Chapter 350 Viral Hepatitis
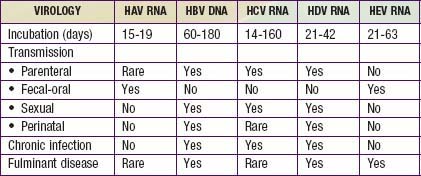
Viral hepatitis continues to be is a major health problem in both developing and developed countries. This disorder is caused by at least 5 pathogenic hepatotropic viruses recognized to date: hepatitis A, B, C, D, and E viruses (Table 350-1). Many other viruses (and diseases) can cause hepatitis, usually as 1 component of a multisystem disease. These include herpes simplex virus (HSV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), varicella-zoster virus, HIV, rubella, adenoviruses, enteroviruses, parvovirus B19, and arboviruses (Table 350-2).
Table 350-2 CAUSES AND DIFFERENTIAL DIAGNOSIS OF HEPATITIS IN CHILDREN
INFECTIOUS
NON-VIRAL LIVER INFECTIONS
AUTOIMMUNE
METABOLIC
TOXIC
ANATOMIC
HEMODYNAMIC
NON-ALCOHOLIC FATTY LIVER DISEASE
From Wyllie R, Hyams JS, editors: Pediatric gastrointestinal and liver disease, ed 3, Philadelphia, 2006, Saunders.
Issues Common to All Forms of Viral Hepatitis
Common Biochemical Profiles in the Acute Infectious Phase
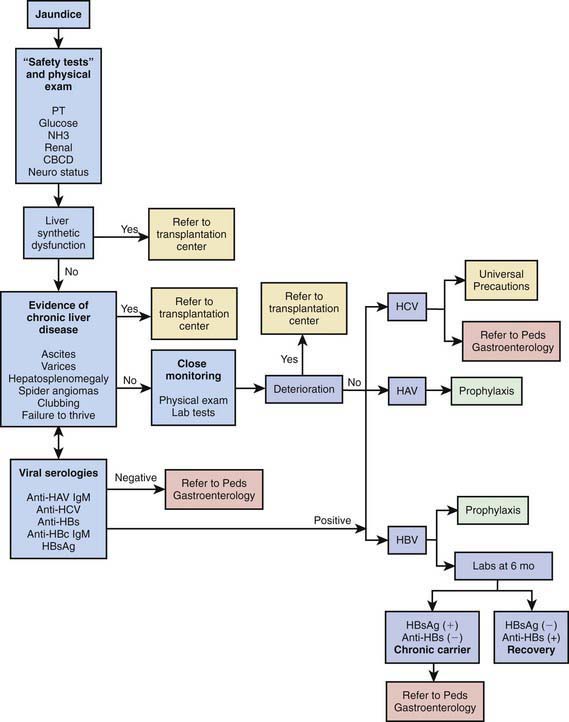
The most important marker of liver injury is altered synthetic function. Monitoring of synthetic function should be the main focus in clinical follow-up to define the severity of the disease. In the acute phase, the degree of liver synthetic dysfunction guides treatment and helps to establish intervention criteria. Abnormal liver synthetic function is a marker of liver failure and is an indication for prompt referral to a transplant center. Serial assessment is necessary because liver dysfunction does not progress linearly. Synthetic dysfunction is reflected by a combination of abnormal protein synthesis (prolonged prothrombin time, high international normalized ratio [INR], low serum albumin levels), metabolic disturbances (hypoglycemia, lactic acidosis, hyperammonemia), poor clearance of medications dependent on liver function, and altered sensorium with increased deep tendon reflexes (hepatic encephalopathy; Chapter 356).
Hepatitis A
Clinical Manifestations
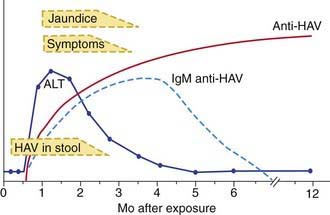
The illness is much more likely to be symptomatic in older adolescents or adults, in patients with underlying liver disorders, and in those who are immunocompromised. It is characteristically an acute febrile illness with an abrupt onset of anorexia, nausea, malaise, vomiting, and jaundice. The typical duration of illness is 7-14 days (Fig. 350-1).
