Chapter 11 What makes phytomedicines unique?
Also, there is a continuum between products which are a (health) food and those which are a potent herbal medicine (called the food–medicine interface). In the area of food research, considerable efforts have been put into understanding the benefits of a ‘balanced diet’ and thus a diet which is composed of a complex combination of mixtures. However, in medicines research in general and specifically in research on herbal medicines systematic research has only been developed since the start of this century (Williamson 2001).
Generally, highly toxic drugs are used as single entities since the dose needs to be very precise, but the natural mixture found in a plant extract may have benefits conferred by some form of interaction between the components. Conversely, there may be toxic ingredients present that do not contribute to the therapeutic efficacy and which are, therefore, undesirable. If the toxic component is also one of the active principles, then the usual assessment of risk:benefit profile applies, but quite often the toxic compound serves no useful purpose and must either be removed or some form of limit test applied. Some examples are discussed in Chapter 10.
Synergistic, multi-factorial and polyvalent effects
• Several compounds affecting a single target, either directly or indirectly: this may include synergy, the metabolism of one active altered by the presence of others in the extract, or the bioavailability of a component changed by the presence of another. These are true interactions and may include pharmacokinetic and pharmacodynamic interactions
• A single compound affecting multiple targets: this is not an interaction between components of a mixture of course, but it helps to explain why a particular herbal medicine (or any drug, for that matter) can be used for different purposes
• Multiple compounds affecting multiple related targets: these are not necessarily interactions, but the result of a number of constituents acting in different ways; however, interactions could certainly also be taking place. There may be cases where the effect of one compound cancels out the effect of another by antagonism and this would not be known unless fractionation of an extract before testing had been carried out.
While here we focus on the unique characteristics of complex mixtures, novel concepts and approaches in pharmacology show ‘the other side of the coin’. Network pharmacology and systems biology are used to represent the different types of relationships between biological entities such as genes, proteins, chemical compounds, and transcription factors. In essence, the single drug single target view is giving way to a more complex understanding of networks within an organism affected by a medicine and the complexity of the intervention (Pujol et al 2010).
Mechanisms of interaction
Interactions can also be classified as:
• pharmacodynamic: where the effects of one drug are altered or added to by the presence of another at the site of action (so-called ‘pharmacological’ interactions)
• pharmacokinetic: processes involving absorption, distribution, metabolism and excretion.
An example of both is provided by Ayurveda, where an ancient combination formula known as ‘Trikatu’ contains black pepper (Piper longum). Pepper contains the alkaloid piperine, which has many useful pharmacological activities (anti-inflammatory, anti-allergic, digestive), which add to the desired effects of the other ingredients in the formula. These could be considered to be pharmacodynamic interactions. However, piperine is also known to increase the bioavailability of a number of drugs by enhancing absorption. Piperine modulates the multidrug transporter P-glycoprotein and influences the efflux of other compounds, both herbal and synthetic drugs, from cells (Najar et al 2010). P-gp (the ‘permeability protein’) is a trans-membrane ATP-binding cassette transporter or pump, which transports various molecules across intra- and extra-cellular membranes, especially in the gut, kidney, liver and blood–brain barrier. It regulates the distribution and bioavailability of many drugs by blocking or facilitating entry into cells. Thus, increased intestinal expression of P-gp will reduce absorption of drugs that are substrates, reducing bioavailability, lowering drug plasma concentrations, and, therefore, reducing efficacy. Inhibition of P-gp will conversely increase plasma concentrations, which may lead to enhanced efficacy, but also, possibly, drug toxicity. Many other natural products are also known to modulate P-gp, and these include curcumin, quercetin, hesperidin and epigallocatechin gallate. They affect the bioavailability of not only other herbal components, but also ‘conventional’ drugs, and this is an untapped therapeutic area which remains to be explored. Inhibition of P-gp by non-toxic, common elements of the diet may even help to delay or inhibit resistance to, for example, antibiotics and anti-cancer drugs.
