CHAPTER 15 Diarrhea
Diarrhea is a universal human experience. The average American has an estimated 0.65 episodes of acute gastrointestinal illness per year.1 For most persons, episodes of diarrhea last a day or two and rapidly subside without medical intervention. For others, diarrhea lasts for more than a few days or is complicated by fever, prostration, or rectal bleeding. Such persons are likely to visit their physicians. Over 3.5 million outpatient visits for diarrhea occur each year.2 Most patients can be managed successfully as outpatients; however, more than 150,000 hospital admissions each year are for gastroenteritis. Over the course of a year, chronic diarrhea (liquid stools for more than four weeks) may occur in 5% of the population and is thus a major cause of disability for Americans.3 In developing countries, acute infectious diarrhea remains an important cause of morbidity and mortality, particularly among children.
DEFINITION
Most patients consider increased fluidity of stool as the essential characteristic of diarrhea.4 Stool consistency is difficult to quantitate and visual scales may be helpful for patients to use in describing their diarrhea.5 Researchers also have used stool frequency or stool weight as a surrogate marker of diarrhea. Three or more bowel movements daily are considered to be abnormal, and the upper limit of stool weight is generally agreed to be 200 g daily in Western countries. Although stool weight often is cited as a “scientific” definition of diarrhea, diarrhea should not be defined solely in terms of fecal weight. Some persons have increased fecal weight as a result of fiber ingestion but do not complain of diarrhea because their stool consistency is normal. For example, stool output can be as great as 300 g when a high-fiber diet is consumed, as is customary in some developing countries. Conversely, about 20% of patients referred for evaluation of diarrhea may have a normal stool weight. Whether this is a result of hyperdefecation (i.e., more frequent passage of formed stool) or a change in consistency (i.e., passage of small-volume loose stools) is unclear.
In a study of the objective determinants of decreased fecal consistency,4 the ability of water-insoluble fecal solids, such as those derived from dietary fiber or bacterial cell walls, to hold or bind fecal water correlated well with fecal consistency. Too little water-holding capacity to bind all the water present resulted in loose stools, but when fecal solids had sufficient water-holding capacity to bind all the water present, stools remained thick or formed. Fecal consistency correlated best with the ratio of the water-holding capacity of insoluble solids to the total amount of water present and not simply to the amount of fecal water, further supporting the concept that stool weight should not be the sole criterion for diarrhea.
Fecal incontinence may be reported as “bad diarrhea” by some patients, especially older adults.6 Although many incontinent patients have loose stools, their major problem is with the mechanisms of continence and not with intestinal fluid or electrolyte absorption. Accordingly, all patients who complain of diarrhea should be asked about the presence of fecal incontinence. If incontinence is frequent, especially in the absence of rectal urgency or loose stools, the patient should be evaluated for incontinence and not for diarrhea (see Chapter 17).
PATHOPHYSIOLOGY
Diarrhea frequently represents a protective response to a variety of intestinal insults and assaults. Normally, the intestine absorbs most of the fluid that it secretes, and intestinal motility provides a favorable milieu for water, electrolyte, and nutrient absorption. When infectious agents, toxins, or other noxious substances are present in the intestine, fluid secretion and motility are stimulated to expel the unwanted material, thereby producing diarrhea. This protective response is valuable acutely but, when chronic, it is inappropriate and no longer serves an adaptive purpose. Historically, diarrhea was thought to be primarily a motility disorder. An improved understanding of intestinal electrolyte transport since 1970 has shifted the emphasis to epithelial function, rather than motility. Clearly, however, epithelial and motor functions are altered in a coordinated fashion to produce diarrhea.7
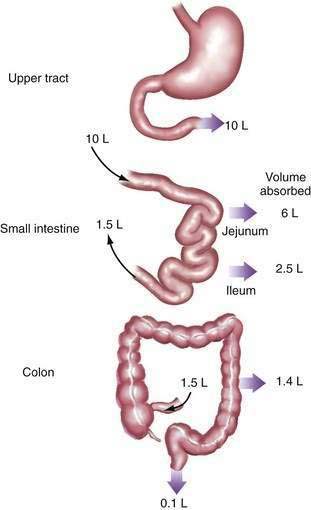
Diarrhea usually is the result of an excess of stool water rather than a decrease in the water-holding capacity of fecal solids, with the implication that water transport within the intestine is abnormal.4 Normally, the small intestine and colon absorb 99% of both oral intake and endogenous secretions from the salivary glands, stomach, liver, and pancreas—a total fluid load of approximately 9 to 10 L daily (Fig. 15-1). Diarrhea results from a disruption of this normally fine-tuned mechanism; reduction of water absorption by as little as 1% can result in diarrhea. Therefore, to understand the pathogenesis of diarrhea, one needs to understand normal water absorption by the intestine and the abnormalities that can impair water absorption.
Water itself is not actively transported but moves across the intestinal mucosa secondary to osmotic forces generated by the transport of solutes—that is, electrolytes and nutrients (see Chapters 99 and 100). The molecular pathways of ion and nutrient transport across the mucosa have been well characterized and are regulated by a complex communication system of extracellular and intracellular messengers that maintain fluid equilibrium throughout a wide range of physiologic conditions. Normally, absorption and secretion take place simultaneously, but absorption is quantitatively greater. Either a decrease in absorption or an increase in secretion leads to additional fluid within the lumen and diarrhea. Disruption of epithelial electrolyte transport or its regulatory system by toxins, drugs, hormones, and cytokines is a major cause of diarrhea.
Diarrhea resulting from disordered electrolyte transport is known as secretory diarrhea, even though it is more commonly caused by reduced absorption than by net secretion.8 Another major cause of diarrhea is ingestion of some poorly absorbed, osmotically active substance (e.g., magnesium ion, lactulose) that retains fluid within the lumen to maintain osmotic equilibration with body fluids, thereby reducing water absorption. Diarrhea resulting from this mechanism is known as osmotic diarrhea (Table 15-1). Few clinical situations produce pure secretory or osmotic diarrhea, but considering some conditions in which one or the other mechanism predominates is useful before considering more complex processes.
| TYPE OF DIARRHEA | CAUSES | EXAMPLES |
|---|---|---|
| Secretory diarrhea | Exogenous secretagogues | Enterotoxins (e.g., cholera) |
| Endogenous secretagogues | Neuroendocrine tumors (e.g., carcinoid syndrome) | |
| Absence of ion transporter | Congenital chloridorrhea | |
| Loss of intestinal surface area | Intestinal resection, diffuse intestinal mucosal disease | |
| Intestinal ischemia | Diffuse mesenteric atherosclerosis | |
| Rapid intestinal transit | Intestinal hurry following vagotomy | |
| Osmotic diarrhea | Ingestion of poorly absorbed agent | Magnesium ingestion |
| Loss of nutrient transporter | Lactase deficiency |
OSMOTIC DIARRHEA
Ingestion of poorly absorbed cations and anions or poorly absorbed sugars or sugar alcohols (e.g., mannitol, sorbitol) accounts for most osmotic diarrheas.9 Ions that are poorly absorbed include magnesium, sulfate, and phosphate. These ions are transported actively by mechanisms that are saturated at low intraluminal ion concentrations and passively by mechanisms that are slow. Together, these processes limit total absorption to a fraction of the amount that can be ingested. Because neither the small intestine nor colon can maintain an osmotic gradient, unabsorbed ions (and their counter ions) that remain in the intestinal lumen obligate retention of water to maintain an intraluminal osmolality equal to that of body fluids (about 290 mOsm/kg). Therefore, approximately 3.5 mL of water (1000 mL/kg divided by 290 mOsm/kg) are retained for every 1 mOsm of retained ions or molecules.9–12
Sugars and sugar alcohols are the other category of substances that cause osmotic diarrhea.12 Monosaccharides, but not disaccharides, can be absorbed intact across the apical membrane of the intestine. When disaccharides such as sucrose and lactose are ingested, absence of the appropriate disaccharidase will preclude absorption of the disaccharide or its component monosaccharides (see Chapter 101). The most common clinical syndrome of disaccharidase deficiency is acquired lactase deficiency, which accounts for lactose intolerance in many adults.13 Lactase is present in the brush border of the small intestine of most immature mammals but disappears in adult mammals, including 70% of adult humans.14 The main exceptions are persons from the northern European gene pool, who typically maintain lactase activity into adult life. Lactase activity often falls with age even in this group, however. Congenital deficiency of lactase is rare and seems to be the result of a mutation in a gene distinct from that for lactase-phlorizin hydrolase (the gene affected in adult lactase deficiency).15 Acquired deficiencies also may be associated with mucosal diseases of the upper small intestine. Congenital sucrase and trehalase deficiencies are rare and prevent the adequate digestion of sucrose (table sugar) and trehalose (a sugar found in mushrooms), respectively. Lactulose is a synthetic disaccharide that cannot be hydrolyzed by the human intestine and is not absorbed intact in more than trace amounts. It thereby causes an osmotic diarrhea when given in sufficient quantity to overwhelm the metabolic capacity of colonic bacteria (about 80 g/day).
The essential characteristic of osmotic diarrhea is that it disappears with fasting or cessation of ingestion of the offending substance. This characteristic has been used clinically to differentiate osmotic diarrhea from secretory diarrhea, which typically continues with fasting. Electrolyte absorption is not impaired in osmotic diarrhea, and electrolyte concentrations in stool water are usually low.10–12
SECRETORY DIARRHEA
Secretory diarrhea has many causes, and the mechanism of this type of diarrhea is always net secretion of anions (chloride or bicarbonate) or net inhibition of sodium absorption.16 The stimuli for secretion arise from the intestinal lumen, subepithelial space, or systemic circulation and substantially alter the messenger systems that regulate ion transport pathways. In some cases, congenital absence of a specific transport molecule limits sodium or chloride absorption and results in diarrhea; in others, lack of sufficient absorptive surface area limits electrolyte, particularly sodium, absorption critically.
The most common cause of secretory diarrhea is infection.16 Enterotoxins from a host of infectious agents (primarily bacteria but also parasites and viruses) interact with receptors that modulate intestinal transport and lead to increased anion secretion. Enterotoxins also may block specific absorptive pathways in addition to stimulating secretion. Most enterotoxins inhibit Na+-H+ exchange in the small intestine and colon, thereby blocking one of the important driving forces for electrolyte and fluid absorption.17,18
Peptides, such as vasoactive intestinal peptide, produced by endocrine tumors, cause secretory diarrhea by stimulating secretion by epithelial cells, as do peptides released from subepithelial neurons and inflammatory cells (see Chapter 32).19 Neurotransmitters such as acetylcholine or serotonin (5-hydroxytryptamine, 5-HT) and other modulators such as histamine and inflammatory cytokines also are potent secretory stimuli.20,21 Most of these endogenous regulators of intestinal transport elicit diarrhea by altering intracellular messengers, such as cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), and calcium, that control specific transport pathways.22 In addition, peptides and other regulators may affect the synthesis, localization, and degradation of individual transport proteins. Exogenous agents, such as drugs and some poisons, lead to secretory diarrhea, presumably by interacting with intracellular regulators or intracellular messengers of the enterocytes.
The absence or disruption of a specific absorptive pathway may cause diarrhea. For example, rare congenital syndromes, such as congenital chloridorrhea and congenital sodium diarrhea, are caused by the absence of a specific transport molecule.23 In chloridorrhea, Cl−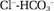 -HCO3− exchange in the ileum and colon is defective, thereby transforming chloride into a poorly absorbed ion. Diarrhea resulting from chloridorrhea can be reduced by limiting oral chloride intake or chloride secretion (i.e., by reducing gastric acid secretion with a proton pump inhibitor) or by stimulating chloride absorption in the colon by enhancing short-chain fatty acid absorption.24 Several transporter defects have been proposed for congenital sodium diarrhea.25
-HCO3− exchange in the ileum and colon is defective, thereby transforming chloride into a poorly absorbed ion. Diarrhea resulting from chloridorrhea can be reduced by limiting oral chloride intake or chloride secretion (i.e., by reducing gastric acid secretion with a proton pump inhibitor) or by stimulating chloride absorption in the colon by enhancing short-chain fatty acid absorption.24 Several transporter defects have been proposed for congenital sodium diarrhea.25
More commonly, a relative shift in the balance of absorptive and secretory pathways may contribute to diarrhea in clinical settings associated with epithelial injury or changes in cell proliferation. For example, viral gastroenteritis and celiac disease may disproportionately compromise transporters (e.g., disaccharidases, Na+-coupled absorption) that mediate absorption on the villous surface, whereas secretory pathways in the crypt are unchanged or increased (see Chapters 104 and 107).
For intestinal fluid and electrolyte absorption to be complete, the intestine must have an adequate surface area and adequate contact time with luminal contents. Substantial loss of surface area, as in celiac disease or inflammatory bowel disease (IBD) or after resective surgery, may compromise water absorption. Even though the reserve absorptive capacity in the small intestine and colon is large, sufficiently long surgical resections inevitably cause diarrhea. In some cases, the problem is temporary, because over time the intestine may improve its capacity for absorption by the process of adaptation.26 Such compensation is impossible following resection of certain segments of the intestine with highly specific absorptive functions that simply cannot be assumed by other segments of the bowel. For example, ileocecal resection is followed by permanent inability to absorb sodium chloride against a concentration gradient27 and, if sufficient ileum is resected, by failure to absorb vitamin B12, intrinsic factor, and normal amounts of conjugated bile acids (see Chapter 103).
Abnormal motility may lead to diarrhea that has secretory and osmotic components.8 For fluid and electrolyte absorption to be complete, the contact time between luminal contents and the epithelium must be sufficient to permit absorption. In some patients, abnormal motility produces intestinal “hurry.”28,29 Because rapid transit prevents adequate time for absorption, diarrhea results despite intact mucosal absorptive capacity, as measured by intestinal perfusion studies during which contact time is maximized by rapid infusion of fluid into the intestine.8 In some patients with intestinal hurry, the oral-cecal transit time may be as short as 10 minutes. Under such circumstances, the diarrhea is exacerbated by malabsorption of nutrients that produces an osmotic component to diarrhea. In disorders such as diabetes mellitus and postvagotomy diarrhea, intestinal hurry has been linked to abnormal enteric nervous system function.30 In other clinical settings, such as amyloidosis, postprandial diarrhea, and irritable bowel syndrome (IBS), enteric nervous system dysfunction is suspected, but unproved.31,32 Many endocrine diarrheas, such as those caused by peptide-secreting tumors or hyperthyroidism, may lead to diarrhea not only by affecting intestinal electrolyte transport, but also by accelerating intestinal motility.33
Conversely, slow intestinal transit may lead to a secretory diarrhea by promoting small intestinal bacterial overgrowth.34,35 Excess bacteria in the small intestine disrupt digestion and may alter electrolyte transport. The best documented example of diarrhea related to this mechanism is scleroderma. Although diabetes mellitus is often suspected of causing diarrhea by slow transit and stasis, as seen in scleroderma, such a pathophysiology is not always established (see Chapter 102).36,37
Evaluation of the role of intestinal motility in the pathogenesis of diarrhea has been limited by the lack of the necessary tools to measure the interactions among motility, propulsive forces, and transit time. Except for intestinal perfusion studies, during which the effect of motility on electrolyte transport is eliminated, no methodology exists to dissociate the effects of intestinal transport and motility on net absorption.8 Thus, consensus has not been achieved on whether too much or too little motility causes diarrhea, nor on how luminal factors may alter intestinal smooth muscle function.
