, pass anteriorly, and are replaced by an aponeurosis as the muscle continues toward the midline—the external oblique, internal oblique, and transversus abdominis muscles; two vertical muscles, near the midline, which are enclosed within a tendinous sheath formed by the aponeuroses of the flat muscles—the rectus abdominis and pyramidalis muscles.
two vertical muscles, near the midline, which are enclosed within a tendinous sheath formed by the aponeuroses of the flat muscles—the rectus abdominis and pyramidalis muscles.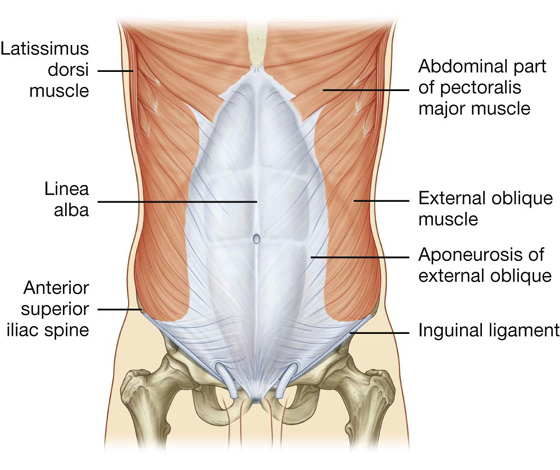
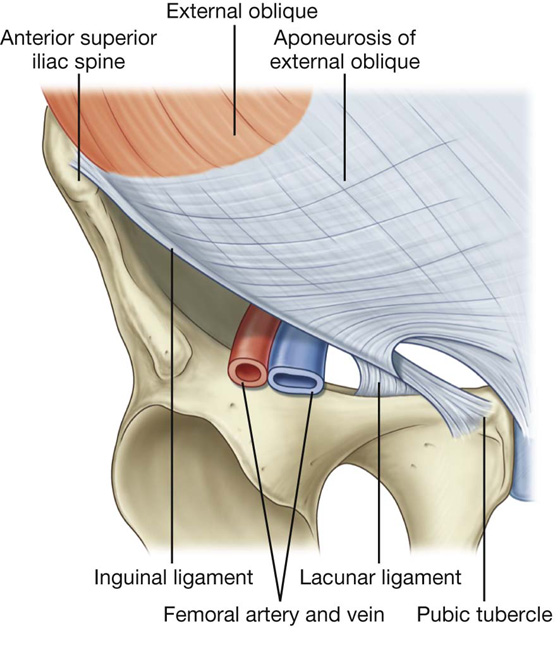
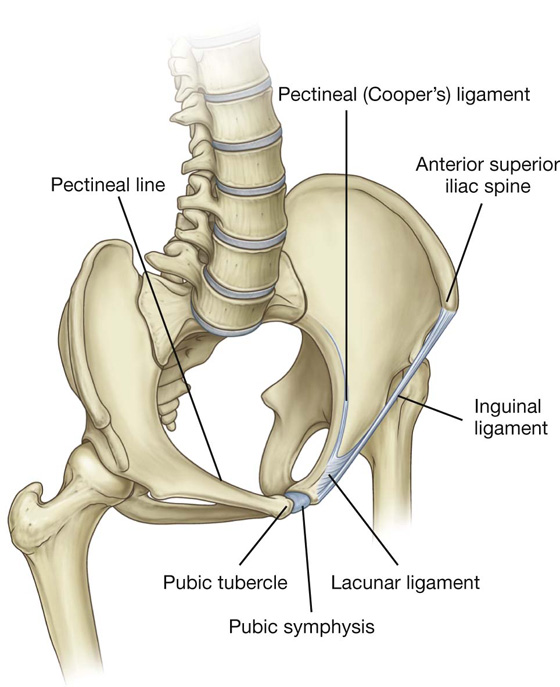
 Additional fibers extend from the lacunar ligament along the pecten pubis of the pelvic brim to form the pectineal (Cooper’s) ligament.
Additional fibers extend from the lacunar ligament along the pecten pubis of the pelvic brim to form the pectineal (Cooper’s) ligament.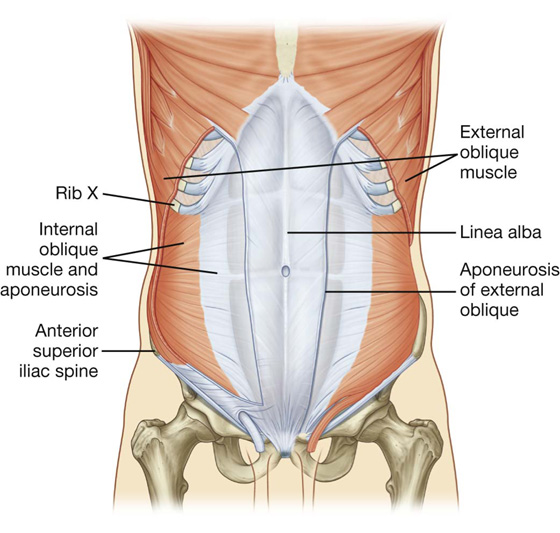
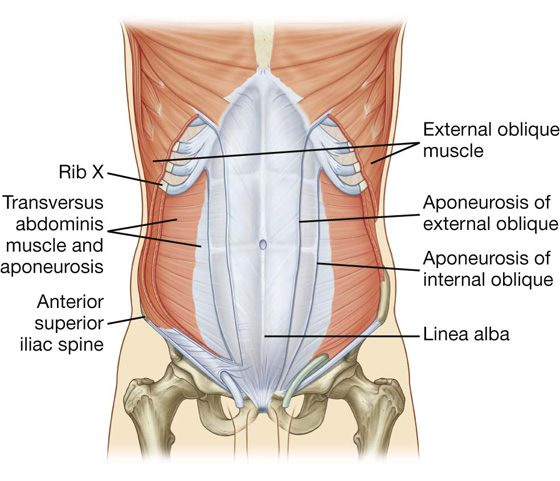
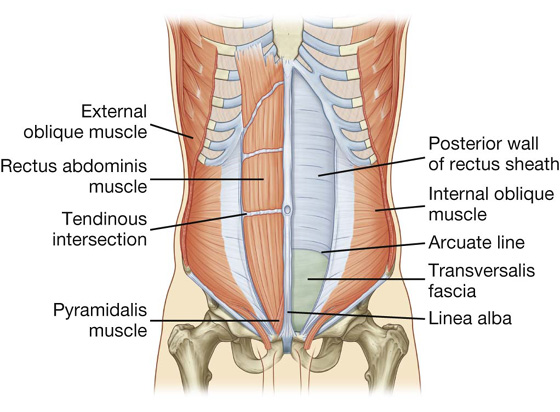
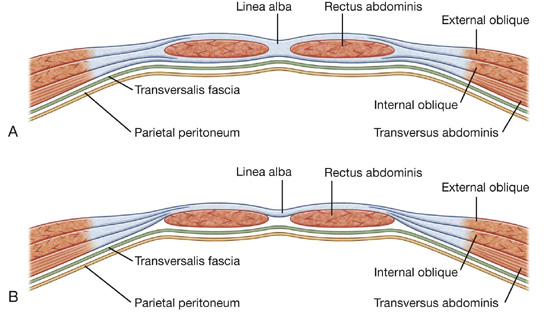
 The anterior wall consists of the aponeurosis of the external oblique and half of the aponeurosis of the internal oblique, which splits at the lateral margin of the rectus abdominis.
The anterior wall consists of the aponeurosis of the external oblique and half of the aponeurosis of the internal oblique, which splits at the lateral margin of the rectus abdominis.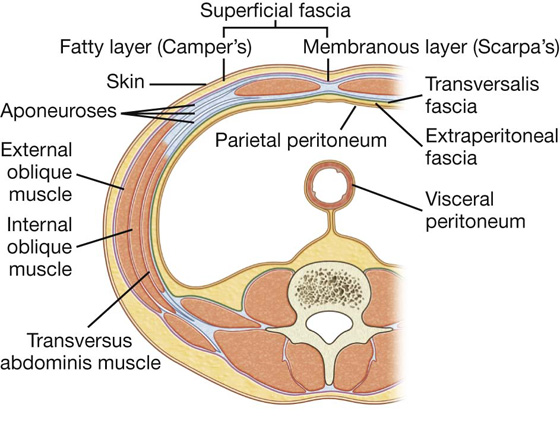
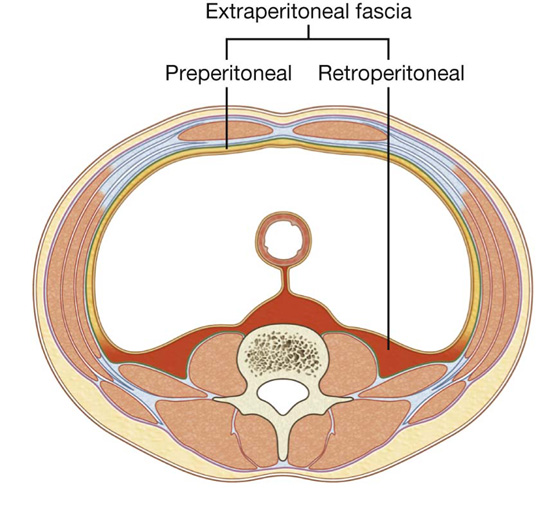
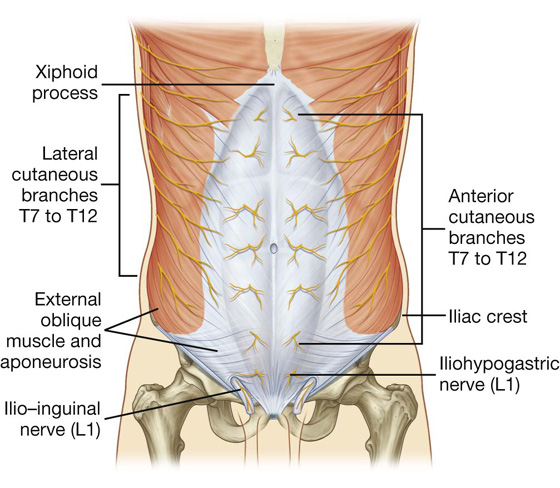
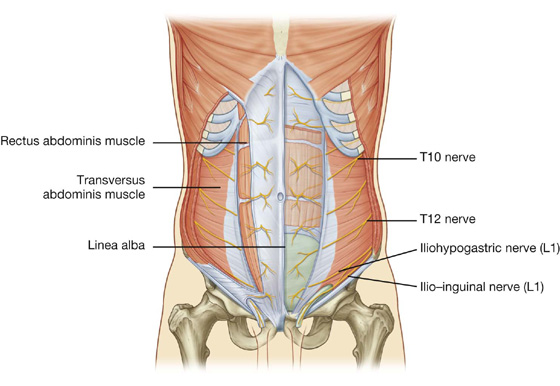
 Additionally, the ilio-inguinal nerve (a branch of L1) supplies the anterior surface of the scrotum or labia majora, and sends a small cutaneous branch to the thigh.
Additionally, the ilio-inguinal nerve (a branch of L1) supplies the anterior surface of the scrotum or labia majora, and sends a small cutaneous branch to the thigh.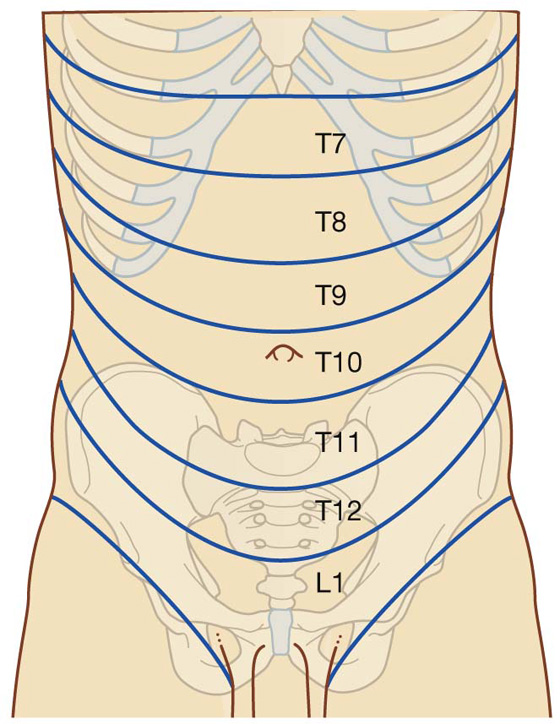
 The superior part of the wall is supplied by branches from the musculophrenic artery, a terminal branch of the internal thoracic artery.
The superior part of the wall is supplied by branches from the musculophrenic artery, a terminal branch of the internal thoracic artery. The inferior part of the wall is supplied by the medially placed superficial epigastric artery and the laterally placed superficial circumflex iliac artery, both branches of the femoral artery.
The inferior part of the wall is supplied by the medially placed superficial epigastric artery and the laterally placed superficial circumflex iliac artery, both branches of the femoral artery.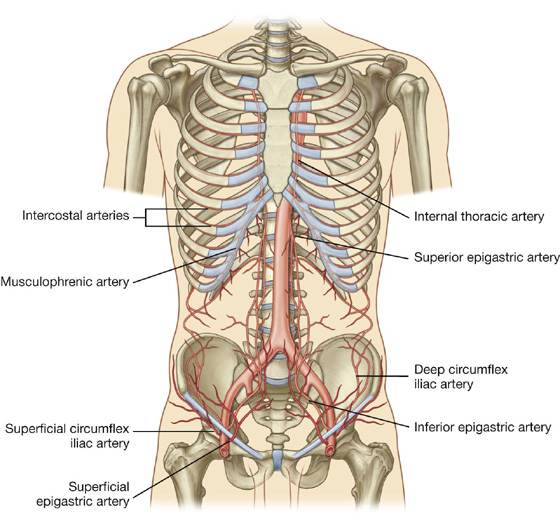
 The superior part of the wall is supplied by the superior epigastric artery, a terminal branch of the internal thoracic artery.
The superior part of the wall is supplied by the superior epigastric artery, a terminal branch of the internal thoracic artery. The inferior part of the wall is supplied by the medially placed inferior epigastric artery and the laterally placed deep circumflex iliac artery, both branches of the external iliac artery.
The inferior part of the wall is supplied by the medially placed inferior epigastric artery and the laterally placed deep circumflex iliac artery, both branches of the external iliac artery.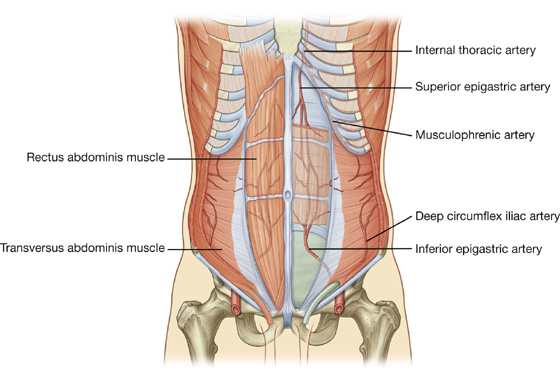
 Superficial lymphatics above the umbilicus pass in a superior direction to the axillary nodes, while drainage below the umbilicus passes in an inferior direction to the superficial inguinal nodes.
Superficial lymphatics above the umbilicus pass in a superior direction to the axillary nodes, while drainage below the umbilicus passes in an inferior direction to the superficial inguinal nodes. Deep lymphatic drainage follows the deep arteries back to parasternal nodes along the internal thoracic artery, lumbar nodes along the abdominal aorta, and external iliac nodes along the external iliac artery.
Deep lymphatic drainage follows the deep arteries back to parasternal nodes along the internal thoracic artery, lumbar nodes along the abdominal aorta, and external iliac nodes along the external iliac artery. The second covering is formed by the musculature of the internal oblique (a covering from the transversus abdominis muscle is not acquired because the processus vaginalis passes under the arching fibers of this abdominal wall muscle).
