Section II Intravenous Infusion Drugs
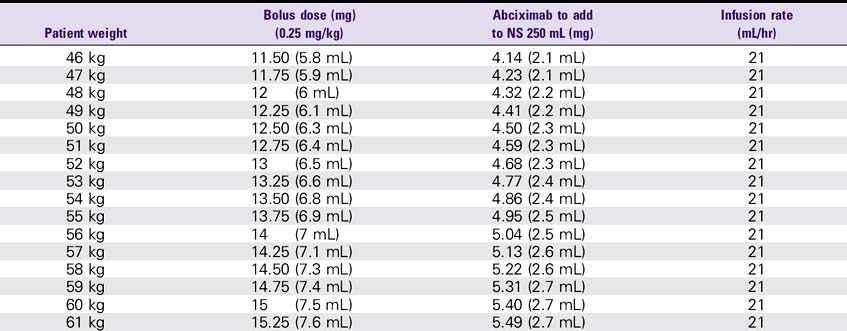
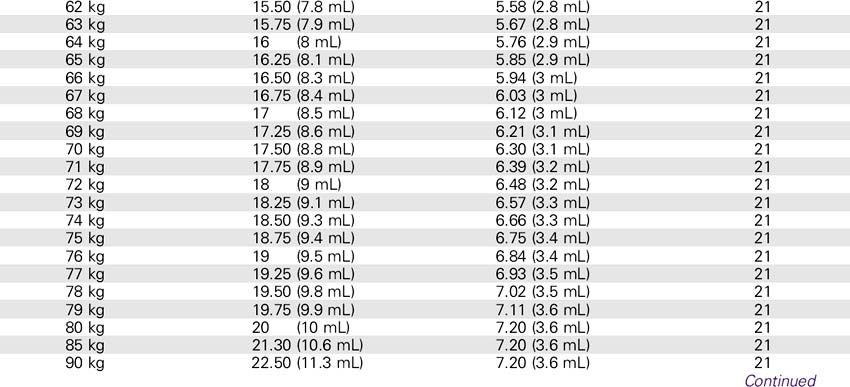
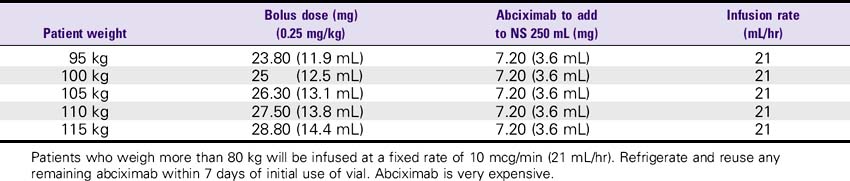
Abciximab (ReoPro)
WARNINGS
1. Increased risk of bleeding, especially in the presence of anticoagulants or thrombolytics.
2. Heparin doses may be reduced during abciximab infusions.
3. Administration is contraindicated in cases of active internal bleeding, a history of GI bleeding within 6 weeks, cardiovascular accident (CVA) within 2 years, thrombocytopenia, or uncontrolled hypertension.
4. Platelet counts, PT, INR, and PTT should be monitored at regular intervals.
INCOMPATIBILITY
Abciximab should be administered in a separate IV line whenever possible. No incompatibilities have been reported with infusion IV fluids or commonly used cardiovascular medications.
NURSING CONSIDERATIONS
1. Use a separate IV site for administration. An infusion pump or controller should be used. Additional filtration is not required if the infusion bag is prepared using at least a 5-micron filter.
2. Watch closely for bleeding or anaphylaxis.
3. Arterial/venous sheaths may be removed while abciximab is infusing, provided that ACT is adequate and direct pressure is held for 30 minutes.
4. Minimize arterial and venous punctures.
5. Platelets may need to be administered if platelet counts drop below 100,000 or as treatment for active bleeding.
Alteplase (Activase)
USES
1. As a thrombolytic for treatment of acute myocardial infarction (AMI), with chest pain duration greater than 20 minutes and onset within less than 12-24 hours, to improve ventricular function.
2. For acute pulmonary embolism (PE), age less than 75 years and within 5 days of thrombus formation.
3. For acute ischemic stroke, age less than 75 years and within the first 3 hours of the onset of symptoms.
SOLUTION PREPARATION
Reconstitute 100-mg vial alteplase in 100 mL sterile water. Final concentration: 1 mg/mL.
DOSE
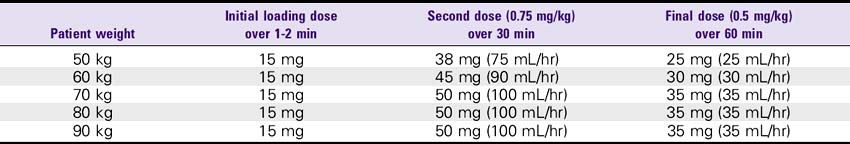
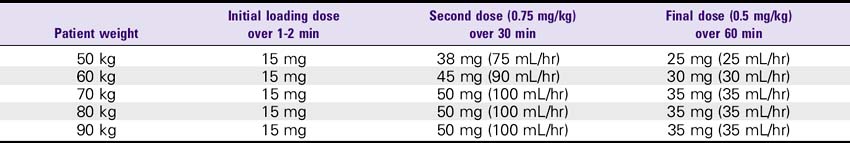
1. AMI (front-loaded dose): Give 15 mg (15 mL) bolus, then infuse.75 mg/kg over 30 minutes (up to 50 mg), then give 0.5 mg/kg over the next 60 minutes (not more than 35 mg). For patients weighing less than 67 kg: Give 15-mg bolus IV push, then 0.75 mg/kg over 30 minutes, then 0.5 mg/kg over 60 minutes.
2. PE: 100 mg over 2 hours, infuse at 50 mL/hr.
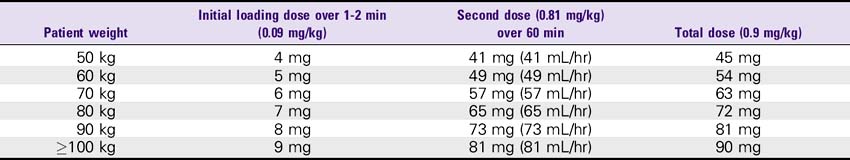
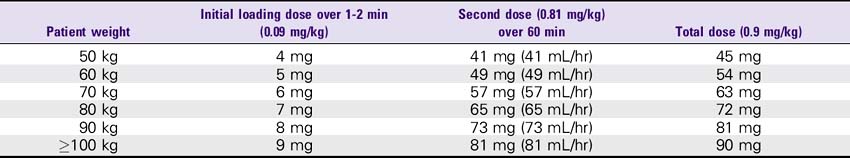
3. Stroke: 0.9 mg/kg (up to 90 mg). Infuse 10% (0.09 mg/kg) as a bolus dose, followed by the remainder (0.81 mg/kg) as a continuous infusion over 60 minutes. Maximum total dose is 90 mg.
WARNINGS
1. Increased risk of bleeding, especially in the presence of anticoagulation.
2. For AMI patients: Heparin doses of 5000-unit bolus and 1000 units/hr or 800 units/hr for patients weighing less than 80 kg may be used. For stroke patients: Start heparin infusion without loading bolus doses.
3. Administration of alteplase is contraindicated in cases of active internal bleeding, history of GI bleeding within 6 weeks, trauma or surgery within 1 month, thrombocytopenia, uncontrolled hypertension (SBP greater than 185, DBP greater than 110, unresponsive to nitrates or calcium antagonists) intracranial neoplasm, arteriovenous malformations, or aneurysm. Also contraindicated with history of cerebrovascular accident (CVA) within 1 month, seizure occurring at the time of stroke, or any suspicion of hemorrhagic stroke.
ADVERSE REACTIONS
1. Bleeding is the major side effect. Intracranial hemorrhage (0.4%-0.87%) or stroke has been reported as a complication. Minor bleeding such as increased bruising, hematuria, GI bleeding, bleeding at the injection site (up to 15.3%), and genitourinary hemorrhage is possible.
COMPATIBILITY
| Dextrose 5% | Metoprolol |
| Lidocaine | Propranolol |
NURSING CONSIDERATIONS
1. Use a separate IV site; do not administer with heparin because of incompatibility.
2. Watch closely for bleeding, particularly within the first hour of administration, or for anaphylaxis.
3. Minimize arterial and venous punctures for at least 24 hours after administration and avoid insertion of foley catheter.
4. Heparin and aspirin (160-325 mg) should be used with alteplase to reduce risk of rethrombosis.
Aminophylline (Theophylline)
DOSE
WARNINGS
1. Toxicity warning: Monitor serum levels to avoid toxicity; normal levels are 5-15 mcg/mL. Incidence of toxicity increases significantly with serum levels greater than 20 mcg/mL, with symptoms including ventricular arrhythmias, convulsions, and death.
2. Patients with decreased ability to clear plasma of aminophylline (e.g., those with impaired liver function, congestive heart failure (CHF), greater than 55 years of age, sustained high fever) are at increased risk of toxicity.
3. Aminophylline may cause arrhythmia; monitor levels if significant changes in HR or rhythm occur. Ventricular arrhythmias will respond to lidocaine.
ADVERSE REACTIONS
1. Adverse reactions rarely occur when serum levels are less than 20 mcg/mL.
2. CNS: Irritability, restlessness, headache, insomnia, reflex hyperexcitability, muscle twitching, convulsions.
3. GI: Nausea, vomiting, epigastric pain, hematemesis, diarrhea; may induce gastroesophageal reflux.
4. Cardiovascular: Palpitations, tachycardia, extrasystoles, hypotension, circulatory failure, ventricular arrhythmias.
5. Other: Tachypnea, proteinuria, fever, hyperglycemia, rash.
NURSING CONSIDERATIONS
Aminophylline Drip Rate Calculation Chart
Theophylline 400 mg/500 mL or 800 mg/1000 mL (Aminophylline concentration: 1 mg/mL)
| Dose | Infusion rate |
|---|---|
| 10 mg/hr | 10 mL/hr |
| 15 mg/hr | 15 mL/hr |
| 20 mg/hr | 20 mL/hr |
| 25 mg/hr | 25 mL/hr |
| 30 mg/hr | 30 mL/hr |
| 35 mg/hr | 35 mL/hr |
| 40 mg/hr | 40 mL/hr |
| 45 mg/hr | 45 mL/hr |
| 50 mg/hr | 50 mL/hr |
| 55 mg/hr | 55 mL/hr |
| 60 mg/hr | 60 mL/hr |
| 65 mg/hr | 65 mL/hr |
| 70 mg/hr | 70 mL/hr |
| 75 mg/hr | 75 mL/hr |
Amiodarone (Cordarone)
Dose
1. Infuse 150 mg over 10 minutes (15 mg/min). Prepare solution with 150 mg amiodarone in 100-mL D5W bag. The initial infusion rate is not greater than 30 mg/min.
