Chapter 39 Vitamins, calcium, bone
Vitamins1 are substances that are essential for normal metabolism but are supplied chiefly in the diet.
Vitamins fall into two groups:
Vitamin A: retinol
Deficiency of retinol leads to xerophthalmia, squamous metaplasia, hyperkeratosis and impairment of the immune system.
Therapeutic uses
Retinol and derivatives provide therapeutic benefit in a number of clinical areas.
Psoriasis
Tazorotene, a topical retinoid, is effective in the treatment of chronic stable plaque psoriasis. Skin irritation is common, making it unsuitable for the treatment of inflammatory forms of psoriasis. The 0.05% cream is better tolerated than 0.1% although less effective. Acitretin is a retinoic acid derivative (t½ 48 h) that is used orally for severe psoriasis. Dose range 25 mg alternate days – 50 mg daily (see p. 262, as well as other disorders of keratinisation).
Acne
Tretinoin is retinoic acid and is used in acne by topical application (see p. 273). Isotretinoin is a retinoic acid isomer (t½ 20 h) given orally for acne (see p. 273). It is also effective for preventing second tumours in patients following treatment for primary squamous cell carcinoma of the head and neck.
Vitamin B complex
Niacin (nicotinic acid, B3)
is converted to nicotinamide, and subsequently to nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADPH), the cofactors that are essential for the oxidation–reduction reactions that comprise tissue respiration. Nicotinamide is used for nutritional purposes. Pellagra resulting from dietary deficiency of niacin is rarely seen in developed countries. Causes other than dietary include alcoholism, carcinoid syndrome and Hartnup disease. Nicotinic acid at pharmacological doses is used for the treatment of some hyperlipidaemias (see p. 444). Adverse effects include peripheral vasodilatation, unpleasant flushing, itching and fainting.
Vitamin D, calcium, parathyroid hormone, calcitonin, bisphosphonates, bone
Vitamin D
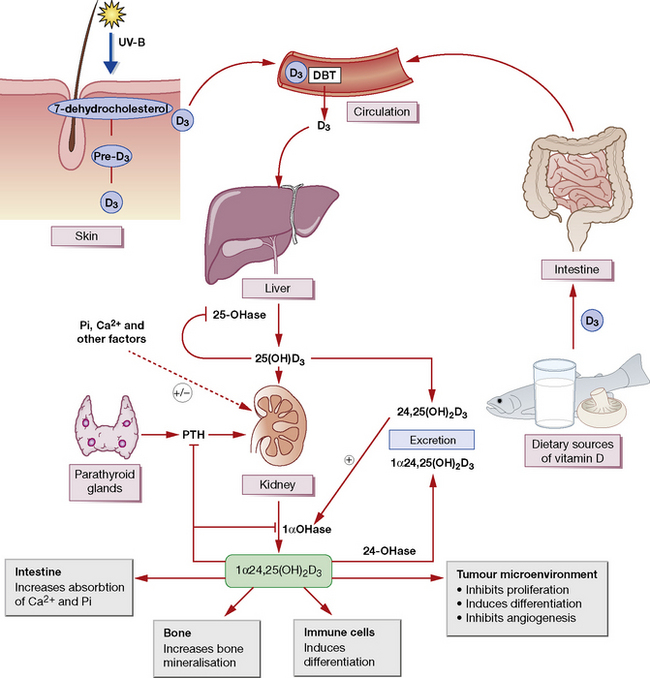
Vitamin D comprises a number of structurally related sterol compounds having similar biological properties (but different potencies) in that they prevent or cure the vitamin D-deficiency diseases, rickets and osteomalacia. The most relevant form of vitamin D is vitamin D3 (colecaciferol). This is made by ultraviolet irradiation of 7-dehydrocholesterol in the skin. It is also absorbed in the intestinal tract; however, few foods contain significant levels of vitamin D (Fig. 39.1). Vitamin D2 (ergocalciferol) is made by ultraviolet irradiation of ergosterol in plants. This is not the naturally occurring form.
Pharmacokinetics
Actions
are complex. Vitamin D promotes the active transport of calcium and phosphate in the gut (increased absorption) and renal tubule (reduced excretion), and thus controls, with PTH, the plasma calcium concentration and the mineralisation of bone (see Fig. 39.1). After a dose of D2 or D3 there is a lag of about 21 h before the intestinal effect begins; this is probably due to the time needed for its metabolic conversion to the more active forms. With the biologically more active calcitriol, the lag is only 2 h.
