Chapter 31 Neoplastic disease and immunosuppression
Neoplastic disease
Cancer treatments and outcomes
Cancers share some common characteristics:
• Growth that is not subject to normal spatial restrictions for that tissue and fails to respond to apoptotic signals (see below) or in which a high proportion of cells are dividing, i.e. there is a high ‘growth fraction’.
• Tendency to spread to other parts of the body (metastasise).
• Less differentiated cell morphology.
• Tendency to retain some characteristics of the tissue of origin, at least initially.
Cancer treatment employs six established principal modalities:
This account describes the main groups of drugs (see p. 510) but it is important to understand the overall context in which systemic therapy is offered to patients.
Systemic cancer therapy
Cancers originating from different organs of the body differ in their initial behaviour and in their response to treatments (Table 31.1). Primary surgery and/or radiotherapy to a localised cancer offer the best chance of cure for patients. Drug treatments offer cure only for certain types of cancer, often characterised by their high proliferative rate, e.g. lymphoma, testicular cancer, Wilms’ tumour. More often, systemic therapy offers prolongation of life from months to many years and associated improvements in quality of life, even if patients ultimately die from their disease.
Table 31.1 Degree of benefit achieved with systemic therapy for common cancers
| Curable: chemosensitive cancers | Improved survival: some degree of chemosensitivity | Equivocal survival benefit: chemoresistant cancers |
|---|---|---|
| Teratoma | Colorectal cancer | Sarcoma |
| Seminoma | Small cell lung cancer | Bladder cancer |
| High-grade non-Hodgkin’s lymphoma | Ovarian cancer | Melanoma |
| Hodgkin’s lymphoma | Breast cancer | Renal cancer Insensitive to cytotoxic chemotherapy but now can be controlled temporarily with oral VEGF2 and mTOR inhibitors |
| Wilms’ tumour | Cervical cancer | Primary brain cancers |
| Acute myeloblastic leukaemia | Endometrial cancer | Nasopharyngeal carcinoma |
| Acute lymphoblastic leukaemia in childhood | Gastro-oesophageal cancer Cholangiocarcinoma and gall bladder cancer | Hepatoma Insensitive to cytotoxic chemotherapy but now can be controlled temporarily with oral VEGF inhibitors |
| Myeloma | ||
| Pancreatic cancer | ||
| Low-grade non-Hodgkin’s lymphoma | ||
| Non-small cell lung cancer | ||
| Adult acute lymphoblastic leukaemia |
Classes of cytotoxic chemotherapy drugs
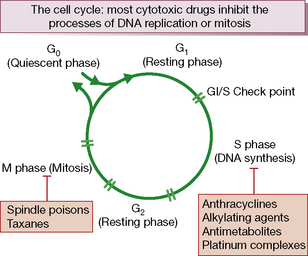
Cytotoxic chemotherapy drugs exert their effect by inhibiting cell proliferation. All proliferating cells, whether normal and malignant, cycle through a series of phases of: synthesis of DNA (S phase), mitosis (M phase) and rest (G1 phase). Non-cycling cells are quiescent in G0 phase (Fig. 31.1).
They are thus all potentially mutagenic. Cytotoxic drugs ultimately induce cell death by apoptosis,2 a process by which single cells are removed from living tissue by being fragmented into membrane-bound particles and phagocytosed by other cells. This occurs without disturbing the architecture or function of the tissue, or eliciting an inflammatory response. The instructions for apoptosis are built into the cell’s genetic material, i.e. ‘programmed cell death’.3
Cytotoxic drugs can be classified as either:
• cell cycle non-specific: these kill cells whether they are resting or actively cycling (as in a low growth fraction cancer such as solid tumours), e.g. alkylating agents, doxorubicin and allied anthracyclines, or
• cell cycle (phase) specific: these kill only cells that are actively cycling, often because their site of action is confined to one phase of the cell cycle, e.g. antimetabolite drugs.
Table 31.2 provides a summary of the key groups of anticancer drugs, their common toxicities and main treatment applications.
