CHAPTER 106 Whipple’s Disease
HISTORY
In 1907, the pathologist George H. Whipple reported in detail, the case of a 36-year-old male physician-missionary who died after a five-year illness involving arthritis, chronic cough, weight loss, and chronic diarrhea.1 At autopsy, Whipple found lipid deposits in the intestinal mucosa as well as in mesenteric and retroperitoneal lymph nodes. Microscopic examination further revealed a large number of macrophages with foamy cytoplasm in the lamina propria of the small intestine. Whipple suspected a disorder of fat metabolism and proposed the term intestinal lipodystrophy for the disease subsequently bearing his name.
In the following decades, only a few cases were reported and the diagnosis uniformly was made at autopsy. The first antemortem diagnosis was made in 1947 based on findings in a mesenteric lymph node removed at laparotomy,2 and the first diagnosis by peroral intestinal biopsy was made in 1958.3 In 1949, Black-Schaffer4 introduced the periodic acid-Schiff (PAS) stain to the histopathologic diagnosis of Whipple’s disease. Inclusions in macrophages stained red using this stain, thus documenting that the intracellular material was glycoprotein rather than lipid.
The first report of successful antibiotic treatment (using chloramphenicol) was published in 1952.5 In 1961, two groups independently visualized bacteria by electron microscopy in affected tissues6,7; subsequent reports confirmed these observations. The bacteria associated with Whipple’s disease are rod-shaped and of uniform size. Consistent positive therapeutic effects were achieved with antibiotic treatment.8 These findings and the positive PAS reaction4 suggested that the disease was unlikely to be a primary disorder of fat metabolism, but rather that it was a bacterial disease; efforts to cultivate this bacterium before 2000 failed to yield reproducible or consistent results.
The nature of the bacterium remained obscure until the early 1990s, when its 16S ribosomal DNA (rDNA) sequence was determined and phylogenetic analysis established the relationship of the bacterium to the actinomycetes.9,10 The name Tropheryma whippelii was introduced,10 and the novel 16S rDNA sequence provided the basis for sensitive diagnostic testing using the polymerase chain reaction (PCR). In situ hybridization experiments showed that the unique bacterial 16S rRNA sequence colocalized with areas of pathology, thus supporting the relevance of the sequence and organism, the presence of which was thereby inferred.11 Further advancement came with successful propagation of the Whipple’s disease bacterium in coculture with human fibroblast cells.12 At that point, the bacterium formally was described as a new species, and its name was modified to Tropheryma whipplei.13 With the availability of adequate amounts of purified genomic DNA, the complete genome sequences of two different bacterial isolates were determined and published in 2003.14,15
EPIDEMIOLOGY
Whipple’s disease is a rare disorder. The first comprehensive epidemiologic survey was performed by Dobbins in 1987,8 compiling information on 696 patients and including 617 published and 79 unpublished cases recorded through 1986. According to this analysis, Whipple’s disease is a sporadic disorder with a predilection for middle-aged white men. Data on age and sex were available for 664 patients; 86% were male, and the mean age at diagnosis was 49 years. Most patients were white; only 10 were African, one was a Native American, three patients were from India, and one was Japanese. Most of the patients originated from Europe (373 patients) or from the United States (246 patients). Within Europe, Germany (114 patients) and France (91 patients) were strongly represented. Relatively few cases originated from South America (11 patients) and Australia (13 patients).
A small epidemiologic study from western Switzerland calculated the incidence of Whipple’s disease to be approximately 0.4 per million of the population per year.16 A similar incidence of 0.4 per million per year was estimated for Germany.17 An epidemiologic analysis of 110 patients with Whipple’s disease in Germany, identified between 1965 and 1995, noted the incidence of cases to be relatively stable over three decades and a relatively even geographic distribution of the patients’ residences.18 There are only a few observations of geographically confined case clusters (up to seven cases).19–21
In recent decades, several studies have indicated a statistically significant increase in the age of patients at diagnosis and an increasing percentage of female patients.18,22,23 Currently, patients are first dianosed at a mean age of 56 years.18 It has been speculated that the increasing use of antibiotics for unrelated complaints may be a contributing factor in delaying the age of onset of Whipple’s disease. In a cohort of 191 patients whose Whipple’s disease was diagnosed between 1992 and 2007, 75% were male and 25% were female.24 There are virtually no cases in children and young adults.
One remarkable epidemiologic feature in Dobbins’ analysis8 was the strong representation of patients with occupations in the farming and building trades, involving outdoor work or frequent contact with animals or soil: of 191 patients for whom data were available, 43 (22%) were farmers and 10 (5%) were carpenters; patients in all farming-related trades accounted for 34% of the total; by comparison, the fraction of farm workers among the total workforce in the analyzed countries was approximately 10%.
MICROBIOLOGY AND GENOMICS
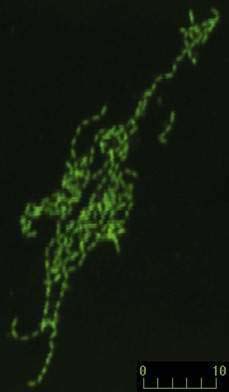
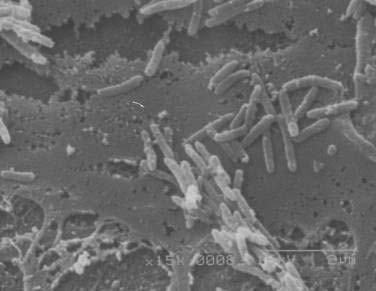
After many unsuccessful attempts to cultivate the bacterium associated with Whipple’s disease, successful propagation of T. whipplei was reported in 2000, using infected heart valve tissue in coculture with human fibroblast cells.12 Seven culture passages were performed, over a period of 285 days, and the 16S rDNA sequence of T. whipplei was detected after each passage. The initial estimate of the doubling time of the bacterium was 18 days, which represents extremely slow growth. Since the initial report, approximately 20 to 30 additional strains of T. whipplei have been isolated from various clinical specimens, including infected heart valves, duodenal biopsy specimens, ocular vitreous fluid, cerebrospinal fluid (CSF), synovial fluid, heparinized blood, mesenteric lymph node tissue, muscle tissue, and feces.25–31 Methods to determine growth and identity of the bacterium in culture include immunofluorescence,25,28 nucleic acid staining (Fig. 106-1),29 endpoint PCR and sequencing,25,28,29 quantitative PCR,27,29 electron microscopy (Fig. 106-2),29 and in situ hybridization.29 Subsequent studies arrived at estimates of shorter bacterial doubling times between 28 hours and four days.27,29,32 A cell-free (axenic) medium has been designed, using data from the genome sequence32; it consists of cell culture medium supplemented with extra amino acids. Despite these experimental advancements, however, culture of T. whipplei at present is feasible only in specialized laboratories and is not suitable for routine diagnostic purposes.
Phylogenetic analysis of the T. whipplei 16S rDNA sequence, initially amplified by broad-range PCR from infected tissue, established that the bacterium is an actinomycete, a member of the class Actinobacteria.9,10 A more detailed analysis places the organism in an intermediate phylogenetic position between the genus Cellulomonas (with the common group A peptidoglycan) and a rare group of actinomycetes (with group B peptidoglycan; i.e., a different linkage of cell wall components).33 Both groups of organisms consist predominantly of environmental bacteria that are found in soil and water and on plants. Nevertheless, the relationships of T. whipplei to any of the other known actinomycetes are quite distant (<92% 16S rRNA sequence similarity).
Differences among strains of T. whipplei first were observed in the 16S-23S rDNA intergenic spacer sequence34,35; seven different 16S-23S rRNA spacer sequence types have been described so far.34–36 One study found the two most common types, 1 and 2, in a similar ratio (≈1 : 2) among patients from the United States, Germany, and Switzerland.34 In any given patient, the same spacer type was found in different anatomic compartments—for example, intestine, blood, CSF34—which argues for systemic dissemination of a single bacterial strain in a patient with Whipple’s disease. Additional variability among strains was found in a 23S rRNA insertion sequence,37 in the groEL heat-shock protein gene,38 and at a series of variable number of tandem repeat (VNTR) loci in T. whipplei.39
Genome sequence information has been used to distinguish strains of T. whipplei. One study found that T. whipplei strains were quite heterogeneous when PCR-amplified sequences of four highly variable genomic loci were compared.40 Another study, based on microarray hybridization of DNA from cultivated isolates, found that genomic divergence of strains is due mostly to differences in members of the novel WiSP (T. whipplei surface protein) family but that aside from these differences, genome content is relatively conserved.31 So far, there is no indication that different strain types are associated with different clinical features or with different geographic locations.
