12
Visual Acuity, Refractive Procedures, and Sudden Vision Loss
Refractive Error
Definition
The state of an eye in which light rays are not properly focused on the retina, resulting in image blur.
Ametropia
A refractive error (e.g., myopia, hyperopia, or astigmatism).
Anisometropia
A difference in refractive error between the two eyes; usually 2D or more.
Astigmatism
Light rays are unequally focused producing two lines rather than a single point because the curvature of the cornea or, less commonly, the curvature of the lens varies in different meridians. If the cornea is steeper in the vertical meridian, it is referred to as “with-the-rule” astigmatism; if it is steeper in the horizontal meridian, it is called “against-the-rule” astigmatism. Astigmatism can also be designated regular (symmetric or asymmetric) or irregular. A cylindrical lens corrects regular astigmatism.
Emmetropia
No refractive error; light rays are focused on the retina, and thus no corrective lens is required for distance vision.
Hyperopia (Farsightedness)
Light rays are focused at a point behind the retina. A “plus” spherical lens is used to correct this refractive error.
Myopia (Nearsightedness)
Light rays from a distant object are focused at a point in front of the retina and those from a near object are focused on the retina. A “minus” spherical lens is used to correct this refractive error.
Presbyopia
Loss of accommodation of the crystalline lens with age; average age of the onset of symptoms is early 40s and this process continues through the early 60s. A “plus” spherical lens (i.e., bifocal “add”) is used to correct this problem.
Etiology
Astigmatism
The curvature of the cornea or, less commonly, the curvature of the lens varies in different meridians. Acquired forms may be caused by disorders of the eyelids (tumor, chalazion, ptosis), cornea (pterygium, limbal dermoid, degenerations, ectasias, surgery), lens (cataract), and ciliary body (tumor).
Hyperopia
The refractive power of the cornea is too weak or the axial length of the eye is too short. Acquired forms may be caused by disorders that decrease the eye’s refractive power (alteration in the lens [posterior lens dislocation, aphakia, diabetes], drugs [chloroquine, phenothiazines, antihistamines, benzodiazepines], poor accommodation [tonic pupil, drugs, trauma], corneal flattening [contact-lens-induced], intraocular silicone oil) or effective length (central serous retinopathy, retrobulbar masses, choroidal tumors).
Myopia
The refractive power of the eye is too strong or the axial length of the eye is too long. Acquired forms may be caused by disorders that increase the eye’s refractive power (alteration in the lens [diabetes, galactosemia, uremia, cataracts, anterior lenticonus, anterior lens dislocation], drugs [sulfonamides, miotic agents], excessive accommodation, corneal steepening [keratoconus, congenital glaucoma, contact-lens-induced]) or effective length (congenital glaucoma, posterior staphyloma, retinopathy of prematurity, scleral buckle surgery). Myopia also increases in the dark (night myopia).
Presbyopia
Loss of lens elasticity or possibly reduced ciliary muscle effectivity. Premature presbyopia may occur with debilitating illness, diphtheria, botulism, mercury toxicity, head injury, cranial nerve III palsy, Adie’s tonic pupil, and tranquilizers.
Epidemiology
Hyperopia is normally present during infancy and early childhood and then declines between ages 8 and 13 years, resulting in emmetropia in most adults. The incidence of refractive errors in the US population is approximately 25% for myopia and 25% for hyperopia; 50% have some degree of astigmatism. After the age of 40 years old, 50% of people have hyperopia. Hyperopia is associated with shallow anterior chambers and narrow angles; myopia is associated with lattice degeneration and retinal detachment.
Symptoms
Decreased vision when not wearing corrective lenses; distant objects are blurry and near objects are clear (myopia), distant and near objects are blurry (hyperopia), or near objects that are blurry become clearer when held further away (presbyopia). May have asthenopia (eye strain) due to sustained accommodative effort (overcorrected myopia, or undercorrected or uncorrected hyperopia; asthenopia may also be produced by convergence insufficiency or accommodative insufficiency [hypothyroidism, anemia, pregnancy, nutritional deficiencies, and chronic illness]). Hyperopia may be asymptomatic in children and young adults, who can compensate enough with accommodation.
Signs
Decreased uncorrected visual acuity that improves with pinhole testing, glasses, or contact lenses; hyperopes may have normal uncorrected visual acuity.
Differential Diagnosis
Normal eye exam except for decreased vision: amblyopia, retrobulbar optic neuropathy, other optic neuropathies (toxic, nutritional), nonorganic (functional) visual loss, rod monochromatism, cone degeneration, retinitis pigmentosa sine pigmento, cortical blindness.
Evaluation
• Consider potential acuity meter (PAM) testing, rigid contact lens overrefraction, and corneal topography (computerized videokeratography) if irregular astigmatism is suspected.
Prognosis
Good except for pathologic myopia (see Chapter 10).
Refractive Surgery Complications
Intraocular Refractive Procedures
Used to correct moderate to high degrees of myopia and hyperopia.