Reduced intestinal blood flow has an important but as yet poorly defined role in diarrhea. Whether mesenteric ischemia has a direct effect on absorption or whether low blood flow prompts secondary responses (e.g., via cytokines or neurotransmitters) that affect fluid transport and produce a secretory diarrhea is not clear. Radiation enteritis also produces an abnormal intestinal microcirculation associated with persistent diarrhea that may be difficult to treat (see Chapters 39 and 114).
COMPLEX DIARRHEA
Although classification of diarrhea as osmotic or secretory may be instructive in thinking about the pathophysiology of diarrhea, cases of pure secretory or pure osmotic diarrhea are uncommon. Most clinically important diarrhea is complex in pathogenesis; rather than being produced by a single pathophysiologic mechanism, several mechanisms are involved. Causes may include the effects of substances released by enteric endocrine cells, cytokines released by local and remote immunologically reactive cells, activity of the enteric nervous system, and peripherally released peptides and hormones (paracrine, immune, neural, and endocrine systems; see Chapter 1).
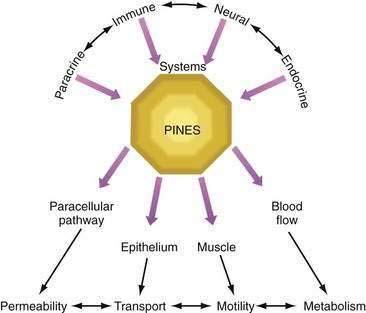
Further complicating the understanding of diarrhea is the recognition that certain mediators affect not only epithelial or muscle function, but also each other. For example, enteric nerves may stimulate mast cells, and products released from mast cells (particularly histamine) may alter enteric neuron functions.38 A single agonist, such as prostaglandin, may have multiple simultaneous effects on epithelial function, muscle contraction, and the paracellular pathway, thereby leading to effects on ion transport, motility, and mucosal permeability.39 Therefore, a number of modulators and effectors contribute to the final clinical picture. A full appreciation of the pathophysiology of diarrhea requires consideration of a regulatory system known as PINES (paracrine, immune, neural, and endocrine system modulators; Fig. 15-2).
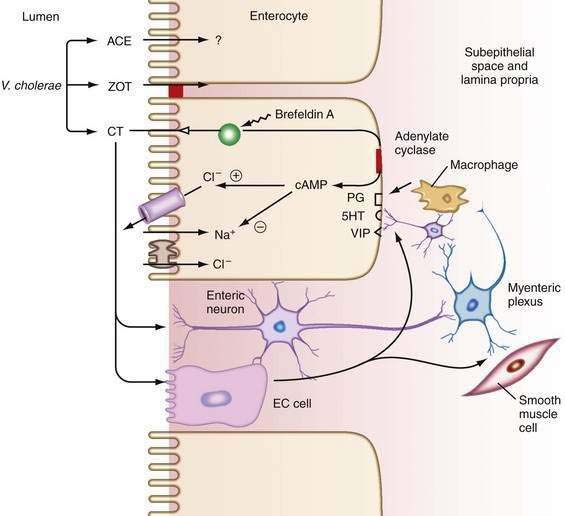
An example of the complexity of the pathophysiology of a diarrheal syndrome is cholera. Cholera is often cited as the paradigm of a pure secretory diarrhea: cholera toxin targets the epithelial cell, increases the second messenger, cAMP, which opens apical chloride channels to stimulate chloride secretion, and results in diarrhea; however, the actual mechanism whereby cholera induces diarrhea is far more complex.40 Cholera toxin stimulates endocrine cells and neural elements that reinforce its direct secretory effect on enterocytes.41 In addition, cholera toxin causes distinct changes in intestinal motility. Other toxins produced by Vibrio cholerae target tight junctions and thereby alter mucosal permeability (Fig. 15-3; see Chapter 107).42
Another example of dysregulation of PINES is IBD.43 Diarrhea in patients with IBD involves more than just exudation into the lumen as a result of destruction of the mucosa. Intact enterocytes are barraged by multiple secretagogues released by immune cells in the intestine and by bacterial toxins that may influence enterocyte function. Although initial models of diarrhea in IBD suggested altered fluid transport driven by chloride secretion, more recent studies have demonstrated that the diarrhea in IBD is mediated by an antiabsorptive effect associated with down-regulation of sodium channels and pumps.44–46 The pathophysiology of diarrhea in IBD is even more complex if we consider the role of luminal bacteria. Bacterial proteins, such as flagellin, may stimulate the production of cytokines, such as interleukin (IL)-8, which further attract inflammatory cells.47 Cytokines and immune cells also may influence tight junction barrier function and enterocyte secretory and absorptive pathways directly.48,49 Conversely, epithelial cells may secrete cytokines, such as IL-6, that enhance polymorphonuclear leukocyte (neutrophil) function.50
IBS is another example of a disorder with a complex pathophysiology. A constellation of factors, such as altered motility,32 bile acid malabsorption,51 and compromised rectal reservoir capacity,52 may aggravate symptoms in patients with IBS. At a more fundamental level, alterations in mast cell or enterochromaffin cell number, serotonin content, and serotonin reuptake and transport may contribute to the development of diarrhea (see Chapter 118).53–55
Complex pathophysiology also may be observed in malabsorption syndromes and functional disorders, particularly those characterized by rapid transit. Failure to absorb carbohydrates may lead to osmotic diarrhea, but failure to absorb long-chain fatty acids may complicate matters by impairing electrolyte absorption by the colon.12,56 Common postprandial functional diarrhea probably involves an interplay between motility and transport functions. Diarrhea caused by food allergy also involves activation of immunologic, paracrine, and neural mechanisms that regulate vascular permeability, electrolyte transport, and motility (see Chapters 9 and 101).57
CLINICAL CLASSIFICATION
ACUTE VERSUS CHRONIC DIARRHEA
The time course of diarrhea can help direct management. Acute diarrhea (<four weeks) usually is caused by an infection, which is generally self-limited or easily treated.58 Most common bacterial and viral causes of diarrhea run their course within seven days. Persistence of an acute diarrheal episode beyond seven days raises the possibility of a protozoal cause, such as giardiasis or cryptosporidiosis. Although few infectious agents (e.g., Aeromonas or Yersinia spp.) cause prolonged diarrhea in an immunocompetent person, chronic diarrhea is usually not caused by an infectious agent. Therefore, when confronted with a patient with chronic diarrhea, the clinician must consider noninfectious causes (see later).
LARGE-VOLUME VERSUS SMALL-VOLUME DIARRHEA
The daily total stool output may also provide hints about the cause. IBS often results in normal or only slightly elevated 24-hour stool weights, whereas diarrhea of other causes may produce greater stool weights. The stool weight can be estimated by the patient’s history: patients with diarrhea that produces dehydration (in the absence of vomiting or limited oral intake) typically have stool weights more than 1000 g and therefore are unlikely to have IBS (see Chapter 118).
OSMOTIC VERSUS SECRETORY DIARRHEA
Distinguishing diarrhea that results from intestinal malabsorption of ingested nonelectrolytes (osmotic diarrhea) from diarrhea that results from malabsorption or secretion of electrolytes (secretory diarrhea) helps separate the small number of cases of osmotic diarrhea from the much larger number of cases of secretory diarrhea. This distinction is based on the measurement of stool electrolyte concentrations.10 In secretory diarrhea, sodium, potassium, and accompanying anions account almost entirely for stool osmolality, whereas in osmotic diarrhea poorly absorbable solutes within the lumen of the intestine account for much of the osmotic activity of stool water (see later discussion). Because osmotic diarrhea is caused by the ingestion of some poorly absorbed substance, it abates with fasting. Secretory diarrhea typically continues during fasting, although stool output may decrease modestly because of reduced endogenous secretions.
WATERY VERSUS FATTY VERSUS INFLAMMATORY DIARRHEA
When diarrhea is chronic (>four weeks), the differential diagnosis can overwhelm even the most experienced clinician. By characterizing stools as watery, fatty, or inflammatory on the basis of simple stool tests, evaluation of the patient can be expedited by limiting the number of conditions that must be considered in the differential diagnosis.3 Watery diarrhea implies a defect primarily in water absorption as a result of increased electrolyte secretion or reduced electrolyte absorption (secretory diarrhea) or ingestion of a poorly absorbed substance (osmotic diarrhea). Fatty diarrhea implies defective absorption of fat and perhaps other nutrients in the small intestine. Inflammatory diarrhea implies the presence of one of a limited number of inflammatory or neoplastic diseases involving the gastrointestinal tract.
EPIDEMIOLOGIC FEATURES
One of the most useful clinical approaches to narrowing the differential diagnosis is to relate diarrhea to its setting. For example, a soccer mom and a backpacker from Nepal conceivably could have the same cause of the diarrhea but are more likely to have different causes. Some common clinical scenarios and the diagnoses that should be considered are shown in Table 15-2.
Table 15-2 Likely Causes of Diarrhea in Well-Defined Patient Groups or Settings
DIFFERENTIAL DIAGNOSIS
Many gastrointestinal and systemic diseases may present with diarrhea. To facilitate the differential diagnosis, the clinician should divide diarrheal diseases into acute and chronic and further subdivide chronic diarrhea by stool characteristics—watery, inflammatory, and fatty (Table 15-3).
| Acute Diarrhea |
Acute diarrhea is defined as lasting less than four weeks, although many cases last shorter than four days.58 The usual cause is infection by bacteria, viruses, protozoa, or multicellular parasites (Table 15-4). Acute diarrhea also can be caused by food poisoning, food allergies, and medications. Diseases that lead to chronic diarrhea may present with an acute onset and therefore must be considered when acute diarrhea becomes persistent (see Chapter 107).
Table 15-4 Infections That Cause Diarrhea
Chronic watery diarrhea may be caused by ingestion of poorly absorbed, osmotically active substances (osmotic diarrhea) or, more commonly, conditions that cause secretory diarrhea. Ingestion of any of a limited number of osmotic agents, such as magnesium, phosphate, and sulfate laxatives, or poorly absorbed carbohydrates, causes osmotic diarrhea. By contrast, chronic secretory diarrhea, in which electrolyte malabsorption leads to retention of fluid within the lumen, is associated with many clinical conditions (see Table 15-3).
Although IBD typically produces diarrhea characterized by the presence of blood and pus, other diseases of inflammation without ulceration, such as microscopic colitis, cause diarrhea with the characteristics of chronic secretory diarrhea. Diarrhea in such cases is thought to be mediated by secretion of cytokines and other inflammatory mediators (see Chapter 124).
Chronic watery diarrhea can also be caused by the ingestion of drugs or poisons (Table 15-5).59–61 Identification of drugs as the cause of diarrhea depends on recognizing that the initiation of drug ingestion and the onset of diarrhea occurred coincidentally. Such a temporal correlation, however, is not always easy to identify and requires a detailed and carefully taken history. The pathophysiology of drug-induced diarrhea is complex and has not been carefully studied. Some drugs may activate specific receptors and transporters; for example, caffeine, like theophylline, may increase intracellular cAMP activity and fluid secretion. Clinically, this phenomenon can be seen in cases of what has been called “Starbucks diarrhea.” Erythromycin interacts with the motilin receptor, thereby stimulating propulsive motor activity in the gastrointestinal tract. Other antibiotics may alter the bacterial flora in the colon and lead to impaired colonic salvage of malabsorbed carbohydrate or overgrowth of toxin-producing Clostridium difficile. Some drugs such as cocaine may interfere with blood flow to the intestine. Chemotherapeutic agents are associated with a high frequency of diarrhea, which may result from disruption of the delicate balance between enterocyte proliferation and apoptosis, leading to what has been termed an apoptotic enteropathy. A diverse group of drugs (e.g., aspirin, mycophenolate mofetil, gold) can incite an inflammatory process in the intestine that may cause diarrhea. The problem of detecting drug-induced diarrhea is more difficult in patients with surreptitious laxative abuse, because these patients deliberately conceal vital information about the cause of their problem (see later discussion of factitious diarrhea).62
Table 15-5 Medications and Toxins Associated with Diarrhea
NSAIDs, nonsteroidal anti-inflammatory drugs.
Another category of chronic watery diarrhea involves disordered motility or dysregulation of intestinal function. Problems such as postvagotomy diarrhea, postsympathectomy diarrhea, diabetic autonomic neuropathy, amyloidosis, and probably diarrhea-predominant IBS belong in this category. In these situations, the diarrhea has the characteristics of a secretory diarrhea, because of primary dysregulation of electrolyte transport or of altered motility that speeds luminal fluid past absorptive sites in the intestine (see Chapters 35, 53, and 118).
Another large category of watery diarrhea is diarrhea caused by endocrine dysfunction. Relatively common endocrine disturbances, such as hyperthyroidism and Addison’s disease, can be complicated by chronic secretory diarrhea. Much rarer endocrine tumors also produce diarrhea, typically by altering electrolyte absorption or speeding intestinal transit. The rarity of these tumors makes the pretest probability of finding these conditions low, especially in the absence of liver metastases, and therefore screening tests often are falsely positive (see later and Chapters 31, 32, and 35).
Other tumors cause watery diarrhea by obstructing bowel, blocking lymphatic drainage, interfering with absorption, or causing electrolyte secretion. Examples of such conditions include colon carcinoma (bowel obstruction), lymphoma (lymphatic obstruction in the small bowel and mesentery), and villous adenomas of the rectum (secretion of a large amount of potassium-rich gelatinous fluid into the lumen). Villous adenomas found more proximally in the colon rarely cause this type of diarrhea (see Chapters 29, 122, and 123).
