Viral Pathogenesis
I Factors Affecting Viral Virulence
1. Species that can be infected by a virus
2. Cells must express surface molecules recognized by a viral attachment protein or other structure.
3. Cells must provide compatible biochemical machinery to replicate virus.
B Routes of viral entry into host cells
• Initial viral replication generally occurs at the site of entry, but some viruses spread to target tissues where major pathologic effects occur.
C Tissue specificity (tropism)
• Consequences of viral infection depend on target organs involved and extent of the damage to these tissues.
1. Local infection without spread (e.g., rhinovirus, other common cold viruses)
• Viruses that manifest symptoms at the initial site of entry cause diseases with short incubation periods and early prodromes.
2. Viremic spread from viral point of entry (e.g., measles, mumps, and chickenpox viruses) results in diseases with longer incubation periods.
• Example: varicella-zoster virus (VZV) is acquired by respiratory route and initiates infection in the lungs. Infection spreads through blood to liver and other organs, initiates a secondary viremia, and then reach the skin to cause classic symptoms of chickenpox. Incubation period is 10 to 30 days.
• Attenuated virus strain that cannot reach or infect its disease-related target organ may lose its virulence (e.g., attenuated live polio vaccine, cannot infect the brain to cause major disease).
• Virus-specific antibodies can block viremic spread to target tissue.
D Support of viral replication by host cells
1. Permissive cells possess all the biochemical machinery needed by a virus to enter the cell and replicate, yielding a productive infection.
2. Nonpermissive cells do not support replication, but they may be transformed by DNA tumor viruses (e.g., Epstein-Barr virus and HPV).
3. Semipermissive cells allow some viral functions to occur or support low levels of replication.
1. Major viral mechanisms for disrupting the structure and functioning of infected cells are summarized in Table 19-1.
TABLE 19-1
| Mechanism | Representative Viruses |
| Inhibition of cellular protein synthesis | Polioviruses, HSV, togaviruses |
| Inhibition of cellular DNA synthesis; degradation of DNA | Herpesviruses |
| Alteration of cell membranes | |
| Glycoprotein insertion | All enveloped viruses |
| Syncytia (giant cell) formation | HSV, varicella-zoster virus, paramyxoviruses, HIV |
| Permeability changes | Togaviruses, herpesviruses |
| Disruption of cytoskeleton | Naked capsid viruses, HSV |
| Formation of inclusion bodies | |
| Negri bodies (cytoplasmic) | Rabies |
| Basophilic (owl’s eye) nuclear bodies | Cytomegalovirus |
| Cowdry type A nuclear bodies | HSV |
| Nuclear basophilic bodies | Adenoviruses |
| Acidophilic perinuclear bodies | Reoviruses |
| Toxicity of virion components | Adenovirus fibers |
| Immunosuppression | HIV, cytomegalovirus, measles virus, influenza virus |
HIV, human immunodeficiency virus; HSV, herpes simplex virus.
2. Appearance of characteristic inclusion bodies in infected cells facilitates laboratory diagnosis of infection by some viruses (e.g., rabies, HSV, and cytomegalovirus).
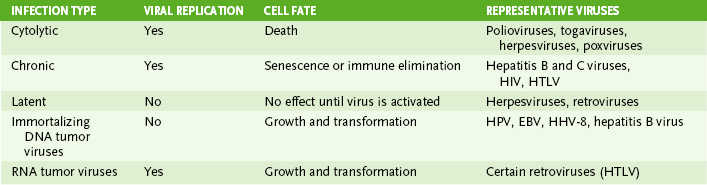
• Classification of viral infections based on the fate of virus-infected cells is shown in Table 19-2.
• Viral replication leads to death of target cell
• Relatively rapid antibody-mediated immune resolution is common.
• Continual production of virions or viral components occurs without cell lysis or immune resolution.
• Virus remains dormant within certain cells until reactivated by stress or immunosuppression to become cytolytic or chronic.
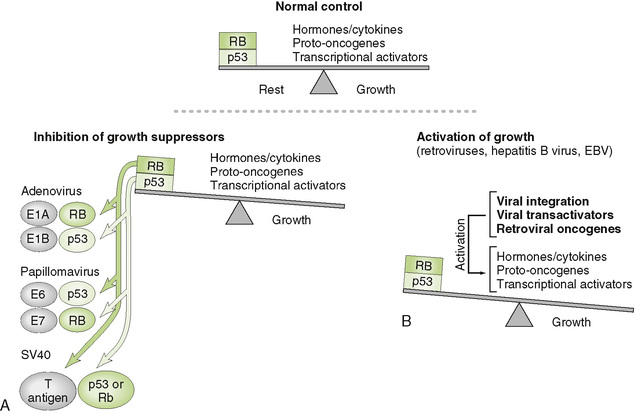
• Persistent infection by tumor viruses promotes uncontrolled cell growth, contributing to transformation of infected cells into cancer cells (Fig. 19-1).
• DNA tumor viruses do not replicate in infected cells (production of virions leading to cell lysis would preclude transformation).