2. Follow with a slow infusion of 360 mg over the next 6 hours (1 mg/min). Prepare solution with 900 mg amiodarone in 500 mL D5W glass bottle.
3. Follow with a maintenance infusion of 540 mg over the remaining 18 hours (0.5 mg/min).
4. After the first 24 hours: 0.5 mg/min continuous infusion. If IV concentration is greater than 2 mg/mL, it should be administered through a central venous catheter with in-line filter. (For greater than 2 mg/mL, administer via central venous catheter only.)
5. If breakthrough episodes of ventricular fibrillation or tachycardia should occur, an additional infusion of 150 mg over 10 minutes may be administered (150 mg in 100 mL D5W).
6. When switching from IV to PO, use the following as a guide:
7. During cardiac arrest, 300 mg (2 ampules 150 mg each) may be given IV push. May repeat 150-mg IV push in 3-5 minutes, up to a maximum cumulative dose of 2.2 g IV in 24 hours.
WARNINGS
1. Although IV amiodarone has been used safely in some patients with acute myocardial infarction (AMI), it is clearly a negative inotrope. Use cautiously in patients with left ventricular dysfunction.
2. Hypotension is the main complication of IV therapy; therefore use with caution in hypotensive patients.
3. Marked cardiomegaly, particularly resulting from myocardiopathy, is a relative contraindication to IV use of amiodarone.
4. Use cautiously in patients with thyroid dysfunction. Amiodarone has been reported to produce hypothyroidism or hyperthyroidism.
5. Because of extensive tissue distribution and prolonged elimination period, the time at which a life-threatening arrhythmia will recur following discontinued therapy or an interaction with subsequent treatment may be unpredictable. Patients must be observed carefully when other antiarrhythmic agents are substituted after amiodarone is stopped.
ADVERSE REACTIONS
1. Cardiovascular: Sinus bradycardia, hypotension, heart block, proarrhythmic effects.
2. Pulmonary: Within the first few weeks, may present with acute onset of nonspecific symptoms (e.g., fever, shortness of breath, and cough). These are probably symptoms of a hypersensitivity reaction associated with an eosinophilic lung infiltrate (e.g., pulmonary fibrosis, interstitial pneumonitis).
3. Thyroid: Interferes with T4 → T3 conversion (hypothyroidism occurs more often than hyperthyroidism).
4. GI: Nausea/vomiting, anorexia, abdominal pain, and constipation.
5. Hepatic: Abnormal liver function tests, especially elevated aminotransferase and alkaline phosphatase levels, ≈25% of patients. Increased prothrombin time (PT)/international normalized ratio (INR).
6. Dermatologic: Allergic rash, photosensitivity, and unusual blue-gray skin discoloration.
7. Neurologic: Tremor, ataxia, peripheral neuropathy, fatigue, and weakness.
8. Ophthalmologic: High occurrence of corneal microdeposits caused by the secretion of amiodarone by the lacrimal gland with accumulation on corneal surface. This does not seem to affect vision and is reversible once the drug is discontinued.
COMPATIBILITY
| Drug | Interaction effect |
|---|---|
| Warfarin | Increased anticoagulation effect |
| Beta-blockers | Beta-blocker effects are enhanced |
| Calcium channel blockers | Additive effects of both drugs are enhanced, resulting in reduced cardiac sinus and AV nodal conduction, and contractility |
| Digoxin | Increased digoxin concentrations, thus increasing toxic potential |
| Flecainide | Increased flecainide concentrations |
| Phenytoin | Increased phenytoin concentrations |
| Procainamide | Increased procainamide concentrations |
| Quinidine | Increased quinidine concentrations, which can cause fatal cardiac arrhythmias |
NURSING CONSIDERATIONS
1. Consult the Amiodarone Drip Rate Calculation Chart to determine the drip rate.
2. Muscle weakness may present a great hazard for ambulation.
4. Monitor ECG and rhythm throughout therapy.
5. Assess patient for signs of lethargy, edema of the hands and feet, weight loss, and pulmonary toxicity (e.g., shortness of breath, cough, rales, fever, pulmonary function tests).
Amiodarone (Cordarone)
| Dose | Concentration | Infusion rate |
|---|---|---|
| 15 mg/min | 150 mg amiodarone /100 mL D5W = 1.5 mg/1 mL D5W | 600 mL/hr |
| 1 mg/min | 900 mg/500 mL D5W = 1.8 mg/1 mL D5W | 33 mL/hr × 6 hr |
| 0.5 mg/min | 900 mg/500 mL D5W = 1.8 mg/1 mL D5W | 17 mL/hr × 18 hr |
| After the first 24 hr 0.5 mg/min | 600 mg/500 mL = 1.2 mg/mL | 25 mL/hr |
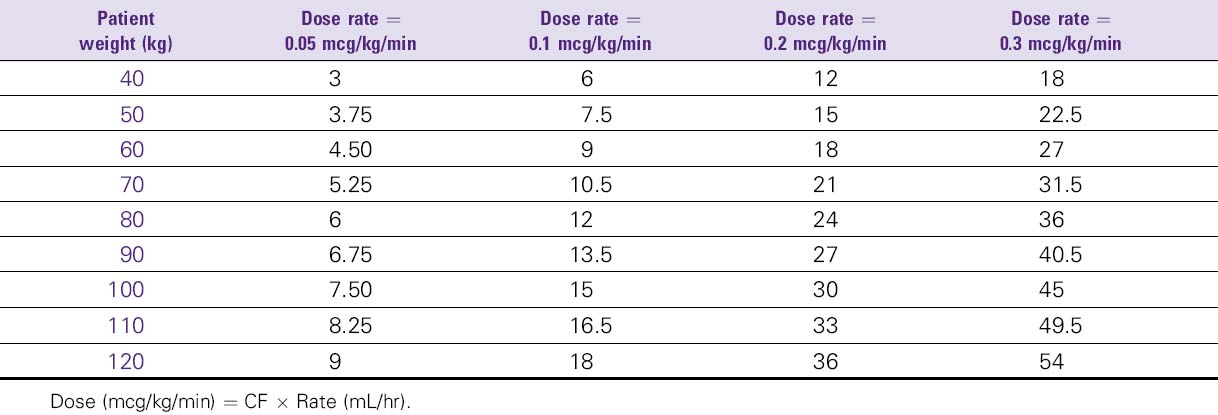
Argatroban (Acova)
WARNINGS
1. Heparin must be discontinued before administration of argatroban.
2. A baseline aPTT should be obtained before initiating therapy.
3. Patients with hepatic impairment require a dosage adjustment.
4. Doses greater than 10 mcg/kg/min should not be administered.
5. Contraindicated in overt bleeding.
6. Hemorrhage can occur at any site in the body; an unexplained fall in hematocrit or blood pressure should be evaluated for bleeding.
7. Use extreme caution in the following instances: Severe hypertension; immediately following lumbar puncture; spinal anesthesia; major surgery, especially involving the brain, spinal cord or eye; hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders and GI lesions such as ulcerations.
NURSING CONSIDERATIONS
1. Monitor therapy using the aPTT. The aPTT typically reaches steady-state effect levels within 1 to 3 hours after initiation.
2. Dose adjustment may be required to attain the target aPTT. Check the aPTT 2 hours after initiation of therapy or dosage adjustment to confirm that the patient has attained the desired therapeutic range (1.5 to 3 times control, usually about 55-80 seconds).
3. For conversion to oral therapy with warfarin, maintain argatroban infusion until INR is greater than 4. Combination therapy with argatroban and warfarin does produce a combined effect on laboratory measurement of INR. Once INR is greater than 4, then stop the infusion, repeat INR 4-6 hours later; if INR is between 2 and 3, maintain warfarin monotherapy.
| HIT patients | HIT patients with renal impairment | HIT patients with hepatic impairment |
|---|---|---|
| Initiate at 2 mcg/kg/min | No dosage adjustment required | Initiate at 0.5 mcg/kg/min |
| Titrate until steady-state aPTT is 1.5 to 3 times baseline value | Titrate until steady-state aPTT is 1.5 to 3 times baseline value |
Atracurium (Tracrium)
USES
Skeletal muscle relaxation during mechanical ventilation to prevent ventilator resistance and/or decreased energy expenditure states. Paralysis of skeletal muscles requires that patients be intubated and on mechanical ventilation.
WARNINGS
1. Sedation should be ordered and given in addition to atracurium because it does not alter the level of consciousness and does not relieve pain.
2. Tracrium drip may be interrupted to assess neurologic status based on physician’s orders. Neurologic assessment will include responsiveness, orientation, extremity movement, and pupillary reaction.
3. If drip is interrupted, patient should be given 10-mg bolus and resume prior rate (mg/hr).
NURSING CONSIDERATIONS
1. Use infusion control device and monitor ECG, respirations, and vital signs continuously.
2. Monitor for malignant hyperthermia.
3. Tachyphylaxis is possible in long-term use.
4. Anticholinesterase reversal agents, endotracheal intubation equipment, and mechanical ventilation equipment should be available.