The last category of chronic watery diarrhea is idiopathic secretory diarrhea. This rubric includes two entities, epidemic secretory diarrhea (also known as Brainerd diarrhea) and sporadic idiopathic secretory diarrhea. Both disorders are protracted but self-limited conditions (see later discussion of idiopathic secretory diarrhea).63,64
Chronic inflammatory diarrhea is the designation for a diverse group of infectious or idiopathic inflammatory and neoplastic processes. Stools are characterized by the presence of mucus and pus and are usually associated with ulceration of the mucosa. Idiopathic IBD, including ulcerative colitis and Crohn’s disease, typically produces such stools. Less commonly, other inflammatory conditions such as diverticulitis or ulcerative jejunoileitis may be associated with blood or pus in the stool, as may infectious diseases that are invasive or ulcerating. Infections that cause chronic inflammatory diarrhea include bacterial infections, such as tuberculosis, yersiniosis, and Clostridium difficile–associated colitis, viral infections that ulcerate, such as cytomegalovirus and herpes simplex virus, and invasive parasitic infections, such as strongyloidiasis. In the immunocompromised person, a broader range of infectious agents should be considered. Noninfectious diseases that cause chronic inflammatory diarrhea include ischemic colitis, and neoplasms, such as colon cancer or lymphoma, that are complicated by ulceration of the mucosa (see Chapters 29, 107 to 112, 114, 115, 117, and 123).
Chronic fatty diarrhea results from malabsorption or maldigestion. Malabsorption syndromes caused by mucosal diseases, such as celiac disease or Whipple’s disease, typically produce fatty diarrhea. Short bowel syndrome or postresection diarrhea can also present with this pattern, although if the resection is relatively limited, the diarrhea may be watery secondary to nutrient or bile-acid malabsorption. Small intestinal bacterial overgrowth causes steatorrhea by deconjugation of bile acids. Mesenteric ischemia affecting the small intestine may impair intestinal absorption of fat, but weight loss is more often attributed to sitophobia (“fear of eating”) because of postprandial pain. Maldigestion as a result of pancreatic exocrine insufficiency or inadequate duodenal bile acid concentration produces steatorrhea. Although fatty, the stools may not be very loose in maldigestive conditions because, in the absence of fat digestion, triglyceride remains intact and has little effect on colonic electrolyte absorption. By contrast, malabsorption in the presence of normal digestion may produce fairly voluminous diarrhea because of the cathartic action of free fatty acids in the colon (see Chapters 59, 101, 103 to 106, and 114).61
EVALUATION OF THE PATIENT
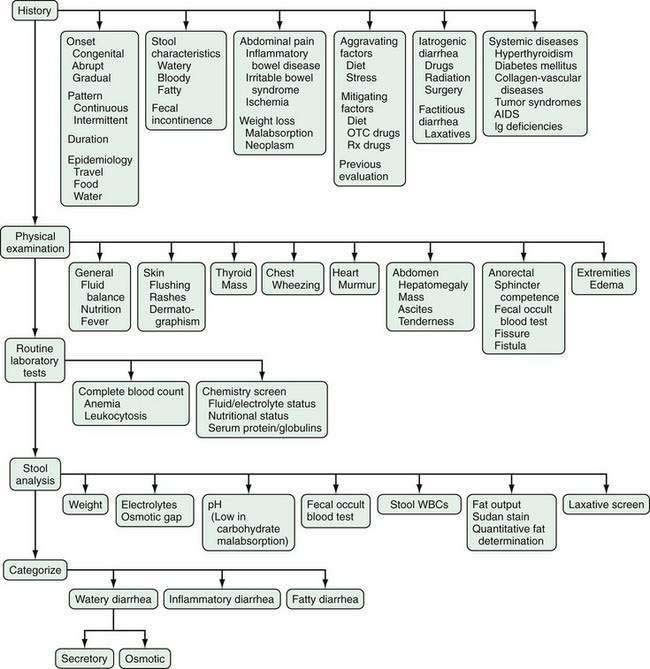
HISTORY
Stool characteristics, such as the presence of blood, mucus, pus, oil droplets, or food particles, are also important. Blood in the stool signals the possibility of malignancy or IBD, although it is often caused by hemorrhoids in patients with frequent evacuations. In patients with acute infectious diarrhea, visible blood in the stool is highly specific for infection with an invasive organism.65 Watery stools suggest an osmotic or secretory process, and the presence of oil or food particles is suggestive of malabsorption, maldigestion, or intestinal hurry. The phenomenon of floating stools generally represents an increase in the gas content rather than the fat content of the stools. The physician should also ask about the relationship of defecation to meals or fasting, passage of stool during the day versus the night, and presence of fecal urgency or incontinence. Urgency and incontinence are not indicative of voluminous diarrhea but suggest a problem with rectal compliance or with the muscles regulating continence. Nocturnal diarrhea that awakens the patient from sleep strongly suggests an organic rather than a functional disorder such as IBS. Other coexisting symptoms such as abdominal pain, flatulence, bloating or gaseous distention, cramps, fever, and weight loss should be noted. Excessive flatus suggests increased fermentation of carbohydrate by colonic bacteria as a result of ingestion of poorly absorbable carbohydrate or malabsorption of carbohydrate by the small intestine.
Because iatrogenic causes of diarrhea, such as drugs, previous surgery, or radiation therapy, are common, the physician should explore the history thoroughly for prior abdominal surgeries and ingestion of prescription drugs and over-the-counter remedies, including nutritional and herbal therapies. The patient’s diet should be reviewed thoroughly because diarrhea may result from ingestion of large quantities of poorly absorbable carbohydrates, such as fructose, or sugar alcohols, such as sorbitol or mannitol, which may be consumed in fruit juices and soda (which contain fructose and high-fructose corn syrup) or as dietetic, sugar-free candies and chewing gums (which contain sorbitol and mannitol).66 Excessive coffee consumption also may be associated with diarrhea.
Epidemiologic clues also should be pursued (see Table 15-2). For example, recent foreign travel, particularly to undeveloped countries, makes the diagnosis of travelers’ diarrhea likely. The globalization of commerce has increased the frequency of once exotic infections in those without grossly obvious exposures.67 The physician also should consider the patient’s residence in a rural or urban environment, the source of the patient’s drinking water, and the patient’s occupation, sexual orientation, and use of alcohol or illicit drugs. Potential secondary gains from illness or a history of attempted weight loss and fixation on body image should raise the possibility of laxative abuse (see later).
The patient’s history is essential in differentiating patients with IBS from those with other functional disorders or organic conditions that cause diarrhea. Current definitions of IBS emphasize the presence of abdominal pain associated with defecation.68 Additional factors that suggest a diagnosis of IBS include a long history that usually extends back to adolescence or young adulthood, passage of mucus, and exacerbation of symptoms by stress. Factors that argue against a diagnosis of IBS include the recent onset of diarrhea, especially in older patients, diarrhea that awakens the patient from sleep, weight loss, the presence of blood in the stool, and stool weights more than 400 to 500 g daily. Abnormal blood test results, such as a low hemoglobin level, low serum albumin concentration, or high erythrocyte sedimentation rate, also argue against a diagnosis of IBS (see Chapter 118).
Painless diarrhea should no longer be considered a form of IBS. The Rome III committee has defined functional diarrhea as “at least 3 months, which need not be consecutive, in the preceding 6 months of liquid (mushy) or watery stools more than three-quarters of the time; and no abdominal pain.”68 Obviously, many patients with chronic diarrhea will not have a readily defined cause of diarrhea identified when first seen and could be characterized as having functional diarrhea. Physicians should not rush to make this diagnosis without exploring alternative possibilities, particularly those that can produce episodic and variable diarrhea, such as small intestinal bacterial overgrowth and carbohydrate malabsorption. Functional diarrhea must also be distinguished from idiopathic secretory diarrhea (see later).
FURTHER EVALUATION OF ACUTE DIARRHEA
Most cases of acute diarrhea are caused by infectious diseases that have a limited course, from a few days to a few weeks, and do not require a physician’s intervention unless the patient’s immune system is compromised or the patient develops complications of volume depletion or other evidence of severe toxicity, including inability to ingest fluid, frequent vomiting, and debilitating muscle or joint pain.58
Stool samples should be examined for white blood cells to identify inflammatory diarrhea.69 The standard method for detecting white blood cells in stool is with a Wright stain and microscopy. The accuracy of the test depends on the experience and skill of the observer, because false-positive and false-negative results are common. Tests for the neutrophil products, calprotectin and lactoferrin, are sensitive and specific for the detection of neutrophils in stool and may be a useful alternative to microscopy.70 Studies have suggested that stool cultures are unlikely to grow pathogenic bacteria in the absence of fecal leukocytes; therefore, a Wright stain of stool or a fecal lactoferrin assay can be used to decide which stool samples should be sent for bacterial culture, thereby minimizing expense. This approach may be of more value in outpatients than in patients hospitalized with diarrhea, because the latter have toxicity or have failed to resolve spontaneously within a few days and must have stool cultures sent. Routine stool cultures are of little use for hospitalized patients in whom acute diarrhea develops while the patient is in the hospital; testing for Clostridium difficile toxin is likely to be more helpful. The diagnostic value of examination of stool for ova and parasites depends on the pretest probability of a parasitic infection and the experience of the observer. Enzyme-linked immunosorbent assays (ELISAs) for giardiasis and cryptosporidiosis and serologic testing for amebiasis are more accurate tests than stool microscopy and should be ordered, even in the absence of fecal leukocytes.71 Patients who have been treated with antibiotics in the preceding three months or those in whom diarrhea develops in an institutional setting should be tested for C. difficile toxin.72 With the increase in community acquired C. difficile infections, physicians should consider this treatable cause of acute diarrhea, even in the absence of a prior history of antibiotic use (see Chapters 107 to 109).
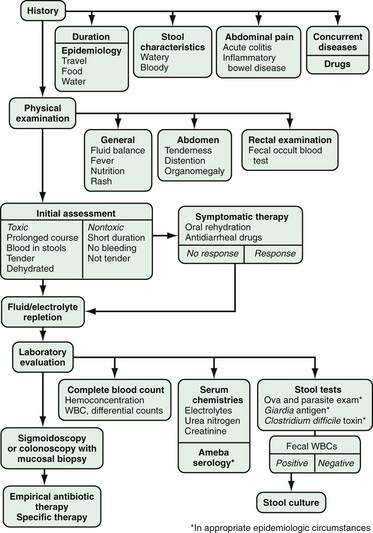
Abdominal radiographs should be obtained in toxic patients to assess for colitis and to look for evidence of ileus or megacolon. Proctoscopy or flexible sigmoidoscopy also should be considered in patients who are clearly toxic with infection, have blood in the stool, or have persistent acute diarrhea. Sigmoidoscopy is probably adequate as an early investigation in such cases of severe acute diarrhea. In patients with AIDS-related diarrhea, colonoscopy is preferable because a substantial proportion of infections and lymphomas may be present only in the right colon,73 although this approach has been called into question.74 If sigmoidoscopy or colonoscopy is done, mucosal biopsy specimens should be obtained, even if the mucosa does not appear to be grossly inflamed, because pathologic examination can identify important clues to facilitate a specific diagnosis.75 An algorithm for the evaluation of patients with acute diarrhea is shown in Figure 15-4.
FURTHER EVALUATION OF CHRONIC DIARRHEA
Because the differential diagnosis of chronic diarrhea is more extensive than that of acute diarrhea, evaluation of patients with chronic diarrhea is more complex.76,77 Initially, the physician should categorize the diarrhea as watery, inflammatory, or fatty (Fig. 15-5). In addition to the history, physical examination, and routine laboratory tests already mentioned, analysis of a stool sample can be used to categorize the diarrhea and thereby limit the number of conditions to be considered in the differential diagnosis. Stool analysis can be obtained on a random sample or a timed collection (i.e., 24-, 48-, or 72-hour stool sample). The value of analyzing a timed collection is that the stool weight and hence the output of stool components, such as fat, can be measured accurately. The daily stool weight is perhaps the best clue to the potential metabolic impact of the diarrhea. In the absence of a timed collection, however, assessments of other stool characteristics on a random, or spot, collection still provide many clues to the correct diagnosis.3 These assessments include stool sodium and potassium concentrations, stool pH, a fecal occult blood test, and an examination of stool for white blood cells or a test for the presence of a surrogate marker, such as fecal lactoferrin or calprotectin.70 In appropriate circumstances, stool samples can also be analyzed for fat content and for laxatives, including magnesium, phosphate, sulfate, bisacodyl, and anthraquinones (see later discussion of factitious diarrhea).
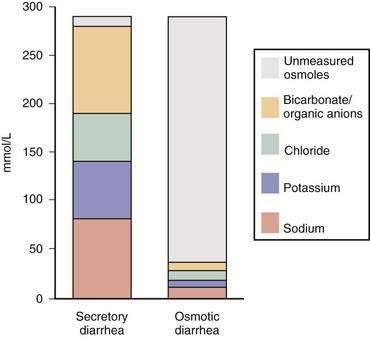
Measurement of stool sodium and potassium concentrations allows the physician to calculate an osmotic gap in stool water. The osmotic gap is calculated by subtracting twice the sum of the sodium and potassium concentrations from 290 mOsm/kg, the osmolality of stool in the body.10 The concentration is doubled to account for anions that accompany these cations. A small osmotic gap (<50 mOsm/kg), which signifies that the osmolality of stool water is attributable mostly to incompletely absorbed electrolytes, is characteristic of secretory diarrhea (Fig. 15-6). On the other hand, a large osmotic gap (>100 mOsm/kg) indicates that much of the stool osmolality is composed of nonelectrolytes. A large gap is characteristic of an osmotic diarrhea, usually resulting from ingestion of some poorly absorbed substance, such as magnesium salts. When the sum of sodium and potassium concentrations doubled is higher than 290 mOsm/kg, ingestion of a poorly absorbed multivalent anion, such as phosphate or sulfate, is likely.10 Such a negative osmotic gap is the result of an excess of cations obligated by multivalent anions. The actual measurement of stool osmolality is of value only in detecting samples that have been contaminated by the addition of water or hypotonic urine. Such samples have an osmolality lower than 290 mOsm/kg. Stool osmolality tends to rise once the stool has been collected because of continuing bacterial fermentation in vitro.12 Therefore, measured osmolality should not be used to calculate the fecal osmotic gap.
The pH of stool water provides useful information about the possibility of carbohydrate malabsorption.10 Carbohydrate that reaches the colon is promptly fermented by the bacterial flora, with release of CO2 and H2 gases and short-chain fatty acids. As a result of fermentation, the pH is acidic, usually dropping to less than 6, a finding that indirectly indicates excess carbohydrate fermentation in the colon.
