Chapter 19 Vertebral Augmentation
 PV (bone cement injection into fractured vertebrae) and PK balloon augmentation followed by bone cement injection into fractured vertebrae are safe and effective procedures used in the treatment of patients with painful VCFs.
PV (bone cement injection into fractured vertebrae) and PK balloon augmentation followed by bone cement injection into fractured vertebrae are safe and effective procedures used in the treatment of patients with painful VCFs. During the PV and PK procedures, careful needle placement is critical with the use of omniplanar fluoroscopic imaging.
During the PV and PK procedures, careful needle placement is critical with the use of omniplanar fluoroscopic imaging. Injection of PMMA must be done slowly and carefully using a viscous opacified cement mixture to avoid extrusion or injection must be halted promptly upon PMMA extrusion.
Injection of PMMA must be done slowly and carefully using a viscous opacified cement mixture to avoid extrusion or injection must be halted promptly upon PMMA extrusion. Overinjection of PMMA should be avoided, particularly with respect to the posterior cortical wall and spinal canal or neural foramen region.
Overinjection of PMMA should be avoided, particularly with respect to the posterior cortical wall and spinal canal or neural foramen region. Any post-procedure neurological abnormality mandates a careful evaluation, including consideration of prompt computed tomography to define the PMMA injection in three dimensions and guide potential therapeutic options, including surgical decompression.
Any post-procedure neurological abnormality mandates a careful evaluation, including consideration of prompt computed tomography to define the PMMA injection in three dimensions and guide potential therapeutic options, including surgical decompression. Vertebroplasty and kyphoplasty are effective, relatively simple procedures to treat VCF-related pain.
Vertebroplasty and kyphoplasty are effective, relatively simple procedures to treat VCF-related pain. These procedures can be safely performed on very frail, medically ill patients with careful attention to positioning, light sedation, copious local anesthetic, and a gentle touch.
These procedures can be safely performed on very frail, medically ill patients with careful attention to positioning, light sedation, copious local anesthetic, and a gentle touch. The exact role of “balloon” versus “no balloon” procedures remains to be evaluated by a randomized controlled trial, and currently mainly expert opinion guides this decision making.
The exact role of “balloon” versus “no balloon” procedures remains to be evaluated by a randomized controlled trial, and currently mainly expert opinion guides this decision making. These procedures involve placing a large (at least 13 g) needle deeply into the spine. Care must be taken not to cause harm due to needle misplacement.
These procedures involve placing a large (at least 13 g) needle deeply into the spine. Care must be taken not to cause harm due to needle misplacement. Osteoporotic patients will suffer new fractures in many cases (up to 25% of patients); these patients MUST be on aggressive anti-osteoporotic therapy, or referred for such care.
Osteoporotic patients will suffer new fractures in many cases (up to 25% of patients); these patients MUST be on aggressive anti-osteoporotic therapy, or referred for such care.Introduction
Vertebral compression fractures (VCFs) can result from a variety of conditions, which include but are not limited to osteoporosis, primary or metastatic spine neoplasms, and some benign bone tumors such as vertebral hemangiomas. Especially with the aging population, osteoporosis remains the most common cause of VCFs.1 Direct care costs of osteoporotic VCFs have been estimated between $12.2 and $17.9 billion a year. VCFs are associated with significant morbidity and mortality, including impairment of daily activities and psychosocial performance. The kyphotic deformities caused by VCFs are associated with pulmonary dysfunction, constipation, and imbalance. Compared with age-matched control subjects, patients with VCFs have a higher mortality rate, increasing with the numbers of fractures as well as the duration of follow-up.1–4