5. No dosage reductions are needed for renal or hepatic insufficiency.
6. The desired level of neuromuscular blockade is usually measured by train of four peripheral neurostimulator assessment. Typically, the goal of therapy is 0/4 train of four (100% blocked) to 2/4 train of four (75% blocked). Usually, this is assessed every 15 minutes during titration and at least every 4 hours during maintenance infusion.
7. A “sedation holiday” or temporary removal of the infusion may be necessary for all patients on therapy for more than 48 hours to assess the patient for continued need and to conduct a neurologic exam.
Atracurium (Tracrium) Drip Rate Calculation Chart
Tracrium 250 mg Added to 225 mL (Concentration: 1 mg/mL or 1000 mcg/mL)
| Patient weight | Dose (0.5 mg/kg/hr) | Infusion rate |
|---|---|---|
| 50 kg | 25 mg/hr | 25 mL/hr |
| 60 kg | 30 mg/hr | 30 mL/hr |
| 70 kg | 35 mg/hr | 35 mL/hr |
| 80 kg | 40 mg/hr | 40 mL/hr |
| 90 kg | 45 mg/hr | 45 mL/hr |
Bivalirudin (Angiomax)
USES
For the replacement of heparin in patients undergoing percutaneous coronary intervention (PCI) who are at a high risk for bleeding or thrombotic complications. Angiomax is a bivalent, direct thrombin inhibitor that provides rapid and reversible anticoagulant activity during PCI.
SOLUTION PREPARATION
DOSE
Dosage Adjustment
Infusion rate should be decreased as follows in patients with moderate to severe renal impairment.
| Maintenance dose | Creatinine clearance |
|---|---|
| 1.4 mg/kg/hr | 30-59 mL/min |
| 0.7 mg/kg/hr | 10-29 mL/min |
| 0.2 mg/kg/hr | <10 mL/min |
WARNINGS
Should not be used in patients with active bleeding disorders or known sensitivity to Angiomax.
NURSING CONSIDERATIONS
1. Sheath removal may be performed using a time-based procedure instead of serial ACT monitoring. Angiomax achieves rapid hemostasis at the groin site with 10-15 minutes of manual pressure and may obviate the need for closure devices.
| Renal function | Time to sheath removal | Estimated Angiomax serum levels |
|---|---|---|
| CrCl ≥30 mL/min | 1 hour | <2 mcg/mL |
| CrCl 10-30 mL/min | 2-2.5 hours | <2 mcg/mL |
2. Patients previously treated with unfractionated heparin prior to arrival in the cath lab can be switched to bivalirudin after heparin has been discontinued for approximately 30 minutes.
3. Low molecular weight heparin (LMWH) should be discontinued for at least 8 hours prior to bivalirudin administration.
Cisatracurium (Nimbex)
USES
Skeletal muscle relaxation during mechanical ventilation to prevent ventilator resistance and/or decreased energy expenditure states. Paralysis of skeletal muscles requires that patients must be intubated and on mechanical ventilation.
WARNINGS
1. Sedation should be ordered and given in addition to the cisatracurium because it does not alter the level of consciousness and does not relieve pain.
2. Nimbex drip may be interrupted to assess neurologic status based on physician’s orders. Neurologic assessment will include responsiveness, orientation, extremity movement, and pupillary reaction.
ADVERSE REACTIONS
Bradycardia (0.4%), hypotension (0.2%), flushing (0.2%), bronchospasm (0.2%), rash (0.1%)
NURSING CONSIDERATIONS
1. Use infusion control device and monitor ECG, respirations, and vital signs continuously.
2. Monitor for malignant hyperthermia.
3. Tachyphylaxis is possible in long-term use.
4. Anticholinesterase reversal agents, endotracheal intubation equipment, and mechanical ventilation equipment should be available.
5. No dosage reductions are needed for renal or hepatic insufficiency.
6. The desired level of neuromuscular blockade is usually measured by train of four peripheral neurostimulator assessment. Typically, the goal of therapy is 0/4 train of four (100% blocked) to 2/4 train of four (75% blocked). Usually, this is assessed every 15 minutes during titration and at least every 4 hours during maintenance infusion.
7. A “sedation holiday” or temporary removal of the infusion may be necessary for all patients on therapy for greater than 48 hours to assess the patient for continued need and to conduct a neurologic exam.
Cisatracurium (Nimbex) Drip Rate Calculation Chart
Nimbex 250 mg in 250 mL (Concentration: 1 mg/mL or 1000 mcg/mL)
| Patient weight | Dose (3.0 mcg/kg/min) | Infusion rate |
|---|---|---|
| 50 kg | 9 mg/hr | 9 mL/hr |
| 60 kg | 11 mg/hr | 11 mL/hr |
| 70 kg | 13 mg/hr | 13 mL/hr |
| 80 kg | 14 mg/hr | 14 mL/hr |
| 90 kg | 16 mg/hr | 16 mL/hr |
Conivaptan (Vaprisol)
USES
Treatment of euvolemic or hypervolemic hyponatremia. An increase in serum sodium concentrations and free water clearance occur.
WARNINGS
1. Conivaptan is contraindicated in patients with hyponatremia associated with hypovolemia.
2. Use with caution in patients with hepatic disease or renal impairment.
3. Additive hypertensive effects are possible with antihypertensive agents.
4. Conivaptan may increase digoxin concentrations, and should not be given with ketoconazole, itraconazole, and fluconazole due to increased conivaptan effects.
5. Ampules should be stored in the original container, protected from light until ready for use. Do not use vials if particulate matter is visible or solution is discolored.
ADVERSE REACTIONS
1. Cardiovascular: Hypertension, orthostatic hypotension, peripheral edema, phlebitis (very common).
2. Dermatologic: Injection site reaction
3. Endocrine metabolic: Hypokalemia, increased thirst.
4. Gastrointestinal: Constipation, diarrhea, vomiting
INCOMPATIBILITIES
LR & NS. Dilute only with D5W. Do not mix or administer with other IV fluids or medications.
NURSING CONSIDERATIONS
1. Diluted solution should be used immediately and administration completed within 24 hours of mixing.
2. Dilute only with D5W injection; do not mix with or administer with LR injection or 0.9% sodium chloride injection.
3. To minimize risk of vascular irritation, administer through central line or large veins and change infusion site every 24 hours (peripheral veins).
4. Frequently monitor serum sodium, electrolytes, urine output and volume status.
5. Carefully monitor neurologic status, in the event that serum sodium rises at an undesirably rapid rate.
6. Frequently monitor vital signs, in the event that patients develop hypovolemia or hypotension.
| Loading dose | Rate |
|---|---|
| 20 mg/100 mL D5W | 200 mL/hr |
| Infusion doses | Rate |
|---|---|
| 20 mg/250 mL D5W | 11 mL/hr |
| 40 mg/250 mL D5W | 11 mL/hr |
Dexmedetomidine (Precedex)
USES
Indicated for short-term use as a sedative for patients undergoing mechanical ventilation in the ICU and cardiac surgery unit (CSU) setting.
SOLUTION PREPARATION
Dilute 2 mL dexmedetomidine into 48 mL NS before IV administration for any purpose.
WARNINGS
1. Patients should be continuously monitored while receiving dexmedetomidine because of clinically significant episodes of bradycardia and sinus arrest.
2. Caution should be exercised when administering dexmedetomidine to patients with advanced heart block.
3. Transient hypertension has been observed primarily during the loading dose in association with the initial peripheral vasoconstrictive effects of dexmedetomidine. Hypotension is possible in hypovolemic patients.
4. Abrupt discontinuation can result in nervousness, agitation, and headaches accompanied by a rapid rise in BP and elevated catecholamine concentrations in the plasma.
ADVERSE REACTIONS
1. Incidence greater than 10%: Hypotension, hypertension, nausea
2. Incidence 1%-10%: Bradycardia, atrial fibrillation, hypoxia, anemia, pain, pleural effusion, infection, leukocytosis, oliguria, pulmonary edema, thirst
3. Incidence less than 1%: Fever, hyperpyrexia, hypovolemia, light anesthesia, pain, rigors, BP fluctuation, heart disorder, aggravated hypertension, dizziness, headache, neuralgia, neuritis, speech disorder, abdominal pain, diarrhea, vomiting, arrhythmias, atrioventricular (AV) block, cardiac arrest, extrasystoles, atrial fibrillation, heart block, T-wave inversion, tachycardia, supraventricular tachycardia, increased GGT, increased SGOT, increased SGPT, acidosis, respiratory acidosis, hyperkalemia, increased alkaline phosphatase, agitation, confusion, delirium, hallucination, illusion, somnolence, anemia, apnea, bronchospasm, dyspnea, hypercapnia, hypoventilation, hypoxia, pulmonary congestion, increased sweating, photopsia, abnormal vision
NURSING CONSIDERATIONS
1. Use administration components made with synthetic or coated natural rubber gaskets.
2. Products should be inspected visually for particulate matter and discoloration before administration.
3. Consult the Dexmedetomidine Drip Rate Calculation Chart to determine the drip rate.
4. Safety and effectiveness of dexmedetomidine has not been evaluated in infusions over 24 hours; however, it has been used for up to 9 days in some patients.
Diltiazem (Cardizem)
DOSE
WARNINGS
1. Diltiazem may prolong atrioventricular (AV) node conduction and rarely may cause second- or third-degree heart block. It is contraindicated in patients with sick sinus syndrome or heart block unless functioning ventricular pacemaker is present.
2. Monitor response and possibly decrease dose in patients with liver failure. Rare instances of acute hepatic injury have occurred with diltiazem injection.
3. May cause hypotension; use with caution in hemodynamically compromised patients and those taking drugs that decrease peripheral resistance, intravascular volume, myocardial contractility, or conduction.
ADVERSE REACTIONS
1. Cardiovascular: Hypotension—treat with saline or raise feet if required. Vasodilation (flushing) may occur. Atrial flutter, atrioventricular (AV) block, bradycardia, chest pain, congestive heart failure (CHF), and ventricular arrhythmias have been reported.
