Airway Clearance Therapy
After reading this chapter you will be able to:
 Describe how normal airway clearance mechanisms work and the factors that can impair their function.
Describe how normal airway clearance mechanisms work and the factors that can impair their function.
 Identify pulmonary diseases associated with abnormal clearance of secretions.
Identify pulmonary diseases associated with abnormal clearance of secretions.
 State the goals and clinical indications for airway clearance therapy.
State the goals and clinical indications for airway clearance therapy.
 Describe the proper technique and potential benefit of each of the following:
Describe the proper technique and potential benefit of each of the following:
 Directed coughing and related expulsion techniques
Directed coughing and related expulsion techniques
 Positive expiratory pressure therapy
Positive expiratory pressure therapy
 High-frequency compression/oscillation methods
High-frequency compression/oscillation methods
 Evaluate a patient’s response to airway clearance therapy.
Evaluate a patient’s response to airway clearance therapy.
 Modify airway clearance therapies on the basis of patient response.
Modify airway clearance therapies on the basis of patient response.
Airway clearance therapy involves the use of noninvasive techniques designed to help mobilize and remove secretions and improve gas exchange.1–6 Previously, airway clearance methods often were grouped under a broad category of techniques called chest physical therapy (CPT). CPT involves not only airway clearance techniques but also various exercise protocols and breathing retraining methods.1,2 This chapter focuses on noninvasive airway clearance. The primary invasive method of airway clearance, suctioning, is discussed in Chapter 33.
Traditionally, airway clearance therapy has involved CPT (postural drainage, percussion, and vibration [PDPV]) combined with cough instruction.6 Over the years, several additional noninvasive airway clearance methods have been developed to augment or replace this traditional approach. These newer techniques include modified breathing and coughing routines, manual hyperinflation, and mechanical devices designed to augment secretion clearance.2
In the past, airway clearance methods were commonly used in medical practice without firm scientific knowledge regarding their effectiveness. One example was to order CPT for essentially all postoperative patients in the hope that its use would prevent the respiratory complications of surgery.7–12 It is now known that this broad application of airway clearance therapy is both ineffective and costly. In an effort to address these types of issues, the American College of Chest Physicians (ACCP) conducted a formal systematic review of the evidence-based practice for nonpharmacologic airway clearance therapies.3 The recommendations of the ACCP are included in this chapter.
As a result of evidence-based initiatives, it has been shown that combining certain airway clearance techniques with exercise can improve lung function in patients with cystic fibrosis (CF).3,5,6 Another review of related research raised questions regarding the effectiveness of CPT in patients with acute and stable chronic obstructive pulmonary disease (COPD), chronic bronchitis, or bronchiectasis.13,14 Successful outcomes in airway clearance techniques require knowledge of normal and abnormal physiology, careful patient evaluation and selection, a clear definition of therapeutic goals, rigorous application of the appropriate evidence-based methods, and ongoing assessment and follow-up.15–18
Physiology of Airway Clearance
Normal Clearance
Normal airway clearance requires a patent airway, a functional mucociliary escalator, and an effective cough.5,19,20 Airways normally are kept open by structural support mechanisms (see Chapter 8) and kept clear by proper function of the ciliated mucosa. The mucociliary clearance mechanism operates from the larynx down to the respiratory bronchioles. The mucus itself originates from the goblet cells and submucosal glands, although Clara cells and tissue fluid transudation also contribute to airway secretions. Ciliated epithelial cells normally move this mucus via a coordinated wave of ciliary motion toward the trachea and larynx, where excess secretions can be swallowed or expectorated.
Although essentially a reserve clearance mechanism, cough is one of the most important protective reflexes.21–23 By ridding the larger airways of excessive mucus and foreign matter, cough assists the normal mucociliary clearance and helps ensure airway patency.
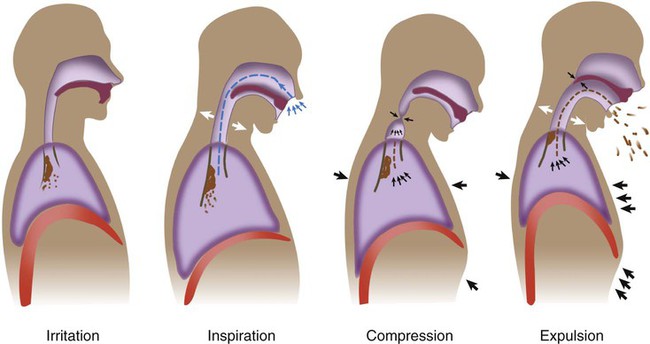
As shown in Figure 40-1, there are four distinct phases to a normal cough: irritation, inspiration, compression, and expulsion. In the initial irritation phase, an abnormal stimulus provokes sensory fibers in the airways to send impulses to the medullary cough center in the brain. This stimulus normally is inflammatory, mechanical, chemical, or thermal. Infection is a good example of cough stimulation caused by an inflammatory process. Foreign bodies can provoke a cough through mechanical stimulation. Chemical stimulation can occur when irritating gases are inhaled (e.g., cigarette smoke). Finally, cold air may cause thermal stimulation of sensory nerves and produce a cough.