The genome of T. whipplei is quite small for a bacterium.14,15 It consists of approximately 926,000 base pairs and is the smallest of all known actinomycete genomes. Its guanine + cytosine (G+C) content of 46% is unusually low for actinomycetes, which generally are organisms with high genomic G+C content. Genome size contraction is believed to have resulted from gene loss during the evolution of T. whipplei and is a general feature of bacteria that occupy a host-dependent ecological niche. This bacterium lacks various metabolic capabilities, including deficiencies in carbohydrate and energy metabolism and amino acid biosynthesis, which makes it dependent on supplies from its host environment.
Two more features of the T. whipplei genome are quite remarkable. A relatively large fraction of its genes is dedicated to the biosynthesis of cell surface molecules, and features of the genome suggest multiple “built-in” mechanisms for antigenic variation involving the WiSP family. These mechanisms are believed to involve VNTR sequences, which are known to be associated with antigenic phase variation in other organisms. There also are two unusual, large genomic regions of noncoding repetitive DNA that are thought to contribute to genetic plasticity.14 A comparative analysis revealed that the two sequenced strains are distinguished by inversion of a large segment of the genome (≈57%).15 WiSP family protein genes at each end of the large segment serve as anchoring points for the inversion. Taken together, these features suggest that interaction of T. whipplei with its host and its evasion of a host immune response are major parts of the organism’s lifestyle; these factors might contribute to its ability to sustain a chronic infection.
PATHOGENESIS AND IMMUNOLOGY
The exact source of infection and the sequence of events leading to bacterial multiplication and pathologic changes are still unclear. Because of the prominence of intestinal manifestations, an oral route of acquisition is assumed,8 but this is unproven. Current concepts hold that once T. whipplei has been acquired, it enters the proximal small intestine where the bacteria invade the mucosa. Evidence for this is provided by electron microscopy.41,42 Fluorescence in situ hybridization demonstrates colocalization of T. whipplei 16S rRNA with areas of pathologic change and indicates that most viable bacteria are extracellular and located just below the epithelial basement membrane in the lamina propria (see later).11 From the intestinal mucosa, bacteria are thought to spread via lymphatics into mesenteric and mediastinal lymph nodes and into the systemic circulation.
Relatively little is known about the natural habitats of T. whipplei. Only humans seem to be affected, with outdoor workers more strongly represented than other professional groups.8 A PCR-based search in effluent from a German sewage treatment plant revealed positive results for T. whipplei DNA in 25 of 38 samples from five different plants43; another study found T. whipplei DNA in 17 of 46 samples from Austrian sewage plants.44 Several studies have reported detection of T. whipplei DNA in saliva, gastric juice, intestinal biopsies, and stool of asymptomatic persons,44–48 whereas several other PCR-based studies of intestinal biopsy samples have provided little or no evidence of infection in persons without the histologic features of Whipple’s disease.49–53 Two studies that reported T. whipplei DNA in the stool of asymptomatic persons found higher rates of positive results in sewage workers than in other groups of people (25% vs. 7% and 12% vs. 4%, respectively).44,48 A concept of asymptomatic healthy carriage of T. whipplei has been proposed but not confirmed.47,48 So far, there is no evidence for person-to-person transmission of T. whipplei,8 and there are only a few reports of the disease in relatives of persons with the disease.54,55 The genome sequence suggests that the organism is highly dependent on nutrients from other sources.14,15 The proposed extracellular location in the villus tips below the intestinal basement membrane,11 a site with rich influx of nutrients, fits these requirements well.
Several abnormalities of immune function have been observed in patients with Whipple’s disease,8,56,57 including transient (i.e., during active disease), as well as persistent (i.e., after therapy) abnormalities; the persistent abnormalities are presumed to serve as predisposing factors for development of disease. Precisely defined immune defects, however, such as the physical or functional absence of specific cell types, mediators, or receptors, have not been identified. Small case series58,59 have described an over-representation of the HLA-B27 haplotype in patients with Whipple’s disease, but others60,61 have not supported this association. Humoral immunity in patients with Whipple’s disease grossly appears to be normal.8
During active disease, reduced CD4/CD8 T-cell ratios (both in the lamina propria and in peripheral blood), reduced proliferation of peripheral T cells to stimulating agents (e.g., phytohemagglutinin, concanavalin A), and reduced delayed-type hypersensitivity reactions to common antigens in skin tests have been obsesrved.56,58,62 This may be a consequence of malnutrition, however, rather than a pre-existing immunological abnormality. One study showed that the monocytes of a patient with Whipple’s disease exhibited an impaired ability to degrade bacterial antigens,63 which is consistent with the prolonged persistence of bacterial remnants in intestinal macrophages after therapy observed in histologic studies of Whipple’s disease.8,64 Other immunologic abnormalities persist after therapy: reduced numbers of peripheral blood monocytes that express the alpha chain of complement receptor 3 (CD11b),56 a reduced capability of peripheral blood monocytes to produce interleukin (IL)-12 on stimulation with bacterial antigens,57 and a dysregulation of mononuclear cell function, such that the components of a Th1-type immune response are reduced and those of a Th2 immune response are increased.65 The latter observation was supported in a study with specific T. whipplei antigen from cultivated bacteria: Duodenal lymphocytes and peripheral blood mononuclear cells from healthy people exhibited robust Th1 type immune reactivity, but those of patients with Whipple’s disease showed reduced or absent T. whipplei-specific Th1 responses.66 Furthermore, it was shown that macrophages from duodenal tissue of a patient with Whipple’s disease exhibited a transcriptional pattern associated with a Th2 immune response.67
Another investigation revealed that IL-16, a cytokine that is constitutively expressed in T cells, mast cells, dendritic cells, and circulating monocytes and that is released during apoptosis, was expressed at high levels and released by macrophages upon infection with T. whipplei; when added to the experimental model, IL-16 promoted T. whipplei replication in both monocytes and macrophages.68 Circulating blood levels of IL-16 and nucleosomes (a marker of apoptosis) also were found to be elevated in patients with active Whipple’s disease compared with patients with treated Whipple’s disease and compared with controls.69
Several reports describe secondary or opportunistic infections in patients with Whipple’s disease,64,70–72 the most common being Giardia lamblia, which is observed in 8% to 12% of patients. Rare cases of infections with Pneumocystis jirovecii, Cryptosporidium parvum, Nocardia spp., Mycobacterium tuberculosis, Serratia marcescens, Candida spp., dermatophytes, and Strongyloides stercoralis also have been recorded. In addition, T. whipplei infection has been detected by PCR in one patient with acquired immunodeficiency syndrome (AIDS).73 A possible role of the immune system in clearing T. whipplei infection was further suggested by the report of a patient without adequate response to antibiotic treatment, who eventually benefited from adjuvant interferon-γ treatment74; however, this effect was not reproduced in other patients. Taken together, all these observations and laboratory findings suggest that there are immunologic factors, including quantitative deficiencies in macrophage activation, microbial phagocytosis, and the regulation of a cellular immune response, that facilitate the occurrence of Whipple’s disease.