2. GI: Constipation, nausea, vomiting, elevated liver function tests.
NURSING CONSIDERATIONS
1. Consult the Diltiazem Drip Rate Calculation Chart to determine the drip rate.
2. Diltiazem should be administered by an IV pump to ensure controlled infusion.
3. Closely monitor and document changes in BP, HR, or in the rhythm strip. Document BP at least every hour.
Diltiazem (Cardizem) Drip Rate Calculation Chart
Diltiazem 125 mg Added to 100 mL (Concentration: 1 mg/mL)
| Dose | Infusion rate |
|---|---|
| 5 mg/hr | 5 mL/hr |
| 6 mg/hr | 6 mL/hr |
| 7 mg/hr | 7 mL/hr |
| 8 mg/hr | 8 mL/hr |
| 9 mg/hr | 9 mL/hr |
| 10 mg/hr | 10 mL/hr |
| 11 mg/hr | 11 mL/hr |
| 12 mg/hr | 12 mL/hr |
| 13 mg/hr | 13 mL/hr |
| 14 mg/hr | 14 mL/hr |
| 15 mg/hr | 15 mL/hr |
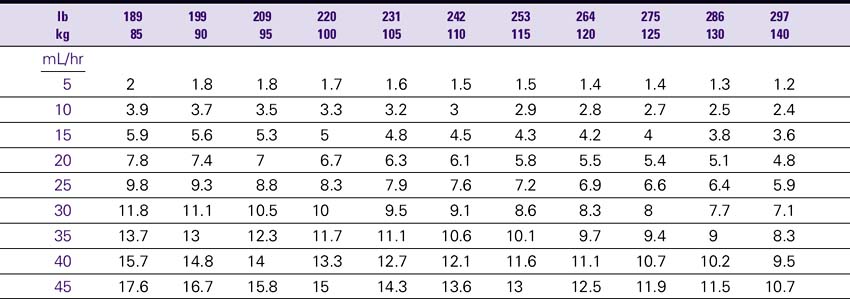
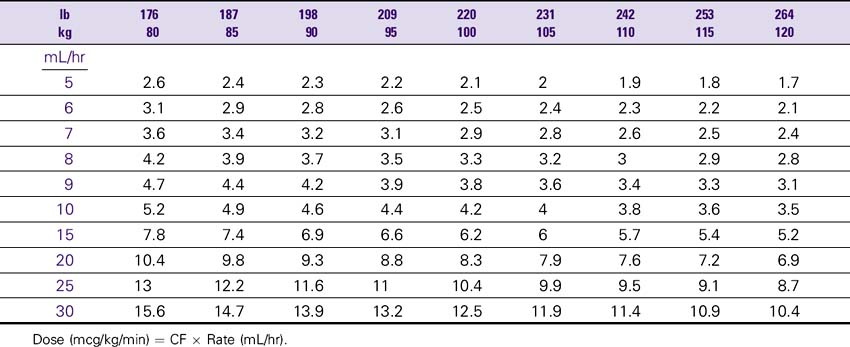
Dobutamine (Dobutrex)
WARNINGS
1. Hypovolemia should be corrected before use.
2. In the presence of atrial fibrillation, digoxin is usually given before dobutamine initiation to prevent development of a rapid ventricular response.
3. Dobutamine is contraindicated in patients with idiopathic hypertropic subaortic stenosis (IHSS).
4. Dobutamine should be used with extreme caution after myocardial infarction (MI) or in patients with marked mechanical obstruction (e.g., severe valvular aortic stenosis).
5. Use with caution in patients receiving halothane or cyclopropane anesthesia.
NURSING CONSIDERATIONS
1. The drip may turn a light pink several hours after mixing, but stability or potency is not altered.
2. An accurate weight should be obtained before administration of dobutamine.
3. Consult the Dobutamine Drip Rate Calculation Chart to determine the drip rate.
4. Except in cardiac arrest situations, dobutamine should be administered via an IV pump to ensure controlled infusion.
5. Closely monitor and document changes in BP, HR, or the rhythm strip. Document BP at least every hour. Pulmonary wedge pressure and cardiac output monitoring is desirable.
Dobutamine (Dobutrex) Drip Rate Calculation Chart—Patient Weight 35–80 kg (77–176lb)
Concentration: 2 mg/mL (500 mg/250 mL)
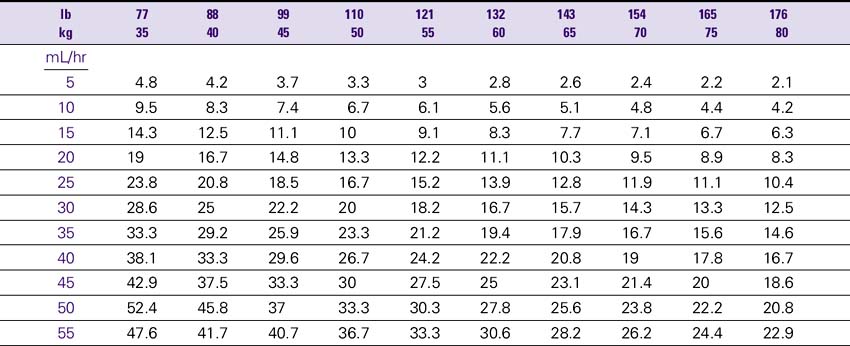
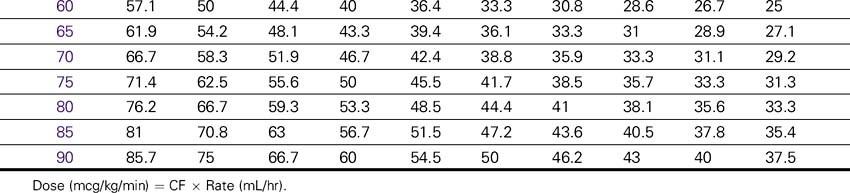
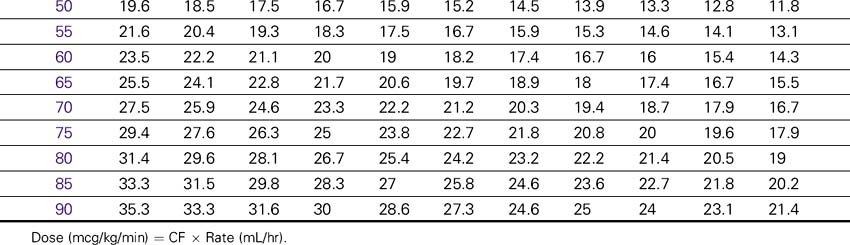
Dopamine (Intropin)
USES
Correction of hemodynamic imbalances present in shock syndrome resulting from myocardial infarction (MI), trauma, septicemia, open heart surgery, renal failure, and chronic cardiac decompensation as in congestive heart failure (CHF).
WARNINGS
1. Dopamine should not be used in patients with pheochromocytoma.
2. Patients who have been treated with MAO inhibitors (e.g., Marplan, Nardil, or Parnate) will require a reduced starting dose of dopamine—usually at least 1/10 of the usual dose.
3. Avoid cyclopropane or halogenated hydrocarbon anesthetics.
4. Extravasation or peripheral ischemia can cause sloughing and necrosis of tissue in the surrounding area. Antidote: The site should be infiltrated with a 10-mL solution containing 5 mg phentolamine (Regitine); a fine hypodermic needle should be used; obtain a physician’s order for the antidote.
NURSING CONSIDERATIONS
1. An accurate weight should be obtained before administration of dopamine.
2. Consult the Dopamine Drip Rate Calculation Chart to determine the drip rate.
3. Except in cardiac arrest situations, dopamine should be administered via an IV pump to ensure controlled infusion.
4. The renal perfusion patient’s BP should be monitored and documented hourly. The hypotensive patient’s BP should be monitored with each increase in dose while dopamine is being incrementally increased. After the desired results are obtained, monitor BP at least hourly and document. Any disproportionate rise or fall in BP should be noted and reported immediately to the physician.
5. Closely document changes in skin color or temperature in the extremities as a monitor for ischemia. Closely document changes in HR, renal output, and signs of reversal of confusion or comatose state as a monitor of drug effectiveness.