The second covering is formed by the musculature of the internal oblique (a covering from the transversus abdominis muscle is not acquired because the processus vaginalis passes under the arching fibers of this abdominal wall muscle).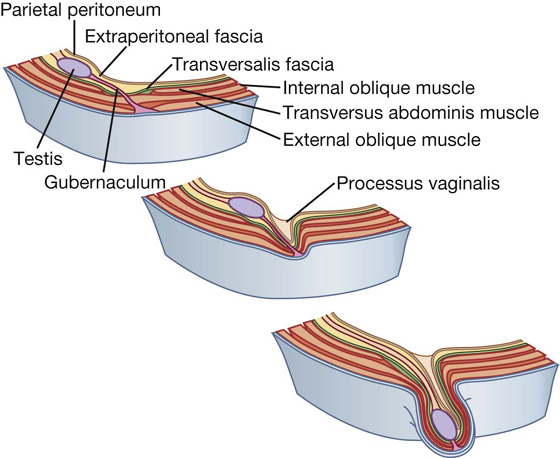
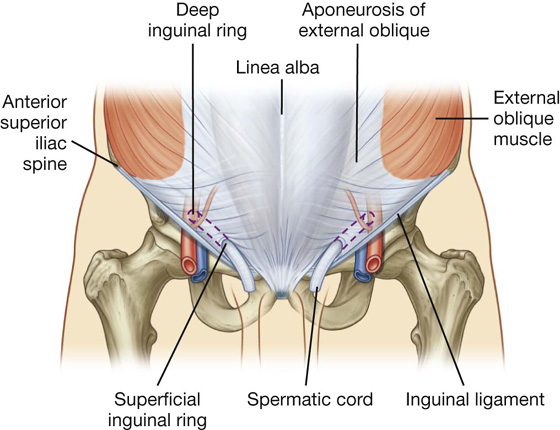
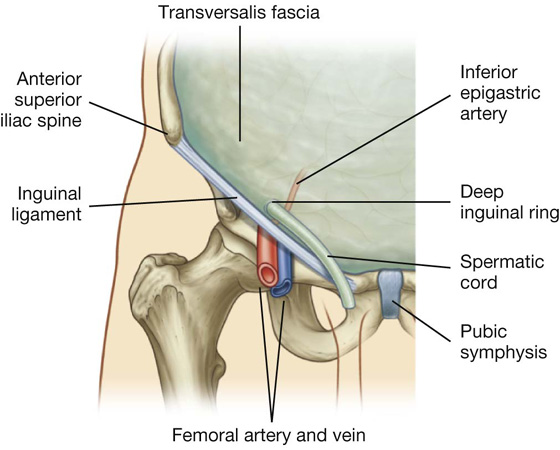
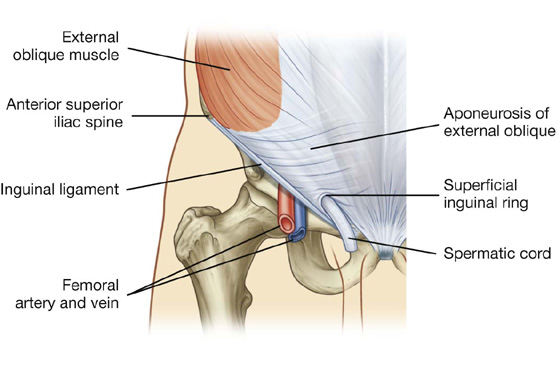
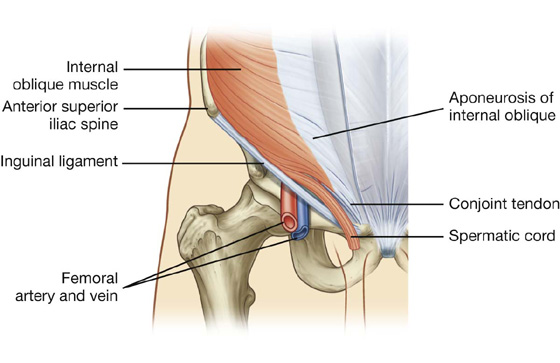
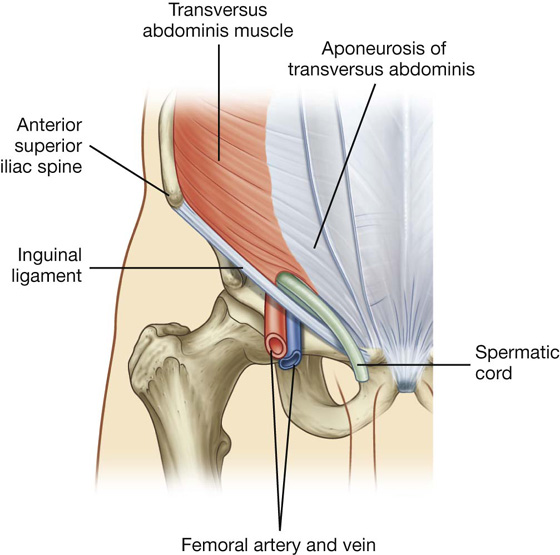
 the spermatic cord in men,
the spermatic cord in men,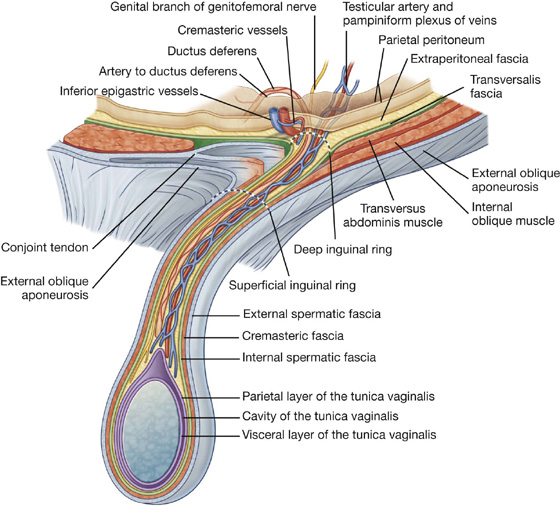
 the ductus deferens,
the ductus deferens, lymphatics, and
lymphatics, and the cremasteric fascia with the associated cremasteric muscle, which is the middle fascial layer and arises from the internal oblique muscle; and
the cremasteric fascia with the associated cremasteric muscle, which is the middle fascial layer and arises from the internal oblique muscle; and In men, the superficial inguinal ring can be easily located by following the spermatic cord superiorly to the lower abdominal wall—the external spermatic fascia of the spermatic cord is continuous with the margins of the superficial inguinal ring.
In men, the superficial inguinal ring can be easily located by following the spermatic cord superiorly to the lower abdominal wall—the external spermatic fascia of the spermatic cord is continuous with the margins of the superficial inguinal ring. The deep inguinal ring, which is the internal opening to the inguinal canal, lies superior to the inguinal ligament, midway between the anterior superior iliac spine and pubic symphysis. The pulse of the femoral artery can be felt in the same position, but below the inguinal ligament.
The deep inguinal ring, which is the internal opening to the inguinal canal, lies superior to the inguinal ligament, midway between the anterior superior iliac spine and pubic symphysis. The pulse of the femoral artery can be felt in the same position, but below the inguinal ligament.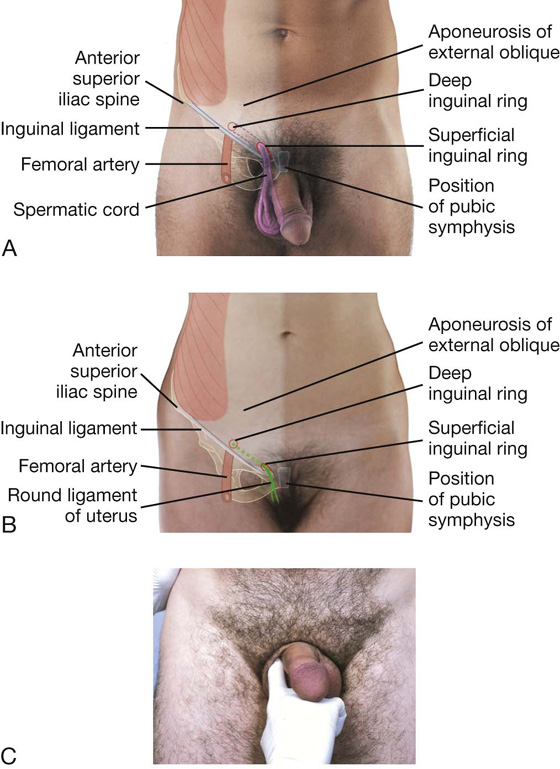
 directly, through the posterior wall of the inguinal canal.
directly, through the posterior wall of the inguinal canal. Inguinal hernias are therefore classified as either indirect or direct and occur more commonly in men than in women possibly because men have a much larger inguinal canal than women.
Inguinal hernias are therefore classified as either indirect or direct and occur more commonly in men than in women possibly because men have a much larger inguinal canal than women.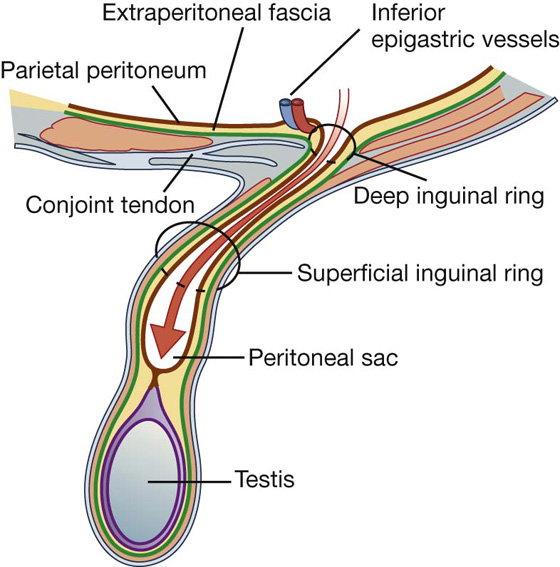
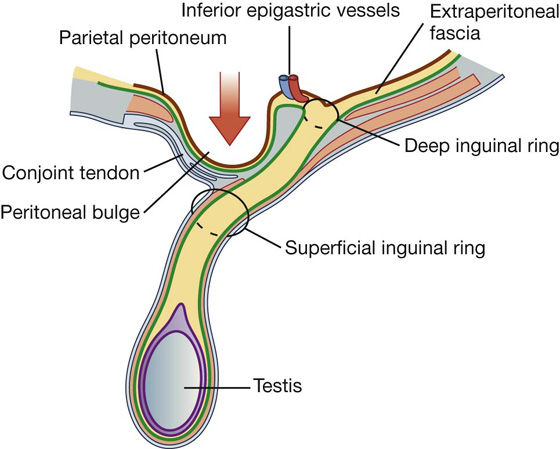
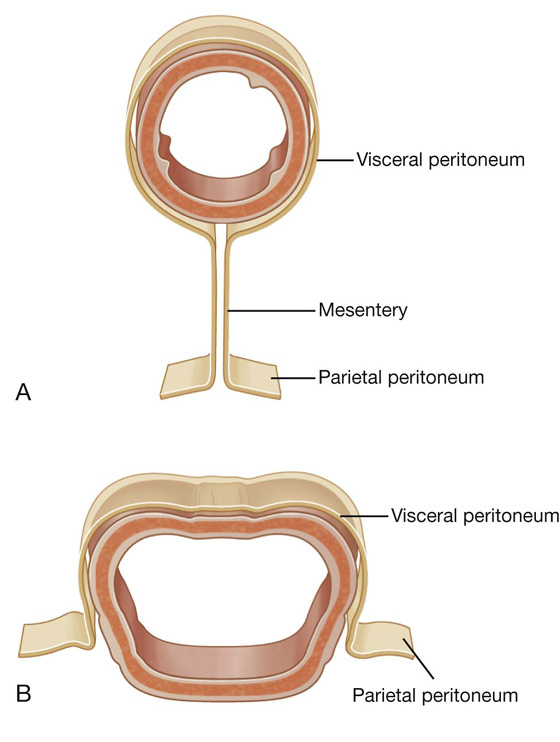
 The greater sac accounts for most of the space in the peritoneal cavity, beginning superiorly at the diaphragm and continuing inferiorly into the pelvic cavity. It is entered once the parietal peritoneum has been penetrated.
The greater sac accounts for most of the space in the peritoneal cavity, beginning superiorly at the diaphragm and continuing inferiorly into the pelvic cavity. It is entered once the parietal peritoneum has been penetrated.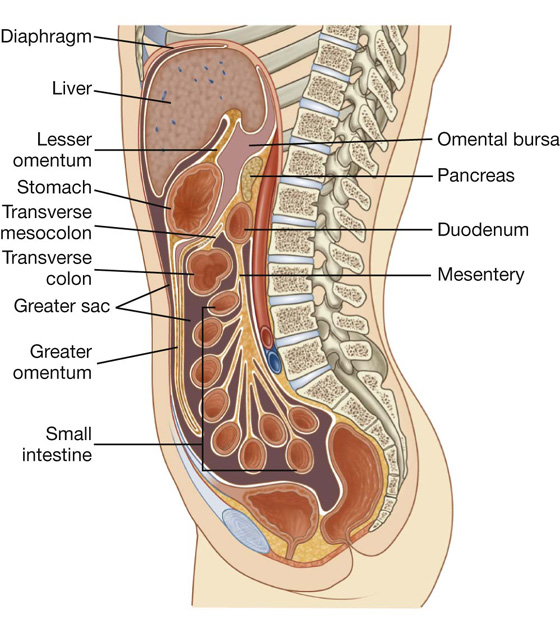
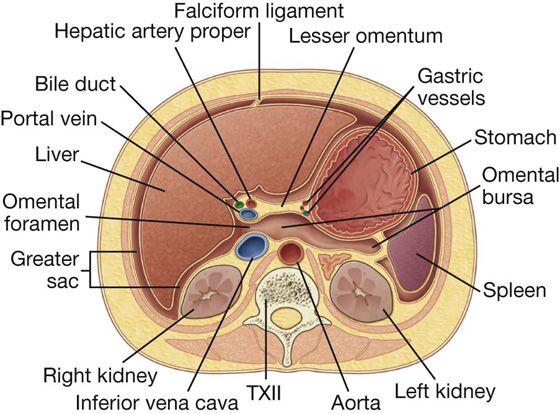
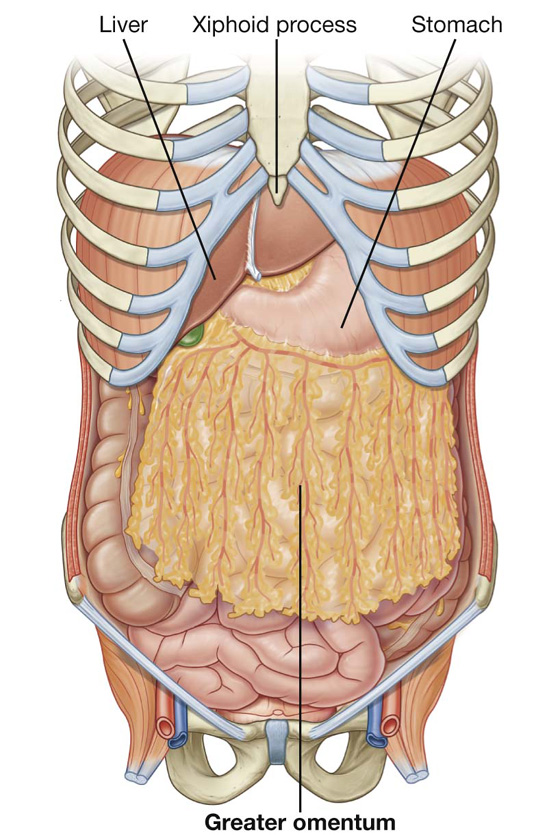
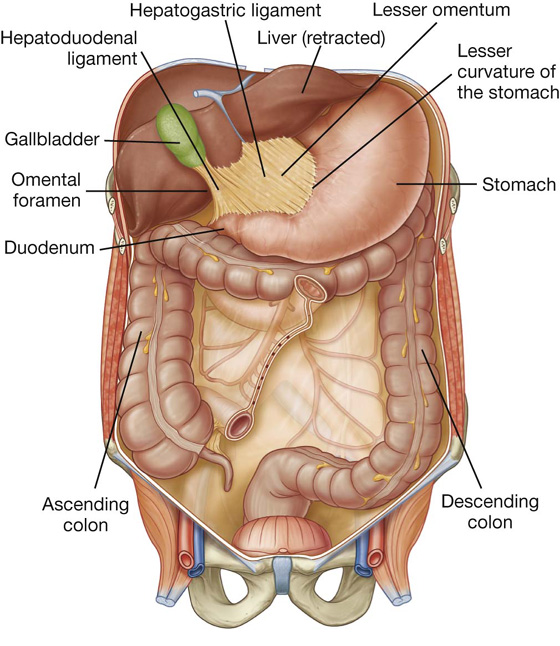
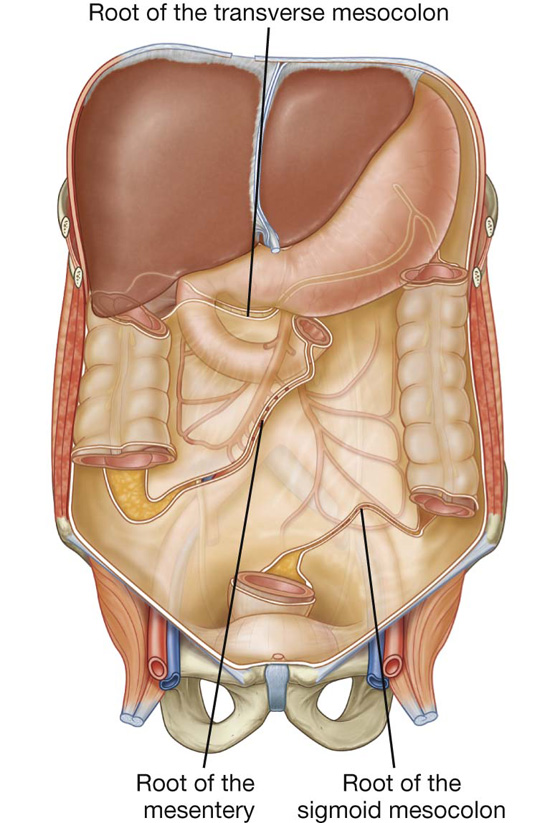
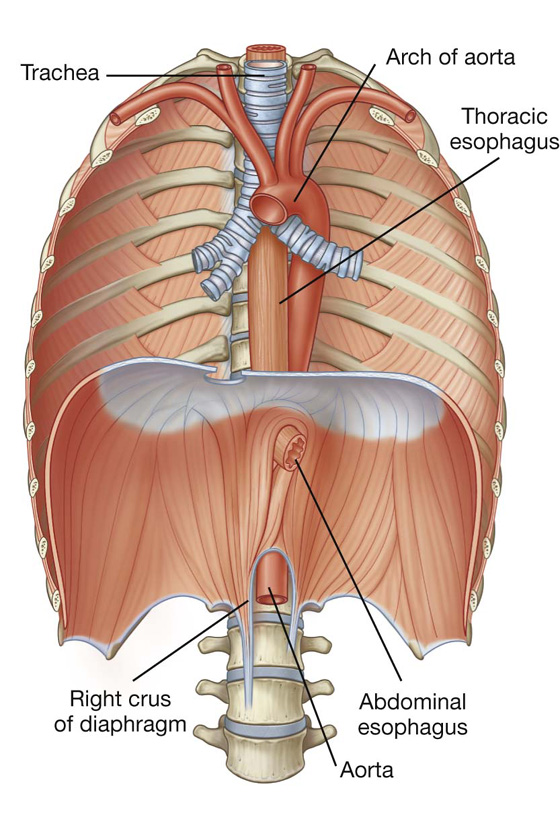
 The anterior vagal trunk consists of several smaller trunks whose fibers mostly come from the left vagus nerve; rotation of the gut during development moves these trunks to the anterior surface of the esophagus;
The anterior vagal trunk consists of several smaller trunks whose fibers mostly come from the left vagus nerve; rotation of the gut during development moves these trunks to the anterior surface of the esophagus; Similarly, the posterior vagal trunk consists of a single trunk whose fibers mostly come from the right vagus nerve, and rotational changes during development move this trunk to the posterior surface of the esophagus.