Refractive Lens Exchange
The noncataractous crystalline lens can be removed and replaced with an intraocular lens implant of appropriate power to correct the resulting aphakia. The main disadvantage of this method is loss of accommodation. This procedure is controversial for myopia because of the risk of retinal detachment in pseudophakic eyes; hyperopic eyes with nanophthalmos may develop choroidal effusions.
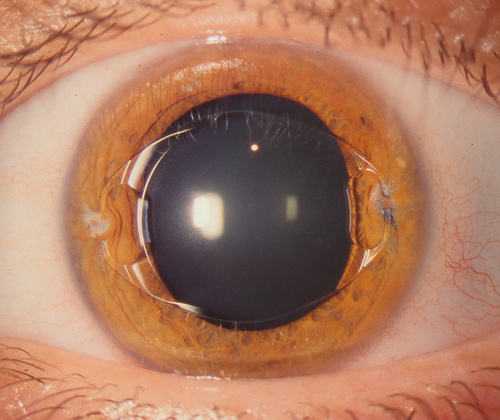
Phakic Intraocular Lens
A lens implant is placed in the anterior chamber, posterior chamber, or fixated to the iris with the optic centered over the pupil. Prophylactic peripheral iridotomies are performed prior to surgery to prevent postoperative pupillary block angle-closure glaucoma (see Chapter 6).
Symptoms
Postoperatively may have photophobia, pain, decreased vision, glare, and halos.
Signs and Complications
Corneal edema (endothelial cell loss), cataract, glaucoma, pupillary block, iridocyclitis, and endophthalmitis; may have residual refractive error. Additional complications of clear lens extraction include posterior capsular opacification, cystoid macular edema, retinal detachment, suprachoroidal hemorrhage, choroidal effusion, retained lens material, and iris damage.
Corneal Refractive Procedures
Incisional
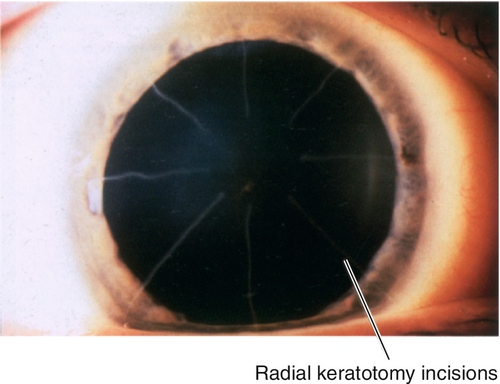
Radial keratotomy (RK)
This procedure corrects low-to-moderate myopia. Deep, radial, corneal incisions are created with a diamond knife to flatten the central cornea. The surgical effect depends on various factors including depth and number of incisions, size of optical zone, patient age and gender, design of diamond blade, and surgeon experience. Radial keratotomy can be combined with astigmatic keratotomy for compound myopia (myopia with astigmatism). It is rarely performed anymore.
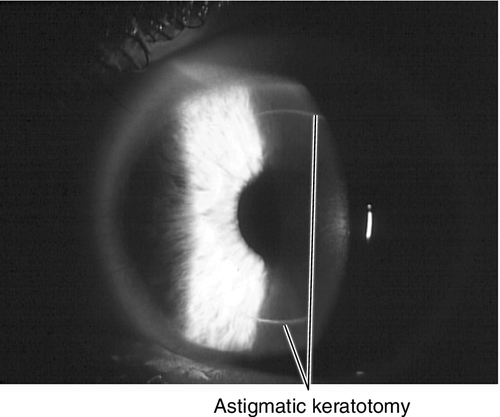
Astigmatic keratotomy
This procedure corrects corneal astigmatism. Deep, midperipheral arcuate or straight incisions (parallel to the limbus) are made (traditionally with a diamond knife and more recently with femtosecond lasers) on the steep corneal meridian to flatten it. As with RK, the surgical effect depends on depth and number of incisions, size of optical zone (usually 7 mm), patient age, and surgeon experience. The incisions must not be > 90° or intersect with RK incisions. This technique is useful after penetrating keratoplasty for high-to-moderate astigmatism.
Limbal relaxing incisions / peripheral corneal relaxing incisions
Similar to astigmatic keratotomy, this technique is used to correct low amounts of astigmatism, usually in association with cataract surgery. Arcuate incisions, 500–600 μm deep with a guarded blade, are placed at the 10–11 mm optical zone concentric to the limbus to correct 1–2D of astigmatism. Because ultrasound is not required, these relaxing incisions can be performed at the time of surgery or in the clinic. Femtosecond lasers allow these incisions to be created at greater depth, at smaller optical zones (8–10 mm), and also intrastromally, with more precise control of each parameter. Corneal topography is recommended prior to performing this procedure.
Symptoms
Postoperatively may have decreased vision (due to undercorrection, overcorrection, or irregular astigmatism), fluctuating vision, difficulty with night vision, halos, glare, starbursts, ghost images, double vision, and foreign body sensation.