CLINICAL FEATURES
Whipple’s disease usually is a systemic infection, and almost any organ or organ system can be affected.8 Manifestations in the intestinal tract are reported most commonly and are largely responsible for the classic clinical features of Whipple’s disease.20,75 In many patients, arthralgias precede intestinal symptoms by several years (1 to 10 years; up to 30 years reported), although it is unclear whether joints are infected at that point; in some cases, low-grade intermittent fever also occurs for years before the diagnosis is made.23,76 More recent reports suggest a wider spectrum of extraintestinal manifestations, most likely reflecting advances in diagnostic procedures. Patients tend to have less-advanced disease at the time of diagnosis, possibly as a result of earlier detection.8,23
SMALL INTESTINE AND LYMPHATIC SYSTEM
Bacterial and macrophage-predominant inflammatory cell infiltration of the small intestinal mucosa and obstruction of mesenteric lymph nodes lead to a malabsorption syndrome with weight loss, diarrhea, and abdominal pain as the dominant signs and symptoms.20,23,75–77 Weight loss in amounts of 5 to 20 kg occurs gradually, usually over a period of at least a year, sometimes resulting in severe cachexia in the terminal stage of untreated disease.8,20,76 Diarrhea can consist of voluminous steatorrheic stools or may be watery.20 Occult gastrointestinal bleeding is not uncommon, and in some cases gross gastrointestinal bleeding occurs.8,20
Abdominal (mesenteric and retroperitoneal) and peripheral lymphadenopathy are common,20,23,76,77 and in some instances, enlarged abdominal lymph nodes have raised the suspicion of malignancy.77 In rare instances, malignant lymphomas have occurred in patients with Whipple’s disease.78–80
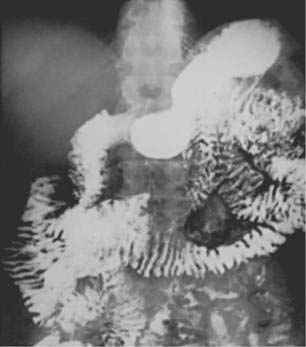
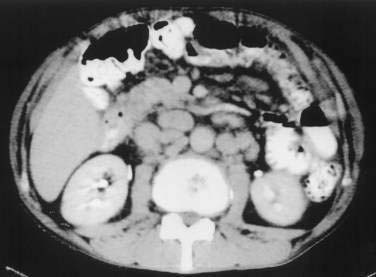
Barium examination of the intestinal tract can reveal nonspecific abnormalities, such as prominent and edematous duodenal and jejunal folds and intestinal dilatation, that also are found in other malabsorption syndromes (Fig. 106-3).8,76 Computed tomography (CT) (Fig. 106-4) or magnetic resonance imaging (MRI) can detect retroperitoneal or para-aortic lymphadenopathy.76,81 Enlarged abdominal lymph nodes have a hypodense appearance on CT scans and are hyperechoic on ultrasonograms.71,82
Laboratory examinations in patients with intestinal Whipple’s disease often reveal an increased erythrocyte sedimentation rate, decreased serum carotene level, decreased serum iron concentration, anemia, decreased serum protein levels, proteinuria, and elevated stool fat content.8,76
CENTRAL NERVOUS SYSTEM
Symptomatic CNS manifestations have been reported in 10% to 43% of patients with intestinal Whipple’s disease with more recent series reporting lower rates.20,23,76,77 Neurologic disease can occur concurrently with intestinal manifestations at the time of diagnosis, but it is more common at the time of clinical relapse, which can occur during or after treatment.8,83 It is thought that bacteria enter the CNS early in the course of disease and that because most drugs do not penetrate the CNS well, the bacteria persist during medical treatment. The result is the impression that intestinal disease goes into remission initially and neurologic disease develops subsequently, even as antibiotics continue to be given. Relapses affecting the CNS are ominous because they tend to be refractory to renewed antibiotic treatment.8,84 Although rare, instances of isolated primary neurologic Whipple’s disease have been reported in patients without intestinal or other manifestations.85,86
Two reviews summarized the neurologic findings of Whipple’s disease in 84 and 122 published cases, respectively.87,88 Common findings are progressive dementia and cognitive changes (28% to 71%), supranuclear ophthalmoplegia (32% to 51%), and altered level of consciousness (27% to 50%); less common are psychiatric symptoms, hypothalamic manifestations (e.g., polydipsia, hyperphagia, insomnia),89 cranial nerve abnormalities, nystagmus, seizures, and ataxia. Two signs that are characteristic of CNS Whipple’s disease are oculomasticatory myorhythmia and oculofacial skeletal myorhythmia,87 each consisting of slow rhythmic and synchronized contractions (≈1/sec) of ocular, facial, or other muscles; they occur in less than 20% of cases,87,88 and they have not yet been documented in other CNS diseases.
Results of neuroimaging (CT or MRI scans) may be normal or reveal mild to moderate brain atrophy or focal lesions without a predilection for specific sites.87,88,90 These abnormalities are not specific for Whipple’s disease, but focal lesions may be used to guide stereotactic biopsies, which in most cases reveal characteristic histology87; MRI appears to be more sensitive than CT scan.88 Results of standard CSF examinations usually are normal, although sometimes there is mild pleocytosis.8,87 CSF cytology often reveals PAS-positive sickle-form particle-containing cells, and PCR often yields positive results for T. whipplei DNA, even in a considerable proportion of neurologically asymptomatic patients.91
CARDIOVASCULAR SYSTEM
Cardiac manifestations of Whipple’s disease include endocarditis, myocarditis, and pericarditis.23 In one autopsy series from the preantibiotic era,75 valvular endocarditis with vegetations was noted in 58% of cases. In contrast, in a more recent series of Whipple’s disease,23 clinically apparent endocarditis was less common (3 of 52 patients). All valves may be affected, but the mitral valve is most commonly pathologically altered, and involvement of the aortic valve leads to the most significant symptoms.8 Some patients require valve replacement. PAS-positive macrophages and bacteria have been documented in native92 and porcine prosthetic93,94 valve tissue and in the myocardium95 by histology and electron microscopy, respectively. Based on PCR testing of excised heart valve tissue, T. whipplei is increasingly being recognized as an agent of “blood culture-negative endocarditis,” even in patients with only minor or no apparent intestinal involvement.94,96–100
MUSCULOSKELETAL SYSTEM
Oligoarthralgias or polyarthralgias, usually involving the ankles, knees, elbows, or fingers, are a common complaint of patients with Whipple’s disease.8 Rheumatoid factor usually is absent. Destructive joint changes or accumulation of synovial fluid are rare, but when they are present, they are accompanied by PAS-positive macrophages (by histology), bacteria (by electron microscopy), or DNA of T. whipplei (by PCR) in synovial tissue or joint fluid.8,101,102 One synovial fluid specimen yielded T. whipplei in culture.30 Sacroiliitis and spondylitis may occur, but ankylosing forms are rare, and there does not seem to be a strong association of these manifestations with HLA-B27.8 Rare manifestations are infectious spondylodiskitis103 and prosthetic joint infection.104
OTHER MANIFESTATIONS
One common feature of Whipple’s disease is skin hyperpigmentation, which has been found in 17% to 66% of patients.20,23,75–77 This finding tends to occur in light-exposed areas of the skin and is unrelated to adrenal dysfunction or hyperbilirubinemia. Histopathologic changes in the skin, however, are extremely rare,105 and the pathophysiology of this hyperpigmentation is unknown.
Ocular manifestations of Whipple’s disease have been described and are diverse, but rare. These include uveitis, vitritis, retinitis, retrobulbar neuritis, and papilledema.8 They usually are associated with CNS disease, and almost all reported patients also had clinical or histologic evidence of intestinal involvement. PAS-positive macrophages, DNA of T. whipplei, and visible bacilli may be detected in vitrectomy specimens.106,107 One case of uveitis has been reported in which the vitreous fluid and one intestinal biopsy specimen yielded positive PCR results, although intestinal histology was normal.107 Another uveitis case was a source for a positive culture.26
Chronic cough was a symptom in Whipple’s original patient and was reported relatively frequently in earlier series75 but infrequently since then.23 Some patients have pleuritis with effusion or granulomatous pulmonary disease resembling sarcoidosis.8
PATHOLOGY
SMALL INTESTINE
On gross inspection, the mucosa of the distal duodenum and jejunum is abnormal in most patients with Whipple’s disease. Whitish-to-yellow plaque-like patches are observed in approximately three quarters of patients (Fig. 106-5); alternatively, the mucosa can appear pale yellow.64,108 Abnormal villus structure and mild mucosal flattening become evident using magnifying optics.
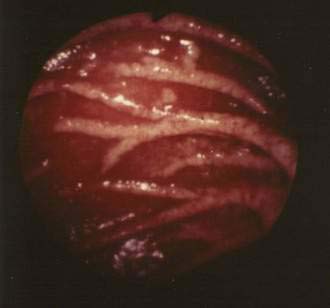
Figure 106-5. Endoscopic view of the distal duodenum in a patient with untreated Whipple’s disease. The plicae circulares are swollen, and the mucosal surface is intact. Numerous whitish patches, reflecting lipid deposits, are present within the mucosa (see Fig. 106-6).
(Courtesy of Hans Jörg Meier-Willersen, MD, Heidelberg, Germany.)
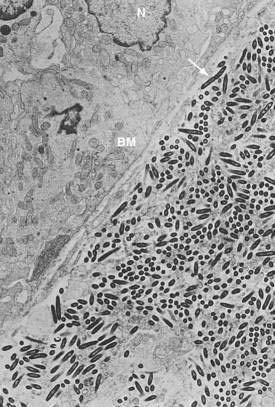
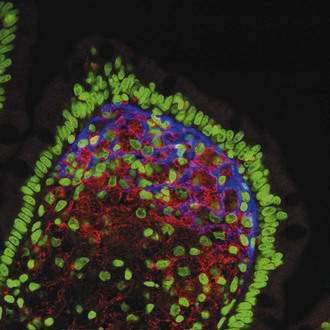
The histopathologic features of intestinal Whipple’s disease are quite distinctive. Viewed with light microscopy, the visible patches reflect lipid deposits or lymphangiectasia, whereas villus distention results from infiltration by macrophages in the lamina propria (Fig. 106-6). The swollen cytoplasm of macrophages appears foamy when stained with hematoxylin-eosin, but numerous granular particles become visible when the PAS stain is used (see Fig. 106-6). These particles correspond to phagolysosomes filled with numerous T. whipplei, and the positive reaction with PAS reflects the glycoprotein content of the bacterial cell walls. Single extracellular bacteria are barely visible with conventional light microscopy because of their small size, but they become evident in the mucosal stroma with high-resolution light microscopy and electron microscopy (Fig. 106-7). The number of bacteria varies greatly among patients.