Indications
Treatment of calcium and bone disorders
Hypercalcaemia
Temporary measures
• Physiological saline solution is important, firstly to correct sodium and water deficit, and secondly to promote sodium-linked calcium diuresis in the proximal renal tubule. Initially, 500 mL 0.9% saline should be given intravenously over 4 h and then adjusted to maintain urine output at 100-150 mls/hour until the plasma Ca2 + level falls below 3.0 mmol/L and the oral intake is adequate. The regimen requires careful attention to fluid and electrolyte balance, particularly in patients with renal insufficiency secondary to hypercalcaemia or heart failure who are unable to excrete excess sodium. The use of furosemide to enhance renal Ca2 + excretion has been largely abandoned owing to the exacerbation of electrolyte disturbances and the increased availability of newer agents.
• Bisphosphonates are the agents of choice in moderate to severe hypercalcaemia, There are a number of bisphosphonates licensed for this indication but pamidronate and zoledronic acid are the most widely used agents. Pamidronate2 is infused according to the schedule in Table 39.1; it is active in a wide variety of hypercalcaemic disorders. A fall in the serum calcium concentration begins within the first day, reaches a nadir in 5–6 days and lasts for 20–30 days. Zoledronic acid has the advantage of being more potent and it can be administered over a shorter time (4 mg over 15 min versus 2 h). It is a convenient regimen for patients with hypercalcaemia of malignancy where repeat courses may be required every 3–4 weeks.
• Calcitonin. When the hypercalcaemia is at least partly due to mobilisation from bone, calcitonin (4 units/kg) can be used to inhibit bone resorption, and may enhance urinary excretion of calcium. The effect develops in a few hours but responsiveness is lost over a few days owing to tachyphylaxis. Calcitonin is not as effective as the bisphosphonates; however, its shorter onset of action makes it a valuable agent for initial management of hypercalcaemia until the peak onset of action of the bisphosphonate at 2–4 days.
• An adrenocortical steroid, e.g. prednisolone 20–40 mg/day orally, is effective in particular situations; it reduces the hypercalcaemia of hypervitaminosis D (which is due to excessive intestinal absorption of calcium either secondary to intoxication or granulomatous disease, e.g. sarcoidosis). Corticosteroid may be effective in the hypercalcaemia of malignancy where the disease itself is responsive, e.g. myeloma of lymphoma. Patients with hyperparathyroidism do not respond.
• Dialysis is quick and effective and is likely to be needed in severe cases or in those with renal failure.
Table 39.1 Treatment of hypercalcaemia with disodium pamidronate
| Calcium (mmol/L) | Pamidronate (mg) |
|---|---|
| < 3.0 | 15–30 |
| 3.0–3.5 | 30–60 |
| 3.5–4.0 | 60–90 |
| > 4.0 | 90 |
Infuse slowly, e.g. 30 mg in 250 mL 0.9% saline over 1 h. Expect a response in 2–4 days.
The above measures are only temporary, giving time to tackle the cause.
Hypercalciuria
In renal stone formers, in addition to general measures (low calcium diet, high fluid intake), urinary calcium may be diminished by a thiazide diuretic (with or without citrate to bind calcium) and oral phosphate (see above). See also Nephrolithiasis, p. 463.
Parathyroid hormone
Parathyroid hormone (PTH) acts chiefly on the kidney, increasing renal tubular reabsorption of calcium and excretion of phosphate; it increases calcium absorption from the gut, indirectly, by stimulating the renal synthesis of 1α,25-vitamin D (see above and Fig. 39.1). PTH increases the rate of bone remodelling (mineral and collagen) and osteocyte activity with, at high doses, an overall balance in favour of resorption (osteoclast activity) with a rise in plasma calcium concentration (and fall in phosphate); but, at low doses, the balance favours bone formation (osteoblast activity).
Bisphosphonates
Indications
• Paget’s disease of bone (see below).
• Hypercalcaemia due to malignancy (see above) and metastatic bone disease Bisphosphonates reduce skeletal fractures and loss of skeletal integrity associated with metastatic bone disease. The best evidence for such a benefit comes from use of intravenous zoledronic acid in patients with metastatic lung cancer, prostate cancer or breast cancer. There is no evidence that bisphosphonates can prevent bone metastases in these cancers.