Similarly, the posterior vagal trunk consists of a single trunk whose fibers mostly come from the right vagus nerve, and rotational changes during development move this trunk to the posterior surface of the esophagus.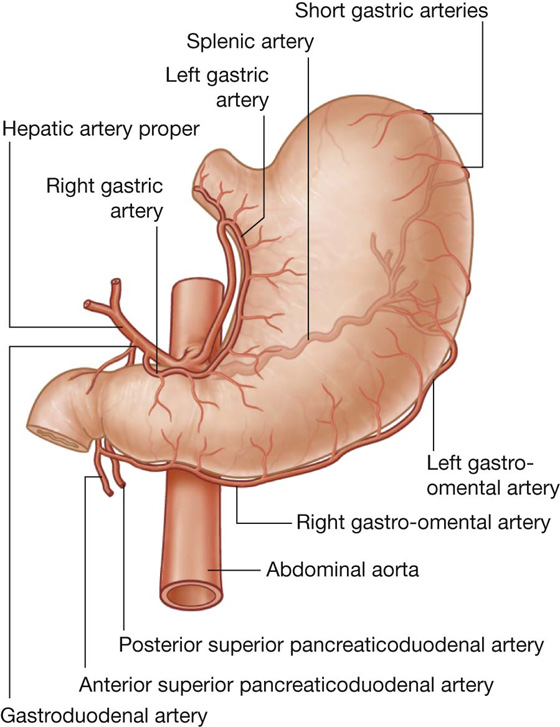
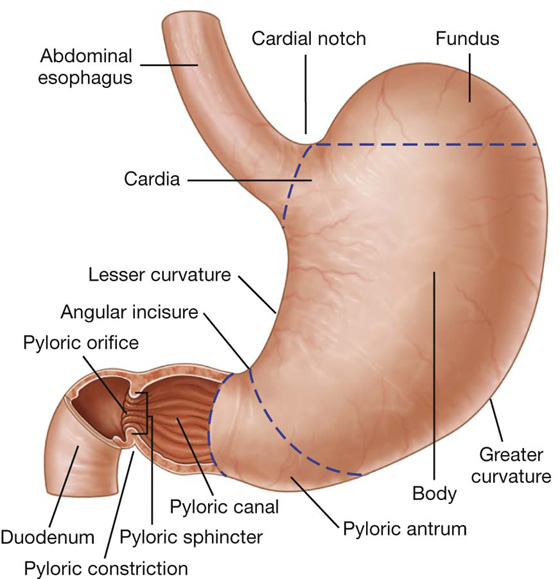
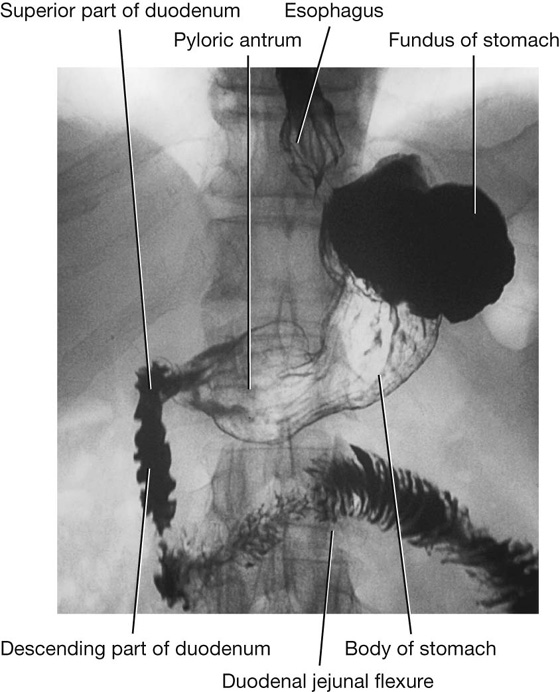
 the greater curvature, which is a point of attachment for the gastrosplenic ligament and the greater omentum;
the greater curvature, which is a point of attachment for the gastrosplenic ligament and the greater omentum;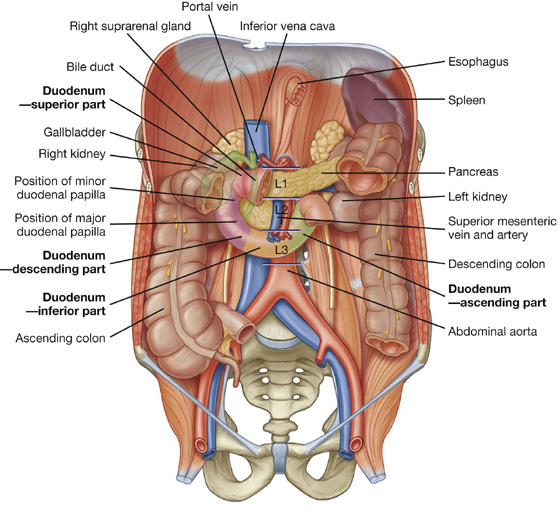
 The superior part (first part) extends from the pyloric orifice of the stomach to the neck of the gallbladder, is just to the right of the body of vertebra LI, and passes anteriorly to the bile duct, gastroduodenal artery, portal vein, and inferior vena cava. Clinically, the beginning of this part of the duodenum is referred to as the ampulla or duodenal cap, and most duodenal ulcers occur in this part of the duodenum.
The superior part (first part) extends from the pyloric orifice of the stomach to the neck of the gallbladder, is just to the right of the body of vertebra LI, and passes anteriorly to the bile duct, gastroduodenal artery, portal vein, and inferior vena cava. Clinically, the beginning of this part of the duodenum is referred to as the ampulla or duodenal cap, and most duodenal ulcers occur in this part of the duodenum. The descending part (second part) of the duodenum is just to the right of midline and extends from the neck of the gallbladder to the lower border of vertebra LIII. Its anterior surface is crossed by the transverse colon, posterior to it is the right kidney, and medial to it is the head of the pancreas. This part of the duodenum contains the major duodenal papilla, which is the common entrance for the bile and pancreatic ducts, and the minor duodenal papilla, which is the entrance for the accessory pancreatic duct, and the junction of the foregut and the midgut just below the major duodenal papilla.
The descending part (second part) of the duodenum is just to the right of midline and extends from the neck of the gallbladder to the lower border of vertebra LIII. Its anterior surface is crossed by the transverse colon, posterior to it is the right kidney, and medial to it is the head of the pancreas. This part of the duodenum contains the major duodenal papilla, which is the common entrance for the bile and pancreatic ducts, and the minor duodenal papilla, which is the entrance for the accessory pancreatic duct, and the junction of the foregut and the midgut just below the major duodenal papilla. The ascending part (fourth part) of the duodenum passes upward on, or to the left of, the aorta to approximately the upper border of vertebra LII and terminates at the duodenojejunal flexure.
The ascending part (fourth part) of the duodenum passes upward on, or to the left of, the aorta to approximately the upper border of vertebra LII and terminates at the duodenojejunal flexure. duodenal branches from the anterior superior pancreaticoduodenal artery (from the gastroduodenal artery),
duodenal branches from the anterior superior pancreaticoduodenal artery (from the gastroduodenal artery), duodenal branches from the posterior superior pancreaticoduodenal artery (from the gastroduodenal artery),
duodenal branches from the posterior superior pancreaticoduodenal artery (from the gastroduodenal artery), duodenal branches from the anterior inferior pancreaticoduodenal artery (from the inferior pancreaticoduodenal artery—a branch of the superior mesenteric artery),
duodenal branches from the anterior inferior pancreaticoduodenal artery (from the inferior pancreaticoduodenal artery—a branch of the superior mesenteric artery), duodenal branches from the posterior inferior pancreaticoduodenal artery (from the inferior pancreaticoduodenal artery—a branch of the superior mesenteric artery), and
duodenal branches from the posterior inferior pancreaticoduodenal artery (from the inferior pancreaticoduodenal artery—a branch of the superior mesenteric artery), and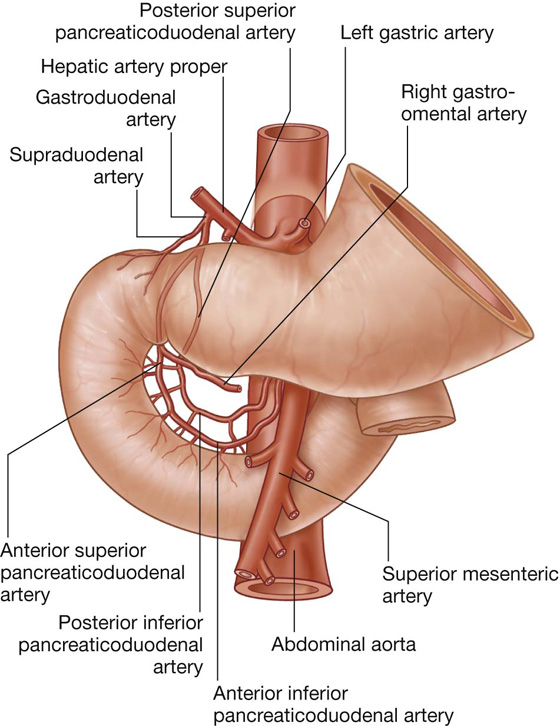
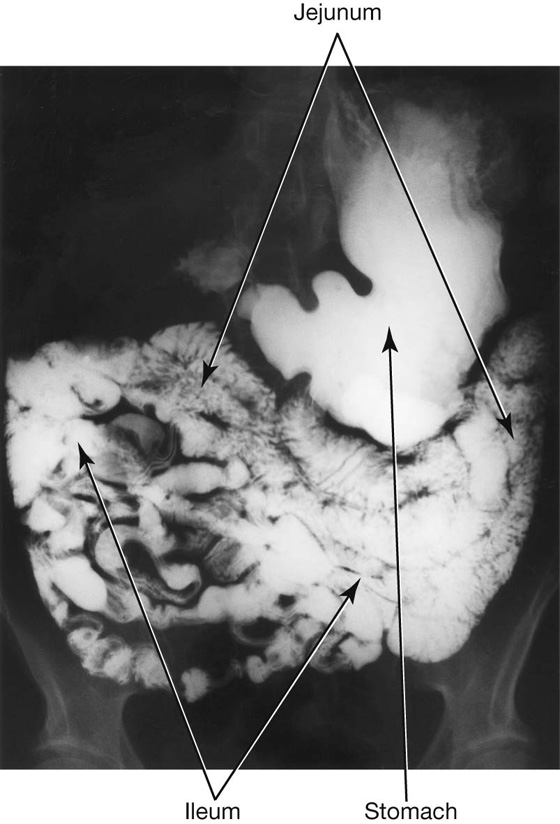
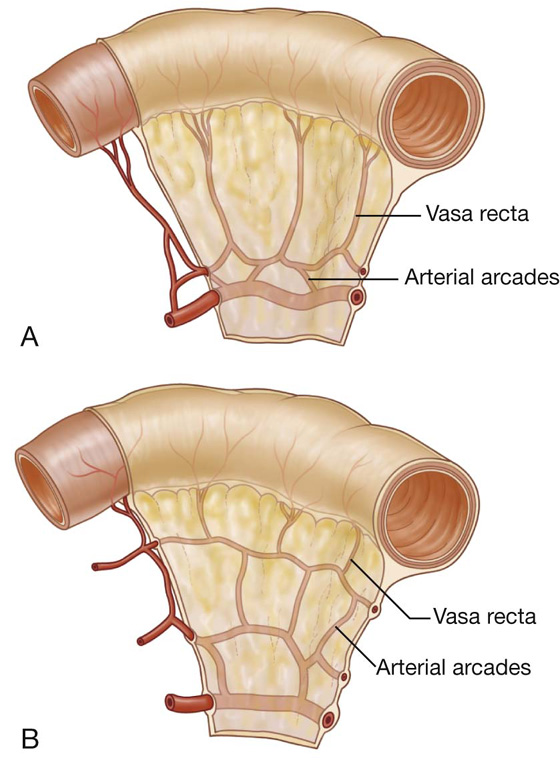
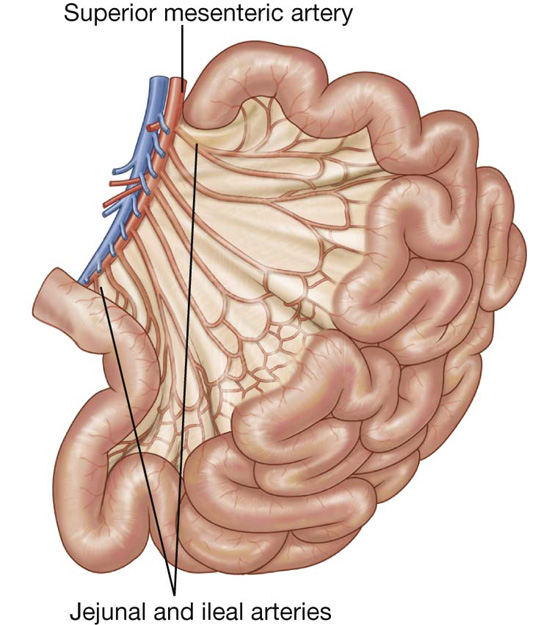
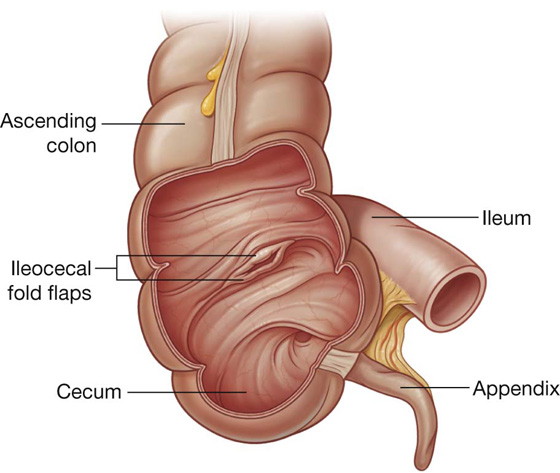
 Posterior duodenal ulcers erode either directly onto the gastroduodenal artery or, more commonly, onto the posterior superior pancreaticoduodenal artery, which can produce torrential hemorrhage and may be fatal in some patients. Treatment may involve extensive upper abdominal surgery with ligation of the vessels or by endovascular means, whereby the radiologist may place a very fine catheter retrograde from the femoral artery into the celiac artery. The common hepatic artery and the gastroduodenal artery are cannulated and the bleeding area may be blocked using small coils, which stem the flow of blood.
Posterior duodenal ulcers erode either directly onto the gastroduodenal artery or, more commonly, onto the posterior superior pancreaticoduodenal artery, which can produce torrential hemorrhage and may be fatal in some patients. Treatment may involve extensive upper abdominal surgery with ligation of the vessels or by endovascular means, whereby the radiologist may place a very fine catheter retrograde from the femoral artery into the celiac artery. The common hepatic artery and the gastroduodenal artery are cannulated and the bleeding area may be blocked using small coils, which stem the flow of blood.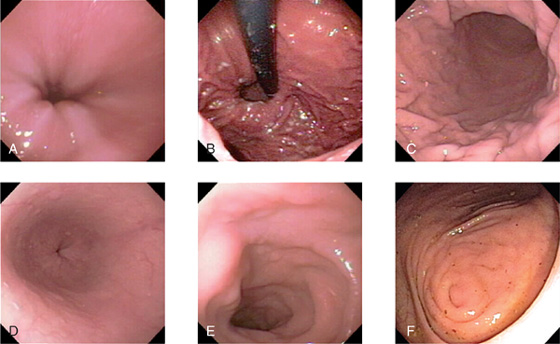
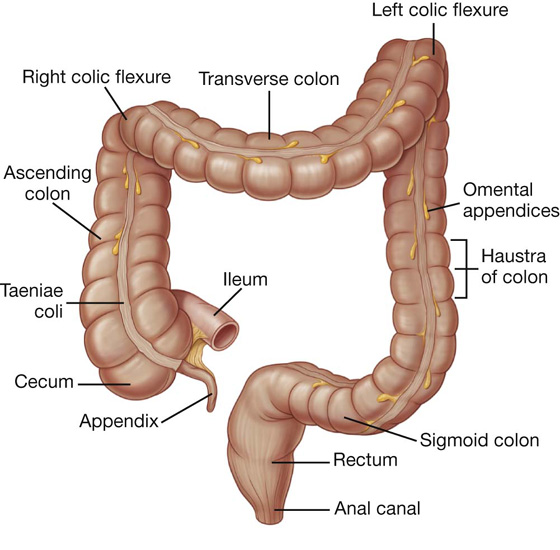
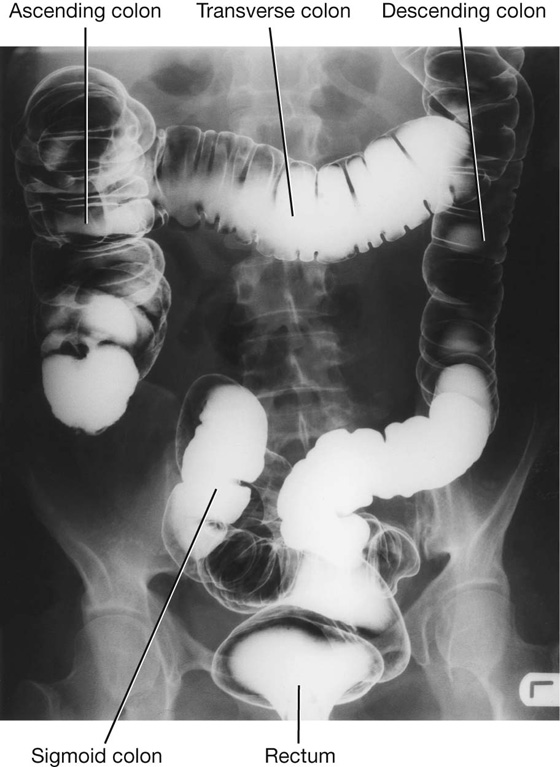
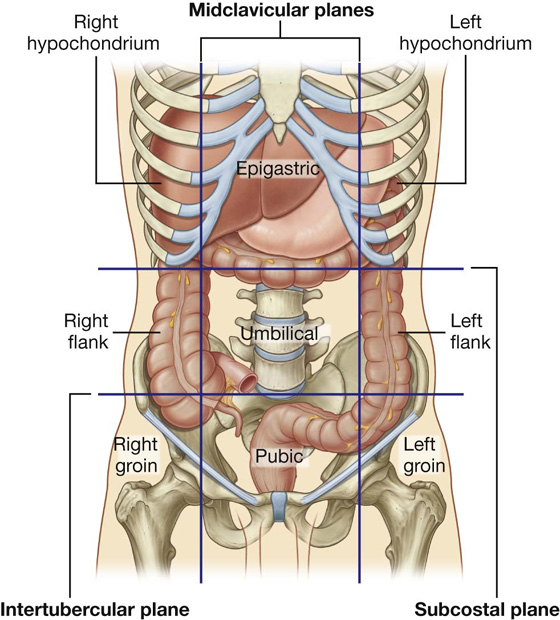
 the segregation of longitudinal muscle in its walls into three narrow bands (the taenia coli), which are primarily observed in the cecum and colon and less visible in the rectum; and
the segregation of longitudinal muscle in its walls into three narrow bands (the taenia coli), which are primarily observed in the cecum and colon and less visible in the rectum; and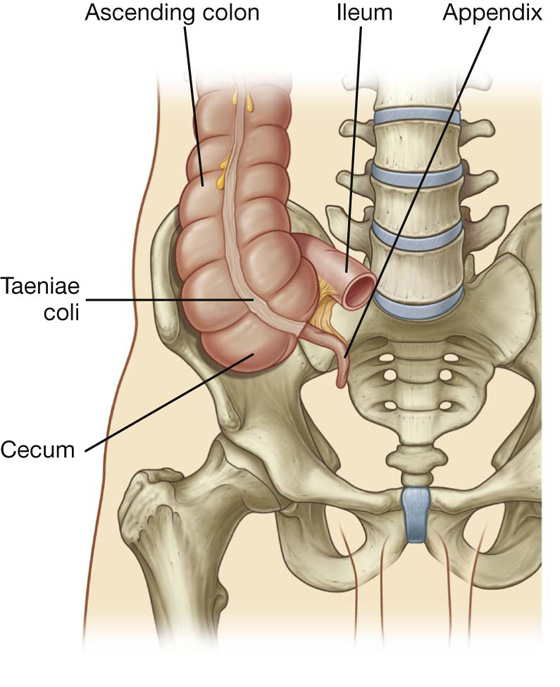
 posterior to the cecum or the lower ascending colon, or both, in a retrocecal or retrocolic position;
posterior to the cecum or the lower ascending colon, or both, in a retrocecal or retrocolic position; anterior to the terminal ileum, possibly contacting the body wall, in a pre-ileal position or posterior to the terminal ileum in a postileal position.
