CHAPTER 35
Biochemical aspects of psychiatric disorders
William J. Marshall; Teifion Davies
CHAPTER OUTLINE
INTRODUCTION: PSYCHIATRY AS A CLINICAL DISCIPLINE
THE CLASSIFICATION OF PSYCHIATRIC DISORDERS
THE AETIOLOGY OF PSYCHIATRIC DISORDERS
BIOCHEMICAL INVESTIGATIONS IN PSYCHIATRIC DISORDERS
PSYCHIATRIC MANIFESTATIONS OF ORGANIC DISEASE
Acute confusional state (delirium)
Post-traumatic stress disorder
ENDOCRINE AND METABOLIC MANIFESTATIONS OF PSYCHIATRIC DISEASE
Abnormalities of the hypothalamo–pituitary–adrenal axis
Abnormalities of the hypothalamo–pituitary–thyroid axis
Abnormalities of the hypothalamo–pituitary–gonadal axis
Abnormalities of growth hormone secretion
Abnormalities of prolactin secretion
METABOLIC COMPLICATIONS OF PSYCHOTROPIC DRUGS
Drugs causing hyperprolactinaemia
Drugs causing hyperglycaemia and hyperlipidaemia
INTRODUCTION: PSYCHIATRY AS A CLINICAL DISCIPLINE
Psychiatry is the branch of medicine that deals with the disturbance, distress and disability arising from those disorders of the nervous system that affect mental functioning. ‘Mental’ functions are those that we regard as distinguishing human beings as persons, and are usually divided into the major domains of cognition (thinking, remembering, planning); perception (awareness of self and environment); mood (feelings, emotions, ‘affect’) and behaviour (objective manifestations of subjective states). Although it is clear that each of these mental functions must be realized in the human brain (and that they may be affected by overt damage to the brain, as in head injury), they have been regarded traditionally as emergent phenomena that are only tenuously dependent upon the biological structures and processes of the brain itself.
This ‘epiphenomenal’ view of mental functions has been reinforced by two separate sets of findings. First, the psychological and neurobiological study of normal mental functions is only beginning to link them to anatomically discrete structures or pathways. Instead, they have been found to rely on distributed systems of activity in many brain areas that appear to interact in a seemingly limitless pattern of complexity. Second, the delineation of abnormal states has been constrained by a failure to identify specific causes, biological markers or even reliable pathognomonic features, with the result that psychiatry has been slow to follow most other medical disciplines away from the description of clinical syndromes (clusters of clinical symptoms and signs) towards a classification of disorders based on aetiology.
Psychiatric diagnosis remains, therefore, near one extreme of a continuum from ‘organic’ (having often localized pathology, discrete (possibly pathognomonic) signs and objective laboratory findings) to ‘non-organic’ (unclear structural pathology, overlapping signs and no confirmatory tests). Psychiatrists’ skills are largely clinical, and consist in eliciting a wide array of information directly from the patient by means of standardized interviewing and observation of behaviours and responses, supplemented by collateral information from informants such as carers, family members and others. Psychiatric decision-making begins with a comparison of this clinical information with what is known of the normal repertoire of mental states (taking into account age, sex and developmental and cultural factors), and proceeds with a systematization of the abnormal features to produce a set of recognized, but potentially overlapping, clusters. These form hypotheses to be tested by further enquiry, again usually direct questioning, so that the relative likelihood that the patient’s presentation is due to one syndrome rather than another is evaluated. The end result of this iterative process is rarely a single clear-cut diagnosis but rather a shortlist of differential diagnoses stratified according to the balance of probabilities on the evidence available. Immediate management will proceed on the basis of the most likely (or most serious in the short term) diagnosis, and will be reassessed iteratively as above and modified as new information, such as response to initial treatment, emerges.
Investigations in psychiatry
The emphasis on clinical information, that is, information gained directly from the patient in the clinic or at the bedside, should not be taken to mean that there is no role for laboratory investigations in psychiatry. Also, a general physical examination should form part of every psychiatric assessment and this or particular features of the patient’s history may lead to consideration of appropriate objective tests. What is true of psychiatry, as of all branches of medicine, is that no investigation should be regarded as ‘routine’: all investigations carry a finite risk of morbidity, and it is important to remember that this can be psychological as well as physical. Therefore, all investigations must be justified and the reasons for their selection or omission should be recorded (see below). It is also of great importance that the rationale for investigation, the intended procedures and potential unwanted effects be discussed with the patient and that his or her consent be sought before proceeding.
The reasons for selecting specific tests will be similar to other branches of medicine: screening (e.g. asymptomatic patients from high-risk population groups); baseline (to exclude or establish the extent of physical disorder, or as a preparation for certain treatments); monitoring (to monitor progression of primary physical disease or of disease secondary to the mental disorder or to monitor therapeutic drugs), and therapeutic (as part of the treatment plan, or to monitor compliance with treatment). A rational plan of investigation should be based on one or more specific purposes, as listed above, and be stratified into: primary (simpler, quicker, cheaper, more likely to gain information); secondary (more complex, expensive or specific) and tertiary (most complex, expensive or reliant on specialist operation). This stratified approach clarifies the medicolegal status of investigations, at least in psychiatric practice: primary level tests should be considered for every patient, and if not performed, the reasons for omission should be recorded; secondary and tertiary level tests should be performed only if indicated by the presentation, by other findings or on specialist advice, and the reasons for their performance recorded.
THE CLASSIFICATION OF PSYCHIATRIC DISORDERS
Psychiatric illnesses are usually referred to as ‘disorders’ rather than ‘diseases’, in part because of the overlap in clinical features between individual conditions and, indeed, with what may be regarded as an extreme of normal behaviour.
