CHAPTER 11 Wound construction
ECCE
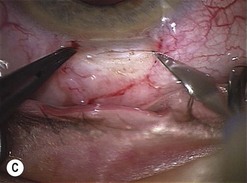
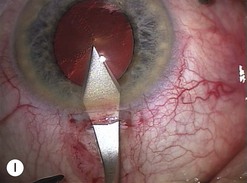
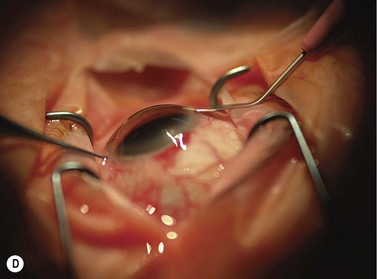
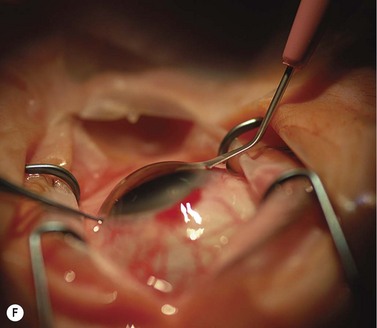
Scleral ECCE wound
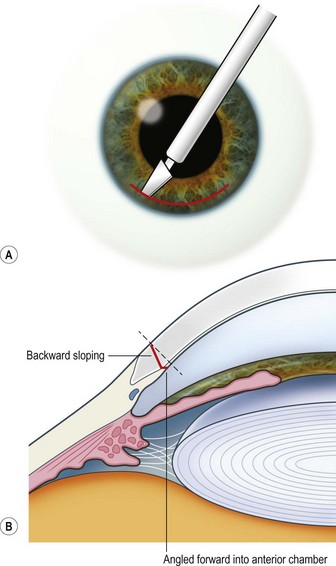
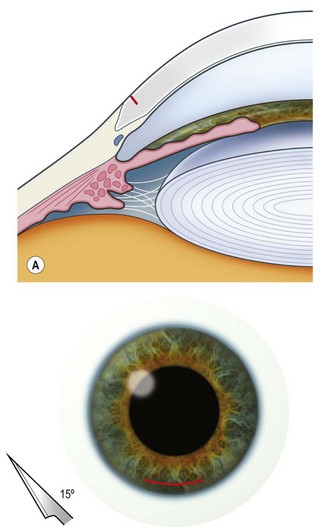
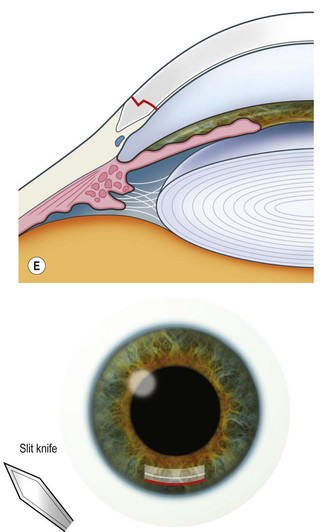
The scleral wound is made around 120°, initially backward sloping and then, when the deeper tissues are reached, a forward entry into the eye is made (Fig. 11.1). More bleeding is likely because of perforating scleral vessels and diathermy is a prerequisite. Careful wound construction and suturing can be rewarded by very little surgically induced astigmatism because of the distance from the optical centre of the cornea and the ability to leave the sutures at the desired tension.
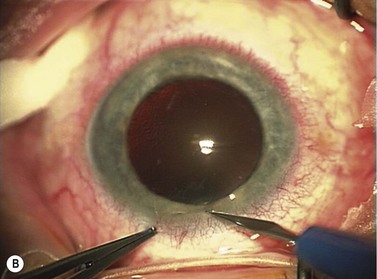
Corneal ECCE wound
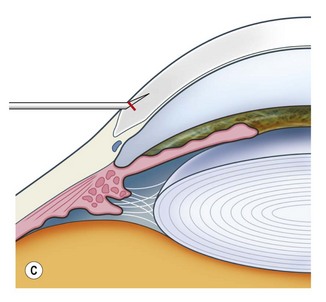
This should be made approximately 1 mm into clear cornea and sloped gently backwards. This ensures that the sutures when placed to close the wound will enter and exit corneal tissue and avoid the need for reflecting the conjunctiva (see Fig. 11.1).
