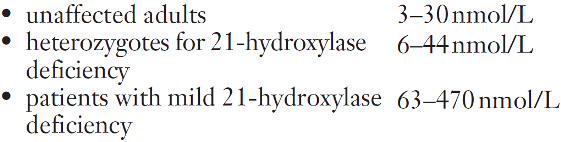CHAPTER 22
Reproductive function in the female
CHAPTER OUTLINE
Plasma concentrations of reproductive hormones
HORMONES REGULATING REPRODUCTIVE FUNCTION
Steroid secretion through the menstrual cycle
Steroid hormone transport and metabolism
Actions of gonadal steroid hormones
Biochemical diagnosis of pregnancy
Diagnosis of ectopic pregnancy
Biochemical monitoring of pregnancy
Screening for fetal malformation
Fetal tissue sampling techniques
Monitoring of maternal and fetal well-being
Biochemical changes during pregnancy
ORAL CONTRACEPTION AND HORMONE REPLACEMENT THERAPY
Metabolic effects of oestrogens
Metabolic effects of progestogens
INTRODUCTION
In both normal males and females, the gonads produce steroid hormones that affect secondary sexual characteristics, the functioning of the reproductive tract and sexual behaviour. Production of gametes and hormones by the gonads is under the control of pituitary glycoprotein hormones. The hypothalamo–pituitary–gonadal axis appears to be universal throughout the vertebrates: small amounts of releasing factors from the hypothalamus elicit the release of larger amounts of glycoprotein gonadotrophins and yet larger increases in gonadal steroids. These steroids in turn influence the rate at which they themselves are produced (feedback control).
PHYSIOLOGY
The ovaries
The human ovaries produce female gametes and steroid sex hormones. Both functions depend largely on the monthly growth and rupture of (usually) a single ovarian follicle.
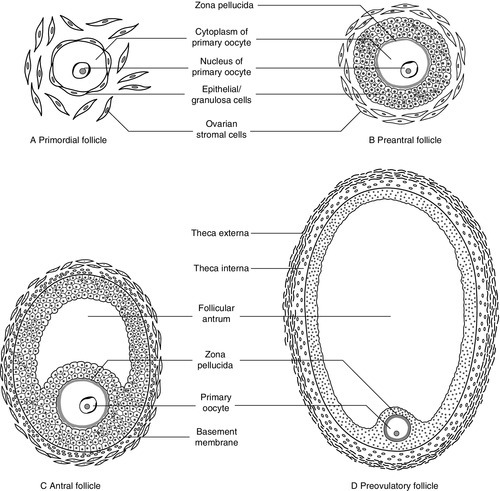
At birth, some 106 immature germ cells are present in the ovaries as primary oocytes, arrested between prophase and metaphase of the first meiotic division. Each primary oocyte is surrounded by a layer of epithelial cells, the whole being known as a primordial follicle (Fig. 22.1). Primary oocytes do not complete meiosis during childhood: in fact the majority of them degenerate.
During childhood, the ovaries remain inactive, but at puberty, a monthly ovarian cycle is established through the interaction of the hypothalamus and pituitary with ovarian follicles and manifests itself by the onset of menstruation.
At puberty, the pulsatile release of gonadotrophin releasing hormone (GnRH) from the hypothalamus stimulates the pituitary production of gonadotrophins. Growth hormone (GH) pulses released by the pituitary also increase in amplitude. This amplification of GH secretion may be regulated by the pubertal increase in the production of androgenic and oestrogenic hormones. These sex steroids stimulate skeletal growth and sexual maturation, augmenting the role of GH in promoting somatic growth and development.
The human menstrual cycle is 23–39 days long. By definition, day 1 of the cycle is the first day of menstrual bleeding. The cycle is divided into follicular (or proliferative) and luteal (or secretory) phases by the event of ovulation. In cycles of different length, it is the duration of the follicular phase that varies; the length of the luteal phase is remarkably constant at 13–15 days.
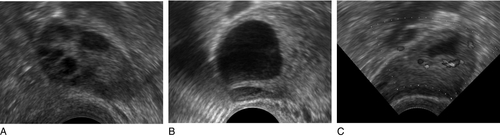
On day one of the cycle, several antral follicles, which are 2–9 mm in diameter, are present in both ovaries. These consist largely of a fluid-filled antrum and can be visualized by ultrasonography (Fig. 22.2A). Each antral follicle has developed from a primordial follicle by proliferation of the epithelial granulosa cells and by the appearance and coalescence of fluid-filled spaces among them (see Fig. 22.1). The earlier stages of follicle development do not require gonadotrophin stimulation, but the ‘recruitment’ of antral follicles from the preantral pool appears to be follicle stimulating hormone (FSH) and anti-Müllerian hormone (AMH) dependent.
FIGURE 22.2 Vaginal ultrasonographic images of the ovary. (A) Polycystic ovary showing multiple antral type peripheral follicles; (B) Pre-ovulatory follicle 20 mm; (C) Corpus luteum with Doppler showing surrounding neovascularity. Vessels show as prominent echoes below the darker collapsed follicle.
Anti-Müllerian hormone is now considered to be the main regulator of early follicular recruitment from the primordial pool. It is produced by small enlarging follicles rather than the primordial follicles themselves. Plasma concentrations of AMH do, however, appear to reflect primordial follicle numbers in humans and rodents.
Through a poorly understood process of selection, one of the apparently identical antral follicles present on day 1 becomes dominant, while the others degenerate. The dominant follicle grows rapidly in the late follicular phase, reaching a maximum diameter of approximately 20 mm (Fig. 22.2B). Outside the basement membrane of the granulosa cell layer, the wall of the dominant follicle consists of the theca interna and theca externa, which have developed from ovarian stromal cells. At ovulation, the follicle collapses, releasing its fluid and the oocyte, which by now has completed the first meiotic division. The follicle subsequently refills with fluid, and blood vessels penetrate the basement membrane and vascularize the granulosa cells for the first time; further proliferation of the granulosa cells transforms what was the follicle into the corpus luteum (Fig. 22.2C). The corpus luteum has a limited life span: in the absence of conception it involutes, another dominant follicle develops and the cycle repeats itself.
Insulin-like growth factor-1 has actions that regulate sex steroids through the control of the plasma concentration of sex hormone binding globulin (SHBG). Although the precise mechanism is unclear, increasing concentrations of GH are believed to exert some effects on circulating insulin concentrations. Growth hormone induces peripheral insulin resistance which leads to compensatory increases in insulin secretion and protein anabolism. Insulin regulates hepatic insulin-like growth factor 1 (IGF-1) through its effects on insulin-like binding protein 1 (IGFBP-1).
Obesity is associated with increased plasma concentrations of insulin. Therefore, if excessive nutrition intake persists during childhood, it is possible that hyperinsulinaemia may lead to lower concentrations of IGFBP-1 and reduced plasma SHBG concentrations, enhancing IGF-1 and sex steroid bioavailability.
Plasma concentrations of reproductive hormones
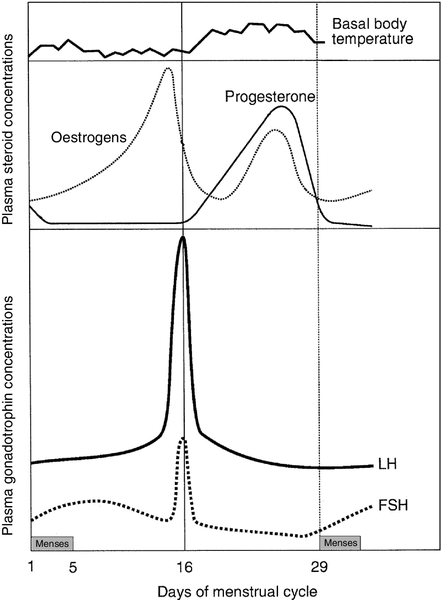
Figure 22.3 illustrates the fluctuations in plasma concentrations of reproductive hormones through a typical menstrual cycle. Changes in the pulsatile release of gonadotrophins in the late luteal phase and early follicular phase appear to bring about the growth of a group of antral follicles, one of which (the dominant follicle) enlarges greatly and secretes increasing amounts of oestrogens (mainly 17β-oestradiol). Although oestrogens generally exert a negative feedback effect on gonadotrophin concentrations, high, rising oestrogen concentrations in the late follicular phase feed back positively on the hypothalamic–pituitary axis, causing a massive release of luteinizing hormone (LH) (the LH ‘surge’) and a smaller release of FSH. The LH surge triggers the resumption of meiosis and follicle rupture with oocyte release. Oestrogen concentrations fall transiently prior to ovulation. The corpus luteum secretes both oestrogens and progesterone; plasma concentrations of both hormones peak in the mid-luteal phase falling in the late luteal phase as, in the absence of conception, the corpus luteum involutes. It is likely that ovarian proteins such as inhibin and activin also play a role in regulating follicle development and steroid synthesis. These proteins and other structurally similar ‘growth factors’ may exert important paracrine effects within the ovary.
Uterine changes
Rising oestrogen concentrations in the follicular phase induce proliferative changes in the endometrium, causing it to thicken. Oestrogens and progesterone (from the corpus luteum) induce secretory changes in the endometrium, preparing it for pregnancy. Once deprived of hormonal support from the corpus luteum, the endometrium is shed and another cycle begins.
Conception
Fertilization usually takes place in the ampullary part of the Fallopian tube, close to the ovary. Implantation occurs 6–7 days after fertilization. Human chorionic gonadotrophin (hCG) production from the conceptus acts to prolong the life of the corpus luteum so that it continues to produce progesterone, thus maintaining the endometrium.
HORMONES REGULATING REPRODUCTIVE FUNCTION
Gonadotrophin releasing hormone is a decapeptide secreted by neurons in the median eminence of the hypothalamus. It is carried in the portal hypophysial vessels from the hypothalamus to the pituitary, where it stimulates the release of LH and FSH. Concentrations of GnRH in the general circulation are very low but are assumed to be reflected by LH concentrations. The release of LH and FSH are pulsatile: the inter-pulse interval decreases from 90 to 60 min during the follicular phase until ovulation, then lengthens to once or twice a day in the late-luteal phase.