Table 31.2 Principal classes of cytotoxic drug, their common toxicities and examples of clinical use
| Drug class | Common toxicities | Examples of clinical use |
|---|---|---|
| Cytotoxic drugs | ||
| Alkylating agents | Nausea and vomiting, bone marrow depression (delayed with carmustine and lomustine), cystitis (cyclophosphamide, ifosfamide), pulmonary fibrosis (especially busulfan). Male infertility and premature menopause may occur. Myelodysplasia and secondary neoplasia | Widely used in the treatment of both haematological and non-haematological cancers, with varying degrees of success |
| Platinum drugs | Bone marrow depression, nausea and vomiting, allergy reaction (esp. carboplatin), nephrotoxicity, hypomagnesaemia; hypocalcaemia; hypokalaemia; hypophosphataemia; hyperuricaemia (all as a consequence of renal dysfunction, primarily associated with cisplatin); Raynaud’s disease; sterility; teratogenesis; ototoxicity (cisplatin); peripheral neuropathy; cold dysaesthesia and pharyngolaryngeal dysaesthesia (oxaliplatin) | Testicular cancers, ovarian cancer; oxaliplatin acts synergistically with 5FU and is licensed in combination with 5FU to treat both advanced and early stages of colorectal cancer |
| Nucleoside analogues, e.g. cytarabine, gemcitabine, fludarabine | Bone marrow depression, mainly affecting platelets; mild nausea and vomiting; diarrhoea; anaphylaxis; sudden respiratory distress with high doses (cytarabine); rash, fluid retention and oedema; profound immunosuppression with fludarabine | Cytarabine is used in haematological regimens; gemcitabine is used for pancreatic cancer, bladder cancer and some other solid tumours; fludarabine is active in chronic lymphatic leukaemia and lymphoma |
| Taxanes | Nausea and vomiting, hypersensitivity reactions, bone marrow depression, fluid retention; peripheral neuropathy; alopecia; arthralgias; myalgias; cardiac toxicity; mild GI disturbances; mucositis | Breast and gynaecological cancers; recent evidence that docetaxel improves survival in advanced prostate cancer |
| Anthracyclines | Nausea and vomiting, bone marrow depression; cardiotoxicity (may be delayed for years); red-coloured urine; severe local tissue damage and necrosis on extravasation; alopecia; stomatitis; anorexia; conjunctivitis; acral (extremities) pigmentation; dermatitis in previously irradiated areas; hyperuricaemia | Common component of many chemotherapy regimens for both haematological and non-haematological malignancies |
| Antimetabolites, e.g. 5-fluorouracil, methotrexate | Nausea and vomiting; diarrhoea; mucositis, bone marrow depression, neurological defects, usually cerebellar; cardiac arrhythmias; angina pectoris, hyperpigmentation, hand–foot syndrome, conjunctivitis | Commonly used in haematological and non-haematological malignancies |
| Topoismerase I inhibitors | Nausea and vomiting; cholinergic syndrome; hypersensitivity reactions; bone marrow depression; diarrhoea; colitis; ileus; alopecia; renal impairment; teratogenic | Irinotecan is effective in advanced colorectal cancer; topotecan is used in gynaecological malignancies |
| Mitotic spindle inhibitors (vinca alkaloids) | Nausea and vomiting; local reaction and phlebitis with extravasation, neuropathy, bone marrow depression; alopecia; stomatitis; loss of deep tendon reflexes; jaw pain; muscle pain; paralytic ileus | Commonly used in haemato-oncology regimens |
| Hormones | ||
| Tamoxifen | Hot flushes; transiently increased bone or tumour pain; vaginal bleeding and discharge; rash; thromboembolism; endometrial cancer | Oestrogen receptor-positive, advanced and early stage breast cancer |
| Aromatase inhibitors | Nausea; dizziness; rash; bone marrow depression; fever; masculinisation | Equivalence with tamoxifen suggested |
| Medroxyprogesterone acetate | Menstrual changes; gynaecomastia; hot flushes; oedema, weight gain; hirsutism; insomnia; fatigue; depression; thrombophlebitis and thromboembolism; nausea; urticaria; headache | Third-line therapy for slowly progressive breast cancer in postmenopausal women |
| Flutamide | Nausea; diarrhoea; gynaecomastia; hepatotoxicity | Prostate cancer |
| Goserelin | Transient increase in bone pain and urethral obstruction in patients with metastatic prostatic cancer; hot flushes; impotence; testicular atrophy; gynaecomastia | Prostate cancer |
| Leuprolelin (LHRH analogue) | Transient increase in bone pain and ureteral obstruction in patients with metastatic prostatic cancer; hot flushes, impotence; testicular atrophy; gynaecomastia; peripheral oedema | Prostate cancer |
| Immunotherapy | ||