Signs and Complications
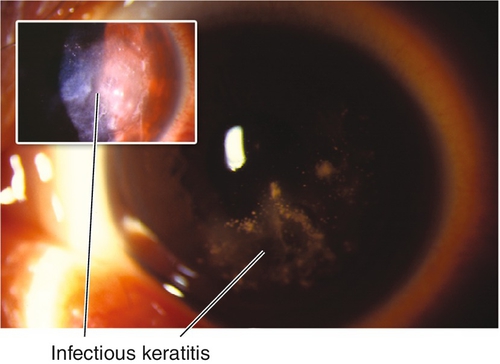
Corneal scarring, infection, or perforation; may have residual refractive error, regression, or progression over time.
Excimer Laser
This ultraviolet laser (193 nm) ablates corneal tissue to correct myopia (central ablation), hyperopia (peripheral ablation), regular astigmatism (eliptical ablation), irregular astigmatism (topography-guided ablation), or reduce higher-order aberrations (wavefront ablation).
Photorefractive keratectomy (PRK)
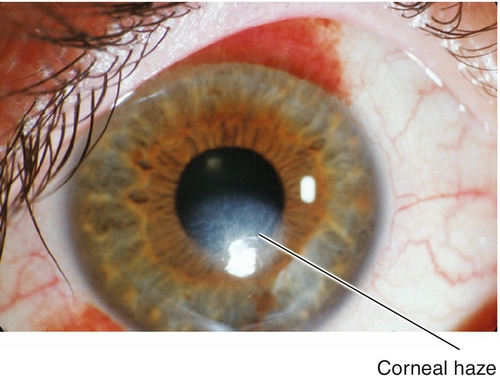
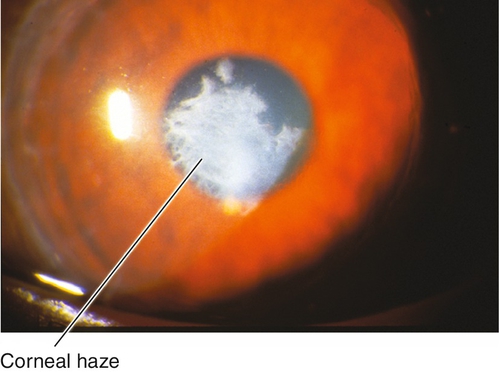
This procedure uses excimer laser ablation to reshape the corneal stroma after epithelial removal (mechanical, alcohol, or laser). The visual recovery period is longer than with LASIK, but final visual outcomes are similar in low and moderate myopia. Patients may experience initial pain due to the epithelial defect, and decreased vision due to corneal haze.
Laser-assisted subepithelial keratectomy and epithelial-laser in-situ keratomileusis
These procedures combine PRK and laser in-situ keratomileusis (LASIK) techniques. A flap composed of only epithelium is created with topical alcohol (LASEK) or a mechanical epithelial separator (epi-LASIK), the epithelial flap is carefully retracted, laser energy is applied to the stromal bed, and the epithelial flap is replaced or excised. This procedure may combine the advantages of PRK and LASIK by decreasing the incidence of pain and haze associated with PRK and risk of flap complications associated with LASIK.
Laser in-situ keratomileusis
This procedure combines automated lamellar keratoplasty and PRK techniques. A mechanical microkeratome or femtosecond laser is first used to cut a partial-thickness hinged corneal flap, which is then folded back. The programmed excimer laser energy is applied to the underlying stromal bed, and the flap is replaced. LASIK allows for faster visual recovery and minimal pain after surgery, but there is a higher risk of complications due to flap-related problems than with PRK.
Symptoms
Postoperatively may have decreased vision (due to undercorrection, overcorrection, irregular astigmatism, or haze), fluctuating vision, difficulty with night vision, halos, glare, starbursts, ghost images, double vision, and variable discomfort (ranging from foreign body sensation to moderate pain).