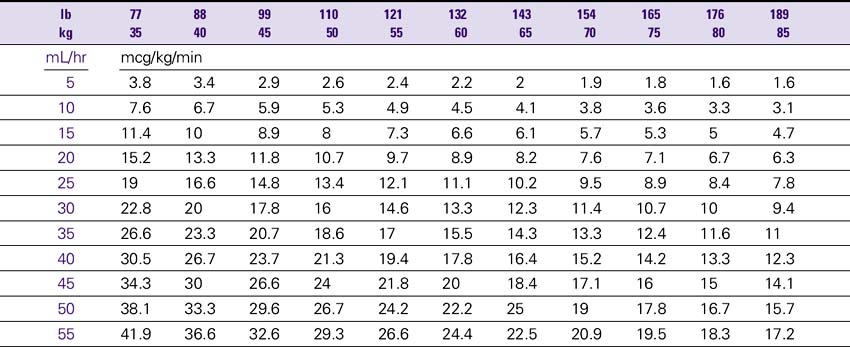
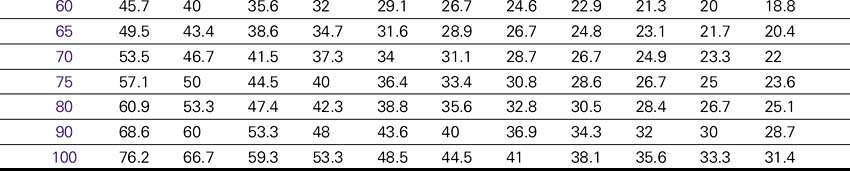
Dopamine (Intropin) Drip Rate Calculation Chart—Patient Weight 35–85 kg (77–189lb)
Concentration: 1.6 mg/mL (400 mg/250 mL)
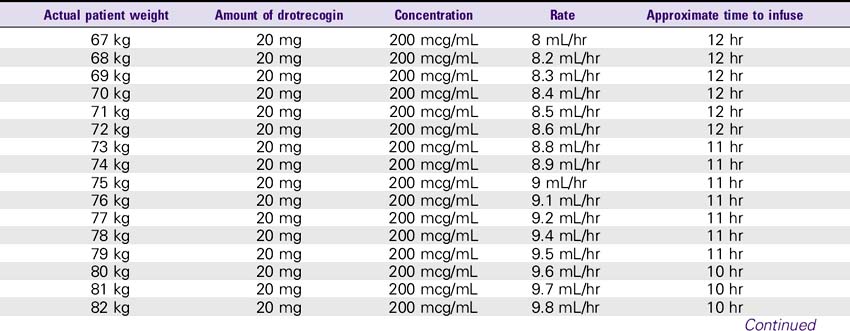
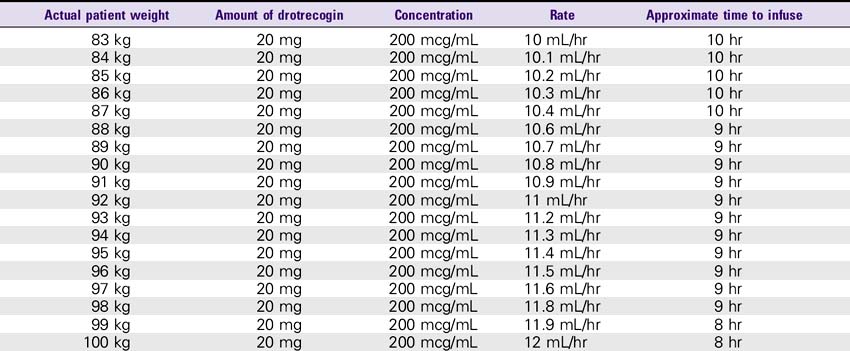
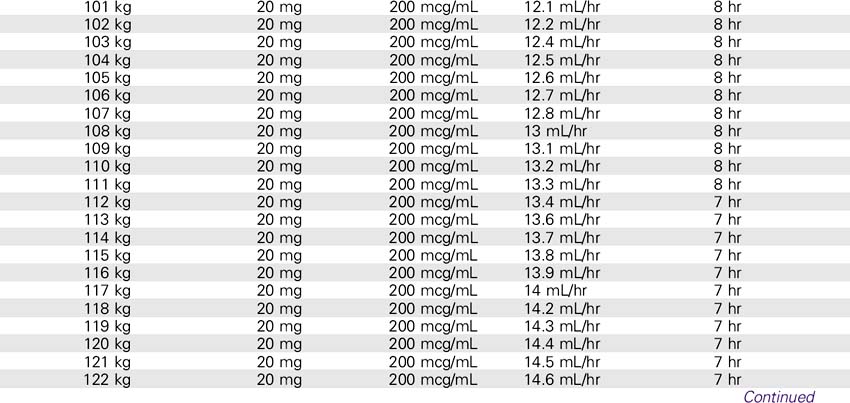
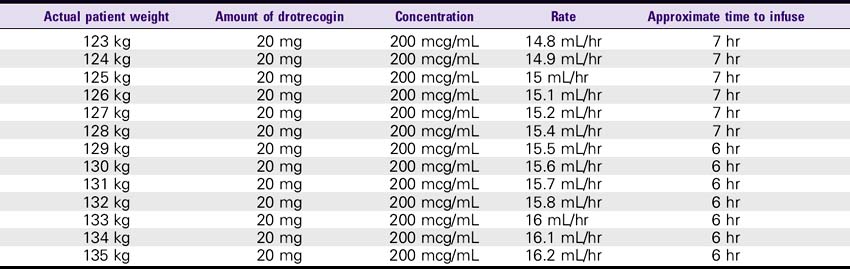
Drotrecogin Alfa (Xigris)
SOLUTION PREPARATION
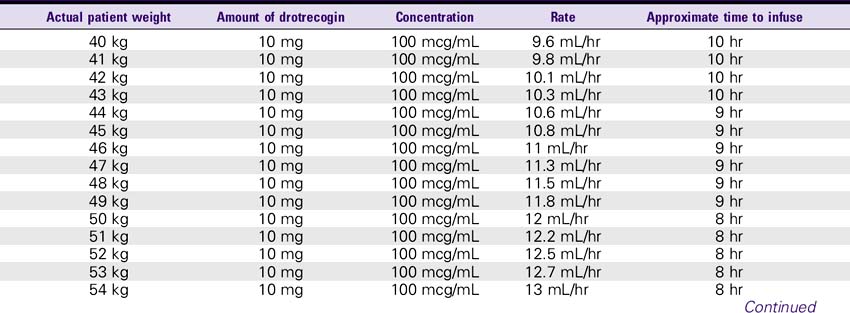
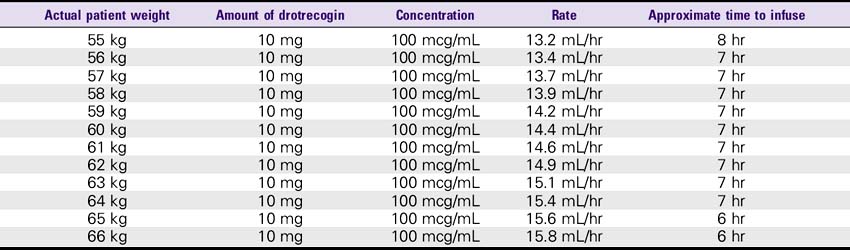
1. Reconstitute the 5-mg vials with 2.5 mL sterile water for injection, USP. The 20-mg vials must be reconstituted with 10 mL of sterile water for injection, USP. This results in a concentration of approximately 2 mg/mL. This solution can be held for only 3 hours.
2. The reconstituted solution must be further diluted with sterile 0.9% sodium chloride injection. The IV solution should be prepared immediately after reconstitution in the vials. IV administration must be completed within 12 hours after the IV solution is prepared.
CONTRAINDICATIONS
2. Hemorrhagic stroke within last 3 months.
3. Platelet count of less than 30,000.
4. Recent intracranial or intraspinal surgery or severe head trauma requiring hospitalization (in last 2 months); trauma patient with increased risk of life-threatening bleeding.
5. Patients with epidural catheters or within 12 hours of epidural catheter removal.
6. Intracranial neoplasm or mass lesion.
7. Hypersensitivity to drotrecogin or any component of the product.
8. Efficacy has not been established in patients with lower risk of death (APACHE II score less than 25).
9. Use caution when giving with other anticoagulants or if PTT/PT are elevated.
WARNINGS
1. Therapeutic heparin (greater than 15,000 units/day) or low molecular weight heparin (LMWH).
2. GI bleed within past 6 months.
3. Administration of thrombolytic therapy within past 3 days.
4. Administration of oral anticoagulants or glycoprotein IIb/IIIa inhibitors within past 7 days.
5. Administration of aspirin greater than 650 mg/day or other platelet inhibitors within past 7 days.
6. Ischemic stroke in past 3 months.
7. Intracranial arteriovenous malformation or aneurysm.
8. Known bleeding diathesis except for acute coagulopathy related to sepsis.
9. Chronic severe hepatic disease.
10. Any other condition in which bleeding constitutes a significant hazard.
NURSING CONSIDERATIONS
1. The aPTT is not reliable for the status of coagulopathy in patients on Xigris, so only the PT is a valuable lab test.
2. Stop infusion 2 hours before invasive procedures. Consider restarting 12 hours after major surgery or immediately after uncomplicated, less invasive procedures.
Epinephrine (Adrenalin) Injection
DOSE
WARNINGS
1. Epinephrine increases myocardia oxygen demand and should not be used in cardiogenic shock.
2. Epinephrine should not be used in traumatic or hemorrhagic shock.
3. Intracardiac epinephrine injection runs the risk of coronary artery laceration, cardiac tamponade, pneumothorax, and intramyocardial injection of the drug.
4. Epinephrine may cause potentially fatal ventricular arrhythmias including fibrillation; use with care in patients with organic heart disease or those receiving other drugs that sensitize the heart.
NURSING CONSIDERATIONS
1. Consult the Epinephrine Drip Rate Calculation Chart to determine the drip rate.
2. Except in cardiac arrest situations, epinephrine should be administered via an IV pump to ensure controlled infusion.
3. The patient’s BP and HR should be monitored every 2-5 minutes until patient is stabilized, then every 15 minutes.
4. Patient should be closely monitored while receiving IV epinephrine. Document BP and HR; observe and report rate and character (regularity and force) of the pulse.
Epinephrine (Adrenalin) Drip Rate Calculation Chart
Epinephrine 1 mg/250 mL (Concentration: 4 mcg/mL)
| Dose | Infusion rate |
|---|---|
| 1 mcg/min | 15 mL/hr |
| 1.5 mcg/min | 22 mL/hr |
| 2 mcg/min | 30 mL/hr |
| 2.5 mcg/min | 37 mL/hr |
| 3 mcg/min | 45 mL/hr |
| 3.5 mcg/min | 52 mL/hr |
| 4 mcg/min | 60 mL/hr |
| 5 mcg/min | 75 mL/hr |
| 6 mcg/min | 90 mL/hr |
| 7 mcg/min | 105 mL/hr |
| 8 mcg/min | 120 mL/hr |
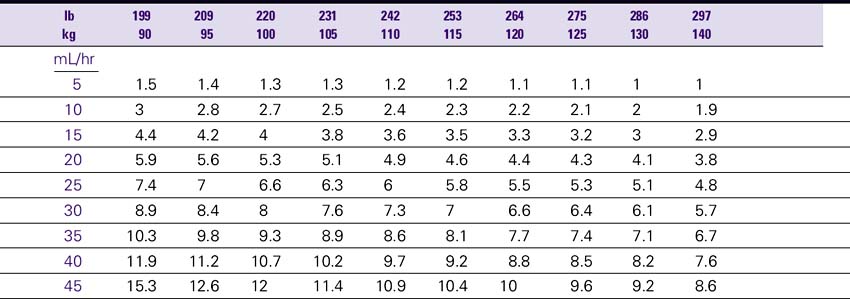
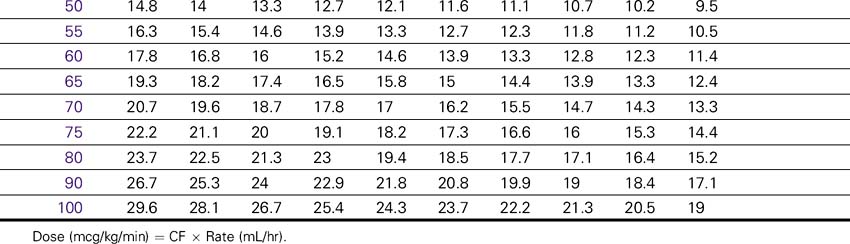
Dose (mcg/kg/min) = CF × Rate (mL/hr).
