57 Subarachnoid Hemorrhage
Clinical Vignette
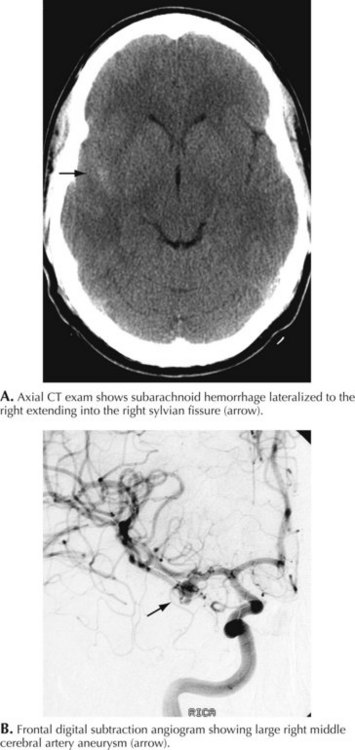
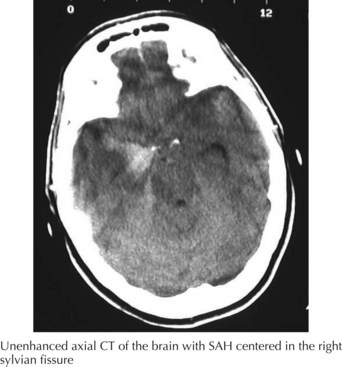
A 66-year-old woman suddenly experienced a terrible temporal pain radiating into her forehead. The headache was so severe that she almost lost consciousness. She became nauseated, vomited, and felt disoriented. Her family called emergency medical services and had her brought to the emergency room. There, she was noted to be arousable but sleepy and confused. She had nuchal rigidity, photophobia, but no focal motor deficit. An unenhanced head computed tomography (CT) showed subarachnoid hemorrhage centered in the right sylvian fissure but no brain parenchymal abnormalities. Angiography demonstrated a ruptured middle cerebral artery aneurysm that was successfully clipped the next morning. The patient’s postoperative course was uneventful (Figs. 57-1 and 57-2).
Clinical Vignette
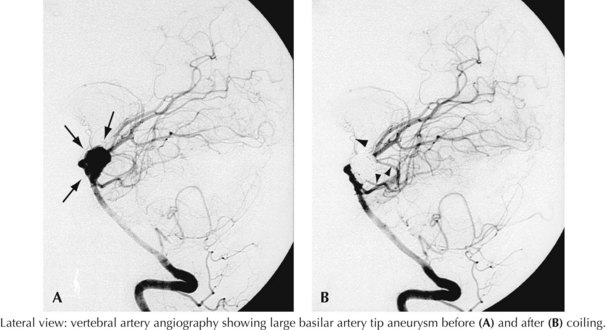
A 44-year-old postal worker presented with severe headache and nuchal rigidity to an emergency room. Unenhanced head CT revealed a subarachnoid hemorrhage centered in the basal cisterns, and further evaluation with catheter angiography revealed a large aneurysm arising from the basilar artery tip. After consultation between the neurosurgeon and the interventional neuroradiologist, the decision to perform endovascular coil embolization was made and the procedure was carried out successfully. The patient recovered fully after a 3-week hospital stay but required a second procedure after 6 months when follow-up angiography demonstrated a reexpansion of the neck of the aneurysm (Figs. 57-3 and 57-4).