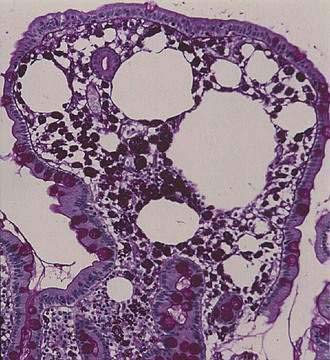
Figure 106-6. Histology of the small intestinal mucosa in the same patient as in Fig. 106-5. A villus is distended by an infiltrate of macrophages that contain periodic acid–Schiff–positive granular particles (type 1 cells) and by lipid droplets. The epithelial layer is intact. Original magnification ×84.
Electron microscopy shows uniformity in size and shape of the bacteria, with an external diameter of 0.2 to 0.25 µm and a length of up to 2.5 µm.42,109 There is an electron-dense outer layer that is not found in other bacteria; some authors have speculated that this unusual membrane may be of host origin.109 Most of the structurally intact bacteria, including dividing forms, are found outside of host cells in the lamina propria (see Fig. 106-7).8,109 In contrast, the intracellular bacteria in macrophages often are found in various stages of degradation. Findings based on fluorescent in situ hybridization using specific T. whipplei 16S rDNA probes support and extend the findings derived from electron microscopy11; the 16S rRNA signal from metabolically active bacteria is found in the intestinal lamina propria, just beneath the basement membrane, but it is absent from the PAS-positive macrophages (Fig. 106-8). Thus, T. whipplei appears to prefer extracellular environments within the host, despite its association with eukaryotic cells. Following the success of T. whipplei laboratory cultivation, antisera have been raised against T. whipplei isolates and have been used for testing small intestine, lymph node, heart, and brain tissue12,110,111; observed staining patterns are similar to those of PAS staining. These T. whipplei-specific antisera did not react with a number of control bacteria and tissues affected by other diseases; however, apart from a few experimental studies, immunohistology has not yet been more widely adopted for routine diagnostic purposes.
Mucosal infiltration with PAS-positive cells usually is diffuse, but patchy lesions may be present in some patients. The inflammatory reaction generally is dominated by macrophages, whereas neutrophils, eosinophils, lymphocytes, and plasma cells are scarcer.64,112 This cellular composition is unusual for an invasive bacterial infection, a feature that suggests a disturbance of mobilization and chemotaxis of leukocytes.64
Variants of the usual histologic findings occur in some patients. These include rare cases with PAS-positive macrophages that are located exclusively in the submucosa and rare cases with epithelioid granulomas in the affected mucosa.64 Taken together, the intestinal histopathology of Whipple’s disease demonstrates some heterogeneity. Although the characteristic lesions are almost invariably present in the proximal small bowel, they might continuously extend as far as to the terminal ileum.75 Occasionally, the diagnosis is first made with ileal biopsies obtained during colonoscopy.
During treatment, the histologic findings in the intestinal mucosa change substantially but slowly, over several months or more.41,64,113 Apart from a continuous decrease in the number of PAS-positive macrophages, the pattern of cellular infiltration of the mucosa changes with time from diffuse to patchy; this feature necessitates multiple biopsies during follow-up endoscopic examinations. Mucosal infiltration shifts from the upper part of the mucosa (i.e., villi) to the lower part of the mucosa (i.e., pericryptal lamina propria) and submucosa. More significantly, the cytologic aspects of the PAS-positive macrophages undergo changes.64 Before treatment, most macrophages have numerous granular particles in the cytoplasm that stain intensely red with PAS (type 1 macrophages; see Fig. 106-6). Within one to six months of treatment, the percentage of type 1 macrophages gradually decreases, and, in parallel, cells with only some coarse granular inclusions and a background of diffusely or finely granular, more faintly PAS-positive cytoplasm (type 2 macrophages) increase in number.
After six to 15 months, most macrophages that are still present have diffuse and faintly PAS-positive material in their cytoplasm but lack granular inclusions (type 3 macrophages). Type 3 macrophages contain only filamentous remnants of bacteria.64 Thus, their positive PAS reaction reflects the presence of glycoprotein residues of degraded bacterial cell walls. Even in adequately treated patients, some type 3 macrophages usually persist, occasionally for more than 10 years; in fact, the finding of type 3 macrophages alone is consistent with intestinal remission. Despite documented clinical remission of intestinal disease, however, some patients still harbor viable T. whipplei and can later develop extraintestinal Whipple’s disease. Thus, the prognostic value of intestinal histology during the follow-up of patients is limited.64
EXTRAINTESTINAL PATHOLOGY
Autopsy reports of untreated patients with Whipple’s disease have revealed involvement of virtually any organ and tissue.75,114 As with intestinal disease, the histologic hallmark of extraintestinal involvement is the presence of intracellular PAS-positive granular particles; the diagnostic significance of these lesions in extraintestinal tissues is limited, however, and additional evidence is required for the diagnosis of Whipple’s disease. Rod-shaped bacteria have been documented by electron microscopy in many extraintestinal organs, including liver, heart, lung, brain, eye, lymph nodes, bone marrow, and spleen.8
Two different types of lymph node lesions are common in Whipple’s disease. Abdominal nodes generally contain lipid deposits that induce a granulomatous foreign body type of reaction.1 Peripheral lymph nodes (inguinal, axillary, cervical) generally do not contain lipids but feature a toxoplasmosis-like lymphadenitis with small clusters of epithelioid macrophages, some of which have PAS-positive particles that correspond to inclusions with T. whipplei. Rarely, and most commonly in the mediastinum, a third type of lymph node reaction may be observed that resembles sarcoidosis.
Whipple’s disease affects diverse regions of the brain. Most commonly, perivascular infiltrates of PAS-positive macrophages are present, as well as tumor-like granulomas of variable size, consisting of glial cells with intensely PAS-positive granular particles.8 Occasionally, granulomas in the ventricular system cause occlusive hydrocephalus. PAS-positive macrophages often can be detected free in the CSF, even in patients without neurologic or psychiatric symptoms (see later).
DIAGNOSIS
The vast majority of patients with Whipple’s disease suffer from involvement of the intestinal tract regardless of the presence or absence of gastrointestinal symptoms.23,76,77 Thus, the primary diagnostic approach to a patient with clinically suspected Whipple’s disease is upper endoscopy (see Fig. 106-5) with mucosal biopsy. To avoid sampling errors in patients with patchy lesions, one should obtain approximately five biopsy specimens from regions as far distal as possible within the small intestine.24 Histologic examination with routine hematoxylin-eosin and PAS stains is usually sufficient to reach a diagnosis (see earlier). In some cases, findings may be corroborated with silver stains,1 but the Gram stain is less useful in this infection. Traditionally, electron microscopy has been used as the gold standard for confirming the diagnosis of Whipple’s disease by showing bacteria of characteristic size and shape.8 More recently, PCR analysis has taken over the role as preferred confirmatory test.49,50
Since the molecular characterization of T. whipplei, a number of PCR-based assays have been developed for diagnostic purposes.10,37,49–51,103,107,115 These assays vary by their target sequences and amplification strategy as well as by the amount of validation and diagnostic experience that is available for them. Newer PCR tests increasingly use the real-time PCR platform.94 In almost all patients with a histologic diagnosis of Whipple’s disease, well-designed and well-standardized PCR assays detect T. whipplei DNA in the intestinal mucosa.49,50 PCR from intestinal biopsies appears unsuitable as a primary diagnostic approach, because Whipple’s disease only rarely is diagnosed by PCR in the setting of histologically negative intestinal biopsies,49–53 and T. whipplei DNA has been detected in unaffected control subjects.45–47 In practical terms, normal intestinal histology in the absence of extraintestinal disease that suggests Whipple’s disease excludes the diagnosis, provided that multiple biopsy specimens are examined.