Vertebroplasty and kyphoplasty are minimally invasive techniques used to treat painful VCF. Vertebroplasty is the percutaneous injection of a vertebral body (VB) with bone cement, generally polymethylmethacrylate (PMMA). PMMA has been used in orthopedics since the late 1960s.5 Percutaneous vertebroplasty (PV) was first reported by a French group in 1987 for the treatment of painful hemangiomas. This case was a “stunner” of a C2 hemangioma, according to the authors. It was painful and got better after cement injection.6,7 Since then, the indications for PV have expanded to include osteoporotic compression fractures, traumatic compression fractures, and painful vertebral metastasis.8,9 Kyphoplasty is a modification of PV. It involves the percutaneous placement of balloons (called “tamps”) into the VB with an inflation and deflation sequence to create a cavity before the cement injection. Percutaneous kyphoplasty (PK) may restore some of the VB height and reduce the kyphotic angulation of the compression fracture before PMMA injection.10
Ideal candidates for PV or PK have activity-related axial pain corresponding to the level of a recent compression fracture. This pain lessens or abates completely with recumbency, sitting still, or both. A complete neurological examination and recent radiographic imaging are mandatory to rule out spinal cord compromise or retropulsed bony fragments in the canal. Magnetic resonance imaging (MRI) shows an increased T2-weighted signal caused by bone edema at the level with a recent fracture. Bone scan has also been used to target the most recent fracture(s) in patients with multiple fractures because uptake of radiotracer has been associated with a higher rate of excellent pain relief compared with PV without correlation with scintigraphy.11,12 Spinal cord compression on MRI (in the absence of neurological findings) is a relative contraindication. There may be cases in which the procedure is still indicated in a patient with a “tight canal” such as this, but the margin of error is very small, so if a small amount of PMMA extrudes, neurological deficits may ensue. If a posterior cortical fracture is suspected on MRI, a computed tomography scan will reveal the bony architecture more precisely.
Procedural Overview and Complication Avoidance
Standards for the safe practice of these techniques have been published by the Society of Interventional Radiology (SIR) in 2003 and recently updated by the Cardiovascular and Interventional Radiological Society of Europe; highlights of this document can be found in Box 19-1.13,14
Box 19-1 Summary of Guidelines for Percutaneous Vertebroplasty and Percutaneous Kyphoplasty According to the Society of Interventional Radiology and Cardiovascular and Interventional Radiological Society of Europe
General anesthesia or monitored anesthesia care (MAC) can be used. If MAC is used, the surgeon must use generous amounts of local anesthetic, especially injected onto the periosteum, where much nociception occurs. Some patients experience discomfort with advancement of the trocars across the posterior cortical margin with balloon inflation (in the case of kyphoplasty) and with PMMA injection. The anesthesiologist must be prepared to “deepen” the MAC during these phases of the procedure. Patient selection is important with consideration to the anesthesia choice. Very anxious or nervous patients may have a better experience with a general anesthetic. Careful consideration must be given to padding the pressure points of this fragile group of patients. After uni- or bipedicular VB access has been obtained, some clinicians proceed directly with injection of PMMA, but others prefer to do venography before cement injection. In theory, venography provides anatomical knowledge of large venous channels’ proximity to the trocar. This information is used to more carefully inject the PMMA. For example, if a small amount of contrast injection reveals a direct spread into a venous channel, the operator may move the trocar before injection or carefully inject relatively solidified PMMA to embolize the large vein before injecting more PMMA into the VB. The literature reveals variable efficacy of the use of venography.15,16 Most groups no longer use venography.