Pulsatile secretion of GnRH is essential to maintain normal function, as continuous infusion causes downregulation of receptors and amenorrhoea. Administration of GnRH, modified to prolong its action, is used to induce therapeutic hypogonadism.
Follicle stimulating hormone, LH and hCG are structurally similar glycoproteins. Each consists of an α- and a β-subunit, which are associated by non-covalent interactions. The α-subunits of FSH, LH and hCG (and of thyroid stimulating hormone, TSH) are identical, while the β-subunits differ, conferring hormonal specificity. The α-subunit consists of 92 amino acids. Gonadotrophins are largely metabolized in the liver; 10–15% is excreted unchanged in the urine.
Follicle stimulating hormone
The β-subunit of FSH contains 118 amino acids. Carbohydrate constitutes 22–25% of the dry weight of FSH and there are four branched, mannose-rich oligosaccharide side chains covalently bound to asparagine residues, two on the α-subunit and two on the β-subunit. The biological role of the carbohydrate is partly to protect the hormone against degradation. Follicle stimulating hormone is synthesized and secreted by gonadotrophic cells of the anterior pituitary, which also synthesize LH.
The primary target of FSH is the granulosa cell of the ovarian follicle. Follicle stimulating hormone stimulates differentiation of immature granulosa cells, induces cytochrome P450-dependent aromatase, induces LH receptors on granulosa cells and increases the binding of FSH by granulosa cells. The circulating half-life of FSH is ~ 4 h. Oestradiol amplifies the actions of FSH on granulosa cells.
Luteinizing hormone
The β-subunit of LH consists of 92 amino acids. Luteinizing hormone has three carbohydrate side chains, carbohydrate accounting for approximately 15% of the dry weight of the hormone. The circulatory half-life of LH is short (20 min), contributing to the pronounced pulsatility of plasma LH concentrations.
Ovarian LH receptors are found on theca cells and on mature granulosa cells. Luteinizing hormone stimulates production of androgens by the theca cells, and of oestradiol and progesterone by mature granulosa cells and corpus luteal cells. Luteinizing hormone regulates steroid biosynthesis by influencing uptake and side chain cleavage of cholesterol.
Human chorionic gonadotrophin
The β-subunit of hCG consists of 145 amino acids. The hormone is synthesized by the syncytiotrophoblastic cells of the placenta. It has a relatively long plasma half-life (24–36 h).
Human chorionic gonadotrophin interacts with the same receptors on luteal cells as does LH. It acts in the first trimester of pregnancy to maintain the corpus luteum and its ability to secrete progesterone, which supports the endometrium. After the first trimester, the placenta takes over the synthesis of progesterone from the corpus luteum. Human chorionic gonadotrophin is detectable in plasma eight days after conception and its concentration peaks around the tenth week of pregnancy.
Human chorionic gonadotrophin also stimulates testosterone synthesis in the testes of male fetuses, providing stimulation for male sexual differentiation.
Inhibin and activin
Inhibin is a glycoprotein that has been isolated from follicular fluid in two forms comprising a common α-subunit and one of two β-subunits, βA (in inhibin A) and βB (in inhibin B). Follicular fluid also contains two dimers of the inhibin β-subunit, which are called activin: activin A is the homodimer of the βA-subunit, activin A-B is the βAβB heterodimer.
Inhibin A and inhibin B have different patterns of circulation during the two phases of the ovarian cycle and play different physiological roles during follicular recruitment, maturation and ovulation. In the luteal phase, inhibin A suppresses FSH secretion. The concentration of inhibin A then decreases significantly as the corpus luteum involutes.
Development of the dominant follicle is characterized by secretion of increasingly large amounts of oestradiol and inhibin A into the circulation. There is evidence that the maintenance of the follicle is affected by intraovarian signalling, with inhibins and activins acting as important paracrine messengers.
During the menstrual cycle, plasma activin A concentrations vary in a biphasic manner, with the highest values occurring at mid-cycle during the luteo-follicular transition and nadirs occurring in the mid-follicular and mid-luteal phases.
Prolactin
Prolactin is a single chain polypeptide comprising 199 amino acids. It shares a high degree of homology with growth hormone and placental lactogen. Its most potent biological form (80–90%) is monomeric; 8–20% is dimeric and 1–5% is macroprolactin. The latter is a complex of monomeric prolactin and IgG and is immunoreactive (i.e. detected in most assay systems) but biologically relatively inactive.
Prolactin is synthesized by lactotroph cells of the anterior pituitary. Dopamine is the principal negative modulator of prolactin secretion; oestrogens and thyroid hormone releasing hormone increase prolactin release.
Prolactin has a variety of actions in vertebrates, playing roles in processes as diverse as osmoregulation and metamorphosis. It also has a direct but variable effect on follicular development and function. Prolactin is luteotrophic in some mammals but not in man. The only definite role of prolactin in women is the postpartum initiation and maintenance of milk production.
Macroprolactinaemia is a relatively common phenomenon in patients with hyperprolactinaemia in clinical practice; to ensure appropriate detection and management, all patients found to have significant hyperprolactinaemia should be screened for macroprolactinaemia (see Chapter 18).
Anti-Müllerian hormone
Anti-Müllerian hormone is a glycoprotein also known as Müllerian inhibitory factor (MIF) (see Chapter 21 for its role in normal and disordered sex development). It belongs to the transforming growth factor b family and is encoded on the short arm of chromosome 19. It acts through two receptors (AMHR1 and 2), which are present on the target organs in the gonads and Müllerian ducts.
Measurement of AMH is used clinically for assessment of ‘ovarian reserve’ and likely response to ovarian stimulation for subfertility. It has largely superseded measurement of basal FSH and inhibin B as a marker of follicular potential, particularly as the concentrations do not change with the menstrual cycle. It can also be used as a tumour marker for granulosa cell malignancies and appears to be an excellent marker for polycystic ovarian syndrome as these patients have significantly elevated concentrations.
REPRODUCTIVE STEROID HORMONES
Structure
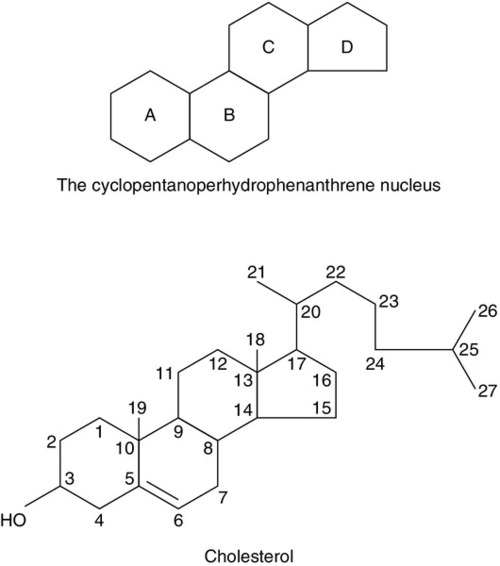
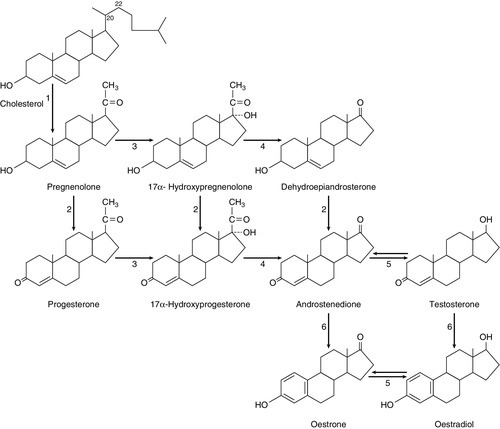
Ovarian steroid hormones are derivatives of cholesterol and contain the cyclopentanoperhydrophenanthrene nucleus (Fig. 22.4). Oestrogens (C-18 steroids), androgens (C-19 steroids) and progestogens (C-21 steroids) contain 18, 19 and 21 carbon atoms, respectively. These compounds and the pathways involved in their biosynthesis are illustrated in Figure 22.5.
FIGURE 22.5 Ovarian steroid biosynthetic pathways. The numbered arrows correspond to the description of biosynthetic enzymes in the text.
Biosynthetic enzymes
The numbers below refer to the enzymes and pathways labelled in Figure 22.5.
2. 3β-Hydroxysteroid dehydrogenase Δ4,5 isomerase. This catalyses both the 3β-hydroxysteroid dehydrogenation and isomerization of the double bond from ring B to ring A. Pregnenolone is thereby converted to progesterone.
3/4. 17α-Hydroxylase/17,20-desmolase. This catalyses the 17α-hydroxylation of pregnenolone and progesterone. The desmolase reaction involves the formation of a peroxide at C-20, epoxidation of the C-17 and C-20 carbons and side chain cleavage to form the C-17 oxosteroids dehydroepiandrosterone (from pregnenolone) and androstenedione (from progesterone). Both reactions are catalysed by the same enzyme. The intermediates are 17α-hydroxypregnenolone and 17α-hydroxyprogesterone, respectively.
5. 17-Oxosteroid reductase. This catalyses the conversion of a 17-oxosteroid to a 17β-hydroxysteroid and vice versa. Androstenedione and oestrone are converted to testosterone and oestradiol, respectively.
6. Aromatase. This converts C-19Δ4,3 oxosteroids to oestrogens by hydroxylation of the C-19 angular methyl group, oxidation and cleavage of the C-19 methyl group as formaldehyde, dehydrogenation of the A ring, and finally conversion of the 3-oxo group to a 3β-hydroxy group. The activity of this enzyme in granulosa cells is regulated by FSH.
Oestrogens are synthesized from androstenedione, the major pathway being through oestrone. Pregnenolone is converted to androstenedione either by the Δ4 pathway (through progesterone) or by the Δ5 pathway (through dehydroepiandrosterone).
The Δ4 pathway is favoured in granulosa/corpus luteal cells, while the Δ5 is favoured in theca cells.
The major secreted oestrogen is 17β-oestradiol; it is in equilibrium with oestrone in the circulation. Oestrone is further metabolized to oestriol, probably mostly in the liver. Oestradiol is the most potent oestrogen of the three and oestriol the least.