Extraintestinal manifestations warrant the examination of specimens from affected sites. Histology and cytology with PAS staining, electron microscopy, and PCR all are useful for this purpose. A small number of cases has been reported with positive PCR results in extraintestinal samples and intestinal histology that is negative for PAS-positive macrophages. Examples of such cases include febrile illness with erythrocyte-associated bacteria, uveitis, endocarditis, and neurologic disease.94,98–100,107,116,117
In an effort to distinguish between true Whipple’s disease and asymptomatic carriage, one study used quantitative real-time PCR on a number of different sample types.117 When both saliva and stool were positive, the sensitivity was 65% and the positive predictive value was 95% for detecting intestinal manifestations. The positive predictive value increased to 100% when the bacterial load was greater than 104 per gram of stool. When PCRs from both stool and saliva were negative, the negative predictive value for intestinal disease was 96% (one of 23 intestinal cases was PCR-negative in both stool and saliva). The sensitivity for diagnosing extraintestinal disease was insufficient. Similarly, PCR from peripheral blood lacked sensitivity in this study, and its diagnostic value previously has been questioned.118
Considering the systemic nature of the disorder, it is important to evaluate commonly involved organ systems on a routine basis whenever a new diagnosis of Whipple’s disease has been established. Ultrasound examination might reveal enlarged mesenteric lymph nodes that have unusually high echogenicity due to lipid deposits.71 Neurologic examination is indicated, including routine sampling of CSF.91 Based on cytologic or PCR analysis of the spinal fluid, 70% of patients with intestinal Whipple’s disease in one study were found to have CNS infection with T. whipplei, even though they had no neurologic or psychiatric symptoms.91 Imaging studies of the brain generally are not helpful in the absence of neurologic symptoms. In selected patients with Whipple’s disease and anemia, wireless capsule endoscopy might detect a site of bleeding in the small intestine. In one case report, capsule endoscopy revealed diffuse disease and discrete areas of bleeding in the middle and distal portions of the jejunum, although the exact reason for the bleeding was not defined.119
During treatment, diagnostic assessments should be repeated at regular intervals. Endoscopic lesions usually resolve within months but can last for up to a year.108 Intestinal histology improves within several months,64 and PCR assays on intestinal biopsy tissues convert to negative within a time range of about one to 12 months after appropriate therapy is instituted.49 Some PAS-positive macrophages can persist for years,64 even while the patient remains in clinical remission (see earlier). Enlarged abdominal lymph nodes can require more than a year to regress and can result in fibrosis. Follow-up examination of the CSF should include PCR analysis.91 As has been documented in a culture-positive case, T. whipplei can persist in a viable state in the CNS despite prolonged administration of antibiotics.29
DIFFERENTIAL DIAGNOSIS
Disorders that mimic the histology of Whipple’s disease are uncommon.17 PAS-positive cells in intestinal biopsies can include mucosal smooth muscle cells that are rich in glycogen or plasma cells that contain immunoglobulin (Russell bodies) in the setting of chronic duodenitis; other disorders include macroglobulinemia, intestinal xanthelasmas, and pseudomelanosis duodeni. Rarely, PAS-positive cells reflect intestinal infection with Mycobacterium avium complex and Rhodococcus equi (in HIV coinfected hosts), or Histoplasma capsulatum. Differentiation from Whipple’s disease usually is possible by means of histochemical stains (e.g., stains for acid-fast bacteria and use of diastase) and by immunocytochemistry.17
Sarcoid-like granulomas are rare in Whipple’s disease but can occur in the stomach,120 small intestine,64,121 liver,122 and lymph nodes. A possible relationship between Whipple’s disease and sarcoidosis remains unresolved.123 By means of PCR analysis, thoracic sarcoidosis124 and intestinal sarcoidosis125 tissues were both found to be negative for T. whipplei DNA.
Most patients with Whipple’s disease have enlarged abdominal lymph nodes.78 Rare cases have been observed of Whipple’s disease associated with metachronous or synchronous malignant lymphomas,78–80 but the relationship of T. whipplei infection to lymphoma, if any, remains unclear.
TREATMENT AND PROGNOSIS
The initial response of Whipple’s disease to antibiotic treatment usually is prompt.126 Diarrhea often resolves within several days, arthralgias often resolve within a few weeks, and significant weight gain occurs within a few months.20 In the 1970s and early 1980s, long-term tetracycline therapy usually was given,23,77 but it became increasingly clear that patients treated in this manner often suffered relapses, many of which affected the CNS.127,128 CNS relapses have a poor prognosis, because they are often refractory to renewed treatment.83 It therefore was suggested that the treatment of Whipple’s disease include antibiotics that cross the blood-brain barrier. Since the mid-1980s, trimethoprim-sulfamethoxazole has been used commonly.83,129
Current recommendations for the treatment of Whipple’s disease are based on observations from numerous case reports,8 several clinical series,23,76,77 and retrospective analyses of antibiotic regimens.83,126 In a retrospective analysis of 88 patients,83 relapses were most common after monotherapy with tetracyclines. Of 49 patients treated with tetracycline alone, 21 relapsed, including nine with CNS relapses. Only a small number of relapses (2 of 15 patients treated), none of which involved the CNS, were observed after initial parenteral treatment with penicillin plus streptomycin, followed by long-term oral tetracycline (the Duke regimen). Tetracyclines and trimethoprim-sulfamethoxazole were compared in another series of 30 patients.126 Trimethoprim-sulfamethoxazole was superior to tetracyclines in inducing remission (12 of 13 vs. 13 of 22 treatment courses, respectively, including remissions after relapse). CNS relapses occurred in two of 22 patients receiving tetracycline and in one of 13 receiving trimethoprim-sulfamethoxazole. Despite its clinical efficacy and ability to cross the blood-brain barrier, several reports indicate that relapses, including CNS relapses, also can occur after use of trimethoprim-sulfamethoxazole.130–132 Some patients appear to have benefited from repeated intravenous courses of third-generation cephalosporins.91
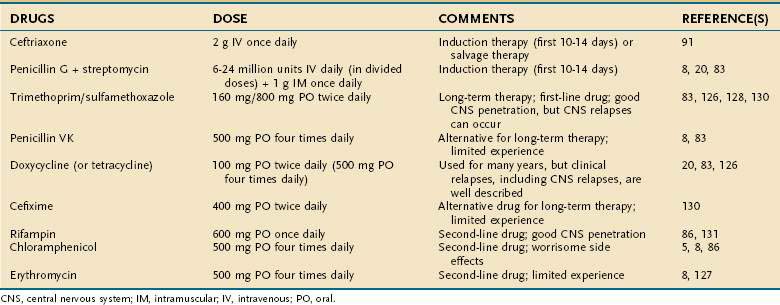
Based on these clinical observations, the current recommendation for treatment of Whipple’s disease is to begin with an induction phase using either penicillin G plus streptomycin or a third-generation cephalosporin (e.g., ceftriaxone) followed by treatment with at least one drug that efficiently crosses the blood-brain barrier (e.g., trimethoprim-sulfamethoxazole) for at least one year. An overview of antibiotic treatments, including suggested doses, is given in Table 106-1.
Data concerning antibiotic susceptibility have become available from in vitro experiments with T. whipplei in culture as well as from genome analysis. Growth in the presence of antibiotics was assessed with real-time PCR.133,134 In coculture with fibroblasts, the bacterium appears sensitive to doxycycline, macrolides, penicillins, rifampin, teicoplanin, and trimethoprim-sulfamethoxazole; variably sensitive to imipenem; and only moderately sensitive or resistant to cephalosporins, fluoroquinolones, and vancomycin.133 In axenic medium, the results were very similar to those in cell culture, except that T. whipplei was sensitive to ceftriaxone and vancomycin.134 Interestingly, the T. whipplei genome lacks the gene for dihydrofolate reductase, which is the target for trimethoprim action,135 so that the susceptibility to trimethoprim-sulfamethoxazole most likely is based solely on its sulfamethoxazole component. Consistent with this, trimethoprim has been found inactive in laboratory experiments.134 Some have proposed treating Whipple’s disease with either sulfamethoxazole or sulfadiazine alone instead of in combination with trimethoprim.136 Another treatment suggestion—to use hydroxychloroquine in combination with doxycycline—came from in vitro experiments showing enhanced bactericidal activity from the known alkalinization of intracellular vacuoles by hydroxychloroquine.133 Clinical experience with this regimen is still limited, and, in addition, the doxycycline component does not penetrate well into the CNS.
Bentley SD, Maiwald M, Murphy LD, et al. Sequencing and analysis of the genome of the Whipple’s disease bacterium Tropheryma whipplei. Lancet. 2003;361:637. (Ref 14.)
Dobbins WOIII. Whipple’s Disease. Springfield, Ill: Charles C Thomas; 1987. (Ref 8.)
Fenollar F, Lepidi H, Raoult D. Whipple’s endocarditis: Review of the literature and comparisons with Q fever, Bartonella infection, and blood culture–positive endocarditis. Clin Infect Dis. 2001;33:1309. (Ref 99.)
Fredricks DN, Relman DA. Localization of Tropheryma whippelii rRNA in tissues from patients with Whipple’s disease. J Infect Dis. 2001;183:1229. (Ref 11.)
La Scola B, Fenollar F, Fournier PE, et al. Description of Tropheryma whipplei gen. nov., sp. nov., the Whipple’s disease bacillus. Int J Syst Evol Microbiol. 2001;51:1471. (Ref 13.)