Double Strength Epinephrine (Adrenalin) Drip Rate Calculation Chart
Epinephrine 2 mg/250 mL (Concentration: 8 mcg/mL)
| Dose | Infusion rate |
|---|---|
| 1 mcg/min | 7.5 mL/hr |
| 1.5 mcg/min | 11 mL/hr |
| 2 mcg/min | 15 mL/hr |
| 2.5 mcg/min | 19 mL/hr |
| 3 mcg/min | 23 mL/hr |
| 3.5 mcg/min | 26 mL/hr |
| 4 mcg/min | 30 mL/hr |
| 5 mcg/min | 38 mL/hr |
| 6 mcg/min | 45 mL/hr |
| 7 mcg/min | 53 mL/hr |
| 8 mcg/min | 60 mL/hr |
Dose (mcg/kg/min) = CF × Rate (mL/hr).
Triple Strength Epinephrine (Adrenalin) Drip Rate Calculation Chart
Epinephrine 3 mg/250 mL (Concentration: 12 mcg/mL)
| Dose | Infusion rate |
|---|---|
| 1 mcg/min | 5 mL/hr |
| 1.5 mcg/min | 7.5 mL/hr |
| 2 mcg/min | 10 mL/hr |
| 2.5 mcg/min | 13 mL/hr |
| 3 mcg/min | 15 mL/hr |
| 3.5 mcg/min | 18 mL/hr |
| 4 mcg/min | 20 mL/hr |
| 5 mcg/min | 25 mL/hr |
| 6 mcg/min | 30 mL/hr |
| 7 mcg/min | 35 mL/hr |
| 8 mcg/min | 40 mL/hr |
Dose (mcg/kg/min) = CF × Rate (mL/hr).
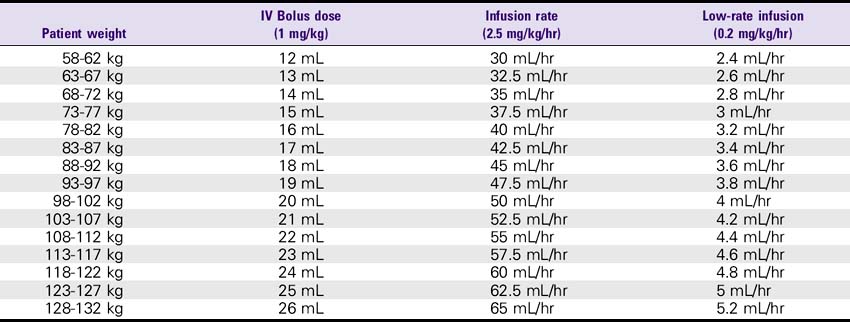
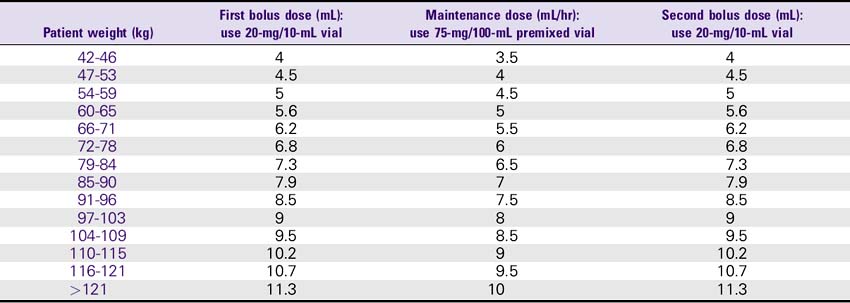
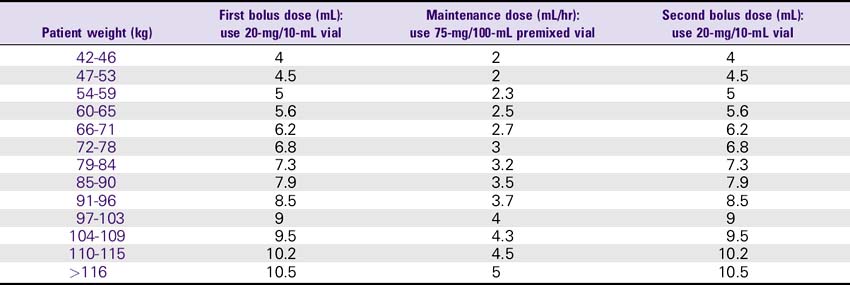
Eptifibatide (Integrilin)
USES
1. A cyclic heptapeptide antagonist of the platelet glycoprotein (GP) IIb/IIIa receptor, which inhibits platelet aggregation.
2. Integrilin, in combination with heparin, is indicated for the treatment of acute coronary syndromes (e.g., unstable angina, non–Q-wave myocardial infarction [MI]), including those patients who are to be managed medically and those undergoing PTCA.
DOSE
1. Acute coronary syndrome: The recommended dose for patients with normal renal function (i.e., creatinine less than 2) is 180 mcg/kg bolus as soon as possible, followed by 2 mcg/kg/min infusion up to the time of discharge, CABG surgery, or 72 hours. For patients with renal insufficiency (i.e., creatinine 2-4), use 180 mcg/kg bolus and 1 mcg/kg/min infusion.
2. Percutaneous coronary intervention (PCI): The recommended dose for patients with normal renal function (i.e., creatinine less than 2) is 180 mcg/kg bolus and 2.0 mcg/kg/min infusion. A second bolus of 180 mcg/kg is given 10 minutes after the first bolus. For patients with renal insufficiency (i.e., creatinine 2-4), use 180-mcg/kg bolus and a 1 mcg/kg/min infusion. A second bolus of 180 mcg/kg is repeated after the first bolus.
WARNINGS
1. Bleeding is the most common complication encountered during therapy with eptifibatide.
2. Use with caution in patients with platelet count less than 150,000/mm3
3. Use with caution in patients with hemorrhagic retinopathy.
4. Caution should be employed when using with other drugs that affect hemostasis.
5. During therapy with eptifibatide, patients should be monitored for potential bleeding. When bleeding cannot be controlled with pressure, infusion of eptifibatide and heparin should be discontinued.
NURSING CONSIDERATIONS
1. Check the patient’s ACT, aPTT, and platelet count at baseline; check again within 6 hours after loading infusion, and check at least daily thereafter during therapy.
2. If patient experiences a platelet decrease to less than 90,000/mm3, additional platelet counts should be performed to exclude pseudothrombocytopenia. If thrombocytopenia is confirmed, eptifibatide and heparin should be discontinued.
3. In PTCA, before pulling the sheath, heparin should be discontinued for 3-4 hours; ACT less than 180 seconds or PTT less than 45 seconds should be documented.
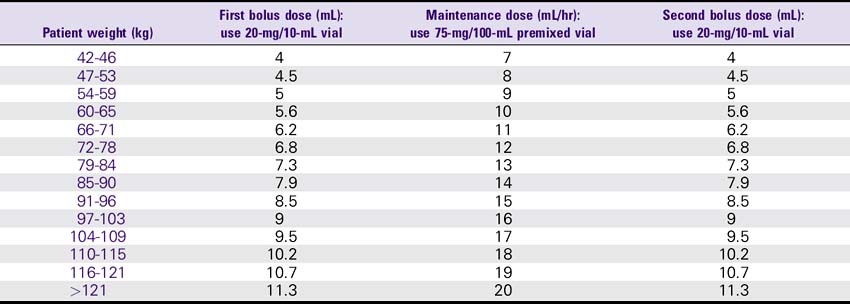
Eptifibatide (Integrilin) Dosing Chart
ESPRIT Trial Dosing for PCI: 180-mcg/kg Bolus, Then 2 mcg/kg/min Infusion, Followed by a Second 180-mcg/kg Bolus 10 Minutes After the First Bolus—Normal Renal Function (Creatinine less than 2)
Esmolol (Brevibloc)
WARNINGS
1. Esmolol is contraindicated in patients with sinus bradycardia or heart block, uncompensated congestive heart failure (CHF), cardiogenic shock, or allergy to beta-blockers.
2. Esmolol should be used with caution in patients with hyperreactive airways, diabetes, hypoglycemia, or renal failure.
3. Esmolol should be administered carefully to avoid extravasation.
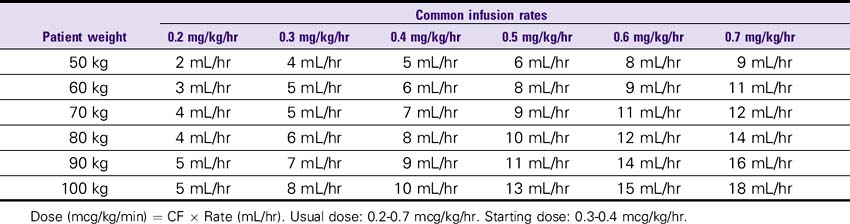
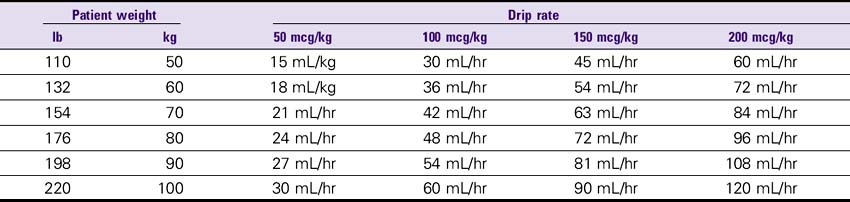
Fenoldopam (Corlopam)
USES
Treatment of severe hypertension and in patients with renal compromise; potential use for congestive heart failure (CHF).