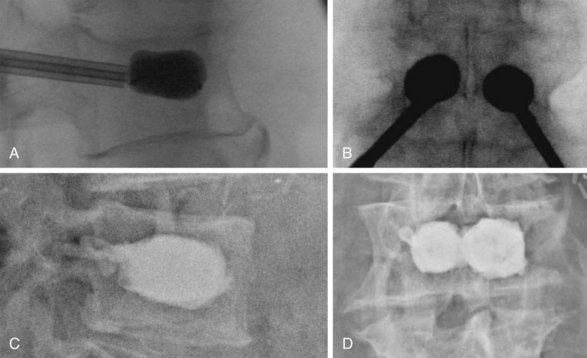
PMMA injection into the VB is undertaken after careful imaging confirming location of the trocar or trocars into the anteromedial portion of the VB. The PMMA should be opacified and beginning to harden to the consistency of toothpaste before injection. Injection can be done by small syringes filled with PMMA or one of several commercially available kits. The injection must be done under live lateral or biplanar fluoroscopic guidance. If PMMA begins to go into a blood vessel or toward the posterior cortical margin, it must be halted immediately. The authors halt cement injection when it spreads to the posterior third of the VB. To minimize PMMA leakage, several groups recommend the use of high-viscosity cement and relatively small volume injection (Fig. 19-1).17–19
Outcomes and Potential Complications
Both PK and PV have a very high acceptance and use rate. There is substantial improvement in pain and significant improvement in function after treatment by either of these techniques. Kyphoplasty improves height of the fractured vertebra and improves kyphosis by more than 50% if performed within 3 months from the onset of the fracture (onset of pain). There is some height improvement, although not as marked, along with significant clinical improvement, if the procedure is performed after 3 months.20
In multiple studies, PV has been shown to provide substantial pain relief or improved mobility in 75% to 92.4% of patients with osteoporotic VCFs and in 50% to 86% of patients with pathologic VCFs secondary to neoplasm.1,8,13
Two studies published in 2009 called the efficacy of PV into question. Although both were randomized controlled trials (RCTs), several problems exist within the methodology of each study. Kallmes et al21 published the larger of the two studies, in which during a 4-year period, 131 patients with VCF from 11 centers were randomized to either PV or a “sham” procedure, which was a medial branch block. Pain scores decreased in both the control and PV groups with no statistically significant difference at 1 month (3.9 ± 2.9 for the PV group vs 4.6 ± 3.0 for the control group; P = .19). The authors did note a trend toward a statistically significant difference in clinically meaningful improvement in pain (64% in the PV group vs. 48% in the control group at 1 month; P = .06). Unfortunately, this study has several flaws. Crossover was significant, with 43% of the control group crossing over to PV but only 12% of the PV crossing over to the control procedure. Patients with back pain and fractures up to 1 year old were included, but chronic back pains with an acute VCF were not specifically excluded. Also, the study had a small sample size, necessitating the liberalization of the inclusion criteria and later decreasing the target enrollment because of difficulty in patient recruitment.21
Another study published at the same time was performed by Buchbinder et al.22 Seventy-eight patients were recruited over a 4-year period at five institutions and randomized to PV or medial branch block. Patients were not allowed to cross over if pain control was inadequate. The PV group had a decrease in pain from 7.4 ± 2.1 preoperatively to 2.6 ± 2.9 at 3 months, and the control group had a decrease from 7.1 ± 2.3 to 1.9 ± 3.3 over the same time period. In addition, no difference was found in disability or quality of life questionnaires between the two groups. The small sample size makes interpretation of the results somewhat difficult, as does the use of a medial branch block for the control group. In addition, the authors do note they also had difficulty recruiting for their study, and approximately 30% of eligible participants declined to participate.22
The superiority of one technique over the other remains controversial and is discussed in the following text. As experience grows with these techniques, various groups are pushing the envelope on indications for the procedure, with the possibility of seeing more complications in this patient group. There are some preliminary data and case series on efficacy in patients with radicular pain, traumatic burst fractures, severe VCF or vertebral plana, cervical spine pathology, and intraoperative PMMA augmentation of pedicle screw fixation spinal stabilization.23–26 In our center, we have pushed “relative” contraindications in cancer patients without increased morbidity even in those with very advanced cancers.27,28
Adjacent-Level Fractures