| BCG (bacille Calmette-Guérin) | Bladder irritation; nausea and vomiting; fever; sepsis, granulomatous pyelonephritis; hepatitis; urethral obstruction; epididymitis; renal abscess | Localised bladder cancer |
| Interferon-α | Fever; chills; myalgias; fatigue; headache; arthralgias, bone marrow depression; anorexia; confusion; depression; psychiatric disorders; renal toxicity; hepatic toxicity; rash | Renal cancer |
| Interleukin-2 | Fever; fluid retention; hypotension; respiratory distress; rash; anaemia, thrombocytopenia; nausea and vomiting; diarrhoea, capillary leak syndrome, nephrotoxicity; myocardial toxicity; hepatotoxicity; erythema nodosum; neuropsychiatric disorders; hypothyroidism; nephrotic syndrome | Renal cancer |
| Trastuzumab (Herceptin) | Fever; chills; nausea and vomiting; pain; hypersensitivity and pulmonary reactions, bone marrow depression; cardiomyopathy; ventricular dysfunction; congestive cardiac failure; diarrhoea | Advanced and early stage breast cancer, combined with cytotoxic chemotherapy |
| Rituximab (MabThera) | Hypersensitivity reaction, bone marrow depression, angioedema, precipitation of angina or arrhythmia with pre-existing heart disease | Non-Hodgkin’s lymphoma |
Adverse effects of cytotoxic chemotherapy
Principal adverse effects are manifest as, or follow damage to, the following:
may occur within hours of treatment or be delayed, and last for several days, depending on the agent. As emetogenicity is largely predictable, preventive action can be taken. The most effective drugs are competitive antagonists of serotonin (5-hydroxytryptamine type 3, 5HT3) receptors, e.g. ondansetron, and corticosteroids such as dexamethasone, which benefit by unknown, multi-factorial mechanisms. Other effective antiemetics include domperidone, metoclopramide, cyclizine and prochlorperazine (see p. 534). Combinations of drugs are frequently used and routes of administration selected as commonsense counsels, e.g. prophylaxis may be oral, but when vomiting occurs the parenteral route and suppositories are available.
Classes of cytotoxic agents
Chemotherapy in clinical practice
Drug use and tumour cell kinetics
Evidence from leukaemia in laboratory animals shows that:
• Survival time is inversely related to the initial number of leukaemia cells, or to the number remaining after treatment.
• A single leukaemia cell is capable of multiplying and eventually killing the host.
• Increased cell cycle (division) time.
• Decrease in the number of cells actively dividing, with more in the resting state (decrease in growth fraction), but with the potential to switch back to a fast growth state.
• Increased cell death within the tumour as it ages.
• Overcrowding of cells leading to necrotic, avascular and hypoxic areas that cannot easily be penetrated by drugs. These are fertile areas for clonal selection of the most robust cancer cells.
Selectivity of drugs for cancer cells is generally low compared with the selectivity shown by antimicrobial agents but it can be substantial, e.g. in lymphoma, where tumour cell kill with some drugs is 10 000 times greater than that of marrow cells. Cell destruction by cytotoxic drugs follows first-order kinetics, i.e. a given dose of drug kills a constant fraction of cells (not a constant number) regardless of the number of cells present. Thus a treatment that reduces a cell population from 1 000 000 to 10 000 (a two-log cell kill) will reduce a cell population of 1000 to 10. Furthermore, cell chemosensitivity within a cancer is not homogeneous owing to random mutations (clonal selection) as the tumour grows, the cells remaining after initial doses being more likely to resist further treatment. Therefore, combining several drugs may be more effective than a single agent given repeatedly to the limit of tolerance.
in combination chemotherapy is influenced by:
• Choosing drugs that act at different biochemical sites in the cell.
• Using drugs that attack cells at different phases of the growth cycle (see Fig. 31.1). ‘CHOP’ (cyclophosphamide, doxorubicin (previously known as hydroxydoxorubicin) vincristine (previously called oncovin) and prednisolone), is a standard combination chemotherapy regimen for non-Hodgkin’s lymphoma. The first three cytotoxic drugs exert their antitumour effect on different aspects of cell proliferation. The antitumour effect of corticosteroid remains unclear.
• The desirability of attaining synchronisation of cell cycling to achieve maximum cell kill. Cells are killed or are arrested in mitosis by vincristine, which is then withdrawn. Cells then enter a new reproductive cycle more or less synchronously, and when most are judged to be in a phase sensitive to a particular phase-specific drug, e.g. methotrexate or cytarabine, it is given.
