The Prevalence of Self-Reported Sensitive Skin in Large Cohorts Identified by Surveys
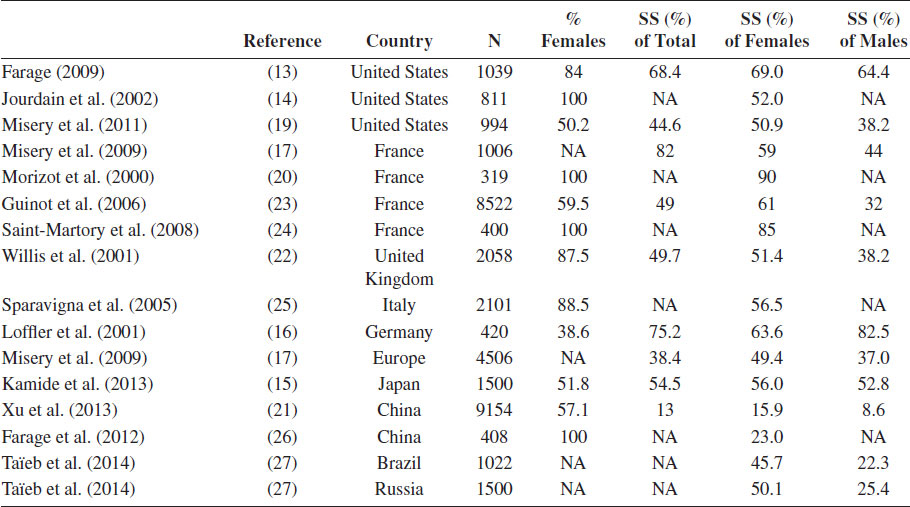
Note: Percentages are either extracted or calculated from the result sections of the original articles. NA, not applicable; SS, subjects with sensitive skin.
Clinical Manifestation
There is no consensus on the symptom profile of sensitive skin. People assessing their skin as sensitive experience unpleasant sensations of the skin, particularly characterized by stinging, burning, or itching sensations (20,24,28). Remarkably, visible signs of skin irritation, as erythema or skin dryness, are absent in many individuals (20,22,24,29,30). Since the symptoms reported by subjects are rather nonspecific, the differentiation from or relationship with dermatological disorders such as irritant contact dermatitis, allergic contact dermatitis, rosacea, physical urticaria, and dermographism, xerosis, (atopic) dermatitis, and photodermatoses may be challenging. Deeper understanding of sensitive skin will enable placing this condition in the context of skin pathology.
Factors impeding the detection and definition of sensitive skin are the frequent absence of clinical signs and subjective character of symptoms (20,31,32) and the heterogeneity thereof (20).
Eliciting Factors
Sensitive skin perceptions are elicited by exogenous and endogenous factors that usually have a considerably low impact on individuals and frequently do not cause skin irritation (13,18,22,23,25).
In order to define the symptom profile and to address specific exogenous and endogenous factors which may elicit skin reactions, nonuniform comprehensive questionnaires were conducted in different populations (18,22,23), showing various risk factors and triggers. Among these factors, the important triggers are cosmetics and soaps (20,21,24–26), environmental climate [temperature changes (14,15,20,21), heat (14,15,20,24,25), cold (14,15,20,25), and dampness (21)], sun exposure (14,21,24), stress or emotions (20,24,25), wind (14,15,25), and friction from clothes (24).
Having a dry or greasy skin (17,21), a history of childhood atopic dermatitis (AD) (17,21,22), being female (21), and having a fair skin type (21) are important host factors increasing the risk of having sensitive skin (21).
Subject Selection in Clinical Studies
To enable conduction of clinical studies in a population with sensitive skin and with nonsensitive skin, assumptions on the definition and inclusion criteria have to be made. Erroneous selection of subjects may possibly lead to formulating wrong conclusions on pathomechanisms. Moreover, since symptoms might be subclinical in situations in which the skin is not challenged, differences in physiology may be minute and difficult to detect, and therefore careful selection of subjects with sensitive skin is of high importance.