Steroid secretion through the menstrual cycle
Before ovulation the granulosa cells are not vascularized. The theca cells produce large quantities of androgens, which diffuse into the granulosa cells and are converted to oestrogens. This concept of cooperation between cell types is called the two-cell theory. Vascularization of the granulosa cells leads to increased progesterone secretion in the luteal phase. The stromal cells of the ovaries secrete small amounts of androgens. This secretion becomes more significant after the menopause when it adds to oestrogen concentrations through peripheral conversion in adipose tissue.
Steroid hormone transport and metabolism
Most of the circulating oestrogens and progesterone is protein bound, loosely to albumin and strongly to globulins: oestrogens are carried by SHBG and progesterone by cortisol-binding globulin. Degradation of both C-18 and C-21 steroids occurs in the liver and involves hydroxylation and water solubilization by conversion to glucuronide and sulphate conjugates. Water-soluble metabolites are excreted in urine and in bile. Quantitative urinary tests were used in the past to assess concentrations of oestrogens and progestogens, but have been superseded by rapid, sensitive and specific serum immunoassays. The major excreted oestrogen and progestogen are oestradiol and pregnanediol, respectively.
Actions of gonadal steroid hormones
Oestrogens
Oestrogens broadly maintain the functions of the reproductive tract, are responsible for the development of secondary sexual characteristics and affect sexual behaviour. Oestrogens cause myometrial hypertrophy, cause the endometrium to thicken in the follicular phase, promote secretion of large amounts of watery cervical mucus around the time of ovulation and maintain vaginal function; after bilateral oophorectomy or the menopause, the uterus and vagina shrink in size.
Oestrogens produce duct growth in the breasts and are responsible for breast enlargement at puberty. Oestrogens, like androgens, increase libido in humans, apparently by a direct action on hypothalamic neurons. In general, oestrogens reduce secretion of LH and FSH (negative feedback) but, in mid-cycle, oestrogens increase LH secretion (positive feedback). In addition to their reproductive role, oestrogens have important systemic effects: they maintain bone density and skin thickness and protect against atheromatous arterial disease in premenopausal females.
Synthetic oestrogen derivatives with agonist actions are used in contraceptive and hormone replacement preparations. Ethinyloestradiol is the most commonly used, particularly in contraceptives; it is potent and, unlike naturally occurring oestrogens, is active when given by mouth.
Anti-oestrogen preparations are also available: clomifene citrate and tamoxifen are non-steroidal triphenylethylene derivatives with mixed oestrogen agonist and antagonist actions. Clomifene citrate is used to induce ovulation, and tamoxifen to treat oestrogen-dependent breast cancers.
Progestogens
Progesterone causes secretory changes in endometrium already primed by oestrogen. It promotes the secretion of smaller amounts of thicker cervical mucus. It is essential for the maintenance of early pregnancy.
Synthetic steroids with progesterone agonist properties are used in contraception and hormone replacement therapy. Those commonly used tend to be derivatives either of 17α-hydroxyprogesterone or of 19-nortestosterone. Both types of derivative are used in combined oral contraceptive preparations: along with ethinyloestradiol, they prevent follicle growth, promote an endometrial reaction unfavourable to implantation and render cervical mucus thick and impenetrable to sperm.
Mifepristone is a derivative of norethisterone that blocks the actions of progesterone peripherally. It induces menstruation when given in the late luteal phase and induces abortion when given in pregnancy.
Androgens
The two main androgens in the female are testosterone and androstenedione. Dehydroepiandrosterone (DHEA) and its sulphate (DHEAS) are less important androgens. During the reproductive years, 90% of DHEA and DHEAS is synthesized by the adrenals and 10% by the ovaries. Androstenedione is derived equally from the ovaries and adrenals. Only 50% of testosterone production is glandular (roughly equal contributions from adrenals and ovaries), the remainder resulting from peripheral metabolism of weaker androgens, mostly in adipose tissue. A total of 99% of testosterone is bound and 1% is free: the great majority (78%) is bound to SHBG. This is a glycoprotein synthesized in the liver, with a carbohydrate content of 34%. It is a high-affinity, low-capacity binding protein. Plasma SHBG concentrations are raised when oestrogen concentrations are high (as in pregnancy) and in hyperthyroidism; concentrations are low in women treated with testosterone. Because SHBG-bound testosterone is relatively inert biologically, it may be useful for laboratories to measure both testosterone and SHBG, to give an indication of how much testosterone is not bound to SHBG (‘free androgen index’).
OLIGO- AND AMENORRHOEA
Oligomenorrhoea is defined as a menstrual cycle length of greater than six weeks but less than six months. Amenorrhoea is complete absence of menstruation or cycle length greater than six months. In these conditions, ovulation does not occur or is very infrequent. Women with oligo- or amenorrhoea may seek medical assistance because their bleeding pattern is abnormal, because of infertility, because of hirsutism/virilism or with a combination of these complaints.
The majority of patients with oligomenorrhoea have the polycystic ovary syndrome. The classic ‘polycystic ovary syndrome’ (PCOS) was described by Stein and Leventhal in 1935; they described an association between polycystic ovaries and oligo- and amenorrhoea in a small series of patients, most of whom were infertile or hirsute. It later became apparent that plasma concentrations of LH and/or androgens are elevated in many, but not all, women with polycystic ovaries; FSH concentrations are normal. However, it should be noted that around a quarter of women who have no gynaecological symptoms have also been found to have polycystic ovaries.
Polycystic ovary syndrome is a syndrome of ovarian dysfunction. It seems most likely that changes in the pattern of gonadotrophin secretion are responsible for the failure of antral follicles to grow and for a dominant follicle to emerge and ovulate (Fig. 22.2A). It is possible to induce follicular growth in patients with PCOS by therapeutic administration of FSH. It is important to recognize that polycystic ovaries and PCOS can occasionally occur as a secondary phenomenon in patients with other endocrine disorders. These include conditions such as congenital adrenal hyperplasia and Cushing syndrome, hyperprolactinaemia and acromegaly.
The revised diagnostic criteria of PCOS require any two of the following to be present:
• clinical and/or biochemical evidence of hyperandrogenism
• polycystic ovaries
and exclusion of other aetiologies (e.g. congenital adrenal hyperplasia, androgen-secreting tumours, Cushing syndrome).
The primary clinical indicator of androgen excess is the presence of hirsutism or acne. The sole presence of androgenic alopecia as an indicator of hyperandrogenism has been less well studied.
Few data are available on the value of routinely measuring androstenedione in hyperandrogenic patients, although it may be somewhat more elevated in patients with non-classic 21-hydroxylase deficiency congenital adrenal hyperplasia than in PCOS. Nonetheless, at present, the paucity of clinical data with androstenedione precludes its recommendation for the routine assessment of hyperandrogenism.
Weight loss can help to regularize menstrual periods with resumption of natural ovulation. If weight loss fails, clomifene, tamoxifen or gonadotrophins may be used to induce ovulation. Metformin may also improve ovulation rates when used with clomifene in patients resistant to clomifene alone.
Cyproterone acetate (an anti-androgenic progestogen) reduces hirsutism and is usually given as part of a combined oral contraceptive pill. The oestrogen component increases the plasma concentration of SHBG, which is often low in women with PCOS. The result is a decrease in the concentration of free testosterone, which also contributes to the reduction in hirsutism and acne. The treatment adopted depends on the desired outcome, for example whether this is to control acne, regularize menstruation or to achieve pregnancy.
Polycystic ovary syndrome is frequently associated with the metabolic syndrome of insulin resistance, hyperinsulinaemia and dyslipidaemia. This topic is discussed in detail in Chapter 15.
Hyperprolactinaemia is another important cause of oligo/amenorrhoea and acts by suppressing LH secretion and inhibiting ovulation. The normal range for plasma prolactin concentration in women is approximately 300–700 mIU/L, although this is dependent on the assay used. It is difficult to define a precise upper limit, but significant disease is unlikely unless the prolactin concentration is > 1000 mIU/L. The possibility that hyperprolactinaemia is secondary to drugs (e.g. opiates, methyldopa, antipsychotics, oestrogens and metoclopramide) must be excluded. Thyrotropin-releasing hormone (TRH) stimulates prolactin secretion, and therefore primary hypothyroidism can cause oligomenorrhoea. Patients found to have unexplained significant hyperprolactinaemia should have pituitary imaging to exclude a macroprolactinoma (a prolactin-secreting adenoma) or a non-functioning tumour (interfering with the normal dopamine suppression of prolactin secretion); the most frequent diagnoses resulting are idiopathic disease (no scan abnormality) or a microadenoma. (See Chapter 18 for further details.)
The medical management of hyperprolactinaemia is with a dopamine agonist. Bromocriptine has been used widely, but cabergoline, a longer acting preparation, is now usually preferred. It is better tolerated and more efficacious than bromocriptine but is associated with psychiatric adverse effects. Quinagolide is another longer acting preparation, and an alternative to cabergoline. This topic is discussed further in Chapter 18. Trans-sphenoidal surgery is an option for some patients.
Primary ovarian failure also causes amenorrhoea. It is diagnosed by the finding of a raised plasma FSH concentration (> 40 IU/L) and is irreversible. Other causes of oligomenorrhoea include androgen-secreting tumours and late onset congenital adrenal hyperplasia: these conditions are discussed later in this chapter, in the account of hirsutism and virilism.
INFERTILITY
Infertility is defined as failure to conceive after 12 months of regular sexual intercourse without contraception. The partner’s semen should be analysed to exclude male subfertility, but the results are often inconclusive; insemination of mature oocytes in vitro establishes the fertilizing capacity of semen more reliably.
Imaging techniques are of considerable importance in the investigation of infertility. Laparoscopy with hydrotubation, hysterosalpingography and hysterocontrast sonography all assess patency of the fallopian tubes, using direct vision, radiography and sonography respectively; intraperitoneal spillage of fluid injected through the cervix excludes tubal blockage. Transvaginal ultrasonography is used to assess ovarian morphology and to monitor ovulation.