Signs and Complications
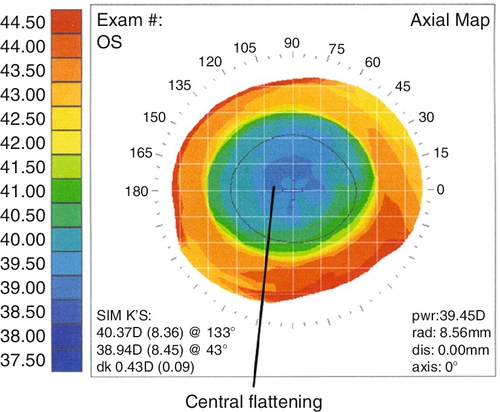
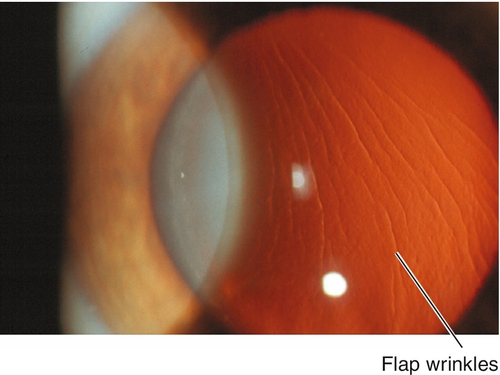
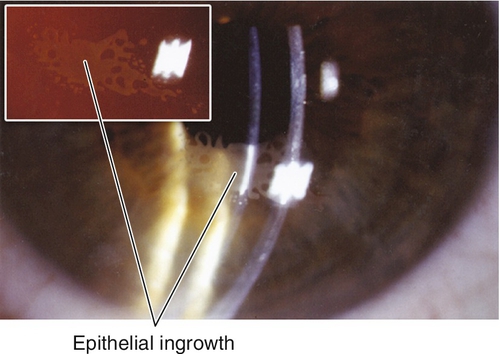
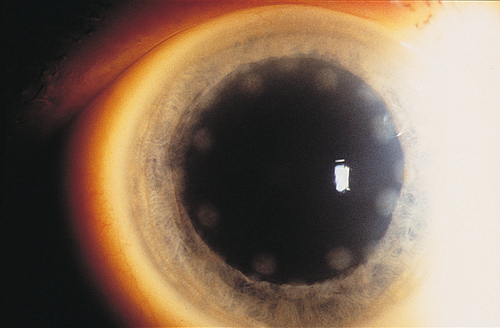
Corneal scarring, infection, keratectasia, central toxic keratopathy, decentration (apparent on corneal topography), or dry eyes; may have residual refractive error, regression over time. Additional LASIK complications include flap striae, epithelial ingrowth, flap trauma, and diffuse lamellar keratitis (DLK, “sands of the Sahara”). Haze from surface ablation may be early (due to delayed epithelial healing) or late (≥ 3 months, due to deeper ablations and UV exposure).
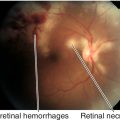
Figure 12-10 Laser in-situ keratomileusis flap striae demonstrating vertically curved wrinkles in the flap.
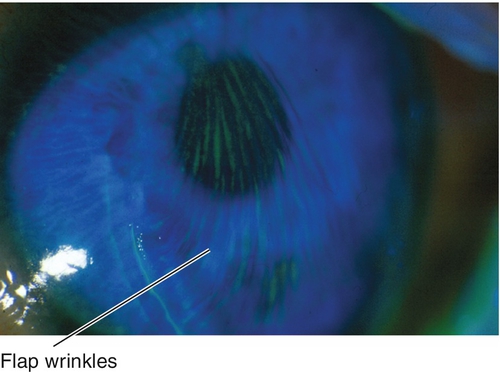
Figure 12-11 Same patient as Figure 12-10 with laser in-situ keratomileusis flap striae demonstrating the curved striae that are enhanced with topical fluorescein and viewed with a blue light.
Implants
Intracorneal inlays
This procedure combines a partial-thickness corneal flap (similar to LASIK) with a thin, contact-lens-like, intrastromal implant that is placed in the central optical zone to correct refractive errors without removing corneal tissue. It may be most useful for treating presbyopia.
Intrastromal corneal ring segments (Intacs)
Polymethylmethacrylate (PMMA) ring segment implants are placed into peripheral corneal channels at two-thirds depth outside the visual axis to correct low-to-moderate myopia by flattening the cornea without cutting or removing tissue from the central optical zone. These channels can be made manually or with a femtosecond laser. Also used to treat mild-to-moderate keratoconus by shifting and stabilizing the cone, allowing for improved contact lens tolerance and best spectacle-corrected visual acuity.
Symptoms
Postoperatively may have decreased vision (due to undercorrection, overcorrection, or irregular astigmatism), fluctuating vision, difficulty with night vision, halos, glare, and foreign body sensation.
Signs and Complications
Implant extrusion, implant decentration, infection, stromal deposits (intrastromal corneal ring segments), flap striae (intracorneal inlays), epithelial ingrowth (intracorneal inlays); may have residual refractive error.
Thermokeratoplasty
Conductive keratoplasty
Radiofrequency energy is applied to the peripheral cornea to shrink collagen tissue and treat low-to-moderate hyperopia through central corneal steepening. A hand-held contact probe with a 450 μm needle directly delivers energy in a ring pattern outside the visual axis. The number of spots determines the amount of central steepening (8 to 32 spots).
Symptoms
Postoperatively may have pain, foreign body sensation, decreased vision (irregular astigmatism, regression), glare, and halos.
Signs and Complications
Corneal scarring in area of application, regression over time; may have residual refractive error.
Refractive Surgery Complications: Evaluation / Management
Evaluation
• Corneal topography (computerized videokeratography).
• Consider rigid contact lens overrefraction if irregular astigmatism is suspected.
Prognosis
Usually good; depends on specific surgical technique and any complications. Hyperopia treatments tend to regress with time.
Vertebrobasilar Insufficiency (Vertebrobasilar Atherothrombotic Disease)
Definition
Vertebrobasilar (posterior) circulation constitutes the arterial supply to the brain stem, cerebellum, and occipital cortex. Impaired blood flow to these areas may manifest in a myriad of symptoms. Transient ischemic attacks (TIAs) in this vascular territory are referred to as vertebrobasilar insufficiency (VBI).