The relative advantages and disadvantages of scleral and corneal ECCE wounds are summed up in Table 11.1.
| Section | Advantages | Disadvantages |
|---|---|---|
| Corneal | Quicker | More astigmatism |
| No bleeding | Endothelial damage | |
| ‘Mechanics easier’ (more stable) | Suture care (loose etc.) | |
| No superior rectus suture needed | Less strong wound | |
| Easier access for trabeculectomy later | ||
| Scleral | Less astigmatism | More bleeding |
| Wound alignment easier | Astigmatism less easily changed (by removing sutures) | |
| Less endothelial damage | More trabecular meshwork damage | |
| Suture care less (below conjunctiva) | Postoperative intraocular pressure rise more likely | |
| Stronger wound | Iris prolapse more likely | |
| May need superior rectus suture | ||
| Trabeculectomy more difficult later | ||
| Takes longer |
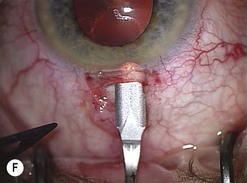
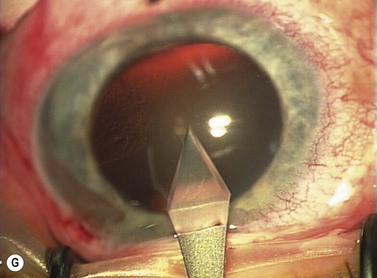
Phaco wounds
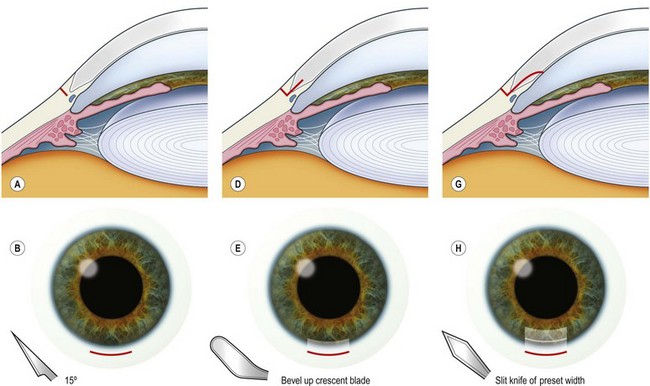
The principle of constructing a phaco wound is the same wherever it is positioned. There should be at least one step in the wound to ensure wound stability1.
Scleral tunnel phaco wound
Corneal tunnel phaco wound
Postoperatively, the corneal stroma at the wound edges should be hydrated as this ensures greater safety from postoperative ingress of fluids into the eye and may well help to reduce the incidence of endophthalmitis2.
How to decide where to place the wound
Wound construction for B-MICS
In order to avoid significant stromal edema the construction of wounds for biaxial micro incision cataract surgery (B-MICS) must be carefully performed with specialized instrumentation. This is covered in some detail in the chapter on Nuclear Disassembly (Chapter 13).
1 May WN, Castro-Combs J, Quinto GG, et al. Standardized Seidel test to evaluate different sutureless cataract incision configurations. J Cataract Refract Surg. 2010;36(6):1011-1017.
2 Vasavada AR, Praveen MR, Pandita D, et al. Effect of stromal hydration of clear corneal incisions: quantifying ingress of trypan blue into the anterior chamber after phacoemulsification. J Cataract Refract Surg. 2007;33(10):1673-1674.