Maiwald M, von Herbay A, Fredricks DN, et al. Cultivation of Tropheryma whipplei from cerebrospinal fluid. J Infect Dis. 2003;188:801. (Ref 29.)
Maizel H, Ruffin JM, Dobbins WOIII. Whipple’s disease: A review of 19 patients from one hospital and a review of the literature since 1950. Medicine (Baltimore). 1970;49:175. (Ref 20.)
Moos V, Kunkel D, Marth T, et al. Reduced peripheral and mucosal Tropheryma whipplei–specific Th1 response in patients with Whipple’s disease. J Immunol. 2006;177:2015. (Ref 66.)
Raoult D, Birg ML, LaScola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med. 2000;342:620. (Ref 12.)
Raoult D, Ogata H, Audic S, et al. Tropheryma whipplei twist: A human pathogenic actinobacteria with a reduced genome. Genome Res. 2003;13:1800. (Ref 15.)
Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med. 1992;327:293. (Ref 10.)
Renesto P, Crapoulet N, Ogata H, et al. Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet. 2003;362:447. (Ref 32.)
Vital Durand D, Lecomte C, Cathébras P, et al. Whipple disease: Clinical review of 52 cases. Medicine (Baltimore). 1997;76:170. (Ref 23.)
von Herbay A, Ditton HJ, Schuhmacher F, Maiwald M. Whipple’s disease: Staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997;113:434. (Ref 91.)
von Herbay A, Maiwald M, Ditton HJ, Otto HF. Histology of intestinal Whipples disease revisited: A study of 48 patients. Virchows Arch. 1996;429:335. (Ref 64.)
1. Whipple GH. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull Johns Hopkins Hosp. 1907;18:382.
2. Oliver-Pascual E, Galan J, Oliver-Pascual A, Castillo E. Un caso de lipodistrofia intestinal con lesiones gangliones mesentericas de granulomatosis lipofagica (enfermedad de Whipple). Rev Esp Enferm Apar Dig. 1947;6:213.
3. Bolt RJ, Pollard HM, Standaert L. Transoral small-bowel biopsy as an aid in the diagnosis of malabsorption states. N Engl J Med. 1958;259:32.
4. Black-Schaffer B. The tinctoral demonstration of a glycoprotein in Whipple’s disease. Proc Soc Exp Biol Med. 1949;72:225.
5. Paulley JW. A case of Whipple’s disease (intestinal lipodystrophy). Gastroenterology. 1952;22:128.
6. Chears WC, Ashworth CT. Electron microscopic study of the intestinal mucosa in Whipple’s disease: Demonstration of encapsulated bacilliform bodies in the lesion. Gastroenterology. 1961;41:129.
7. Yardley JH, Hendrix TR. Combined electron and light microscopy in Whipple’s disease. Bull Johns Hopkins Hosp. 1961;109:80.
8. Dobbins WOIII. Whipple’s Disease. Springfield, Ill: Charles C Thomas; 1987.
9. Wilson KH, Blitchington R, Frothingham R, Wilson JAP. Phylogeny of the Whipple’s disease–associated bacterium. Lancet. 1991;338:474.
10. Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med. 1992;327:293.
11. Fredricks DN, Relman DA. Localization of Tropheryma whippelii rRNA in tissues from patients with Whipple’s disease. J Infect Dis. 2001;183:1229.
12. Raoult D, Birg ML, LaScola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med. 2000;342:620.
13. La Scola B, Fenollar F, Fournier PE, et al. Description of Tropheryma whipplei gen. nov., sp. nov., the Whipple’s disease bacillus. Int J Syst Evol Microbiol. 2001;51:1471.
14. Bentley SD, Maiwald M, Murphy LD, et al. Sequencing and analysis of the genome of the Whipple’s disease bacterium Tropheryma whipplei. Lancet. 2003;361:637.
15. Raoult D, Ogata H, Audic S, et al. Tropheryma whipplei twist: A human pathogenic actinobacteria with a reduced genome. Genome Res. 2003;13:1800.
16. Salomoni I. [Whipple’s disease in Western Switzerland between 1960 and 1983: Observation of a case, comparison of a series of 11 cases diagnosed in Western Switzerland between 1960 and 1983 with data from the literature]. Rev Med Suisse Romande. 1984;104:655.
17. von Herbay A. Morbus Whipple: histologische Diagnostik nach der Entdeckung von Tropheryma whippelii. Pathologe. 2001;22:82.
18. von Herbay A, Otto HF, Stolte M, et al. Epidemiology of Whipple disease in Germany: Analysis of 110 patients diagnosed in 1965-95. Scand J Gastroenterol. 1997;32:52.
19. Capron JP, Thevenin A, Delamarre J, et al. Whipple’s disease: Study of three cases and epidemiological and radiological remarks. Lille Med. 1975;20:842.
20. Maizel H, Ruffin JM, Dobbins WOIII. Whipple’s disease: A review of 19 patients from one hospital and a review of the literature since 1950. Medicine (Baltimore). 1970;49:175.
21. Lopatin RN, Grossman ET, Horine J, et al. Whipple’s disease in neighbors. J Clin Gastroenterol. 1982;4:223.
22. Ectors NL, Geboes KJ, Devos RM, et al. Whipple’s disease: A histological, immunocytochemical, and electron-microscopic study of the small-intestinal epithelium. J Pathol. 1994;172:73.
23. Vital Durand D, Lecomte C, Cathébras P, et al. Whipple disease: Clinical review of 52 cases. Medicine (Baltimore). 1997;76:170.
24. von Herbay A. Whipple’s Disease Online [Internet]. [cited 2009 May 20] Available from http://www.WhipplesDisease.net
25. Raoult D, La Scola B, Lecocq P, et al. Culture and immunological detection of Tropheryma whippelii from the duodenum of a patient with Whipple disease. JAMA. 2001;285:1039.
26. Drancourt M, Raoult D, Lepidi H, et al. Culture of Tropheryma whippelii from the vitreous fluid of a patient presenting with unilateral uveitis. Ann Intern Med. 2003;139:1046.
27. Masselot F, Boulos A, Maurin M, et al. Molecular evaluation of antibiotic susceptibility: Tropheryma whipplei paradigm. Antimicrob Agents Chemother. 2003;47:1658.
28. Fenollar F, Birg ML, Gauduchon V, Raoult D. Culture of Tropheryma whipplei from human samples: A 3-year experience (1999 to 2002). J Clin Microbiol. 2003;41:3816.
29. Maiwald M, von Herbay A, Fredricks DN, et al. Cultivation of Tropheryma whipplei from cerebrospinal fluid. J Infect Dis. 2003;188:801.
30. Puéchal X, Fenollar F, Raoult D. Cultivation of Tropheryma whipplei from the synovial fluid in Whipple’s arthritis. Arthritis Rheum. 2007;56:1713.
31. La MV, Crapoulet N, Barbry P, et al. Comparative genomic analysis of Tropheryma whipplei strains reveals that diversity among clinical isolates is mainly related to the WiSP proteins. BMC Genomics. 2007;8:349.
32. Renesto P, Crapoulet N, Ogata H, et al. Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet. 2003;362:447.
33. Maiwald M, Ditton HJ, von Herbay A, et al. Reassessment of the phylogenetic position of the bacterium associated with Whipple’s disease and determination of the 16S-23S ribosomal intergenic spacer sequence. Int J Syst Bacteriol. 1996;46:1078.
34. Maiwald M, von Herbay A, Lepp PW, Relman DA. Organization, structure, and variability of the rRNA operon of the Whipple’s disease bacterium (Tropheryma whippelii). J Bacteriol. 2000;182:3292.
35. Hinrikson HP, Dutly F, Nair S, Altwegg M. Detection of three different types of Tropheryma whippelii directly from clinical specimens by sequencing, single-strand conformation polymorphism (SSCP) analysis and type-specific PCR of their 16S-23S ribosomal intergenic spacer region. Int J Syst Bacteriol. 1999;49:1701.
36. Geissdörfer W, Wittmann I, Röllinghoff M, et al. Detection of a new 16S-23S rRNA spacer sequence variant (type 7) of Tropheryma whippelii in a patient with prosthetic aortic valve endocarditis. Eur J Clin Microbiol Infect Dis. 2001;20:762.
37. Hinrikson HP, Dutly F, Altwegg M. Evaluation of a specific nested PCR targeting domain III of the 23S rRNA gene of “Tropheryma whippelii” and proposal of a classification system for its molecular variants. J Clin Microbiol. 2000;38:595.
38. Morgenegg S, Dutly F, Altwegg M. Cloning and sequencing of a part of the heat shock protein 65 gene (hsp65) of “Tropheryma whippelii” and its use for detection of “T. whippelii” in clinical specimens by PCR. J Clin Microbiol. 2000;38:2248.