A small increase in adjacent-level fractures was noticed with long-term follow-up. This phenomenon is similar with both PV and PK.29 Subsequent VCFs may occur at adjacent or remote levels (compared with the treated “vertebroplastied” level). The reported rate of new fractures varies from 7% to 20% over a 1-year follow-up, with sooner refracture occurring at the adjacent levels.30,31 This suggests a local unfavorable biomechanical situation in some patients who have adjacent-level fracturing and ongoing disease process (usually osteoporosis) in the nonadjacent fracture group. A large database of 106 patients who underwent 212 PV procedures was evaluated over a 3-year follow-up period by Kim et al.32 They noted 72 new fractures over the 3-year follow-up (7.9%). The 1-year fracture-free rate was 93% by Kaplan-Meier analysis. The mean fracture-free interval was 32 months, with adjacent fractures being predicted by location in the thoracolumbar junction and greater height restoration. A more recent retrospective study by Tseng et al31 reviewed 852 patients who underwent 1131 PV procedures and a minimum of a 2-year follow-up. Of these patients, 16.6% experienced new VCFs of which 62% were adjacent level fractures. The adjacent-level fractures occurred earlier than the nonadjacent-level fractures (71.9 ± 71.8 days vs. 286.8 ± 232.8 days).31 There may be certain anatomical configurations in the fracture that predispose to adjacent-level fracture, such as intraosseous clefts.33 In addition, whereas leakage of cement into the intravertebral disc and lower body mass index appear to predispose patients to adjacent-level fracture, nonadjacent-level fractures are associated with decreased segmental mobility.34 Also, certain patient populations may be at higher risk for future fractures, including those with osteoporosis, previous vertebral fracture, and organ transplant recipients.4,31,35 In all series examining this phenomenon, outcomes of pain relief after PV or PK for the adjacent-level fracture are excellent. It should be noted that up to 20% of patients with untreated VCF have a subsequent VCF within 1 year.36
Complications
Complications are rare but can be serious. The exact incidence is unknown. Most case series report asymptomatic PMMA extrusion rates of around 10% to 15%.10,37 SIR divides complications for these techniques into two categories: minor and major. Minor complications are those considered to require no therapy and having no consequence, such as PMMA extrusion into the disc. Major complications are those requiring therapy, including an unplanned increase in the level of care needed or ongoing permanent sequelae (i.e., PMMA into the spinal canal with neurological deficit). SIR noted published complication rates for major complications to be less than 1%, except in those with neoplastic involvement of the vertebrae, in whom the reported level of major complications is less than 5%.13
PMMA can flow out of the VB posteriorly into the spinal canal or neural foramina or anteriorly into the paraspinous veins with systemic consequences. There are case reports of nerve root and spinal cord compression from extravertebral PMMA.38,39 There have been several reports of minimally symptomatic pulmonary emboli, one case of cardiovascular collapse requiring pulmonary embolectomy, one lethal pulmonary embolus, and one case of paradoxical cerebral arterial PMMA emboli.40–43 The literature suggests that there may be less PMMA leak with PK versus PV.44
Infectious complications, although rare, have been reported. There have been several reports of osteomyelitis requiring corpectomy.45 Meticulous attention to sterile technique is warranted, including preoperative intravenous antibiotic administration. Most have abandoned the older technique of adding tobramycin powder to the PMMA because of uncertainty over both the efficacy and potential impact on the PMMA properties.
Complications have been reported with both procedures (vertebroplasty and kyphoplasty); a review of U.S. Food and Drug Administration safety data revealed 58 reported complications from 1999 through 2003 of approximately 200,000 procedures performed. These were approximately evenly divided among PV and PK, with more cases of pedicle fracture and spinal cord compression in PK than PV.46 This voluntary reporting system is almost certainly flawed and most likely underreports the overall incidence of complications. As further studies are performed, a more complete risk-to-benefit ratio can be defined.