Abnormal Clearance
Any abnormality that alters airway patency, mucociliary function, strength of the inspiratory or expiratory muscles, thickness of secretions, or effectiveness of the cough reflex can impair airway clearance and cause retention of secretions.19,20,22,23 In addition, some therapeutic interventions, especially interventions used in critical care, can result in abnormal clearance.
In the presence of pathogenic organisms, retention of secretions can lead to infection. Infectious processes provoke an inflammatory response and the release of chemical mediators. These chemical mediators, including leukotrienes, proteases, and elastases, can damage the airway epithelium and increase mucus production, resulting in a vicious cycle of worsening airway clearance.20
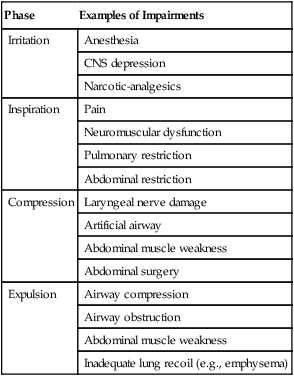
Compounding these problems may be failure of the cough reflex. In patients with retained secretions, interference with any one of the four phases of cough can result in ineffective airway clearance. Table 40-1 provides examples of factors that can impair the normal cough reflex.
TABLE 40-1
Mechanisms Impairing Cough Reflex
| Phase | Examples of Impairments |
| Irritation | Anesthesia |
| CNS depression | |
| Narcotic-analgesics | |
| Inspiration | Pain |
| Neuromuscular dysfunction | |
| Pulmonary restriction | |
| Abdominal restriction | |
| Compression | Laryngeal nerve damage |
| Artificial airway | |
| Abdominal muscle weakness | |
| Abdominal surgery | |
| Expulsion | Airway compression |
| Airway obstruction | |
| Abdominal muscle weakness | |
| Inadequate lung recoil (e.g., emphysema) |
As indicated in Box 40-1, additional factors can impair airway clearance in critically ill patients with artificial airways, the most important of which is the airway itself.16 The presence of the tube in the trachea increases mucus secretion, whereas the cuff of the tube mechanically blocks the mucociliary escalator. In addition, movement of the tube tip and cuff can cause erosion of the tracheal mucosa and impair mucociliary clearance further. Lastly, endotracheal tubes impair the compression phase of the cough reflex by preventing closure of the glottis (see Table 40-1).
Although suctioning is used to aid secretion clearance, it too can cause damage to the airway mucosa and impair mucociliary transport. Inadequate humidification can cause thickening or inspissation of secretions, mucous plugging, and airway obstruction. High fractional inspired oxygen (FiO2) concentrations can impair mucociliary clearance, either directly or by causing acute tracheobronchitis. Several common drugs, including some general anesthetics and narcotic-analgesics, can depress mucociliary transport. Lastly, several diseases commonly seen in critically ill patients are associated with poor secretion clearance; these are discussed in the following sections.3,12
Diseases Associated With Abnormal Clearance
Several diseases are associated with abnormal clearance, including diseases affecting airway patency, composition and production of mucus, ciliary structure and function, and normal cough reflex.3,20,24 Internal obstruction or external compression of the airway lumen can impair airway clearance. Examples include foreign bodies, tumors, and congenital or acquired thoracic anomalies such as kyphoscoliosis. Internal obstruction also can occur with mucus hypersecretion, inflammatory changes, or bronchospasm, further narrowing the lumen. Examples include asthma, chronic bronchitis, and acute infections.
Diseases that alter normal mucociliary clearance can also cause retention of secretions. CF is a common disorder in this category. In CF, the solute concentration of the mucus is altered because of abnormal sodium and chloride transport.25 This alteration increases the viscosity of mucus and impairs its movement up the respiratory tract. Although less common, there are several conditions in which the respiratory tract cilia do not function properly.26 These ciliary dyskinetic syndromes also can contribute to ineffective airway clearance.
Chronic airway inflammation and infection can lead to bronchiectasis, a common finding in CF and ciliary dyskinetic syndromes. In bronchiectasis, the airway is permanently damaged, dilated, and prone to constant obstruction by retained secretions.14 Other conditions that can lead to bronchiectasis include chronic obstructive lung diseases, foreign body aspiration, and obliterative bronchiolitis.27
As previously discussed, any condition that affects the four components of an effective cough also alters airway clearance. Mucociliary function may be normal, but without an effective cough, mucous plugs, obstruction, and atelectasis can occur. The most common conditions affecting the cough reflex are musculoskeletal and neurologic disorders, including muscular dystrophy, amyotrophic lateral sclerosis, spinal muscular atrophy, myasthenia gravis, poliomyelitis, and cerebral palsy (see Chapter 29).
General Goals and Indications
The primary goal of airway clearance therapy is to help mobilize and remove retained secretions, with the ultimate aim to improve gas exchange, promote alveolar expansion, and reduce the work of breathing. Box 40-2 lists general indications for airway clearance therapy.3,5,12 More specific indications are described as each airway clearance technique is discussed.