There are two principal systems for classifying psychiatric disorders. One is provided by the Diagnostic and Statistical Manual (DSM) of the American Psychiatric Association. The current edition (DSM-5), published in 2013, includes 18 groups of disorders, including personality disorders, together with a group of conditions that are deemed to require further research before they can be accepted for clinical use.
The World Health Organization’s (WHO) International Classification of Diseases (10th edition) (ICD-10) is more widely used outside the USA. The ICD-10 classification of mental and behavioural disorders is shown in Table 35.1. The number of psychiatric disorders that are recognized is continuing to increase as new conditions are described and others are subdivided and reclassified. The number of psychiatric diagnoses listed in DSM-II in 1968 was 182, and this had slightly more than doubled to 365 in DSM-IV (1994). Although the number appears not to have been significantly increased in DSM-5, several conditions have been sub-divided and others unified and there is an effective overall increase.
TABLE 35.1
A classification of psychiatric disorders
| Classification | Examples or notes |
| Organic disorders | Conditions with a structural (e.g. dementia) or functional (e.g. delirium in fever) basis |
| Mental and behavioural disorders due to psychoactive substance use | Includes alcohol and illicit or prescribed drug misuse |
| Schizophrenia and delusional disorders | Delusions are firmly held but abnormal beliefs; hallucinations are clear perceptions without an objective stimulus |
| Mood (affective) disorders | Depression, bipolar disorder (including mania) etc. |
| Neurotic, stress-related and somatoform disorders | Physical and/or psychological manifestations of anxiety are central. Includes conditions that suggest pathology in an organ or organs, but where none can be demonstrated |
| Behavioural syndromes | Having a psychological, physical or physiological cause |
| Disorders of personality and behaviour | Developmental disorders that do not have an obvious psychiatric or organic cause |
| Mental retardation |
Psychiatric disorders have traditionally been divided into neuroses (in which the symptoms, principally anxiety, vary only in severity from those of normal behaviour) and psychoses, with features such as delusions (an abnormal belief, e.g. of persecution) or hallucinations (perceptions lacking an objective stimulus) and typically with little insight. However, this is not to suggest that neuroses are less harmful in their effect. There are also conditions that have features that overlap these definitions (e.g. anorexia nervosa). The classification of some mental disorders as neuroses therefore has little practical value.
Psychiatric disorders are common: the overall prevalence in the general population is estimated to exceed 20%, comprising mainly depression, anxiety and adjustment disorders (e.g. grief reactions) and contribute to about 30% of consultations with family doctors; in hospitals, organic disorders (e.g. delirium, particularly in the elderly) are more common. The major psychoses (by any definition) (e.g. schizophrenia) are less common (< 5% in total), but form a major part of the work of many psychiatrists.
There are several subspecialties within psychiatry. These include child psychiatry, old age psychiatry, substance misuse and forensic psychiatry.
This chapter primarily concerns the clinical biochemical aspects of psychiatric disorders and those general medical disorders that have psychiatric manifestations.
THE AETIOLOGY OF PSYCHIATRIC DISORDERS
The causation of most psychiatric disorders is multifactorial. Biological (e.g. genetic, organic), psychological and behavioural (e.g. abuse in childhood, emotional trauma), and social and environmental factors (e.g. social isolation) are all involved.
Alterations in neurotransmission are undoubtedly a key mechanism in schizophrenia and affective disorders, although the cause of this remains uncertain. In schizophrenia, for example, there is considerable evidence to implicate dopaminergic pathways, with increased activity in the subcortical and limbic regions of the brain and reduced activity in the prefrontal cortical regions. The drugs that are used in the treatment of schizophrenia all have effects on dopaminergic neurotransmission. Decreased monoamine activity has long been considered to be an important mechanism in depression, a notion that is supported by the efficacy of monoamine oxidase inhibitors in its management. Many addictive drugs have effects on neurotransmission: for example, cocaine blocks the reuptake of dopamine in the brain and benzodiazepines bind to receptors for γ-aminobutyric acid, an important inhibitory neurotransmitter.
Until recently, it has been difficult to study biochemical activity in the brain. Measurements of neurotransmitters and their metabolites made in peripheral blood, blood in veins draining the brain and in cerebrospinal fluid provide only indirect information about brain activity and none on localization. However, the techniques of positron emission tomography (PET) and single-proton emission tomography (SPET) are beginning to shed light on the molecular basis of psychiatric disorders. Functional MRI measures cerebral blood flow, which is related to neuronal activity. And there can be little doubt that studies on the molecular genetics of receptors and enzymes involved in neurotransmission will contribute to a greater understanding of the biochemical disturbances that underlie psychiatric disorders.
BIOCHEMICAL INVESTIGATIONS IN PSYCHIATRIC DISORDERS
Although laboratory investigations do not have a specific role in the diagnosis of the majority of psychiatric disorders, they are important for several reasons. First, because many physical diseases can give rise to symptoms that occur in psychiatric disorders (e.g. psychosis and delirium in systemic lupus erythematosus), it is frequently important to exclude an organic cause in a patient presenting with an apparent psychiatric disorder (albeit the results of such investigations are usually negative). Features suggesting an organic cause for an apparent psychiatric disorder include late age of onset, with no previous history or family history of psychiatric disorder, and no psychological or social precipitating factor. Second, organic illnesses may be complicated by psychiatric disorders. For example, panic disorder, generalized anxiety, social phobia and depression, occur more frequently in patients with irritable bowel syndrome (IBS) than in the general population, and antidepressant and anxiolytic medication has been shown to benefit significant numbers of patients with refractory IBS. Another connection between organic illness and psychiatric disorders is that the latter may cause both metabolic and endocrine disturbances (e.g. amenorrhoea in anorexia nervosa).