Tests to predict ovulation are required in women who have long cycles (> 35 days). The LH surge that precedes follicle rupture can be detected by qualitative urine tests based on monoclonal antibodies. Commercially available point-of-care tests are very sensitive and are increasingly being used. Measuring serum progesterone concentration in the mid-luteal phase (i.e. seven days after time of expected ovulation) is a well-established method for assessing ovulation. Values in conception cycles have been found to range between 28 and 53 nmol/L; 30 nmol/L is generally taken as the lower limit of normal for mid-luteal phase progesterone. Blood sampling before or after the mid-luteal peak can give misleadingly low values. Sampling can be timed correctly by taking serial specimens and identifying the mid-luteal phase specimen retrospectively. It is the specimen taken seven days before the first day of the next menstrual period. (The classic day 21 sample will only be the mid-luteal phase if the woman has a regular 28-day cycle.) Progesterone is secreted in a pulsatile fashion during the luteal phase, concentrations varying by as much as a factor of three; when sampling coincides with a trough, the plasma progesterone concentration may appear abnormally low.
Basal plasma concentrations of FSH and LH are measured routinely in infertile women. A single specimen is usually taken during the early follicular phase on day 1–3 of the cycle. Interpretation of apparently abnormal early follicular phase gonadotrophin concentrations must take into account the pulsatile nature of release of both hormones. For LH, the pulse interval in the early follicular phase is 65–90 min, with peak and trough concentrations varying by as much as a factor of five. Plasma FSH concentrations do not fluctuate so widely – by a factor of two at the most. Elevated FSH concentrations in women with normal-length menstrual cycles indicate diminished fertility and possibly incipient ovarian failure. In patients undergoing assisted conception, early follicular phase plasma FSH concentrations are inversely related to the number of oocytes obtained and to the probability of conception. Measurement of AMH, however, is now preferred, as it gives a more accurate reflection of ovarian reserve and likely response to ovarian stimulation, and is not dependent on the day of the cycle. Measurement of early follicular phase LH is of less certain diagnostic value; concentrations tend to be abnormally high in patients with PCOS. Misleadingly high values are obtained when sampling coincides with a pulse peak. Basal concentrations of prolactin and thyroid hormones are unlikely to be of diagnostic value unless the length of the menstrual cycle is abnormal.
Increasingly, subfertility, whatever its cause, is treated using assisted conception techniques. Modern superovulation regimens promote the development of more than one follicle and include the use of gonadotrophin releasing hormone analogues, follicle stimulating hormone and human chorionic gonadotrophins.
Oocytes are collected by ultrasound-directed follicle aspiration. They are then either incubated with sperm (in vitro fertilization, IVF) or directly injected (intracytoplasmic sperm injection, ICSI). The resulting embryos are then transferred to the uterine cavity.
The major risk of superovulation regimens is ovarian hyperstimulation syndrome (OHSS). This generally occurs in the luteal phase or during early pregnancy and is triggered by either exogenous or endogenous hCG. Patients with polycystic ovaries, more than 20–25 follicles in both ovaries or with a high (> 6000 pmol/L) or rapidly rising oestradiol (doubling on consecutive days) prior to hCG administration are most at risk. Mild OHSS is common (20–30% of stimulated cycles) and leads to abdominal distension, enlarged ovaries, nausea and vomiting and is treated conservatively. Severe OHSS (< 2%), caused by increased vascular permeability and fluid shift out of the intravascular space, presents with ascites, increased blood viscosity, oliguria, thromboembolism and respiratory problems and can result in multi-organ failure. Biochemical changes include hyponatraemia from hypersecretion of antidiuretic hormone, hyperkalaemia, hypoalbuminaemia, abnormal liver enzymes and high plasma creatinine.
HIRSUTISM AND VIRILISM
Hirsutism means excessive hair growth in sites usually associated with male sexual maturity, that is, the face, lower abdomen (giving the appearance of a male escutcheon), anterior thigh, mid-chest and periareolar region. It can be associated with acne and oily skin. Virilism refers to more extreme manifestations of androgen exposure, including temporal hair recession, clitoromegaly, increased muscle mass, breast atrophy and deepening of the voice.
Dihydrotestosterone derived from testosterone acts on hair follicles to stimulate growth of terminal hair (long, coarse and pigmented) rather than vellus hair (short, fine and poorly pigmented). Once stimulated, the hair follicle remains responsive to low-dose weak androgen exposure because it has attained the ability to convert weak androgens to testosterone.
Polycystic ovary syndrome is responsible for hirsutism in a substantial proportion of patients. Rarer causes are discussed below. In many cases, however, no cause can be diagnosed; such hirsutism is termed idiopathic.
In idiopathic hirsutism, total plasma testosterone concentrations are normal, but subtle androgen disorders can generally be identified. Plasma androstenedione concentrations may be elevated and SHBG reduced; free testosterone concentration may be raised even though total testosterone is within normal limits.
The investigation of hirsutism should determine whether androgen concentrations are indeed elevated and to what degree, and rule out serious disease (particularly malignancy) of the ovaries or adrenals. Grossly elevated testosterone concentrations (> 5 nmol/L) and hirsutism of sudden onset (especially if accompanied by virilism) suggest malignancy.
Computed axial tomography (CT) or magnetic resonance imaging (MRI) is used to visualize adrenal tumours. Laboratory investigations used to evaluate hirsutism include measurements of serum cortisol and 17α-hydroxyprogesterone, in addition to testosterone, androstenedione and SHBG; steroid hormone concentrations following overnight dexamethasone suppression or ACTH stimulation may also be useful. Congenital adrenal hyperplasia is a recognized cause of hirsutism. The most frequent type is 21-hydroxylase deficiency, which results in elevated plasma concentrations of 17α-hydroxyprogesterone and increased synthesis of adrenal androgens. Testosterone concentrations are significantly elevated and ACTH stimulation is followed by an excessive increase in 17α-hydroxyprogesterone (see Appendix 22.1, below, for protocol). In Cushing syndrome, there is typically loss of the normal diurnal rhythm in plasma cortisol concentrations and failure of suppression in the overnight dexamethasone suppression test.
Although 90% of DHEAS is normally of adrenal origin, elevated DHEAS concentrations in hirsutism do not confirm an adrenal source of the excess: elevated DHEAS concentrations may also be found in polycystic ovary syndrome. However, a DHEAS concentration of more than twice the upper limit of normal does suggest the possibility of an adrenal tumour. Suppression of elevated androgen concentrations in hirsutism by dexamethasone might be expected to confirm an adrenal source; however, selective venous catheterization studies have demonstrated that elevated ovarian androgens can also be suppressed by dexamethasone in patients with hirsutism.
Once the rarer causes of hirsutism (Box 22.1) have been excluded, treatment consists of medication to suppress androgen secretion or counter its effects. Combined oral contraceptives and the anti-androgen cyproterone acetate are widely used. Medical treatment can be supplemented by cosmetic techniques such as shaving, waxing and electrolysis.
PREGNANCY
Introduction
Pregnancy is associated with marked hormonal changes in the maternal circulation that facilitate the metabolic, vascular and immunological adjustments necessary for the fetus to thrive. Abnormal concentrations of these hormones, or of other plasma constituents of fetoplacental origin, may indicate gestational pathology. The metabolic changes associated with pregnancy are relatively short lived and thus are rarely harmful to the healthy mother.
Prior to the widespread availability of high-resolution ultrasound scanning, the diagnosis of early pregnancy failure (such as spontaneous abortion and ectopic pregnancy), fetal malformation and fetal growth disturbances relied solely on clinical evaluation backed by tests on maternal blood. Although these indirect and often inaccurate methods have largely been replaced by ultrasound technology, biochemical assays still form an important part of the screening of many pregnancies.
Biochemical diagnosis of pregnancy
Human chorionic gonadotrophin
Human chorionic gonadotrophin (hCG) can be detected in maternal blood 7–9 days after conception (i.e. 22–24 days after the last menstrual period in women with regular 28-day cycles) and in urine 1–2 days later. The biochemical diagnosis of pregnancy is routinely made by a monoclonal antibody enzyme immunoassay that detects urinary hCG concentrations as low as 25 IU/L. The plasma concentration of hCG in normal pregnancy rises steeply to a peak at ten weeks of gestation and then falls and remains at a lower concentration for the remainder of pregnancy. This fall at the end of the first trimester is peculiar to hCG, as concentrations of the other chemical products of the fetoplacental unit rise with increasing length of gestation.
High sensitivity assays have shown that hCG is present in the plasma at low concentrations (< 5 IU/L) prior to conception. The biochemical diagnosis of pregnancy should only be made when plasma hCG concentration exceeds 25 IU/L or if a lower concentration doubles within two days. In cases where exogenous hCG has been given to induce ovulation, hCG estimations should be delayed by up to 14 days to allow clearance of the administered hCG.
The use of quantitative serum hCG estimations in the diagnosis of ectopic pregnancy is discussed below.
Apart from its place in the diagnosis of pregnancy, other well-established roles for hCG estimation are in the diagnosis and monitoring of gestational trophoblastic disease and as a marker for some tumours (see Chapter 42). The diagnosis of gestational trophoblastic disease is usually made ultrasonographically or histologically after an episode of vaginal bleeding in early pregnancy, though the condition may present after a term pregnancy when persistently high concentrations of hCG may help in making the diagnosis. After the uterus has been surgically evacuated, persistence or recurrence of the condition is monitored by serial quantitative hCG assays. The response of gestational trophoblastic disease to chemotherapy can be monitored in a similar fashion.
Human chorionic gonadotrophin measurement also plays a part in screening for Down syndrome. This topic is discussed on p. 443.
Diagnosis of ectopic pregnancy
Ectopic pregnancy poses a considerable hazard to the mother (95% are tubal: a ruptured tubal pregnancy can cause severe intra-abdominal bleeding), so that accurate diagnosis of this condition is essential. The symptoms and signs are notoriously variable and definitive diagnosis frequently depends on laparoscopy. However, use of additional investigations, such as measurement of hCG concentration, may help to reduce the number of ‘unnecessary’ laparoscopies and allow medical or conservative treatment.