Osteoporosis
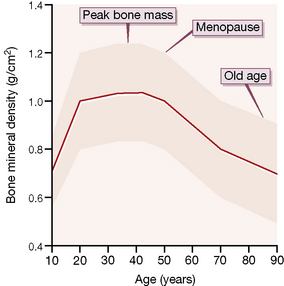
Osteoporosis is a disease characterised by increased skeletal fragility, low bone mineral density (less than 2.5 standard deviations below the mean for young people; Fig. 39.2) and deterioration of bone microarchitecture. It occurs most commonly in post-menopausal women and patients taking long-term corticosteroid. Exclude underlying causes such as hyperthyroidism, hyperparathyroidism and hypogonadism (in both sexes) before treatment is initiated.
Pharmacotherapy
Selective oestrogen receptor modulator
Raloxifene is effective for both the prevention and treatment of osteoporosis; it reduces the incidence of vertebral but not of non-vertebral fractures. It is probably less effective than bisphosphonates but no direct comparisons have been made. Raloxifene reduces the risk of breast cancer (see p. 517) but there is a three-fold increase in the risk of venous thromboembolic disease.
Parathyroid hormone
PTH increases bone resorption but the synthetic PTH, teriparatide, administered daily (20 micrograms, subcutaneously), stimulates bone formation and reduces the risk of vertebral and non-vertebral fractures. However, in a large randomised controlled trial, teriparatide alone did not reduce the risk of hip fracture. It is indicated for severe post-menopausal osteoporosis or where bisphosphonates have proved to be ineffective.3
Armitage J.M., Bowman L., Clarke R.J., et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. J. Am. Med. Assoc.. 2010;303:2486–2494.
Bone H.G., Hosking D., Devogelaer J.P., et al. Ten years’ experience with alendronate for osteoporosis in post-menopausal women. N. Engl. J. Med.. 2004;350:1189–1199.
El-Kadiki A., Sutton A.J. Role of multivitamins and mineral supplements in preventing infections in elderly people: systematic review and meta-analysis of randomised controlled trials. Br. Med. J.. 2005;330:871–876.
Farford B., Prescutti R.J., Moraghan T.J. Nonsurgical management of primary hyperparathyroidism. Mayo Clin. Proc.. 2007;82:351–355.
Holick M.F. Vitamin D deficiency. N. Engl. J. Med.. 2007;357:266–281.
Kalantar-Zadeh K., Shah A., Duong U., et al. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int.. 2010;78(Suppl. 117):S10–S21.
Lambrinoudaki I., Christodoulakos G., Botsis D. Bisphosphonates. Ann. N. Y. Acad. Sci.. 2006;1092:403–407.
Osterhues A., Holzgreve W., Michels K.B. Shall we put the world on folate? Lancet. 2009;374:959–961.
Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287.
Ralston S.H., Langston A.L., Reid I.R. Pathogenesis and management of Paget’s disease of bone. Lancet. 2008;372:155–163.
Rosen C.J. Vitamin D insufficiency. N. Engl. J. Med.. 2011;364:248–254.
Sambrook P., Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018.
Steddon S.J., Cunningham J. Calcimimetics and calcilytics – fooling the calcium receptor. Lancet. 2005;365:2237–2239.
Whyte M.P. Paget’s disease of bone. N. Engl. J. Med.. 2006;355:593–600.
1 The term was coined by Casimir Funk in 1912 from the Latin vita meaning life and the (mistaken) belief that the organic compounds involved were amines. See: Hardy A 2004 Historical keywords. Vitamin. Lancet 364:323.
2 Formerly called aminohydroxypropylidenediphosphonate disodium, APD.
3 In a pivitol 19-month trial, teriparatide increased bone mineral density in the spine and femoral neck, and rates of new vertebral fractures and non-vertebral fractures were 5% and 6.3% compared with 14.3% and 9.7% respectively for placebo (Neer R M, Arnaud C D, Zanchetta J R et al 2001 Effect of parathyroid hormone (1–34) on fractures and bone mineral density in post-menopausal women with osteoporosis. New England Journal of Medicine 344(19):1431–1441).
4 This term refers to the various disorders of bone and mineral metabolism that occur as a result of chronic kidney disease, i.e. hyperparathyroid bone disease, osteomalacia, osteoporosis and osteosclerosis. Also referred to as renal osteodystrophy.