Airway Clearance Therapy for Acute Conditions
Patients with acute conditions in whom airway clearance therapy may be indicated include (1) acutely ill patients with copious secretions; (2) patients in acute respiratory failure with clinical signs of retained secretions (audible abnormal breath sounds, deteriorating arterial blood gases, chest radiographic changes); (3) patients with acute lobar atelectasis; and (4) patients with  abnormalities owing to lung infiltrates or consolidation.15–17 In treating acute respiratory conditions, inhaled bronchodilator therapy before airway clearance therapy may improve the overall effectiveness of the treatment both by opening the airways and by increasing the mucociliary activity. For acute pulmonary infections, inhaled antibiotics after airway clearance therapy can lead to improved deposition of the antibiotic.2 Acute conditions for which airway clearance therapy is probably not indicated include (1) acute exacerbations of COPD, (2) pneumonia without clinically significant sputum production, and (3) uncomplicated asthma.3,5
abnormalities owing to lung infiltrates or consolidation.15–17 In treating acute respiratory conditions, inhaled bronchodilator therapy before airway clearance therapy may improve the overall effectiveness of the treatment both by opening the airways and by increasing the mucociliary activity. For acute pulmonary infections, inhaled antibiotics after airway clearance therapy can lead to improved deposition of the antibiotic.2 Acute conditions for which airway clearance therapy is probably not indicated include (1) acute exacerbations of COPD, (2) pneumonia without clinically significant sputum production, and (3) uncomplicated asthma.3,5
Airway Clearance Therapy for Chronic Conditions
Airway clearance therapy has proved effective in aiding secretion clearance and improving pulmonary function in chronic conditions associated with copious sputum production, including CF, bronchiectasis, and chronic bronchitis in certain patients.3,10,15–17 Generally, sputum production must exceed 25 to 30 ml/day for airway clearance therapy to improve secretion removal significantly.
Airway Clearance Therapy to Prevent Retention of Secretions
Airway clearance therapy has been used as a preventive mode of respiratory care in various disorders. Current evidence is inclusive regarding the benefits of this approach. The best-documented preventive uses of airway clearance therapy include (1) body positioning and patient mobilization to prevent retained secretions in acutely ill patients and (2) CPT combined with physical activity to maintain lung function in patients with CF.3,4,6,8,28 Other preventive applications of airway clearance therapy have not proved to be useful.3,5
Determining the Need for Airway Clearance Therapy
Box 40-3 lists the key factors that must be considered when assessing a patient’s need for airway clearance therapy.3,15–17 Physical findings such as a loose, ineffective cough, labored breathing pattern, decreased or bronchial breath sounds, coarse inspiratory and expiratory crackles, tachypnea, tachycardia, or fever may indicate a potential problem with retained secretions. A chest radiograph often shows atelectasis and areas of increased density in such cases.
Airway Clearance Methods
Chest Physical Therapy
CPT (percussion, postural drainage, and vibration) has long been considered a standard of care in patients with CF and in some other patients with specific pulmonary conditions. Evidence suggests that these therapies benefit mucus transport and assist in the expectoration of secretions.3,11,21
Postural drainage involves the use of gravity and mechanical energy to help mobilize secretions. The various body positions assumed are intended to drain secretions from each of the patient’s lung segments into the central airways, where they can be removed by cough or suctioning.1,2,15,29–31 This drainage is accomplished by simply placing the segmental bronchus to be drained in a more vertical position, permitting gravity to assist in the process. Positions generally are held for 3 to 15 minutes (longer in special situations) and modified as the patient’s condition and tolerance warrant.15 Cough methods are used with postural drainage therapy and are discussed separately.
To guide practitioners in applying postural drainage techniques, the American Association for Respiratory Care (AARC) has developed and published a clinical practice guideline on postural drainage. Excerpts from the AARC guideline, including indications, contraindications, hazards and complications, assessment of need, assessment of outcome, and monitoring, appear in Clinical Practice Guideline 40-1.15
Postural drainage is most effective in conditions characterized by excessive sputum production (>25 to 30 ml/day). For maximum effect, head-down positions should exceed 25 degrees below horizontal.1,2,30–33 Positions can be modified if a patient’s condition presents a contraindication to treatment.2 Postural drainage is not likely to succeed unless and until adequate systemic and airway hydration is ensured.34,35 In patients in critical care, including patients on mechanical ventilation, postural drainage should be performed every 4 to 6 hours as indicated. In spontaneously breathing patients, frequency should be determined by assessing patient response to therapy.
Technique
To avoid gastroesophageal reflux and the possibility of aspiration, treatment times should be scheduled before or at least 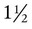 to 2 hours after meals or tube feedings.36,37 If the patient assessment indicates that pain may hinder treatment implementation, the clinician also should consider coordinating the treatment regimen with prescribed pain medication.
to 2 hours after meals or tube feedings.36,37 If the patient assessment indicates that pain may hinder treatment implementation, the clinician also should consider coordinating the treatment regimen with prescribed pain medication.