Transvaginal ultrasound enables the diagnosis of intrauterine pregnancy at a time when the plasma hCG concentration is around 1000 IU/L. The absence of a visible embryonic sac within the uterus in these circumstances makes an ectopic pregnancy the likely diagnosis. The rate of increase in plasma hCG concentrations may provide useful additional information. Early in a normal pregnancy, these usually double every two days, while the rise in ectopic pregnancy is, in general, less. Progesterone measurements have also been used in the investigation of possible ectopic pregnancy. A plasma progesterone concentration of > 25 nmol/L is suggestive of a viable intrauterine pregnancy; however, recent guidelines from the National Institute for Health and Care Excellence (NICE) in the UK suggest that progesterone measurement should no longer be used in this context. Low or falling concentrations of progesterone and hCG may allow expectant management if symptoms are minimal, as this suggests that the pregnancy is failing and will resolve spontaneously. Surgery can also be avoided in patients with hCG < 5000 IU/L and minimal symptoms, as they can be treated with the folate antagonist, methotrexate, as long as hCG monitoring is available. Pain and haemodynamic instability are indications for laparoscopy.
In clinical practice, the diagnosis of ectopic pregnancy depends on a high index of suspicion, measurement of serum hCG concentration, pelvic ultrasound and, when indicated, laparoscopy.
Biochemical monitoring of pregnancy
Spontaneous abortion
Many studies have reported low concentrations of various hormones and placental proteins in patients with early spontaneous abortion. However, these studies have often been performed after the onset of bleeding and their findings may have been the result, rather than the cause, of the pregnancy failure. Plasma progesterone concentrations have, for example, been reported to be low in these circumstances, but administration of progestogens to women with threatened abortions has not been shown to prevent pregnancy loss.
Screening for fetal malformation
Prior to the widespread availability of high-resolution ultrasound scanners, the antenatal detection of fetal malformation was based on maternal biochemical screening. To some extent, ultrasound has made this screening less important, but these tests may focus attention on the high-risk pregnancy so that an appropriate diagnostic procedure can be performed.
Biochemical screening
Screening for Down syndrome (trisomy 21) in the past has depended entirely on maternal age and past history of chromosomal abnormalities in offspring and has resulted in some form of invasive tissue sampling (chorionic villus sampling or amniocentesis) being offered to essentially all pregnant women over a locally agreed age, usually 35 years. (The risk of a Down syndrome pregnancy is 1:1528 at maternal age 20, 1:1351 at 25, 1:909 at 30, 1:384 at 35 and 1:112 at age 40.) This approach was unsatisfactory because, although the risk of trisomy 21 increases with maternal age, 70% of Down infants are born to women below the age of 35. In addition, many women over 35 decline the offer of invasive tissue sampling because of the risk of miscarriage.
The observation in 1984 that maternal plasma α-fetoprotein (AFP) concentrations were lower in the presence of fetal Down syndrome than in unaffected pregnancies, led to its use in screening for this condition. Subsequently, maternal plasma concentrations of hCG were found to be elevated and concentrations of unconjugated oestriol decreased when the fetus had Down syndrome. The assay of these substances in maternal plasma at around 16 weeks of gestation has become known as the ‘triple test’ and, in combination with maternal age and weight, gives a risk score of the fetus having Down syndrome.
The chosen cut-off for a ‘positive’ screen will govern the detection and false positive rates. A commonly chosen cut-off is a triple test result representing a risk of 1 in 250 or higher, corresponding approximately with the risk for a maternal age of 35 years alone. At this cut-off, there is a detection rate of 61% and a false positive rate of 5%.
Maternal biochemical screening for Down syndrome is not diagnostic of the condition, but allows a better selection of a high-risk group (to whom diagnostic amniocentesis may be offered) than use of maternal age alone. However, screening does not detect other, more serious trisomies, for which older women are also at increased risk. The detection and analysis of free fetal DNA in the maternal plasma may, in future, obviate the need for invasive testing and reduce the risk of fetal loss.
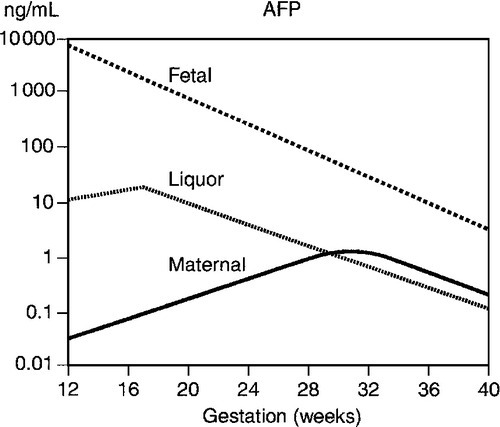
α-Fetoprotein is one of the two major proteins in the fetal circulation. The AFP concentration in fetal plasma greatly exceeds that in amniotic fluid and maternal plasma. Thus, even minor leakages from the conceptus result in readily detectable rises in maternal plasma AFP concentrations. This formed the basis of maternal screening in the second trimester for a range of fetal malformations, e.g. neural tube defects, but these are now reliably diagnosed by ultrasound. Several situations can give rise to false positive results, the most important of which is an incorrect estimation of gestational age, as maternal plasma AFP rises steeply at 16–20 weeks (Fig. 22.6). Multiple pregnancies are also associated with higher concentrations.
FIGURE 22.6 Changes in α-fetoprotein (AFP) concentration with gestation in maternal and fetal plasma and amniotic fluid liquor.
Ultrasound
While ultrasound examination allows the diagnosis of the majority of significant structural fetal anomalies, most genetic disorders cannot be diagnosed by this means and require tissue sampling techniques to identify an affected fetus. The majority of these procedures are performed for fetal karyotyping, but despite the increasing use of tissue sampling techniques such as amniocentesis and chorionic villus sampling (CVS) over the last 20–30 years, there has not been a significant reduction in the birth incidence of infants with abnormal karyotypes.
Current screening practice
An important advance in the antenatal diagnosis of Down syndrome was the recognition of the nuchal translucency (NT) sign. This is the appearance on ultrasound of increased fluid at the back of the fetal neck in affected fetuses and can be detected late in the first trimester. Its specificity and sensitivity are enhanced if it is combined with other investigations such as measurement of hCG and pregnancy-associated plasma protein A (PAPP-A; the ‘combined test’). Pregnancy-associated plasma protein A is a zinc metalloproteinase that acts on insulin-like growth factor binding protein 4, decreasing its affinity for IGF-1 and IGF-2, and thus regulating IGF activity in certain tissues. The combined test detects 90% of affected pregnancies with a screen positive rate of 2%, if a cut-off risk of 1:150 is chosen.
For women presenting only in the second trimester, the recommended screening tests are either the ‘triple test’, involving measurement of hCG, AFP and oestriol or the ‘quadruple test’ – the triple test with the addition of inhibin A. Other available tests are: the ‘integrated test’ (NT and serum measurements) or, if NT is not available, measurements on maternal serum alone (‘the serum integrated test’) (see Box 22.2). The integrated test (if good quality NT measurement is available) performs better than the combined test but is more difficult to administer. The serum integrated test performs less well.
Women undergoing screening should appreciate that positive tests require confirmation (diagnosis) by an invasive procedure: before 12 weeks of gestation, this is by chorionic villus sampling, thereafter by amniocentesis.
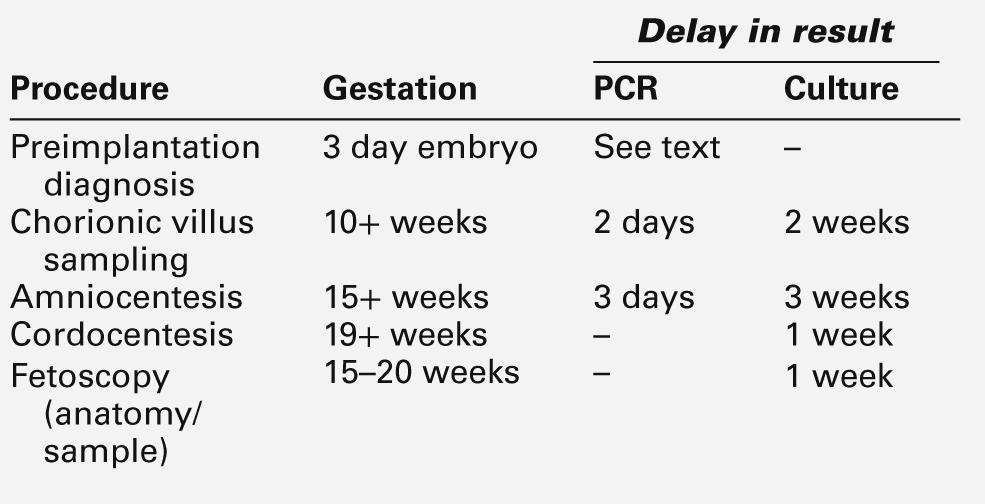
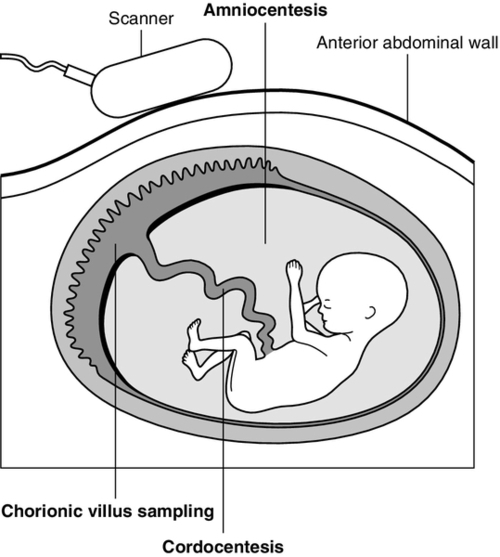
Fetal tissue sampling techniques
The prenatal diagnosis of many chromosomal and genetic abnormalities requires tissue sampling. Various techniques are available (see Table 22.1 and Fig. 22.7) but, with few exceptions, if a diagnostic test is available for the condition under investigation, any of the methods of sampling may be employed. The differences between the techniques are primarily the gestation at which the test may be performed and the delay between sampling and the result. The list of inherited disorders that can be diagnosed by DNA studies is growing rapidly. However, in many cases, these conditions are not due to single gene abnormalities, or disease-specific gene probes are not available, and family studies may be necessary to evaluate individual cases to assess their suitability for prenatal diagnosis. In vitro fertilization has also opened up the possibility of preimplantation genetic diagnosis from one or two cell biopsy of the embryo, using comparative genomic hybridization or single nucleotide polymorphism arrays. It is beyond the scope of this book to discuss fetal tissue sampling techniques in detail, and this section only summarizes the essential features.