• Avoidance of cross-resistance (see below) between drugs. In some instances, use of one drug regimen followed by another rather than using them simultaneously in combination avoids drug resistance and improves therapeutic efficacy. For example, epirubicin given for four cycles followed by CMF (concomitant cyclophosphamide, methotrexate and 5-fluorouracil) for four cycles has largely replaced CMF alone as standard adjuvant chemotherapy for breast cancer, because the outcome is better.
• Non-overlapping toxicity profiles. Before establishing a combination regimen, Phase 1 trials (see p. 40) are undertaken, frequently fixing the dose of one drug while escalating the dose of another, in small cohorts of carefully monitored patients, so that toxicity and patient safety can be monitored.
• Empirical evidence of efficacy against a particular tumour type. The antitumour activity of platinum complexes was a chance finding (see below).
• Enhanced cell killing in preclinical models when drugs are combined. Oxaliplatin on its own has limited cytotoxicity against colorectal cancer cell lines in vitro and in mouse xenograft models, but its combination with 5FU confers a more than additive, i.e. synergistic, killing effect on tumour cells.
Improving efficacy of chemotherapy
Methods that potentially widen the narrow therapeutic index of cytotoxic agents include:
• Regional (as opposed to systemic) administration of drugs: intrathecal, intra-arterial into liver and isolated limb perfusion.
• Selective organ delivery of drug by altered formulation e.g. Caelyx is a formulation comprising high concentrations of doxorubicin encased in liposomes. This also alters toxicity profiles (e.g. less renal but more mucosal toxicity).
• High-dose (bone marrow ablative) chemotherapy is feasible by harvesting stem cells prior to drug exposure, and returning the cells to the patient on completion of treatment. This is a successful strategy in haematological malignancies but failed trials in almost all solid tumours.
• Circadian rhythms exist in cell metabolism and proliferation, and those of leukaemic cells differ from normal leucocytes. The time of day at which therapy is administered can theoretically influence the outcome (chronomodulation).
• In large solid tumours, the fraction of cells multiplying rapidly is often relatively small. In ovarian cancer, for example, patients benefit from debulking surgery (cytoreduction) prior to cytotoxic drug therapy.
Hazards to staff handling cytotoxic agents
Certain chemotherapy regimens require the simultaneous administration of intrathecal methotrexate and intravenous vincristine. In the UK until recently, each drug was presented in similar bolus volumes and the drug-filled syringes appeared very alike except that the syringe for intrathecal administration had a red stopper. Nevertheless, from 1985 in the UK inadvertent intrathecal administration of vincristine occurred on 14 occasions: 10 patients died and the remainder suffered paralysis.4
Endocrine therapy
Hormonal influence on cancer
put it to her husband and herself as to whether she should have performed the operation of removal of the [fallopian] tubes and ovaries. Its nature was fully explained to them both, and also that it was a purely experimental one … She readily consented … as she knew and felt her case was hopeless. [Eight months after operation] all vestiges of her previous cancerous disease had disappeared. [The surgeon concluded, after treating two further cases, that there may be ovarian influences in breast cancer and added that] whether [this is] accepted or not, I am sure I shall be acquitted of having acted thoughtlessly or recklessly.5
In 19416 it was shown that prostatic cancer with metastases was made worse by androgen and made better by oestrogen (diethylstilbestrol).
Hormonal agents
is androgen dependent and metastatic disease can be helped by orchidectomy, or by pituitary suppression of androgen secretion with a gonadorelin (LHRH) analogue, e.g. buserelin, goserelin, leuprorelin or triptorelin (see p. 615). These cause a transient stimulation of luteinising hormone and thus testosterone release, before inhibition occurs; some patients may experience exacerbation of tumour effects, e.g. bone pain, spinal cord compression. Where this can be anticipated, prior orchidectomy or anti-androgen treatment, e.g. with cyproterone or flutamide, is protective.
Immunotherapy
• Non-specific stimulation of active immunity with vaccines, e.g. BCG (bacille Calmette–Guérin7) instilled into the urinary bladder for bladder cancer. Other approaches involve the injection of tumour cells or tumour cell extracts combined with an immune stimulant such as BCG.