Third, psychotropic medication can cause metabolic abnormalities, and particularly given the high prevalence of psychiatric disorders, and hence the use of psychotropic drugs, clinical biochemists must be cognizant of these, so that, for example, the finding of a high serum prolactin concentration in a patient being treated with an antipsychotic drug should not lead automatically to a search for a pituitary tumour. Fourth, substance abuse may involve the clinical biochemist both analytically and in relation to the metabolic disturbances that may ensue. While it is beyond the scope of this book to discuss the role of the laboratory in the detection of substance abuse, the metabolic complications associated with some of the more frequently encountered substances are discussed in Chapter 40.
Table 35.2 summarizes the biochemical and other laboratory investigations that may be of value in excluding an organic cause for an apparent ‘psychiatric presentation’; the ensuing sections of this chapter describe the major psychiatric manifestations of organic diseases and the metabolic changes that can occur in psychiatric disorders and as a result of their treatment.
TABLE 35.2
Laboratory investigations to exclude organic disease in patients with psychiatric symptoms
| Investigation | Rationale |
| Full blood count | Anaemia can lead to cerebral hypoxia, causing confusion; macrocytosis may be due to vitamin B12 deficiency, a cause of dementia, and to a high alcohol intake; chronic anaemia may contribute to depression |
| Acute phase proteins | High erythrocyte sedimentation rate (ESR) suggests systemic illness, e.g. infection, malignancy, that may cause delirium; C-reactive protein (CRP) elevation may indicate abrupt onset of inflammation |
| Creatinine, ‘electrolytes’, estimated glomerular filtration rate (eGFR), calcium, liver function tests | Electrolyte disorders and renal and hepatic failure can cause delirium; they may result from behavioural disturbance secondary to psychiatric disorder |
| Blood gases | Hypocapnia can mimic anxiety (but note that, more frequently, hyperventilation secondary to anxiety or a panic attack can cause hypocapnia); hypercapnia in respiratory failure can cause delirium |
| Glucose | Hypoglycaemia can mimic anxiety (and, when chronic, may lead to behavioural disturbance and trigger referral to a psychiatrist); hyperglycaemia can cause delirium |
| Thyroid function tests | See text |
| Syphilis serology | General paresis (a late manifestation of neurosyphilis), presenting with dementia, tremor and upper motor neuron signs, can develop 5–15 years after primary infection, if this is not treated |
| Blood cultures | To detect occult sepsis |
| Blood alcohol | A frequent cause of and exacerbating feature in psychiatric presentations |
Other laboratory investigations that may be valuable (though are less frequently required) include measurement of heavy metals, carboxyhaemoglobin (for carbon monoxide poisoning), antinuclear factor (for cerebral lupus erythematosus) etc.
PSYCHIATRIC MANIFESTATIONS OF ORGANIC DISEASE
Acute confusional state (delirium)
Delirium is a complex syndrome of altered consciousness, manifest by decreased awareness of the environment and inattention, together with cognitive impairment, with memory deficit, disorientation, decreased perception and language disturbance. There may, in addition, be emotional disturbances (e.g. anxiety, irritability) and psychomotor changes (e.g. agitation or retardation). Symptoms tend to fluctuate, confusion typically being greater at night or in unfamiliar surroundings (e.g. a hospital ward).
The causes include:
• metabolic and endocrine (see below)
• infection (systemic, particularly with high fever and meningeal/cerebral)
• vascular (e.g. cerebral haemorrhage and infarction)
• toxic (e.g. drugs, both intoxication and withdrawal, and toxins, e.g. carbon monoxide)
• neoplasia (primary cerebral tumours, cerebral metastases and paraneoplastic syndromes)
• trauma (e.g. subdural haematoma, cerebral contusion)
• miscellaneous (e.g. post-ictal, postoperative).
The metabolic and endocrine causes are summarized in Table 35.3. When one of these is the cause of acute confusion, there will often be other clinical features of, or clues to, the condition, but if this is not the case, the laboratory investigations performed beyond a standard ‘biochemical profile’ and full blood count (with differential) should reflect the relative frequency of the conditions. It should be noted that in the elderly, almost any acute illness can present with acute confusion, and that the elderly may be at increased risk of developing confusion in response to any causative condition as a result of poor nutrition, visual or auditory impairment, or incipient dementia. The tendency for elderly people to be taking multiple therapeutic drugs coupled with age-related changes in the rates of metabolism and excretion of drugs is another important factor.
TABLE 35.3
Metabolic and endocrine causes of delirium (acute confusional state)
| Cause | Notes |
| Cerebral hypoxia | Secondary to, for example, cardiac failure, respiratory failure, hypotension, severe anaemia |
| Hypo-/hyperglycaemia | Hypoglycaemia can present with anxiety and, when chronic, with behavioural disturbance (see Chapter 17) |
| Hyponatraemia | Dependent on the rate of fall as well as the absolute value of the sodium concentration |
| Hypo-/hypercalcaemia | |
| Renal failure | |
| Liver failure | |
| Adrenal failure | |
| Hyper-/hypothyroidism | Depression is a far more frequent psychiatric manifestation of hypothyroidism |
| Hyperpyrexia | Causing rhabdomyolysis as in malignant hyperthermia or the ‘neuroleptic malignant syndrome’ due to central dopamine blockade |
| Acute porphyria | |
| Vitamin deficiencies(thiamin, niacin and vitamin B12) | |
| Drugs | Including alcohol (ethanol), illicit agents and a wide range of therapeutic drugs, particularly in the elderly |
Sepsis and drug intoxication or withdrawal are particularly common causes of delirium. Sepsis may increase the risk of drug-induced delirium by increasing the permeability of the blood–brain barrier. For drugs that are protein-bound, a low plasma albumin concentration may also increase toxicity by increasing the proportion of the drug present in the unbound form.