A performed systematic literature review on objective measurements on sensitive skin identified studies including subjects by means of perception, for example, burning, stinging, itching, and general skin discomfort (33). This self-reported skin sensitivity was determined (i) by sensory skin reactions following application of specific stimuli or (ii) by questionnaire. Subsequently, the relation of the group characteristics of the selected populations and the selected study population with objective measurements were studied, both at baseline and following specific stimulations.
Subject Selection and Provocation by Stimuli
Many research groups select sensitive subjects and nonsensitive subjects as a control group by evaluation of sensory discomfort after application of a chemical agent on the skin. Lactic acid is one of the most frequently used agents (34–43), and the lactic acid stinging test (LAST) was previously even proposed as the best diagnostic test available for sensitive skin (44). LAST identifies “stingers”—subjects perceiving sensations of stinging after application of lactic acid on the nasolabial fold—which are assumed to correlate with sensitive skin. A detailed evaluation of studies including subjects by self-perceived sensitive skin and using LAST as a provocative method to enhance skin reactions shows that in subjects with sensitive skin, higher stinging scores are observed (45), time to onset and peaking of stinging response are shorter (45), and the overall stinging scores are higher (46,47). However, the test is subjective in nature and lacks sensitivity, 59.9–80% (27,45,48) of subjects with sensitive skin report a positive reaction to LAST, and specificity, 66.7% of nonsensitive subjects experience this reaction (48) for sensitive skin. Thus, lactic acid mainly elicits some, but not all key symptoms in the clinical profile of sensitive skin, predominantly skin stinging and slightly burning. This might be the reason for the insufficient sensitivity. In addition to this, studies have shown that a positive response to one substance does not predict a reaction to another substance (40,45,49–52), questioning the validity of using a single substance to test skin sensitivity. Next to using lactic acid as a challenge, studies of sensitive skin reactions and sensations have been performed using a wide range of chemical agents. They include but are not limited to sodium lauryl sulfate (SLS), capsaicin, menthol, benzoic acid, trans-cinnamic acid, octane, cumene, methyl nicotinate and acetyl-β-methylcholine chloride (vasodilators), ethanol, allergens, occlusion, cocamidopropyl betaine and benzalkonium chloride (surfactants), and balsam of Peru. Furthermore, material interactions (53) and electrical provocations (54) followed by noninvasive measurements of the biophysical parameters of the skin have been appraised in people reporting perceptions of sensitive skin to quantify sensory irritation and to reveal the underlying mechanisms of sensitive skin. Many provocations used in experiments resulted in sensory skin reactions, but, again, these might not be specifically addressing the sensitive skin.
Subject Selection by Questionnaire
An increasing number of studies select subjects by means of self-perceived sensitive skin inventoried by questionnaires, using different definitions of sensitive skin, as explained in the “Epidemiology” section. Some research groups included solely the question, “Do you have a sensitive skin, yes or no?” In one study, skin sensitivity was scored on a labeled magnitude scale, permitting both semantic descriptors and a continuum of intensity rankings to compare individuals (55). In contrast, other research groups conducted extensive nonuniform questionnaires addressing sensory, objective symptoms and additionally encompassing numerous potentially eliciting factors by means of inclusion of subjects with sensitive skin and with nonsensitive skin.
A ten-item questionnaire was developed by Misery et al. (56). With this questionnaire, researchers made an attempt to establish standardized patient questionnaires, which might allow scoring in a reproducible manner on a “sensitive scale” and might be suited for monitoring the evolution of the skin condition (56). However, only a selected population with sensitive skin diagnosed in a private clinic was included in this study, limiting the application of the developed scale as a diagnostic tool for selecting sensitive and nonsensitive subjects for research purposes.