Diagnosis
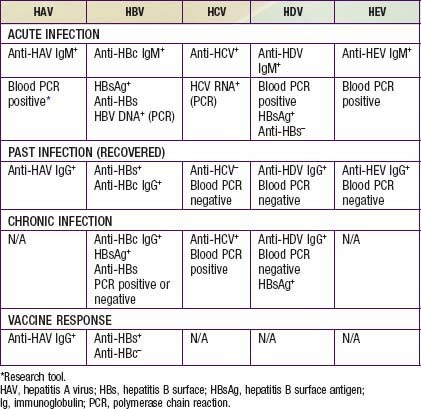
Acute HAV infection is diagnosed by detecting antibodies to HAV, specifically, anti-HAV (IgM) by radioimmunoassay or, rarely, by identifying viral particles in stool. A viral polymerase chain reaction (PCR) assay is available for research use (Table 350-3). Anti-HAV is detectable when the symptoms are clinically apparent, and it remains positive for 4-6 mo after the acute infection. A neutralizing anti-HAV (IgG) is usually detected within 8 wk of symptom onset and is measured as part of a total anti-HAV in the serum. Anti-HAV (IgG) confers long-term protection.
Prevention
Immunoglobulin
Indications for intramuscular administration of immunoglobulin (IG) (0.02 mL/kg) include pre-exposure and postexposure prophylaxis (Table 350-4).
| PRE-EXPOSURE PROPHYLAXIS (TRAVELERS TO ENDEMIC REGIONS) | ||
|---|---|---|
| Age | Exposure | Dose |
| <1 yr of age | Expected <3 mo | Ig 0.02 mL/kg |
| Expected 3-5 mo | Ig 0.06 mL/kg | |
| Expected long term | Ig 0.06 mL/kg at departure and every 5 mo thereafter | |
| ≥1 yr of age | Healthy host | HAV vaccine |
| Immunocompromised host, or one with chronic liver disease or chronic health problems | HAV vaccine and Ig 0.02 mL/kg | |
| POSTEXPOSURE PROPHYLAXIS* | |
|---|---|
| Exposure | Recommendations |
| ≤2 wk since exposure | < 1 y of age: IG 0.02 mL/kg Immunocompromised host, or host with chronic liver disease or chronic health problems: IG 0.02 mL/kg and HAV vaccine >1 yr and healthy host: HAV vaccine, IG remains optional Sporadic non-household or close contact exposure: prophylaxis not indicated* |
| >2 wk since exposure | None |
Ig, immunoglobulin.
* Decision for prophylaxis in non-household contacts should be tailored to individual exposure and risk.
Vaccine
The availability of two inactivated, highly immunogenic, and safe HAV vaccines has had a major impact on the prevention of HAV infection. Both vaccines are approved for children >1 yr of age. They are administered intramuscularly in a 2-dose schedule, with the 2nd dose given 6-12 mo after the 1st dose. Seroconversion rates in children exceed 90% after an initial dose and approach 100% after the 2nd dose; protective antibody titer persists at least for 10 yr. The immune response in immunocompromised persons, older patients, and those with chronic illnesses may be suboptimal; in those patients, combining the vaccine with IG for pre- and postexposure prophylaxis is indicated. HAV vaccine may be administered simultaneously with other vaccines. A combination HAV and HBV vaccine is currently undergoing trials in adults. For healthy persons >1 yr of age, vaccine is preferable to immunoglobulin for pre-exposure and postexposure prophylaxis (see Table 350-3).
