CHAPTER 39 COMPLICATIONS OF PULMONARY AND PLEURAL INJURY
It has been stated that chest trauma is the primary cause of death in up to 25% of fatalities following traumatic injury, and a major contributing factor in another 25%, although as few as 5%–15% require acute operative intervention. Based on these generalizations, it is accepted that overall chest injury is common, acute operative intervention uncommon, and a significant, although ill-defined, number of thoracic operations are performed for delayed complications. The actual incidence of each of these varies from center to center based on ratio of blunt to penetrating admissions as well as overall volume. The two most common complications, persistent air leak and empyema, occur roughly in 5% of patients admitted who have required tube thoracostomy.
PULMONARY
Persistent Air Leak
Persistent air leak in a ventilated patient without a discrete lesion is better thought of as an alveolar-pleural fistula rather than a BPF. Clearly, the underlying lung injury affects outcome, with alveolar-pleural leak in adult respiratory distress syndrome patients being associated with up to 80% mortality. Whatever the underlying anatomy, air leak in ventilated patients can be a significant marker of increased mortality. Pierson and colleagues reviewed the course of 39 patients (out of a population of 1700 mechanically ventilated patients) who presented with air leak lasting more than 24 hours, of whom 27 were trauma patients. The risk factors for mortality correlated with the following: air leak not present on admission or shortly thereafter (45% early vs. 94% if developed later); leak greater than 500 ml per breath (57% if less vs. 100% if greater); and post–chest trauma (56% for trauma admissions vs. 92% for nontrauma admissions). These findings illustrate that while the course in trauma admissions is more benign, it still represents a major concern. On the other hand, the air leak itself is rarely the cause of death. These air leaks can lead to persistent or even tension pneumothorax that compromises ventilation. Pleural tubes (at times multiple) may be required. Less commonly, air leak is significant enough to affect oxygenation. The primary treatment is to minimize alveolar pressure, using end-inspiratory plateau pressure as an (admittedly crude) reflection of this. Ideally, the end-inspiratory plateau pressure should be less than 30 cm H2O. The most common method of attaining this is to combine low tidal volume and permissive hypercapnia. Alternative methods if this approach fails are high-frequency jet ventilation or independent lung ventilation. It should be stressed that high-frequency jet ventilation, although used successfully in patients with central airway disruption and in the operating room, does not reduce mean airway pressure consistently, nor does it uniformly reduce air leak or improve oxygenation. Thus, it should not be used routinely in patients with alveolar-pleural fistula. A temporizing technique is to isolate the lobe that is the primary source of leak bronchoscopically. This is done by sequentially occluding bronchi with a Swan-Ganz or other balloon catheter. If this results in elimination or significant reduction in air leak, occlusive material (Gelfoam, fibrin glue, blood mixed with tetracycline, etc.) can be injected. In most cases, the air leak will diminish as airway pressure decreases. Surgery can be performed, but in the setting of diffuse parenchyma injury, lung inflammation, severe emphysema, and/or steroids, the risk is that staple lines will fail and the leak will be exacerbated. If surgery is felt to be needed, reinforced staple lines (i.e., with bovine strips, etc.), apical tents (mobilizing the apical pleura so that it falls onto the area of resection), and/or anatomic lobectomy (if predominantly one lobe) should be considered.
Pneumonia
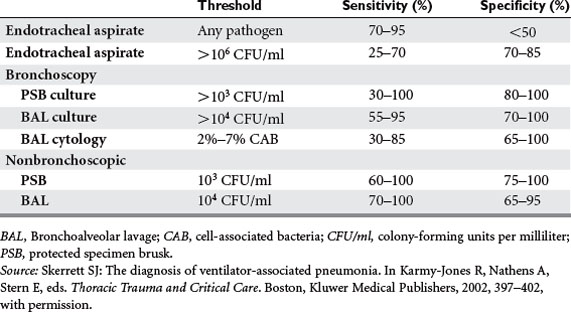
Pneumonia may be the most common complication of chest trauma. Risk factors include aspiration, need for ventilation, direct injury, pulmonary contusion, and persistent atelectasis secondary to pain. The incidence is as low as 6% in nonintubated patients to as high as 44% in ventilated patients. Despite the high incidence, there are no data supporting prophylactic antibiotics. Of all patients admitted with a diagnosis of pulmonary contusion, nearly 50% will develop pneumonia, barotrauma, and/or major atelectasis. One-fourth will progress to adult respiratory distress syndrome. Ventilatorassociated pneumonia (VAP), defined as pneumonia arising more than 48 hours after initiation of mechanical ventilation, is difficult to define and diagnose. At our institution, we have found that the incidence in patients ventilated for more than 7 days ranges from 20% to 30%. Clinical suspicion is often raised by new infiltrates, recurring fever, rising leukocytes, and/or a change in endotracheal secretions. However, distinguishing between colonization and infection may require specialized techniques. Quantitative cultures obtained from a variety of approaches increase the specificity (although perhaps with reduced sensitivity) of endobronchial cultures, and each institution must define cut-off values based on whether the patient is already receiving antibiotics (Table 1).