39. Maiwald M, Lepp PW, Relman DA. Analysis of conserved non-rRNA genes of Tropheryma whipplei. Syst Appl Microbiol. 2003;26:3.
40. Li W, Fenollar F, Rolain JM, et al. Genotyping reveals a wide heterogeneity of Tropheryma whipplei. Microbiology. 2008;154:521.
41. Dobbins WOIII, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol. 1967;51:225.
42. Dobbins WOIII, Kawanishi H. Bacillary characteristics in Whipple’s disease: An electron microscopic study. Gastroenterology. 1981;80:1468.
43. Maiwald M, Schuhmacher F, Ditton HJ, von Herbay A. Environmental occurrence of the Whipple’s disease bacterium (Tropheryma whippelii). Appl Environ Microbiol. 1998;64:760.
44. Schöniger-Hekele M, Petermann D, Weber B, Müller C. Tropheryma whipplei in the environment: Survey of sewage plant influxes and sewage plant workers. Appl Environ Microbiol. 2007;73:2033.
45. Street S, Donoghue HD, Neild GH. Tropheryma whippelii DNA in saliva of healthy people. Lancet. 1999;354:1178.
46. Ehrbar HU, Bauerfeind P, Dutly F, et al. PCR-positive tests for Tropheryma whippelii in patients without Whipple’s disease. Lancet. 1999;353:2214.
47. Amsler L, Bauernfeind P, Nigg C, et al. Prevalence of Tropheryma whipplei DNA in patients with various gastrointestinal diseases and in healthy controls. Infection. 2003;31:81.
48. Fenollar F, Trani M, Davoust B, et al. Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. J Infect Dis. 2008;197:880.
49. von Herbay A, Ditton HJ, Maiwald M. Diagnostic application of a polymerase chain reaction assay for the Whipple’s disease bacterium to intestinal biopsies. Gastroenterology. 1996;110:1735.
50. Ramzan NN, Loftus E, Burgart LJ, et al. Diagnosis and monitoring of Whipple disease by polymerase chain reaction. Ann Intern Med. 1997;126:520.
51. Müller C, Petermann D, Stain C, et al. Whipple’s disease: Comparison of histology with diagnosis based on polymerase chain Reaction in four consecutive cases. Gut. 1997;40:425.
52. Pron B, Poyart C, Abachin E, et al. Diagnosis and follow-up of Whipple’s disease by amplification of the 16S rRNA gene of Tropheryma whippelii. Eur J Clin Microbiol Infect Dis. 1999;18:62.
53. Maiwald M, von Herbay A, Persing DH, et al. Tropheryma whippelii DNA is rare in the intestinal mucosa of patients without other evidence of Whipple disease. Ann Intern Med. 2001;134:115.
54. Gross JB, Wollaeger EE, Sauer WG, et al. Whipple’s disease: A report of four cases, including two in brothers, with observations on pathologic physiology, diagnosis, and treatment. Gastroenterology. 1959;36:65.
55. Dykman DD, Cuccherini BA, Fuss IJ, et al. Whipple’s disease in a father-daughter pair. Dig Dis Sci. 1999;44:2542.
56. Marth T, Roux M, von Herbay A, Fuerle GE. Persistent reduction of complement receptor-3 alpha-chain expressing mononuclear blood cells and transient inhibitory serum factors in Whipple’s disease. Clin Immunol Immunopathol. 1994;72:217.
57. Marth T, Neurath M, Cuccherini BA, Strober W. Defects of monocyte interleukin 12 production and humoral immunity in Whipple’s disease. Gastroenterology. 1997;113:442.
58. Feurle GE, Dörken B, Schopf E, Lenhard V. HLA-B27 and defects in the T-cell system in Whipple’s disease. Eur J Clin Invest. 1979;9:385.
59. Dobbins WO. HLA antigens in Whipple’s disease. Arthritis Rheum. 1987;30:102.
60. Bai JC, Mota AH, Maurino E, et al. Class I and class II HLA antigens in a homogeneous Argentinean population with Whipple’s disease: Lack of association with HLA-B27. Am J Gastroenterol. 1991;86:992.
61. Olivieri I, Brandi G, Padula A, et al. Lack of association with spondyloarthritis and HLA-B27 in Italian patients with Whipple’s disease. J Rheumatol. 2001;28:1294.
62. Martin FF, Vilseck J, Dobbins WO, et al. Immunological alterations in patients with treated Whipple’s disease. Gastroenterology. 1972;63:6.
63. Bjerknes R, Odegaard S, Bjerkvig R, et al. Whipple’s disease: Demonstration of a persisting monocyte and macrophage dysfunction. Scand J Gastroenterol. 1988;23:611.
64. von Herbay A, Maiwald M, Ditton HJ, Otto HF. Histology of intestinal Whipple’s disease revisited: A study of 48 patients. Virchows Arch. 1996;429:335.
65. Marth T, Kleen N, Stallmach A, et al. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple’s disease. Gastroenterology. 2002;123:1468.
66. Moos V, Kunkel D, Marth T, et al. Reduced peripheral and mucosal Tropheryma whipplei–specific Th1 response in patients with Whipple’s disease. J Immunol. 2006;177:2015.
67. Desnues B, Lepidi H, Raoult D, Mege JL. Whipple’s disease: Intestinal infiltrating cells exhibit a transcriptional pattern of M2/alternatively activated macrophages. J Infect Dis. 2005;192:1642.
68. Desnues B, Raoult D, Mege JL. IL-16 is critical for Tropheryma whipplei replication in Whipple’s disease. J Immunol. 2005;175:4575.
69. Benoit M, Fenollar F, Raoult D, Mege JL. Increased levels of circulating IL-16 and apoptosis markers are related to the activity of Whipple’s disease. PLoS ONE. 2007;2:e494.
70. Bassotti G, Pelli MA, Ribacchi R, et al. Giardia lamblia infestation reveals underlying Whipple’s disease in a patient with longstanding constipation. Am J Gastroenterol. 1991;86:371.
71. Meier-Willersen HJ, Maiwald M, von Herbay A. Morbus Whipple in Assoziation mit opportunistischen Infektionen. Dtsch Med Wochenschr. 1993;118:854.
72. Fenollar F, Lepidi H, Gerolami R, et al. Whipple’s disease associated with giardiasis. J Infect Dis. 2003;188:828.
73. Maiwald M, Meier-Willersen HJ, Hartmann M, von Herbay A. Detection of Tropheryma whippelii DNA in a patient with AIDS. J Clin Microbiol. 1995;33:1354.
74. Schneider T, Stallmach A, von Herbay A, et al. Treatment of refractory Whipple’s disease with interferon-gamma. Ann Intern Med. 1998;129:875.
75. Enzinger FM, Helwig EB. Whipple’s disease: A review of the literature and report of fifteen patients. Virchows Arch. 1963;336:238.
76. Fleming JL, Wiesner RH, Shorter RG. Whipple’s disease: Clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients. Mayo Clin Proc. 1988;63:539.
77. von Herbay A, Otto HF. Whipple’s disease: A report of 22 patients. Klin Wochenschr. 1988;66:533.
78. von Herbay A, Otto HF. Abdominale Lymphome beim Morbus Whipple. Dtsch Med Wochenschr. 1989;114:2028.
79. Gillen CD, Coddington R, Monteith PG, Taylor RH. Extraintestinal lymphoma in association with Whipple’s disease. Gut. 1993;34:1627.
80. Gruner U, Goesch P, Donner A, Peters U. Whipple disease and non-Hodgkin lymphoma. Z Gastroenterol. 2001;39:305.
81. MacDermott RP, Shephard JAO. Whipple’s disease: Case record 37-1997. N Engl J Med. 1997;337:1612.
82. Albrecht T. Computertomographie abdominaler Lymphome bei Morbus Whipple. Rofo Fortschr Geb Röntgenstr Neuen Bildgeb Verfahr. 1994;160:487.
83. Keinath RD, Merrell DE, Vlietstra R, Dobbins WOIII. Antibiotic treatment and relapse in Whipple’s disease: Long-term follow-up of 88 patients. Gastroenterology. 1985;88:1867.
84. Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8:899.
85. Johnson L, Diamond I. Cerebral Whipple’s disease: Diagnosis by brain biopsy. Am J Clin Pathol. 1980;74:486.
86. Adams M, Rhyner PA, Day J, et al. Whipple’s disease confined to the central nervous system. Ann Neurol. 1987;21:104.
87. Louis ED, Lynch T, Kaufmann P, et al. Diagnostic guidelines in central nervous system Whipple’s disease. Ann Neurol. 1996;40:561.
88. Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: Report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81:443.