Hepatitis B
Clinical Manifestations
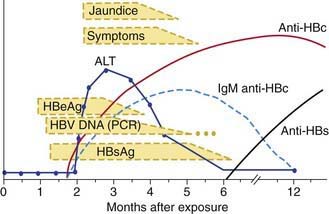
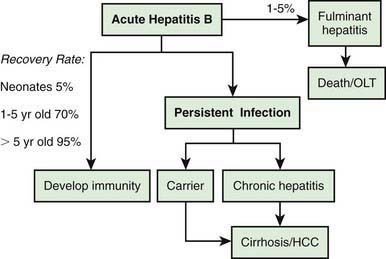
Many acute cases of HBV infection in children are asymptomatic, as evidenced by the high carriage rate of serum markers in persons who have no history of acute hepatitis. The usual acute symptomatic episode is similar to that of HAV and HCV infections but may be more severe and is more likely to include involvement of skin and joints (Fig. 350-2). The first biochemical evidence of HBV infection is elevation of serum ALT levels, which begin to rise just before development of fatigue, anorexia, and malaise, which occurs about 6-7 wk after exposure. The illness is preceded in a few children by a serum sickness–like prodrome marked by arthralgia or skin lesions, including urticarial, purpuric, macular, or maculopapular rashes. Papular acrodermatitis, the Gianotti-Crosti syndrome, can also occur. Other extrahepatic conditions associated with HBV infections in children include polyarteritis, glomerulonephritis, and aplastic anemia. Jaundice, which is present in ∼25% of acutely infected patients, usually begins ∼8 wk after exposure and lasts for ∼4 wk.
Diagnosis
The serologic profile of HBV infection is more complex than for HAV infection and differs depending on whether the disease is acute or chronic (Fig. 350-3). Several antigens and antibodies are used to confirm the diagnosis of acute HBV infection (see Table 350-3). Routine screening for HBV infection requires assay of ≥3 serologic markers (HBsAg, anti-HBc, anti-HBs). HBsAg is the first serologic marker of infection to appear and is found in almost all infected persons; its rise closely coincides with the onset of symptoms. Persistence of HBsAg beyond 6 mo defines the chronic infection state. During recovery from acute infection, because HBsAg levels fall before symptoms wane, IgM antibody to HBcAg (anti-HBc IgM) might be the only marker of acute infection. Anti-HBc IgM rises early after the infection and remains positive for many months before being replaced by anti-HBc IgG, which then persists for years. Anti-HBc is therefore a valuable serologic marker of acute HBV infection. Anti-HBs marks serologic recovery and protection. Only anti-HBs is present in persons immunized with hepatitis B vaccine, whereas both anti-HBs and anti-HBc are detected in persons with resolved infection. HBeAg is present in active acute or chronic infection and is a marker of infectivity. The development of anti-HBe marks improvement and is a goal of therapy in chronically infected patients. HBV DNA can be detected in the serum of acutely infected patients and chronic carriers. High DNA titers are seen in patients with HBeAg, and they typically fall once anti-HBe develops.
Prevention
Hepatitis B Immunoglobulin
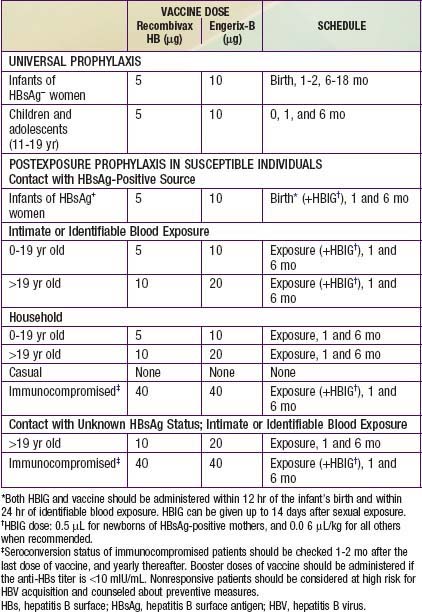
HBIG is indicated only for specific postexposure circumstances and provides only temporary protection (3-6 mo) (Table 350-5). It plays a pivotal role in preventing perinatal transmission when administered within 12 h of birth.
Universal Vaccination
Current HBV vaccination recommendations are as follows (see Table 350-5):
Postexposure Prophylaxis
Recommendations for postexposure prophylaxis for prevention of hepatitis B infection depend on the conditions under which the person is exposed to HBV (see Table 350-5). Vaccination should never be postponed if written records of the exposed person’s immunization history are not available, but every effort should still be made to obtain those records.
