CHAPTER 44
Forensic biochemistry
Robert J. Flanagan; Sarah Belsey; Terhi Launiainen
CHAPTER OUTLINE
INTRODUCTION
Forensic biochemistry can be defined as the application of biochemical assays in the service of the courts. Examples include DNA analysis for human identification and methods for the detection of trace evidence, such as the Kastle–Mayer (phenolphthalein/hydrogen peroxide) and luminol reactions used to detect the presence of blood. This chapter, however, focuses on laboratory measurements rather than methods. Many of these are standard laboratory procedures, while others are specific to forensic work.
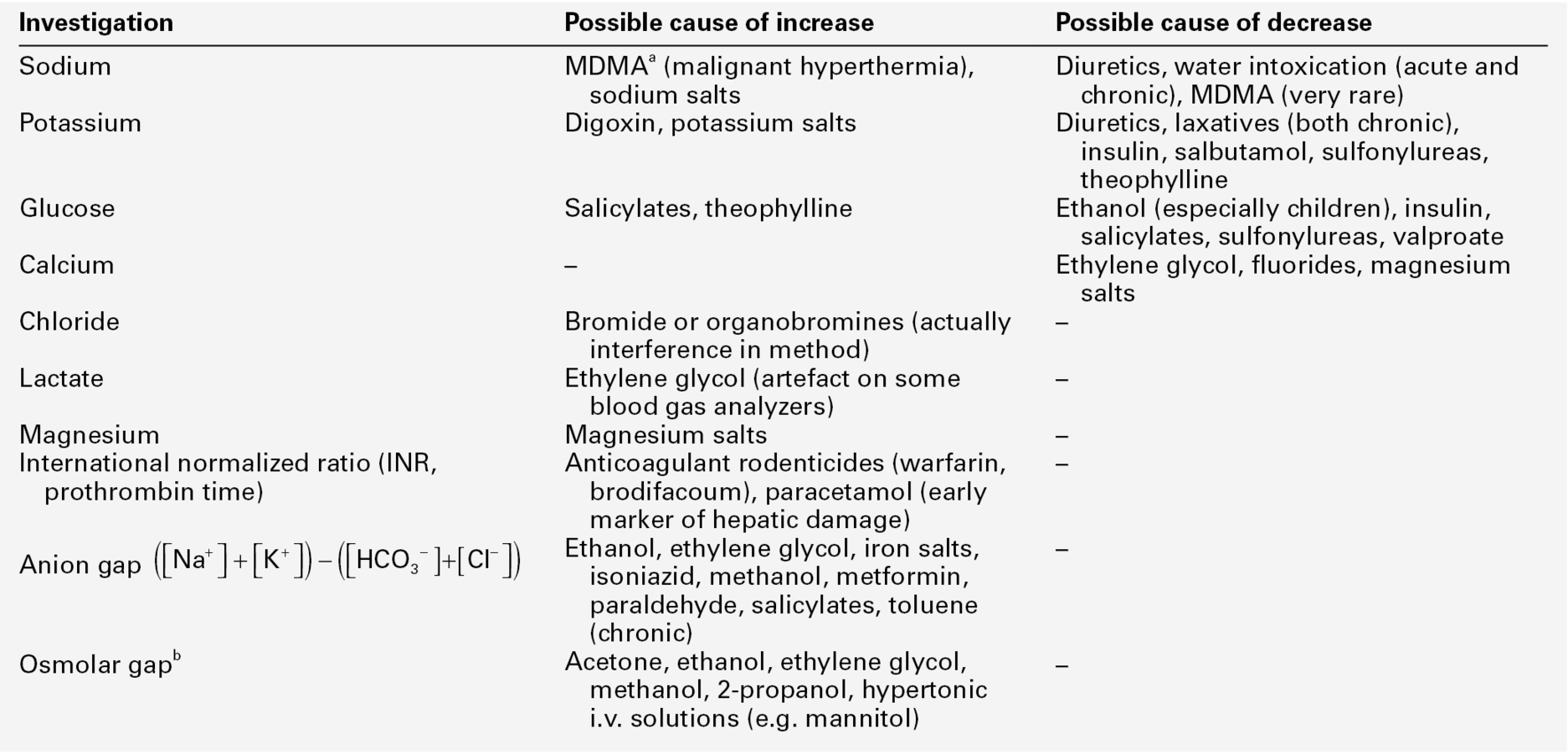
The range of forensic situations that the clinical biochemistry laboratory may be asked to help with is wide and, as in all such work, the results of tests can usually be properly interpreted only when considered together with all the available evidence. Post-mortem biochemistry has a role in investigating the cause of death in some apparently natural deaths, including both diabetic and alcoholic ketoacidosis, deaths that may have involved a prolonged stress response such as hypothermia, as well as in the diagnosis of disease processes such as early myocardial infarction, which may be difficult to diagnose by physical examination. There is clearly considerable overlap between clinical and forensic toxicology, in that some endogenous substances can be used as poisons (e.g. sodium chloride, potassium chloride and insulin) and in some instances, suspicion of poisoning may be aroused by abnormal biochemical results (see Table 44.1).
TABLE 44.1
Some laboratory investigations commonly requested on blood samples that may arouse suspicion of poisoning
Forensic biochemistry has an important role in the investigation of deaths and serious injuries occurring in hospital. Assault committed within hospital may involve poisoning, and can range from murder (intent to kill), through manslaughter (culpable homicide) and attempted murder, to malicious poisoning (usually of a child or an elderly relative). Iatrogenic poisoning may range from relatively minor drug administration errors, to catastrophes such as asphyxia caused by an anaesthetic error. The results of an analysis, or residual or unused samples, or even apparatus used in giving the drug, may be required by officers acting on behalf of the courts. Therefore, in all cases, careful specimen labelling and handling, reporting and laboratory record-keeping is important. The standard of completion of request forms and sample labelling is still very poor in some hospitals. This can cause many problems if samples are required by the police or coroner. Knowledge of the limitations of the analytical methods used is also important – an enzymatic ethanol assay is not as selective as headspace gas chromatography, for example.