Fecal occult blood testing and examination of stool for leukocytes allow one to identify inflammatory diarrhea resulting from colitis or malignancy. Other diarrheal conditions that cause occult bleeding include lymphoma of the small intestine, celiac disease (fecal occult blood in 50% of cases), and refractory sprue (fecal occult blood in 70% of cases).78
Stool fat output can be measured quantitatively by chemical means on a timed (48- to 72-hour) collection or estimated qualitatively by use of a Sudan stain on a random specimen. Steatorrhea is defined as excessive loss of fat in the stool (more than 7 g, or 9% of intake, for 24 hours). This definition, however, may not be valid for the diagnosis of fat malabsorption or maldigestion in all patients with chronic diarrhea. In one study,79 diarrhea induced with laxatives produced mild steatorrhea in 35% of normal subjects. In patients with diarrhea, fat excretion in the range of 7 to 14 g/24 hr has a low specificity for the diagnosis of defective fat absorption. Fat excretion more than 14 g/24 hr, however, strongly indicates a problem with fat absorption.79 Fat intake during a quantitative collection should be estimated from diet diaries, because patients with diarrhea frequently have anorexia or early satiation that may reduce their fat intakes substantially, thereby reducing fat excretion. For a valid study, patients should consume 70 to 100 g of fat daily for a few days before and during the timed collection. Measurement of fat excretion as a measure of malabsorption also can be compromised by ingestion of the lipase inhibitor orlistat or the fat substitute olestra.80
When only a random sample of stool is available, qualitative estimation of fat excretion by means of a Sudan stain of a fecal smear may be helpful.81 Semiquantitative methods can be applied to measure the number and size of fat globules, and these methods produce results that correlate well with quantitative collections (see Chapter 101).
In patients in whom surreptitious laxative ingestion is suspected, stool water can be analyzed for laxatives by chemical or chromatographic methods. As currently done commercially, this analysis is subject to error.82 If positive, the test for the laxative should be repeated on another stool sample to confirm the finding before confronting the patient with this discovery.
Stool samples can also be assayed with a chemical test for carbohydrate (anthrone reagent)12 and for α1-antitrypsin clearance to detect protein-losing enteropathy.83 These tests have limited clinical usefulness and should not be used routinely for the initial evaluation of a patient with chronic diarrhea.
Chronic Watery Diarrhea
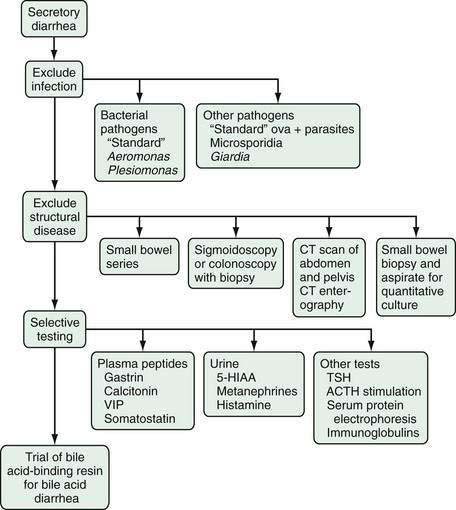
Secretory diarrhea has a broad differential diagnosis, as indicated previously (see Table 15-3), and a wide investigative net must be cast to identify a specific cause (Fig. 15-7).
Infection should be excluded by stool culture for bacteria and special tests for other organisms. The patient’s human immunodeficiency virus (HIV) status should be clarified at this point, because patients with AIDS are more likely than others to have an infectious cause of chronic diarrhea (see Chapter 33).84 Although most bacteria that cause diarrhea are cleared spontaneously within four weeks, some organisms, such as Aeromonas and Plesiomonas, may produce chronic diarrhea.85,86 Special culture techniques may be needed to detect these organisms. Special techniques are also required to find other pathogens. For example, coccidia and microsporidia require special microbiologic techniques such as polymerase chain reaction methodology for optimal detection.87 Giardiasis and cryptosporidiosis are sometimes difficult to diagnose by a standard examination for ova and parasites, and ELISA testing for Giardia antigen and cryptosporidium antigen should be ordered.71 Examination of mucosal biopsy specimens with special stains or electron microscopy may be needed to find pathogens.
Small intestinal bacterial overgrowth may result in secretory diarrhea, presumably caused by toxins, as well as fatty diarrhea caused by bile salt deconjugation (see later). The glucose-hydrogen breath test (see later) can be used to screen for this condition, but the gold standard for diagnosis of small intestinal bacterial overgrowth remains quantitative culture of a small bowel aspirate, if available (see Chapter 102).88 Structural diseases, such as short bowel syndrome, gastrocolic or enteroenteric fistula, mucosal diseases, IBD, and tumors, including lymphomas, should be sought by means of radiographic and endoscopic techniques. Small bowel radiographs remain an important method for detecting structural small bowel diseases. Computed tomography (CT) or magnetic resonance imaging (MRI), particularly CT or MRI enterography, is of value for detecting not only small bowel and colonic diseases, but also diseases extrinsic to the bowel that can cause diarrhea, such as pancreatic tumors.
Visualization and biopsy of the mucosa of the small bowel by endoscopy or enteroscopy can be valuable, although whether push enteroscopy adds much to standard esophagogastroduodenoscopy for the evaluation of diffuse small bowel diseases is uncertain.89 Diseases that may be detected by small intestinal biopsy include celiac disease, Crohn’s disease, giardiasis, intestinal lymphoma, eosinophilic gastroenteritis, tropical sprue, Whipple’s disease, lymphangiectasia, abetalipoproteinemia, amyloidosis, mastocytosis, and various infectious processes. Patients with many of these disorders usually, but not always, present with steatorrhea (see Chapters 27, 29, 35, 101, 104 to 109, and 111).
The role of wireless capsule endoscopy in the diagnosis of diarrhea caused by small bowel disease is evolving rapidly.90 Subtle lesions not appreciated by other diagnostic modalities can be seen, and new methods of deep enteroscopy (double-balloon and spiral overtube enteroscopy) allow access to and biopsy of most of these abnormalities (see Chapter 19). Sigmoidoscopy or colonoscopy can be used to visualize the colon and permit directed biopsies. Because colonic causes of chronic secretory diarrhea tend to produce diffuse changes throughout the colon, sigmoidoscopy usually is adequate for this purpose.91 Colonoscopy is preferable if the patient is older (and thus requires screening for colon cancer), has blood in the stool, is suspected of having right colonic or ileal disease, or has AIDS.73,92 Chronic disorders that can be diagnosed by inspection of the colonic mucosa include pseudomelanosis coli, polyps, tumors, Crohn’s disease, ulcerative colitis, amebiasis, and nonspecific ulceration. All patients with undiagnosed chronic secretory diarrhea should have mucosal biopsy specimens obtained from the colon, even when the mucosa appears grossly normal.3 Random biopsies should include multiple samples from several locations to give the pathologist the best chance of making a diagnosis. Diseases in which the colonic mucosa appears normal endoscopically, but which can be diagnosed histologically, include microscopic colitis (lymphocytic and collagenous colitis, see later), amyloidosis, granulomatous infections, and schistosomiasis (see Chapters 35, 110 to 112, and 124). The diagnostic yield of colonoscopy or sigmoidoscopy with biopsy in patients referred for chronic diarrhea is approximately 15% to 30%.
The next level of investigation is selective testing for diarrhea caused by peptide-secreting tumors, an intellectually interesting form of chronic watery diarrhea that is quite rare. The pretest probability of having a peptide-secreting tumor in a patient with chronic diarrhea is so low that screening these patients with a panel of serum peptide levels is far more likely to produce a false-positive than a true-positive result.93 Testing for elevated serum peptide levels or urinary metabolites of endocrine mediators, such as 5-hydroxyindoleacetic acid (5-HIAA), metanephrine, and histamine, should be limited to those patients who have chronic diarrhea with symptoms and signs consistent with a tumor syndrome, such as flushing or a large hard liver in carcinoid syndrome, ulcer disease suggestive of Zollinger-Ellison syndrome, headache, flushing, and urticaria pigmentosa in mastocytosis, or patients who have a CT scan that shows a tumor.19 Scintigraphy using radiolabeled octreotide, especially combined with positron-emission tomography (PET) or CT, also can be used to identify peptide-secreting tumors (see Chapters 31 and 32).
More common endocrinologic diseases that cause diarrhea are diabetes mellitus, hyperthyroidism, and Addison’s disease. In many cases, other symptoms and signs, such as an enlarged thyroid or skin pigmentation characteristic of Addison’s disease, suggest the presence of these conditions. Blood glucose, thyroid-stimulating hormone, and serum cortisol levels before and after injection of an adrenocorticotropic hormone analog should be measured selectively in patients who might have one of these conditions. Other blood tests that may be relevant in evaluating secretory diarrhea include serum protein electrophoresis and immunoglobulin electrophoresis. Selective immunoglobulin A (IgA) deficiency may present with recurrent intestinal infections such as giardiasis, whereas combined variable immune deficiency can be associated with a variety of puzzling intestinal findings that sometimes mimic celiac disease.94 Testing for HIV and HIV-2 may be appropriate (see Chapters 33 and 35).
Osmotic diarrhea has a much more limited differential diagnosis, and its evaluation is much simpler (Fig. 15-8).9 For practical purposes, osmotic diarrhea is caused by one of three conditions—ingestion of exogenous magnesium, consumption of poorly absorbable carbohydrates, or carbohydrate malabsorption. Ingestion of other osmotically active substances is unusual. Fortunately, these conditions can be differentiated by taking a careful history and performing simple stool tests.
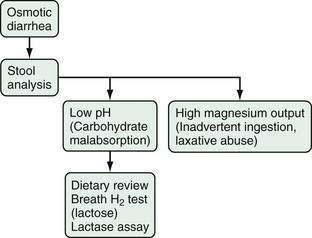
Figure 15-8. Algorithm for the evaluation of chronic osmotic diarrhea.
(From Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464-86.)
Magnesium can be measured directly in stool water by atomic absorption spectrophotometry.11 Excretion of more than 15 mmol (30 mEq) of magnesium daily or concentrations in stool water of more than 44 mmol/L (90 mEq/L) strongly suggests magnesium-induced diarrhea. The ingestion may be intentional, as in a patient with surreptitious laxative ingestion, or accidental, as in a patient who uses magnesium-containing antacids or mineral supplements.
Ingestion of poorly absorbed carbohydrates or carbohydrate malabsorption typically leads to a low fecal pH because of bacterial fermentation in the colon. A fecal pH lower than 6 is highly suggestive of carbohydrate malabsorption.10,12 More generalized malabsorption that involves fecal loss of amino acids and fatty acids in addition to carbohydrate may produce a somewhat higher pH (e.g., pH = 6 to 7.5). Isolated carbohydrate malabsorption is usually caused by ingestion of a poorly absorbable carbohydrate, such as lactose in a person with lactase deficiency. Other common causes include ingestion of poorly absorbed sugar alcohols that are used as artificial sweeteners, such as sorbitol or mannitol, or excessive ingestion of sugars with a limited absorption capacity, such as fructose.66 Therapeutic use of inhibitors of carbohydrate absorption, such as acarbose, may also lead to carbohydrate malabsorption.95 Because fermentation produces not only short-chain fatty acids that acidify the stool, but also carbon dioxide and hydrogen, complaints of gas and bloating by the patient are clinical clues to the presence of carbohydrate malabsorption, although these symptoms are fairly nonspecific (see Chapters 16, 100, and 101).96
Once the clinical picture or stool analysis suggests carbohydrate malabsorption, a careful review of the patient’s diet may indicate the likely source. In some persons, a hydrogen breath test with lactose as the sugar substrate can confirm lactose intolerance as the diagnosis.97 In this test, a previously fasting patient ingests 25 g of lactose dissolved in water, and exhaled breath is assayed for hydrogen content at baseline and at intervals for several hours. Because hydrogen is not a normal product of human metabolism, any increase in breath hydrogen concentration is the result of bacterial fermentation and indicates that unabsorbed lactose has reached the colon. This principle also has been applied to the assessment of sucrase deficiency after administration of a sucrose load and to the assessment of fructose malabsorption after a fructose load.98 Breath hydrogen testing has been adapted to detect small intestinal bacterial overgrowth with the use of glucose, a substrate that ordinarily should be absorbed completely before reaching the colon.88 Lactulose, a nonabsorbable but easily fermented disaccharide, also has been used to detect small intestinal bacterial overgrowth, but because of the wide variability of intestinal transit time, use of lactulose for this purpose is problematic. Lactulose can be used as a substrate for determining the oral-cecal transit time. Breath hydrogen testing after administration of d-xylose has been advocated as a screening test for generalized intestinal malabsorption.99 For most purposes breath hydrogen testing provides only supportive evidence when the pretest likelihood of a particular diagnosis is high (see Chapter 101).
Chronic Inflammatory Diarrhea
Patients with chronic diarrhea and white blood cells or blood in the stool are classified as having inflammatory diarrhea. These characteristics indicate that the mucosa is disrupted and inflamed. Diagnostic considerations include IBD, infections, pseudomembranous enterocolitis, mesenteric ischemia, radiation enteritis, and neoplasia. Because these conditions may produce a secretory diarrhea without markers of inflammation in the stool, they must be considered in the differential diagnosis of secretory diarrhea as well (see Chapters 39, 104 to 112, 114, 115, and 121 to 124).
Sigmoidoscopy or colonoscopy should be undertaken to look initially for structural changes, because colitis is a common cause of inflammatory diarrhea and a neoplasm can be life threatening (Fig. 15-9). Sigmoidoscopy can detect most causes of inflammatory diarrhea but can miss disorders localized to the right colon and ileum. Because preparation for this test is simpler than that for colonoscopy and the frequency of complications is lower, sigmoidoscopy is preferred by some physicians. Others prefer to examine the entire colon and terminal ileum in patients with inflammatory diarrhea, especially if occult blood is detected in the stool.92 The choice of test depends on the circumstances of the individual patient. For example, a patient older than 50 years who has not undergone previous screening for colon cancer would benefit by undergoing colonoscopy in this setting. Whichever test is selected, biopsy specimens must be obtained from the colon to aid in making the correct diagnosis.
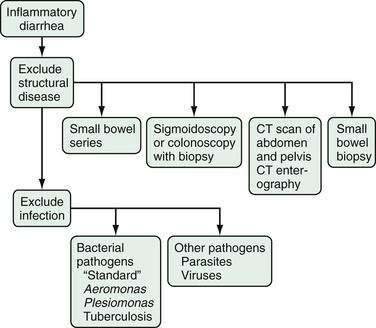
Figure 15-9. Algorithm for the evaluation of chronic inflammatory diarrhea. CT, computed tomography.
(From Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464-86.)
Infection can cause chronic inflammatory diarrhea or aggravate existing inflammatory diarrhea caused by ulcerative colitis or Crohn’s disease. The pathogens most likely to cause chronic inflammatory diarrhea are C. difficile, cytomegalovirus, Entamoeba histolytica, Yersinia spp., and Mycobacterium tuberculosis. C. difficile and cytomegalovirus are notorious for causing exacerbations of IBD (see Chapters 111 and 112).100 In addition to biopsies, appropriate cultures and serologic tests should be obtained to exclude these infections.
Chronic Fatty Diarrhea
Steatorrhea implies the disruption of fat solubilization, digestion, or absorption in the small intestine. The evaluation of chronic fatty diarrhea is designed to distinguish maldigestion, inadequate luminal breakdown of triglyceride, from malabsorption, inadequate mucosal transport of the products of digestion (see Chapter 101).