Necrotizing Lung Infection
The cause(s) of lung abscess in the surgical intensive care unit (ICU) population include aspiration, complications of pneumonia, retained foreign body, septic emboli, and/or infected traumatic injury. More specific etiologies in the trauma population include aspiration (with or without bronchial obstruction), infected pneumatocoele, infected site of resection (in particular emergent tractotomy), and late complications of ventilatorassociated pneumonia. As a whole, these are less common in trauma patients than nontrauma patients. Of 45 thoracotomies performed at our institution over 7 years for abscess, necrotizing pneumonia, and lung gangrene, only 4 were in patients initially admitted after traumatic injury.
Over the three decades of approximately the 1950s through the 1970s, a number of advances reduced the mortality rates from approximately 50% to 10%. These advances included recognizing the importance of antibiotics, the role of aspiration, the need for pulmonary toilet (including liberal use of bronchoscopy), and finally the benefit in selected patients of operative intervention. Subsequently, the major addition to the armamentarium has been image-guided catheter drainage as an intermediate category between medical and surgical management. Percutaneous catheter drainage can be performed even in ventilated patients and has reduced the number of thoracotomies required. While there is always concern about the risk of empyema and/or bronchopleural fistula, the former can be usually easily managed by chest drainage, and the latter is rarely so significant as to impair oxygenation. Some patients will require thoracotomy, which, in the trauma population, usually results from persistent sepsis and inability or incomplete drainage, hemoptysis, or persistent or major bronchopleural fistula (see Table 1). The two primary operations are lobectomy for large central cavities, or debridement (plus muscle flap to help close the space) for smaller peripheral cavities. At operation, there are several technical points that can help reduce complications: prevent aspiration by isolating the affected lung before posterolateral positioning; expose the main pulmonary artery early in the case so that control can be achieved should hemorrhage result; place a nasogastric tube or esophagoscope in the esophagus because the anatomy may be obliterated; and refrain from resecting small abscesses (<2 cm) that are in otherwise viable parenchyma. Air leak is not uncommon, and, as will be discussed under the empyema section, a residual space can be managed with continuous postoperative irrigation.
Retained Parenchyma Missiles
The need for removal of parenchyma foreign objects is based on the risk of developing complications, which appear to be more common with irregularly shaped missiles compared with smooth objects. The University of Heidelberg reviewed the course of 55 patients who had retained bullets. Thirty-four experienced recurrent hemoptysis (single episode in eight). A Finnish review of 502 patients over several years noted that 20% developed complications requiring surgery. These included chronic bronchitis (39), lung abscess (31), bronchiectasis (5), empyema (24), and/or bronchopleural fistula (10). Many contemporary recommendations are based on data from World War II and in the two decades following, including the aforementioned studies. In World War II, early removal of retained missiles was associated with 0.9% mortality, while late removal of symptomatic objects was associated with 7.3% mortality. However, it was noted then and subsequently that waiting 2–6 weeks to allow parenchyma inflammation to resolve was also associated with easier removal, with reduced complications, notably less bleeding, air leak, and empyema. The technique of removal obviously depends on the location and nature of the missile. Peripheral objects can be removed by wedge resection. Deeper objects can be retrieved via tractotomy or occasionally lobectomy if there is significant associated destruction, necrosis, or infection. Uncommonly, over time central foreign objects can erode into the bronchi, leading to obstructive pneumonitis and abscess formation. These may be retrieved endoscopically, while severe tissue destruction, if present, mandates lobectomy. Alternatively, these objects may migrate peripherally, resulting in empyema. These can be retrieved and the empyema managed by VATS or thoracotomy.
PLEURAL
Empyema
Empyema has been described as having three stages. The first, usually within 1–7 days, is referred to as the “acute” or “serous” phase. This distinction is important because at this stage there is the best chance for draining the thin, exudative fluid by simple thoracostomy. There have been attempts to treat this early stage by simple aspiration. Evidence of vigorous inflammation (pH<7.0) almost universally predicts failure of this technique. Tapping may be appropriate in patients who have complex effusions, with or without loculations, but who have other potential sites of infection. However managed, it is imperative that complete drainage be achieved, or failing that, early operative drainage is performed before progressive pleural obliteration occurs, characteristic of progression to the second or “subacute” phase, and thence the final or “chronic” phase. Palpation at the time of thoracostomy and/or loculations noted on CT can alert the surgeon to the presence of loculations that would indicate simple tube drainage will fail.