Measuring synergy
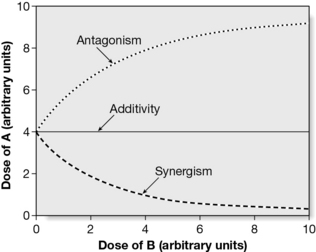
The general understanding of synergy is that it is an effect seen by a combination of substances that is greater than would have been expected from a consideration of individual contributions. It does, however, have a precise mathematical definition, which may depend on the method used to prove it. Such methods have been discussed extensively (Berenbaum 1989, Spelman et al 2006, Wagner 2009, Wagner and Ulrich-Merzenich 2009, Williamson 2001) and the isobole method is generally accepted as the method of choice. It is independent of the mechanism of action and applies under most conditions; it makes no assumptions as to the behaviour of each agent and is, therefore, applicable to multiple component mixtures. An isobole is an ‘iso-effect’ curve, in which a combination of ingredients (da,db) is represented by a point on a graph, the axes of which are the dose axes of the individual agents (Da and Db). If there is no interaction, the isobole (the line joining the points representing the combination to those on the dose axes representing the individual doses with the same effect as the combination) will be a straight line. If synergy is present, the dose of the combination needed to produce the same effect will be less than that for the individual components and the curve will be ‘concave up’. The opposite applies for antagonism, which produces a ‘concave down’ isobole, as shown in Fig. 11.1.
Multiple pharmacological effects in a single plant
Ispaghula, Plantago ovata
Ispaghula, or psyllium husk, is (paradoxically) effective in both constipation and diarrhoea. The laxative effect is achieved principally through its fibre content, but the reason why it is more effective in chronic constipation than other types of fibre may be due to the fact that the seed also contains constituents with gut stimulatory properties, mediated partly through cholinergic activation, which is likely to enhance the laxative effect. Interestingly, it also contains gut inhibitory constituents, which could provide a scientific explanation for the traditional use of ispaghula in diarrhoea. In addition to gut stimulatory and inhibitory constituents, ispaghula also contains anti-amoebic constituents explaining its traditional use in amoebic dysentery, thus demonstrating multiple effects, some supporting and some opposing a particular activity, in one medicinal plant (Gilani and Rahman 2005).
Liquorice, Glycyrrhiza glabra
In Traditional Chinese Medicine (TCM), liquorice (Glycyrrhiza glabra) is added to many formulae as a synergistic agent, to enhance activity and detoxify, and it demonstrates a number of instances of synergism not only with its own constituents, but also with other herbs. For example, blood levels of glycyrrhizin are lower, due to reduced absorption, if it is taken as part of an extract or mixture rather than as an isolated compound. Whole extracts of liquorice inhibit angiogenesis, granuloma formation and fluid exudation in inflammation, as does isoliquiritin, whereas glycyrrhizin and glycyrrhetinic acid tend to promote angiogenesis (reviewed in Williamson 2001). The mechanism of the antiinflammatory effect has recently been further investigated on both cyclooxygenase (COX) and lipoxygenase (LOX) products, using models of lipopolysaccharide (LPS)-induced prostaglandin E2 (PGE2), calcimycin-induced thromboxane (TXB2), and leukotriene (LTB4) release, in murine macrophages and human neutrophil cells. The whole plant extract, and isolated glabridin, inhibited the release of PGE2 and TXB2 (COX products) and LTB4 (a LOX product), while isoliquiritigenin exerted an inhibitory effect only against COX products and failed to suppress LOX products (Chandrasekaran et al 2010). Glycyrrhizin failed to exhibit any inhibitory effects on both COX and LOX products in this model, although it has previously shown anti-inflammatory activity by inhibiting the generation of reactive oxygen species by neutrophils. However, glycyrrhizin has also recently been shown to attenuate LPS-induced acute lung injury by inhibiting cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase expression. In this case, the elevated concentrations of pro-inflammatory cytokines interleukin 1β and tumour necrosis factor (TNF)-α in bronchoalveolar fluid, caused by LPS administration, were significantly inhibited by glycyrrhizin pre-treatment, as was the concentration of nitric oxide (NO) in lung tissues (Ni et al 2011). These examples illustrate the complexity of the effects involved in even a single herbal extract, and it may be that this is found much more commonly than has been previously thought. Liquorice has many other beneficial effects, and is still the subject of intensive research for its potential use as an anticancer, antiviral, smooth-muscle relaxant, antipsoriatic and even memory enhancing agent, and it is likely that even more mechanisms and interactions will be discovered.
