Chapter 1 Visual System
The Eye
Anatomic Features
The eye is a special sense organ made up of three coats, or tunics, as follows:
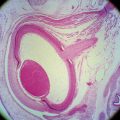
Within this globe are three spaces: the anterior chamber, posterior chamber, and vitreous chamber. The crystalline lens is located in the region of the posterior chamber (Figure 1-1).
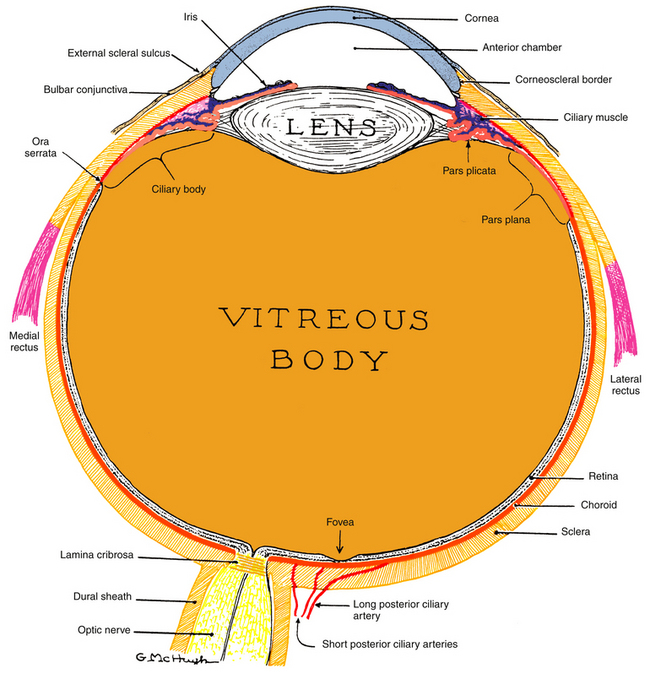
Figure 1-1 The visual system.
(From Kronfeld PC: The human eye, Rochester, NY, 1943, Bausch & Lomb Press.)
The interior of the eye is made up of three chambers. The anterior chamber is bounded in front by the cornea and posteriorly by the iris and anterior surface of the lens. The posterior chamber lies behind the iris and surrounds the equator of the lens, separating it from the ciliary body. The anterior and posterior chambers are continuous with one another through the pupil, and both contain aqueous humor that is produced by the ciliary body. The aqueous humor provides nourishment for the surrounding structures, particularly the cornea and lens. The vitreous chamber, which is the largest space, lies adjacent to the inner retinal layer and is bounded in front by the lens. This chamber contains a gel-like substance, the vitreous humor.
Anatomic Directions and Planes
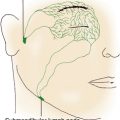
Anatomy is an exacting science, and specific terminology is basic to its discussion. The following anatomic directions should be familiar (Figure 1-2):
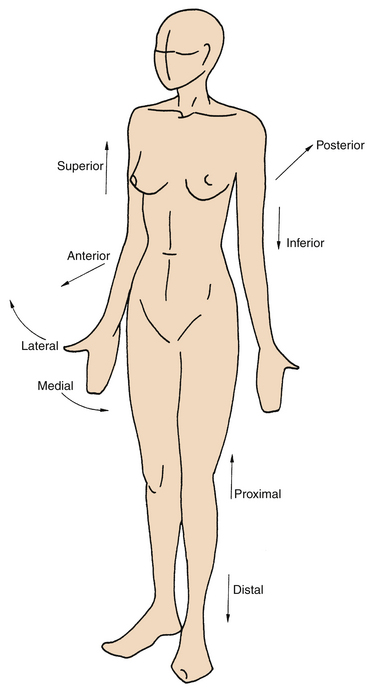
Figure 1-2 Anatomic directions.
(From Palastanga N, Field D, Soames R: Anatomy and human movement, Oxford, UK, 1989, Butterworth-Heinemann.)
The following planes are used in describing anatomic structures (Figure 1-3):
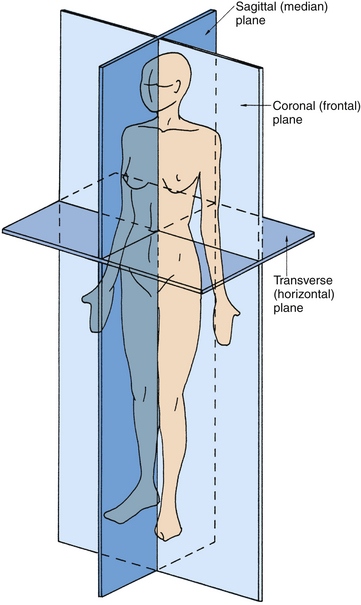
Figure 1-3 Anatomic planes.