The primary treatment of empyema is to both completely drain the thorax and to permit full lung expansion. There are several “local” considerations that may impact operative approach and outcomes (Table 2). Predominant among these are whether loculations and/or a restrictive visceral peel have formed. In the acute setting, particularly when clinical signs suggest active infection, the primary goal is simply to drain the pleura. Evidence of loculations suggests that simple tube drainage will fail. Alternative approaches could include image-directed catheter placement, thoracoscopic drainage, and “mini” or full thoracotomy. Thrombolytic therapy has been advocated as an alternative to operative intervention, but current data suggest that when compared with thoracoscopic approaches as primary intervention, thrombolytic therapy is associated with a higher failure rate, increased length of stay, and greater cost. Thrombolytic therapy is a reasonable alternative in patients who are deemed at high risk for operative intervention, and whose loculations may be diaphanous. In essence, these criteria would be in the uncommon scenario of a patient who is clinically infected and frail but not yet intubated, as once a patient is on the ventilator, the primary complication of operative approaches (respiratory failure) has already occurred. Thrombolytic therapy does have a role in the early postoperative period after operative decortication residual loculated fluid collections are present. In this setting, the fibrinous adhesions are “soft” and may be lysed.
The residual pleural space remains a problem, requiring a flexible approach, depending primarily on whether the lung is capable of expanding (Table 3). In the trauma population, the primary reason for failure of lung expansion is visceral peel, while in nontraumatic empyema, the etiology is relatively equally divided among visceral peel, parenchyma consolidation, and/or space after lung resection.
Table 3 Managing Residual Space
Aguilar MM, Battistella TD, Owings JT, Su T. Posttraumatic empyema: risk factor analysis. Arch Surg. 1997;132:647-650.
Bayfield MS, Spotnitz WD. Fibrin sealant in thoracic surgery: pulmonary applications, including management of bronchopleural fistula. Chest Surg Clin North Am. 1996;6:567-584.
Boyd AD, Glassman LR. Trauma to the lung. Chest Surg Clin North Am. 1997;7:263-284.
Eddy AC, Luna GK, Copass M. Empyema thoracis in patients undergoing emergent closed tube thoracostomy for thoracic trauma. Am J Surg. 1989;157:494-497.
Hagan JL, Hardy JD. Lung abscess revisited: a survey of 184 cases. Ann Surg. 1983;197:755-762.
Karmy-Jones R, Jurkovich GJ. Blunt chest trauma. Curr Prob Surg. 2004;41:211-380.
Karmy-Jones R, Vallieres E, Harrington R. Surgical management of necrotizing pneumonia. Clin Pulm Med. 2003;10:17-25.
Dulchavesky SA, Ledgerwood AM, Lucas CE. Management of chylothorax after blunt chest trauma. J Trauma. 1988;28:1400-1401.
Meyer DM, Jessen ME, Wait MA, Estera AS. Early evacuation of traumatic retained hemothoraces using thoracoscopy: a prospective, randomized trial. Ann Thorac Surg. 1997;64:1396-1400.
Moon WK, Im JG, Ycon KM, et al. Complications of Klebsiella pneumonia: CT evaluation. J Comput Assist Tomogr. 1995;19:176-181.
Pierson DJ, Horton CA, Bates PW. Persistent bronchopleural air leak during mechanical ventilation: a review of 39 cases. Chest. 1986;90:321-323.
Richardson JD, Carrillo E. Thoracic infection after trauma. Chest Surg Clin North Am. 1997;7:401-428.
Schamaun M. Postoperative pulmonary torsion: report of a case and survey of the literature including spontaneous and posttraumatic torsion. Thorac Cardiovasc Surg. 1994;42:116-121.
Schamaun M, von Buren U, Pirozynski W. Massive lung necrosis in. Klebsiella pneumonia. Schweiz Med Wochenser. 1980;110:223-225.
Schermer CR, Matteson BD, Demarest GB3rd, et al. A prospective evaluation of video-assisted thoracic surgery for persistent air leak due to trauma. Am J Surg. 1999;177:480-484.
Skerrett SJ. The diagnosis of ventilator-associated pneumonia. In: Karmy-Jones R, Nathens A, Stern E, editors. Thoracic Trauma and Critical Care. Boston: Kluwer Medical Publishers; 2002:397-402.
Stathopoulos G, Chrysikopoulou E, Kalogeromitros A, et al. Bilateral traumatic pulmonary pseudocysts: case report and literature review. J Trauma. 2002;53:993-996.
Taskinen SO, Salo JA, Halttunen PE, Sovijarvi AR. Tracheobronchial rupture due to blunt chest trauma: a follow-up study. Ann Thorac Surg. 1989;48:846-849.
Velmahos GC, Demetriades D, Chan L, et al. Predicting the need for fhoracoscopic evacuation of residual traumatic hemothorax: chest radiograph is insufficient. J Trauma. 1999;46:65-70.
Vogt-Moykopf MD, Krumhaar D. Treatment of intrapulmonary shell fragments. Surg Gynecol Obstet. 1966;123:1233-1236.
Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest. 1997;111:1548-1551.