Before positioning, the procedure (including adjunctive techniques) should be explained to the patient. As necessary, clothing around the waist and neck should be loosened. Also, the clinician should inspect any monitoring leads, intravenous tubing, and oxygen (O2) therapy equipment connected to the patient and, if necessary, make adjustments to ensure continued function during the procedure. Because postural drainage positioning predisposes patients to arterial desaturation, pulse oximetry should be considered if hypoxemia is suspected.15 Before starting the procedure, the clinician should measure the patient’s vital signs and auscultate the chest. These simple assessments serve as baseline measurements for monitoring the patient’s response during the procedure and can assist in determining outcomes.
Figure 40-2 depicts the primary positions used to drain the various lung lobes and segments. Generally, to obtain the proper head-down position, the clinician must lower the head of the bed by at least 16 to 18 inches to achieve the desired 25-degree angle. In the ambulatory care setting, a tilt table can be used in lieu of a hospital bed. A tilt table allows precise positioning at head-down angles up to 45 degrees. When angles this large are used, shoulder supports must be provided to prevent the patient from sliding off the tilt table.
When the patient is in position, the clinician confirms the patient’s comfort and ensures proper support of all joints and bony areas with pillows or towels. The indicated position is maintained for a minimum of 3 to 15 minutes if tolerated and longer if good sputum production results.2 Between positions, pauses for relaxation and breathing control are useful and can help prevent hypoxemia. Because postural drainage therapy can increase O2 consumption, critically ill patients at risk for hypoxemia should be given supplemental O2 during the procedure.32
During the procedure, the patient is continually observed for any untoward effects or complications (see Clinical Practice Guideline 40-1). Moderate changes in vital signs are expected during treatment; however, as indicated in Table 40-2, significant problems may require immediate intervention.15
TABLE 40-2
Complications of Postural Drainage Therapy and Recommended Interventions
| Complication | Action to Be Taken/Possible Intervention |
| Hypoxemia | Administer higher FiO2 during procedure if potential for or observed hypoxemia exists. If patient becomes hypoxemic during treatment, administer 100% O2, stop therapy immediately, return patient to original position, and consult physician |
| Increased intracranial pressure | Stop therapy, return patient to original resting position, and consult physician |
| Acute hypotension during procedure | Stop therapy, return patient to original resting position, and consult physician |
| Pulmonary hemorrhage | Stop therapy, return patient to original resting position, and call physician immediately. Administer O2 and maintain an airway until physician responds |
| Pain or injury to muscles, ribs, or spine | Stop therapy that appears directly associated with pain or problem, exercise care in moving patient, and consult physician |
| Vomiting and aspiration | Stop therapy, clear airway and suction as needed, administer O2, maintain airway, return patient to previous resting position, and contact physician immediately |
| Bronchospasm | Stop therapy, return patient to previous resting position, and administer or increase O2 delivery while contacting physician. Administer physician-ordered bronchodilators |
| Arrhythmias | Stop therapy, return patient to previous resting position, and administer or increase O2 delivery while contacting physician |
Outcome Assessment
Specific outcome criteria indicating a positive response to postural drainage are listed in the AARC clinical practice guideline excerpts that appear in Clinical Practice Guideline 40-1. Generally, achievement of one or more of these outcomes indicates that the therapy is meeting its objectives and should be continued. Not all criteria are required to justify continuing postural drainage.
Because secretion clearance is affected by patient hydration, the clinician may need to wait for at least 24 hours after optimal systemic hydration has been achieved to see any evidence of increased sputum production. In the interim, tracheobronchial clearance can be enhanced in some patients by the provision of bland aerosol therapy with an unheated jet nebulizer. Depending on the specific application, other aerosol generators may be deployed to deliver bland aerosol.34,38
Percussion and Vibration
Percussion and vibration involve application of mechanical energy to the chest wall by the use of either hands or various electrical or pneumatic devices. Both methods are designed to augment secretion clearance.3 In theory, percussion should help loosen secretions from the tracheobronchial tree, making them easier to remove by coughing or suctioning. Vibration should aid movement of secretions toward the central airways during exhalation.
The effectiveness of percussion and vibration as an adjunct to postural drainage is controversial. This controversy is partly due to the fact that there is no consensus regarding what represents the “right” force or frequency for either technique.1,5 In addition, because percussion and vibration are often only a part of the treatment regimen in most clinical studies, it is hard to draw conclusions about the effect of these methods alone.3 Both the types of patients studied and the outcome measures used often differ across studies. In studies that focus on patients who produce copious secretions, percussion generally is found to be effective in increasing sputum production.39 When the outcome measure is not the volume of secretions produced, results are less positive.3,5
For these reasons, and in light of current knowledge, the routine use of these methods cannot be justified. However, because percussion and vibration may increase the volume of sputum production in some patients, the addition of these techniques to postural drainage may be appropriate in selected cases, especially if postural drainage alone fails to mobilize secretions.15
Manual Percussion and Vibration
The clinician performs manual percussion with his or her hands in a cupped position, with fingers and thumb closed (Figure 40-3). This position traps a cushion of air between the hand and chest wall.2 The striking force may be against the bare skin, although a thin layer of cloth, such as a hospital gown or bed sheet, does not significantly impair transmission of the energy wave and is more comfortable for the patient.