One practical problem is that samples may be collected, assays performed and results reported before the need for forensic investigation has become apparent. Another problem is that all that may be available are specimens collected after death (see Box 44.1), although in general it is information on an analyte’s concentration prior to, or at the time of death that is required. In this case, the likelihood of agonal or post-mortem change, and indeed, sample contamination during collection, must be taken into account when interpreting results. An associated problem is that there are often no reference ranges for fluids such as vitreous humour, pericardial fluid, or synovial fluid since such samples are, for practical purposes, rarely available during life, except from laboratory animals. Method validation is also compromised by this same lack of reference material. Furthermore, the time needed for analyte equilibration between plasma and, for example, vitreous humour during life remains unknown.
Of course the laboratory, too, can be the subject of forensic investigation if laboratory error of whatever nature comes under scrutiny of the courts (see Box 44.2). Examples here include the use of inappropriate analyses, delayed analyses, sample mix-ups and errors in reporting, including the use of inappropriate units. In all cases, it is best to write out units in full, for example ‘milligrams per litre’, rather than using symbols, when producing reports for the courts. The laboratory should provide clear guidance as to the significance of a result, especially when different units may be used. Patients have died when a paracetamol result reported in mg/L has been assumed to be in mmol/L. Mass units (SI) should be used for drugs except for lithium, thyroxine and methotrexate, where molar units should be employed. For metals/trace elements and for alcohol (ethanol), either molar or mass units may be employed. However, for forensic purposes, including the regulations governing occupational lead exposure, mass units are often the rule and the laboratory should remember this when providing interpretation of results. For clinical purposes, ethanol is often reported as mass units per litre (mg/L), but for forensic purposes, at least in the UK, ethanol is still reported as mg/100 mL (mg%).