(From Palastanga N, Field D, Soames R: Anatomy and human movement, Oxford, UK, 1989, Butterworth-Heinemann.)
Because the globe is a spherical structure, references to locations can sometimes be confusing. In references to anterior and posterior locations of the globe, the anterior pole (i.e., center of the cornea) is the reference point. For example, the pupil is anterior to the ciliary body (see Figure 1-1). When layers or structures are referred to as inner or outer, the reference is to the entire globe unless specified otherwise. The point of reference is the center of the globe, which would lie within the vitreous. For example, the retina is inner to the sclera (see Figure 1-1). In addition, the term sclerad is used to mean “toward the sclera,” and vitread is used to mean “toward the vitreous.”
Refractive Conditions
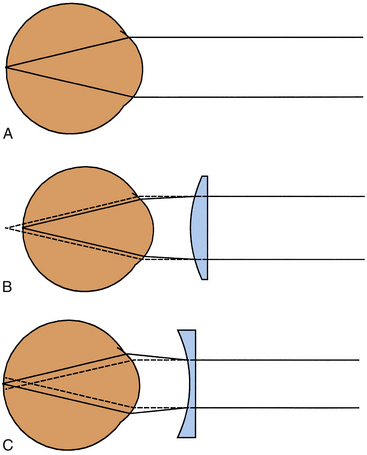
If the refractive power of the optical components of the eye, primarily the cornea and lens, correlate with the distances between the cornea, lens, and retina so that incoming parallel light rays come into focus on the retina, a clear image will be seen. This condition is called emmetropia (Figure 1-4, A). No correction is necessary for clear distance vision. In hyperopia (farsightedness), the distance from the cornea to the retina is too short for the refractive power of the cornea and lens, thereby causing images that would come into focus behind the retina (Figure 1-4, B). Hyperopia can be corrected by placing a convex lens in front of the eye to increase the convergence of the incoming light rays. In myopia (nearsightedness), because the lens and cornea are too strong or, more likely, the eyeball is too long, parallel light rays are brought into focus in front of the retina (Figure 1-4, C). Myopia can be corrected by placing a concave lens in front of the eye, causing the incoming light rays to diverge.
Ophthalmic Instrumentation
The outside of the globe and the eyelids can be assessed with a biomicroscope. This combination of an illumination system and a binocular microscope allows stereoscopic views of various parts of the eye. Particularly beneficial is the view of the transparent structures, such as the cornea and lens. A number of auxiliary instruments can be used with the biomicroscope to measure intraocular pressure and to view the interior of the eye.
Basic Histologic Features
Epithelial Tissue
Epithelial tissue often takes the form of sheets of epithelial cells that either cover the external surface of a structure or that line a cavity. Epithelial cells lie on a basement membrane that attaches them to underlying connective tissue. The basement membrane can be divided into two parts: the basal lamina, secreted by the epithelial cell, and the reticular lamina, a product of the underlying connective tissue layer.1 The free surface of the epithelial cell is the apical surface, whereas the surface that faces underlying tissue or rests on the basement membrane is the basal surface.
Connective Tissue
The fibers found in connective tissue include flexible collagen fibers with high tensile strength, delicate reticular fibers, and elastic fibers, which can undergo extensive stretching. Collagen fibers are a major component of much of the eye’s connective tissue. These fibers are composed of protein macromolecules of tropocollagen that have a coiled helix of three polypeptide chains. The individual polypeptide chains can differ in their amino acid sequences, and the tropocollagen has a banded pattern because of the sequence differences.2 Collagen is separated into various types on the basis of such differences, and several types are components of ocular connective tissue structures.
Muscle Tissue
Muscle tissue is contractile tissue and can be classified as striated or smooth, voluntary or involuntary. Striated muscle has a regular pattern of light and dark bands and is subdivided into skeletal and cardiac muscle. Skeletal muscle is under voluntary control, whereas cardiac muscle is controlled involuntarily. The structure of skeletal muscle and the mechanism of its contraction are discussed in Chapter 10.