Clinical examples of synergy and polyvalent action
Ginkgo, Ginkgo biloba
The ginkgolides are known to be platelet-activating factor (PAF) antagonists, a mechanism of anti-inflammatory activity, and a synergistic interaction between ginkgolides A and B has been shown using an in vitro platelet aggregation test. A positive interaction was shown by an isobole curve using a 50% mixture of the two. Furthermore, the presence of the other ginkgolides and the ginkgoflavones also had an effect on the overall activity: a mixture of ginkgolides A, B and C, at a dose of 100–240 mg, generated a PAF-antagonizing effect in humans which was equivalent to a dose of 120 mg of a standardized ginkgo extract containing only 6–7 mg of ginkgolides, together with bilobalide and flavonol glycosides (Wagner 1999). However, the ginkgo flavones are also anti-inflammatory, the combination being considered additive and possibly synergistic in effect as well as increasing blood circulation to the brain, and a total ginkgo extract acts as an antioxidant activity in brain preparations. Clinical studies have shown ginkgo to be effective in improving cognitive function as well as the early stages of dementia; the preparation used being a total extract not just the flavonoids. This suggests polyvalent as well as synergistic activity
Ginkgo, taken in combination with other herbs, also shows synergistic-like interactions: a double-blind, cross-over trial using 20 young, healthy volunteers tested ginseng (Panax ginseng) with ginkgo extract and found it to be more effective in improving cognitive function than either alone. Cognitive performance was assessed in three studies, using ‘serial threes’ or ‘serial sevens’, which are arithmetic tests involving subtracting from a random number. Although single treatments showed improvements, the combination produced a significant and sustained improvement, especially in the ‘serial sevens’ test (Scholey and Kennedy 2002).
Cannabis, Cannabis sativa
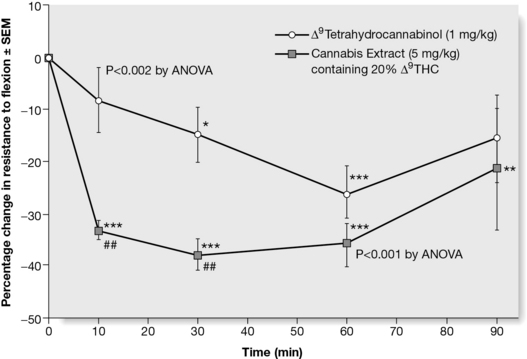
Cannabis has potential as a therapeutic agent in chronic conditions such as rheumatoid arthritis, HIV infection and multiple sclerosis. Documented reports of interactions within the herb include the fact that levels of tetrahydrocannabinol (THC) in the brain can be elevated by cannabidiol, and it is known that THC taken alone can induce anxiety, which can be attenuated by the presence of cannabidiol in the herb. There is additional evidence to show that the effect of the herb is both qualitatively and quantitatively different to that of isolated THC (Wilkinson et al 2003). The herb extract is a better antispastic agent than THC alone, as measured in an immunogenic model of multiple sclerosis (Fig. 11.2). The graph shows that the extract acts more rapidly than isolated THC. The rest of the extract has no effect in this system, suggesting that in this case, the effect of THC is enhanced by the presence of other compounds in the extract, but there is no additive effect.
Establishing the active pharmaceutical ingredient (API) of a product
Other reasons for not isolating individual constituents
• Unstable constituents: Sometimes the presence of all of the components isolated from the plant material, which may include antioxidants for example, may ‘protect’ the actives from decomposition. Examples of herbs in which this is thought to take place include valerian (Valeriana spp.), garlic (Allium sativum), ginger (Zingiber officinalis) and hops (Humulus lupulus).
• Unknown active constituents: For some herbs, even those which are widely used, the actives may not have been completely identified. This is in fact very common, and as can be seen from the example of liquorice discussed earlier, even for a herb with a very long history, new effects for old compounds are continually being discovered. Other examples include raspberry leaf (Rubus idaeus), chasteberry (Vitex agnus castus), passion flower (Passiflora incarnata), hawthorn (Crataegus spp.) and many others.
• A range of actives identified: It is unusual for a plant to contain only one active constituent. Even for cannabis, where there is only one significant psychoactive ingredient, tetrahydrocannabinol, other constituents of the herb may enhance its activity, as shown in Fig. 11.2. Other examples include Echinacea, devil’s claw (Harpagophytum procumbens), artichoke (Cynara scolymus), St John’s wort (Hypericum perforatum), liquorice (Glycyrrhiza glabra) and many others.
Of course, more than one of these situations may apply within a single herb.