Anxiety
Anxiety is a normal response to a threat, but in the anxiety disorders, the response is out of proportion to any real danger and causes suffering and behavioural disturbance.
The anxiety disorders comprise three conditions: generalized anxiety disorder, in which symptoms are persistent, and two conditions in which they are episodic – panic disorder, in which there is no apparent stimulus, and phobic anxiety disorders, in which anxiety stems from fear of situations, for example going out alone or being in crowded spaces (agoraphobia), or objects, for example spiders. All types can be associated with somatic symptoms, including chest pain, palpitation, dyspnoea (typically a feeling of not being able to take a full breath), paraesthesiae and sweating, but these are particularly associated with panic disorder. They may lead to the patient seeking urgent medical advice because they fear a serious illness, for example a heart attack. Patients with generalized anxiety disorder may seek medical advice because of bowel disturbance.
The somatic symptoms in the acute anxiety disorders are due to a combination of increased sympathetic activity, release of catecholamines and hyperventilation. The latter causes falls in arterial PCO2 and hydrogen ion concentration (rise in pH) with normal bicarbonate and normal PO2, reducing the plasma concentration of ionized calcium and increasing neuromuscular excitability. Typically, symptoms can be provoked by overbreathing and abated by rebreathing into a paper (not plastic, which risks asphyxiation) bag. It is not usually difficult to distinguish hyperventilation in anxiety from hyperventilation in response to acidosis (e.g. ketoacidosis) or in pulmonary disease (e.g. asthma, pulmonary oedema) but, if necessary, arterial blood gases can be measured.
Hypocapnia can also cause anxiety. The symptoms produced (e.g. chest pain) may lead sufferers to believe that they have serious disease, inducing anxiety and setting up a vicious circle.
The differential diagnosis of anxiety disorders includes drug dependence and withdrawal, hyperthyroidism, hypoglycaemia, hypoparathyroidism and phaeochromocytoma.
Thyroid function tests should always be performed in patients presenting with an apparent primary anxiety disorder. Anxiety, insomnia, emotional lability and difficulty concentrating are frequently present in patients with hyperthyroidism and the symptoms may occasionally be sufficiently severe to suggest hypomania. It may be difficult to distinguish between an anxiety state and mild hyperthyroidism: features suggesting the latter include eye signs, the presence of a goitre and proximal myopathy. Tremor is often present in both conditions, but the hands tend to be warm and moist in hyperthyroidism as a result of the hyperdynamic circulation, but cold and clammy in anxiety disorder. Resting pulse rate is usually normal in anxiety disorders but increased in hyperthyroidism. The anxiety symptoms of hyperthyroidism usually respond to treatment with β-blockers.
The diagnosis of hypoglycaemia is discussed in detail in Chapter 17, and of phaeochromocytoma in Chapter 38. Measurement of fasting blood glucose concentration during an attack is a simple matter; measuring plasma or urinary catecholamines or their metabolites is not, but phaeochromocytoma, although rare, should be considered when there are predominant sympathetic symptoms (e.g. palpitation, sweating) and no obvious precipitating factor can be identified.
Dementia
Dementia – a condition characterized by a progressive decline in cognitive function (particularly loss of short-term memory) without confusion or loss of arousal – is common, particularly in the elderly, in whom Alzheimer disease and cerebrovascular disease (usually diffuse, small vessel disease) are the major causes. Metabolic and endocrine conditions are uncommon causes. A treatable cause should be sought through laboratory investigations and imaging, particularly in younger patients. The former should include a full blood count, erythrocyte sedimentation rate (ESR) and standard biochemical renal- liver- and bone-related tests, thyroid function tests, measurement of serum vitamin B12 concentration and serology for systemic lupus erythematosus and syphilis (see Table 35.2). In individuals with a history of alcohol abuse, thiamin deficiency should be considered but is usually diagnosed on the basis of the therapeutic response to an intravenous bolus of thiamin. Heavy metal and carbon monoxide poisoning may need to be considered if there is possibility of exposure. A syndrome of ‘pseudodementia’ may arise in severe depression; this should be sought clinically as investigations are likely to be normal.
Considerable effort continues to be invested in research to determine the cause of Alzheimer disease and to identify early markers of the disease that could direct early intervention. To date, however, although various candidate markers have been investigated, none has been identified and the role of laboratory investigations is confined to eliminating treatable causes of dementia.
The pathological hallmarks of Alzheimer disease are senile plaques and neurofibrillary tangles throughout the cerebral cortex but particularly in the hippocampus. The first stage in the development of plaques is the deposition of β-amyloid protein, which is produced through the proteolysis of amyloid precursor protein (APP). This protein is coded for on chromosome 21, which may explain why individuals with Down syndrome (trisomy 21) are prone to the early development of Alzheimer-like changes in the CNS. Neurofibrillary tangles develop as a result of the aggregation of tau protein driven by its undergoing hyperphosphorylation, but the underlying cause of either of these processes remains unknown. As the disease develops, diffuse cerebral atrophy ensues. The most obvious functional abnormality in Alzheimer disease is a reduction in cerebral cholinergic activity. This has led to the development of treatments using cholinesterase inhibitors, but thus far, these are of limited benefit, and that only in early disease.