III Antiviral Host Response and Immunopathogenesis
• Resolution of viral infections requires elimination of extracellular virions and destruction of virus-infected cells.
A Key components of antiviral response (see Chapter 4, Fig. 4-6)
1. Interferons (IFN-α and IFN-β) secreted by infected cells induce an antiviral state in neighboring noninfected cells, thus limiting spread of infection within host tissues.
2. Natural killer cells activated by IFN kill infected cells.
3. Antibodies have a major role in resolution of lytic infections.
• Antibody can inactivate (neutralize) or help eliminate (opsonize) free virions.
• Antibody is essential for controlling viruses that are spread by viremia.
4. TH1 inflammatory and cell-mediated cytolytic responses are essential for resolution of nonlytic and enveloped virus infections because virus may not kill the infected cells.
B Pathologic effects of antiviral response
• The immune response to viral infections is responsible for many of the clinical manifestations of these infections, especially those that are nonlytic (Table 19-3).
TABLE 19-3
| Pathologic Effect | Immune Mediators | Representative Viruses |
| Flu-like symptoms | Interferons, other cytokines | Respiratory viruses, viremia-inducing viruses |
| Delayed-type hypersensitivity and inflammation | T cells, macrophages, cytokines | Enveloped viruses |
| Immune complex deposition | Antibody, complement, macrophages | Hepatitis B virus |
| Hemorrhage | T cells, antibody, complement | Dengue virus |
| Postinfection cytolysis | T cells | Enveloped viruses |
C Viral escape from immune responses
• Viruses employ various mechanisms to evade or reduce host antiviral responses, thereby increasing their spread and persistence in the host.
1. Formation of syncytia, which permit direct cell-to-cell spread of virions, protecting them from antibody
2. Initiation of latency in certain cells
3. Antigenic shift in viral proteins that induce immune response
4. Lymphocyte destruction or injury
5. Inhibition of IFN antiviral activity
6. Inactivation of complement (e.g., HSV)
7. Release from infected cells of viral antigen that can block antibody neutralization of virions
IV Clinical Course of Viral Diseases
• Viral infections may be asymptomatic or symptomatic. Site of disease manifestation and time needed for damage to occur determine the incubation period (Table 19-4).
TABLE 19-4
Incubation Periods of Common Viral Infections
| Incubation Period | Disease (Virus) |
| Very short (<1 wk) | Acute respiratory disease (adenoviruses), common cold (rhinoviruses), croup and bronchiolitis (parainfluenza virus), influenza |
| Short (1-3 wk) | Chickenpox, measles, mumps, rubella |
| Long (4-21 wk) | Hepatitis B, mononucleosis (Epstein-Barr virus), rabies, warts (papillomaviruses) |
| Very long (1-10 yr) | Acquired immunodeficiency syndrome (human immunodeficiency virus) |
1. Diseases that manifest at the site of entry generally have incubation periods of less than 1 week.
2. Diseases that manifest in tissues distant from the site of entry have incubation periods ranging from about 1 week to several months (rabies).
3. Diseases that result from immunopathogenesis or slow accumulation of tissue damage (e.g., neuronal) may have incubation periods ranging from several weeks (HBV and Epstein-Barr virus) to years (acquired immunodeficiency syndrome [AIDS]).
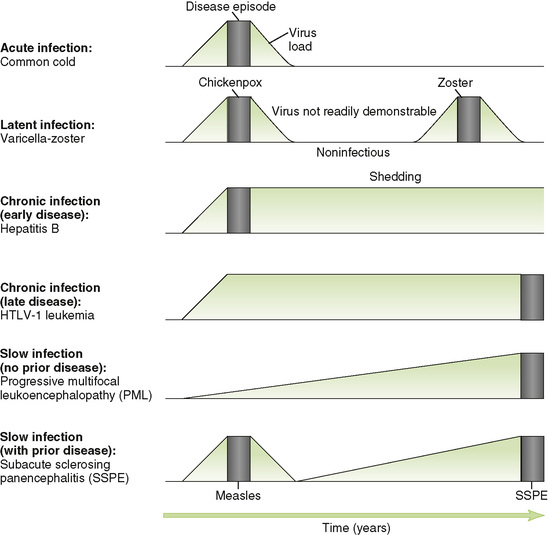
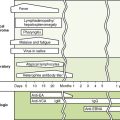
B Acute versus persistent infections (Fig. 19-2)
C Conventional slow infections
• These infections, which all involve neuronal tissue, are characterized by a very slow increase in viral load over a long period of time and gradual progression once symptoms appear.
1. Progressive multifocal leukoencephalopathy caused by JC virus usually occurs in immunocompromised individuals.
2. Subacute sclerosing panencephalitis is a rare late complication due to defective measles virus in the brain.
3. HTLV-1 initial infection is asymptomatic or causes mononucleosis-like disease, and then 25 to 30 years later, it causes adult acute T cell lymphocytic leukemia (ATLL).