The clinician rhythmically strikes the chest wall in a waving motion, using both hands alternately in sequence with the elbows partially flexed and wrists loose (see Figure 40-3). Slower, more relaxing rates are better tolerated by the patient and the clinician. It is not a difficult technique to master, but practice is needed to determine the appropriate force and to maintain a rhythmic pattern.
Vibration sometimes is used together with percussion but is limited to application during exhalation. It may also be used as an alternative to percussion in acutely ill patients with chest wall discomfort or injury. To vibrate the chest wall, the clinician lays one hand on the patient’s chest over the involved area and places the other hand on top of the first (Figure 40-4). Alternatively, the clinician places the hands on either side of the chest. After the patient takes a deep breath, the clinician exerts slight to moderate pressure on the chest wall and initiates a rapid vibratory motion of the hands throughout expiration.
Mechanical Percussion and Vibration
Various electrical and pneumatic devices have been developed to generate and apply the energy waves used during percussion and vibration. Typically, these devices have both a frequency and a percussion force control (Figure 40-5). Most units provide frequencies up to 20 to 30 cycles per second, or 20 to 30 Hz. Noise, excess force, and mechanical failure all are potential problems. Electrical devices also pose a potential shock hazard.
These devices have the advantage of reducing fatigue on the caregiver and can deliver consistent rates, rhythms, and impact forces.1,2 However, there is no firm evidence that such devices are more effective than manual techniques. For this reason, the selection of manual or mechanical methods should be left to the patient in consultation with the care provider.40
Coughing and Related Expulsion Techniques
Directed Cough
Although cough may stimulate mucociliary activity, it has little direct effect on secretion clearance in individuals who do not produce sputum.40,41 In patients with copious secretions, directed coughing is as good at clearance as more complicated methods.7,42–45 However, coughing is most effective in clearing secretions from the central, and not peripheral, airways.46 To help practitioners apply this important technique, the AARC has developed and published a clinical practice guideline on directed cough. Excerpts from the AARC guideline, including indications, contraindications, hazards and complications, assessment of need, assessment of outcome, and monitoring, appear in Clinical Practice Guideline 40-2.16
Standard Technique
Good patient teaching is crucial in developing an effective directed cough regimen. The three most important aspects involved in patient teaching are (1) instruction in proper positioning, (2) instruction in breathing control, and (3) exercises to strengthen the expiratory muscles.7 These activities are modified according to the patient’s underlying clinical problem.
Modifications in Technique
To help enhance expulsion, the patient should exhale with moderate force through pursed lips, while bending forward. This forward flexion of the thorax enhances expiratory flow by upward displacement of the abdominal contents. After three or four repetitions of this maneuver, the patient is encouraged to bend forward and initiate short staccato-like bursts of air. This technique relieves the strain of a prolonged hard cough, and the staccato rhythm at a relatively low velocity minimizes airway collapse. This technique has a modification called huffing, whereby the patient is instructed to make the sound of “huff, huff, huff” rapidly with the mouth open.3 Alternatively, either FET or autogenic drainage (AD) may be used in these patients. Both of these clearance mechanisms are discussed later.
Patients with neuromuscular conditions present a special challenge in cough management. These patients typically are unable to generate the forceful expulsion needed to move secretions toward the trachea.24 If this problem results in retained secretions, there are only three options: (1) placement of an artificial airway and removal of secretions by tracheobronchial suctioning (see Chapter 33), (2) manually assisted cough, and (3) MIE.
Manually assisted cough is external application of pressure to the thoracic cage or epigastric region, coordinated with forced exhalation.16,22 In this technique, the patient takes as deep an inspiration as possible, assisted as needed by the application of positive pressure via a self-inflating bag or intermittent positive pressure breathing device. At the end of the patient’s inspiration, the clinician begins exerting pressure on the lateral costal margins or epigastrium. This pressure increases the force of compression throughout expiration; this mimics the normal cough mechanism by generating an increase in the velocity of the expired air and may be helpful in moving secretions toward the trachea, where they can be removed by suctioning. Manually assisted cough with pressure to the lateral costal margins is contraindicated in patients with osteoporosis or flail chest.16 Manually assisted cough using epigastric pressure is contraindicated in unconscious patients with unprotected airways, in pregnant women, and in patients with acute abdominal pathology, abdominal aortic aneurysm, or hiatal hernia.16
Forced Expiratory Technique
FET is a modification of the normal directed cough. FET, or huff cough, consists of one or two forced expirations of middle to low lung volume without closure of the glottis, followed by a period of diaphragmatic breathing and relaxation.46,47 The goal of this method is to help clear secretions with less change in pleural pressure and less likelihood of bronchiolar collapse. To help keep the glottis open during FET, the patient is taught to phonate or “huff” during expiration. The period of diaphragmatic breathing and relaxation following the forced expiration is essential in restoring lung volume and minimizing fatigue.
Comparative clinical studies on the effectiveness of FET have shown favorable results. Generally, FET results in increased sputum production, especially when combined with postural drainage.7,48 The technique is particularly useful in patients prone to airway collapse during normal coughing, such as patients with COPD, CF, or bronchiectasis. However, FET requires that patients generate high expiratory airflow, which may not be attainable in intubated patients with respiratory failure.