Most cases of Alzheimer disease are sporadic, but a small number show autosomal dominant inheritance, and are due to mutations in the genes coding for APP or presenilins 1 and 2. Genetic factors also impinge on sporadic Alzheimer disease: some 80% of patients possess at least one ε4 allele of the gene coding for apolipoprotein E (the usual allele being ε3). However, the basis of this association remains uncertain.
Depression
Introduction
Depression is one of the commonest psychiatric disorders. Its typical features are low mood, loss of interest and enjoyment (anhedonia), reduced energy and increased fatigue; other common features include psychomotor retardation, poverty of movement and disordered ideation (e.g. hypochondriasis, feelings of unworthiness). Somatic symptoms, particularly headache and fatigue, are common. In severe cases, auditory hallucinations, delusions and suicidal ideation may be present. Recurrent distinct episodes are common, although some patients experience a chronic, low-grade depression (dysthymia). Depression can occur on its own (unipolar disorder) or (less frequently) be a feature of bipolar affective disorder (manic depressive psychosis) with episodes of depression or hypomania interspersed with periods of normality.
Depression can develop as a natural response to physical illness (depressive adjustment disorder) but is typically not as severe as depression arising without precipitating factors. If the associated illness is self-limiting, it may only be transient. However, the boundary between depressive adjustment disorder and depressive illness is not clear-cut, and depressive symptoms arising in a patient with physical illness should not be assumed to be a response to the illness. Indeed, because some depressive symptoms can be features of organic illness, the possibility of depression should be actively considered. Up to a third of patients with physical illness have depressive symptoms, and major depression is present in up to 15%, the prevalence correlating with the number of physical symptoms. Untreated comorbid depression worsens the outcome in physical illness (e.g. cardiovascular disease, diabetes, arthritis). Indicators of possible depressive disorder include: failure to adjust to the illness; reduced physical functioning; slower recovery than might be expected, and a reduction in social activity.
Depression and thyroid function
The differential diagnosis includes several other psychiatric disorders, and depression can coexist with other disorders, for example panic disorder. The most frequent organic disease that may be mistaken for depression is hypothyroidism, although apathy is more common than true depressive symptoms in the latter. Other features include inattentiveness and loss of short-term memory. Frank psychosis due to hypothyroidism (‘myxoedema madness’) is now rare. Hypothyroidism is a common condition, particularly in the elderly; its manifestations can be protean and it should be excluded by measuring thyroid stimulating hormone (TSH) and free thyroxine concentrations in any patient presenting with depression or a decline in cognitive function. The psychiatric manifestations improve with thyroxine replacement treatment, although sometimes the cognitive dysfunction persists, perhaps because of a deleterious effect of chronic thyroid hormone deficiency on the CNS. Depression that is refractory to treatment may show a pattern of ‘sick euthyroidism’ (low plasma TSH and free thyroxine concentrations, see Chapter 19), and the depression may respond to thyroxine treatment; thyroid function should be reassessed when the depression improves as there is unlikely to be primary hypothyroidism. In some patients, the initiation of thyroxine treatment can temporarily exacerbate the psychiatric symptoms or even, in susceptible individuals (e.g. those with a family history of affective disorder), precipitate a manic episode. It should be remembered that, particularly in elderly people, hyperthyroidism occasionally presents atypically (apathetic hyperthyroidism) and may be mistaken for depression. Thyroid disease is discussed in detail in Chapter 19.
Depression and adrenal function
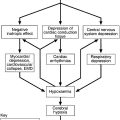
Depression is a common feature of Cushing syndrome, whether pituitary dependent or independent or the result or administration of exogenous steroids (though this can also cause euphoria). That it is cortisol, rather than corticotrophin (adrenocorticotrophic hormone, ACTH) that is the cause of the depressive symptoms is suggested by the absence of any excess of depression in patients with Nelson syndrome (in which ACTH concentrations are high but cortisol is low). Furthermore, metyrapone, which inhibits the synthesis of cortisol and causes increased concentrations of ACTH, has a beneficial effect on depression in Cushing syndrome. Depression in Cushing syndrome is more frequent in women and in older age groups, and its severity shows some correlation with the severity of other features of the condition. The acute administration of high doses of corticosteroids (e.g. prednisolone 30 mg/day) can lead to hyperphagia, insomnia and euphoria, and their sudden withdrawal can precipitate depression.
Patients with depression often fail to show suppression of plasma cortisol concentration in the overnight dexamethasone suppression test (see Chapter 18) and (though less frequently) in the formal low-dose test, in which dexamethasone 0.5 mg is administered 6-hourly for 48 h.
Depression can lead to accelerated loss of bone mineral density and increased risk of fracture: the hypercortisolaemia is likely, in part, to be responsible for this.
Depression can also be a feature of Addison disease. The symptoms may pre-date the classic physical signs of the condition but respond to cortisol replacement treatment.
Depression in the metabolic syndrome and diabetes
There is a well recognized association between diabetes mellitus and depression. Dr Thomas Willis, a 17th century British physician, considered that diabetes was caused by ‘sadness or long sorrow’. One study has reported a prevalence of major depression in diabetes of 9.3%, compared with 6.1% in the general population. The presence of depression in diabetes is associated with poorer glycaemic control and poorer outcome but appropriate psychiatric intervention can reduce the morbidity associated with diabetes. Both diabetes and depression are risk factors for cardiovascular disease; the risk in patients with depression is approximately twice that in those without.
It seems more likely that diabetes predisposes to depression than vice versa. A nine-year prospective study of people with or without depression showed no excess risk of diabetes in those with depression. Further, treatment of depression in patients with diabetes has no effect on glycaemic control. How diabetes predisposes to depression is uncertain, but insulin resistance as part of the metabolic response to the psychosocial stress arising from having a chronic illness may, in part, be responsible.