Nerve Tissue
Nerve tissue contains two types of cells: neurons, which are specialized cells that react to a stimulus and conduct a nerve impulse, and neuroglia, which are cells that provide structure and metabolic support. The neuron cell body is the perikaryon, which has several cytoplasmic projections. The projections that conduct impulses to the cell body are dendrites (usually several), and the (usually single) projection that conducts impulses away from the cell body is an axon. Schwann cells encircle nerve fibers and can secrete a lipoprotein material, myelin, that then surrounds the Schwann cell covering the fiber. Nerve fibers thus are either myelinated or unmyelinated; myelinization improves impulse conduction speed.3
Neuroglial cells outnumber neurons by a ratio of 10 to 50:1, depending on the location.3 Neuroglial cells include astrocytes, oligodendrocytes, and microglia. Astrocytes provide a framework that gives structural support and contributes to the nutrition of neurons. Oligodendrocytes produce myelin in the central nervous system, where there are no Schwann cells. Microglia possess phagocytic properties and increase in number in areas of damage or disease.3
Brief Review of Human Cellular Physiology
Fluid and solute transport across the cell membrane can occur passively by diffusion that occurs when molecules pass from a higher to a lower concentration down the concentration gradient; no energy is expended. Channel proteins within the cell membrane create water-filled passages linking the intracellular and extracellular spaces. A million ions per second may flow through such a channel and tens of millions of ions per second can enter or exit a cell.4 These channels facilitate ion movement across the lipid bilayer and move ions, also without the expenditure of energy. Channels may be specific for ion type, molecule size, or charge (i.e., Na+ channels, K+ channels, cation channels). Channels can be gated, opening and closing in response to certain stimulants (i.e., voltage-gated, chemical-gated, ligand-gated). In facilitated diffusion, carrier proteins bind to substrates that they carry across the membrane (these are slower and selective but can carry larger molecules); they never form a direct connection between the intracellular and extracellular environments. Molecules such as glucose and amino acids are moved in this way. Active transport mechanisms use energy. Transporters and co-transporters move substance against the concentration gradient and need a steady supply of ATP. Transporting epithelia are polarized and the apical and basal membranes have differing properties; both often contain ion channels; however, the Na+/K+ ATPase pumps are generally located in the basolateral membranes. Aquaporins are bidirectional channels composed of major intrinsic proteins that specifically allow water passage but may not allow other materials to pass through the channel. Aquaporins are numerous in ocular tissues: cornea, lens, ciliary body epithelia, and retina. Membranes containing aquaporins have 100 times greater water permeability than membranes without them.5 Aquaporins may have functions other than transport; some may regulate cell migration processes and some may have a role in neural signal transduction.6
Intercellular Junctions
Intercellular junctions join epithelial cells to one another and to adjacent tissue; some are named by their type and some by their shape. Protein components of intercellular junctions include cell adhesion molecules, transmembrane proteins (occludin, claudin), junctional adhesion molecules, and associated cytoplasmic proteins.7 Junctions between cells or with connective tissue can have additional functions other than adhesion. Physical changes, such as pressure and biochemical or pharmaceutical factors, can modulate junctions and alter the junctional proteins. This allows information about changes in the extracellular environment to be relayed to the cell interior affecting intracellular processes.
In a tight (occluding) junction, the outer leaflet of the cell membrane of one cell comes into direct contact with its neighbor. Ridgelike elevations on the surface of the cell membrance fuse with complementary ridges on the surface of a neighboring cell.8 As the paired strands meet, the neighboring cell membranes are fused.9 The fibers of tight junctions are connected to the cytoskeleton within the cell.
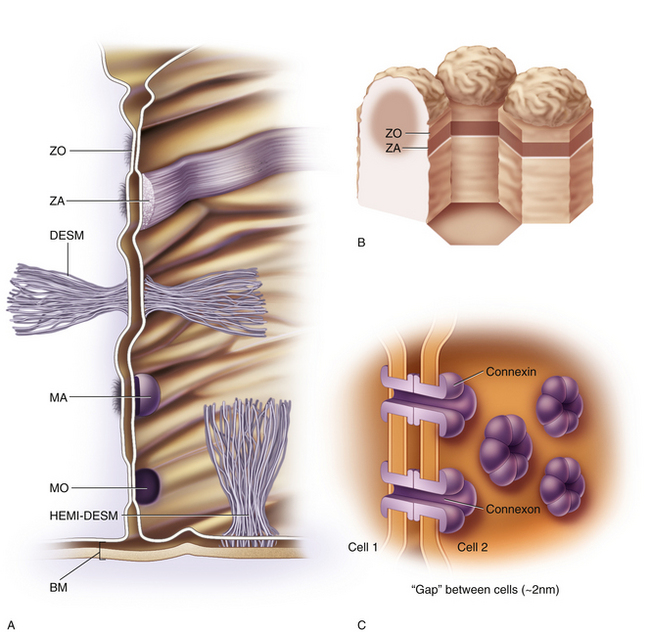
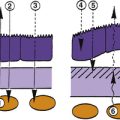