• Specific immunisation strategies, where tumour-specific and tumour-associated antigens have been identified. Melanomas, for example, possess melanoma differentiation antigens (tyrosinase, gp100, MART1) as well as tumour-associated antigens (MAGE, BAGE, GAGE series of major histocompatibility complex (MHC)-associated peptides and a family of lipoproteins known as gangliosides). Both DNA and whole-protein vaccines derived from these antigens are being evaluated in melanoma, but results to date have been clinically disappointing.
• Interleukins that stimulate proliferation of T lymphocytes and activate natural killer cells; interleukin-2 is used in metastatic renal cell carcinoma with marginal effectiveness.
• Interferons. Interferon-α is used for chronic granulocytic leukaemia, hairy cell leukaemia, renal cell carcinoma and Kaposi’s sarcoma.
Thalidomide was withdrawn in the 1960s following evidence of its teratogenic effects, including some on fetal limb development (see p. 63). These very effects prompted the notion that suppression of cell proliferation might actually provide benefit. Investigation revealed that thalidomide possessed immunomodulatory properties, anti-inflammatory actions, direct effects on tumour cells and their microenvironment, and actions on angiogenesis (see below). Thalidomide and analogues designed to reduce toxicity (immunomodulatory drugs: lenalidomide) have a therapeutic role in myeloma and are synergistic with dexamethasone and chemotherapeutic agents.
Development of anticancer drug therapy
In general, anticancer drugs develop from:
• Chance discovery: cisplatin. In the 1960s, scientists studying the effect of an electric current on bacteria cultured in a Petri dish noted that the cells stopped dividing, instead forming long filamentous structures. Further investigation revealed that the inhibitor of cell division was in fact an ion formed in solution from the platinum electrodes used in the experiment. The platinum complex, cis-diammine-dichloroplatinum (II), later known as cisplatin, was isolated and subsequently developed for its potential to kill cancer cells. When given to patients with a variety of different types of cancer, germ cell tumours in particular were found to possess remarkable sensitivity to cisplatin treatment, and this drug remains in use for treating such patients today.8
• Analogues. Severe vomiting, renal and nerve damage, and deafness limit the therapeutic efficacy of cisplatin. Carboplatin and oxaliplatin, second- and third-generation compounds derived from cisplatin, combine enhanced toxicity towards cancer cells with improved tolerance.
• Mass screening programmes. See Chapter 3.
• Rational drug design. Academic institutions and commercial biotechnology companies involved in experimental therapeutics study the cancer process to identify key (‘target’) genes or gene products that regulate aspects of carcinogenesis and then try to find ways of blocking the function of these targets (Table 31.3). Unlike conventional cytotoxic chemotherapy, many of these agents are thus more cancer-cell selective and thus cytostatic. In other words, a targeted biological agent may prevent tumour growth or progression and delay recurrence, but may not induce rapid tumour shrinkage, hitherto the key conventional endpoint for evaluating cytotoxic drugs. Some examples follow to illustrate the opportunities created by this type of approach.
Table 31.3 Some novel molecular targets being exploited in anticancer drug developmenta
| Target | Drugb | Examples of current clinical use |
|---|---|---|
| Her2/neu | Trastuzumab (Herceptin) | Advanced and early stage breast cancer Advanced gastric cancer |
| CD20 | Rituximab (MabThera) | Non-Hodgkin’s lymphoma |
| EGFR | Cetuximab (Erbitux) | Improves survival in advanced colorectal cancer Lung cancer and head and neck cancer (in combination with radiotherapy) |
| EGFR | Gefitinib (Iressa) | Advanced non-small cell lung cancer |
| EGFR | Erlotinib (Tarceva) | Advanced non-small cell lung cancer, advanced pancreatic cancer |
| VEGF | Bevacizumab (Avastin) | Advanced colorectal cancer; trials confirm some efficacy in non-small cell lung cancer and renal cancer |
| Bcr-abl | Imatinib (Glivec) | Chronic myeloid leukaemia |
| c-kit | Imatinib (Glivec) | Gastrointestinal stromal tumours |
| Raf/MAPK | Sorafenib | Hepatocellular and renal cancer Clinical trials ongoing in breast cancer, GIST and melanoma |
| Cyclin-dependent kinase | Flavopiridol | Undergoing clinical trials |
| mTor (a key regulator of cell cycle progression) | Everolimus and temsirolimus | Renal Pancreatic neuroendocrine tumours |
| Proteasome inhibitor | Bortezomib | Active in myeloma; trials of combination therapy in progress |
a Drugs at various stages in the process of obtaining a licence for use in the UK.
b The suffix ‘mab’ identifies a monoclonal antibody, whereas ‘nib’ identifies a tyrosine kinase inhibitor.