SAMPLES AND SAMPLING
Information recorded on the sample container at the time the sample is collected should include the names (first and family or last name) and date of birth, patient or post-mortem number and the date and time of collection. This information, together with details of the collection site in post-mortem work, and the sample type (including a note of any preservative), and any other appropriate information, should be recorded on an accompanying assay request form (see Box 44.3). The date and time of receipt of all specimens by the laboratory should be recorded and a unique identifying number assigned in each case. Any residual specimen should be kept securely at − 20 °C or below until investigation of the incident has been concluded.
In forensic work, it is important to be able to guarantee the identity and integrity of the specimen from when it was collected through to the reporting of the results, although this ideal is often not attained in normal clinical laboratory practice. ‘Chain of custody’ is a term used to refer to the process used to maintain and document the history of the specimen (see Box 44.4). Procedures for appropriate storage of samples that may be required for forensic analysis and for authorizing and documenting the release of such samples on request of a coroner, for example, must also be in place. Ideally, samples should be protected during transport by the use of tamper-evident seals and should be submitted in person to the laboratory by the coroner’s officer or other investigating personnel. If storage is to be at − 5 to − 70 °C, basic precautions to preserve sample integrity, including labelling, must be undertaken (glass tubes will break if over-full when frozen). The requirements of the UK Human Tissue Act, or other relevant legislation on the retention and storage of pathological samples, must be met.
POISONING WITH ENDOGENOUS AGENTS
Many analytes of toxicological interest (and their metabolites) also occur naturally in the body and hence, reference ranges, ‘cut-offs’ or other means of delineating an exogenous source for the compound(s) of interest must be adopted. Examples include acetone, carbon monoxide (measured as % carboxyhaemoglobin) ethanol, γ-hydroxybutyrate (GHB), insulin, iron, potassium, sodium and testosterone.
Testosterone continues to be abused by athletes and this remains the most common adverse finding declared by World Anti-Doping Agency accredited laboratories; the practice is usually detected by the demonstration of an elevated testosterone:epitestosterone ratio and by procedures to ensure that such a finding is not ‘natural’ for a particular athlete. Numerous other endogenous agents have been used in an attempt to enhance performance or to mask use of other agents in sport but further discussion of this topic is beyond the scope of this chapter.
γ-Hydroxybutyrate
γ-Hydroxybutyrate (GHB), and its precursors γ-butyrolactone (GBL) and 1,4-butanediol, are used to improve athletic performance, as intoxicants and sometimes in assault such as drug-facilitated sexual assault (which includes rape). As with alcohol, voluntary ingestion always has to be considered (Box 44.5). γ-Hydroxybutyrate exposure may also arise from unexpected sources (Box 44.6). The substance is practically odourless and tasteless and has a short plasma half-life (20 min or so), making covert administration hard to detect. Quantitation is needed as GHB is an endogenous compound. A urine cut-off of 10 mg/L is recommended in attempting to differentiate endogenous GHB excretion from deliberate GHB/GBL administration. Unusually, the urine cut-off seems more reliable than a measure of plasma GHB, but by ~ 12 h after GHB ingestion, the concentration in urine is usually < 10 mg/L. For post-mortem blood, the cut-off is generally taken as 50 mg/L because of the likelihood of post-mortem GHB production.
Insulin
Endogenous insulin is co-secreted with C-peptide. In theory, therefore, in suspected administration of exogenous insulin, which lacks C-peptide, the ratio of the two analytes could be useful as an indicator of the source of the insulin. However, commercially available immunoassays have highly variable responses to different types of insulin making the insulin:C-peptide ratio extremely difficult to interpret. An insulin-degrading enzyme (IDE) is widely distributed in tissues including erythrocytes, hence use of haemolysed samples, including post-mortem samples, is likely to give false results. Use of different anticoagulants to preserve samples is also associated with differences in plasma insulin results. The presence of hepatic or renal insufficiency, or of endogenous anti-insulin or anti-proinsulin antibodies (analogous to the analytical problems with digoxin assay if anti-digoxin Fab antibody fragments have been given) may be further possible sources of error (see also Chapter 17).