The major causes of maldigestion are pancreatic exocrine insufficiency (e.g., chronic pancreatitis) and lack of bile (e.g., advanced primary biliary cirrhosis). Mucosal diseases (e.g., celiac disease) are the usual causes of malabsorption. The absolute amount of steatorrhea and the fecal fat concentration (grams of fat/100 g of stool) provide clues to the cause of steatorrhea.101 The degree of steatorrhea tends to be higher with maldigestion (as in pancreatic insufficiency)—often more than 30 g fat/day—than with mucosal disease, because of the greater disruption of fat assimilation with maldigestion. Additionally, the fecal fat concentration tends to be higher with maldigestion than with mucosal disease because mucosal disease often is associated with poor fluid and electrolyte absorption, so that the stool fat content is diluted by unabsorbed water. Also, because fat digestion usually is intact in mucosal disease, triglycerides are broken down to fatty acids in the small intestine and pass into the colon, where they inhibit electrolyte and water absorption and thus further dilute the fat content of stool.56 By contrast, maldigestion caused by pancreatic and biliary disorders typically reduces triglyceride hydrolysis and does not result in delivery of excess fatty acids to the colon or inhibition of fluid and electrolyte absorption. Therefore, the unabsorbed fat is dispersed in a smaller stool volume and is thus more concentrated. A fecal fat concentration more than 9.5 g/100 g in a patient with suspected maldigestion strongly suggests a pancreatic or biliary cause of steatorrhea.
The further evaluation of patients with chronic fatty diarrhea is relatively straightforward (Fig. 15-10). The first step is to look for a structural problem involving the small bowel or pancreas. The evaluation may include small bowel radiography or endoscopy with small bowel biopsy, CT, and MR cholangiopancreatography. When a small bowel biopsy is performed, luminal contents should be aspirated and a sample sent for quantitative culture to exclude small intestinal bacterial overgrowth. Because celiac disease is the most common cause of mucosal disease that leads to malabsorption, tissue transglutaminase antibodies and endomysial antibodies should be determined (see Chapters 102 and 104).102
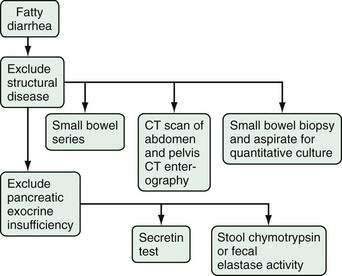
Figure 15-10. Algorithm for the evaluation of chronic fatty diarrhea.
(From Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464-86.)
If no intestinal abnormalities are discovered or radiographic evidence of chronic pancreatitis is detected, abnormal pancreatic exocrine function should be considered. Available tests of pancreatic function all have limitations. The secretin stimulation test, in which exogenous secretin is used to stimulate the pancreas and bicarbonate output is measured by aspiration of duodenal contents, is the most time-honored of these tests but is rarely performed because of its complexity.103 For the test to be done properly, the duodenum and stomach have to be intubated and the samples obtained from the duodenum need to be analyzed promptly. Attempts to update the secretin stimulation test by combining it with endoscopic retrograde cholangiopancreatography have been attempted, but these modifications have not been adopted widely.104 Determination of pancreatic enzyme concentrations in stool has been advocated as a simpler screening test for pancreatic exocrine insufficiency. Direct measurement of stool chymotrypsin activity has poor sensitivity and specificity in patients with chronic diarrhea.105 Measurement of fecal elastase has only somewhat better reliability.106 In reality, the best way to determine pancreatic exocrine insufficiency may be a therapeutic trial of pancreatic enzyme supplementation. If such a trial is conducted, high doses of enzymes should be prescribed and some objective measurement, such as fecal fat excretion or weight gain, should be monitored to assess response (see Chapter 59).107
Inadequate bile salt solubilization of dietary fat can usually be inferred from the patient’s history or physical examination (e.g., cholestatic jaundice, ileal resection, or a known enterocolic fistula). If proof of the mechanism is required, analysis of a postprandial duodenal aspirate can demonstrate reduced conjugated bile acid concentrations. This test may not be available outside specialized centers, and a therapeutic trial of exogenous conjugated bile acids may be the best way of establishing the diagnosis. Supplementation with bile acids reduces steatorrhea and can often improve the patient’s nutritional status without aggravating diarrhea.108
TREATMENT
The most important treatment of diarrhea is to ensure that fluid and electrolyte deficits are replenished with intravenous fluids or oral rehydration therapy. Although oral rehydration therapy is a convenient, inexpensive therapeutic option in industrialized countries, its major impact has been in decreasing the morbidity and mortality from cholera and other infectious diarrheas in less developed countries.109 Because nutrient absorption enhances sodium and fluid absorption in the jejunum, even when other forms of sodium absorption are impaired, orally ingested saline solutions that contain glucose, amino acids, or more complex nutrients that can be hydrolyzed at the brush border or intraluminally will be absorbed readily. Although the earliest oral rehydration solutions used glucose to accelerate sodium absorption, cereal-based oral rehydration solutions are now thought to be superior.110 Modifications to the formula have included hypo-osmolarity and use of amylase-resistant starch to enhance production of short-chain fatty acids in the colon, thereby stimulating colonic water and electrolyte absorption.111,112 Although oral rehydration solutions increase fluid and electrolyte absorption, they are not designed to reduce stool output, and stool weight actually may increase with use of these solutions. Use of oral rehydration solutions is precluded in patients who are vomiting frequently. Most sport drinks (e.g., Gatorade) are designed to replenish modest electrolyte losses from sweat and do not contain enough sodium to replace losses in diarrhea adequately. These solutions can be used if additional sources of sodium and absorbable nutrients (e.g., pretzels or crackers) are ingested concomitantly. Solutions that approximate the World Health Organization oral rehydration solution or cereal-based rehydration solutions more closely are available commercially (e.g., Rehydralyte, Resol, Ricalyte).
EMPIRICAL THERAPY OF ACUTE DIARRHEA
Because infection is a frequent cause of acute diarrhea, empirical trials of antibiotic therapy are often considered by physicians.113 If the prevalence of bacterial or protozoal infection is high in a community or a specific situation, empirical use of an antibiotic is logical, as in the treatment of travelers’ diarrhea with a fluoroquinolone or rifaximin, even without bacteriologic proof of infection.114 Antibiotic therapy also is often used empirically for more severely ill patients while bacterial culture results are pending. This approach has been called into question with the observation that patients in whom hemolytic-uremic syndrome develops in response to infection with Escherichia coli are more likely than those without hemolytic-uremic syndrome to have received empirical antibiotic therapy, although the occurrence of the syndrome may relate to the specific antibiotic and dose prescribed.115 Experts also advise against empirical antibiotic treatment of salmonellosis unless enteric fever is present.116 For patients with persistent diarrhea (lasting more than one week), an empirical trial of metronidazole or nitazoxanide for a protozoal infection is sometimes considered (see Chapters 107 and 109).117
Nonspecific antidiarrheal agents can reduce stool frequency, stool weight, and coexisting symptoms, such as abdominal cramps (Table 15-6). Opiates such as loperamide or diphenoxylate with atropine frequently are prescribed.118 The concern that these antiperistaltic agents slow the clearance of pathogens from the intestine has largely not been substantiated. Intraluminal agents, such as bismuth subsalicylate (Pepto-Bismol) and adsorbents (e.g., kaolin), also may help reduce the fluidity of bowel movements. Racecadotril, a drug that inhibits enkephalinase and thereby increases the effects of endogenous opiates on the mu opiate receptor, is available for the treatment of acute diarrhea in some countries.119
| DRUG CLASS | AGENT | DOSE |
|---|---|---|
| Opiates (mu opiate receptor selective) | Codeine | 15-60 mg four times daily |
| Diphenoxylate | 2.5-5 mg four times daily | |
| Loperamide | 2-4 mg four times daily | |
| Morphine | 2-20 mg four times daily | |
| Tincture of opium | 2-20 drops four times daily | |
| Enkephalinase inhibitor (delta opiate receptor effects) | Racecadotril* (acetorphan) | 1.5 mg/kg three times daily |
| Alpha-2 adrenergic agonist | Clonidine | 0.1-0.3 mg three times daily |
| Somatostatin analog | Octreotide | 50-250 µg three times daily (subcutaneously) |
| Bile acid-binding resin | Cholestyramine | 4 g one to four times daily |
| Colesevelam | 3 tabs twice daily | |
| Colestipol | 4 g one to four times daily | |
| Fiber supplements | Calcium polycarbophil | 5-10 g daily |
| Psyllium | 10-20 g daily |
EMPIRICAL THERAPY OF CHRONIC DIARRHEA
Symptomatic treatment with an opiate often is necessary in patients with chronic diarrhea because specific treatment may not be available.118 Potent opiates such as codeine, opium, or morphine are underused in the management of these patients, largely because of fear of abuse. In fact, these agents are rarely abused by patients with chronic diarrhea, especially if a few simple measures are taken. First, the patient needs to be informed about the abuse potential of the medication and should be warned not to increase the dose without consulting the physician. Second, the dose should be low initially and titrated up until efficacy is achieved. Third, use of the opiate should be monitored closely, and the prescription should not be refilled until an interval appropriate with the anticipated usage has passed.
Other agents that are sometimes used as nonspecific antidiarrheal agents include octreotide and clonidine. Octreotide, a somatostatin analog, has been shown to improve diarrhea in patients with the carcinoid syndrome and other endocrinopathies, dumping syndrome, chemotherapy-induced diarrhea, and AIDS.120 The benefit in other diarrheal diseases is less clear. Clonidine, an α-adrenergic agent that has effects on intestinal motility and transport,121 may have a special role in diabetic diarrhea, but its hypotensive effect limits its usefulness in many patients with diarrhea.122
Interest in and evidence to support the use of probiotics, ostensibly good bacteria (e.g., certain strains of lactobacilli), as therapy for diarrhea has been increasing. By modifying the colonic flora, these agents may stimulate local immunity and speed the resolution of travelers’ diarrhea, antibiotic-associated diarrhea, and infantile diarrhea.123–125 Herbal remedies for diarrhea include those containing berberine (goldenseal, barberry), which appears to stimulate fluid and electrolyte absorption, and arrowroot, the mechanism of which is unknown.126,127
Stool-modifying agents such as psyllium alter stool consistency but do not reduce stool weight.128,129 They can be helpful in patients with coexisting fecal incontinence and in some patients with low stool weights (see earlier). The change from watery to semiformed stools may be sufficient to alleviate symptoms. In addition, pectin may delay transit through the proximal intestine and increase luminal viscosity, thus serving as an adjunctive empirical treatment.
SELECTED DIARRHEAL SYNDROMES
IRRITABLE BOWEL SYNDROME AND FUNCTIONAL DIARRHEA
Undoubtedly, the most common diagnosis made in patients with chronic diarrhea is IBS, yet only a fraction of patients with chronic diarrhea actually meet the current criteria for the diagnosis of IBS, for which abdominal pain is a central feature68,130 and diagnostic criteria have changed. In the past, painless diarrhea was considered to be a subtype of IBS, but such patients are now excluded from a diagnosis of IBS and are labeled as having functional diarrhea instead.
Diarrhea in patients with IBS and functional diarrhea tends to be variable and sometimes alternates with periods of constipation.68 When measured, daily stool output is relatively low, typically less than 400 g/24 hr. Consistency varies from loose to soft and rarely is watery. The diarrhea does not awaken patients from sleep. Rectal urgency and fecal incontinence may be pronounced, especially during periods of psychological stress. Weight loss and evidence of chronic illness are uncommon (see Chapter 118). In some patients, IBS seems to be a late consequence of acute gastroenteritis.131
When typical features of IBS are absent, other diagnoses should be considered. An alternative diagnosis is carbohydrate malabsorption which can produce diarrhea of variable severity, depending on the amount of the malabsorbed carbohydrate consumed.132 In addition, cramps, excessive flatus, and bloating may be present with carbohydrate malabsorption. A carefully taken dietary history and a stool pH value lower than 6 distinguish this disorder from IBS. Caution should be exercised when using breath testing to confirm lactose intolerance in a patient with chronic diarrhea.133 Another condition that can simulate IBS is small intestinal bacterial overgrowth. The diagnosis of this condition is complex and controversial (see earlier).88 Bile acid–induced diarrhea also can vary in severity, depending on the rate of delivery of bile acids to the colon. Response to a therapeutic trial of a bile acid–binding resin may be a reasonable diagnostic test for this condition (see later). Celiac disease is another diagnosis that often is considered but is found in only 3% to 5% of patients who meet criteria for a diagnosis of IBS.134 Most patients with other causes of chronic diarrhea have been misdiagnosed as IBS at some point before the correct diagnosis is discovered.
MICROSCOPIC COLITIS
The clinical and pathologic spectrum of microscopic colitis has been reviewed in the 2000s (see Chapter 124).135,136 The interrelationship between the different histologic diagnoses is not entirely clear. Because their clinical presentations are so similar and their pathologic appearances differ only in the presence or absence of a thickened subepithelial collagen table, current usage now includes lymphocytic colitis and collagenous colitis as histologic subtypes of microscopic colitis. Therefore, microscopic colitis is defined as a syndrome of watery diarrhea characterized by a normal colonoscopic appearance and histologic changes of lymphocytic-plasmacytic inflammation in the lamina propria and intraepithelial lymphocytosis, with or without thickening of the subepithelial collagen table.
Microscopic colitis is a fairly common cause of chronic diarrhea of obscure origin in the general population, as well as at referral centers. At a tertiary referral center, microscopic colitis was discovered in 10% of patients with chronic diarrhea, with an even division between the lymphocytic and collagenous subtypes.93 A population-based epidemiologic study in Sweden has shown that the annual incidence of microscopic colitis is similar to that of Crohn’s disease, and the disorder was diagnosed in 10% of patients who presented with chronic nonbloody diarrhea.137 The keys to making the diagnosis are remembering to obtain biopsies of normal-appearing colonic mucosa in patients who present with chronic watery diarrhea, taking a sufficient number of biopsy specimens (6 to 10) to avoid sampling error, and having a skilled pathologist review the biopsy slides.
Like idiopathic IBS, the cause or causes of microscopic colitis remain unknown. The disease occurs most frequently in middle-aged women and often occurs in association with autoimmune diseases, such as arthritis and hypothyroidism. A history of nonsteroidal anti-inflammatory drug use is frequent. Most fascinating is the tight linkage of lymphocytic and collagenous colitis with human leukocyte antigen (HLA)-DQ2 and HLA-DQ1,3 (including the HLA-DQ1,3 subtypes HLA-DQ1,7, HLA-DQ1,8, and HLA-DQ1,9), as in celiac disease, suggesting the possibility that similar immune mechanisms are involved in the pathogenesis of microscopic colitis and celiac disease.138 In fact, microscopic colitis has been associated with celiac disease and may be a cause of persistent diarrhea in patients with celiac disease who are treated with a gluten-free diet.139 Gluten is almost certainly not the causative antigen in microscopic colitis, however, because many patients with celiac disease continue to have histologic evidence of lymphocytic colitis despite treatment with a gluten-free diet, and elimination of gluten from the diet is not effective in other patients with microscopic colitis.140 Bacterial antigens in the colonic lumen, rather than dietary antigens, might play an important pathogenic role in microscopic colitis.