Targeted biological therapies
Passive immunotherapy using monoclonal antibodies raised against specific tumour-associated antigens on the cell surface
Targeted antibodies have the advantage of high cancer specificity and relatively low host toxicity.
• Rituximab, an anti-CD20 monoclonal antibody, for the treatment of low-grade follicular lymphomas and for use in combination with CHOP (see above) for high-grade lymphoma, as these tumours carry the antigen CD20 on the cell surface.
• Significant over-expression of the Her2/neu (erbB2) cell surface growth factor receptor occurs in approximately 20% of breast cancers and gastric cancers and is associated with a far more aggressive form of breast cancer compared with non-Her2-expressing tumours. Trastuzumab (Herceptin), a humanised monocolonal antibody, binds specifically to the Her2/neu receptor, blocking its function in regulating intracellular processes, including cell proliferation. In combination with conventional cytotoxic chemotherapy, trastuzumab significantly improves the survival of patients with advanced or early breast cancer, compared with cytotoxic chemotherapy alone. A series of key adjuvant trials conducted across Europe and the USA showed that trastuzumab combined with chemotherapy provided the biggest survival gains ever recorded for this disease.9,10,11 Potential cumulative cardiac dysfunction with trastuzumab is dose limiting.
• Her2 is a member of the epidermal growth factor receptor (EGFR) family. EGFRs are highly expressed by about 85% of colorectal cancers and are important in regulating cell proliferation. Another monoclonal antibody, cetuximab (Erbitux), blocks EGFR function and is used for selected colorectal cancers. It was subsequently found that this drug had no benefit in approximately 40% of all colorectal cancers with a mutation in the K-RAS oncogene. The mutation constitutively activates the cell pathway downstream of the EGFR receptor, so blocking it with cetuximab has no effect. This is an important example of personalising medicine to the individual cancer type and selecting which agents are suitable in individual cancers. It is also crucial in the design and selection of patients for future evolutions of this therapy in colorectal cancer, as patients with K-RAS mutations would only get the unwanted effects and absolutely no benefit if treated with EGFR inhibitors. Limiting toxicities for this class of drugs are fatigue, rash, mucositis and hypomagnesaemia.
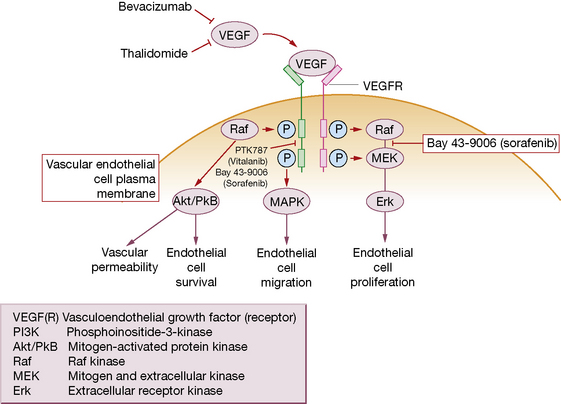
• Vasculoendothelial growth factor (VEGF) is a major angiogenic signal regulator for new blood vessel formation (angiogenesis). Angiogenesis, a process that is common to all cancers, is vital for the growth and establishment of secondary tumours to grow beyond 1–2 mm when diffusion of nutrients becomes insufficient to maintain tumour growth. Blockade of VEGF and its receptor is a successful strategy for treating several types of neoplasm. The monoclonal antibody bevacizumab (Avastin) improves survival or slows tumour growth significantly, when combined with cytotoxic chemotherapy for advanced colorectal, lung and breast cancers. This novel approach evokes a range of adverse drug reactions that differ from those of conventional cytotoxics: hypertension, proteinuria, bleeding, and increased risk of thromboembolic events.
Radioimmunotherapy
Monoclonal antibodies targeted against epitopes12 on tumour cells, e.g. rituximab against CD20 in lymphoma, are conjugated to radionuclides such as yttrium-90 (ibritumomab) or iodine-131 (tositumomab) to deliver radiation directly to the cellular target; they produce durable responses in patients resistant to chemotherapy and unconjugated antibody.