A tight junction that forms a zone or belt around the entire cell, joining it with each of the adjacent cells is called a zonula occludens (ZO) (Figure 1-5). In these zones, row on row of interwining ridges effectively occlude the intercellular space. A substance cannot pass through a sheet of epithelium whose cells are joined by ZO by passing between the cells, but must pass through the cell.In stratified epithelia, whose surface layer is constantly being sloughed and replaced from below, ZO, if present, will be located in the surface layer. The components of the tight junction are found in increasing numbers as a cell moves from its origin in the basal layer until, finally when the cell reaches the surface, its occluding junction is complete.10 The complex formed by the junctional proteins in the ZO can be affected in some diseases, causing in a breakdown in the barrier function, allowing a pathway to open through the network. Currently, researchers are developing pharmaceuticals that will cause a temporary disruption of the barrier, and that would allow other drugs or substances to pass through the intercellular route. In some instances, ridges in a tight junction are fewer and discontinuous, resulting in a “leaky epithelium.”8
A zonula adherens (ZA), an intermediate junction, is a similarly-shaped zone. However, the adjacent plasma membranes are separated, leaving a narrow intercellular space that contains a glycoporotein material causing cell adhesion but allowing intercellular passage.12 ZA junctions produce relatively firm adhesions. Adjacent to the adhering junction are fine microfilaments that extend from a plaque just inside the membrane to filaments of the cytoskeleton, contributing to cell stability.8 A terminal bar consists of a zonula occludens and a zonula adherens side by side, with the tight junction lying nearest the cell apex.1,8
A desmosome is a strong, spotlike attachment between cells (see Figure 1-5). A dense disc or plaque is present within the cytoplasm adajcent to the plasma membrane at the site of the adherence. Hairpin loops of cytoplasmic filaments called tonofilaments extend from the disc into the cytoplasm and link to keratin filaments in the cytoskelton, contributing to cell stability. Other filaments, transmembrane linkers, cadherins, extend from the plaque across the intercellular space, holding the cell membranes together and forming a strong bond.12 The intercellular space contains an acid-rich mucoprotein that acts as a strong adhesive.8
A hemidesmosome provides a strong connection between the cell and its basement membrane and underlying connective tissue. It contains similar intracellular components; the protein complex extends through the cell membrane to attach to keratin in the basement membrane. Bundles of filaments join the intracellular plaque to underlying connective tissue matrix, often attaching to a plaque embedded in the connective tissue.10
Gap junctions are formed by a group of (usually six)proteins, called connexins, that span the cell membrane and unite with connexins of a neighboring cell, forming a channel called a connexon (see Figure 5-1).13 These narrow channels allow rapid cell-to-cell communication, i.e., passage of small molecules and ions from one cell to another. A group of cells with such connection act like a syncytium, that is, a single cell with multiple nuclei.
1. Krause W.J., Cutts J.H. Epithelium. In: Krause W.J., Cutts J.H., editors. Concise text of histology. Baltimore: Williams & Wilkins; 1981:27.
2. Copenhaver W.M., Kelly D.E., Wood R.L. The connective tissues. In: Copenhaver W.M., Kelly D.E., Wood R.L., editors. Bailey’s textbook of histology. ed 17. Baltimore: Williams & Wilkins; 1978:142.
3. Krause W.J., Cutts J.H. Nervous tissue. In: Krause W.J., Cutts J.H., editors. Concise text of histology. Baltimore: Williams & Wilkins; 1981:137.
4. Mergler S., Pleyer U. The human corneal endothelium: new insights into electrophysiology and ion channels. Prog Retin Eye Res. 2007;26:359-378.
5. Agre P., Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72-78.
6. Verkman A.S., Ruiz-Ederra J., Levin M.H. Functions of aquaporins in the eye. Prog Retin Eye Res. 2008;27:420-433.
7. Teranishi S., Kimura K., Kawamoto K., et al. Protection of human corneal epithelial cells from hypoxia-induced disruption of barrier function by keratinocyte growth factor. Invest Ophthalmol Vis Sci. 2008;49:2432-2437.
8. Copenhaver W.M., Kelly D.E., Wood R.L. Epithelium. In: Copenhaver W.M., Kelly D.E., Wood R.L., editors. Bailey’s textbook of histology. ed 17. Baltimore: Williams & Wilkins; 1978:103.
9. Yoshida Y., Ban Y., Kinoshita S. Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Invest Ophthalmol Vis Sci. 2009;50:2103-2108.
10. Suzuki K., Tanaka T., Enoki M., et al. Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2000;41:2495-2500.
11. Delamere N.A. Ciliary body and ciliary epithaliu. In: Fischbarg J., editor. The biology of the eye. Amsterdam, The Netherlands: Elsevier; 2006:127-148.
12. Fawcett D.W., Jensh R.P. Bloom & Fawcett concise histology. London: Chapman & Hall; 1997. p 19
13. Shurman D.L., Glazewski L., Gumpert A., Zieske J.D., Richard G. In vivo and in vitro expression of connexins in the human corneal epithelium. Invest Ophthalmol Vis Sci. 2005;46:1957-1965.