Active Cycle of Breathing Technique
To emphasize that FET always should include breathing exercises, the originators of this technique modified the procedure and renamed it the active cycle of breathing technique (ACBT).2,20 ACBT consists of repeated cycles of breathing control, thoracic expansion, and FET (Box 40-4). Breathing control involves gentle diaphragmatic breathing at normal tidal volumes for 5 to 10 seconds with relaxation of the upper chest and shoulders. This phase is intended to help prevent bronchospasm. The thoracic expansion exercises involve deep inhalation, approaching vital capacity, with relaxed exhalation, which may be accompanied by percussion, vibration, or compression. The thoracic expansion phase is designed to help loosen secretions, improve the distribution of ventilation, and provide the volume needed for FET. The subsequent FET moves secretions into the central airways. Postoperative patients may require splinting at the thoracic or abdominal incision site. Patients with extreme airway sensitivity are susceptible to airway irritation during ACBT.
Although ACBT can be performed in the sitting position, it is most beneficial when combined with postural drainage. As an added benefit, ACBT seems to minimize or prevent the O2 desaturation so common during postural drainage, at least in patients with CF.49 When ACBT is compared with similar methods of secretion clearance, studies indicate that ACBT can provide comparable results in terms of both sputum production and distribution of ventilation.5,6,20,50,51 ACBT is not useful with young children (<2 years old) or critically ill patients.
Autogenic Drainage
Autogenic drainage (AD) is another modification of directed coughing, designed as an airway clearance mechanism that can be performed independently by trained patients.3,20 During AD, the patient uses diaphragmatic breathing to mobilize secretions by varying lung volumes and expiratory airflow in three distinct phases (Figure 40-6).20 For maximum benefit, the patient should be in the sitting position. Patients are taught to control their expiratory flows to prevent airway collapse while trying to achieve a mucous “rattle” rather than a wheeze. Coughing should be suppressed until all three breathing phases are completed.
In patients with CF, AD provides sputum clearance comparable to PDPV but is less likely to produce O2 desaturation. In addition, AD seems to be tolerated better by patients and has the advantage of being performed without assistance and in one position.3,7,52–55
Mechanical Insufflation-Exsufflation
The use of MIE devices (also called cough-assist device or “coughlator”) has experienced a resurgence more recently, especially in patients with certain neuromuscular disorders (Figure 40-7).3,24 The reason is growing evidence that MIE helps prevent respiratory complications in patients with neuromuscular disorders by helping them generate sufficient expiratory flow rates needed for effective secretion clearance.56–61
MIE via an oronasal interface is effective, provided that there is no fixed airway obstruction or glottic collapse during exsufflation. For patients with severe restrictive disease who have not been taking deep breaths, insufflation pressures should be increased gradually to avoid chest wall muscle strains. Abdominal distention is infrequent and reduced by decreasing insufflation, not exsufflation, pressures. The effectiveness of MIE in persons with airway obstruction caused by disorders such as COPD is less clear, and MIE may be detrimental.3,55
Positive Airway Pressure Adjuncts
PAP adjuncts are used to help mobilize secretions and treat atelectasis. As adjuncts for airway clearance, these methods are never used alone; they are always combined with directed cough or other airway clearance techniques.17 One of three different approaches can be used: (1) CPAP, (2) EPAP, and (3) PEP.
The AARC has developed and published a clinical practice guideline on the use of PAP adjuncts with airway clearance therapy to guide practitioners in applying these techniques. Excerpts from the AARC guideline, including indications, contraindications, hazards and complications, assessment of need, assessment of outcome, and monitoring, appear in Clinical Practice Guideline 40-3.17 Use of these methods to treat atelectasis is reviewed in Chapter 39. The following discussion focuses on the use of PEP therapy as an adjunct in secretion clearance.
Most clinical studies of PEP therapy involved patients with CF, although its use in COPD and in preventing postoperative atelectasis has also been investigated.3,62 Generally, compared with other airway clearance methods (PDPV, AD, ACBT) in patients with CF, PEP therapy provides comparable mucociliary clearance, with the added advantages of being potentially self-administered and cost-effective.3,63–66 Patients generally prefer PEP over other methods. However, PEP therapy does not appear to be as useful in enhancing lung clearance in chronic bronchitis.67 In regard to the prevention of postoperative atelectasis, studies provide conflicting results.61–63 In addition, PEP therapy cannot be used in young children (<3 years old).