Whatever the cause, mucosal inflammation is largely responsible for the diarrhea of microscopic colitis. Colonic perfusion studies have shown that absorption of water and salt is impaired in lymphocytic colitis and collagenous colitis.141 In vitro studies using human colon specimens have demonstrated decreases in sodium chloride absorption accompanied by changes in diffusion and the function of tight junctions.142 Colonic water absorption correlates inversely with the cellularity of the lamina propria, but not with the thickness of the collagen table. Net secretion of water and salt by the colon is not noted frequently, however. Typical stool weights of 500 to 1000 g/24 hr are consistent with little or no fluid absorption by the colon. Bile acid malabsorption also may play a role in the pathogenesis of diarrhea in this condition.143
A Cochrane analysis of interventions for treating collagenous colitis has concluded that budesonide has the best evidence for efficacy.144 Less well-established agents for the treatment of collagenous colitis include bismuth subsalicylate, mesalamine, and prednisone. Studies of budesonide in the treatment of lymphocytic colitis are fewer in number, and the overall evidence in support of a benefit is weaker.145
Microscopic colitis remits in most patients with time, and symptomatic therapy with an antidiarrheal drug may be an appropriate option.146 Bile acid sequestrants also may improve diarrhea in patients with this condition.143
POSTSURGICAL DIARRHEA
Diarrhea after Gastric Surgery
For many years, peptic ulcer was treated surgically by vagotomy with pyloroplasty or antrectomy. The introduction of highly selective vagotomy in the 1980s led to a decrease in the frequency of postoperative diarrhea. The more traditional surgeries are still done for obstructing or malignant ulcer disease. In addition, the use of gastric bypass surgery for the treatment of obesity has increased dramatically. This operation and other bariatric surgeries are commonly associated with digestive symptoms, including diarrhea.147 Diarrhea also can occur as a complication of laparoscopic antireflux surgery, presumably because of accidental vagotomy.148
The most common syndrome seen after gastric surgery is dumping syndrome, a condition characterized by postprandial flushing, hypotension, diarrhea, and hypoglycemia (see Chapter 53). This syndrome results from unregulated gastric emptying, osmotic shifts, and the rapid release of peptide hormones from the intestine149 and can be treated successfully with a modified diet, antidiarrheal drugs, and the somatostatin analog octreotide.150
Diarrhea after Bowel Resection
Loss of intestinal surface area promotes malabsorption of fluid, electrolytes, or nutrients, depending on the portion of the bowel resected. The bowel is endowed with an excess of surface area for absorption under ordinary circumstances. Diarrhea develops after resection of the small intestine that leaves insufficient surface area for normal absorption, so-called short bowel syndrome (see Chapter 103).151 The process of intestinal adaptation may improve intestinal electrolyte absorption with time but cannot overcome defects in specialized functions.152 For example, removal of the ileocecal area limits the ability of the intestine to absorb sodium against its electrochemical gradient, a defect that cannot be compensated for elsewhere in the gastrointestinal tract (see Chapter 99).27 Similarly, resection of the terminal ileum results in a permanent reduction in vitamin B12 absorption, thereby necessitating parenteral vitamin B12 replacement. Ileal resection also may compromise conjugated bile acid absorption and result in bile acid–mediated fluid and electrolyte secretion by the colon.153 Various approaches can be used to correct nutritional deficiencies and intestinal dysfunction in patients with short bowel syndrome (see Chapter 103).
Ileostomy Diarrhea
Normally, 1 to 1.5 L of fluid enters the colon from the small intestine each day; an ileostomy diverts this fluid from the body. Adaptation eventually results in a decrease in flow from the small intestine to an average of 750 mL daily, provided that the patient has a sufficient length of functioning small bowel. This excess daily fluid loss usually is readily overcome by an increase in oral intake by the patient. Patients with ileostomies tolerate abnormally increased fluid losses poorly, however, and are at risk of dehydration under such circumstances. Ileostomy diarrhea is said to be present when fluid losses exceed 1000 mL daily (see Chapter 113).154
Causes of ileostomy diarrhea include stomal stenosis, partial small bowel obstruction, small intestinal bacterial overgrowth, recurrent IBD, recurrent tumor proximal to the stoma, medication-associated diarrhea, and intraperitoneal infection. In most cases, however, no specific cause is identified. A special circumstance occurs in patients with an ileal pouch formed to create a continent ileostomy or an ileoanal anastomosis after colectomy for ulcerative colitis, in whom inflammation of the pouch, so-called pouchitis, caused by bacterial overgrowth or recurrent IBD may develop.155 This condition is treated with antibiotics, such as metronidazole, with probiotics, such as Lactobacillus spp., or with an anti-inflammatory drug, such as mesalamine.
Postcholecystectomy Diarrhea
Postcholecystectomy diarrhea is relatively common, occurring in up to 20% of patients after cholecystectomy.156,157 It usually occurs shortly after cholecystectomy, but the onset may be delayed, perhaps in response to some additional unknown disturbance that develops over time. The conventional explanation for postcholecystectomy diarrhea relates to changes in the enterohepatic cycling of bile acids, but the evidence in support of this mechanism is limited. The gallbladder provides a reservoir for bile acids at night when they are not needed for digestion of fat. When the gallbladder is removed, the enterohepatic cycling of bile acids continues at night, and a substantial portion of the bile acid pool remains within the small bowel at all times. Every 90 minutes during fasting, the migrating myoelectric complex passes through the small intestine and rapidly sweeps intestinal contents, including much of the bile acid pool in these cases, past the specialized absorptive sites in the ileum and into the colon. Increased concentrations of bile acids in the colonic lumen may inhibit fluid and electrolyte absorption and accelerate transit. Alternatively, bacterial deconjugation of bile acids in the small bowel may increase and thereby decrease ileal absorption. Some, but not all, studies have confirmed increased fecal bile acid excretion in patients with postcholecystectomy diarrhea.
BILE ACID–INDUCED DIARRHEA
The importance of bile acid malabsorption as a mechanism for producing chronic secretory diarrhea in the absence of ileal resection or disease is controversial.98,153,158 Bile acid malabsorption has been well described as the mechanism of diarrhea when ileal disease or resection allows excessive amounts of conjugated bile acid to enter the colon. Concentrations of bile acid in the colon higher than 3 to 5 mmol/L can inhibit electrolyte absorption and stimulate secretion by the colonic mucosa.159
How often this mechanism produces chronic watery diarrhea when there is no overt ileal disease or resection is unclear. In rare congenital cases, primary bile acid malabsorption is caused by mutations in the ileal sodium-dependent bile acid transporter gene (see Chapter 64), but most cases of adult-onset bile acid malabsorption are not associated with a discrete molecular defect.160–162 Accelerated transit through the small bowel and colon could account for impaired ileal bile acid absorption in the absence of ileal disease or molecular defects.163 Studies from Europe and the United States have indicated that bile acid malabsorption is common in patients with idiopathic chronic diarrhea. Reports of the therapeutic effect of bile acid–sequestering resins in this setting, however, are discrepant. In many European studies, a high proportion of patients with idiopathic secretory diarrhea responded to therapeutic doses of bile acid–sequestering resins, whereas in American studies no consistent beneficial effect has been observed.
DIARRHEA IN HOSPITALIZED PATIENTS
Diarrhea is a side effect of many medications, including those frequently used in hospitalized patients (see Table 15-5).59 Antibiotic therapy is particularly likely to cause diarrhea by at least two main mechanisms, impairing carbohydrate metabolism by the colonic bacterial flora and facilitating overgrowth of C. difficile and production of toxins by the bacteria.164 In some cases, erythromycin produces diarrhea by its motilin-like effect on gastrointestinal transit.
Impaired bacterial metabolism can cause diarrhea by allowing carbohydrates and associated water to remain in the intestinal lumen.165 Ordinarily, all dietary fiber and about 20% of wheat starch evade digestion and absorption and reach the colon. Colonic bacteria ferment these carbohydrates to short-chain fatty acids, hydrogen, and carbon dioxide. These fermentation products and associated water are rapidly absorbed by the colon, and therefore diarrhea does not result. By contrast, when an antibiotic kills some of the normal colonic flora, fermentation decreases; undigested fiber and carbohydrates as well as water are retained in the lumen, thereby leading to an osmotic diarrhea. In some persons, intestinal transit may be modified by illness or by other drugs given concomitantly, thus leading to greater delivery of carbohydrate to the colon and further aggravating the diarrhea. Such diarrhea should subside when the patient is fasting.
C. difficile-related diarrhea is a more serious concern.166 Hospitalized patients and residents of nursing homes are likely to be colonized by this organism; approximately 20% of hospitalized patients become colonized with C. difficile. Physical proximity and poor hand hygiene are major factors in its spread within institutions. Factors precipitating diarrhea in this condition include antibiotic therapy, chemotherapy, and altered immunity, including reduced gastric acidity resulting from administration of a proton pump inhibitor.167 C. difficile-related disease ranges in severity from modest diarrhea to life-threatening colitis, particularly when patients are colonized with a virulent strain of C. difficile (see Chapter 108). Patients may have severe pain, abdominal tenderness, and marked polymorphonuclear leukocytosis, with an increased percentage of immature forms. In less severely ill patients suspected of having this infection, stool should be tested for C. difficile toxin and, if the result is positive, appropriate antibiotic therapy should be initiated (see Chapter 108). In more severely ill patients, analysis of stool samples for toxin may not be sufficient,168 and sigmoidoscopy is performed to identify the characteristic findings of pseudomembranous colitis. Occasionally, the colitis is evident only more proximally in the colon, and colonoscopy is required to confirm the diagnosis. Therapy with metronidazole or vancomycin often suppresses the diarrhea, but recurrence is common because the organism forms spores. Probiotics, toxin-absorbing resins, and vaccines are being studied as ways to reduce recurrence.72
Diarrhea also may be a complication of enteral nutrition, although it is often the result of coexisting problems.169 Tube feeding, although more physiologic than parenteral nutrition, is still different from the normal presentation of nutrients to the intestine by oral ingestion of food, and the regulatory system of the gastrointestinal tract may not adapt to tube feeding. Some tube feeding formulas are hypertonic and may induce diarrhea by a mechanism similar to that of dumping syndrome. In such cases, a change in formula to one that is isotonic may be of benefit. In other cases, slowing the rate of infusion and thereby decreasing the delivery of nutrients to the intestine may be helpful, but this approach may be of limited value if the patient’s nutritional needs are not being met at the slower rate of infusion. Addition of an antidiarrheal agent such as loperamide or tincture of opium to the tube feeding may be necessary, although this approach has limitations, especially for patients in whom ileus is a risk (see Chapter 120).
Intestinal ischemia may develop in some hospitalized patients, especially those with hypotension or shock.170 These patients are at risk of developing bloody diarrhea caused by ischemic colitis or more profound diarrhea if small intestinal ischemia develops (see Chapter 114).
The risk of fecal impaction is increased in older adults, patients on prolonged bowel rest, and those taking constipating drugs. Paradoxical or overflow diarrhea with incontinence may be the first clue to an impaction. Hospitalized patients in whom diarrhea develops should undergo a digital rectal examination to exclude fecal impaction.171
FACTITIOUS DIARRHEA
Surreptitious laxative abuse should be considered for persons in whom diarrhea remains undiagnosed, especially in those who fit into one of the following four categories (Table 15-7): (1) patients with anorexia nervosa or bulimia who use laxatives as a way of adjusting body weight (see Chapter 8); (2) those who use laxative-induced illness for secondary gain, such as disability income, or to generate concern in other persons; (3) patients with Munchausen’s syndrome, who feign illness to confound physicians; and (4) persons who are being poisoned with laxatives by their caregivers.172,173
| GROUP | CHARACTERISTICS |
|---|---|
| Bulimia | Usually adolescent to young women; concerned about weight or manifesting an eating disorder; may binge eat, vomit, or exercise excessively to neutralize excessive food intake |
| Munchausen syndrome | Patients who relish being a diagnostic challenge; may undergo extensive testing repeatedly |
| Polle’s syndrome (Munchausen’s syndrome by proxy) | Dependent child or adult poisoned with laxatives by parent or caregiver to show effectiveness as caregiver; may have history of sibling who died with chronic diarrhea |
| Secondary gain | Patients may have disability claim pending; illness may induce concern or caring behavior in others |
A high index of suspicion is required for detecting laxative abuse. Physicians usually assume that patients are being truthful, but up to 15% of patients who undergo an evaluation for chronic diarrhea may be abusing laxatives surreptitiously.174 Clues to laxative abuse may be found during the evaluation for chronic diarrhea. For example, hypokalemia may suggest ingestion of stimulant laxatives, such as senna. Detection of pseudomelanosis coli, a brownish pigmentation of the colonic mucosa, suggests chronic ingestion of anthracene laxatives, such as senna or cascara (see Chapter 124). The presence of a large fecal osmotic gap suggests magnesium ingestion.
In patients who belong to one of the groups listed in Table 15-7 and in those with diarrhea that remains undiagnosed after evaluation, stool samples should be analyzed for laxatives with standardized methods. Most laxatives can be detected by spectrophotometry or chromatography, but the accuracy of commercial analysis has been called into question.82 Because some patients exaggerate stool volume by adding urine or water, stool osmolality should be measured as well; a value lower than 290 mOsm/kg suggests dilution of the stool with water or hypotonic urine. Admixture of stool with hypertonic urine often leads to an impossibly high fecal osmolality (typically, >600 mOsm/kg) and to a negative fecal osmotic gap because of high concentrations of sodium and potassium in the urine. A negative fecal osmotic gap also can be noted when phosphate or sulfate osmotic laxatives are ingested. In today’s legal environment, unauthorized room searches for laxatives should not be conducted.
Few outcome studies of the effect of discovery of laxative abuse are available. In one study of 11 patients seen at the Cleveland Clinic, 6 said that they were improved and 5 claimed no benefit; 4 of these 5 unimproved patients sought further medical attention elsewhere for chronic diarrhea.175
IDIOPATHIC SECRETORY DIARRHEA
When an exhaustive evaluation fails to reveal a cause of chronic diarrhea and stool analysis suggests a secretory diarrhea, the diagnosis of idiopathic secretory diarrhea is made. This condition often starts suddenly in a previously healthy person and is differentiated from the many similar acute diarrheal illnesses by persisting beyond four weeks. It occurs in two forms, epidemic and sporadic.63,64
The epidemic form of secretory diarrhea occurs in outbreaks seemingly linked to contaminated food or drink.63,176–180 The initial description of this condition resulted from an outbreak in Brainerd, Minnesota, thus giving this condition its common appellation, Brainerd diarrhea. Several outbreaks have been described in the literature in different communities and even on a cruise ship. Although the epidemiology suggests an infectious cause, no causative agent has been identified in these outbreaks.