Chemo-immunotherapy
Most therapeutic antibodies are monoclonal immunoglobulin (Ig) G antibodies produced in mammalian cell lines by recombinant DNA technology. Genetic engineering alters the molecular structure of key immunogenic portions of the antibody to generate ‘humanised’ chimeric13 antibodies that avoid rejection by the human immune system (but hypersensitivity reactions may occur).
Signal transduction inhibitors
• Imatinib (Glivec, Gleevec) blocks the dysregulated tyrosine kinase hyperactivity produced by the Philadelphia chromosome (bcr-abl) that occurs in chronic myeloid leukaemia and some cases of acute lymphoblastic leukaemia; clinical trials support its therapeutic efficacy. This is an important example of a drug designed precisely to address the biological abnormality that causes a disease. By serendipity, ii imatinib also blocks an oncogenelled c-kit (CD-117) and has now revolutionised treatment of a rare cancer called GIST (gastrointestinal stromal tumour) that hitherto had no known systemic treatment options (both chemo- and radiotherapy primary resistant), and frequently carries a c-kit mutation.
• Tyrosine kinase inhibitors of VEGF receptor and its downstream effector pathways are also in clinical trial (see Fig. 31.2 and Table 31.3).
• The EGFR tyrosine kinase inhibitors, gefitinib (Iressa) and erlotinib (Tarceva), now find use for a variety of EGFR-expressing tumours, specifically in lung cancer, that have a mutation in the EGFR gene, with marked benefit, although cure remains elusive.
Targeting the cell cycle
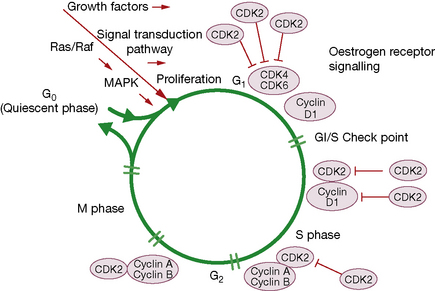
Recent advances in molecular biology have shown that the cell cycle is regulated by a series of proteins that include cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors (Fig. 31.3). Aberrations in these proteins are implicated in uncontrolled progression through the cell cycle (and hence in carcinogenesis), but also represent a new set of therapeutic targets for anticancer therapy, e.g. flavopiridol, which has cyclin-dependent kinase inhibitor activity, and rapamycin, which inhibits mTor (mammalian target of rapamycin), another key regulator of cell cycle progression. Arsenic trioxide modulates cell growth and differentiation, and induces remission in relapsed refractory acute promyelocytic leukaemia in part through apoptosis induction and down-regulation of Bcl-2.
Chemoprevention of cancer
Chemical interventions to reduce cancer risk may be an option for the population as a whole, or for groups at high risk of a specific cancer. Retrospective epidemiological and association studies suggest large effects of certain dietary manipulation but prospective interventional studies have rarely been confirmatory and have even shown harm. The best example is supplemental vitamins, derivatives and dietary micronutrients that may inhibit the development of cancers in the laboratory, e.g. β-carotene, isotretinoin, folic acid, ascorbic acid, α-tocopherol. In a trial of antioxidant supplementation with ascorbic acid, vitamin E, β-carotene, selenium and zinc over 7.5 years, the total cancer incidence was lower in men (who had a lower baseline antioxidant status) than in women.14 Subsequent large-scale randomised trials with supplementation have demonstrated the opposite effect with an unexpectedly higher incidence of new cancers.
Immunosuppression
• Autoimmune, collagen, connective tissue and inflammatory disorders including systemic lupus erythematosus, rheumatoid arthritis, chronic active hepatitis, inflammatory bowel disease, glomerulonephritis, nephrotic syndrome, some haemolytic anaemias and thrombocytopenias, uveitis, myasthenia gravis, polyarteritis, polymyositis, Behçet’s syndrome.
• Organ or tissue transplantation: to prevent immune rejection.
• Cytotoxic cancer chemotherapeutic agents are immunosuppressive because they interfere with mononuclear cell multiplication and function. But they are generally too toxic for the above purposes and the following are principally used for intended immunosuppression:
With the exception of ciclosporin and tacrolimus, all of the above cause non-specific immunosuppression, so that the general defences of the body against infection are impaired.
Adrenal steroids destroy lymphocytes, reduce inflammation and impair phagocytosis (see Ch. 35).