DOSE
1. Severe hypertension: Initial, 0.1 mcg/kg/min; may be increased in increments of 0.05-0.2 mcg/kg/min until target BP is achieved; average rate, 0.25-0.5 mcg/kg/min; usual length of treatment is 1-6 hours with tapering of 12% every 15-30 minutes.
2. Renal perfusion: Initial, 0.05 mcg/kg/min infusion; titrate to a maximum renal perfusion range of 0.1-0.3 mcg/kg/min.
ADVERSE REACTIONS
1. Cardiovascular: Hypotension, edema, tachycardia, facial flushing, asymptomatic T-wave flattening, flutter (atrial), chest pain, angina in patients with history of unstable angina.
3. GI: Nausea, vomiting, diarrhea, xerostomia.
4. Ocular: Intraocular pressure (increased), blurred vision.
5. Other: Increases in portal pressure in cirrhotic patients.
6. The most common side effects are headache, flushing, nausea, and hypotension, each reported in more than 5% of patients.
NURSING CONSIDERATIONS
1. Monitor BP, HR, ECG, renal/hepatic function.
2. No known contraindications.
4. Corlopam contains sodium metabisulfite, which may cause allergic-type reactions, including anaphylactic symptoms, in susceptible people. Corlopam also may cause tachycardia, hypokalemia, or an increase in intraocular pressure.
Haloperidol (Haldol)
USES
For rapid tranquilization of the agitated ICU/cardiac care unit (CCU) patient, haloperidol may be administered IV.
SOLUTION PREPARATION
Haldol may be administered by IV push. Haldol is available as 5 mg/mL in 1-mL and 10-mL vials.
DOSE
1. The usual initial dose is 0.5-2 mg IV for mild agitation, 5-10 mg IV for moderate agitation, and 10+ mg for severe agitation.
2. For subsequent doses: Double the previous dose every 15-30 minutes until the agitation is resolved.
3. When sedation is achieved, maintain the patient by repeating the last dose at 30-minute intervals PRN.
4. Maximum dose: 50-100 mg Haldol given alone or 20 mg Haldol with 1 mg lorazepam (Ativan) IV.
WARNINGS
1. Haldol should not be administered to patients with alcohol or barbiturate withdrawal or brain injury in the past 3-4 days.
2. Contraindicated in patients with narrow angle glaucoma and parkinsonism.
3. Use with caution in patients with cardiovascular disease, seizures, hypotension, CNS depression, or severe liver disease.
NURSING CONSIDERATIONS
1. Observe the patient for onset of drug action 5-30 minutes after the dose is administered.
2. Observe the patient for possible side effects: Laryngospasm—treat with epinephrine 0.1-0.25 mg or 1-2.5 mL IV slowly (keep syringe available at bedside, 1:10,000 concentration, 10 mL syringe); treat extrapyramidal effects with Benadryl IV.
3. The patient’s BP should be monitored closely to observe for hypotension.
Heparin
USES
1. Prophylaxis and treatment of venous thrombosis and its extension; pulmonary embolism, peripheral arterial embolism, atrial fibrillation with embolization.
2. Diagnosis and treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation [DIC]).
3. As an adjunct in the treatment of coronary occlusion with acute myocardial infarction (AMI).
4. Adjuvant therapy with trombolytic agents and antiplatelet glycoprotein GP IIb/IIIa receptor antagonists.
5. For prevention of cerebral thrombosis in stroke patients (usually without loading doses or any bolus doses).
WARNINGS
1. Hemorrhage can occur at virtually any site in patients receiving heparin. An unexplained fall in hematocrit, fall in BP, or any other unexplained symptom should lead to serious consideration of a hemorrhagic event.
2. Use heparin with extreme caution in disease states in which there is increased danger of hemorrhage (e.g., dissecting aneurysm, increased capillary permeability, severe hypertension, spinal anesthesia, GI ulceration, diverticulitis or ulcerative colitis, and liver disease with impaired hemostasis).
3. Thrombocytopenia may occur in patients receiving heparin; baseline and regular platelet monitoring is recommended.
ADVERSE REACTIONS
1. Hemorrhage: Bleeding can usually be controlled by withdrawing the drug. NOTE: 1 mg of protamine will neutralize 100 units of heparin.
2. Hypersensitivity: Chills, fever, urticaria and rarely asthma, nausea/vomiting, shock or anaphylactoid reactions.
3. Thrombocytopenia: May occur 2-12 days after therapy is begun; platelet counts should be done on a regular basis while patient is receiving heparin infusion.
4. Other: Cutaneous necrosis, vasospastic reactions with pain, ischemia and cyanosis in affected limb, suppressed aldosterone synthesis.
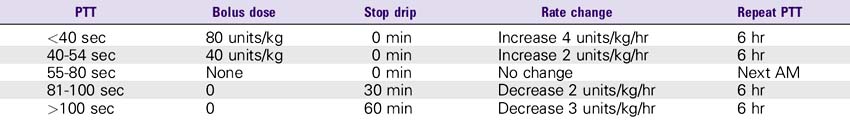
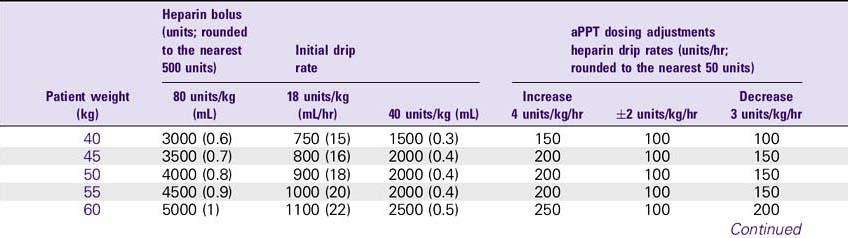
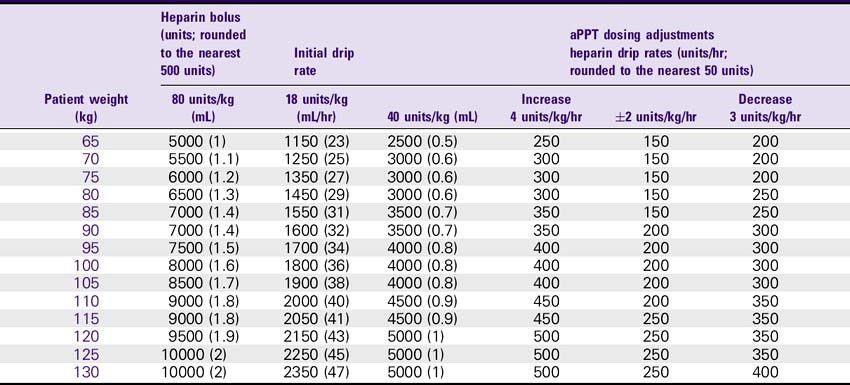
NURSING CONSIDERATIONS
1. Consult the Heparin Drip Rate Calculation Chart to determine drip rate.
2. Heparin should be administered via an IV pump to ensure controlled infusion.
3. Monitor patients for signs/symptoms of hemorrhage; contact physician if symptoms occur.
Heparin Drip Rate Calculation Chart
Heparin 25,000 units/500 mL (Concentration: 50 units/mL)
| Dose | Infusion rate |
|---|---|
| 800 units/hr | 16 mL/hr |
| 900 units/hr | 18 mL/hr |
| 1000 units/hr | 20 mL/hr |
| 1100 units/hr | 22 mL/hr |
| 1200 units/hr | 24 mL/hr |
| 1300 units/hr | 26 mL/hr |
| 1400 units/hr | 28 mL/hr |
| 1500 units/hr | 30 mL/hr |
| 1600 units/hr | 32 mL/hr |
| Height | Males (kg) | Females (kg) |
|---|---|---|
| 5’0″ | 50 | 45.5 |
| 5’1″ | 52.3 | 47.8 |
| 5’2″ | 54.6 | 50 |
| 5’3″ | 56.9 | 52.4 |
| 5’4″ | 59.2 | 54.7 |
| 5’5″ | 61.5 | 57 |
| 5’6″ | 63.8 | 59.3 |
| 5’7″ | 66.1 | 61.6 |
| 5’8″ | 68.4 | 63.9 |
| 5’9″ | 70.7 | 66.2 |
| 5’10″ | 73 | 68.5 |
| 5’11″ | 75.3 | 70.8 |
| 6’0″ | 77.6 | 73.1 |
| 6’1″ | 80 | 75.4 |
| 6’2″ | 82.2 | 77.7 |
| 6’3″ | 84.5 | 80 |
| 6’4″ | 86.8 | |
| 6’5″ | 89.1 | |
| 6’6″ | 91.4 |
Ibutilide (Corvert)
WARNINGS
1. Observe patients with continuous ECG monitor for at least 4 hours after infusion.
2. Potentially fatal arrhythmias (e.g., ventricular tachycardia), usually in association with torsades de pointes (QT prolongation), may occur with ibutilide (up to 1.7% incidence of arrhythmias in treated patients).
INCOMPATIBILITY/INTERACTIONS
1. Avoid use with other antiarrhythmic agents (disopyramide, quinidine, and procainamide) due to increased toxicity. Also, amiodarone and sotalol should be avoided due to their potential to prolong refractoriness.
2. Avoid use with phenothiazines, tricyclic and tetracyclic antidepressants, and nonsedating antihistamines (astemizole) due to QT prolongation.
NURSING CONSIDERATIONS
1. Monitor QT intervals. Discontinue infusion if ventricular tachycardia or prolongation of QT occurs.
2. Ibutilide will usually not affect cardiac output, mean pulmonary arterial pressure, or pulmonary capillary wedge pressure (PCWP).