Etiology
Thrombus, emboli, hypertension, arrhythmias, arterial dissection, hypercoagulable states, and subclavian steel syndrome. Approximately one-fourth of strokes and TIAs occur in the vertebrobasilar distribution. MRI studies suggest that 40% of patients with vertebrobasilar TIAs have evidence of brain-stem infarction.
Epidemiology
Male predilection (2 : 1); occurs in elderly, usually in seventh to eighth decades.
Symptoms
Vertigo is the hallmark of vertebrobasilar ischemia. Additionally, bilateral transient blurring or dimming of vision (seconds to minutes), photopsias (flashes of light), diplopia, unilateral weakness, sensory loss, ataxia, nystagmus, facial numbness, dysarthria, hoarseness, dysphagia, hearing loss, and vertigo; history of drop attacks (syncope) can occur.
Signs
Usually none; may have small, paracentral, congruous, homonymous visual field defects.
Differential Diagnosis
Amaurosis fugax, migraine, papilledema, temporal arteritis, labyrinthitis, vestibular neuronitis, benign paroxysmal positional vertigo.
Evaluation
• Check visual fields.
• Lab tests: Complete blood count (CBC), erythrocyte sedimentation rate (ESR), and blood glucose (hypoglycemia).
• Check blood pressure.
• Head and orbital magnetic resonance imaging (MRI).
• Cervical spine radiographs (cervical spondylosis).
• Medical consultation for complete cardiovascular evaluation, including electrocardiogram, echocardiogram, duplex and Doppler scans of carotid and vertebral arteries.
• Neurology consultation.
Prognosis
Usually poor.
Migraine
Definition
A neurologic syndrome characterized by recurrent attacks lasting 4–72 hours, often with unilateral throbbing moderate-to-severe headache, sensory changes, gastrointestinal symptoms, and mood disturbances. Five stages have been described: prodrome (premonitory symptoms 24–72 hours before other symptoms), aura (transient neurologic symptoms, usually visual), headache, resolution, and recovery. Often migraine is divided into the following categories: without aura, with aura, variant, and complicated. The formal migraine classification (according to the International Headache Society, second edition [IHS-2]) is:
Migraine without Aura (80%)
“Common” migraine; headache may or may not be preceded by a poorly defined prodrome of mood fluctuations (elation, hyperexcitability, food cravings, irritability, restlessness, depression, feeling of impending doom), excessive energy, fatigue, frequent yawning, and gastrointestinal disturbances (anorexia, nausea, and emesis).
Migraine with Aura
Aura (transient neurologic symptoms) develops over 5–20 minutes and lasts < 60 minutes; most commonly visual (bilateral) consisting of scintillations, zigzag lines (fortification phenomenon), spreading scotomas, tunnel vision, micropsia, blurred vision, and altitudinal field loss; can also be vertigo, sensory or motor disturbances, fainting, confusion, or possibly auditory hallucinations. Headache, nausea, photophobia, phonophobia, emesis, irritability, and malaise usually follow immediately after or within an hour; other symptoms include diarrhea, vertigo, tremors, sweating, and chills. Subtypes include:
Typical aura with migraine headache (15–20%)
“Classic” migraine; gradual aura lasting less than 1 hour.
Typical aura with nonmigraine headache
Headache does not satisfy criteria for migraine headache.
Typical aura without headache (5%)
“Acephalgic” migraine; aura (usually visual) without headache. Often occurs in older individuals with a history of classic migraine; 25% have a family history of migraine.
Hemiplegic migraine
Familial or sporadic; rare. Unilateral motor weakness or partial paralysis.
Basilar-type migraine (Bickerstaff syndrome)
Aura mimics vertebrobasilar artery insufficiency with brain-stem or bilateral occipital lobe symptoms (i.e., blindness, diplopia, vertigo, difficulty with speech or coordination). Rare; typically occurs in teenage girls and young women.
Childhood Periodic Syndromes
Recurrent disorders in children with migraine features that are usually precursors of migraine. These include: cyclical vomiting (recurrent episodes of nausea and emesis [at least 5 within 5 days]), abdominal migraine (recurrent attacks of midline abdominal pain for up to 72 hours with nausea, emesis, anorexia, and / or pallor), and benign paroxysmal vertigo of childhood (recurrent vertigo attacks, commonly with emesis or nystagmus).
Retinal Migraine
“Ocular” migraine; rare; monocular scotoma or visual loss for minutes to an hour due to retinal or optic nerve ischemia.
Complications of Migraine
This category includes: chronic migraine (headache for more than 15 days in a month), status migrainosus (headache lasting longer than 72 hours despite treatment, continuous or with brief interruptions [< 4 hours]), persistent aura without infarction (aura lasting longer than 72 hours), migrainous infarction (stroke during a migraine or neurologic deficit lasting longer than 1 week with corresponding lesion on neuroimaging), and migraine-triggered seizures (“migralepsy”; seizure during or immediately after a migraine).