Pathomechanisms and Objective Readouts
Kligman et al. (57) used a novel nomenclature for sensitive skin by proposing different subtypes of sensitive skin defined by clinical presentation or possible underlying etiology as follows: (i) subjective irritation refers to an irritant response without visible clinical signs; (ii) neurosensory irritation signifies neutrally mediated responses such as itching, stinging, burning, and tightness; (iii) chemosensory relates to sensory responses induced by chemicals in contrast to physical, mechanical, and environmental factors; and (iv) psychophysical irritation implies a psychological component. Willis et al. (22) described several clinical forms depending on the intensity of clinical symptoms: (i) Very sensitive skin is dry or fatty and bitterly reacting to both exogenous factors, that is, cosmetic products and environmental factors, and endogenous features. The clinical symptoms are acute and permanent, and both factors trigger determining psychological reactions. (ii) Environmentally sensitive skin is skin that is often clear, dry, and thin and essentially reactive to environmental factors, that is, heat and rapid temperature changes, with frequent bouts of flushing. (iii) Cosmetically sensitive skin is essentially reactive to cosmetics. This intolerance is lighter and often limited to some identifiable cosmetic products. Farage and Maibach also proposed a heterogeneous phenomenon with multiple etiologic aspects (58); Richters et al. (33) critically appraised the evidence of objective measurements at baseline and following skin provocations. This research group chose an approach by including studies which had selected sensitive subjects based on perception. The translation from skin perceptions to objective measurements was made, and it was shown that the strongest evidence exists for the role of an impaired skin barrier in sensitive skin. This impaired barrier might cause sensory perceptions, and vascular reactivity might develop subsequently.
However, strong evidence and consistency on pathways underlying sensitive skin is still lacking at this moment.
Skin Barrier Impairment
Many research groups focused on the skin barrier function and measured transepidermal water loss (TEWL) as a parameter of skin barrier function. In some studies, a higher TEWL was observed in the unchallenged skin of subjects with sensitive skin compared to that in subjects with nonsensitive skin (16,29,37,59). In stingers, a higher TEWL was also measured compared to that in nonstingers (34,37,38,41,52). Additionally, a higher TEWL at baseline seems to correlate with stronger TEWL increase following provocation (34): plastic occlusion resulted in significantly longer evaporation halflife time in subjects with sensitive skin (59), and a trend to increased recovery time of TEWL following SLS stimulation was also observed. A low hydration of the stratum corneum is also associated with an impaired skin barrier. A significantly lower stratum corneum hydration in facial areas in subjects with sensitive skin (47) and in stingers (34) and a nonsignificantly lower stratum corneum hydration in facial areas in subjects with sensitive skin were found (37). Challenging the skin chemically resulted in a significantly lower stratum corneum hydration in stingers compared to that in nonstingers (41). Clinically, a dryer skin is also observed (25). In contrast to these findings, other studies found no difference in stratum corneum hydration between subjects with sensitive skin and subjects with nonsensitive skin (16,29) or between stingers and nonstingers (34,37,41) either challenged or unchallenged. The skin of stingers with self-reported sensitive skin appears to be rougher since Fast Fourier transform evaluation of tapes after tape-stripping the stratum corneum in 243 stingers reveals lower contents of cells (42). This implies a more irregular, rougher, and possibly less hydrated skin as well as impaired intercorneocyte adhesion. Objectively measured skin elasticity and distensibility show no correlation with hypersensitivity (43).
The literature is insufficient and inconclusive with regard to the role of sebum secretion in sensitive skin perceptions. No differences were observed with respect to sebum and surface pH between sensitive and nonsensitive subjects (43,47) and between stingers and nonstingers (34). However, in one study, significantly lower sebum and higher surface pH were measured in subjects with sensitive skin compared to those in subjects with nonsensitive skin (9).
Hypersensitivity: Neurologic Aspects
Sensitive skin has a predominantly subjective character, as stinging, burning, itching, and sensations of tightness are reported, implying that the neurons of subjects with a sensitive skin dysfunction easily respond to mild stimuli. Stander et al. also addressed the role of the neural system and neuromediators in skin sensitivity (60). Differences in pain perceptions were observed in subjects with sensitive skin by functional magnetic resonance imaging (46). Skin discomfort induced by lactic acid leads to the activation of different parts of the cerebral cortex. Quatresooz et al. also measured the electrical current perception threshold (CPT) in subjects with reactive and nonreactive skin and concluded that some subjects with reactive skin and a lower CPT showed a higher density of mast cells in the dermis (54). This is in line with the findings of Kim et al., who detected a lower CPT to 5 Hz electric current on the forearm in subjects with sensitive skin compared to CPT in subjects with nonsensitive skin (38). Five hertz is a selective stimulator of the C fibers of sensory nerves (38). C fibers are unmyelinated fibers playing a role in the perception of pain, itch, and warmth. Additionally, an inverse correlation was shown between the clinical detection thresholds of capsaicin and sensitive skin indexed by questionnaire (61). When the skin comes in contact with capsaicin, noxious heat, or low pH, transient receptor potential vanilloid 1 is activated (62,63) and results in nociceptor-mediated burning pain. An overexpression of this receptor could play a key role in the pathomechanism of sensitive skin, as inhibition results in reduced burning sensation following capsaicin application (64).