Plasma C-peptide is stable for only ~ 2–3 weeks at − 20 °C and for up to 6 months at − 80 °C, whereas plasma insulin is more stable (~ 5 h at room temperature, ~ 1 week at 4 °C and several months at − 20 °C). On the other hand, C-peptide is not degraded by IDE. Anti-insulin antibodies bind proinsulin via its insulin moiety and greatly retard its clearance from the circulation. Because of cross-reaction with proinsulin in some C-peptide immunoassays, proinsulin bound to anti-(pro)insulin antibodies can interfere.
Murder or suicide by insulin is difficult to diagnose and to prove (see Box 44.7). Immunohistochemical demonstration or measurement of elevated insulin concentration in tissue around an injection site compared with a control site can help support the diagnosis. Unlike most purely immunological methods, liquid chromatography-tandem mass spectrometry (LC-MS/MS) may allow differentiation of human insulin from its synthetic derivatives. Besides its use in doping control, the method has been applied to post-mortem material related to an insulin poisoning case.
Magnesium
Poisoning with magnesium is rare, and usually follows intravenous administration. Confusion over units is not uncommon (see Box 44.8). Clinical features of magnesium overdose may include drowsiness, unconsciousness, loss of muscle tone and respiratory and cardiac arrest. Plasma magnesium in healthy adults ranges from 0.7 to 1.3 mmol/L. Features of toxicity are reported at plasma magnesium concentrations of 3.5–5.0 mmol/L, and cardiorespiratory arrest may occur at concentrations of > 8.5 mmol/L. During life, magnesium is distributed within cells (approximate cell:plasma distribution ratio 4:1), hence whole blood concentrations are somewhat higher than plasma concentrations. Doses of magnesium sulphate of up to 4 g (16 mmol magnesium ion) are sometimes given for control of convulsions.
Sodium
Hypernatraemia from sodium chloride (common salt) poisoning must be distinguished from hypernatraemia owing to dehydration from fluid loss or, very rarely, low fluid intake. This is especially important because sodium chloride poisoning may be the result of deliberate administration of large amounts of salt by a third party – salt is hardly ever encountered in self-poisoning episodes nowadays, although there were many deaths, especially in children, when saline emetics were used in an attempt to induce vomiting.
When a patient presents with hypernatraemia, diabetes insipidus and renal disease have to be excluded before salt poisoning can be diagnosed. Hypernatraemia caused by disease processes may be associated with obvious polyuria and polydipsia. Negative water balance can occur easily. This reduces solute excretion and induces hypernatraemic dehydration. Finally, there are rare individuals who develop hypodipsic hypernatraemia (see p. 44). A water deprivation test may be required if this disorder is suspected in life.
Common criteria used to diagnose salt (sodium chloride) poisoning focus on hypernatraemia, with high urinary concentrations of sodium and chloride. However, high urinary concentrations of sodium alone cannot distinguish salt poisoning from dehydration. The medical and legal implications of the two conditions are fundamentally different, so reliable ways to distinguish between them are needed. Be this as it may, both salt poisoning and dehydration (caused by neglect) may lead to criminal or civil action. Fractional excretions (the proportions of sodium and water filtered at the glomerulus that subsequently reach the urine), calculated from the sodium and creatinine concentrations of paired plasma and associated (‘spot’) urine samples, can distinguish the two situations (see Box 44.9). The values should be > 2% in an individual who has been poisoned with salt and is volume replete and < 1% when dehydrated but with viable renal tubules. Note that the units of measurement used must be the same for plasma and urine (plasma creatinine is usually reported in μmol/L, while urine creatinine is often reported in mmol/L).