3. Hypokalemia and hypomagnesemia should be corrected prior to use because prolonged QT and arrhythmias are more common.
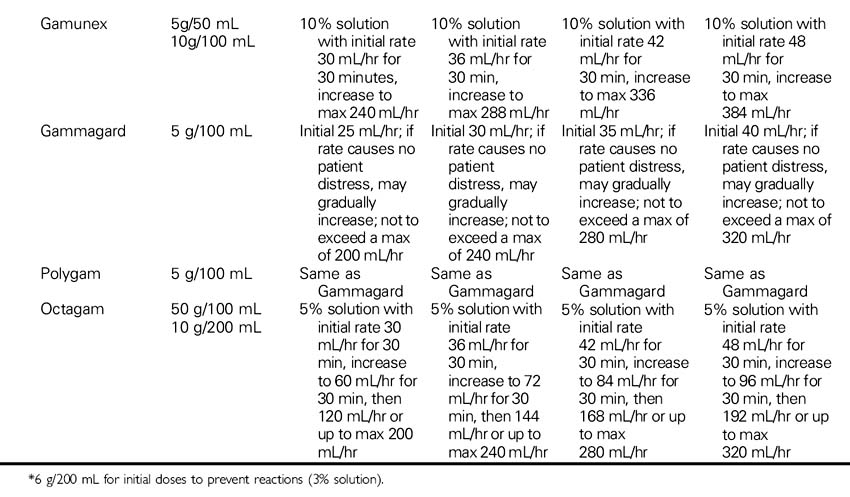
Immune Globulin Intravenous (Carimune, Gamimune, Gammagard, Gammar-P, Gamunex, Iveegam, Octagam, Panglobulin, Polygam, Sandoglobulin, Venoglobulin-S)
USES
For treatment of immunodeficiency syndromes (e.g., hypogammaglobulinemia, agammaglobulinemia), idiopathic thrombocytopenic purpura (ITP), chronic lymphocytic leukemia (CLL), refractory dermatomyositis or polymyositis, autoimmune diseases (e.g., myasthenia gravis or severe rheumatoid arthritis), and Guillain-Barré syndrome.
NURSING CONSIDERATIONS
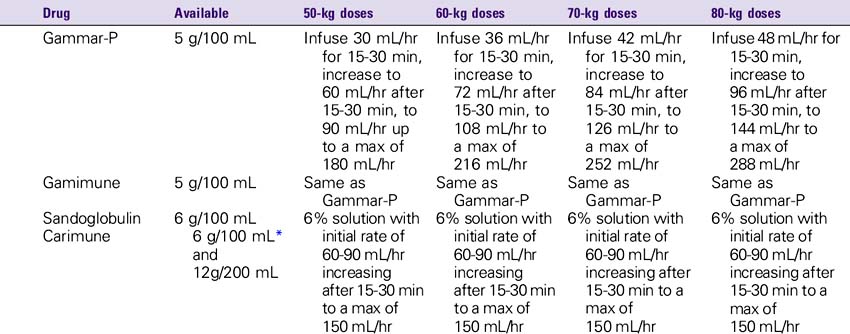
1. Monitor the patient closely for signs of anaphylaxis during the initial dose and at the beginning of the infusion.
2. Consult the Immune Globulin Drip Rate Calculation Chart to determine the drip rate.
3. Use caution in the elderly and in patients with diabetes, preexisting renal disease, or volume depletion. To minimize risk of renal dysfunction, administer at the lowest rate of infusion and lowest concentration that is practical.
Inamrinone (Inocor)
Previously known as amrinone; name changed to avoid confusion with amiodarone.
DOSE
1. Initial bolus of 0.75 mg/kg given slowly over 2-3 minutes; may be repeated in 30 minutes if necessary.
2. Maintenance infusion between 5-10 mcg/kg/min.
3. Total daily dose, including boluses and cumulative infused dose, usually should not exceed 10 mg/kg. (Higher doses, up to 18 mg/kg/day, have been used in a limited number of patients.)
WARNINGS
1. May aggravate symptoms of idiopathic hypertrophic subaortic stenosis.
2. Patients who have received large doses of diuretics may require cautious administration of fluids and electrolytes to ensure sufficient cardiac filling pressure for Inocor to be effective.
3. Inamrinone-induced increases in cardiac output, with resulting diuresis, may require a dose reduction of diuretics to prevent excessive potassium loss.
4. Inamrinone is not recommended for patients with acute myocardial infarction (AMI).
5. Contraindications: Patients sensitive to bisulfates (e.g., asthmatics).
6. Increased plasma concentrations of inamrinone may occur in patients with compromised liver or renal function.
7. Excessive hypotension has occurred when used concomitantly with disopyramide (Norpace).
ADVERSE REACTIONS
1. Thrombocytopenia: Appears to be dose dependent; consider dosage reduction or discontinue inamrinone.
4. May increase AV conduction.
6. Hepatotoxicity: Manifested by elevations in liver function tests and clinical symptoms suggestive of idiosyncratic hypersensitivity reaction.
7. Hypersensitivity reactions (e.g., pericarditis, pleuritis, ascites, jaundice, myositis, vasculitis).
NURSING CONSIDERATIONS
1. Depending on the patient’s status, an accurate weight should be obtained before the administration of inamrinone.
2. Consult the Inamrinone Drip Rate Calculation Chart to determine the drip rate.
3. Except in cardiac arrest situations, inamrinone should be administered via an IV pump to ensure controlled infusion.
4. BP should be measured every 15 minutes until stable, then at least every hour as long as drip remains in use.
5. Physician should order platelet count before inamrinone administration and daily thereafter.
6. Closely monitor and document changes in central venous pressure (CVP), HR, urine output, body weight, and clinical signs and symptoms of CHF (e.g., orthopnea, dyspnea, fatigue).
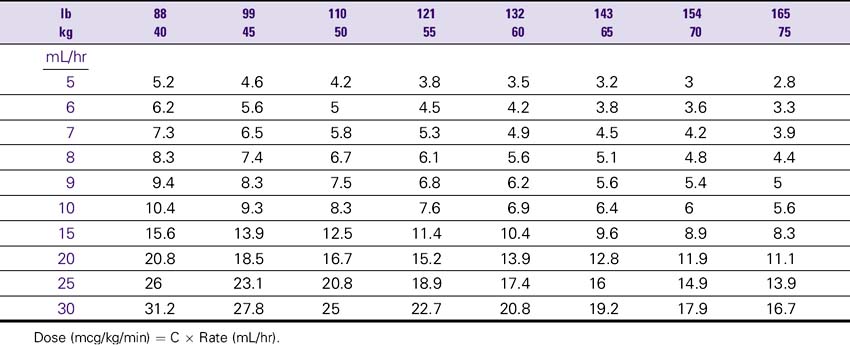
Inamrinone (Inocor) Drip Rate Calculation Chart—Patient Weight 40-75 kg (88-165 lb)
Concentration: 2.5 mg/mL (500 mg/200 mL Total Volume)
Infliximab (Remicade)
USES
1. Infliximab is a chimeric IgG1 monoclonal antibody that binds to human tumor necrosis factor alpha (TNF-α).
2. In combination with methotrexate, it is indicated for the treatment of rheumatoid arthritis.
3. It is also indicated as treatment for moderate to severe active Crohn’s disease and fistulizing Crohn’s disease.
SOLUTION PREPARATION
1. Infliximab is a sterile, white, lyophilized powder for IV infusion. It is in a single-use 20-mL vial of 100 mg. Infliximab should be stored in the refrigerator at 2°-8° C (36°-46° F).
2. Reconstitute with 10 mL of sterile water for injection. Swirl the vial to dissolve the powder; do not shake. Let stand for 5 minutes. Dilute to 250 mL with 0.9% sodium chloride. Mix gently. Infuse over a minimum of 2 hours, with an in-line, sterile, nonpyrogenic, low-protein binding filter of less than 1.2 mcm pore. The infusion should be started within 3 hours of preparation.
WARNINGS
NURSING CONSIDERATIONS
1. Monitor patients for signs and symptoms of infection while they are on infliximab.
2. Inspect the solution for foreign particles or discoloration before infusion. The solution should be clear to yellow colored, opalescent, with few translucent particles.
Infliximab (Remicade) Dosing Chart
(Due to the high cost, doses are usually rounded to the nearest whole vial size to avoid waste)
| Rheumatoid arthritis (Final concentration: 3 mg/kg dose, 0.4-4 mg/mL) | |
| Patient weight | Dose (mg) (per NS 250 mL) |
| ≤33 kg | 100 mg |
| 34-67 kg | 200 mg |
| 68-100 kg | 300 mg |
| 101-133 kg | 400 mg |
| Crohn’s disease (Final concentration: 5 mg/kg dose, 0.4-4 mg/mL) | |
| Patient weight | Dose (mg) (per NS 250 mL) |
| 21-40 kg | 200 mg |
| 41-60 kg | 300 mg |
| 61-80 kg | 400 mg |
| 81-100 kg | 500 mg |
| 101-120 kg | 600 mg |
Infliximab (Remicade) Initial Infusion Rates for Each 250-mL Bag*
| Time | Rate |
|---|---|
| Start-15 min | 10 mL/hr |
| 15-30 min | 20 mL/hr |
| 30-45 min | 40 mL/hr |
| 45-60 min | 80 mL/hr |
| 60-90 min | 150 mL/hr |
| 90-120 min | 250 mL/hr |
Insulin Drip
SOLUTION PREPARATION
Final concentration: 100 units/250 mL (2 units/5 mL).
For intensive insulin therapy in critical care mix 100 units (1 mL) regular insulin in 100 mL NS.
WARNINGS
Monitor blood sugar as ordered; hypoglycemia is possible if sugars are controlled too rapidly.
NURSING CONSIDERATIONS
1. Consult the Insulin Drip Rate Calculation Chart to determine the drip rate.
2. Insulin should be administered via an IV pump to ensure controlled infusion.
Insulin Drip Rate Calculation Chart
Insulin 100 units in 250 mL (Concentration: 2 units/5 mL)
| Dose | Infusion rate |
|---|---|
| 10 units/hr | 25 mL/hr |
| 15 units/hr | 38 mL/hr |
| 20 units/hr | 50 mL/hr |
| 25 units/hr | 63 mL/hr |
| 30 units/hr | 75 mL/hr |