anterior to the terminal ileum, possibly contacting the body wall, in a pre-ileal position or posterior to the terminal ileum in a postileal position.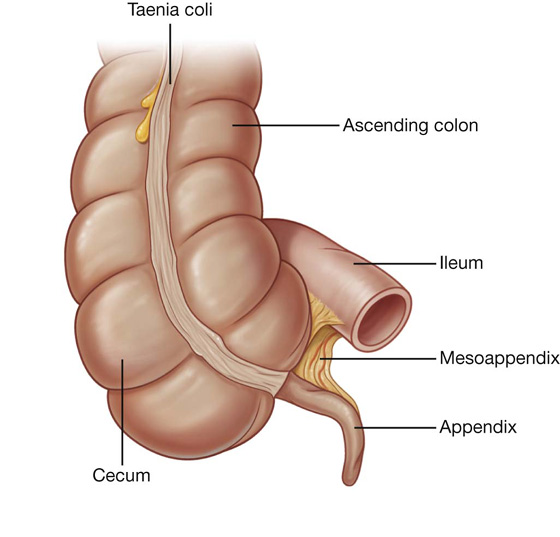
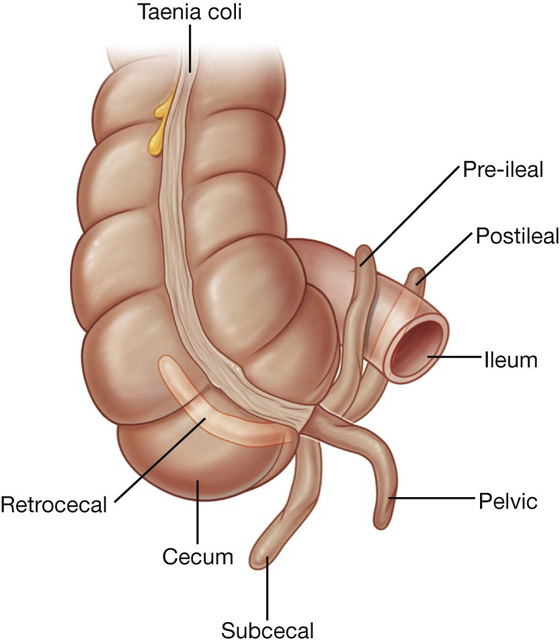
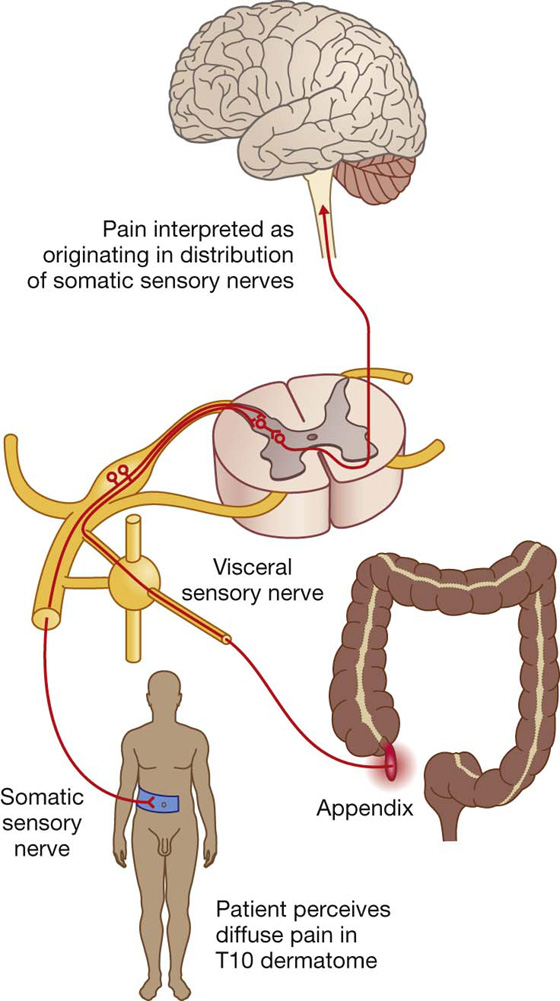
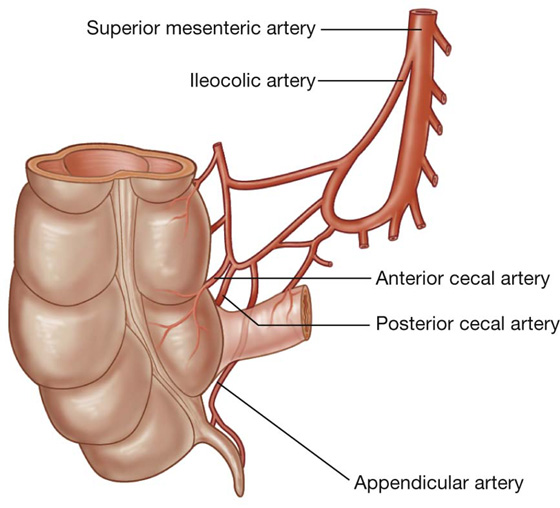
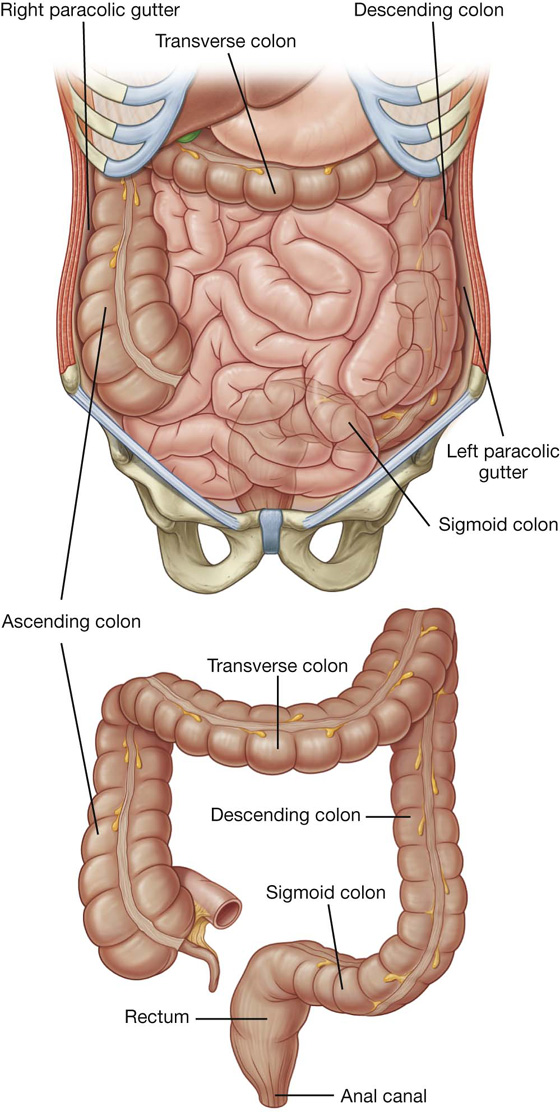
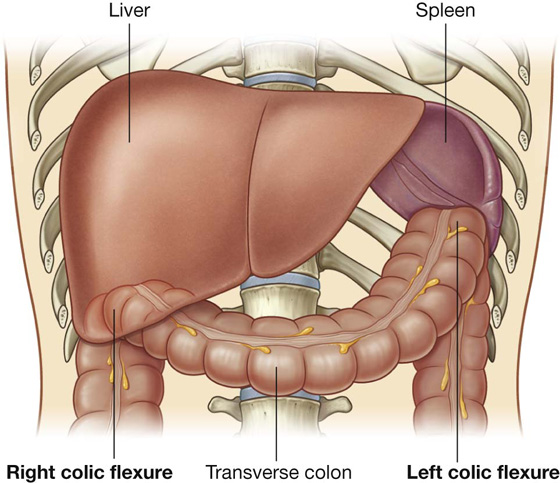
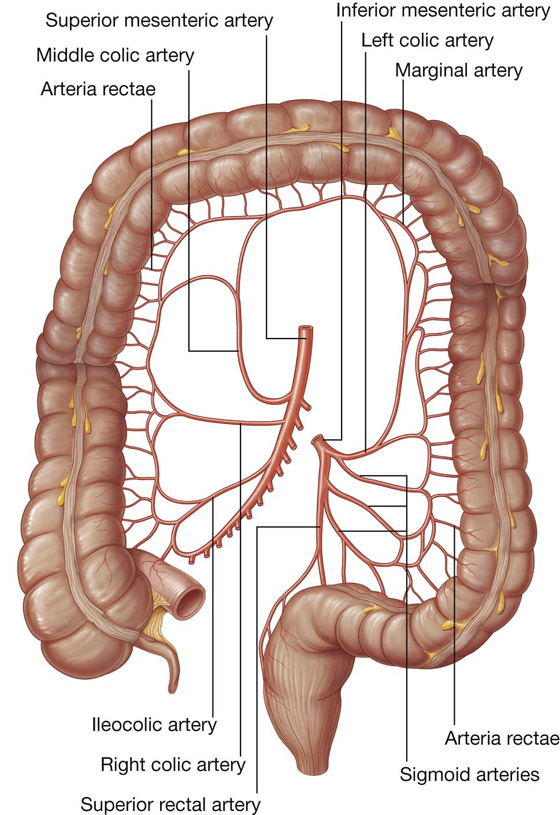
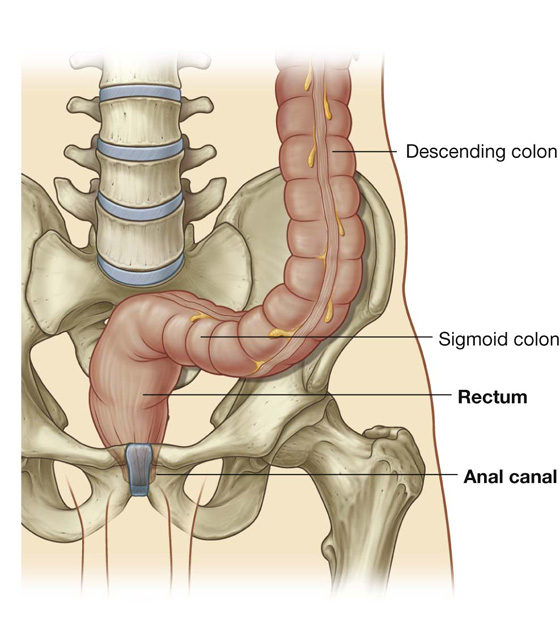
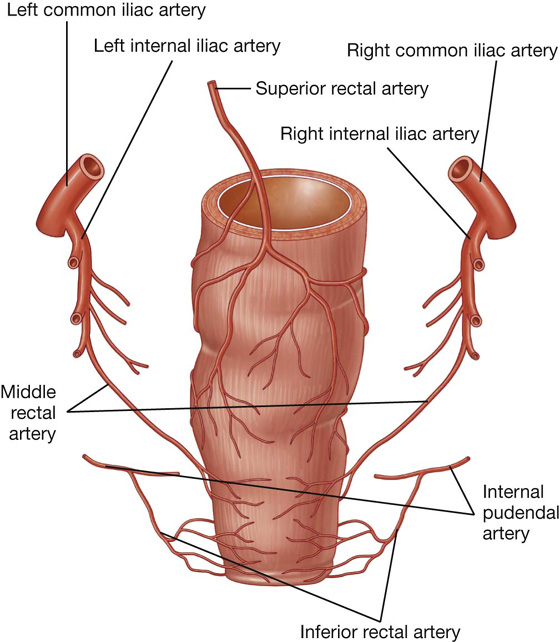
 Mechanical obstruction is caused by an intraluminal, mural, or extrinsic mass, which can be secondary to a foreign body, obstructing tumor in the wall, or extrinsic compression from an adhesion, or an embryological band.
Mechanical obstruction is caused by an intraluminal, mural, or extrinsic mass, which can be secondary to a foreign body, obstructing tumor in the wall, or extrinsic compression from an adhesion, or an embryological band. A functional obstruction is usually due to an inability of the bowel to peristalse, which again has a number of causes, and most frequently is a postsurgical state due to excessive intraoperative bowel handling. Small bowel obstruction is typically caused by adhesions following previous surgery, and history should always be sought for any operations or abdominal interventions (e.g., previous appendectomy). Other causes include bowel passing into hernias (e.g., inguinal), and bowel twisting on its own mesentery (volvulus). Large bowel obstruction is commonly caused by a tumor. Other potential causes include hernias and inflammatory diverticular disease of the sigmoid colon.
A functional obstruction is usually due to an inability of the bowel to peristalse, which again has a number of causes, and most frequently is a postsurgical state due to excessive intraoperative bowel handling. Small bowel obstruction is typically caused by adhesions following previous surgery, and history should always be sought for any operations or abdominal interventions (e.g., previous appendectomy). Other causes include bowel passing into hernias (e.g., inguinal), and bowel twisting on its own mesentery (volvulus). Large bowel obstruction is commonly caused by a tumor. Other potential causes include hernias and inflammatory diverticular disease of the sigmoid colon.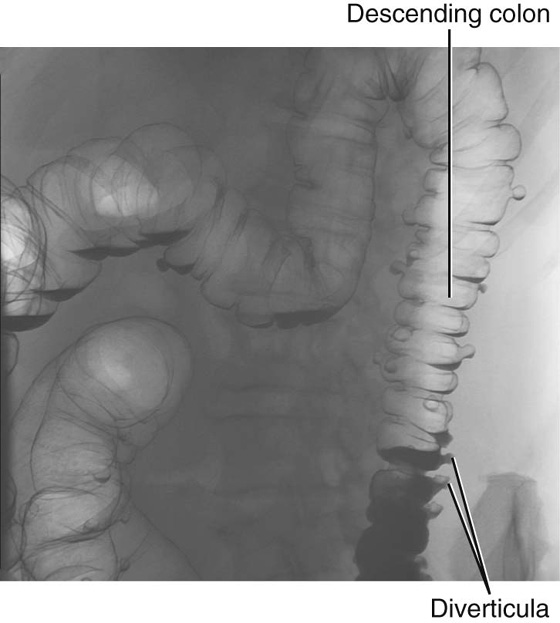
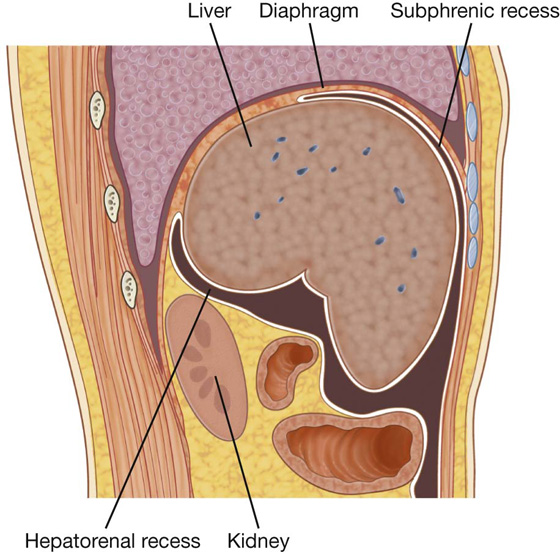
 The hepatorenal recess is a part of the peritoneal cavity on the right side between the liver and the right kidney and right suprarenal gland.
The hepatorenal recess is a part of the peritoneal cavity on the right side between the liver and the right kidney and right suprarenal gland.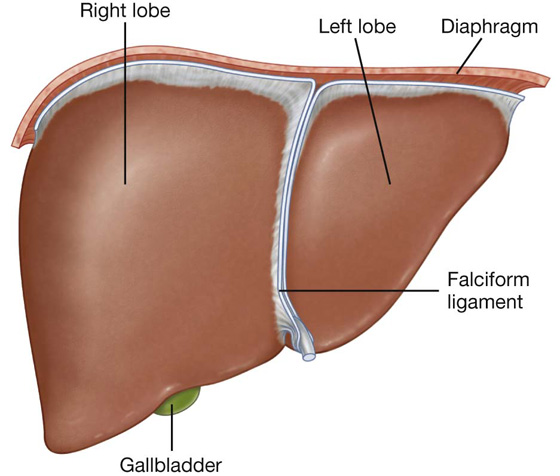
 esophagus,
esophagus, lesser omentum,
lesser omentum, gallbladder,
gallbladder, right colic flexure,
right colic flexure, right transverse colon,
right transverse colon, right kidney, and
right kidney, and right suprarenal gland.
right suprarenal gland.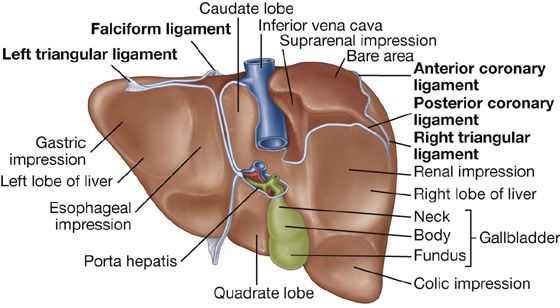
 The anterior boundary of the bare area is indicated by a reflection of peritoneum—the anterior coronary ligament;
The anterior boundary of the bare area is indicated by a reflection of peritoneum—the anterior coronary ligament; The posterior boundary of the bare area is indicated by a reflection of peritoneum—the posterior coronary ligament;
The posterior boundary of the bare area is indicated by a reflection of peritoneum—the posterior coronary ligament; Where the coronary ligaments come together laterally, they form the right and left triangular ligaments.