POST-MORTEM BIOCHEMISTRY
Gradients that are maintained by active processes during life, such as that between intra- and extracellular potassium begin to break down soon after the occurrence of hypoxic or anoxic damage, hence the possibility of both terminal and post-mortem change has to be evaluated when interpreting results. Most deaths that become the subject of post-mortem investigation occur outside hospital and it may be some days before the body is found and samples collected for analysis. Therefore, blood samples are invariably haemolysed to a greater or lesser degree and the likelihood of other changes, such as loss of labile analytes (e.g. glucose and insulin), is high (see Box 44.10). It should be remembered that most clinical reference values are established for plasma or serum, and not haemolysed whole blood.
Vitreous humour
Vitreous humour is preferred to blood for most post-mortem biochemistry (Table 44.2), since it is thought to be far less susceptible to autolytic change, is less likely to be subject to post-mortem contamination by diffusion of drugs or other poisons that may be present at high concentration in the thorax or abdomen at death, and lies within the relatively protected environment of the eye socket. After death, however, potassium quickly leaks from the retina and hence vitreous potassium is not a reliable indicator of ante-mortem plasma potassium and is of minimal value in the diagnosis of exogenous potassium administration. The possibility of concurrent vitreous disease confounding the results must also be remembered.
When collecting vitreous humour, ideally both eyes should be sampled independently and the results reported separately. Potassium concentrations may differ by up to 2.3 mmol/L between the two eyes (in samples from non-putrefied bodies). Specimen contamination with retinal cells is a recognized source of falsely raised vitreous potassium concentrations. Hence, aspiration must be gentle to avoid contamination with retinal fragments as far as possible. Differences may also be due to inappropriate sample handling. Vitreous humour is viscous, hence may require treatment such as centrifugation, heating, dilution or addition of hyaluronidase, to facilitate accurate pipetting. A vitreous potassium concentration > 15 mmol/L suggests gross post-mortem decomposition.
Vitreous sodium and chloride concentrations may fall after death, at rates of up to 1 mmol/L per hour, whereas potassium increases at a rate of 0.14–0.19 mmol/L per hour. If the potassium concentration is < 15 mmol/L, then the sodium and chloride concentrations are thought likely to reflect the situation at death. Urea and creatinine are relatively stable in post-mortem specimens. If vitreous sodium, chloride and urea are > 155, > 115 and > 10 mmol/L, respectively, this may indicate ante-mortem dehydration. If the urea concentration is > 20 mmol/L and creatinine > 200 μmol/L with sodium and chloride concentrations within the normally accepted range, this indicates that uraemia was present before death.
A number of biochemical tests have been advocated for particular purposes, but remain little used (see Table 44.3). Attempts to employ the rate of rise of vitreous humour potassium concentration, in order to calculate the time of death, have largely been abandoned because of the inherent uncertainty of this method, even when vitreous humour hypoxanthine is also analysed, with the aim of increasing the accuracy of the procedure. Use of synovial fluid, as an alternative to vitreous humour, for potassium measurement for time-of-death estimation, has also been advocated. It has been claimed that regression analysis of vitreous humour hypoxanthine, potassium and urea concentrations can give a reliable time-of-death estimate, taking into account the cause of death. There are reports of immunochemical detection of glucagon in pancreatic cells and of calcitonin in thyroid C-cells being used to indicate time of death, but these methods are experimental at best. Serial monitoring of C-reactive protein (CRP), procalcitonin, interleukin-6, interleukin-1β, soluble interleukin-2 receptor or lipopolysaccharide binding protein in the hours after death, has been suggested as an aid to the diagnosis of sepsis, but has not been widely adopted.