Etiology
Although not completely elucidated, mounting evidence supports the role of neurogenic peptides in migraine formation, such as serotonin and dopamine, in the brain. These vasoactive neuropeptides stimulate an inflammatory cascade with the release of endothelial cells, mast cells, and platelets that produce vasodilation and a perivascular reaction. The susceptibility to headaches in migraineurs is due to a baseline state of hyperexcitability in the cerebral cortex, particularly the occipital area. The aura is caused by cortical spreading depression (a wave of neuronal excitation followed by suppression with blood vessel constriction and dilation); the headache is due to neurochemical release causing inflammation and blood vessel dilation. Dopamine stimulation may play a role, particularly in the prodrome. The serotonin receptor (5-HT) is believed to be the most important receptor in the headache pathway.
Epidemiology
Affects approximately 18% of women and 6% of men in the USA. Can occur at any age; family history of migraine in 70%, or a history of motion sickness is common. Commonly triggered by foods, alcohol, caffeine withdrawal, drugs (nitroglycerine, reserpine, hydralazine, ranitidine, estrogen), hormonal changes (menstruation, ovulation, oral contraceptives, hormone replacement), sleep alteration, exertion, stress, fatigue, and head trauma (also fasting, strong odors, flickering lights, sun glare, smoky or noisy environments, altitude, and weather changes). Associated with epilepsy, familial dyslipoproteinemias, Tourette syndrome, hereditary essential tremor, ischemic stroke, depression, asthma, patent foramen ovale. Migraine often demonstrates maternal inheritance and occurs more frequently in patients with mitochondrial disorders. Familial hemiplegic migraine has been mapped to chromosomes 19p13 and 1q.
Symptoms
May have prodrome, aura, headache, or other neurologic, gastrointestinal, or emotional symptoms (see above).
Signs
Usually none; may have visual field defect, cranial nerve palsy, or other neurologic deficits.
Differential Diagnosis
Serious: Meningitis, subarachnoid hemorrhage, temporal arteritis, malignant hypertension, intracranial tumor, arteriovenous malformation, vertebrobasilar artery insufficiency, aneurysm, subdural hematoma, transient ischemic attack, occipital or temporal lobe seizure, cerebrovascular accident.
Others: Tension or cluster headaches, trigeminal neuralgia, temporomandibular joint syndrome, cervical spondylosis, herpes zoster ophthalmicus, sinus or dental pathology, uveitis, angle-closure glaucoma, after lumbar puncture, nonorganic, caffeine withdrawal, carbon monoxide inhalation, and nitrite exposure.
Evaluation
• Check visual fields.
• Check blood pressure and temperature.
• Consider lumbar puncture for suspected meningitis.
• Neuroimaging for complicated migraines or migraines with neurologic deficits.
• Medical or neurology consultation.
Prognosis
Good.
Convergence Insufficiency
Definition
Decreased fusional convergence with near fixation, a remote near point of convergence and a low accommodation convergence/accommodation (AC / A) ratio.
Etiology
Idiopathic but possibly innervational in origin; may be induced by drugs, trauma, or prismatic effect of glasses (base-out).
Epidemiology
Prevalence is estimated at 3–5%; typically occurs in teenagers and young adults, rare before 10 years of age; slight female predilection. Common cause of asthenopia (eye strain); aggravated by anxiety, illness, or lack of sleep. Often associated with an exophoria or intermittent exotropia at near.
Symptoms
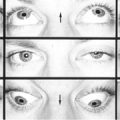
Asthenopia, diplopia, blurred vision, headaches, difficulty reading.
Signs
Inability to maintain fusion at near (patient closes one eye while reading to relieve visual fatigue), distant near point of convergence, reduced fusional convergence amplitudes; possible exophoria at near without exotropia (especially after prolonged reading).
Differential Diagnosis
Presbyopia, refractive error (uncorrected hyperopia, overcorrected myopia), accommodative insufficiency.
Evaluation
Prognosis
Usually good.
Accommodative Excess (Accommodative Spasm)
Definition
A clinical state of excessive accommodation (lens focusing); spasm of the near reflex is the triad of excess accommodation, excess convergence, and miosis.
Etiology
Often triggered by stress or prolonged reading.
Symptoms
Blurred distance vision, headache, brow ache, eye strain at near; may have diplopia.
Signs
Abnormally close near point, miosis, and normal amplitude of accommodation; relief of symptoms with cycloplegia and cycloplegic refraction uncovers more hyperopia than on manifest refraction.
Differential Diagnosis
Iridocyclitis, uncorrected refractive errors (usually hyperopes, but also astigmats and myopes), pseudomyopia (acquired myopia from systemic disorders, drugs, forward lens movement [see Myopia section]).
Evaluation
Prognosis
Good.
Functional Visual Loss
Definition
Visual abnormality not attributable to any organic disease process (nonphysiologic).
Malingering
Fabrication of the existence or extent of a disorder for secondary gain.
Hysteria
Subconscious (not willful) expression of symptoms.