Where the coronary ligaments come together laterally, they form the right and left triangular ligaments. The quadrate lobe is visible on the anterior part of the visceral surface of the liver and is bounded on the left by the fissure for ligamentum teres and on the right by the fossa for the gallbladder. Functionally it is related to the left lobe of the liver.
The quadrate lobe is visible on the anterior part of the visceral surface of the liver and is bounded on the left by the fissure for ligamentum teres and on the right by the fossa for the gallbladder. Functionally it is related to the left lobe of the liver. The caudate lobe is visible on the posterior part of the visceral surface of the liver. It is bounded on the left by the fissure for the ligamentum venosum and on the right by the groove for the inferior vena cava. Functionally, it is separate from the right and the left lobes of the liver.
The caudate lobe is visible on the posterior part of the visceral surface of the liver. It is bounded on the left by the fissure for the ligamentum venosum and on the right by the groove for the inferior vena cava. Functionally, it is separate from the right and the left lobes of the liver.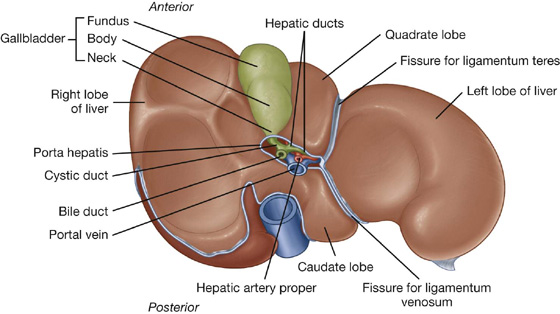
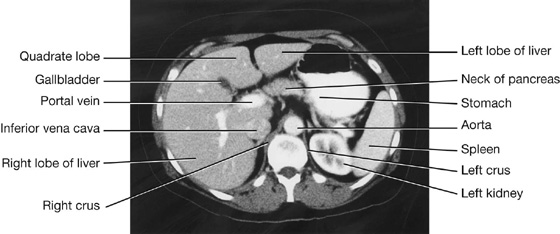
 the right hepatic artery from the hepatic artery proper (a branch of the common hepatic artery from the celiac trunk), and
the right hepatic artery from the hepatic artery proper (a branch of the common hepatic artery from the celiac trunk), and the left hepatic artery from the hepatic artery proper (a branch of the common hepatic artery from the celiac trunk).
the left hepatic artery from the hepatic artery proper (a branch of the common hepatic artery from the celiac trunk).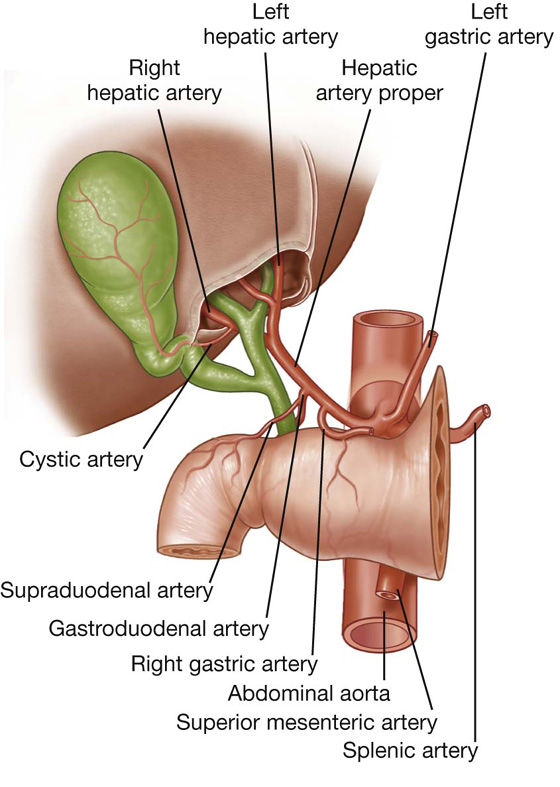
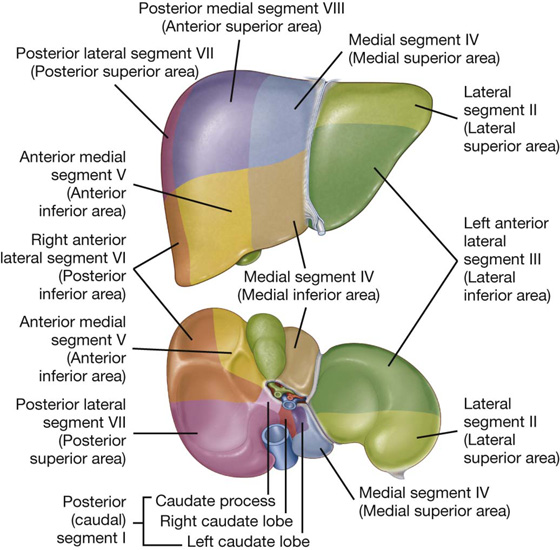
 a major part in the fossa (body of gallbladder), which may be against the transverse colon and the superior part of the duodenum; and
a major part in the fossa (body of gallbladder), which may be against the transverse colon and the superior part of the duodenum; and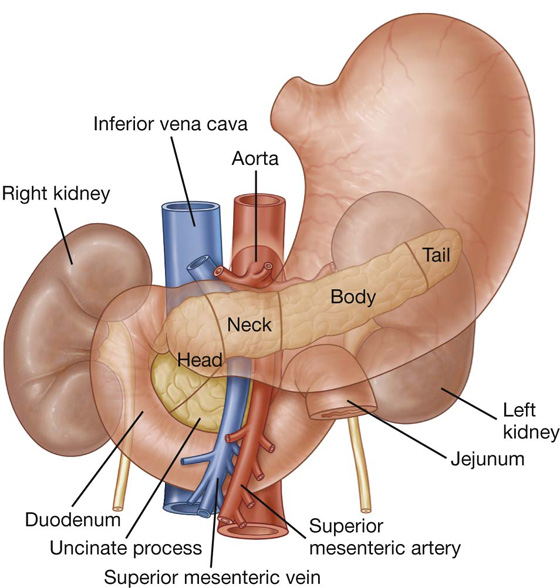
 Projecting from the lower part of the head is the uncinate process, which passes posterior to the superior mesenteric vessels.
Projecting from the lower part of the head is the uncinate process, which passes posterior to the superior mesenteric vessels. The neck of pancreas is anterior to the superior mesenteric vessels. Posterior to the neck of the pancreas, the superior mesenteric and the splenic veins join to form the portal vein.
The neck of pancreas is anterior to the superior mesenteric vessels. Posterior to the neck of the pancreas, the superior mesenteric and the splenic veins join to form the portal vein.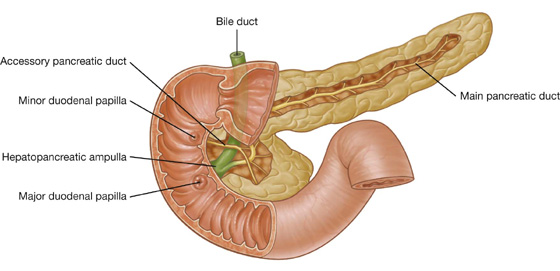
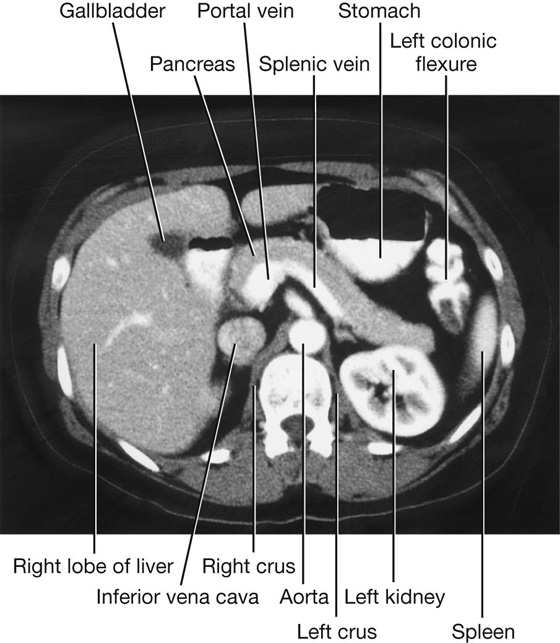
 One branch continues to the left, through the head of the pancreas, and may connect with the pancreatic duct at the point where it turns inferiorly.
One branch continues to the left, through the head of the pancreas, and may connect with the pancreatic duct at the point where it turns inferiorly. A second branch descends into the lower part of the head of pancreas, anterior to the pancreatic duct, and ends in the uncinate process.
A second branch descends into the lower part of the head of pancreas, anterior to the pancreatic duct, and ends in the uncinate process. anterior inferior pancreaticoduodenal artery from the inferior pancreaticoduodenal artery (a branch of the superior mesenteric artery), and
anterior inferior pancreaticoduodenal artery from the inferior pancreaticoduodenal artery (a branch of the superior mesenteric artery), and posterior inferior pancreaticoduodenal artery from the inferior pancreaticoduodenal artery (a branch of the superior mesenteric artery).
posterior inferior pancreaticoduodenal artery from the inferior pancreaticoduodenal artery (a branch of the superior mesenteric artery).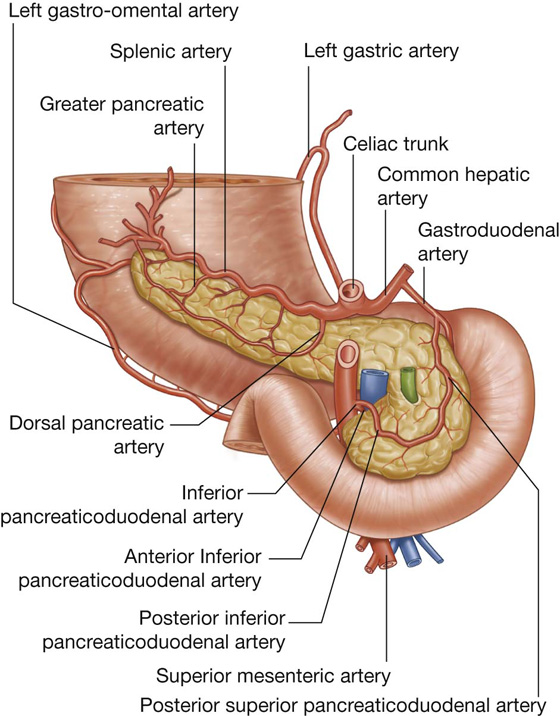
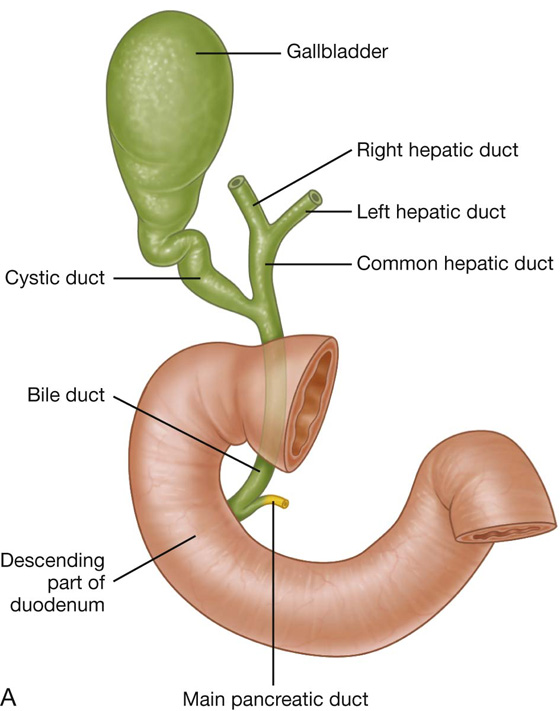
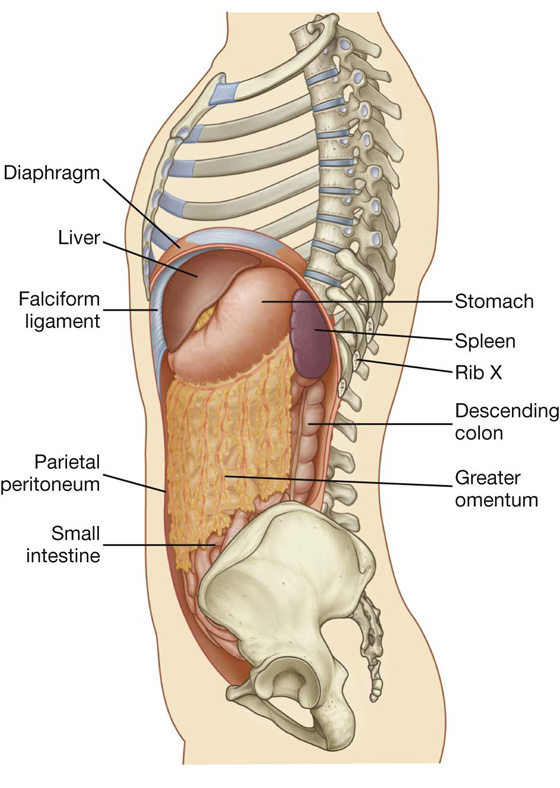
 greater curvature of the stomach by the gastrosplenic ligament, which contains the short gastric and gastro-omental vessels; and
greater curvature of the stomach by the gastrosplenic ligament, which contains the short gastric and gastro-omental vessels; and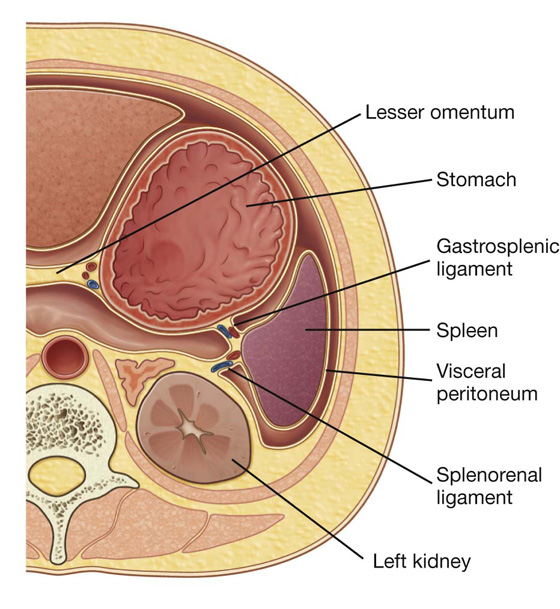
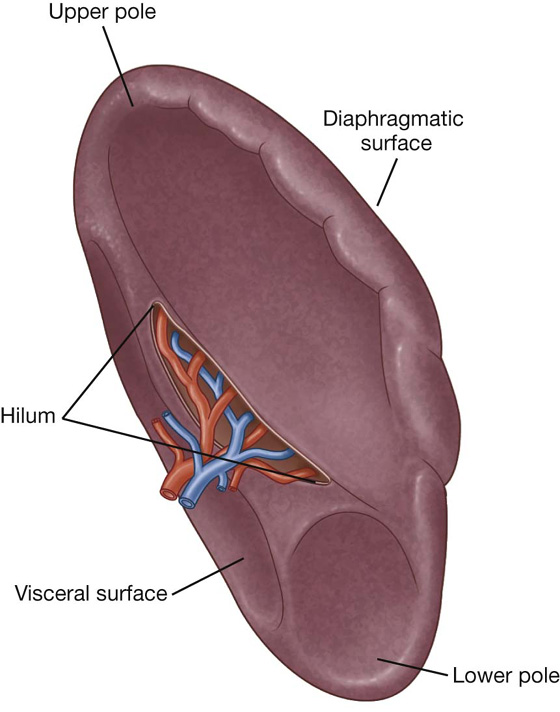
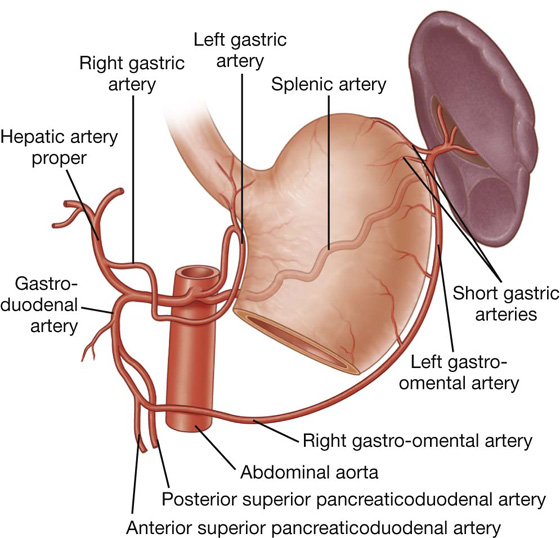
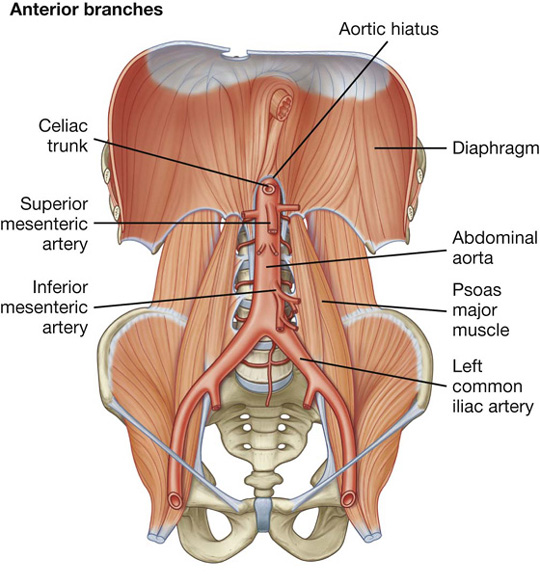
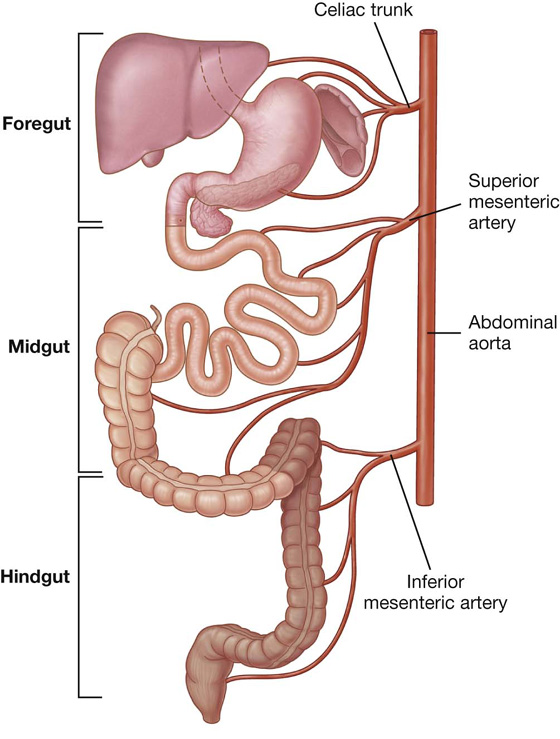
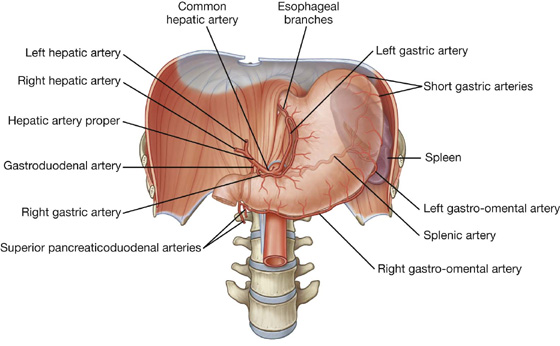
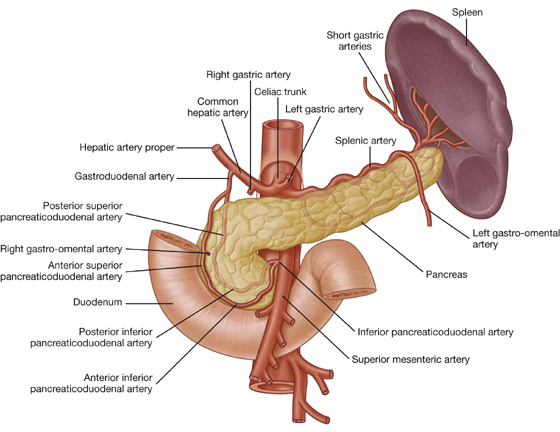
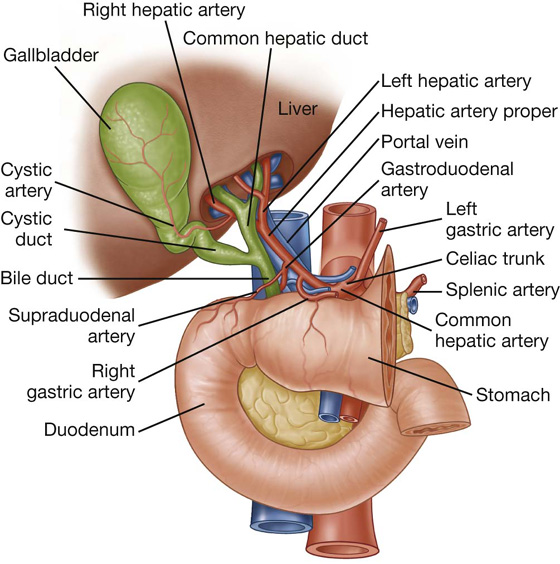
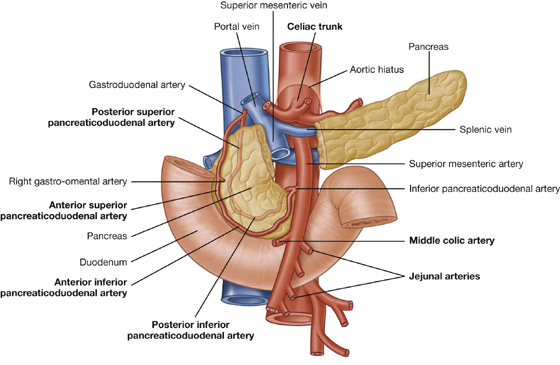
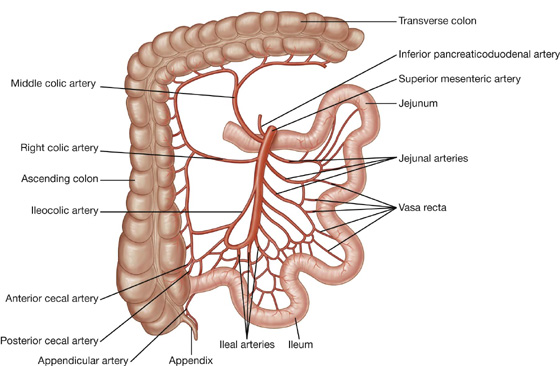
 The superior branch passes upward along the ascending colon to anastomose with the right colic artery.