Chorionic villus sampling
As the fetus and the placenta both develop from the same early blastocyst, their genetic make-up is identical in the vast majority of cases. Thus, chromosomal and DNA analysis of the placenta (chorionic villi) will provide information about the fetus. The post-procedure spontaneous miscarriage rates for transabdominal and transcervical CVS are comparable at approximately 2.5%, not significantly different from the background rate of loss. Potential sources of diagnostic inaccuracy from CVS are maternal cell contamination and placental mosaicism.
Amniocentesis
Traditionally, amniocentesis was usually performed at 15–16 weeks of gestation, but it can be performed earlier. Results of cell culture become available 2–4 weeks later (though a preliminary result may be available within hours from direct examination); the associated fetal loss rate is approximately 1%. Amniocentesis can be used for biochemical assessment of the liquor for fetal lung maturity (now rarely used in the UK). The production of the surfactants lecithin and sphingomyelin by the fetal lung are almost equal until ~ 32 weeks of gestation, after which the lecithin concentration increases more rapidly than that of sphingomyelin. A lecithin–sphingomyelin ratio of > 2 indicates a low risk of the baby developing respiratory distress syndrome after birth. Amniocentesis can also be used for the monitoring of haemolytic disease of the fetus by determining the Rhesus genotype on uncultured amniocytes and measuring the optical density of the liquor at 450 nm as an indication of bilirubin concentration.
Cordocentesis
Fetal blood can be obtained from 19 weeks of gestation by cordocentesis, an outpatient procedure that does not require maternal sedation or the use of fetal paralysis. After visualization of the insertion of the umbilical cord into the placenta by real-time ultrasound, a needle is passed into the umbilical cord through the (locally anaesthetized) maternal abdomen. The fetal loss rate after cordocentesis is approximately 1%.
Monitoring of maternal and fetal well-being
The increased accuracy and availability of ultrasound-based biophysical tests of fetal well-being has resulted in a marked decline in the use of biochemical tests, except for a few clinical situations, for example serum uric acid measurements in a patient with pre-eclampsia, and ‘liver function’ tests and serum bile acid measurements in women with obstetric cholestasis.
Pre-eclampsia is the occurrence of proteinuria and hypertension in pregnancy; peripheral oedema is often present. Untreated, it may progress to life-threatening severe hypertension, renal failure and convulsions (eclampsia) or be complicated by the haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. An early biochemical feature of pre-eclampsia is an increase in plasma urate concentrations, as a result of increased renal tubular reabsorption of urate secondary to decreased renal perfusion. However, this is an unreliable indicator, as urate concentrations rise in the last trimester of normal pregnancies.
Cholestasis of pregnancy is discussed in more detail in Chapter 14. The term includes acute fatty liver of pregnancy (of which hyperuricaemia is also a feature) and HELLP syndrome.
Intrapartum fetal monitoring
The widespread use of fetal heart rate (FHR) monitoring in labour resulted in an increase in the number of operative deliveries, frequently delivering a neonate without any sign of asphyxia. As a result, fetal scalp blood sampling was introduced in order to improve the specificity of FHR monitoring alone. A variety of studies have demonstrated that, when interpreting FHR patterns, variability and accelerations are the features that correlate most closely with fetal scalp pH or lactate concentration. The presence of normal variability or accelerations is indicative of normal oxygenation, whereas the presence of reduced variability or decelerations makes poor oxygenation and hence acidosis, more likely. Although there is good evidence that continuous FHR monitoring can detect a low fetal pH, acidosis, especially in well-grown term fetuses, does not correlate well with fetal death or cerebral injury. Indeed, a number of studies suggest that only 8–15% of cases of cerebral palsy are associated with events occurring during labour and, even in these, the link is not proven as causal.
Biochemical changes during pregnancy
Pregnancy is a major endocrine event in the female lifespan, involving wide-ranging and often considerable changes in the metabolism of various hormones. Concentrations of oestrogens, progesterone, testosterone and prolactin all increase steadily through gestation, while those of LH and FSH decrease. Sharp increases in the concentrations of hormone-binding globulins such as SHBG are seen, reaching a plateau by the end of the first trimester. In addition, placental hormones such as hCG and human placental lactogen (hPL) appear in the maternal circulation within the first few weeks following conception. Pregnancy does not usually alter thyroid function to a significant extent, but changes in individual indices of thyroid function are often seen, such as increases in plasma concentrations of total thyroxine (T4) secondary to an increase in thyroxine binding globulin. Free T4 and free tri-iodothyronine (fT3) concentrations may rise slightly in early pregnancy (as a result of the thyrotrophic action of hCG), but later fall to within, or just below, the non-pregnant reference ranges. Thyroid stimulating hormone concentrations tend to fall in early pregnancy (sometimes to undetectable concentrations), but later return to normal.
Associated with the changes in gonadotrophins and steroid hormones, pregnancy affects several metabolic processes in various tissues and organs, in turn altering the concentrations of many maternal plasma constituents. In some instances, these changes are apparently unrelated to the needs of mother or fetus. Because the use of standard reference ranges is often inappropriate during pregnancy, an appreciation of the metabolic effects of pregnancy is desirable.
An important consideration when interpreting the effects of pregnancy on blood components is the associated change in plasma volume. Total body water increases by about 7 L during pregnancy, with an increase in extracellular fluid being responsible for at least half of this increase. Marked alterations in renal haemodynamics accompany this increase in extracellular fluid. Both glomerular filtration rate (GFR) and renal plasma flow increase by up to 50% above pre-pregnancy values, changes that are compensated for by changes in tubular reabsorption. Plasma creatinine and urea concentrations decrease during pregnancy, and increases in creatinine clearance (paralleling the increase in GFR) become apparent four weeks after conception, peaking 9–11 weeks later. Renal function may deteriorate markedly during pregnancy in women with pre-existing renal disease or a history of pre-eclampsia in a previous pregnancy, and monitoring of plasma urate and creatinine concentrations is advisable.
Plasma proteins
Pregnancy induces widespread changes in plasma protein concentrations, owing both to the effect of changes in plasma volume and to specific hormone-induced changes in protein synthesis and degradation. In general, total protein concentrations are slightly reduced, with those of albumin falling by approximately 15% and those of globulins (especially acute phase proteins) often increasing. Two to three-fold increases in the concentrations of α1-antitrypsin, fibrinogen and caeruloplasmin are often seen during the later stages of gestation. Prealbumin is a notable exception as plasma concentrations fall during pregnancy. Plasma immunoglobulin concentrations are rarely affected by pregnancy.
Pregnancy may induce slight increases in the plasma activity of aminotransferases such as aspartate aminotransferase. Substantial increases in alkaline phosphatase activities occur, reaching a peak of 2–3 times normal adult values during the third trimester. This increase is largely due to the presence of the placental isoenzyme in maternal plasma. Although changes in the fetal isoenzyme have been related to problems of fetal development, this has proved of little diagnostic use owing to the wide range of isoenzyme activities seen in normal pregnancy.
Plasma lipids and lipoproteins
The hyperlipidaemia of pregnancy is well recognized. By the third trimester, fasting plasma triglyceride concentrations are increased 2–3 times, with lesser increases being seen in total cholesterol, phospholipids and non-esterified fatty acids. These increases result from a complicated sequence of changes in the major plasma lipoprotein classes, low density lipoproteins (LDL) and high density lipoproteins (HDL). LDL concentrations rise steadily, but level off during the last ten weeks of pregnancy, whereas those of HDL initially increase, but level off (and may even fall) approximately half way through pregnancy; this may be a result of the onset of the insulin resistance that is typical of pregnancy. Serial measurement of hormones during gestation indicates that the hyperlipidaemia of pregnancy is influenced not only by oestradiol and progesterone, but also by insulin and hPL.
Glucose tolerance
Fasting plasma glucose concentrations usually fall slightly during pregnancy, whereas postprandial concentrations increase. Pregnancy may lead to a deterioration in glucose tolerance, which is subtle in the majority of women, probably due to insulin resistance induced by elevated concentrations of sex hormones. In patients with pre-existing type 1 diabetes mellitus, this may result in an increased requirement for exogenous insulin. The insulin requirement should thus be monitored closely (by regular measurements of blood glucose and glycated haemoglobin) in order to reduce the incidence of fetal malformation, macrosomia and intrauterine death, conditions that are all associated with poor glycaemic control.
The precise nature of the defect in gestational glucose intolerance remains controversial. Patients who are initially free from diabetes but develop gestational diabetes may either revert to normal glucose tolerance after pregnancy (in which case they remain at risk of developing diabetes in the future) or remain frankly diabetic. Although screening for gestational diabetes has been advocated (based on factors such as obesity, strong family history of diabetes mellitus or previous delivery of a baby weighing 4 kg or more), most ‘at-risk’ patients do not develop glucose intolerance and those that do often have low risk factor scores. The presence of glycosuria is similarly of little use, as the majority of pregnant women will have glycosuria at some stage of their pregnancy owing to their lowered renal threshold, rather than glucose intolerance per se.
Consequently, routine measurement of blood glucose has been advocated in all pregnancies. While this will indeed detect patients with gestational glucose intolerance, controversy exists concerning the indications for subsequent formal glucose tolerance testing, as well as the stage of gestation at which such testing should take place. In addition, there is no agreement as to what constitutes an abnormal tolerance test profile, as the World Health Organization (WHO) criteria are based on non-pregnant individuals and other criteria are purely statistical (e.g. values more than two standard deviations above the mean), rather than relating to the outcome of pregnancy. In the UK, the National Institute for Health and Care Excellence (NICE) has produced guidelines, including for the selection of patients for formal glucose tolerance testing. Gestational diabetes, and the management of maternal diabetes during pregnancy are discussed in Chapters 15 and 16.