Ciclosporin
Hazards of immunosuppressive drugs
Impaired immune responses render the subject more liable to bacterial, viral and fungal infections. Treat all infection early and vigorously (using bactericidal drugs where practicable); use human γ-globulin to protect when there is exposure to virus infections, e.g. measles, varicella. Patients who have not had chickenpox and are receiving therapeutic (as opposed to replacement) doses of corticosteroid are at risk of severe chickenpox; they should receive varicella zoster immunoglobulin if there has been contact with the disease within the previous 3 months and in some cases prophylactic antivirals such as aciclovir (see p. 213).
A useful general account by several authors covering all aspects of understanding cancer therapy appears in Medicine. 2004;32:1–37.
Arribas J. Matrix metalloproteases and tumor invasion. N. Engl. J. Med.. 2005;352:2020–2021.
El-Shanawany T., Sewell W.A.C., Misbah S.A., Jolles S. Current uses of intravenous immunoglobulin. Clin. Med. (Northfield Il). 2006;6:356–359.
Greenwald P. Cancer chemoprevention. Br. Med. J.. 2002;324:714–718.
Kaur R. Breast cancer: personal account. Lancet. 2005;365:1742.
Khan S., Sewell W.A.C. Oral immunosuppressive drugs. Clin. Med. (Northfield Il). 2006;6:252–355.
Koon H., Atkins M. Autoimmunity and immuno-therapy for cancer. N. Engl. J. Med.. 2006;354:758–760.
Krause D.S., Van Etten R.A. Tyrosine kinase as targets for cancer therapy. N. Engl. J. Med.. 2005;353:172–187.
Renehan A.G., Booth C., Potten C.S. What is apoptosis, and why is it important? Br. Med. J.. 2001;322:1536–1538.
Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med.. 2004;350:1655–1664.
Rosenberg S.A., Yang J.C., Restifo N.P., et al. Cancer immunotherapy: moving beyond current vaccines. Nat. Med.. 2004;10:909–915.
Veronesi U., Boyle P., Goldhirsch A., et al. Breast cancer. Lancet. 2005;365:1727–1741.
Wasan H.S., Bodmer W.F. The inherited susceptibility to cancer. In: Franfs. S., Teich N. The Molecular and Cellular Biology of Cancer. Oxford University Press: Oxford, 1996. (Chapter 4)
Wooster R., Weber B. Breast and ovarian cancer. N. Engl. J. Med.. 2003;348:2339–2347.
1 Although not in strict accord with the definition of Chapter 12, the word ‘chemotherapy’ is generally used in connection with oncology and it would be pedantic to avoid it. It arose because malignant cells can be cultured and the disease transmitted by inoculation, as with bacteria. The more precise term ‘cytotoxic chemotherapy’ is adopted here.
2 Greek: apo, off; ptosis, a falling.
3 Makin G, Dive C 2001 Apoptosis and cancer chemotherapy. Trends in Cell Biology 11:S22–26. (Dysregulated apoptosis is also involved in the pathogenesis of many forms of neoplastic disease, notably lymphomas; understanding its mechanisms and the defective processes offers scope for novel approaches to the treatment of cancer.)
4 As a consequence of the death of a patient following intrathecal administration of vincristine, two inexperienced doctors were charged with manslaughter (Dyer C 2001 Doctors suspended after injecting wrong drug into spine. British Medical Journal 322:257).
5 Beatson G T 1896 Lancet ii:104, 162.
6 Huggins C et al 1941 Cancer Research 1:293.
7 An attenuated strain of Mycobacterium bovis used to prepare the BCG vaccine for immunisation against tuberculosis.
8 Rosenberg B, Van Camp L, Trosko J E et al 1969 Platinum compounds: a new class of potent antitumour agents. Nature 222:385–386.
9 Bursetin H J 2005 The distinctive nature of HER2-positive breast cancers. New England Journal of Medicine 353:1652–1654.
10 Piccard-Gebhart M J, Procter M, Leyland-Jones B et al for the Herceptin Adjuvant (HERA) Trial Study Team 2005 Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New England Journal of Medicine 353:1659–1672.
11 Romond E H, Perez E A, Bryant J et al 2005 Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New England Journal of Medicine 353:1673–1684.
12 The simplest form of an antigenic determinant, on a complex antigenic molecule, that can combine with antibody or T-cell receptor (Stedman’s Medical Dictionary).
13 Composed of seemingly incompatible parts of different origin.
14 Hercberg S, Galan P, Preziosi P et al 2004 Randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 164:2335–2342.