Metabolomics and the future of research on phytomedicines
Solving such challenges posed by complex mixtures has preoccupied scientists for decades. Since the early 1990s hyphenated techniques have allowed scientists to identify many of the metabolites in an extract in a relatively short period of time. A range of novel approaches, and, most importantly, metabolomics, now offer unique opportunities for understanding such complex mixtures, and their effects. Metabolomics has been defined as ‘both the qualitative and quantitative analysis of all metabolites in an organism’, or: the metabolome represents the collection of all metabolites in a biological organism, which are the end products of its gene expression (Daviss 2005). In essence, this allows an understanding of the complete profile of extractable metabolites from an organism or in this case a phytomedicine. It is intricately linked with multivariate data analysis – a technique for analysing data sets with a large number of variables. In case of NMR of crude extracts, patterns can be visualized and interpreted. This can be done in a comparative manner distinguishing, for example, between differences between relatively similar extracts or it can be linked with, for example, a specific (generally in vitro) biological activity. Ultimately this enables the construction of a complex database of the metabolome (cf. Verpoorte et al 2007). For example, Harris et al (2007) developed a method for the analysis of Vaccinium angustifolium Ait. (lowbush blueberry), used as an over-the-counter treatment of diabetic symptoms. Using such an approach™ that focused on phenolic metabolites, the major metabolites found in the ethanol extracts of leaf, stem, root and fruit were analysed and compared. This allowed a detailed understanding of the complex characteristics of these blueberry extracts.
Berenbaum M. What is synergy? Pharmacol. Rev.. 1989;41:93-141.
Chandrasekaran C.V., Deepak H.B., Thiyagarajan P., Kathiresan S., Sangli G.K., Deepak M., et al. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine. 2010. [Epub ahead of print 2010 Sep 21]
Daviss B. Growing pains for metabolomics. The Scientist. 2005;19:25-28.
Gilani A.H., Rahman A.U. Trends in ethnopharmacology. J. Ethnopharmacol.. 2005;100:43-49.
Harris C.S., Burt A.J., Saleem A., et al. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem. Anal.. 2007;18:161-169.
Najar I.A., Sachin B.S., Sharma S.C., Satti N.K., Suri K.A., Johri R.K. Modulation of P-glycoprotein ATPase activity by some phytoconstituents. Phytother. Res.. 2010;24:454-458.
Ni Y.F., Kuai J.K., Lu Z.F., et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J. Surg. Res. 2011;165:e29-e35. [Epub 2010 Nov 10]
Scholey A.B., Kennedy D.O. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum. Psychopharmacol.. 2002;17:35-44.
Spelman K., Duke J.A., Bogenschutz-Godwin M.J. The synergy principle at work with plants, pathogens, insects, herbivores and humans. In: Cseke L.J., Kirakosyan A., Kaufman P.B., Warber S., Duke J.A., Brielman H.L., editors. Natural products and plants. New York: CRC Press, 2006.
Pujol A., Mosca R., Farrés J., Aloy P. Unveiling the role of network and systems biology in drug discovery – review article. Trends Pharmacol. Sci.. 2010;31:115-123.
Verpoorte R., Choi Y.H., Kim H.K. NMR-based metabolomics at work in phytochemistry. Phytochem. Rev.. 2007;6:3-14.
Wagner H. New targets in the phytopharmacology of plants. In: Herbal medicine, a concise overview for healthcare professionals. Oxford: Butterworth-Heinemann; 1999:34-42.
Wagner H. Synergy research: a new approach to evaluating the efficacy of herbal mono-drug extracts and their combinations. Nat. Prod. Commun.. 2009;4:303-304.
Wagner H., Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97-110.
Wilkinson J.D., Whalley B.J., Baker D., et al. Medicinal cannabis: is Δ-9 THC responsible for all its effects? J. Pharm. Pharmacol.. 2003;55:1687-1694.
Williamson E.M. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401-409.
Schmid B., Lüdtke R., Selbmann H.K., et al. Efficacy and tolerability of a standardised willow bark extract in patients with osteoarthritis: a randomised, placebo-controlled, double-blind trial. Phytother. Res.. 2001;15:344-350.
Ulrich-Merzenich G., Panek D., Zeitler H., Wagner H., Vetter H. New perspectives for synergy research with the “omic”-technologies. Phytomedicine. 2009;16:495-508.