Epidemiology
Twenty percent have coexistent organic disease. Malingering is usually associated with a financial incentive (i.e., legal action or compensation claim).
Symptoms
Decreased vision (monocular or binocular, usually very vague about quality of symptoms), diplopia, metamorphopsia, oscillopsia.
Signs
Decreased visual acuity (20 / 20 to no light perception), abnormal visual field (usually inconsistent or has characteristic pattern); may have voluntary nystagmus, gaze palsy, or blepharospasm. Malingerer is often uncooperative and combative, hysteric is often indifferent but cooperative; otherwise normal examination (especially pupils, optokinetic nystagmus response, fundus).
Differential Diagnosis
Functional visual loss is a diagnosis of exclusion; must rule out organic disease, especially amblyopia, early keratoconus, anterior basement membrane dystrophy, early cataracts, central serous retinopathy, early Stargardt’s disease, retinitis pigmentosa sine pigmento, rod monochromatism, cone degeneration, retrobulbar optic neuritis, optic neuropathy, and cortical blindness.
Evaluation
• Check visual fields (monocular and binocular); beware tunnel vision, spiraling fields, crossing isopters.
• Consider corneal topography (computerized videokeratography).
• Consider electroretinogram, visual evoked response, fluorescein angiogram, or neuroimaging in difficult cases or if unable to prove normal vision and visual field.
Prognosis
No improvement in up to 30%.
Transient Visual Loss (Amaurosis Fugax)
Definition
Unilateral transient visual loss due to ischemia. This is a type of TIA also called temporary monocular blindness (TMB).
Etiology
Usually embolic from carotid artery or cardiac valvular disease; also due to arrhythmias, coagulation disorders, hyperviscosity syndromes, vasospasm, hypotension, and rarely an orbital mass.
Symptoms
Monocular, brief, reversible dimming or loss of vision (typically 2–5 minutes).
Signs
Usually none; may have visible emboli in retinal vessels.
Differential Diagnosis
Vertebrobasilar artery insufficiency, migraine, temporal arteritis, papilledema, pseudopapilledema (optic nerve drusen).
Evaluation
• Check visual fields.
• Medical consultation for complete cardiovascular evaluation including electrocardiogram, echocardiogram, and carotid Doppler studies.
Prognosis
Depends on etiology; 1-year risk of cerebrovascular accident is 2%.
Amblyopia
Definition
Unilateral or bilateral loss of best corrected vision in an otherwise anatomically normal eye. Classified as mild (better than 20/40), moderate (20/40–20/80), and severe (20/ 100–20/400) based on visual acuity.
Etiology
Reduced visual input or abnormal binocularity during visual development (up to 6–8 years old). Various types:
Strabismic
Most common form of amblyopia; develops in a child when the visual input of a deviated eye is inhibited in order to prevent diplopia and visual confusion.
Refractive
Anisometropic
Due to unequal, uncorrected refractive errors between the two eyes, which causes a constant defocusing of the image; usually requires greater than 3D of myopic, greater than 1D of hyperopic, and greater than 1.5D of astigmatic anisometropia. May have associated strabismus (strabismic anisometropia).
Isoametropic
Bilateral amblyopia due to large, but equal, uncorrected refractive errors; usually requires greater than 8D of myopia, greater than 5D of hyperopia, and greater than 2.5D of astigmatism.
Deprivation
Uncommon; usually caused by congenital or acquired visual impairment such as cataract, corneal opacity, or ptosis; often results in significant loss of vision. Occlusion amblyopia (reverse amblyopia) is a rare form of deprivation amblyopia resulting from excessive treatment of amblyopia and is usually transient and reversible.
Epidemiology
Present in approximately 2% of general population. Increased risk with developmental delay, prematurity, ocular trauma, and positive family history. Strabismic and anisometropic account for more than 90% of amblyopia; isoametropic and deprivation are rare, each accounting for 1–2% of amblyopia.
Symptoms
Variable decreased vision.
Signs
Decreased visual acuity, which often improves with single-letter testing (due to crowding phenomenon: difficulty distinguishing optotypes that are close together) or is stable with neutral-density filters (strabismic amblyopia), decreased contrast sensitivity, reduced stereopsis; may have strabismus, nystagmus, ptosis, cataract, corneal opacity, small relative afferent pupillary defect.
Differential Diagnosis
Functional visual loss, retinal or optic nerve disorder.
Evaluation
• Complete eye exam with attention to vision with neutral-density filters and single letters, cycloplegic refraction, retinoscopy, contrast sensitivity, stereopsis, pupils, motility, lids, cornea, lens, and ophthalmoscopy. Accurate diagnosis is difficult in children < 3 years old.
• Consider imaging studies of the retina and optic nerve to rule out organic etiology.