Vascular Responses
Next to neurosensory signs, objective signs such as skin redness are also frequently reported by individuals with sensitive skin, implying a key role for vascular or even inflammatory responses in the pathomechanism of sensitive skin. As a readout for erythema visual assessment (35,37,40,47), colorimetry and spectrophotometry (29,35,38,41,48,65) and laser Doppler flowmetry are used (16,29,37–39,41,65). At baseline, in one study, significant differences in skin redness were found; a lower a* value was found in self-reported sensitive skin compared to that in nonsensitive skin (29). The literature is inconclusive on differences in skin redness between sensitive and nonsensitive subjects following stimulation of the skin. With respect to endothelium markers (CD31), no different quantity is found between subjects with reactive skin and low CPT and subjects with less reactive skin and high CPT (54). Lactic acid, SLS, capsaicin, and cumene application did not result in higher erythema responses in subjects with sensitive skin compared to subjects with nonsensitive skin (29,38,41). In contrast, octane, acetyl-β-methylcholine chloride, methyl nicotinate, and allergen patch testing did result in relatively stronger erythema responses in sensitive subjects (1,41,47). A proposed explanation for vascular reactivity is that when the skin barrier function is impaired in subjects with sensitive skin, subsequently higher concentrations of chemical agents can be reached, eliciting vascular responses more strongly (66).
Allergic Predisposition
AD is considered to be a predisposing factor for the development of sensitive skin, since more individuals with sensitive skin report to have atopy compared to nonsensitive subjects (17,22,67). Stinging is also frequently reported in AD (67). Subjects with AD are more prone to develop skin irritation following application of a patch with SLS (68–72).
However, sensitive subjects selected by LAST seem not to develop stronger objective skin irritation responses following the application of SLS (40,49). A correlation was only demonstrated by Lammintausta et al. showing stronger increase in laser Doppler blood flow (51). Histologically, increased numbers of mast cells are found in subjects with reactive skin and a low CPT (54). This, in combination with an impaired skin barrier function, implies the potential of sensitive skin pathophysiology with that of AD, which should be explored in future studies.
Limitations
Many clinical studies are observational studies, mainly or solely comprising the female gender. Relatively young populations are often included, with a mean age younger than 40 years (14,29,34,37–40,42,47,48, 54,65,73). Many studies unfortunately lack statistical power, since only small populations are included and potential selection bias, confounders, and information bias are frequently not addressed. Many authors do not specify age, ethnicity, skin type, or concomitant skin diseases. Skin diseases are extraneous variables affecting skin sensitivity, especially variables such as skin barrier function and clinical inflammatory parameters. Abnormal physiological pathways such as, impaired skin barrier function and increased inflammatory responses in AD or psoriasis should be excluded from explorative studies on the pathomechanism of sensitive skin. The existing heterogeneity of study designs reported so far complicates the comparison of results; various selection methods have been used which are different natures, and many provocations have been performed. In addition, different body sites are stimulated (40), which are not comparable with respect to skin sensitivity; different time points and devices have been applied; and studies have been performed under various conditions with respect to environmental climate.
Future Perspectives
Since sensitive skin is highly prevalent in the Western world and has an impact on the quality of life, understanding of the physiological reality and managing sensitive skin is of high importance. Approaches focused on identifying pathomechanisms causing perceptions of skin discomfort designated as sensitive skin could enable an evidence-based diagnosis and rational interventions for patients with sensitive skin, implementing personalized medicine, highly aspired in these modern times. New approaches in this research field might encompass highly reproducible in vivo skin models in order to enable studying skin reactions in subjects with sensitive skin. By eliciting skin responses, the mechanisms underlying sensitive skin might be enhanced and become measurable; moreover, when elicited in a standardized way, skin reactions in subjects with different conditions will be comparable.