The clinical procedure for PAP therapy is presented in Box 40-5.67 Equipment can be easily assembled from available parts in most respiratory care departments. Single-use commercial devices are available for purchase (Figure 40-8). Regardless of the equipment used, it is essential to monitor actual airway pressures (as opposed to set or intended pressures).68
Common strategies for PEP therapy vary from three to four times daily, with frequency determined by assessment of patient response. During acute exacerbations, therapy should be performed at decreasing intervals rather than extending the length of the therapy sessions. Aerosol drug therapy may be added to a PEP session using either an in-line hand-held nebulizer or a metered dose inhaler attached to the one-way valve inlet of the system (see Figure 40-8).67 The combination of aerosol drug therapy with PEP seems to improve the efficacy of bronchodilator administration, probably because of better distribution to the peripheral airways.62,67
High-Frequency Compression/Oscillation
As applied to airway clearance, oscillation refers to the rapid vibratory movement of small volumes of air back and forth in the respiratory tract. At high frequencies (12 to 25 Hz), these oscillations act as a physical “mucolytic,” enhancing cough clearance of secretions.69 There are two general approaches to oscillation: external (chest wall) application and airway application. External application is often called high-frequency chest wall compression (HFCWC). Airway application of oscillation methods includes (1) the flutter valve and (2) intrapulmonary percussive ventilation (IPV).
High-Frequency Chest Wall Oscillation
High-frequency chest wall oscillation is accomplished by using a two-part system: (1) a variable air-pulse generator and (2) a nonstretch inflatable vest that covers the patient’s entire torso (Vest Airway Clearance System [Figure 40-9]).2,70 Small gas volumes are alternately injected into and withdrawn from the vest by the air-pulse generator at a fast rate, creating an oscillatory motion against the patient’s thorax. Typically, RTs perform 30-minute therapy sessions at oscillatory frequencies between 5 Hertz (Hz) and 25 Hz. Depending on need and response, one to six therapy sessions may occur per day.

Compression frequency and flow bias (inspiratory vs. expiratory) determine the effectiveness of therapy. Additionally, the oscillation frequency used affects both patient comfort and efficacy.71–73 The current recommendation is to identify individually the frequency that produces optimal results and patient comfort.
Clinical studies of high-frequency chest wall oscillation and HFCWC have shown mixed results. Several studies concluded that when used in patients with CF, the two techniques are equivalent to other airway clearance techniques as measured by improved spirometry or sputum production.3,70,71 Studies in other populations have shown some improvement, as measured by patient perception, increased compliance, or outcome.70,73,74
An alternative device to the vest, the Hayak oscillator (United Hayek Industries, Inc, San Diego, CA), uses a chest shield (turtle shell) strapped to the anterior chest wall. The shell is connected by a large hose to a negative/positive pressure generator that can provide oscillations at frequencies up to 15 Hz. This device is primarily considered a form of providing negative pressure ventilation, and research evaluating its effectiveness as an airway device is limited.75
Intrapulmonary Percussive Ventilation
IPV is an airway clearance technique that uses a pneumatic device to deliver a series of pressurized gas minibursts at rates of 100 to 225 cycles per minute (1.6 to 3.75 Hz) to the respiratory tract, usually via a mouthpiece (Figure 40-10). The device was approved by the U.S. Food and Drug Administration (FDA) in 1993 and is marketed as the Intrapulmonary Percussive Ventilator (Percussionaire, Sandpoint, Idaho).

Comparative studies show that IPV is equivalent to other airway clearance strategies in improving short-term pulmonary function test results and enhancing sputum expectoration in patients with CF.3,33,70,71,76 The therapy is well tolerated by stable patients and appears to offer an alternative in patients with chest wall complications.77 The manufacturer lists potential adverse side effects as sore ribs, fatigue, stress, and irritation.71
Mobilization and Physical Activity
Immobility is a major factor contributing to retention of secretions. Early mobilization and frequent position changes are now standard preventive interventions for atelectasis and pneumonia in postoperative patients.28,78–80 Adding systematic physical activity to mobilization and coughing can enhance mucus clearance further.4,44,53
Frequent movement and physical activity also improve overall aeration and  matching.12,79 In addition, frequent movement and daily physical activity can improve patients’ general fitness, functional capacity, self-esteem, and quality of life within the limitations of their pulmonary disease or deficit.28
matching.12,79 In addition, frequent movement and daily physical activity can improve patients’ general fitness, functional capacity, self-esteem, and quality of life within the limitations of their pulmonary disease or deficit.28
Frequent movement and physical activity can be fatiguing and result in O2 desaturation among patients with significant pulmonary impairment.12 For these reasons, it is probably wise to conduct an exercise evaluation on an ambulatory patient with severe lung disease being considered for exercise therapy (see Chapter 50). In addition, young children and patients with neuromuscular limitations may not be good candidates for airway clearance via exercise but should be evaluated for other forms of physical activity.
Airway Oscillating Devices
Airway oscillating devices produce PEP with oscillations in the airway during expiration. These devices are believed to work based on the principle of collateral ventilation, which suggests that airflow can occur between adjacent lung segments through the canals of Lambert and through the pores of Kohn. PEP studies have shown it to be as effective as other forms of airway clearance.81,82 Patients appear to prefer techniques such as PEP that promote independence; this is important because it has been shown that compliance to airway clearance techniques and exercise is often poor.83
A popular approach to PEP therapy is the flutter valve. It combines the techniques of EPAP with high-frequency oscillations (HFOs) at the airway opening. The valve consists of a pipe-shaped device with a heavy steel ball sitting in an angled “bowl” (Figure 40-11). The pipe bowl is covered by a perforated cap. When the patient exhales actively into the pipe, the ball creates a positive expiratory pressure of between 10 cm H2O and 25 cm H2O. At the same time, the pipe angle causes the ball to flutter back and forth at about 15 Hz. When the valve is properly used, the oscillations created are transmitted down into the airways. Patients can control the pressure by changing their expiratory flows. Changing the angle of the device alters the oscillations.