Hepatitis C
Epidemiology
Risk factors for HCV transmission in the United States previously included blood transfusion as the most common route of infection, but, with the current screening practices, the risk of HCV transmission is now about 0.001% per unit transfused. Illegal drug use with exposure to blood or blood products from HCV-infected persons accounts for more than half of adult cases in the United States. Sexual transmission, especially through multiple sexual partners, is the second most common cause of infection. Other risk factors include occupational exposure; ∼10% of new infections has no known transmission source. In children, perinatal transmission is the most prevalent mode of transmission (see Table 350-1). Perinatal transmission occurs in up to 5% of infants born to viremic mothers. HIV co-infection and high viremia titers (HCV RNA positive) in the mother can increase the transmission rate to 20%. The incubation period is 7-9 wk (range, 2-24 wk).
Clinical Manifestations
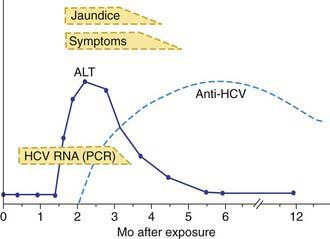
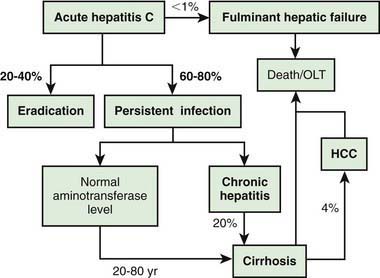
Acute HCV infection tends to be mild and insidious in onset (Fig. 350-4; see also Table 350-1). ALF rarely occurs. HCV is the most likely hepatotropic virus to cause chronic infection (Fig. 350-5). Of affected adults, <15% clear the virus; the rest develop chronic hepatitis. In pediatric studies, 6-19% of children achieved spontaneous sustained clearance of the virus during a 6 yr follow-up.
Diagnosis
Clinically available assays for detection of HCV infection are based on detection of antibodies to HCV antigens or detection of viral RNA (see Table 350-3); neither can predict the severity of liver disease.
Hepatitis D
Epidemiology
HDV can cause an infection at the same time as the initial HBV infection (co-infection), or HDV can infect a person who is already infected with HBV (super-infection). Transmission usually occurs by intrafamilial or intimate contact in areas of high prevalence, which are primarily developing countries (see Table 350-1). In areas of low prevalence, such as the United States, the parenteral route is far more common. HDV infections are uncommon in children in the United States but must be considered when ALF occurs. The incubation period for HDV super-infection is about 2-8 wk; with co-infection, the incubation period is similar to that of HBV infection.
Diagnosis
HDV has not been isolated and no circulating antigen has been identified. The diagnosis is made by detecting IgM antibody to HDV; the antibodies to HDV develop ∼2-4 wk after co-infection and ∼10 wk after a super-infection. A test for anti-HDV antibody is commercially available. PCR assays for viral RNA are available as research tools (see Table 350-2).
Hepatitis E
Epidemiology
Hepatitis E is the epidemic form of what was formerly called non-A, non-B hepatitis. Transmission is fecal-oral (often waterborne) and is associated with shedding of 27-34 nm particles in the stool (see Table 350-1). The highest prevalence of HEV infection has been reported in the Indian subcontinent, the Middle East, Southeast Asia, and Mexico, especially in areas with poor sanitation. The mean incubation period is ∼40 days (range, 15-60 days).
Diagnosis
Recombinant DNA technology has resulted in development of antibodies to HEV particles, and IgM and IgG assays are available to distinguish between acute and resolved infections (see Table 350-3). IgM antibody to viral antigen becomes positive after ∼1 wk of illness. Viral RNA can be detected in stool and serum by PCR.