Processes set off may be studied in a dynamic fashion by exploring parameters at several moments in time following the stimulus. In addition, a better understanding of the morphology and physiology of reactions might be established by exploration of different potential mechanisms applying different perspectives: clinical, biophysical, and immunohistochemical. These methodologies could be complementary and could enable decisively answering questions on the contribution of physiological impairments, and a suitable definition can be established. The hypothesis of multiple etiologies underlying sensitive skin would not be farfetched as also proposed by Farage and Maibach (58). An impaired skin barrier function is strongly hypothesized in literature to be a key player in the pathomechanism of sensitive skin. However, consistent evidence is lacking. Immunohistochemistry could function as a reference in order to validate or investigate in detail the stratum corneum. This enables further research by noninvasive analysis of the skin barrier, for example. Focus should be given to the quantification of components of the stratum corneum, encompassing ceramides, lipid composition, and natural moisturizing factor. Advanced biophysical techniques, such as confocal Raman spectroscopy, can accurately quantify these components in a noninvasive way. However, of note, differences in biophysical measurements do not ensure differences in skin sensitivity since these parameters have high interindividual variability in the general population. Additional parameters measuring the same process could strengthen specific pathways and might explain previously described inconsistencies in study outcomes.
Next to a different perspective on measurements, clinical studies require new methods on the selection of subjects. By including “extreme subjects” with subjectively severe sensitive skin and subjects with subjectively no complaints of sensitive skin, potential differences might become highlighted. It would be interesting when the selection of subjects would become more uniform and comparable. Quantification of perceptions of the sensitive subject could enable the comparison. Translation of these perceptions to biophysical properties and detailed histological data might be the best approach to measuring this dominantly subjective skin condition. One of the criteria which should be included in the selection tool is skin reaction to multiple stimuli, to prevent measuring irritant or allergic contact dermatitis or heat intolerance, for example. Perception-based selection of subjects through a questionnaire spanning a range of provocations, including those of chemical, mechanical, and thermal origin and including multiple signs and symptoms, might be a more valid and applicable selection tool than selection by reaction to one chemical agent skin provocation.
Currently, scientists are still being challenged by this relatively unexplored phenomenon. An integral approach is recommended to unravel the sensitive skin phenomenon, taking into the picture the clinical, biophysical, and histological hallmarks of this condition. A new step in diagnosis and treatment of the condition could be taken only by conducting cross-disciplinary, collaborative research, including experts’ contribution in dermatology, cosmetic sciences, psychology, and biophysics.
REFERENCES
1. Berardesca E, Cespa M, Farinelli N, Rabbiosi G, Maibach H. In vivo transcutaneous penetration of nicotinates and sensitive skin. Contact Dermatitis. 1991;25(1):35–8.
2. Berardesca E, Maibach HI. Sensitive and ethnic skin: A need for special skin-care agents? Dermatologic Clinics. 1991;9(1):89–92.
3. Draelos ZD. Sensitive skin: Perceptions, evaluation, and treatment. American Journal of Contact Dermatitis. 1997;8(2):67–78.
4. Issachar N, Gall Y, Borell MT, Poelman MC. pH measurements during lactic acid stinging test in normal and sensitive skin. Contact Dermatitis. 1997;36(3):152–5.
5. Issachar N, Gall Y, Borrel MT, Poelman MC. Correlation between percutaneous penetration of methyl nicotinate and sensitive skin, using laser Doppler imaging. Contact Dermatitis. 1998;39(4):182–6.
6. Mills Jr OH, Berger RS. Defining the susceptibility of acne-prone and sensitive skin populations to extrinsic factors. Dermatologic Clinics. 1991;9(1):93–8.
7. Muizzuddin N, Marenus KD, Maes DH. Factors defining sensitive skin and its treatment. American Journal of Contact Dermatitis. 1998;9(3):170–5.
8. Paquet F, Pierard-Franchimont C, Fumal I, Goffin V, Paye M, Pierard GE. Sensitive skin at menopause: Dew point and electrometric properties of the stratum corneum. Maturitas. 1998;28(3):221–7.
9. Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38(6):311–5.