89. Lieb K, Maiwald M, Berger M, Voderholzer U. Insomnia for 5 years. Lancet. 1999;354:1966.
90. Verhagen WIM, Huygen PLM, Dalman JE, Schuurmans MMJ. Whipple’s disease and the central nervous system: A case report and a review of the literature. Clin Neurol Neurosurg. 1996;98:299.
91. von Herbay A, Ditton HJ, Schuhmacher F, Maiwald M. Whipple’s disease: Staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997;113:434.
92. Jeserich M, Ihling C, Holubarsch C. Aortic valve endocarditis with Whipple disease. Ann Intern Med. 1997;126:920.
93. Ratliff NB, McMahon JT, Naab TJ, Cosgrove DM. Whipple’s disease in the porcine leaflets of a Carpentier-Edwards prosthetic mitral valve. N Engl J Med. 1984;311:902.
94. Dreier J, Szabados F, von Herbay A, et al. Tropheryma whipplei infection of an acellular porcine heart valve bioprosthesis in a patient who did not have intestinal Whipple’s disease. J Clin Microbiol. 2004;42:4487.
95. Silvestry FE, Kim B, Pollack BJ, et al. Cardiac Whipple disease: Identification of Whipple bacillus by electron microscopy in the myocardium of a patient before death. Ann Intern Med. 1997;126:214.
96. Célard M, de Gevigney G, Mosnier S, et al. Polymerase chain reaction analysis for diagnosis of Tropheryma whippelii infective endocarditis in two patients with no previous evidence of Whipple’s disease. Clin Infect Dis. 1999;29:1348.
97. Elkins C, Shuman TA, Pirolo JS. Cardiac Whipple’s disease without digestive symptoms. Ann Thorac Surg. 1999;67:250.
98. Gubler JGH, Kuster M, Dutly F, et al. Whipple endocarditis without overt gastrointestinal disease: Report of four cases. Ann Intern Med. 1999;131:112.
99. Fenollar F, Lepidi H, Raoult D. Whipple’s endocarditis: Review of the literature and comparisons with Q fever, Bartonella infection, and blood culture–positive endocarditis. Clin Infect Dis. 2001;33:1309.
100. Marín M, Muñoz P, Sánchez M, et al. Tropheryma whipplei infective endocarditis as the only manifestation of Whipple’s disease. J Clin Microbiol. 2007;45:2078.
101. Rubinow A, Canoso JJ, Goldenberg DL, et al. Arthritis in Whipple’s disease. Isr J Med Sci. 1981;17:445.
102. O’Duffy JD, Griffing WL, Li CY, et al. Whipple’s arthritis: Direct detection of Tropheryma whippelii in synovial fluid and tissue. Arthritis Rheum. 1999;42:812.
103. Altwegg M, Fleisch-Marx A, Goldenberger D, et al. Spondylodiscitis caused by Tropheryma whippelii. Schweiz Med Wochenschr. 1996;126:1495.
104. Frésard A, Guglielminotti C, Berthelot P, et al. Prosthetic joint infection caused by Tropheryma whippelii (Whipple’s bacillus). Clin Infect Dis. 1996;22:575.
105. Balestrieri GP, Villanacci V, Battocchio S, et al. Cutaneous involvement in Whipple’s disease. Br J Dermatol. 1996;135:666.
106. Williams JG, Edward DP, Tessler HH, et al. Ocular manifestations of Whipple disease: An atypical presentation. Arch Ophthalmol. 1998;116:1232.
107. Rickman LS, Freeman WR, Green WR, et al. Uveitis caused by Tropheryma whippelii (Whipple bacillus). N Engl J Med. 1995;332:363.
108. Geboes K, Ectors N, Heidbuchel H, et al. Whipple’s disease: Endoscopic aspects before and after therapy. Gastrointest Endosc. 1990;36:247.
109. Silva MT, Macedo PM, Moura Nunes JF. Ultrastructure of bacilli and the bacillary origin of the macrophagic inclusions in Whipple’s disease. J Gen Microbiol. 1985;131:1001.
110. Baisden BL, Lepidi H, Raoult D, et al. Diagnosis of Whipple disease by immunohistochemical analysis: A sensitive and specific method for the detection of Tropheryma whippleii (the Whipple bacillus) in paraffin-embedded tissue. Am J Clin Pathol. 2002;118:742.
111. Lepidi H, Costedoat N, Piette JC, et al. Immunohistological detection of Tropheryma whipplei (Whipple bacillus) in lymph nodes. Am J Med. 2002;113:334.
112. Ectors N, Geboes K, Devos R, et al. Whipple’s disease: A histological, immunocytochemical and electron-microscopic study of the immune response in the small intestinal mucosa. Histopathology. 1992;21:1.
113. Dvorak AM. Ultrastructural monitoring of progress of Whipple’s disease therapy. Dig Dis Pathol. 1989;2:81.
114. Sieracki JC, Fine G. Whipple’s disease: Observations on systemic involvement: II. Gross and histologic observations. Arch Pathol. 1959;67:81.
115. Fenollar F, Fournier PE, Robert C, Raoult D. Use of genome selected repeated sequences increases the sensitivity of PCR detection of Tropheryma whipplei. J Clin Microbiol. 2004;42:401.
116. Lowsky R, Archer GL, Fyles G, et al. Diagnosis of Whipple’s disease by molecular analysis of peripheral blood. N Engl J Med. 1994;331:1343.
117. Fenollar F, Laouira S, Lepidi H, et al. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: Usefulness of saliva and stool specimens for first-line screening. Clin Infect Dis. 2008;47:659.
118. Marth T, Fredricks D, Strober W, Relman DA. Limited role for PCR-based diagnosis of Whipple’s disease from peripheral blood mononuclear cells. Lancet. 1996;348:66.
119. Fritscher-Ravens A, Swain CP, von Herbay A. Refractory Whipple’s disease with anaemia: First lessons from capsule endoscopy. Endoscopy. 2004;36:659.
120. Ectors N, Geboes K, Wynants P, Desmet V. Granulomatous gastritis and Whipple’s disease. Am J Gastroenterol. 1992;87:509.
121. Babaryka I, Thorn L, Langer E. Epithelioid cell granulomata in the mucosa of the small intestine in Whipple’s disease. Virchows Arch. 1979;382:227.
122. Saint-Marc Girardin MF, Zafrani ES, Chaumette MT, et al. Hepatic granulomas in Whipple’s disease. Gastroenterology. 1984;86:753.
123. Donaldson RM. Whipple’s disease: Rare malady with uncommon potential. N Engl J Med. 1992;327:346.
124. von Herbay A, Ditton HJ, Schuhmacher F, et al. Molecular screening of DNA of Tropheryma whippelii in sarcoidosis [abstract]. Pathol Res Pract. 1997;193:87.
125. Abdelmalek MF, Procop GW, Mitchell PS, Persing DH. Lack of association of sarcoidosis and intestinal lymphoma with T. whippelii infection [abstract]. Gastroenterology. 1998;114:G1410.
126. Feurle GE, Marth T. An evaluation of antimicrobial treatment for Whipple’s disease: Tetracycline versus trimethoprim-sulfamethoxazole. Dig Dis Sci. 1994;39:1642.
127. Knox DL, Bayless TM, Pittman FE. Neurologic disease in patients with treated Whipple’s disease. Medicine (Baltimore). 1976;55:467.
128. Feurle GE, Volk B, Waldherr R. Cerebral Whipple’s disease with negative jejunal histology. N Engl J Med. 1979;300:907.
129. Ryser RJ, Locksley RM, Eng SC, et al. Reversal of dementia associated with Whipple’s disease by trimethoprim-sulfamethoxazole: Drugs that penetrate the blood-brain barrier. Gastroenterology. 1984;86:745.
130. Cooper GS, Blades EW, Remler BF, et al. Central nervous system Whipple’s disease: Relapse during therapy with trimethoprim-sulfamethoxazole and remission with cefixime. Gastroenterology. 1994;106:782.
131. Singer R, von Herbay A, Willig F. Successful treatment of cerebral Whipple’s disease with rifampicin. Med Klinik. 1995;90:117.
132. Garas G, Cheng WS, Abrugiato R, Forbes GM. Clinical relapse in Whipple’s disease despite maintenance therapy. J Gastroenterol Hepatol. 2000;15:1223.
133. Boulos A, Rolain JM, Raoult D. Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob Agents Chemother. 2004;48:747.
134. Boulos A, Rolain JM, Mallet MN, Raoult D. Molecular evaluation of antibiotic susceptibility of Tropheryma whipplei in axenic medium. J Antimicrob Chemother. 2005;55:178.
135. Cannon WR. Whipple’s disease, genomics, and drug therapy. Lancet. 2003;361:1916.
136. Knaapen HK, Barrera P. Therapy for Whipple’s disease. J Antimicrob Chemother. 2007;60:457.