Sporadic idiopathic secretory diarrhea affects persons in a fashion identical to that of the epidemic form but does not seem to be acquired easily by family members or others.64 Many affected persons give a history of travel, but to destinations not usually associated with travelers’ diarrhea. Diarrhea begins abruptly and reaches its maximum intensity soon after onset. Weight loss of up to 20 pounds is characteristic and almost always occurs within the first few months of illness and not thereafter. Empirical trials of antibiotics and bile acid–binding resins are ineffective. Nonspecific opioid antidiarrheal agents may provide symptomatic improvement.
DIARRHEA OF OBSCURE ORIGIN
Physicians sometimes fail to make a specific diagnosis in patients with chronic diarrhea, despite an elaborate evaluation, and may refer these patients to centers interested in this condition. Common diagnoses resulting from reevaluation of these patients are shown in Table 15-8.
Table 15-8 Frequent Diagnoses in Patients with Diarrhea of Obscure Origin
Although unusual or obscure conditions that require special tests might be expected to predominate in this group of patients, most of the eventual diagnoses are straightforward and could have been made sooner.93 Fecal incontinence and iatrogenic diarrhea could be recognized with a careful history. Surreptitious laxative ingestion and microscopic colitis could be diagnosed with an appropriate index of suspicion and testing (e.g., laxative screen and colonic biopsy, respectively). Bile acid–induced diarrhea, small intestinal bacterial overgrowth, pancreatic exocrine insufficiency, and carbohydrate malabsorption could be discovered with a detailed history and a properly conducted therapeutic trial. Peptide-secreting tumors are rare, but serum peptide assays and scanning techniques (e.g., CT scanning and octreotide scanning) are widely available. Failure to make a diagnosis typically results from failure to appreciate the evidence at hand and to think through the differential diagnosis of chronic diarrhea.
DuPont AW, Sellin JH. Ileostomy diarrhea. Curr Treat Options Gastroenterol. 2006;9:39-48. (Ref 154.)
Fernandez-Banares F, Esteve M, Salas A, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am J Gastroenterol. 2007;102:2520-8. (Ref 98.)
Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116:1464-86. (Ref 3.)
Fine KD, Seidel RH, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc. 2000;51:318-26. (Ref 91.)
Floch MH, Walker WA, Guandalini S, et al. Recommendations for probiotic use-2008. J Clin Gastroenterol. 2008;42(Suppl 2):S104-8. (Ref 125.)
Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932-40. (Ref 72.)
Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-91. (Ref 68.)
McMahan ZH, DuPont HL. Review article: The history of acute infectious diarrhoea management—from poorly focused empiricism to fluid therapy and modern pharmacotherapy. Aliment Pharmacol Ther. 2007;25:759-69. (Ref 113.)
Murphy C, Hahn S, Volmink J. Reduced osmolarity oral rehydration solution for treating cholera. Cochrane Database Syst Rev 2004; (4):CD003754. (Ref 111.)
Schiller LR. Review article: Anti-diarrhoeal pharmacology and therapeutics. Aliment Pharmacol Ther. 1995;9:87-106. (Ref 118.)
Schiller LR. Secretory diarrhea. Curr Gastroenterol Rep. 1999;1:389-97. (Ref 16.)
Schiller LR. Management of diarrhea in clinical practice: Strategies for primary care physicians. Rev Gastroenterol Disord. 2008;7(Suppl 3):S27-38. (Ref 77.)
Sellin JH. A practical approach to treating patients with chronic diarrhea. Rev Gastroenterol Disord. 2007;7(Suppl 3):S19-26. (Ref 76.)
Theilman NM, Guerrant RL. Clinical practice. Acute infectious diarrhea. N Engl J Med. 2004;350:38-47. (Ref 58.)
Westergaard H. Bile acid malabsorption. Curr Treat Options Gastroenterol. 2007;10:28-33. (Ref 153.)
1. Roy SL, Scallan E, Beach MJ. The rate of acute gastrointestinal illness in developed countries. J Water Health. 2006;4(Suppl 2):31-69.
2. Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver disease, 2006. Am J Gastroenterol. 2006;101:2128-38.
3. Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116:1464-86.
4. Wenzl HH, Fine KD, Schiller LR, Fordtran JS. Determinants of decreased fecal consistency in patients with diarrhea. Gastroenterology. 1995;108:1729-38.
5. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-4.
6. Roach M, Christie JA. Fecal incontinence in the elderly. Geriatrics. 2008;63:13-22.
7. Spiller R. Role of motility in chronic diarrhoea. Neurogastroenterol Motil. 2006;18:1045-55.
8. Fordtran JS, Santa Ana CA, Morawski SG, et al. Pathophysiology of chronic diarrhoea: Insights derived from intestinal perfusion studies in 31 patients. Clin Gastroenterol. 1986;15:477-90.
9. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989;84:1056-62.
10. Eherer AJ, Fordtran JS. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
11. Fine KD, Santa Ana CA, Fordtran JS. Diagnosis of magnesium-induced diarrhea. N Engl J Med. 1991;324:1012-17.
12. Hammer HF, Fine KD, Santa Ana CA, et al. Carbohydrate malabsorption: Its measurement and its contribution to diarrhea. J Clin Invest. 1990;86:1936-44.
13. Naim HY. Molecular and cellular aspects and regulation of intestinal lactase-phlorizin hydrolase. Histol Histopathol. 2001;16:553-61.
14. Lomer MC, Parkes GC, Sanderson JD. Review article: Lactose intolerance in clinical practice-myths and realities. Aliment Pharmacol Ther. 2008;15:93-103.
15. Jarvela I, Sabri Enattah N, Kokkonen J, et al. Assignment of the locus for congenital lactase deficiency to 2q21, in the vicinity of but separate from the lactase-phlorizin hydrolase gene. Am J Hum Genet. 1998;63:1078-85.
16. Schiller LR. Secretory diarrhea. Curr Gastroenterol Rep. 1999;1:389-97.
17. Janecki AJ. Why should a clinician care about the molecular biology of transport? Curr Gastroenterol Rep. 2000;2:378-86.
18. Lucas ML. A reconsideration of the evidence for Escherichia coli Sta (heat stable) enterotoxin-driven fluid secretion: A new view of Sta action and a new paradigm for fluid absorption. J Appl Microbiol. 2001;90:7-26.
19. Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-92.
20. Cooke H. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77-80.
21. Hansen MB, Witte AB. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. 2008;193:311-23.
22. Barrett KE. New ways of thinking about (and teaching about) intestinal epithelial function. Adv Physiol Educ. 2008;32:25-34.
23. Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda). 2008;23:104-14.
24. Canani RB, Terrin G, Cirillo P, et al. Butyrate as an effective treatment of congenital chloride diarrhea. Gastroenterology. 2004;127:630-4.
25. Muller T, Wijmenga C, Phillips AD, et al. Congenital sodium diarrhea is an autosomal recessive disorder of sodium/proton exchange but unrelated to known candidate genes. Gastroenterology. 2000;119:1506-13.
26. Jenkins AP, Thompson RP. Mechanisms of small intestinal adaptation. Dig Dis. 1994;12:15-27.
27. Arrambide KA, Santa Ana CA, Schiller LR, et al. Loss of absorptive capacity for sodium chloride as a cause of diarrhea following partial ileal and right colon resection. Dig Dis Sci. 1989;34:193-201.
28. Guirl MJ, Hogenauer C, Santa Ana CA, et al. Rapid intestinal transit as a primary cause of severe chronic diarrhea in patients with amyloidosis. Am J Gastroenterol. 2003;98:2219-25.
29. Sellin JH, Hart R. Glucose malabsorption associated with rapid intestinal transit. Am J Gastroenterol. 1992;87:584-9.
30. Shakil A, Church RJ, Rao SS. Gastrointestinal complications of diabetes. Am Fam Physician. 2008;77:1697-702.
31. Petre S, Shah IA, Gilani N. Review article: Gastrointestinal amyloidosis—clinical features, diagnosis and therapy. Aliment Pharmacol Ther. 2008;27:1006-16.
32. Chey WY, Jin HO, Lee MH, et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-506.
33. Alam MJ. Chronic refractory diarhoea: A manifestation of endocrine disorders. Dig Dis. 1994;12:46-61.
34. Rana SV, Bhardwaj SB. Small intestinal bacterial overgrowth. Scand J Gastroenterol. 2008;43:1030-7.
35. Jones MP, Bratten JR. Small intestinal motility. Curr Opin Gastroenterol. 2008;24:164-72.
36. Virally-Monod M, Tielmans D, Kevorkian JP, et al. Chronic diarrhoea and diabetes mellitus: Prevalence of small intestinal bacterial overgrowth. Diabetes Metab. 1998;24:530-6.
37. Sellin JH, Chang EB. Therapy insight: Gastrointestinal complications of diabetes—pathophysiology and management. Nat Clin Pract Gastroenterol Hepatol. 2008;5:162-71.
38. Santos J, Yates D, Guilarte M, et al. Stress neuropeptides evoke epithelial responses via mast cell activation in the rat colon. Psychoneuroendocrinology. 2008;33:1248-56.
39. Mohajer B, Ma TY. Eicosanoids and the small intestine. Prostaglandins Other Lipid Mediat. 2000;61:125-43.
40. De Haan L, Hirst TR. Cholera toxin: A paradigm for multi-functional engagement of cellular mechanisms. Mol Membr Biol. 2004;21:77-92.
41. Sellin JH. Cholera: Old story, new endings. Curr Gastroenterol Rep. 1999;1:375-6.
42. Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214-22.
43. Martinez-Augustin O, Romero-Calvo I, Suarez MD, et al. Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;15:114-27.
44. Khan I, Siddique I, Al-Awadi FM, Mohan K. Role of Na+/H+ exchanger isoform-1 in human inflammatory bowel disease. Can J Gastroenterol. 2003;17:31-6.
45. Amasheh S, Barmeyer C, Koch CS, et al. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology. 2004;126:1711-20.
46. Zeissig S, Bergann T, Fromm A, et al. Altered ENaC expression leads to impaired sodium absorption in the noninflamed intestine in Crohn’s disease. Gastroenterology. 2008;134:1436-47.
47. Gewirtz AT, Simon POJr, Schmitt CK, et al. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99-109.
48. Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551-63.
49. Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: Pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379-83.
50. Sitaraman SV, Merlin D, Wang L, et al. Neutrophil-epithelial crosstalk at the intestinal luminal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861-9.
51. Fernandez-Banares F, Esteve M, Salas A, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci. 2001;46:2231-8.
52. Distrutti E, Salvioli B, Azpiroz F, Malagelada JR. Rectal function and bowel habit in irritable bowel syndrome. Am J Gastroenterol. 2004;99:131-7.
53. Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-9.
54. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-64.
55. Kapeller J, Houghton LA, Monnikes H, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967-77.
56. Ramakrishna BS, Mathan M, Mathan VI. Alteration of colonic absorption by long-chain unsaturated fatty acids: Influence of hydroxylation and degree of unsaturation. Scand J Gastroenterol. 1994;29:54-8.
57. Strobel S, Hourihane JO. Gastrointestinal allergy: Clinical symptoms and immunological mechanisms. Pediatr Allergy Immunol. 2001;12(Suppl 14):43-6.
58. Theilman NM, Guerrant RL. Clinical practice. Acute infectious diarrhea. N Engl J Med. 2004;350:38-47.
59. Abraham B, Sellin JH. Drug-induced diarrhea. Curr Gastroenterol Rep. 2007;9:365-72.
60. Cappell M. Colonic toxicity of administered drugs and chemicals. Am J Gastroenterol. 2004;99:1175-90.
61. Pusztaszeri MP, Genta RM, Cryer BL. Drug-induced injury in the gastrointestinal tract: Clinical and pathologic considerations. Nat Clin Pract Gastroenterol Hepatol. 2007;4:442-53.
62. Pollok RC, Banks MR, Fairclough PD, Farthing MJ. Dilutional diarrhoea: Under-diagnosed and over-investigated. Eur J Gastroenterol Hepatol. 2000;12:609-11.
63. Mintz E. A riddle wrapped in a mystery inside an enigma: Brainerd diarrhoea turns 20. Lancet. 2003;362:2037-8.
64. Afzalpurkar RG, Schiller LR, Little KH, et al. The self-limited nature of chronic idiopathic diarrhea. N Engl J Med. 1992;327:1849-52.
65. Bardhan PK, Beltinger J, Beltinger RW, et al. Screening of patients with acute infectious diarrhoea: Evaluation of clinical features, faecal microscopy, and faecal occult blood testing. Scand J Gastroenterol. 2000;35:54-60.
66. Choi YK, Kraft N, Zimmerman B, et al. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233-8.
67. Cabada MM, White ACJr. Travelers’ diarrhea: An update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473-9.
68. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-91.
69. Savola KL, Baron EJ, Tomkins LS, Passaro DJ. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. J Clin Microbiol. 2001;39:266-9.
70. Kane SV, Sandborn WJ, Rufo PA, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309-14.
71. Church D, Miller K, Lichtenfeld A, et al. Screening for Giardia/Cryptosporidium infections using an enzyme immunoassay in a centralized regional microbiology laboratory. Arch Pathol Lab Med. 2005;129:754-9.
72. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932-40.
73. Bini EJ, Cohen J. Diagnostic yield and cost-effectiveness of endoscopy in chronic human immunodeficiency virus-related diarrhea. Gastrointest Endosc. 1998;48:354-61.
74. Kearney DJ, Steuerwald M, Koch J, Cello JP. A prospective study of endoscopy in HIV-associated diarrhea. Am J Gastroenterol. 1999;94:596-602.
75. Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Mucosal biopsy diagnosis of colitis: Acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology. 1994;107:755-63.
76. Sellin JH. A practical approach to treating patients with chronic diarrhea. Rev Gastroenterol Disord. 2007;7(Suppl 3):S19-26.
77. Schiller LR. Management of diarrhea in clinical practice: Strategies for primary care physicians. Rev Gastroenterol Disord. 2008;7(Suppl 3):S27-38.
78. Fine KD. The prevalence of occult gastrointestinal bleeding in celiac sprue. N Engl J Med. 1996;334:1163-7.
79. Fine KD, Fordtran JS. The effect of diarrhea on fecal fat excretion. Gastroenterology. 1992;102:1936-9.
80. Balasekaran R, Porter JL, Santa Ana CA, Fordtran JS. Positive results on tests for steatorrhea in persons consuming olestra potato chips. Ann Intern Med. 2000;132:279-82.