The superior branch passes upward along the ascending colon to anastomose with the right colic artery. The colic branch crosses to the ascending colon and passes upward to supply the first part of the ascending colon.
The colic branch crosses to the ascending colon and passes upward to supply the first part of the ascending colon. Anterior and posterior cecal branches, arising either as a common trunk or as separate branches, supply corresponding sides of the cecum.
Anterior and posterior cecal branches, arising either as a common trunk or as separate branches, supply corresponding sides of the cecum. The ileal branch passes to the left and ascends to supply the final part of the ileum before anastomosing with the superior mesenteric artery.
The ileal branch passes to the left and ascends to supply the final part of the ileum before anastomosing with the superior mesenteric artery.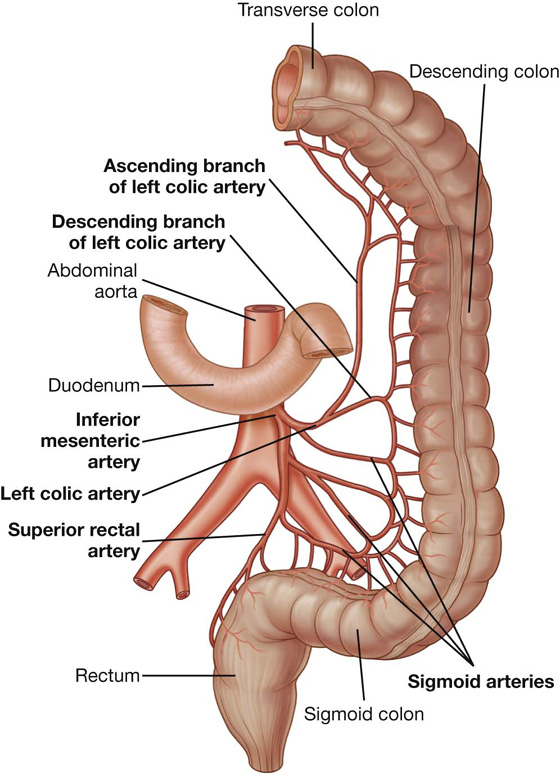
 The ascending branch passes anteriorly to the left kidney, then enters the transverse mesocolon, and passes superiorly to supply the upper part of the descending colon and the distal part of the transverse colon; it anastomoses with branches of the middle colic artery.
The ascending branch passes anteriorly to the left kidney, then enters the transverse mesocolon, and passes superiorly to supply the upper part of the descending colon and the distal part of the transverse colon; it anastomoses with branches of the middle colic artery. The descending branch passes inferiorly, supplying the lower part of the descending colon and anastomoses with the first sigmoid artery.
The descending branch passes inferiorly, supplying the lower part of the descending colon and anastomoses with the first sigmoid artery.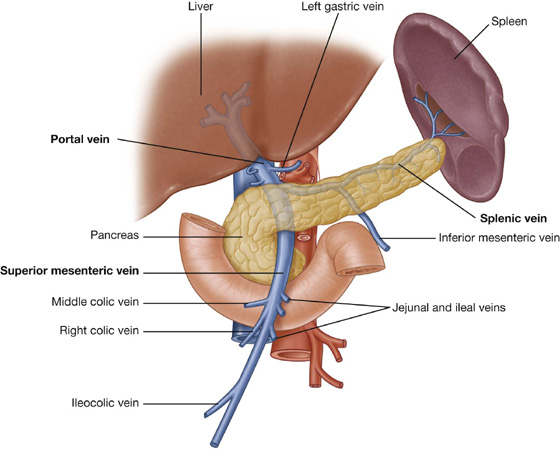
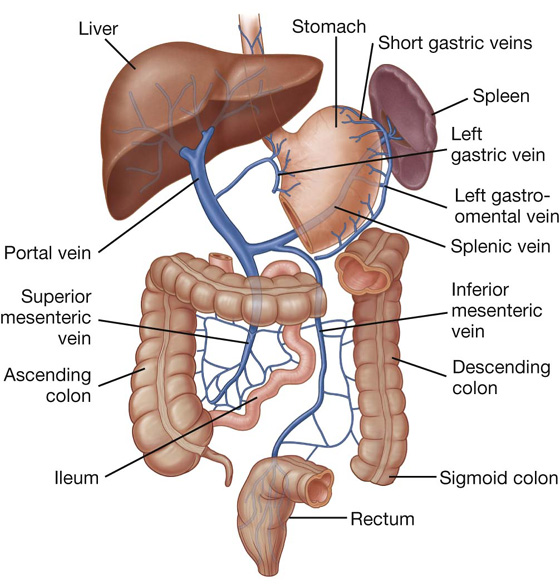
 the anterior and posterior inferior pancreaticoduodenal veins, which pass alongside the arteries of the same name; the anterior superior pancreaticoduodenal vein usually empties into the right gastro-omental vein, and the posterior superior pancreaticoduodenal vein usually empties directly into the portal vein.
the anterior and posterior inferior pancreaticoduodenal veins, which pass alongside the arteries of the same name; the anterior superior pancreaticoduodenal vein usually empties into the right gastro-omental vein, and the posterior superior pancreaticoduodenal vein usually empties directly into the portal vein. the gastroesophageal junction around the cardia of the stomach—where the left gastric vein and its tributaries form a portosystemic anastomosis with tributaries to the azygos system of veins;
the gastroesophageal junction around the cardia of the stomach—where the left gastric vein and its tributaries form a portosystemic anastomosis with tributaries to the azygos system of veins; the anus—the superior rectal vein of the portal system anastomoses with the middle and inferior rectal veins of the systemic venous system; and
the anus—the superior rectal vein of the portal system anastomoses with the middle and inferior rectal veins of the systemic venous system; and the anterior abdominal wall around the umbilicus—the para-umbilical veins anastomose with veins on the anterior abdominal wall.
the anterior abdominal wall around the umbilicus—the para-umbilical veins anastomose with veins on the anterior abdominal wall.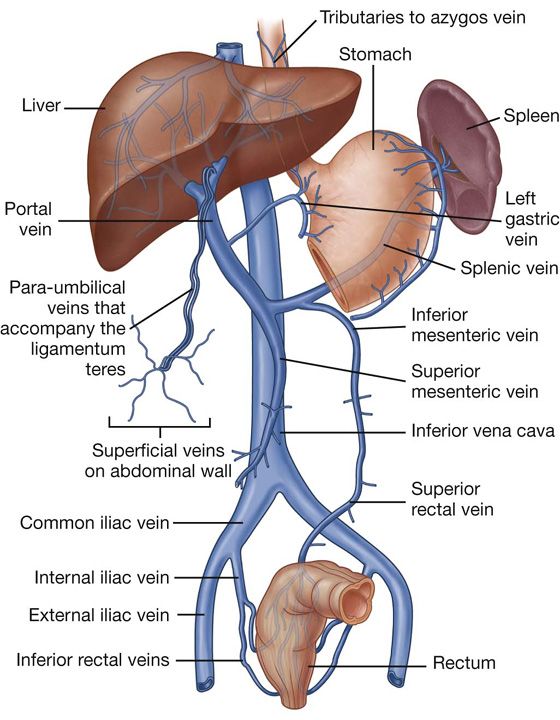
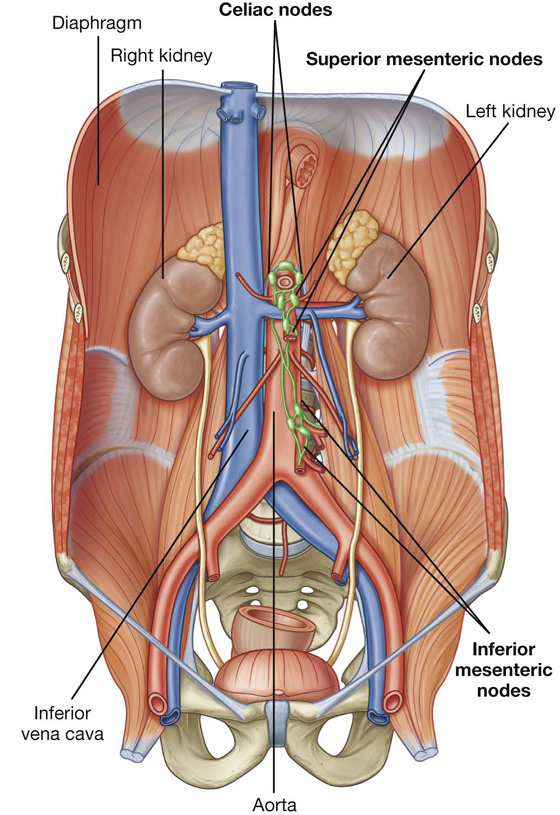
 Extrinsic innervation involves receiving motor impulses from, and sending sensory information to, the central nervous system;
Extrinsic innervation involves receiving motor impulses from, and sending sensory information to, the central nervous system; Intrinsic innervation involves the regulation of digestive tract activities by a generally self-sufficient network of sensory and motor neurons (the enteric nervous system).
Intrinsic innervation involves the regulation of digestive tract activities by a generally self-sufficient network of sensory and motor neurons (the enteric nervous system).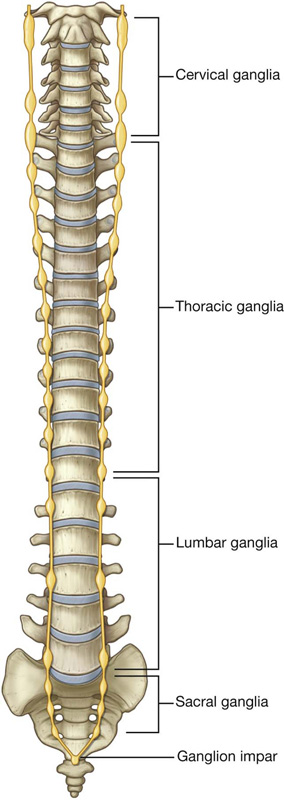
 The thoracic, lumbar, and sacral splanchnic nerves carry preganglionic sympathetic fibers from the sympathetic trunk to ganglia in the prevertebral plexus, and also visceral afferent fibers.
The thoracic, lumbar, and sacral splanchnic nerves carry preganglionic sympathetic fibers from the sympathetic trunk to ganglia in the prevertebral plexus, and also visceral afferent fibers. The pelvic splanchnic nerves (parasympathetic root) carry preganglionic parasympathetic fibers from anterior rami of S2, S3, and S4 spinal nerves to an extension of the prevertebral plexus in the pelvis (the inferior hypogastric plexus or pelvic plexus).
The pelvic splanchnic nerves (parasympathetic root) carry preganglionic parasympathetic fibers from anterior rami of S2, S3, and S4 spinal nerves to an extension of the prevertebral plexus in the pelvis (the inferior hypogastric plexus or pelvic plexus). The greater splanchnic nerve arises from the fifth to the ninth (or tenth) thoracic ganglia and travels to the celiac ganglion in the abdomen (a prevertebral ganglion associated with the celiac trunk).
The greater splanchnic nerve arises from the fifth to the ninth (or tenth) thoracic ganglia and travels to the celiac ganglion in the abdomen (a prevertebral ganglion associated with the celiac trunk). The lesser splanchnic nerve arises from the ninth and tenth (or tenth and eleventh) thoracic ganglia and travels to the aorticorenal ganglion.
The lesser splanchnic nerve arises from the ninth and tenth (or tenth and eleventh) thoracic ganglia and travels to the aorticorenal ganglion. The least splanchnic nerve arises from the twelfth thoracic ganglion and travels to the renal plexus.
The least splanchnic nerve arises from the twelfth thoracic ganglion and travels to the renal plexus.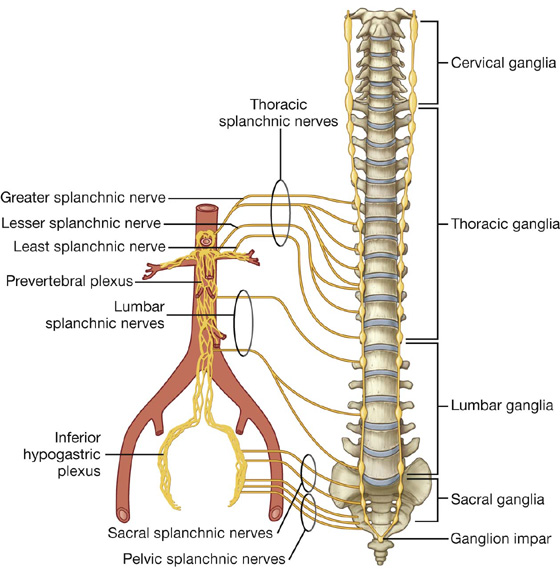
 The aortic plexus consists of nerve fibers and associated ganglia on the anterior and lateral surfaces of the abdominal aorta extending from just below the origin of the superior mesenteric artery to the bifurcation of the aorta into the two common iliac arteries. The major ganglion in this plexus is the inferior mesenteric ganglion at the root of the inferior mesenteric artery.