Approach to Acute or Chronic Hepatitis
Identifying deterioration of the patient with acute hepatitis and the development of ALF is a major contribution of the primary pediatrician (Fig. 350-6). If ALF is identified, the clinician should immediately refer the patient to a transplant center; this can be lifesaving.
Chronic hepatitis can be a stigmatizing disease for children and their families. The pediatrician should offer, with proactive advocacy, appropriate support for them as well as needed education for their social circle. Scientific data and information about support groups are available for families on the websites for the American Liver Foundation (www.liverfoundation-ne.org) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (www.naspghan.org), as well as through pediatric gastroenterology centers.
Centers for Disease Control and Prevention. Hepatitis A vaccination coverage among children aged 24–35 months—United States, 2006 and 2007. MMWR. 2009;58:689-694.
Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the advisory committee on immunization practices (ACIP). MMWR. 2007;56:1080-1084.
Ciocca M, Moreira-Silva SF, Alegria S, et al. Hepatitis A as an etiologic agent of acute liver failure in Latin America. Pediatr Infect Dis J. 2007;26:711-716.
Koslap-Petraco MB, Shub M, Judelsohn R. Hepatitis A: disease burden and current childhood vaccination strategies in the united states. J Pediatr Health Care. 2008;22:3-11.
Lieberman JM, Word BM, et al. Universal hepatitis A vaccination in the United States: a call for action. Pediatr Infect Dis J. 2008;27:287-291.
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685-1694.
Cooke GS, Main J, Thursz MR. Treatment for hepatitis B. BMJ. 2010;340:87-90.
D’Antiga L, Aw M, Mieli-Vergani G, et al. Combined lamivudine/interferon alpha treatment in immunotolerant children perinatally infected with hepatitis B: a pilot study. J Pediatr. 2006;148:228-233.
Kao JH, Chen DS. Hepatitis B vaccination: to boost or not to boost? Lancet. 2005;366:1337-1338.
Lai CL, Yuen MF. Chronic hepatitis B—new goals, new treatment. N Engl J Med. 2008;359(23):2488-2491.
The Medical Letter. Telbivudine (Tyzeka) for chronic hepatitis B. Med Lett. 2007;49:11-12.
Murray KF, Shah U, Mohan N, et al. Chronic hepatitis. J Pediatr Gastroenterol Nutr. 2008;47:225-233.
Shah U, Kelly D, Chang MH, et al. Management of chronic hepatitis B in children. J Pediatr Gastroenterol Nutr. 2009;48:399-404.
Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med. 2009;150:104-109.
Blackard JT, Shata MT, Shire NJ, Sherman KE. Acute hepatitis C virus infection: a chronic problem. Hepatology. 2008;47(1):321-331.
Bortolotti F, Verucchi G, Camma C, et al. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroentrology. 2008;134:1900-1907.
Fernandez-Rodriguez CM, Alonso S, Martinez SM, et al. Peginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: outcomes and factors predicting response. Am J Gastroenterol. 2010;105:2164-2172.
Hoofnagle JH. A step forward in therapy for hepatitis C. N Engl J Med. 2009;360(18):1899-1901.
Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321-332.
McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838.
McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa 2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580-592.
Milazzo L, Antinori S. STAT-C: a full revolution or just a step forward. Lancet. 2010;376:662-663.
Muir AJ, Shiffman ML, Zaman A, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822-832.
Nash KL, Bentley I, Hirschfield GM. Managing hepatitis C virus infection. BMJ. 2009;339:37-42.
Rodriguez-Torres M, Jeffers LJ, Sheikh MY, et al. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;360:257-267.
Emerson SU, Purcell RH. Hepatitis E. Pediatr Infect Dis J. 2007;26:1147-1148.
Holmberg SD. Hepatitis E vaccine: not a moment too soon. Lancet. 2010;376:849-850.
Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817.
Kunilholm MH, Purcell RH, McQuillan GM, et al. Epidemiology of hepatitis E virus in the United States: results from the third national health and nutrition examinstion survey, 1988–1994. JID. 2009;200:48-56.
The Lancet. Hepatitis E vaccine: why wait? Lancet. 2010;376:845.
Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895-902.