The major underlying pathogenic factor in type 2 diabetes is resistance to the actions of insulin. Insulin resistance also underlies the metabolic syndrome (see Chapter 15). This syndrome comprises a range of abnormalities, including visceral obesity, dyslipidaemia, hyperglycaemia and hypertension that are risk factors for both type 2 diabetes and cardiovascular disease. The prevalence of the metabolic syndrome in patients with diabetes is high. Several other abnormalities have been described in both depression and the metabolic syndrome, including hypercortisolaemia, abnormal autonomic nervous system function (e.g. increased resting heart rate with reduced heart rate variability), endothelial dysfunction, increased platelet reactivity and evidence of persistent inflammation.
Plasma concentrations of nutritional factors such as 25-hydroxyvitamin D and ω-3 fatty acids have been found to be consistently low in depressed patients, the former being linked to reduced activity and exposure to sunlight, the latter to modulation of cytokine activity leading to increased hypothalamo–pituitary–adrenal and inflammatory activity. There is no evidence of clinical benefit from dietary supplementation.
Post-traumatic stress disorder
This syndrome can arise as a result of exposure to any severe psychological trauma; physical trauma may also be involved. Examples of causes include experience of natural disasters, war, terrorism, witnessing violent death, torture and rape. Its features include:
• recurrent, intrusive recollections of the event (‘flashbacks’)
• feelings of detachment and isolation (avoidant symptoms)
• persistent symptoms of increased arousal (e.g. exaggerated startle reflex).
The severity of the condition in individuals is dependent both on factors related to the event itself (e.g. its severity, the proximity of the individual to it) and on the personality of the individual, any history of psychiatric illness and other factors.
Endocrine abnormalities associated with the post-traumatic stress disorder include decreased cortisol secretion secondary to decreased secretion of ACTH (despite increased secretion of corticotrophin releasing hormone, CRH). The ACTH response to CRH is reduced, and that to dexamethasone increased, suggesting enhanced negative feedback. This contrasts with the elevated cortisol and reduced sensitivity to dexamethasone that is characteristic of several other psychiatric disorders. The urinary excretion of catecholamines is increased and there is evidence of increased CNS noradrenaline (norepinephrine) and serotonin activity, these two neurotransmitters being known to have a role in storing and retrieval of memory. Some patients with post-traumatic stress disorder have increased plasma concentrations of thyroid hormones and an enhanced TSH response to TRH (thyrotrophin releasing hormone), yet are clinically euthyroid.
Functional magnetic resonance imaging (fMRI) of the brain suggests features in common between post-traumatic stress disorder and obsessive–compulsive disorder.
Schizophrenia
The history of schizophrenia research is littered with the discovery, and subsequent abandonment, of putative biomarkers of the disorder or its aetiology. Recent interest has focused on elevated plasma concentrations of inflammatory cytokines found in patients with both first episode psychosis and chronic schizophrenia. Acute inflammation has been reported to increase the relapse rate of schizophrenic symptoms, and the widespread direct and indirect influences of cytokines on brain neurotransmitter pathways and neural plasticity suggest a mechanism. Augmentation of antipsychotic treatment with the anti-inflammatory drugs acetylsalicylic acid (aspirin) and celecoxib (a cyclooxygenase-2 inhibitor) has been advocated and may improve treatment response in some patients.
Despite the major functional abnormalities that may be present in patients with schizophrenia, significant metabolic disturbances are relatively uncommon. Basal concentrations of most hormones are normal in schizophrenia (elevations in vasopressin are an exception), although the TSH and gonadotrophin responses to their respective releasing hormones are typically blunted. However, significant metabolic abnormalities may develop as a consequence of treatment with psychotropic drugs, as described later in this chapter. Furthermore, substance abuse is frequent in patients with schizophrenia and this may cause metabolic abnormalities, e.g. hyponatraemia with amfetamines (particularly methylenedioxymethamfetamine, MDMA, ‘ecstasy’), hypoglycaemia with ethanol (see Chapter 17) and decreased secretion of gonadotrophins with opioids.
ENDOCRINE AND METABOLIC MANIFESTATIONS OF PSYCHIATRIC DISEASE
The hypothalamus is central to the regulation of the endocrine system and the sympathetic nervous system, and is involved in behavioural responses, so it is not surprising that many psychiatric diseases affect endocrine function. Not all do, however: schizophrenia (see above) is a notable exception.
Abnormalities of the hypothalamo–pituitary–adrenal axis
Abnormalities of cortisol secretion are common in psychiatric disease. Approximately 50% of patients with depression have hypercortisolaemia (though with preservation of the normal circadian variation) secondary to increased secretion of CRH and hence ACTH, although the response of ACTH secretion to an intravenous bolus of CRH is reduced (in contrast to patients with Cushing disease, in whom it is typically exaggerated). Enhanced vasopressin secretion may also contribute to the increased secretion of ACTH in depression.
The reason for the increased CRH drive is unclear. It may be a result of impairment of the normal inhibitory feedback of cortisol on the hypothalamus, but this could be part of the underlying disorder or a response to it.
Individuals with alcohol dependence may develop features of Cushing syndrome (pseudo-Cushing) but these usually resolve rapidly on withdrawal of alcohol.
Similarity between the features of chronic fatigue syndrome and adrenal insufficiency have prompted research into endocrine function in the former condition. Plasma cortisol concentrations tend to be slightly lower than normal, although adrenal responsiveness to ACTH is not impaired, and the ACTH and cortisol responses to insulin-provoked hypoglycaemia are normal. Treatment with low doses (5–10 mg/day) of hydrocortisone has been shown to be of benefit in some patients.