TABLE 44.3
Post-mortem biochemistry: some little used/unvalidated tests
| Analyte | Matrix | Suggested role in diagnosis of: |
| Adrenaline:noradrenaline ratio | Urine | Hypothermia |
| Chymase | Blood | Anaphylactic shock |
| Chromogranin A | Serum, cerebrospinal fluid | Hypothermia |
| C-reactive protein (CRP) | EDTA blood, liver | Recent infection, trauma, burns, ketoacidosis, necrosis. Diagnosis of sepsis if measured soon after death |
| Fructosamine | Vitreous humour | Diabetic ketoacidosis |
| Hypoxanthine | Vitreous humour | Time of death |
| Lactate | Vitreous humour | If very high, may indicate lactic acidaemia (but may be formed perimortem) |
| Thyroglobulin, free tri-iodothyronine (fT3) | Blood | Neck trauma (e.g. strangulation) |
| Troponin | Pericardial fluid | Myocardial damage present prior to microscopic changes |
The measurement of markers of heart muscle damage (e.g. myoglobin, creatine kinase, troponin) as an adjunct to searching for evidence of microscopic changes in samples of myocardium post-mortem, has been investigated with the aim of improving the diagnosis of ante-mortem ischaemic damage. However, the only post-mortem fluid with which there has been some success in this approach, has been pericardial fluid; concurrent post-mortem changes limiting the value of fluids such as blood. Unfortunately, as microscopy has always been viewed as the definitive method for the diagnosis of ante-mortem heart damage, the use of biochemical markers in cases where death has occurred prior to microscopic changes becoming apparent has not been fully investigated. Hence, the interpretation of results remains problematic.
SPECIFIC DIAGNOSTIC PROBLEMS
Anaphylaxis/anaphylactoid reactions
Many drugs may precipitate anaphylaxis or anaphylactoid reactions. Mast-cell tryptase is an indicator of anaphylactic shock. Measurement of blood chymase has also been suggested in this context. Although a raised serum or plasma tryptase activity can supply important supporting evidence in the diagnosis of anaphylaxis, it cannot be used as the sole criterion for the post-mortem diagnosis of anaphylaxis as a cause of death, since there is overlap with results from people who die from causes other than anaphylaxis.
Diabetes