Clinical Presentation
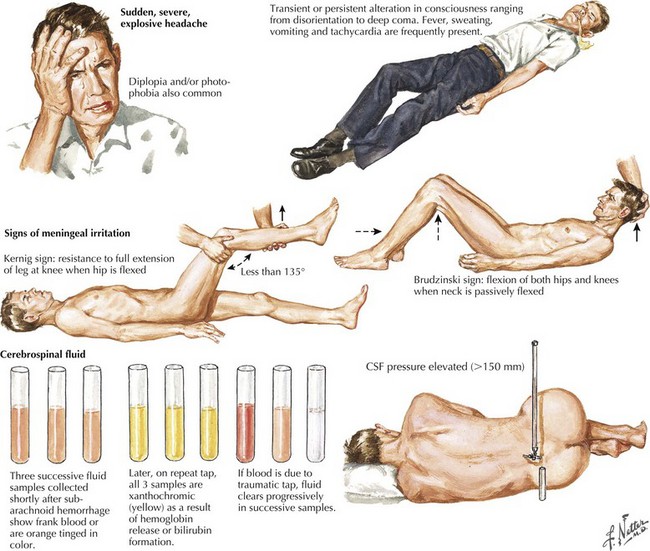
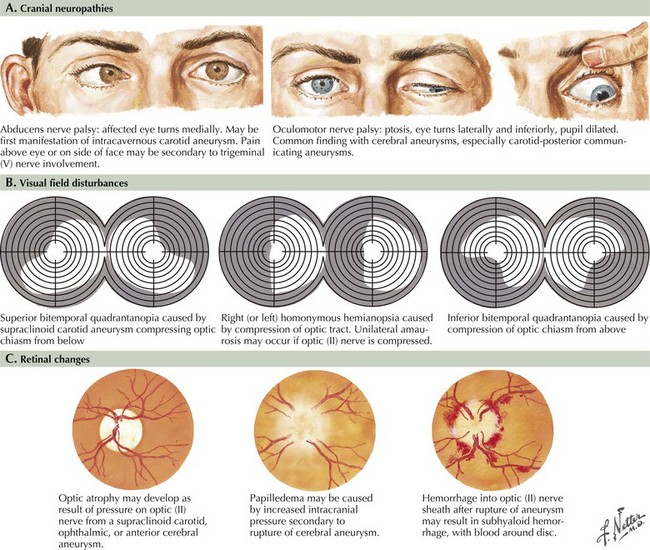
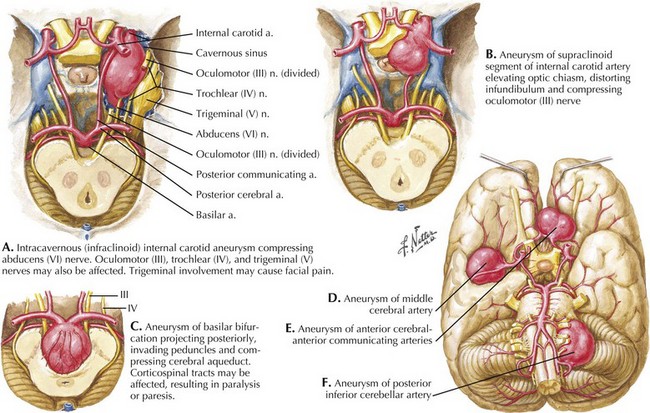
Seizure-like activity may be observed. The incidence of true seizure activity in patients with SAH is estimated at 20%. Seizures in SAH are most commonly associated with middle cerebral artery (MCA) and anterior communicating artery (ACA) aneurysmal rupture causing intracerebral hematomas. Unfortunately, many patients recall having a sentinel hemorrhage or warning leak with a fleeting but severe headache within the 2–3 weeks before the major ictus. This headache is somewhat milder and usually not associated with meningismus; it is often ignored until the catastrophic return of a major rupture. When evaluating patients with SAH or sudden severe headache, special attention should be focused on the level of consciousness, focal neurologic signs such as hemiparesis or cranial nerve palsies, and signs of meningismus (Fig. 57-5). Meningismus frequently occurs, associated with nuchal rigidity. Brudzinski’s maneuver is an excellent means of evaluating meningismus; the examiner flexes the patient’s neck, precipitating hip flexion, knee flexion, and hamstring pain. Diplopia (due to abducens or oculomotor nerve palsies) and visual loss (chiasmal or optic nerve involvement) are caused by either cranial nerve compression from the aneurysmal dome or aneurysmal rupture and increased intracranial pressure (Fig. 57-6).