Other changes
Mild proteinuria may accompany the changes in plasma protein concentrations seen during pregnancy. However, this may be an early warning of a threat to mother and fetus if it develops into pre-eclampsia or eclampsia, where increasing proteinuria is associated with maternal hypertension. In the past, serial quantitative assessments of proteinuria using dipsticks were used. Detection of significant proteinuria is now more usually carried out by measuring a protein to creatinine ratio in a random urine sample. A value of < 30 g/mol helps to rule out significant proteinuria in hypertensive pregnant women. However, severity of the disease is not always related to absolute values.
Labour
The factors that lead to the onset of labour in humans appear to be complex, with a combination of biochemical and physical changes such as uterine stretch. In the first trimester, progesterone from the corpus luteum maintains uterine quiescence until the placenta takes over. In many species, it is falling concentrations of progesterone that initiate labour. This is not the case in humans, although inhibiting progesterone action does increase uterine activity and ripens the cervix. The uterus is primed for activity by a gradual rise in plasma oestrogen concentration and corticotrophin-releasing hormone (CRH) over a period of many weeks. The uterus then becomes sensitive to stimulants including oxytocics, prostaglandins and CRH. These appear to create a biochemical inflammatory-like reaction in the uterus.
Prostaglandins and oxytocin play central roles in human labour by stimulating myometrial contractility. Arachidonic acid is found in amniotic fluid and fetal membranes. This is converted by cyclooxygenase to prostaglandins. Clinical use of this process is seen with digital ‘sweeping of the membranes’ to enhance enzyme activity prior to therapeutic induction of labour, for which slow-release prostaglandin formulations are commonly used.
Oxytocin is a nonapeptide secreted by the posterior pituitary. Administration of oxytocin in pregnancy provokes uterine contractions and is used in the induction and acceleration of labour. Its exact role in the initiation and maintenance of normal human labour is not fully understood, particularly since normal parturition has occurred in women with oxytocin deficiency secondary to pituitary disease.
Corticotrophin-releasing hormone is a 41 amino acid peptide produced in the hypothalamus but in pregnancy it is also synthesized in the placenta and membranes. The concentration of free CRH rises markedly in the last three weeks of pregnancy. It promotes fetal cortisol production and prostaglandin production in the fetal membranes.
Pre-term labour (onset prior to 37 completed weeks of pregnancy) is the commonest cause of later disability and perinatal death. A major contributor to neonatal morbidity is lung dysfunction owing to lack of alveolar surfactant. Corticosteroid administration to the mother at least 24 h before delivery substantially improves outcome. Diagnosis of the onset of pre-term labour can be difficult but important, as tocolytics may delay delivery long enough for steroids to have an effect. An adjunct to assessing the likelihood of the onset of labour is the use of fetal fibronectin measurement. Fibronectins are large (450 kD) glycoproteins found in the cervical tissue, membranes and amniotic fluid. They act as an adhesive between the cells of the membranes and uterine lining and ‘leak’ out into the vagina if this is disrupted, as happens in pre-term labour. A bedside monoclonal antibody swab test for fibronectin is now available, which is poor at predicting the onset of labour but has a negative predictive value of 99% that delivery will not occur within 14 days. Its use substantially reduces the need and cost for treatment or transfer of patients.
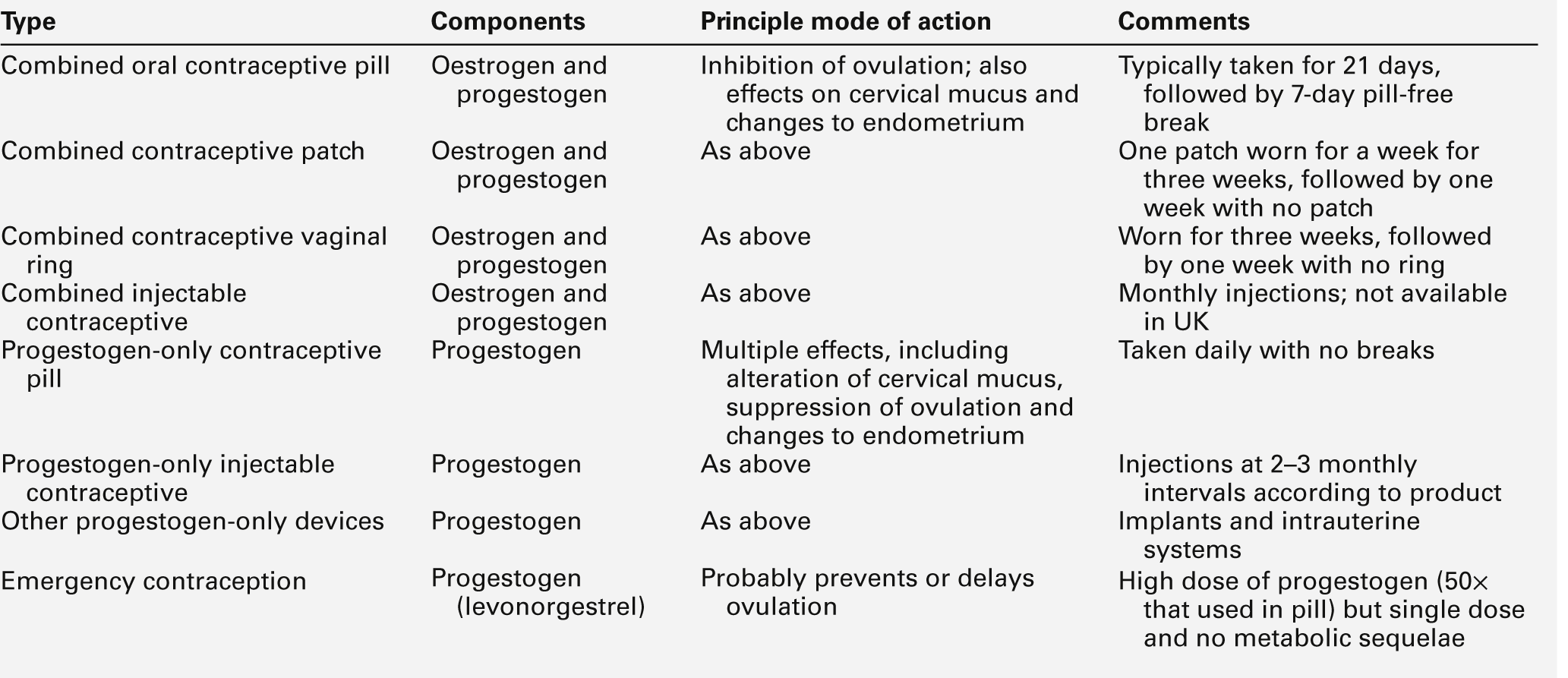
ORAL CONTRACEPTION AND HORMONE REPLACEMENT THERAPY
Introduction
Worldwide, many women are taking gonadal steroids (oestrogens and progestogens) as oral contraceptives (OCs) or sex hormone replacement therapy (HRT). Gonadal steroids have the potential to influence many metabolic systems and hence biochemical variables in the blood.
Methods for contraception can be divided into hormonal and non-hormonal methods. The latter include sterilization, barrier methods, the use of spermicides and natural methods. These do not have metabolic effects (although development of antisperm antibodies can affect the success of vasectomy reversal). Hormonal methods involve treatment of the woman (usually) with hormones to prevent conception, and have the potential to cause secondary metabolic disturbances.
Hormonal methods of contraception are summarized in Table 22.2. The most widely used are combined oral preparations of oestrogen and progestogen. Both components can have metabolic effects, in particular affecting lipid and glucose metabolism. Older combined oral contraceptives used relatively high doses of oestrogen, which could increase the concentration of thyroxine binding globulin, and hence increase total T4 (but not free T4) concentrations, but this is not a significant problem with modern combined oral contraceptives, in which lower doses of oestrogens are used.
There is an important difference between the oestrogen used in OCs and that used in HRT. If suppression of ovulation is required, a synthetic oestrogen (almost always ethinyloestradiol) is necessary. In contrast, HRT requires lower doses of oestrogen, as the objective is to reverse the adverse effects of oestrogen deficiency. For this purpose, ‘natural’ (non-alkylated) oestrogens such as 17β-oestradiol, oestradiol valerate and conjugated equine oestrogens have proved adequate, despite their ineffectiveness as contraceptive agents.
Contraceptive progestogens are used in order to increase contraceptive efficacy in combined regimens, on their own in the ‘mini-pill’ or to control menstrual bleeding. In contrast, HRT progestogens are used to prevent endometrial proliferation, hyperplasia and neoplasia. Again, the net dose of progestogen used in HRT is lower than that required for OC. Because natural progesterone is poorly absorbed when given orally, various synthetic progestogens have been developed. In the UK, the progestogens most commonly used in OC are levonorgestrel, norethisterone, gestodene and desogestrel, the latter two being novel derivatives chosen for their relatively low androgenicity. For postmenopausal HRT, levonorgestrel and norethisterone are most commonly used.
The majority of oral gonadal steroid therapies in current use combine an oestrogen with a progestogen. Progestogen-only oral contraceptives use very low doses of steroids and have comparatively little effect on plasma components.
Metabolic effects of oestrogens
Oestrogens suppress the secretion of FSH by the pituitary and hence suppress follicular maturation. The oestrogen in most combined oral contraceptives is ethinyloestradiol, at a daily dose between 15 and 50 μg, but typically 30–35 μg. Oestrogens tend to increase plasma triglyceride concentrations, reduce LDL-cholesterol and increase HDL-cholesterol concentrations. Oestrogens are prothrombotic, but their net effects on lipid metabolism tend to be anti-atherogenic. They have a negligible effect on glucose tolerance.
Metabolic effects of progestogens
The contraceptive effect of progestogens is related to suppression of LH secretion (tending to suppress ovulation) and to effects on cervical mucus that make it hostile to sperm, and on the endometrium that reduces the likelihood of successful implantation. Several different progestogens are used in combined oral contraceptives; they all lack the 19-methyl group that is present in progesterone and differ mainly (but not exclusively) in their C-17 substituents, whether or not they have a C-21 carbon and in the presence or absence of a methyl or ethyl group in the C-18 position. They include norethindrone, one of the first to become available for this purpose; norgestrel and levonorgestrel (‘second-generation’ progestogens), and the ‘third generation’ progestogens, desogestrel, gestodene and norgestimate. The metabolic effects of progestogens are largely dependent on their androgenicity. Levonorgestrel, gestodene, norgestimate and desogestrel (all 19-nor compounds, that is, lacking a 19-methyl group) have androgenic as well as progestogenic activity, and tend to increase plasma LDL-cholesterol and reduce HDL-cholesterol concentrations. The effects of progestogens on glucose tolerance are considered below.