10. Simion AF, Rau AH. Sensitive skin: What is it and how to formulate for it. Cosmetics & Toiletries. 1994;109:43s.
11. Bernstein ET. Cleansing of sensitive skin; with determination of the pH of the skin following use of soap and a soap substitute. The Journal of Investigative Dermatology. 1947;9(1):5–9.
12. Maibach HI, Lammintausta K, Berardesca E, Freeman S. Tendency to irritation: Sensitive skin. Journal of the American Academy of Dermatology. 1989;21(4 Pt 2):833–5.
13. Farage MA. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clinical and Experimental Dermatology. 2009;34(8):e521–30.
14. Jourdain R, de Lacharriere O, Bastien P, Maibach HI. Ethnic variations in self-perceived sensitive skin: Epidemiological survey. Contact Dermatitis. 2002;46(3):162–9.
15. Kamide R, Misery L, Perez-Cullell N, Sibaud V, Taïeb C. Sensitive skin evaluation in the Japanese population. The Journal of Dermatology. 2013;40(3):177–81.
16. Loffler H, Dickel H, Kuss O, Diepgen TL, Effendy I. Characteristics of self-estimated enhanced skin susceptibility. Acta Dermato-Venereologica. 2001;81(5):343–6.
17. Misery L, Boussetta S, Nocera T, Perez-Cullell N, Taïeb C. Sensitive skin in Europe. Journal of the European Academy of Dermatology and Venereology. 2009;23(4):376–81.
18. Misery L, Myon E, Martin N, Verriere F, Nocera T, Taïeb C. Sensitive skins in France: An epidemiological approach. [French]. Annales de Dermatologie et de Vénéréologie 2005;132(5):425–9.
19. Misery L, Sibaud V, Merial-Kieny C, Taïeb C. Sensitive skin in the American population: Prevalence, clinical data, and role of the dermatologist. International Journal of Dermatology. 2011;50(8):961–7.
20. Morizot F, Guinot C, Lopez J, LaFus I, Tschachler E. Sensitive skin: Analysis of symptoms, perceived causes and possible mechanisms. Cosmetics & Toiletries. 2000;115:83–9.
21. Xu F, Yan S, Wu M, Li F, Sun Q, Lai W et al. Self-declared sensitive skin in China: A community-based study in three top metropolises. Journal of the European Academy of Dermatology and Venereology. 2013;27(3):370–5.
22. Willis CM, Shaw S, De Lacharriere O, Baverel M, Reiche L, Jourdain R et al. Sensitive skin: An epidemiological study. The British Journal of Dermatology. 2001;145(2):258–63.
23. Guinot C, Malvy D, Mauger E, Ezzedine K, Latreille J, Ambroisine L et al. Self-reported skin sensitivity in a general adult population in France: Data of the SU.VI.MAX cohort. Journal of the European Academy of Dermatology and Venereology. 2006;20(4):380–90.
24. Saint-Martory C, Roguedas-Contios AM, Sibaud V, Degouy A, Schmitt AM, Misery L. Sensitive skin is not limited to the face. British Journal of Dermatology. 2008;158(1):130–3.
25. Sparavigna A, Di Pietro A, Setaro M. “Healthy skin”: Significance and results of an Italian study on healthy population with particular regard to “sensitive” skin. International Journal of Cosmetic Science. 2005;27(6):327–31.
26. Farage M, Mandl C, Berardesca E, Maibach H. Sensitive skin in China. Journal of Cosmetics, Dermatological Sciences and Applications. 2012;2(3):184–95.
27. Taïeb C, Auges M, Georgescu V, Perez Cullell N, Misery L. Sensitive skin in Brazil and Russia: An epidemiological and comparative approach. European Journal of Dermatology. 2014;24(3):372–6.
28. Misery L, Myon E, Martin N, Consoli S, Boussetta S, Nocera T et al. Sensitive skin: Psychological effects and seasonal changes. Journal of the European Academy of Dermatology and Venereology. 2007;21(5):620–8.
29. Diogo L, Papoila AL. Is it possible to characterize objectively sensitive skin? Skin Research and Rechnology. 2010;16(1):30–7.
30. Primavera G, Berardesca E. Sensitive skin: Mechanisms and diagnosis. International Journal of Cosmetic Science. 2005;27(1):1–10.