Clinical trials of the flutter valve have produced mixed results in patients with CF compared with existing methods of airway clearance (PDPV or ACBT).71,84,85 However, the flutter device can decrease viscoelasticity of mucus within the airways, modifying mucus and allowing it to be cleared more easily by cough.86 Two studies have shown that the use of the flutter valve in patients with hyperproductive disorders showed equivalent or moderately improved pulmonary function values and secretion clearance.87,88 Given that the flutter valve is readily accepted by patients, is inexpensive, is fully portable, and does not require caregiver assistance (after instruction is provided), additional clinical trials seem justified.85
Other devices include the RC-Cornet (Respan Products, Inc, Toronto, Canada), Pare Respiratory Equipment, and the Acapella (Smiths Medical ASD, Inc./Portex, Kent, UK). The RC-Cornet (currently available in Europe) appears to have an advantage over the flutter valve because it is position-independent and provides a more constant pressure and flow rate throughout expiration. The Acapella and the flutter valve have similar performance characteristics, but the Acapella appears to offer some advantages. It can customize, based on clinical needs, both the frequency and the flow resistance by adjusting the dial. Also, it can be used in any posture, including sitting, standing, or reclining.89
Selecting Airway Clearance Techniques
Selection Factors
Box 40-6 specifies the key factors clinicians should consider when selecting an airway clearance strategy. Motivation is crucial to routine performance of any procedure, especially for chronically ill patients in ambulatory or home care settings. No airway clearance strategy is successful if it is abandoned by the patient. Likewise, no routine strategy is likely to be followed without substantial results. In this regard, increased sputum production, although frequently shown not to be related to improved pulmonary function, is one of the few real outcomes clinicians can use to motivate the patient and gain his or her ongoing cooperation.
Because patients reject methods that are fatiguing, this should be considered in method selection. In addition, the patient’s disease either may suggest the best approach or may impose certain limitations that preclude using a particular method. Patients with some neuromuscular diseases may be unable to engage in therapeutic exercise. Lastly, cost is a critical factor in selecting all treatment strategies. Selecting the least expensive strategy is acceptable if the strategy is also effective. Increasingly, these strategies include therapies that are either self-administered or provided by unskilled caregivers outside the acute care setting. In this context, effective patient or caregiver education plays an increasingly important role (see Chapter 49).
Protocol-Based Airway Clearance
Numerous RT-driven protocols have been published for airway clearance therapy. All of these protocols involve rigorous assessment of the patient both to establish preliminary need and to determine continuation of or modification in therapy. Figure 40-12 is an algorithm used in one such protocol. Changes in therapy occur throughout and are based on the patient’s response and the evaluation of the clinician.



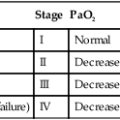
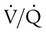 ) imbalances.
) imbalances.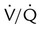 abnormalities caused by unilateral lung disease
abnormalities caused by unilateral lung disease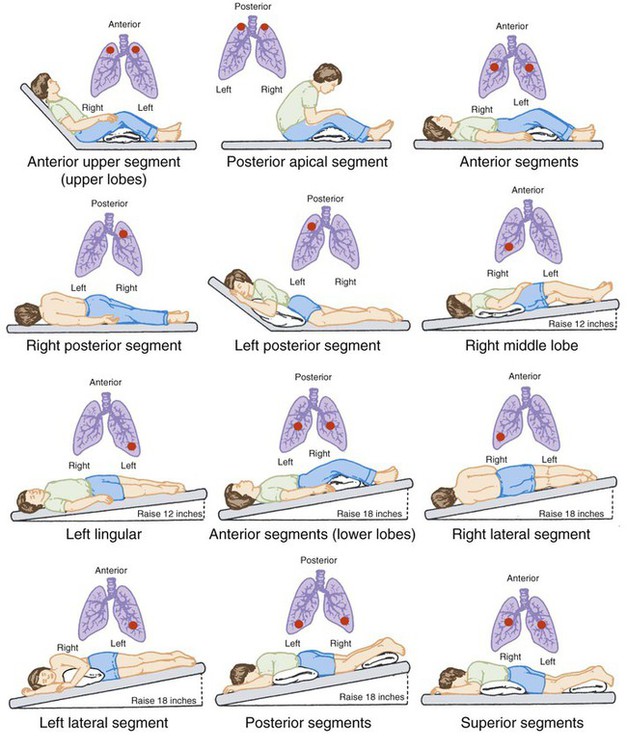








 imbalance, promote atelectasis and shunting, and increase the incidence of infection.
imbalance, promote atelectasis and shunting, and increase the incidence of infection. matching, and improves pulmonary function.
matching, and improves pulmonary function.