The aortic plexus consists of nerve fibers and associated ganglia on the anterior and lateral surfaces of the abdominal aorta extending from just below the origin of the superior mesenteric artery to the bifurcation of the aorta into the two common iliac arteries. The major ganglion in this plexus is the inferior mesenteric ganglion at the root of the inferior mesenteric artery. The superior hypogastric plexus contains numerous small ganglia and is the final part of the abdominal prevertebral plexus before the prevertebral plexus continues into the pelvic cavity.
The superior hypogastric plexus contains numerous small ganglia and is the final part of the abdominal prevertebral plexus before the prevertebral plexus continues into the pelvic cavity.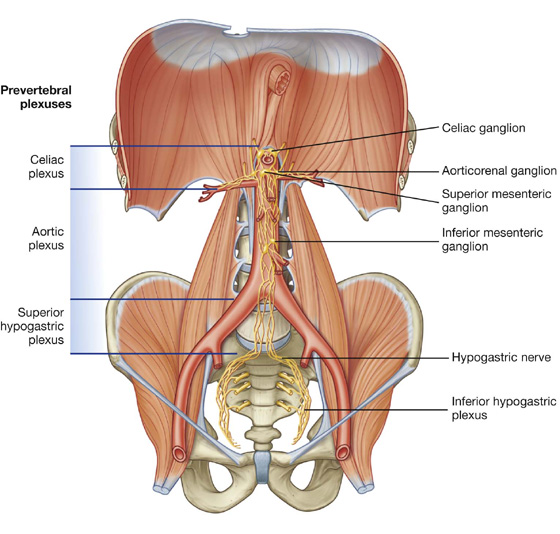
 preganglionic sympathetic and visceral afferent fibers from the thoracic and lumbar splanchnic nerves, and
preganglionic sympathetic and visceral afferent fibers from the thoracic and lumbar splanchnic nerves, and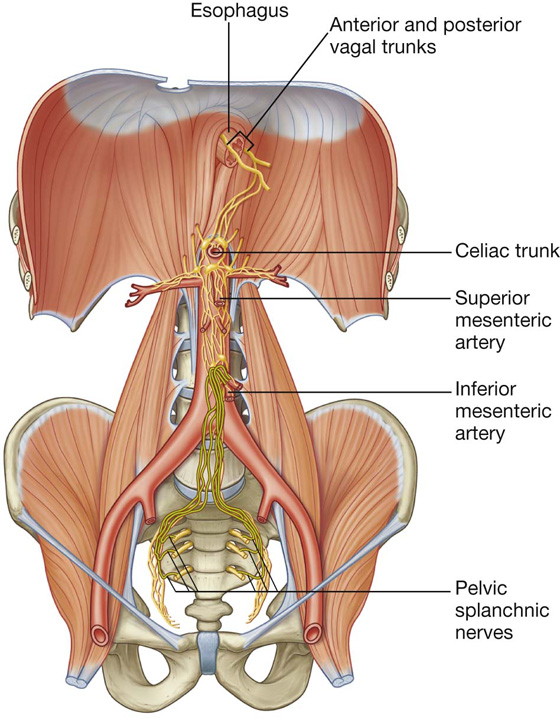
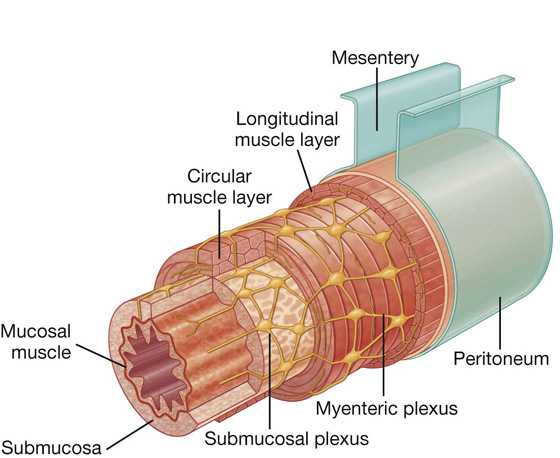
 A preganglionic sympathetic fiber originating at the T6 level of the spinal cord enters an anterior root to leave the spinal cord.
A preganglionic sympathetic fiber originating at the T6 level of the spinal cord enters an anterior root to leave the spinal cord. At the level of the intervertebral foramen, the anterior root (which contains the preganglionic fiber) and a posterior root join to form a spinal nerve.
At the level of the intervertebral foramen, the anterior root (which contains the preganglionic fiber) and a posterior root join to form a spinal nerve. Outside the vertebral column, the preganglionic fiber leaves the anterior ramus of the spinal nerve through the white ramus communicans.
Outside the vertebral column, the preganglionic fiber leaves the anterior ramus of the spinal nerve through the white ramus communicans. Entering the sympathetic trunk, the preganglionic fiber does not synapse, but passes through the trunk, and enters the greater splanchnic nerve.
Entering the sympathetic trunk, the preganglionic fiber does not synapse, but passes through the trunk, and enters the greater splanchnic nerve. The greater splanchnic nerve passes through the crura of the diaphragm and enters the celiac ganglion.
The greater splanchnic nerve passes through the crura of the diaphragm and enters the celiac ganglion. The postganglionic fiber joins the plexus of nerve fibers surrounding the celiac trunk and continues along its branches.
The postganglionic fiber joins the plexus of nerve fibers surrounding the celiac trunk and continues along its branches. The postganglionic fiber travels through the plexus of nerves accompanying the branches of the celiac trunk supplying the stomach and eventually reaches its point of distribution.
The postganglionic fiber travels through the plexus of nerves accompanying the branches of the celiac trunk supplying the stomach and eventually reaches its point of distribution. This input from the sympathetic system may modify the activities of the gastrointestinal tract controlled by the enteric nervous system.
This input from the sympathetic system may modify the activities of the gastrointestinal tract controlled by the enteric nervous system.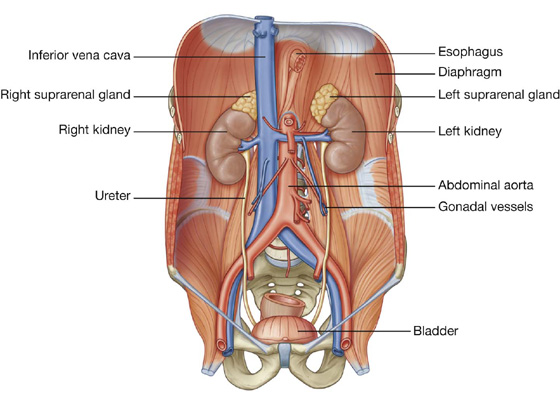
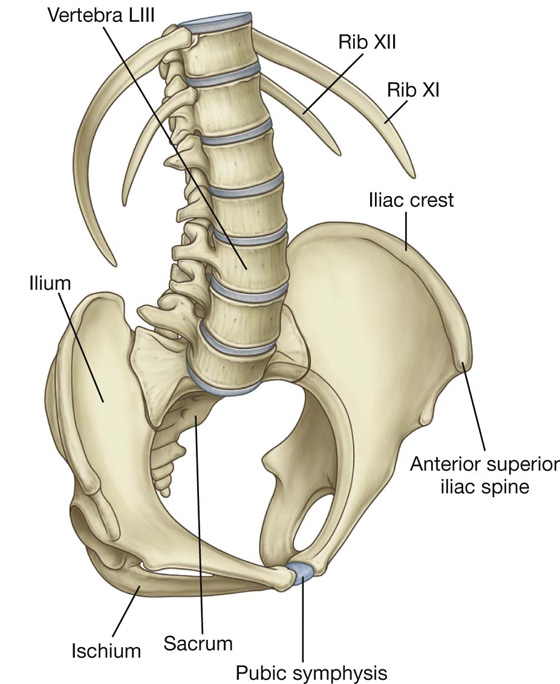
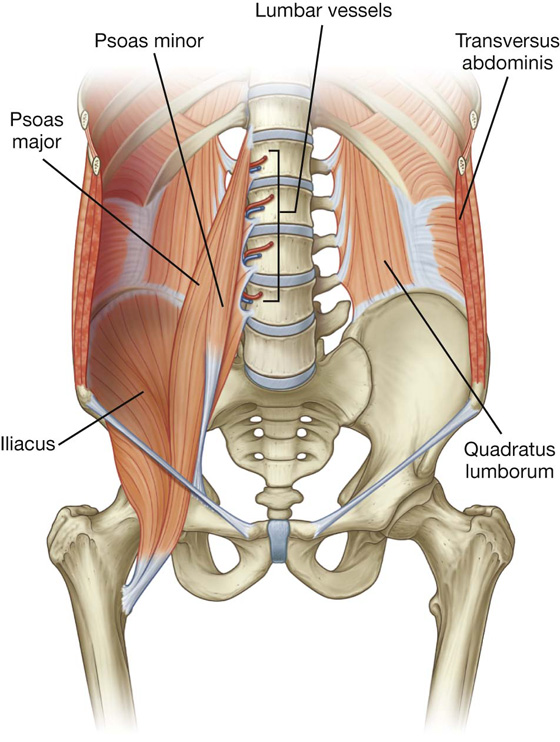
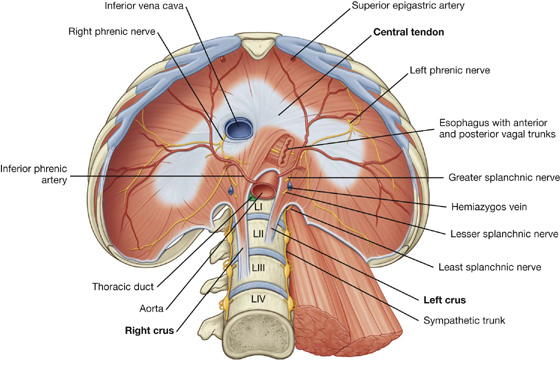
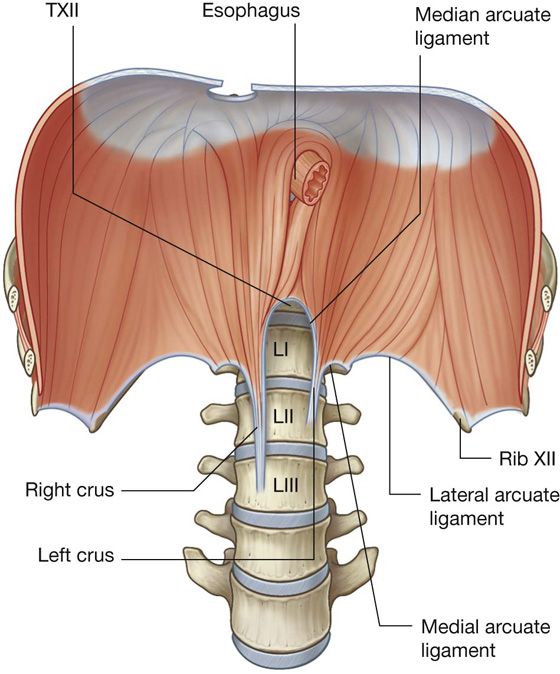
 The aorta passes posterior to the diaphragm and anterior to the vertebral bodies at the lower level of vertebra TXII; it is between the two crura of the diaphragm and posterior to the median arcuate ligament, just to the left of midline.
The aorta passes posterior to the diaphragm and anterior to the vertebral bodies at the lower level of vertebra TXII; it is between the two crura of the diaphragm and posterior to the median arcuate ligament, just to the left of midline. Accompanying the aorta through the aortic hiatus is the thoracic duct and, sometimes, the azygos vein.
Accompanying the aorta through the aortic hiatus is the thoracic duct and, sometimes, the azygos vein. The esophagus passes through the musculature of the right crus of the diaphragm at the level of vertebra TX, just to the left of the aortic hiatus.
The esophagus passes through the musculature of the right crus of the diaphragm at the level of vertebra TX, just to the left of the aortic hiatus. Passing through the esophageal hiatus with the esophagus are the anterior and posterior vagal trunks, the esophageal branches of the left gastric artery and vein, and a few lymphatic vessels.
Passing through the esophageal hiatus with the esophagus are the anterior and posterior vagal trunks, the esophageal branches of the left gastric artery and vein, and a few lymphatic vessels. The left phrenic nerve passes through the muscular part of the diaphragm just anterior to the central tendon on the left side.
The left phrenic nerve passes through the muscular part of the diaphragm just anterior to the central tendon on the left side. Passing posterior to the medial arcuate ligament, on either side, are the sympathetic trunks and the least splanchnic nerves.
Passing posterior to the medial arcuate ligament, on either side, are the sympathetic trunks and the least splanchnic nerves. Other vessels and nerves (i.e., the musculophrenic vessels and intercostal nerves) also pass through the diaphragm at various points.
Other vessels and nerves (i.e., the musculophrenic vessels and intercostal nerves) also pass through the diaphragm at various points.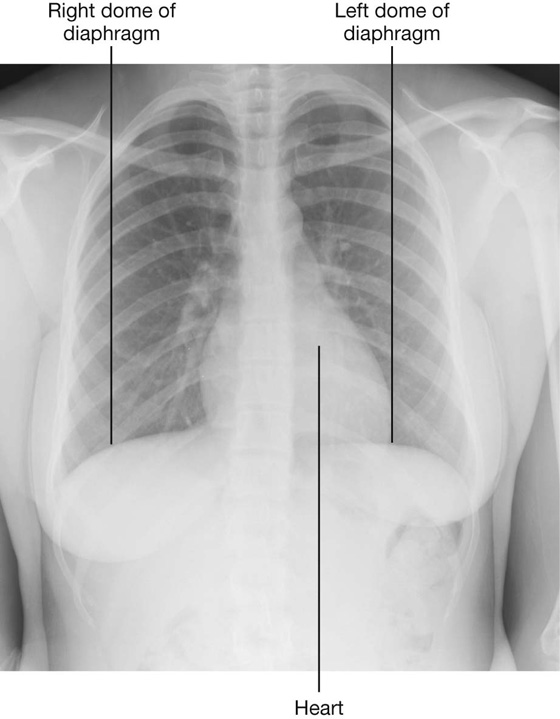
 the liver on the right, with some contribution from the right kidney and the right suprarenal gland, and
the liver on the right, with some contribution from the right kidney and the right suprarenal gland, and the fundus of the stomach and spleen on the left, with contributions from the left kidney and the left suprarenal gland.
the fundus of the stomach and spleen on the left, with contributions from the left kidney and the left suprarenal gland. superiorly, the musculophrenic and pericardiacophrenic arteries, both branches of the internal thoracic artery, and the superior phrenic artery, a branch of the thoracic aorta, supply the diaphragm;
superiorly, the musculophrenic and pericardiacophrenic arteries, both branches of the internal thoracic artery, and the superior phrenic artery, a branch of the thoracic aorta, supply the diaphragm; through an opening on the left when the pleuroperitoneal membrane fails to close the pericardioperitoneal canal (Bochdalek’s hernia).
through an opening on the left when the pleuroperitoneal membrane fails to close the pericardioperitoneal canal (Bochdalek’s hernia).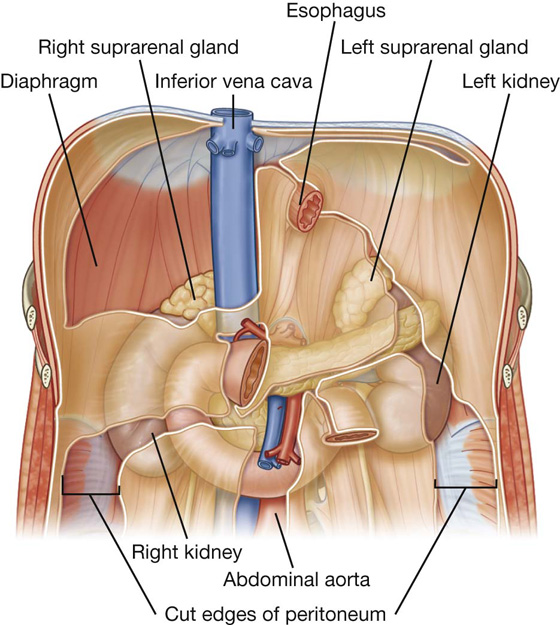
 Moving inferiorly, a large part of the rest of the upper part of the anterior surface is against the liver and is separated from it by a layer of peritoneum.
Moving inferiorly, a large part of the rest of the upper part of the anterior surface is against the liver and is separated from it by a layer of peritoneum. The inferior pole of the kidney, on its lateral side, is directly associated with the right colic flexure and, on its medial side, is covered by a segment of the intraperitoneal small intestine.
The inferior pole of the kidney, on its lateral side, is directly associated with the right colic flexure and, on its medial side, is covered by a segment of the intraperitoneal small intestine.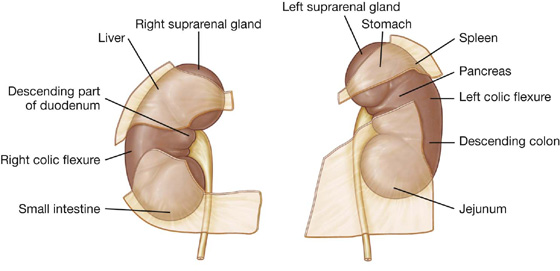
 On its lateral side, the lower half of the kidney is covered by the left colic flexure and the beginning of the descending colon, and, on its medial side, by the parts of the intraperitoneal jejunum.