31. Farage MA, Katsarou A, Maibach HI. Sensory, clinical and physiological factors in sensitive skin: A review. Contact Dermatitis. 2006;55(1):1–14.
32. Green BG. Measurement of sensory irritation of the skin. American Journal of Contact Dermatitis. 2000;11(3):170–80.
33. Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacology and Physiology. 2015;28(2):75–83.
34. An S, Lee E, Kim S, Nam G, Lee H, Moon S et al. Comparison and correlation between stinging responses to lactic acid and bioengineering parameters. Contact Dermatitis. 2007;57(3):158–62.
35. Berardesca E, Abril E, Serio M, Cameli N. Effects of topical gluco-oligosaccharide and collagen tripeptide F in the treatment of sensitive atopic skin. International Journal of Cosmetic Science. 2009;31(4):271–7.
36. de Campos Dieamant G, Velazquez Pereda MDL, Eberlin S, Nogueira C, Werka RM, Queiroz MLS. Neuroimmunomodulatory compound for sensitive skin care: In vitro and clinical assessment. Journal of Cosmetic Dermatology. 2008;7(2):112–9.
37. Distante F, Rigano L, D’Agostino R, Bonfigli A. Intra- and inter-individual differences in sensitive skin. Cosmetics & Toiletries. 2002;117(7):39–46.
38. Kim SJ, Lim SU, Won YH, An SS, Lee EY, Moon SJ et al. The perception threshold measurement can be a useful tool for evaluation of sensitive skin. International Journal of Cosmetic Science. 2008;30(5):333–7.
39. Lee E, An S, Lee TR, Kim HK. Development of a novel method for quantitative evaluation of sensory skin irritation inhibitors. Skin Research and Technology. 2009;15(4):464–9.
40. Marriott M, Holmes J, Peters L, Cooper K, Rowson M, Basketter DA. The complex problem of sensitive skin. Contact Dermatitis. 2005;53(2):93–9.
41. Schliemann S, Antonov D, Manegold N, Elsner P. Sensory irritation caused by two organic solvents—Short-time single application and repeated occlusive test in stingers and non-stingers. Contact Dermatitis. 2011;65(2):107–14.
42. Sparavigna A, Pietro A, Setaro M. Sensitive skin: Correlation with skin surface microrelief appearance. Skin Research and Technology. 2006;12(1):7–10.
43. Vijver van de LPL, Boelsma E, Rausch-Goldbohm RA, Roza L. Subjective skin condition and its association with objective skin measurements. Cosmetics & Toiletries. 2003;118(7):45–54.
44. Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. Journal of the Society of Cosmetic Chemists. 1977;28:197–209.
45. Bowman JP, Floyd AK, Znaniecki A, Kligman AM, Stoudemayer T, Mills OH. The use of chemical probes to assess the facial reactivity of women, comparing their self-perception of sensitive skin. Journal of Cosmetic Science. 2000;51:67–273.
46. Querleux B, Dauchot K, Jourdain R, Bastien P, Bittoun J, Anton JL et al. Neural basis of sensitive skin: An fMRI study. Skin Research and Technology. 2008;14(4):454–61.
47. Roussaki-Schulze AV, Zafiriou E, Nikoulis D, Klimi E, Rallis E, Zintzaras E. Objective biophysical findings in patients with sensitive skin. Drugs under Experimental and Clinical Research. 2005;31 Suppl:17–24.
48. Cho HJ, Chung BY, Lee HB, Kim HO, Park CW, Lee CH. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. Journal of Dermatology. 2012;39(3):295–300.
49. Basketter DA, Griffiths HA. A study of the relationship between susceptibility to skin stinging and skin irritation. Contact Dermatitis. 1993;29(4):185–8.
50. Coverly J, Peters L, Whittle E, Basketter DA. Susceptibility to skin stinging, nonimmunologic contact urticaria and acute skin irritation: Is there a relationship? Contact Dermatitis. 1998;38(2):90–5.
51. Lammintausta K, Maibach HI, Wilson D. Mechanisms of subjective (sensory) irritation: Propensity to non-immunologic contact urticaria and objective irritation in stingers. Dermatosen in Beruf und Umwelt: Occupation and Environment. 1988;36(2):45–9.