Blood and vitreous humour glucose concentrations generally fall rapidly after death and are thus an unreliable guide to ante-mortem glucose concentration. However, since glucose undergoes anaerobic glycolysis to lactate, some investigators have suggested that measuring the sum of vitreous humour glucose and lactate may give a better estimation of the glucose concentration at the time of death. A potentially complicating factor is that lactate may increase perimortem. Moreover, some drugs that are themselves unstable in biological systems, for example ethanol and insulin, may cause fatal hypoglycaemia, hence compounding the difficulties that may be encountered in establishing a cause of death.
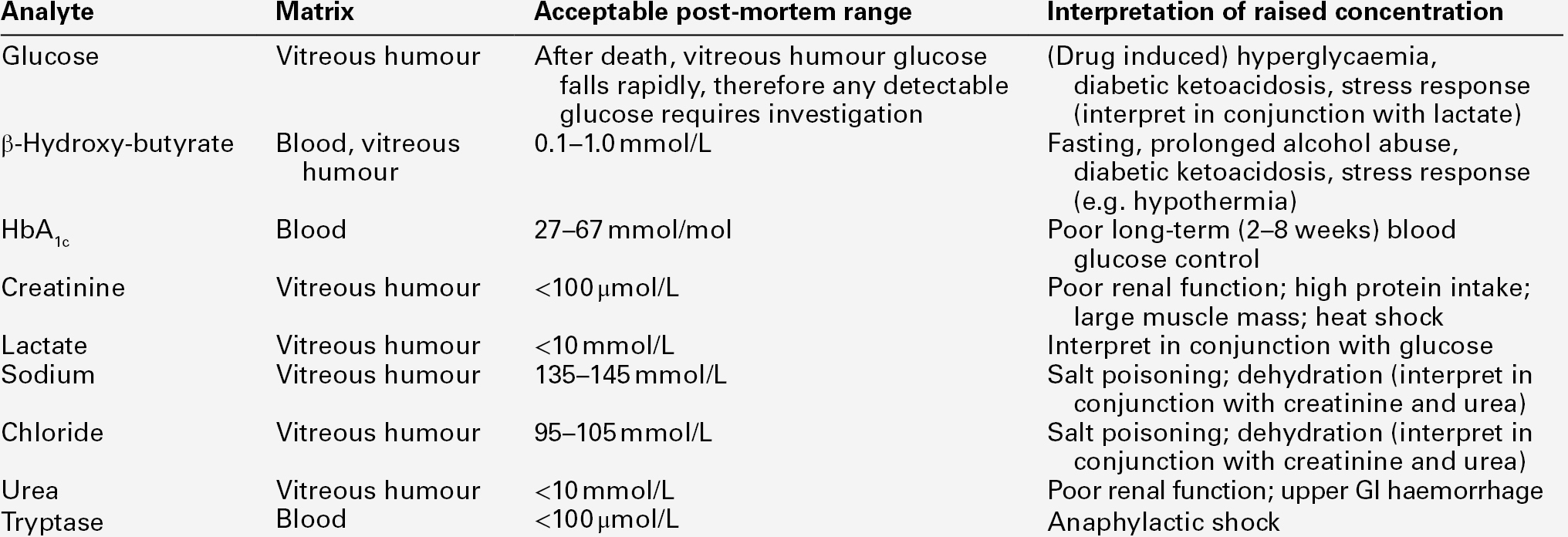
An elevated post-mortem blood HbA1c concentration might indicate poor glucose control during life. This suggestion may be supported by an elevated vitreous humour glucose concentration. In patients with type 1 diabetes, the presence of ketones (principally acetone and its metabolite 2-propanol, but also acetoacetate) may indicate the presence of diabetic ketosis prior to death, especially if considered together with the blood β-hydroxybutyrate (BHB) concentration. Schemes to aid interpretation of vitreous glucose concentrations, similar to that in Figure 44.1, are often cited, but even these may not give clear guidance as to the likely situation perimortem in some cases.
FIGURE 44.1 Aid to the interpretation of post-mortem vitreous humour glucose and blood β-hydroxybutyrate concentrations. *Requires confirmation from vitreous humour sodium and urea/creatinine.
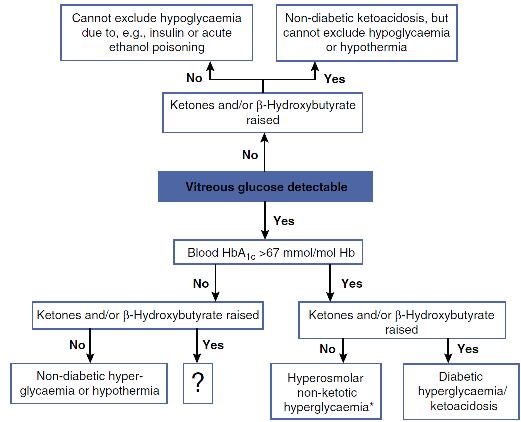
Measurement of ketones in blood or vitreous humour is recommended in unexplained deaths in chronic alcoholic as well as diabetic patients. Prolonged poor nutrition and use of alcohol (common in chronic alcoholics) promote the accumulation of ketones (acetone, butanone) and BHB, and often, elevated ketone concentrations (acetone > 20 mg/L) are the only notable post-mortem finding. A practical scheme for investigating such deaths is given in Figure 44.2.
FIGURE 44.2 Suggested scheme for investigation of unexpected deaths in patients in whom diabetes or alcoholism is a possibility.
Drowning
Haemodilution is likely in victims of fresh-water drowning, but not in those drowned in salt water. Thus, concentrations of ethanol, drugs or other analytes measured in the blood of victims of fresh-water drowning may be misleadingly low. Conversely, haemoconcentration is likely if a cadaver has been dehydrated, for example by heat or by mummification. In all such cases, measurement of blood haemoglobin may give an estimate of the magnitude of haemodilution/concentration, if it has not been degraded by heat or by prolonged storage.