Prognosis
Depends on severity and duration of amblyopia, age at which appropriate treatment is initiated (the younger the better), compliance with treatment, and type of amblyopia (poor for deprivation and high anisometropic, best for strabismic). Amblyopia responds to refractive correction alone in up to 77%. Occlusion therapy is successful in 73% after 1 year. The Amblyopia Treatment Studies (ATS) showed that, for children 3–7 years old, 6 hours of daily patching is as effective as full-time patching for severe amblyopia, and 2 hours is as effective as 6 hours of daily patching or twice-weekly atropine for moderate amblyopia. For children 7–12 years old, 2–6 hours of daily patching and atropine can improve amblyopia even if it has been previously treated. For children 13–17 years old, 2–6 hours of daily patching may improve amblyopia that has not been previously treated. The outcome of occlusion treatment is better in children < 6 years old than in those > 6 years old. After successful treatment, recurrence of amblyopia occurs in 25% within the first year (majority within 6 months) after discontinuation of occlusion therapy and is more common in children younger than 10 years old, with strabismic amblyopia, and when therapy is stopped abruptly and not tapered. Recurrence can be as high as 53% after 3 years.
Cortical Blindness (Cortical Visual Impairment)
Definition
Rare syndrome of bilateral blindness with intact pupillary light response due to widespread damage to the occipital lobes.
Etiology
Cerebrovascular accident (bilateral occipital lobe infarction), rarely neoplasia.
Symptoms
Complete visual loss; rarely, denies blindness (Anton’s syndrome), visual hallucinations.
Signs
Normal eye exam (including pupillary response) except for no light perception vision in both eyes; may demonstrate the Riddoch phenomenon (ability to perceive moving, but not static, objects) and “blind sight” (intact primitive mechanism that may allow navigation around objects).
Differential Diagnosis
Functional visual loss.
Evaluation
• Head and orbital CT scan or MRI.
• Medical and neurology consultation.
Prognosis
Poor.
Visual Pathway Lesions
Definition
Lesion of the visual pathway anywhere from the optic nerve to the occipital lobes (see Chapter 11 for Optic nerve lesions).
Etiology
Most commonly vascular or neoplastic; also due to trauma, infection, multiple sclerosis, sarcoidosis, and other rarer causes.
Symptoms
Asymptomatic or may notice decreased vision or visual field loss.
Signs
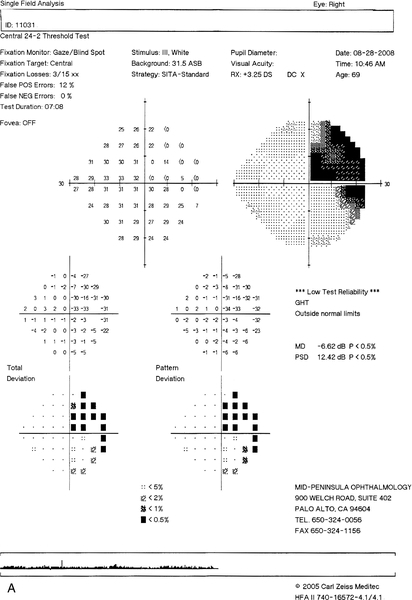
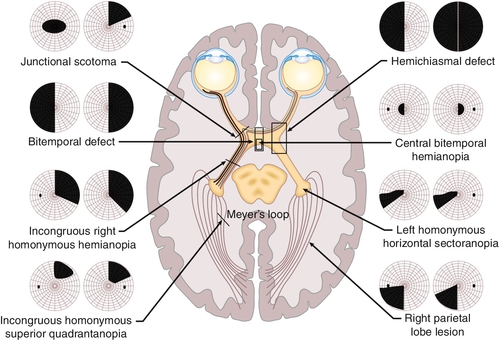
Normal or decreased visual acuity, visual field defect (usually a hemianopia or quadrantanopia [see Fig. 12-17]); may have relative afferent pupillary defect (if the lesion is anterior to the optic chiasm), may have associated endocrine abnormalities or other neurologic deficits.
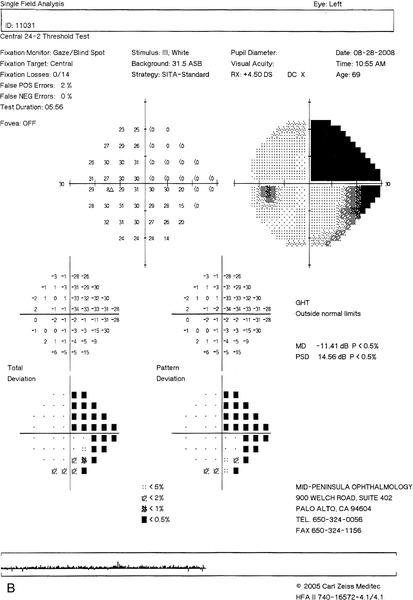
Figure 12-17 Humphrey visual fields of a patient with a stroke demonstrating an incongruous right homonymous hemianopic field defect that is denser superiorly.
Differential Diagnosis
Amaurosis fugax, migraine, temporal arteritis, vertebrobasilar artery insufficiency, functional visual loss.
Evaluation
• Check visual fields (defect helps localize the area of involvement).
• Head and orbital CT scan or MRI.
• Medical and neurology consultation.
Prognosis
Usually poor, but depends on etiology.