81. Fine KD, Ogunji F. A new method of quantitative fecal fat microscopy and its correlation with chemically measured fecal fat output. Am J Clin Pathol. 2000;113:528-34.
82. Shelton JH, Santa Ana CA, Thompson DR, et al. Factitious diarrhea induced by stimulant laxatives: Accuracy of diagnosis by a clinical reference laboratory using thin layer chromatography. Clin Chem. 2007;53:85-90.
83. Strygler B, Nicar MJ, Santangelo WC, et al. Alpha1-antitrypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology. 1990;99:1380-7.
84. Wei SC, Hung CC, Chen MY, et al. Endoscopy in acquired immunodeficiency syndrome patients with diarrhea and negative stool studies. Gastrointest Endosc. 2000;51:427-32.
85. von Graevenitz A. The role of Aeromonas in diarrhea: A review. Infection. 2007;35:59-64.
86. Sack DA, Chowdhury KA, Huq A, et al. Epidemiology of Aeromonas and Plesiomonas diarrhoea. J Diarrhoeal Dis Res. 1988;6:107-12.
87. Muller A, Bialek R, Kamper A, et al. Detection of microsporidia in travelers with diarrhea. J Clin Microbiol. 2001;39:1630-2.
88. Schiller LR. Evaluation of small bowel bacterial overgrowth. Curr Gastroenterol Rep. 2007;9:373-7.
89. Lin S, Branch MS, Shetzline M. The importance of indication in the diagnostic value of push enteroscopy. Endoscopy. 2003;35:315-21.
90. Mazzarolo S, Brady P. Small bowel capsule endoscopy: A systematic review. South Med J. 2007;100:274-80.
91. Fine KD, Seidel RH, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc. 2000;51:318-26.
92. Shah RJ, Fenoglio-Preiser C, Bleau BL, Giannella R. Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea. Am J Gastroenterol. 2001;96:1091-5.
93. Schiller LR, Rivera LM, Santangelo W, et al. Diagnostic value of fasting plasma peptide concentrations in patients with chronic diarrhea. Dig Dis Sci. 1994;39:2216-22.
94. Kalha I, Sellin JH. Common variable immunodeficiency and the gastrointestinal tract. Curr Gastroenterol Rep. 2004;6:377-83.
95. Spengler M, Schmitz H, Landen H. Evaluation of the efficacy and tolerability of acarbose in patients with diabetes mellitus: A postmarketing surveillance study. Clin Drug Invest. 2005;25:651-9.
96. Suarez FL, Saviano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1-4.
97. Di Stefano M, Missanelli A, Miceli E, et al. Hydrogen breath test in the diagnosis of lactose malabsorption: Accuracy of new versus conventional criteria. J Lab Clin Med. 2004;144:313-18.
98. Fernandez-Banares F, Esteve M, Salas A, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am J Gastroenterol. 2007;102:2520-8.
99. Casellas F, de Torres I, Malagelada JR. Improved screening for intestinal villous atrophy by D-xylose breath test. Dig Dis Sci. 2000;45:18-22.
100. Rodemann JF, Dubberke ER, Reske KA, et al. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:339-44.
101. Bo-Linn GW, Fordtran JS. Fecal fat concentration in patients with steatorrhea. Gastroenterology. 1984;87:319-22.
102. Catassi C, Fasano A. Is this really celiac disease? Pitfalls in diagnosis. Curr Gastroenterol Rep. 2008;10:466-72.
103. Wormsley KG. Further studies of the response to secretin and pancreozymin in man. Scand J Gastroenterol. 1971;6:343-50.
104. Moolsintong P, Burton FR. Pancreatic function testing is best determined by the extended endoscopic collection technique. Pancreas. 2008;37:418-21.
105. Goldberg DM. Proteases in the evaluation of pancreatic function and pancreatic disease. Clin Chim Acta. 2000;291:201-21.
106. Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: A review. Clin Chem Lab Med. 2002;40:325-32.
107. Petersen JM, Forsmark CE. Chronic pancreatitis and maldigestion. Semin Gastrointest Dis. 2002;13:191-9.
108. Gruy-Kapral C, Little KH, Fordtran JS, et al. Conjugated bile acid replacement therapy for short-bowel syndrome. Gastroenterology. 1999;116:15-21.
109. Hartling L, Bellemare S, Wiebe N, et al. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev 2006; (3):CD004390.
110. Fontaine O, Gore SM, Pierce NF. Rice-based oral rehydration solution for treating diarrhoea. Cochrane Database Syst Rev 2000; (2):CD001264.
111. Murphy C, Hahn S, Volmink J. Reduced osmolarity oral rehydration solution for treating cholera. Cochrane Database Syst Rev 2004; (4):CD003754.
112. Ramakrishna BS, Subramanian V, Mohan V, et al. A randomized controlled trial of glucose versus amylase-resistant starch hypo-osmolar oral rehydration solution for adult acute dehydrating diarrhea. PLoS ONE. 2008;3:e1587.
113. McMahan ZH, DuPont HL. Review article: The history of acute infectious diarrhoea management-from poorly focused empiricism to fluid therapy and modern pharmacotherapy. Aliment Pharmacol Ther. 2007;25:759-69.
114. Cabada MM, White ACJr. Travelers’ diarrhea: An update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473-9.
115. Panos GZ, Betsi GI, Falagas ME. Systematic review: Are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157:H7 infection? Aliment Pharmacol Ther. 2006;24:731-42.
116. Crum-Cianflone NF. Salmonellosis and the gastrointestinal tract: More than just peanut butter. Curr Gastroenterol Rep. 2008;10:424-31.
117. Cohen SA. Use of nitazoxanide as a new therapeutic option for persistent diarrhea: A pediatric perspective. Curr Med Res Opin. 2005;21:999-1004.
118. Schiller LR. Review article: Anti-diarrhoeal pharmacology and therapeutics. Aliment Pharmacol Ther. 1995;9:87-106.
119. Szajewska H, Ruszczynski M, Chmielewska A, Wieczorek J. Systematic review: Racecadotril in the treatment of acute diarrhoea in children. Aliment Pharmacol Ther. 2007;26:807-13.
120. Szilagyi A, Shrier I. Systematic review: The use of somatostatin or octreotide in refractory diarrhea. Aliment Pharmacol Ther. 2001;15:1889-97.
121. Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Studies of the antidiarrheal action of clonidine: Effects on motility and intestinal absorption. Gastroenterology. 1985;89:982-8.
122. Fedorak RN, Field M, Chang EB. Treatment of diabetic diarrhea with clonidine. Ann Intern Med. 1985;102:197-9.
123. Quigley EM. What is the evidence for the use of probiotics in functional disorders. Curr Gastroenterol Rep. 2008;10:379-84.
124. Surawicz CM. Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2008;42(Suppl 2):S64-70.
125. Floch MH, Walker WA, Guandalini S, et al. Recommendations for probiotic use—2008. J Clin Gastroenterol. 2008;42(Suppl 2):S104-8.
126. Berberine. Altern Med Rev. 2000;5:175-7.
127. Cooke C, Carr I, Abrams K, Mayberry J. Arrowroot as a treatment for diarrhoea in irritable bowel syndrome patients: A pilot study. Arq Gastroenterol. 2000;37:20-4.
128. Eherer AJ, Santa Ana CA, Porter J, Fordtran JS. Effect of psyllium, calcium polycarbophil, and wheat bran on secretory diarrhea induced by phenolphthalein. Gastroenterology. 1993;104:1007-12.
129. Washington N, Harris M, Mussellwhite A, Spiller RC. Moderation of lactulose-induced diarrhea by psyllium: Effects on motility and fermentation. Am J Clin Nutr. 1998;67:317-21.
130. Adeniji OA, Barnett CB, Di Palma JA. Durability of the diagnosis of irritable bowel syndrome based on clinical criteria. Dig Dis Sci. 2004;49:572-4.
131. Dupont AW. Post-infectious irritable bowel syndrome. Curr Gastroenterol Rep. 2007;9:378-84.
132. Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765-71.
133. Pimentel M, Kong Y, Park S. Breath testing to evaluate lactose intolerance in irritable bowel syndrome correlates with lactulose testing and may not reflect true lactose malabsorption. Am J Gastroenterol. 2003;98:2700-4.
134. Locke GR3rd, Murray JA, Zinsmeister AR, et al. Celiac disease serology in irritable bowel syndrome and dyspepsia: A population-based case-control study. Mayo Clin Proc. 2004;79:476-82.
135. Nyhlin N, Bohr J, Eriksson S, Tysk C. Microscopic colitis: A common and an easily overlooked cause of chronic diarrhea. Eur J Intern Med. 2008;19:181-6.
136. Fernandez-Banares F, Salas A, Esteve M, et al. Collagenous and lymphocytic colitis. Evaluation of clinical and histological features, response to treatment, and long-term follow-up. Am J Gastroenterol. 2003;98:340-7.
137. Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: A common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut. 2004;53:346-50.
138. Fine KD, Do K, Schulte K, et al. High prevalence of celiac sprue-like HLA-DQ genes and enteropathy in patients with the microscopic colitis syndrome. Am J Gastroenterol. 2000;95:1974-82.
139. Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830-8.
140. Fine KD, Lee EL, Meyer RL. Colonic histopathology in untreated celiac sprue or refractory sprue: Is it lymphocytic colitis or colonic lymphocytosis? Hum Pathol. 1998;29:1433-40.
141. Lee E, Schiller LR, Fordtran JS. Quantification of colonic lamina propria cells by means of a morphometric point-counting method. Gastroenterology. 1988;94:409-18.
142. Burgel N, Bojarski C, Mankertz J, et al. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433-43.
143. Ung KA, Kilander A, Nilsson O, Abrahamsson H. Long-term course in collagenous colitis and the impact of bile acid malabsorption and bile acid sequestrants on histopathology and clinical features. Scand J Gastroenterol. 2001;36:601-9.
144. Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database Syst Rev 2008; (2):CD003575.
145. Chande N, McDonald JW, MacDonald JK. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev 2008; (2):CD006096.
146. Bonner GF, Petras RE, Cheong DM, et al. Short- and long-term follow-up of treatment for lymphocytic and collagenous colitis. Inflamm Bowel Dis. 2000;6:85-91.
147. Potoczna N, Harfmann S, Steffen R, et al. Bowel habits after bariatric surgery. Obes Surg. 2008;18:1287-96.
148. Oelschlager BK, Quiroga E, Parra JD, et al. Long-term outcomes after laparoscopic surgery. Am J Gastroenterol. 2008;103:280-7.
149. Tack J. Gastric motor disorders. Best Pract Res Clin Gastroenterol. 2007;21:633-44.
150. Penning C, Vecht J, Masclee AA. Efficacy of depot long-acting release octreotide in severe dumping syndrome. Aliment Pharmacol Ther. 2005;22:963-9.
151. Misiakos EP, Macheras A, Kapetanakis T, Liakakos T. Short bowel syndrome: Current medical and surgical trends. J Clin Gastroenterol. 2007;41:5-18.
152. Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130(Suppl 1):S93-9.
153. Westergaard H. Bile acid malabsorption. Curr Treat Options Gastroenterol. 2007;10:28-33.
154. DuPont AW, Sellin JH. Ileostomy diarrhea. Curr Treat Options Gastroenterol. 2006;9:39-48.
155. Pardi DS, Sandborn WJ. Systematic review: The management of pouchitis. Aliment Pharmacol Ther. 2006;23:1087-96.
156. Fisher M, Spillas DC, Tong LK. Diarrhoea after laparoscopic cholecystectomy: Incidence and main determinants. A N Z J Surg. 2008;78:482-6.
157. Sauter GH, Moussavian AC, Meyer G, et al. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732-5.
158. Robb BW, Matthews JB. Bile salt diarrhea. Curr Gastroenterol Rep. 2005;7:379-83.
159. Hofmann AF, Hagey LR. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461-83.
160. Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J Clin Invest. 1997;99:1880-7.
161. Montagnani M, Love MW, Rossel P, et al. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scan J Gastroenterol. 2001;36:1077-80.
162. Balesaria S, Pell RJ, Abbott LJ, et al. Exploring possible mechanisms for primary bile acid malabsorption: Evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhea. Eur J Gastroenterol Hepatol. 2008;20:413-22.
163. Sadik R, Abrahamsson H, Ung KA, Stotzer PO. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99:711-18.
164. McFarland LV. Antibiotic-associated diarrhea: Epidemiology, trends and treatment. Future Microbiol. 2008;3:563-78.
165. Clausen MR, Bonnen H, Tvede M, Mortensen PB. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology. 1991;101:1497-504.
166. DuPont HL, Garey K, Caeiro JP, Jiang ZD. New advances in Clostridium difficile infection: Changing epidemiology, diagnosis, treatment and control. Curr Opin Infect Dis. 2008;21:500-7.
167. Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-95.
168. Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: A systematic review. Lancet Infect Dis. 2008;8:777-84.
169. Luft VC, Beghetto MG, de Mello ED, Polanczyk CA. Role of enteral nutrition in the incidence of diarrhea among hospitalized adult patients. Nutrition. 2008;24:528-35.
170. Sreenarasimhaiah J. Diagnosis and management of ischemic colitis. Curr Gastroenterol Rep. 2005;7:421-6.
171. Roach M, Christie JA. Fecal incontinence in the elderly. Geriatrics. 2008;63:13-22.
172. Savino AC, Fordtran JS. Factitious disease: Clinical lessons from case studies at Baylor University Medical Center. Proc Bayl Univ Med Cent. 2006;19:195-208.
173. Phillips SF. Surreptitious laxative abuse: Keep it in mind. Semin Gastrointest Dis. 1999;10:132-7.
174. Bytzer P, Stokholm M, Andersen I, et al. Prevalence of surreptitious laxative abuse in patients with diarrhoea of uncertain origin: A cost-benefit analysis of a screening procedure. Gut. 1989;30:1379-84.
175. Slugg PH, Carey WD. Clinical features and follow-up of surreptitious laxative users. Cleve Clin Q. 1984;51:167-71.
176. Osterholm MT, MacDonald KL, White KE, et al. An outbreak of a newly recognized chronic diarrhea syndrome associated with raw milk consumption. JAMA. 1986;256:484-90.
177. Parsonnet J, Trock SC, Bopp CA, et al. Chronic diarrhea associated with drinking untreated water. Ann Intern Med. 1989;110:985-91.
178. Mintz ED, Weber JT, Guris D, et al. An outbreak of Brainerd diarrhea among travelers to the Galapagos Islands. J Infect Dis. 1998;177:1041-5.
179. Kimura AC, Mead P, Walsh B, et al. A large outbreak of Brainerd diarrhea associated with a restaurant in the Red River Valley, Texas. Clin Infect Dis. 2006;43:55-61.
180. Vugia DJ, Abbott S, Mintz ED, et al. A restaurant-associated outbreak of Brainerd diarrhea in California. Clin Infect Dis. 2006;43:62-4.