It has been suggested that haemodilution in fresh-water drowning produces a lower chloride concentration in left heart blood as compared with right heart blood, while in salt water drowning, haemoconcentration and chloride ion absorption is said to produce the opposite result. High concentrations of magnesium in left heart blood compared with right heart blood may reflect magnesium absorption from salt water. Trace element analysis has been proposed as an aid to discriminate fresh-water drowning from salt-water drowning, but seems to offer poor discriminatory power and other (non-biochemical) investigations, notably looking for species of diatoms, are more helpful in such cases.
Hypothermia/hyperthermia
Cold may be a significant factor in a death, but there are no specific biochemical markers that can be used to confirm a diagnosis of fatal hypothermia. Indications of ante-mortem cold stress include high urinary adrenaline and noradrenaline concentrations and Wischnewsky spots (small gastric mucosal bleeds). An increased adrenaline:noradrenaline ratio may be a better indicator of ante-mortem hypothermia than the separate catecholamine results. Ketone and glucose concentrations may also be elevated, and in persistent hypothermia, there may be electrolyte disturbances and metabolic acidosis. A role for measurement of chromogranin A has been postulated.
Fatal hyperthermia (heat stroke) often involves multiple organ dysfunction, including skeletal muscle damage without marked inflammatory responses. It has been suggested that isolated elevation of serum creatinine may help in diagnosis.
Inflammation
Blood CRP concentration peaks within 6 h of a stimulus and CRP is stable in post-mortem samples. Liver is said to be a good alternative specimen if blood is not available. Interpretation of results can be difficult, however, as there are many causes of a raised blood CRP in addition to inflammation (see Table 44.3).
Sudden death
Sudden unexpected death in infancy (SUDI, also known as sudden infant death syndrome (SIDS), Table 44.4), may be an acute manifestation of an inborn error of metabolism, particularly a fat oxidation defect, and therefore these should be excluded. Sudden arrhythmic death syndrome (also known as sudden adult death syndrome (SADS)) is thought likely often to have a cardiac origin such as a fatal arrhythmia, but a full toxicological analysis is required to exclude recent use of not only illicit drugs (such as cocaine and other stimulants), but also therapeutic drugs (e.g. tricyclic antidepressants and antipsychotics) that may increase the risk of a fatal cardiac arrhythmia.
TABLE 44.4
Some biological samples required when investigating sudden unexpected death in infancy (SUDI)
| Sample (volume) | Handling | Test |
| Blood (serum) (1–2 mL) |
Centrifuge, store serum at − 20 °C | Toxicology |
| Urine (20 mL if possible) | Store at − 20 °C | Toxicology and specialized tests for inherited metabolic diseases |
| Blood from Guthrie card | Ensure circle is filled. Do not put in plastic bag | Tests for inherited metabolic diseases |
| Skin biopsy | After discussion with paediatrician and laboratory | Tests for inherited metabolic disease, e.g. fibroblast enzyme activity |
| Muscle biopsy | After discussion with paediatrician and laboratory | If history suggests mitochondrial disorder |
Further details: Royal College of Pathologists and Royal College of Paediatrics and Child Health (2004).
Further reading
Up-to-date reviews on post-mortem biochemistry
Practical guidance on post-mortem samples and sampling
Royal College of Pathologists and Royal College of Paediatrics and Child Health. Sudden unexpected death in infancy. A multiagency protocol for care and investigation. London: RCP and RCPCH; 2004. http://www.rcpath.org/NR/rdonlyres/30213EB6-451B-4830-A7FD-4EEFF0420260/0/SUDIreportforweb.pdf [Accessed 30.09.13].
Clinical and post-mortem diagnosis of disorders of glucose metabolism
McGuire LC, Cruickshank AM, Munro PT. Alcoholic ketoacidosis. Emerg Med J. 2006;23:417–420.
Reviews on post-mortem diagnosis of anaphylaxis, hyperthermia/hypothermia and sepsis
Tsokos M. Post-mortem diagnosis of sepsis. Forensic Sci Int. 2007;165:155–164.