Diagnostic Approach
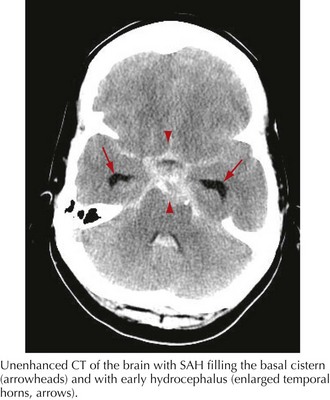
The clinical diagnosis of SAH is best confirmed with brain CT (Fig. 57-7). Its sensitivity is highest in the first 24 hours after headache onset. A mild hemorrhage may wash away within 24 hours but approximately 50% of severe SAHs are still visible on CT 1 week after the ictus, and only one third are seen after 2 weeks. CT confirms the presence of SAH and frequently highlights associated issues such as hydrocephalus, intraparenchymal hematoma, intraventricular hemorrhage, or subdural hemorrhage.
Whenever the clinical suspicion of SAH exists but CT is negative, a lumbar puncture must be performed. A nontraumatic tap is crucial. When the presence of blood in the CSF does not clear between the first and fourth tubes, this is particularly suggestive of SAH (See Fig. 57-5). However, a more sensitive indicator is CSF xanthochromia, which represents lysis of erythrocytes with degradation of heme products into bilirubin within the CSF. This frequently renders the CSF a yellowish color within 1–3 hours after an SAH, and often persists for approximately 2–3 weeks.
To ensure proper communication, predict outcomes, and guide management, a clinical grade for each SAH is needed. Several grading scales are available; the most widely used is the Hunt–Hess scale—a five-tiered description of the patient’s state and an indicator of prognosis (Table 57-1).
| Grade | Description |
|---|---|
| 1 | Asymptomatic, or mild headache and slight nuchal rigidity |
| 2 | Moderate to severe headache, nuchal rigidity, no neurologic deficits other than cranial nerve palsies. |
| 3 | Mild focal deficit, lethargy, confusion |
| 4 | Stupor, hemiparesis, central neurologic signs |
| 5 | Deep coma, decerebrate rigidity, moribund appearance |
Pathophysiology
Intracranial Aneurysms
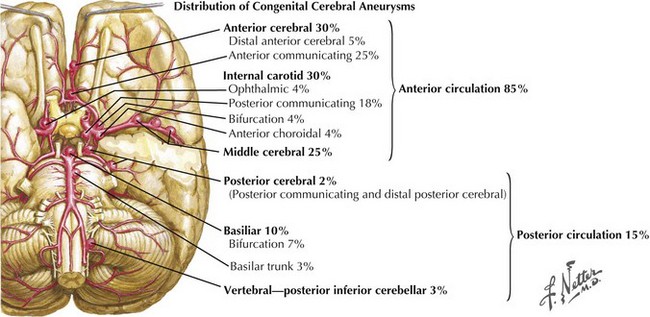
Intracranial aneurysms characteristically occur at branch points of major cerebral arteries. Almost 85% of aneurysms are found in the anterior circulation and 15% within the posterior circulation (Fig. 57-8). Overall, the most common sites are the anterior communicating artery followed by the posterior communicating artery and the middle cerebral artery bifurcation. Within the posterior circulation, the most preponderant site is at the top of the basilar artery bifurcation into the posterior cerebral arteries.
Aneurysms are frequently classified according to size, with small being less than 10 mm, large 10–25 mm, and giant aneurysms larger than 25 mm. At presentation, most aneurysms are small, with only 2% found to be giant. Giant aneurysms are more likely to cause compressive symptoms on the optic chiasm, cranial nerves, and brainstem depending on location. Rarely involvement of tributary vessels, either due to aneurysmal expansion or cavitary clot, may lead to ischemic symptoms as well (Fig. 57-9). Although controversy remains regarding the association of size and the incidence of rupture, 7 mm seems to be the minimal size at the time of rupture. Overall, ruptured aneurysms tend to be larger than unruptured aneurysms.