Metabolic effects of contraceptives
Effects of hormonal contraceptives on lipid metabolism and risk of vascular disease
The overall effect of combined oral contraceptives on lipid metabolism depends on the type and dose of oestrogen and progestogen and on the presence of any genetic tendency to hyperlipidaemia. In general, the effects of the oestrogen and progestogen tend to cancel each other out, though most modern agents tend slightly to reduce plasma LDL-cholesterol and increase HDL-cholesterol concentrations. They may also slightly increase triglyceride concentrations. Although it is not necessary to monitor plasma lipid concentrations in most women, this may be appropriate if there is known hyperlipidaemia, especially hypertriglyceridaemia.
It is noteworthy that in women who do not smoke cigarettes and who are normotensive, there is no evidence of an increase in the relative risk of cardiovascular disease associated with long-term use of combined oral contraceptives. Even in women who smoke or are hypertensive, the absolute risk, though higher, remains very low. The relative risk of ischaemic stroke is slightly increased, but the absolute risk remains very low. The risk of haemorrhagic stroke is unaffected in women below the age of 35, but slightly increased in older women.
The relative risk of venous thromboembolic disease is increased in users of combined oral contraceptives, although the absolute risk remains very low, and is certainly lower than that associated with pregnancy. The mechanisms involved are complex, but appear primarily to be the result of prothrombotic effects on the coagulation mechanism.
Contraindications to the use of the combined oral contraceptives include a personal history of stroke, myocardial infarction or venous thromboembolism, severe hypertension and heavy cigarette smoking.
There is no evidence of increased risk of venous thrombosis, cardiovascular or cerebrovascular disease in women using progestogen-only contraceptives.
Effects of oral contraceptives on glucose homoeostasis and diabetes
Any adverse effect of oral contraceptives on glucose tolerance appears to be related to the progestogen component. Progestogens antagonize the effects of insulin and increase the area under the curve of insulin response to oral glucose tolerance tests, but the effects on glucose tolerance, though statistically significant, are not clinically so.
There is no need to avoid combined preparations in patients with uncomplicated diabetes. Progestogen-only oral contraceptives are safe in patients with type 1 diabetes and can be recommended in many patients for whom combined products are contraindicated (e.g. heavy smokers, severe hypertension). However, longer-acting progestogens, such as levonorgestrel, should be avoided in patients with type 2 diabetes.
Other metabolic effects of oral contraceptives
Hypertension may be exacerbated in OC users owing to an increase in the synthesis of angiotensinogen. However, most oral contraceptive users adapt their vasoactive controls and aldosterone production to compensate for the increase in blood pressure, and there is a poor correlation between the metabolic changes and actual changes in blood pressure.
Metabolic effects of injectable contraceptives
The metabolic effects of combined oral contraceptives in which the hormones are delivered other than orally are broadly similar to those seen with oral agents.
The metabolic effects of injectable progestogen contraceptives are minimal, with the exception that there is concern that depot medroxyprogesterone acetate may have an adverse effect on bone mineral density, probably related to a reduction in plasma oestradiol concentrations. The decrease in density appears reversible but whether there is still a later increased risk of osteoporosis is unknown and thus prolonged use, or use in women with other risk factors for osteoporosis, should be avoided. There is no evidence of significant adverse metabolic effects with progestogen-only implants or intrauterine delivery devices.
Although emergency contraceptives contain high doses of progestogens, exposure to the agent (levonorgestrel) is short-lived and has no adverse metabolic consequences.
Hormone replacement therapy
The primary aim of hormone replacement therapy in postmenopausal women is usually to reduce the clinical features of oestrogen deficiency, which include hot flushes, depression, vaginal dryness and consequent dyspareunia. Particularly in women who undergo a premature menopause, a secondary aim may be to reduce the risk of long-term consequences of oestrogen deficiency, particularly osteoporosis. In seeking to achieve these aims, it is clearly also important to avoid potentially harmful consequences.
Although menopausal symptoms are caused by oestrogen deficiency, oestrogen replacement should be accompanied by cyclical or continuous progestogen replacement in order to prevent endometrial hyperplasia: there is a clear association between the use of unopposed oestrogen treatment and the development of endometrial carcinoma. Women who have undergone hysterectomy can safely be treated with oestrogen alone.
Metabolic effects of the menopause
The onset of the menopause is accompanied by an increase in the plasma concentrations of LDL-cholesterol and triglycerides; HDL concentrations fall slightly, but with a rise in the HDL3 fraction and a fall in the anti-atherogenic HDL2 fraction. These changes are all pro-atherogenic and are thought to be important factors in the increase in the prevalence of cardiovascular disease that occurs following the menopause.
Pancreatic insulin secretion tends to decline and, although there may be an increase in the half-life of insulin in the plasma, insulin sensitivity, and hence glucose tolerance, tend to decline. The distribution of body fat tends towards android rather than gynoid. Increases occur in the plasma concentrations of proteins involved in blood coagulation, including fibrinogen and antithrombin III, and there is a reduction in fibrinolytic activity. All these factors are likely to contribute to the increased risk of cardiovascular disease against a background of the increasing risk associated with advancing age.
Metabolic effects of HRT
The metabolic effects of HRT depend upon the steroids used, their doses and the route of administration, but in summary, oestrogen replacement tends to reduce LDL-cholesterol concentrations. Oestrogens also increase the clearance of remnant particles and reduce the concentration of lipoprotein(a). Although, when used alone, oestrogen replacement may increase HDL concentration, the effects of progestogens may counter this, depending on the androgenicity of the progestogen used. Non-androgenic agents may have little effect or even contribute to an increase in HDL-cholesterol. Conjugated equine oestrogens tend to increase plasma triglyceride concentrations, but oral oestradiol has a lesser effect and transdermal oestradiol reduces triglycerides.
Adverse consequences of hormone replacement therapy
Cyclical, combined oestrogen and progestogen replacement restores cyclical bleeding in the majority of women and is a common cause for them to abandon HRT. However, there are also possible long-term consequences. The most important of these include a small but significant increased in the risk of venous thromboembolism. There is no increase in the risk of endometrial cancer or ovarian cancer. The risk of breast cancer does increase in women who use combined HRT for more than five years, although only very slightly, but the effect declines after cessation of HRT and almost disappears after a further five years.
Hormone replacement therapy and heart disease
The increase in the risk of coronary heart disease following the menopause, together with the metabolic effects of HRT described above, suggested that HRT might be beneficial in the prevention of coronary disease. A major problem in adducing evidence to support this notion is the relatively small number of women who develop coronary disease in the years immediately following the menopause, necessitating the recruitment of large numbers of women into trials to provide the necessary statistical power. Although early epidemiological studies suggested that HRT reduces the risk of coronary disease, meta-analyses of primary prevention trials suggest that HRT confers either no, or only a very small, degree of protection against cardiovascular death in younger women, and none in older women. The results of a large secondary prevention study showed no benefit. Indeed, one trial has indicated that HRT may cause an increase in cardiovascular events in older women in the first few years after starting treatment. The results of clinical trials thus appear to conflict with those of observational studies, and the reason for this remains uncertain. Thus HRT is not recommended for use solely for the primary or secondary prevention of coronary heart disease.
Hormone replacement therapy and osteoporosis
The increased rate of bone loss associated with oestrogen deficiency is a major cause of morbidity and mortality in older women (see Chapter 31); this is mainly due to the increased risk of hip fracture. Evidence from numerous trials is that HRT, whether with oestrogen alone or combined oestrogen and progestogen, increases bone mineral density and reduces the risk of hip fracture. However, although significant, the effect is small, and, except in women who sustain a premature menopause, HRT is not recommended solely for the prevention of fractures. Tibolone, a synthetic steroid with androgenic, oestrogenic and progestogenic properties, has also been shown to increase bone mineral density in postmenopausal women with osteoporosis.
ACKNOWLEDGEMENT
I would like to thank Irfana Koita-Kazi, John Waterstone, John H. Parsons, Mike Savvas and William J. Marshall who wrote this chapter for previous editions of the book.
Further reading
National Institute for Health and Care Excellence (NICE). Antenatal care: routine care for the healthy pregnant woman. NICE Clinical Guideline 62 2008. http://publications.nice.org.uk/antenatal-care-cg62 [Accessed October 2013].
Homepage providing access to extensive information on antenatal care. (As of April 2013, NICE was re-named the National Institute for Health and Care Excellence.)
NHS Fetal anomaly screening program, http://fetalanomaly.screening.nhs.uk/onlineresources; [Accessed October 2013].
Online modules and training resources for screening for fetal anomalies and Down syndrome.
APPENDIX 22.1 ACTH STIMULATION TEST FOR THE DIAGNOSIS OF CONGENITAL ADRENAL HYPERPLASIA
Perinatal diagnosis of congenital adrenal hyperplasia can be confirmed by a single assay of 17α-hydroxyprogesterone (17-OHP) in a capillary blood sample. In less clear-cut cases, it may be necessary to measure concentrations of 17-OHP before and following stimulation. Consequently, 17-OHP concentrations are measured before and 60 min after an intravenous injection of 0.25 mg ACTH. The expected values 1 h after ACTH are as follows:
For further details of the diagnosis of heterozygotes and the late onset form of 21-hydroxylase deficiency, see New et al. (1983, below). A test assessing the ACTH and 17-OHP responses to corticotrophin releasing hormone (CRH) has been described that appears to distinguish classic from late onset congenital adrenal hyperplasia.
References
Moreira AC, Elias LL. Pituitary-adrenal responses to corticotrophin-releasing hormone in different degrees of adrenal 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism 1992;74:198–203.
New MI, Lorenzen F, Lerner AJ et al. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. Journal of Clinical Endocrinology and Metabolism 1983;57:320–6.