Plasma cortisol concentrations are frequently decreased in patients with post-traumatic stress disorder; these patients often demonstrate increased sensitivity to the suppressive effects of dexamethasone on cortisol secretion. This contrasts with the findings in many other psychiatric disorders, in which hypercortisolaemia is accompanied by reduced sensitivity to dexamethasone.
The ACTH response to CRH is blunted in some patients with anxiety disorders but plasma cortisol concentrations are normal. Hypothalamo–pituitary–adrenal function has been reported to be normal in patients with schizophrenia.
Abnormalities of the hypothalamo–pituitary–thyroid axis
Patients with depression frequently demonstrate abnormalities of thyroid function. Up to 20% of patients with depression have been reported to have ‘sick euthyroidism’ (see above) or subclinical hypothyroidism, with normal thyroxine concentrations but slightly elevated concentrations of TSH secondary to increased secretion of TRH and with increased TSH responsiveness to TRH. This pattern is more common in patients with bipolar disorder. Other patients (more particularly those with unipolar depression) have a blunted TSH response to TRH. However, this is not specific to depression, having been reported in schizophrenia and alcoholism as well. Treatment with antidepressants leads to normalization of thyroid function tests, although it is of interest that treatment with tri-iodothyronine apparently enhances their efficacy in some patients, both increasing the rate at which they become effective and the overall response. This suggests that there may be a specific link between thyroid function and mood disorder rather than to the symptoms just being a consequence of reduced cerebral metabolism. Tri-iodothyronine is widely used by psychiatrists for this purpose.
Patients with other acute and chronic psychiatric illnesses may demonstrate the features of the sick euthyroid syndrome (see Chapter 19), and patients with anorexia nervosa typically demonstrate the abnormalities characteristic of starvation, with a low plasma thyroxine concentration, increased concentration of reverse tri-iodothyronine and a reduced TSH response to TRH.
Abnormalities of the hypothalamo–pituitary–gonadal axis
The secretion of gonadotrophin releasing hormone (GnRH) is impaired at low body weights (see Chapter 18), and oligo-/amenorrhoea is frequently reported by women with anorexia nervosa. Amenorrhoea is often a feature of moderate to severe depression, and may result from diminution of the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. Pulsatile release of both FSH and LH takes place during slow-wave (i.e. non-REM) sleep (SWS), but this phase of sleep is shortened or absent in moderate to severe depression. Testosterone has been reported to have an anti-depressant effect in depressed hypogonadal men.
Abnormalities of growth hormone secretion
Anorexia nervosa typically also causes increases in plasma growth hormone concentration with exaggerated responses to growth hormone releasing hormone (GHRH) but decreased concentrations of insulin-like growth factor 1 (IGF-1), probably due to a combination of weight loss and increased concentrations of glucocorticoids
Two major abnormalities of growth hormone secretion have been described in depression: hypersecretion during daytime and reduced secretion during sleep. As growth hormone is also secreted from the pituitary in bursts during SWS, the latter abnormality may arise from the reduction of SWS that is found in depression.
Abnormalities of prolactin secretion
Anxiety is a common cause of hyperprolactinaemia, and blood for prolactin measurement should always be drawn under conditions that minimize stress.
Nocturnal plasma prolactin concentrations are increased in some patients with schizophrenia, but elevated prolactin concentrations in this condition are usually a consequence of treatment with dopamine receptor antagonists (see below).
Prolactin secretion is usually normal in depressive illness, although responsiveness to stimuli such as L-tryptophan may be diminished.
Other metabolic abnormalities
Several psychiatric disorders can lead to abnormalities of fluid, electrolyte and acid–base balance. The respiratory alkalosis that can occur as a result of hyperventilation in anxiety states has been mentioned above. Abnormalities associated with drug abuse are discussed in relation to individual drugs in Chapter 40. Examples include hypophosphataemia in alcoholism and alcohol withdrawal, and potassium and magnesium depletion in patients abusing diuretics or laxatives. Severe hypokalaemia can develop in anorexia nervosa secondary to chronic starvation; this may be exacerbated by vomiting and laxative abuse. Patients with bulimia are particularly prone to developing potassium depletion with hypochloraemic alkalosis as a result of self-induced vomiting. Hypophosphataemia may also occur but is particularly associated with refeeding (see p. 211). Multiple deficiencies of mineral nutrients can occur in both conditions, and in depression. Psychogenic polydipsia can cause profound hyponatraemia and may be difficult to distinguish from diabetes insipidus, as the chronically high urine output can lead to ‘medullary washout’ with a secondary decrease in the concentrating capacity of the kidneys (see Chapter 4).
METABOLIC COMPLICATIONS OF PSYCHOTROPIC DRUGS
Lithium
Lithium is a valuable mood stabilizing drug, widely used in the management of bipolar disorder, both for prophylaxis and in the treatment of acute episodes (particularly of mania). However, it has the potential to cause severe adverse effects. These are directly related to its plasma concentration, and monitoring of plasma concentrations every three months is mandatory (see Chapter 39).
Lithium reduces the responsiveness of the distal parts of the nephron to vasopressin (antidiuretic hormone), resulting in a reduced capacity to concentrate the urine. This effect is demonstrable in most patients being treated with the drug but is rarely of clinical significance, although a few patients develop nephrogenic diabetes insipidus. This is partially reversible if lithium is withdrawn. Lithium is 95% excreted by the kidneys and dehydration decreases the glomerular filtration rate, so increasing the concentration of the drug and potentially exacerbating the toxic effect. However, provided that acute toxicity is avoided, lithium rarely causes long-term renal damage.