Management
Complications of the Ruptured Aneurysm
Technical Aspects of Surgical Clipping
Craniotomy
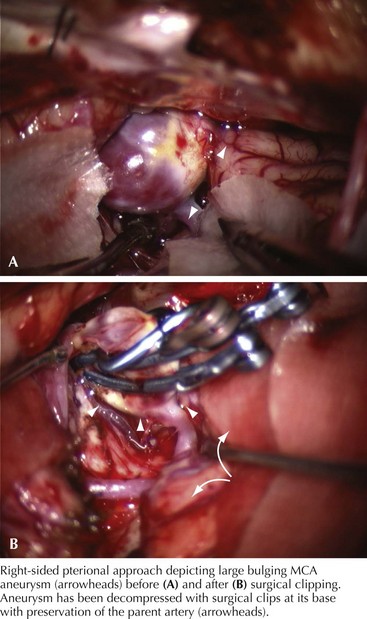
Different approaches are used depending on the location, size, and shape of the aneurysm. Most anterior circulation aneurysms are done via a pterional craniotomy, a fundamental approach in aneurysm surgery. As its name implies, it centers on the pterion and encompasses both the frontal and temporal bone removal to expose the sphenoid wing. After the dura is opened, the frontal and temporal lobes are seen in the region of the sylvian fissure. The surgical microscope is brought into the field to accomplish the remaining surgery with magnified vision (Fig. 57-10).
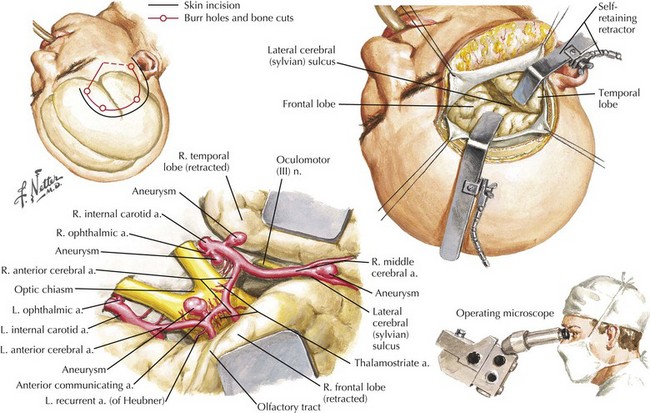
Figure 57-10 Frontotemporal Approach for Internal Carotid, Ophthalmic, Anterior Communicating, and Middle Cerebral Aneurysms.
Posterior circulation aneurysms can be approached posteriorly (Fig. 57-11), via a pterional-transsylvian route or subtemporal route, depending on the projection and height of the basilar bifurcation. Other more complex approaches such as orbito-zygomatic skull base approaches and far-lateral approaches can also be employed for posterior circulation aneurysms as needed. A detailed discussion of surgical approaches is beyond the scope of this chapter.
Endovascular Therapy
The shape, size, and neck diameter of the aneurysm in relation to the parent vessel determine whether or not its dome can be successfully occluded. Larger or odd-shaped aneurysms with wide necks are more difficult to occlude completely. In ISAT the proportions of completely, subtotally (with remnants at the neck), and incompletely occluded aneurysms at follow-up were 66%, 26%, and 8%, respectively. It is not clear what the risk of rebleeding is from a small neck remnant following either endovascular coil embolization or rarely surgical clipping. Post-clip angiography shows that only 4–10% of aneurysms have any major remnant while coiling achieves complete aneurysm occlusion in only 50% of cases. When near-complete occlusions are included, this level increases to 85–90%. Another limitation to endovascular procedures is an approximately 16–32% aneurysm recurrence depending on the location, degree of compaction, and morphology of the aneurysm when primarily treated (Fig. 57-12). Patients may require repeated treatment or even surgical intervention with removal of the coils.
Allen GS, Ahn HS, Preziosi TJ, et al. Cerebral arterial vasospasm—a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308:619-624. This study was conducted in intact neurologically stable patients. Extrapolating these findings to those presenting with deficits and critically ill patients requires caution
Bederson JB, Ward I, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742-2750.
Eskridge J, Song J. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi-detachable coils: results of the food and drug administration multi-center clinical trial. and participants. J Neurosurg. 1998;89:81-86.
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risk of surgical intervention. N Engl J Med. 1998;339:1725-1733.
International Subarachnoid Hemorrhage Aneurysm Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267-1274. Pivotal randomized study that has established the use of endovascular coiling as a less invasive, safe and efficacious alternative to surgical clipping
MacDonald RL, Wallace MC, Kestle JR. The role of angiography following aneurysm surgery. J Neurosurg. 1993;79:826-832.