History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
Findings
Chest Radiograph
Findings
Echocardiogram
Findings
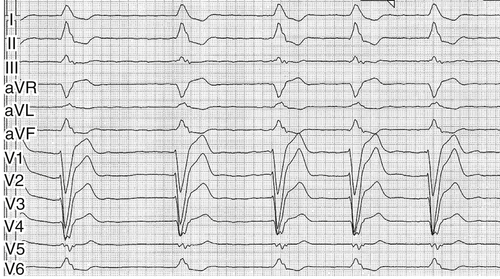
FIGURE 2-1 Surface 12-lead electrocardiogram, recording speed 50 mm/sec (see text for interpretation).
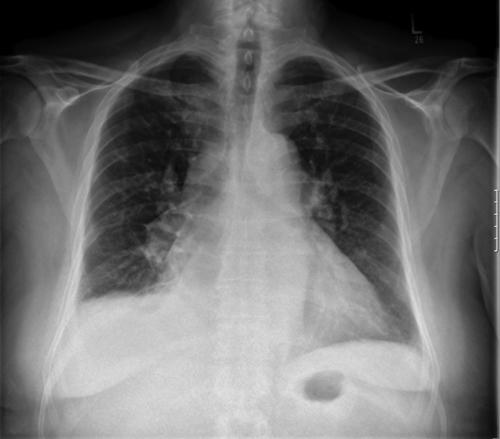
FIGURE 2-2 Chest radiograph, posteroanterior view (see text for interpretation).
Focused Clinical Questions and Discussion Points
Question
Discussion
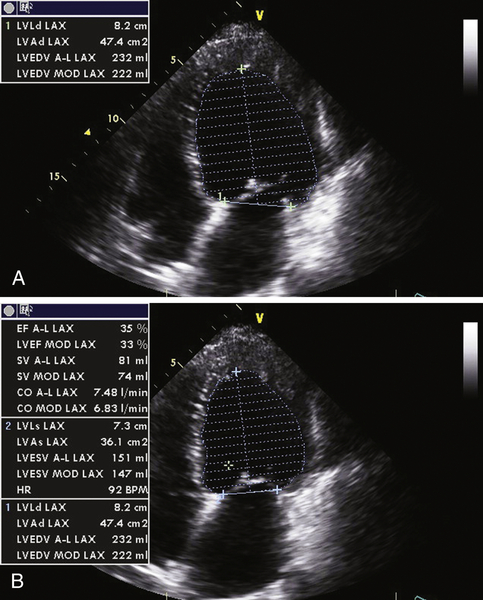
FIGURE 2-3 Two-dimensional transthoracic echocardiographic still-frame images from the 4-chamber view during diastole (A) and systole (B) (see text for interpretation).
Question
Discussion
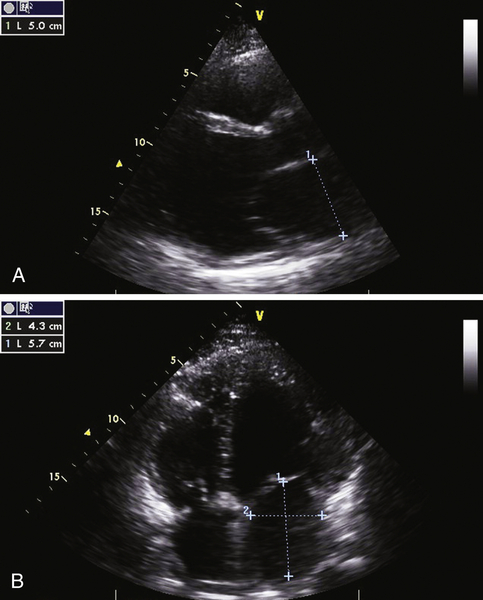
FIGURE 2-4 Two-dimensional transthoracic echocardiographic still-frame images from the parasternal long axis (A) and the 4-chamber view (B) (see text for interpretation).
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
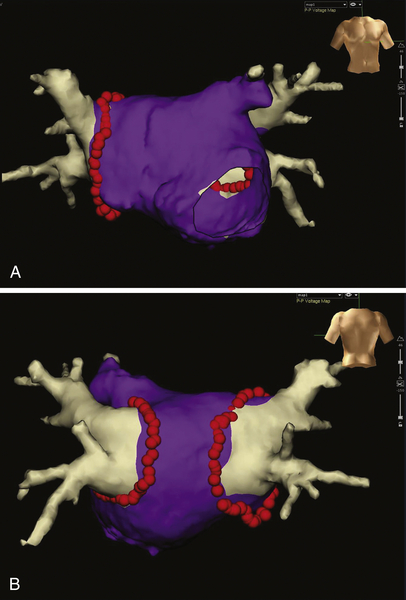
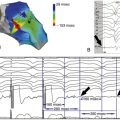
FIGURE 2-5 Anterior-posterior (A) and posterior-anterior (B) view of a three-dimensional model of the left atrium and the pulmonary veins acquired by preprocedural computed tomography and registered into the electroanatomic mapping system. Red dots indicate the circumferential ablation line, purple areas represent bipolar voltages with an amplitude greater than 0.5 mV (normal voltage by definition).
Findings
Selected References
1. Brignole M., Auricchio A., Baron-Esquivias G. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–2329.
2. Calkins H., Kuck K.H., Cappato R. et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606.
3. Dickstein K., Bogale N., Priori S. et al. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–2460.
4. Dickstein K., Vardas P.E., Auricchio A. et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12:1526–1536.
5. Gasparini M., Auricchio A., Regoli F. et al. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression: the importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006;48:734–743.
6. Gasparini M., Auricchio A., Metra M. et al. Multicentre Longitudinal Observational Study (MILOS) Group: Long-term survival in patients undergoing cardiac resynchronization therapy: the importance of performing atrio-ventricular junction ablation in patients with permanent atrial fibrillation. Eur Heart J. 2008;29:1644–1652.
7. Gasparini M., Steinberg J.S., Arshad A. et al. Resumption of sinus rhythm in patients with heart failure and permanent atrial fibrillation undergoing cardiac resynchronization therapy: a longitudinal observational study. Eur Heart J. 2010;31:976–983.
8. Khan M.N., Jaïs P., Cummings J. et al. PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785.
9. Koplan B.A., Kaplan A.J., Weiner S. et al. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53:355–360.
10. Melenovsky V., Hay I., Fetics B.J. et al. Functional impact of rate irregularity in patients with heart failure and atrial fibrillation receiving cardiac resynchronization therapy. Eur Heart J. 2005;26:705–711.
11. Upadhyay G.A., Choudhry N.K., Auricchio A. et al. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008;52:1239–1246.