52. Wu Y, Wang X, Zhou Y, Tan Y, Chen D, Chen Y et al. Correlation between stinging, TEWL and capacitance. Skin Research and Technology. 2003;9(2):90–3.
53. Farage MA, Maibach H. Cumulative skin irritation test of sanitary pads in sensitive skin and normal skin population. Cutaneous and Ocular Toxicology. 2007;26(1):37–43.
54. Quatresooz P, Pierard-Franchimont C, Pierard GE. Vulnerability of reactive skin to electric current perception—A pilot study implicating mast cells and the lymphatic microvasculature. Journal of Cosmetic Dermatology. 2009;8(3):186–9.
55. Robinson MK, Perkins MA. Evaluation of a quantitative clinical method for assessment of sensory skin irritation. Contact Dermatitis. 2001;45(4):205–13.
56. Misery L, Jean-Decoster C, Mery S, Georgescu V, Sibaud V. A new ten-item questionnaire for assessing sensitive skin: The Sensitive Scale-10. Acta Dermato-Venereologica. 2014;94(6):635–9.
57. Kligman AM, Sadiq I, Zhen Y, Crosby M. Experimental studies on the nature of sensitive skin. Skin Research and Technology. 2006;12(4):217–22.
58. Farage MA, Maibach HI. Sensitive skin: Closing in on a physiological cause. Contact Dermatitis. 2010;62(3):137–49.
59. Pinto P, Rosado C, Parreirao C, Rodrigues LM. Is there any barrier impairment in sensitive skin? A quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Research and Technology. 2011;17(2):181–5.
60. Stander S, Schneider SW, Weishaupt C, Luger TA, Misery L. Putative neuronal mechanisms of sensitive skin. Experimental Dermatology. 2009;18(5):417–23.
61. Jourdain R, Bastien P, de Lacharriere O, Rubinstenn G. Detection thresholds of capsaicin: A new test to assess facial skin neurosensitivity. Journal of Cosmetic Science. 2005;56(3):153–66.
62. Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–24.
63. Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annual Review of Neuroscience. 2006;29:135–61.
64. Kueper T, Krohn M, Haustedt LO, Hatt H, Schmaus G, Vielhaber G. Inhibition of TRPV1 for the treatment of sensitive skin. Experimental Dermatology. 2010;19(11):980–6.
65. Aramaki J, Kawana S, Effendy I, Happle R, Loffler H. Differences of skin irritation between Japanese and European women. British Journal of Dermatology. 2002;146(6):1052–6.
66. Lee BH, Park CK, Kim HO, Jo HJ, Park CW, Lee CH. The skin irritations of corrosive and noncorrosive irritants in patients with sensitive skin. Korean Journal of Dermatology. 2007;45(6):551–9.
67. Pons-Guiraud A. Sensitive skin: A complex and multifactorial syndrome. Journal of Cosmetic Dermatology. 2004;3(3):145–8.
68. Agner T, Serup J. Sodium lauryl sulphate for irritant patch testing—A dose–response study using bioengineering methods for determination of skin irritation. The Journal of Investigative Dermatology. 1990;95(5):543–7.
69. Cowley NC, Farr PM. A dose–response study of irritant reactions to sodium lauryl sulphate in patients with seborrhoeic dermatitis and atopic eczema. Acta Dermato-Venereologica. 1992;72(6):432–5.
70. Nassif A, Chan SC, Storrs FJ, Hanifin JM. Abnormal skin irritancy in atopic-dermatitis and in atopy without dermatitis. Archives of Dermatology. 1994;130(11):1402–7.
71. Basketter DA, Miettinen J, Lahti A. Acute irritant reactivity to sodium lauryl sulfate in atopics and non-atopics. Contact Dermatitis. 1998;38(5):253–7.
72. Loffler H, Effendy I. Skin susceptibility of atopic individuals. Contact Dermatitis. 1999;40(5):239–42.
73. Sahlin A, Edlund F, Loden M. A double-blind and controlled study on the influence of the vehicle on the skin susceptibility to stinging from lactic acid. International Journal of Cosmetic Science. 2007;29(5):385–90.