Lithium can also interfere with thyroid function and cause primary hypothyroidism, the risk of this being greater if thyroid autoantibodies are present. For these reasons, plasma creatinine (and hence estimated glomerular filtration rate (eGFR), thyroid stimulating hormone (TSH) concentrations should be measured before starting lithium treatment and at regular intervals (e.g. 4–6 monthly) thereafter.
Drugs causing hyperprolactinaemia
Prolactin secretion by the anterior pituitary is inhibited by dopamine, and given the fact that several antipsychotic drugs (e.g. phenothiazines, butyrophenones) are dopamine (D2 receptor) antagonists, it is not surprising that these drugs are a frequent cause of hyperprolactinaemia. Metoclopramide and domperidone, two widely used antiemetic drugs, also have this effect. Prolactin concentration may be increased to as much as 2500 mU/L, well into the range seen in patients harbouring microprolactinomas. Clinical features of hyperprolactinaemia are, however, relatively uncommon, but amenorrhoea, galactorrhoea and breast enlargement may occur in women, and gynaecomastia and impotence in men. Hyperprolactinaemia is less frequent with the newer second generation antipsychotics, for example olanzapine and quetiapine (though it is seen with risperidone), whose actions are either non-dopaminergic or have higher affinity for 5-hydroxytryptamine (serotonin) receptors than dopamine receptors.
Drugs causing hyponatraemia
Several drugs used in the management of patients with psychiatric disease can interfere with water homoeostasis (probably through stimulating vasopressin secretion) and lead to hyponatraemia. They include selective serotonin reuptake inhibitors (e.g. paroxetine) and carbamazepine, primarily an anticonvulsant but also used in the prophylaxis of bipolar disorder. In the majority of patients, any resultant hyponatraemia is only mild and is asymptomatic, but there is a risk of significant water retention leading to intoxication, and serum sodium concentration (and less frequently osmolality) should be monitored in patients being treated with these drugs.
Drugs causing hyperglycaemia and hyperlipidaemia
The prevalence of diabetes mellitus is higher in patients with schizophrenia in comparison with the general population, but the introduction of chlorpromazine in 1956 caused a significant further increase, and the term ‘chlorpromazine diabetes’ was used to describe this. The mechanism appears to be insulin resistance. Newer second generation antipsychotics can also cause hyperglycaemia and hypertriglyceridaemia, and sometimes hypercholesterolaemia, albeit less frequently. Clozapine is particularly implicated, followed by olanzapine; metabolic abnormalities are less common with risperidone and quetiapine, and least with aripiprazole. Weight gain is frequent with both clozapine and olanzapine. It is recommended that glycated haemoglobin (HbA1C) and lipid concentrations should be measured at inception of treatment with any antipsychotic, and repeated twice yearly in patients on clozapine (they also require to be under surveillance for agranulocytosis, a potentially lethal adverse effect) and olanzapine, and annually for other antipsychotics.
Drugs interfering with hepatic function
Many drugs can cause mild hepatic abnormalities, usually manifest as an increase in plasma aminotransferase and/or alkaline phosphatase activity. Examples include chlorpromazine, haloperidol and some tricyclic antidepressants. Chlorpromazine can cause cholestasis, and this may persist after the drug has been discontinued. The hepatotoxicity of valproate is a particular problem. This drug, primarily used as an anticonvulsant, has become one of the most frequently used mood-stabilising drugs. Several cases of fatal hepatotoxicity have been reported. Patients present with lethargy, anorexia, nausea, and jaundice are found to have hyperammonaemia (although elevated ammonia concentrations have been reported in patients taking valproate in the absence of overt toxicity). Unfortunately, this reaction is an idiosyncratic one, being dependent neither on the dose nor the concentration of the drug, so that therapeutic monitoring of concentrations is of no value in preventing it.
Many drugs used in psychiatric practice affect plasma protein binding (notably carbamazepine) or the hepatic cytochrome enzyme system, causing changes in the availability of other drugs utilizing the same pathway. The result may be to reduce the therapeutic effects of a drug or increase its adverse effects.
FUTURE DEVELOPMENTS
Simple ‘mono-neurotransmitter’ models of psychiatric disorders are increasingly untenable and, along with them, simple distinctions between mental and physical illness. Evidence is accumulating of the interplay of neuroendocrine and inflammatory processes in many psychiatric disorders, and that some disorders that cluster epidemiologically (depression and cardiovascular disease) might not be independent but manifestations of the same pathogenetic process. Although it seems unlikely that measurements of individual or even groups of simple analytes in peripheral blood will ever become of value in the diagnosis or management of the major psychiatric disorders, it is possible to envisage an increasing role for the clinical biochemist in psychiatry. Pharmacogenetics – the study of the genetic influence on responsiveness to drugs – has the potential to facilitate a more tailored approach to prescribing, in relation both to the selection and dosage of drugs. And, given that some psychiatric disorders are clearly at least in part of genetic origin, the identification of genes conferring susceptibility to these conditions will enable the development of microarray-based tests to identify individuals at high risk. These could then be targeted for intervention to manage other risk factors, or possibly prophylactic drug treatment.
CONCLUSION
At present, most psychiatric disorders are diagnosed clinically. The purpose of biochemical investigations in patients with psychiatric symptoms is to rule out an organic cause. However, many psychiatric disorders can give rise to metabolic and endocrine abnormalities, as can treatment with psychotropic drugs.
Further reading
Osborn DPJ, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: Systematic review and metaanalysis. BMC Psychiatry. 2008. ;8:84. http://www.biomedcentral.com/1471-244X/8/84.
An extensive review of the occurrence and features of metabolic syndrome in major psychiatric disorder, with emphasis on the psychoses.