On its lateral side, the lower half of the kidney is covered by the left colic flexure and the beginning of the descending colon, and, on its medial side, by the parts of the intraperitoneal jejunum.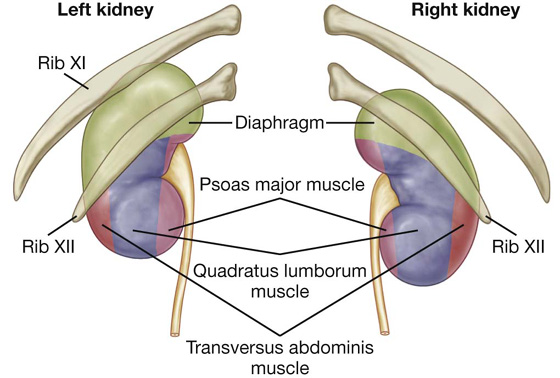
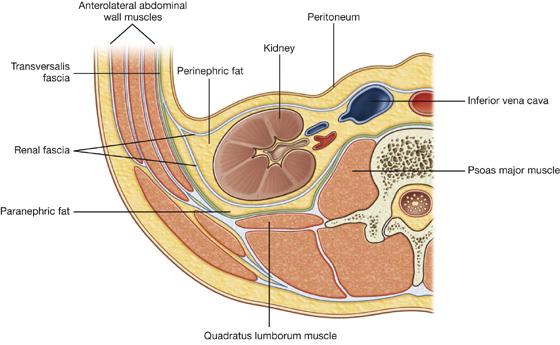
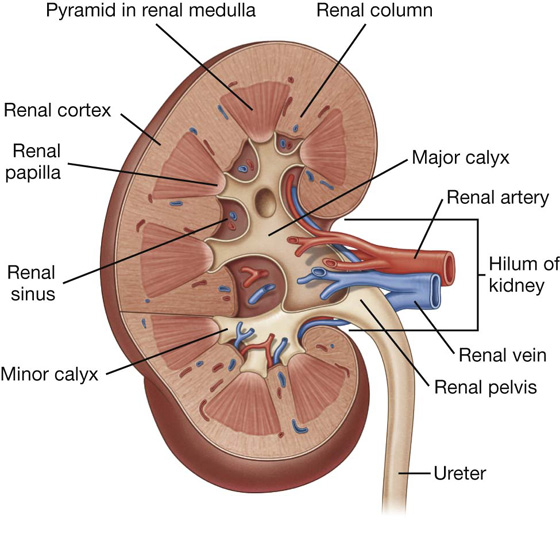
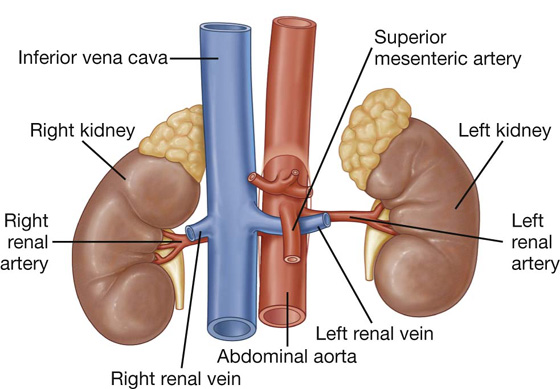
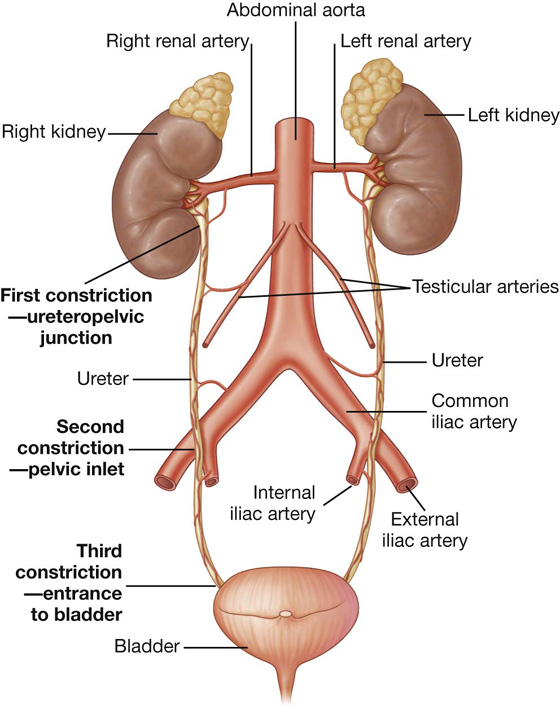
 The middle part may receive branches from the abdominal aorta, the testicular or ovarian arteries, and the common iliac arteries.
The middle part may receive branches from the abdominal aorta, the testicular or ovarian arteries, and the common iliac arteries. In the pelvic cavity, the ureters are supplied by one or more arteries from branches of the internal iliac arteries.
In the pelvic cavity, the ureters are supplied by one or more arteries from branches of the internal iliac arteries. The inferior part of each ureter drains to lymph nodes associated with the external and internal iliac vessels.
The inferior part of each ureter drains to lymph nodes associated with the external and internal iliac vessels.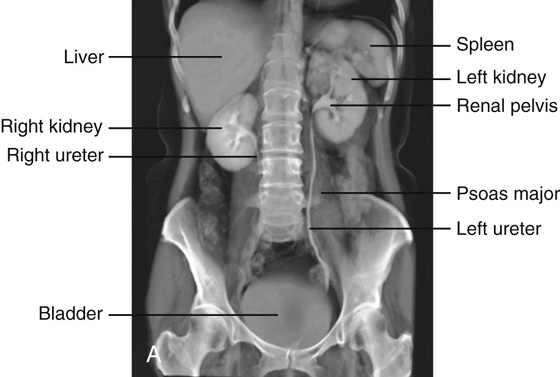
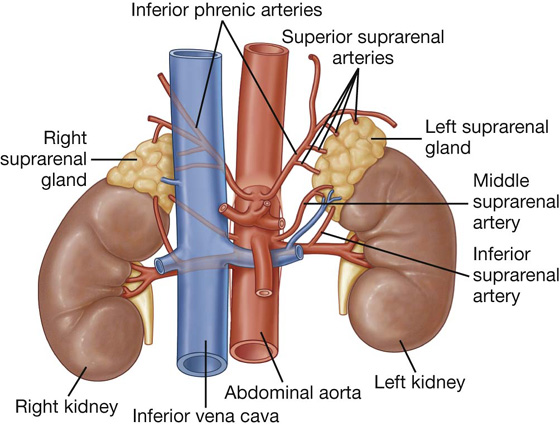
 A middle branch (middle suprarenal artery) to the suprarenal glands usually arises directly from the abdominal aorta.
A middle branch (middle suprarenal artery) to the suprarenal glands usually arises directly from the abdominal aorta. Inferior branches (inferior suprarenal arteries) from the renal arteries pass upward to the suprarenal glands.
Inferior branches (inferior suprarenal arteries) from the renal arteries pass upward to the suprarenal glands.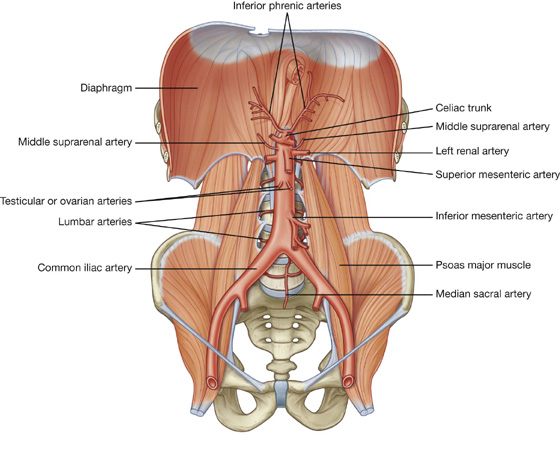
 Anterior to the abdominal aorta, as it descends, are the pancreas and splenic vein, the left renal vein, and the inferior part of the duodenum.
Anterior to the abdominal aorta, as it descends, are the pancreas and splenic vein, the left renal vein, and the inferior part of the duodenum. On its right side are the cisterna chyli, thoracic duct, azygos vein, right crus of the diaphragm, and the inferior vena cava.
On its right side are the cisterna chyli, thoracic duct, azygos vein, right crus of the diaphragm, and the inferior vena cava. terminal branches.
terminal branches. the middle suprarenal arteries—small, lateral branches of the abdominal aorta arising just above the renal arteries that are part of the multiple vascular supply to the suprarenal gland,
the middle suprarenal arteries—small, lateral branches of the abdominal aorta arising just above the renal arteries that are part of the multiple vascular supply to the suprarenal gland, the renal arteries—lateral branches of the abdominal aorta that arise just inferior to the origin of the superior mesenteric artery between vertebrae LI and LII, and supply the kidneys, and
the renal arteries—lateral branches of the abdominal aorta that arise just inferior to the origin of the superior mesenteric artery between vertebrae LI and LII, and supply the kidneys, and the testicular or ovarian arteries—anterior branches of the abdominal aorta that arise below the origin of the renal arteries, and pass downward and laterally on the anterior surface of the psoas major muscle.
the testicular or ovarian arteries—anterior branches of the abdominal aorta that arise below the origin of the renal arteries, and pass downward and laterally on the anterior surface of the psoas major muscle.
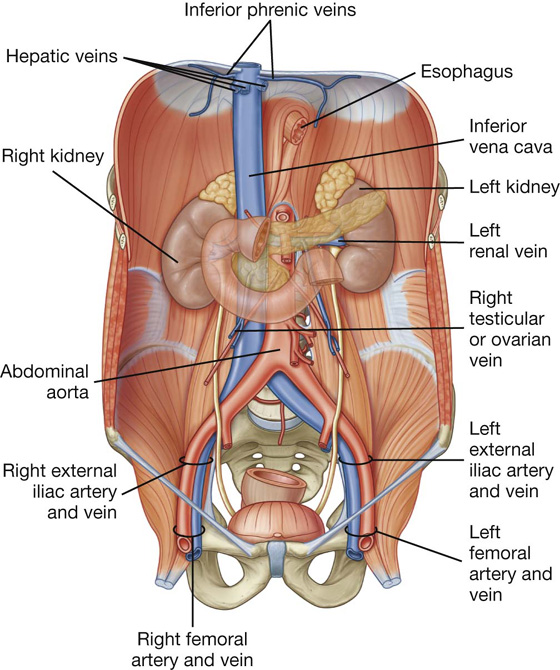
 common iliac veins,
common iliac veins, lumbar veins,
lumbar veins, renal veins,
renal veins, right suprarenal vein,
right suprarenal vein, inferior phrenic veins, and
inferior phrenic veins, and hepatic veins.
hepatic veins. The fifth lumbar vein generally drains into the iliolumbar vein, a tributary of the common iliac vein.
The fifth lumbar vein generally drains into the iliolumbar vein, a tributary of the common iliac vein.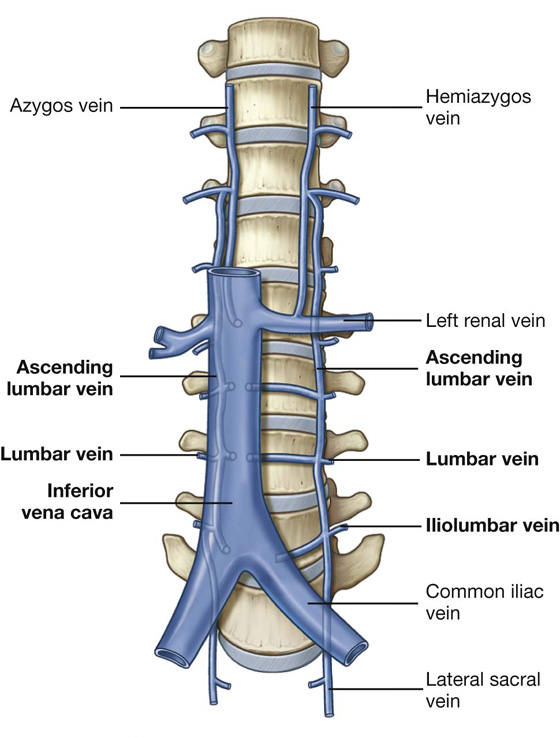
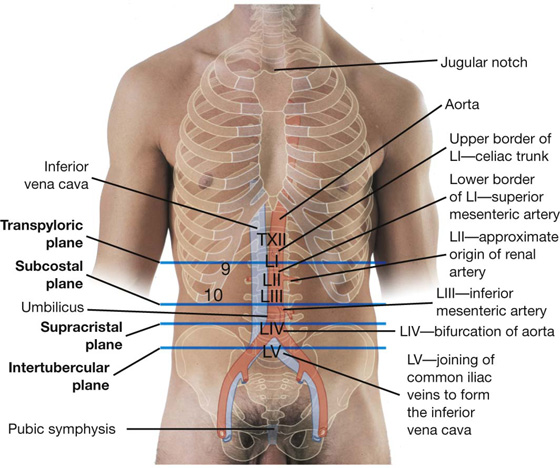
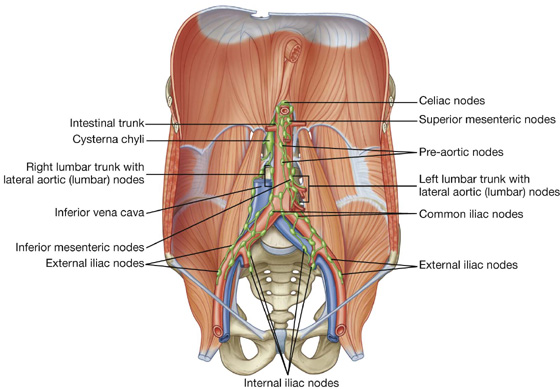
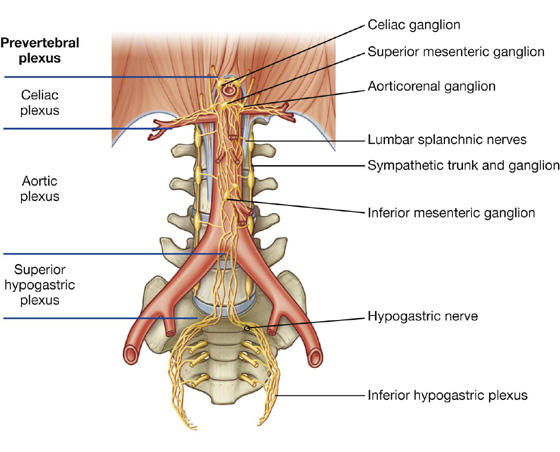
 Beginning at the diaphragm and moving inferiorly, the initial accumulation of nerve fibers is referred to as the celiac plexus—this subdivision includes nerve fibers associated with the roots of the celiac trunk and superior mesenteric artery.
Beginning at the diaphragm and moving inferiorly, the initial accumulation of nerve fibers is referred to as the celiac plexus—this subdivision includes nerve fibers associated with the roots of the celiac trunk and superior mesenteric artery.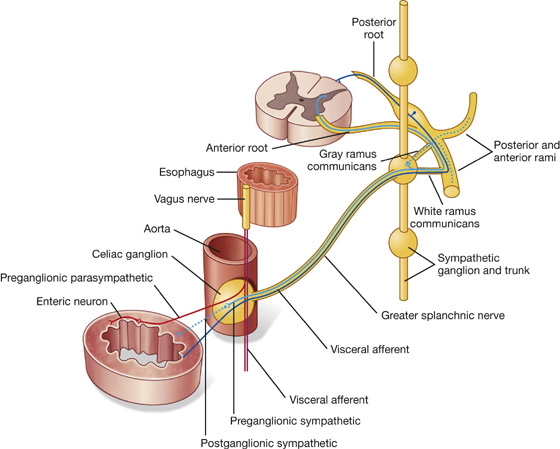
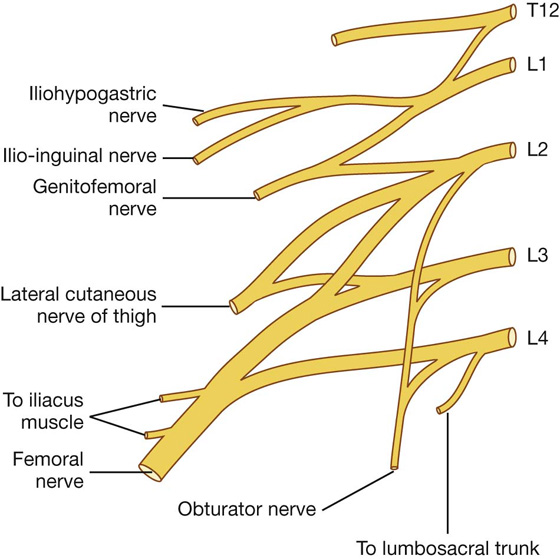
 anterior—genitofemoral nerve,
anterior—genitofemoral nerve, medial—obturator nerve, or
medial—obturator nerve, or lateral—iliohypogastric, ilio-inguinal, and femoral nerves, and the lateral cutaneous nerve of the thigh.
lateral—iliohypogastric, ilio-inguinal, and femoral nerves, and the lateral cutaneous nerve of the thigh.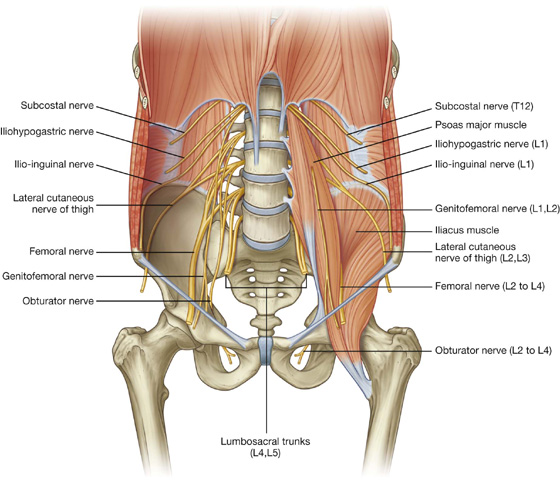
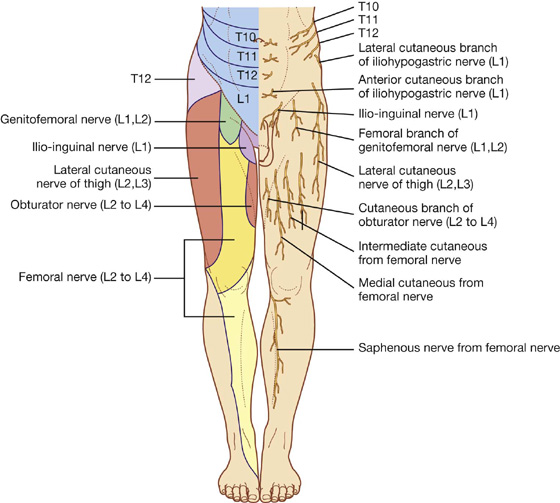
 In men, innervates the cremasteric muscle and terminates on the skin in the upper anterior part of the scrotum; and
In men, innervates the cremasteric muscle and terminates on the skin in the upper anterior part of the scrotum; and In women, accompanies the round ligament of the uterus and terminates on the skin of the mons pubis and labium majus.
In women, accompanies the round ligament of the uterus and terminates on the skin of the mons pubis and labium majus. muscular branches to obturator externus, pectineus, adductor longus, gracilis, adductor brevis, and adductor magnus muscles,
muscular branches to obturator externus, pectineus, adductor longus, gracilis, adductor brevis, and adductor magnus muscles, medial and intermediate cutaneous nerves supplying the skin on the anterior surface of the thigh, and
medial and intermediate cutaneous nerves supplying the skin on the anterior surface of the thigh, and