Vertebroplasty versus Kyphoplasty
Both PV and PK have been shown to be effective to reduce the pain associated with VCFs (Box 19-2). These procedures have low complication rates and tend to have islands of supporters (i.e., clinicians who always favor one over the other regardless of clinical circumstances). One study has been published in the past few years addressing PV versus PK. Liu et al47 enrolled 100 patients with confirmed osteoporotic fractures at the thoracolumbar junction and randomized them to PV or PK. There was no difference in preoperative visual analog scale (VAS) pain score, VB height, or kyphotic wedge angle. Postoperative VAS pain scores (2.6 ± 0.6 for both PK and PV) were significantly different from preoperative pain scores (8.0 ± 0.8 for PK; 7.9 ± 0.7 for PV) but were not statistically different between PV and PK at any time in the postoperative period up to 6 months. Although no difference in pain relief was noted, there was a statistically significant increase in both VB height and reduction of the kyphotic wedge angle in the PK compared with PV. In PK, the VB height increased from 1.13 ± 0.22 cm to 2.04 ± 0.41 cm, and the kyphotic wedge angle decreased from 17. 0 degrees ± 7.3 degrees to 9.0 degrees ± 5.7 degrees. This effect was less pronounced in PV, in which the VB height increased from 1.01 ± 0.22 cm to 1.32 ± 0.26 cm and the wedge angle decreased from 15.5 degrees ± 4.2 degrees to 12.2 degrees ± 3.6 degrees.47 Currently, the authors favor the use of PK in circumstances in which the patient has a collapse of more than 20% of vertebral height and a fracture age of less than 3 months in hopes of restoring vertebral height and maintaining functional anatomy when possible. Deramond et al48 have stated that PK may be preferable over PV in patients with severe or multiple wedge deformity that has developed in the past 3 weeks.
Advances in Technology
Coblation
Patients with spine tumors have an increased risk of perioperative complications, and in the setting of limited life expectancy, this can lead to an unacceptable quality of life.49 Using less invasive techniques to treat VCF and other spinal lesions can potentially help avoid this problem. As described in the introduction, many patients with spinal tumors have VCF. Although PV and PK are highly beneficial in this patient population, additional therapies have been developed that pain physicians can use for treatment of the tumor and to potentially improve the efficacy of PV or PK.
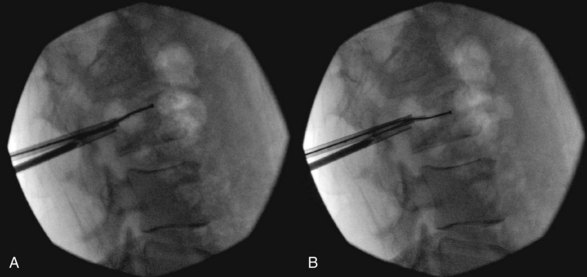
Plasma-mediated radiofrequency ablation (coblation) is currently used in procedures such as tonsillectomy, disc decompression, and tendon and cartilage debridement in which dissolution of the tissue is necessary. A plasma field is created by passing radiofrequency waves through a conducting medium such as saline, creating a precisely focused plasma field composed of ionized particles. This field, formed immediately adjacent to the tip of the device, causes dissolution of soft tissue at the relatively low temperatures of 40° to 70° C.50 Coblation has the potential to increase safety and efficacy in a number of ways. Such a device allows for cavity creation in the VB with little mechanical disturbance of tissue outside of the plasma field. Minimizing disturbance of the rest of the VB could allow for vertebral augmentation in cases of retropulsion of tumor or cortical wall that would otherwise be a contraindication to PV or PK.51 Dissolution of the surrounding soft tissue also allows for destruction of spinal primary or metastatic tumors and increases the efficacy of chemotherapy or radiation therapy by cytoreduction. This is accomplished by removing and replacing the coblation device at different positions of the clock, typically at the 12, 2, 4, 6, 8, and 10 o’clock positions, thus forming a void in the VB larger than the device itself (Fig. 19-2). In conventional PK for treatment of tumor-related VCF, the tumor is pressed against the healthy bone, forming a rim of tissue between the cement and bone. If the practitioner were to choose to perform PK in addition to coblation, removal of the tumor can allow for better interdigitation of the cement in the VB.52 In addition, the relatively low temperatures of the plasma field causes less heat transfer to the VB and assists with hemostasis in the VB.52
Cortoss Cement
PMMA-based cements have a long history of safety and efficacy in the orthopedic community, and their use in PV was a natural extension of their use in arthroplasty. The usual PMMA formulations were modified to allow their use in PV, but doing so has introduced some problems. Because the cement must be injected through small-bore needles, the liquid monomer component was increased to decrease viscosity. Increasing the liquid monomer component has the unfortunate consequence of lowering the compressive strength of the cured polymer and increases the setting time of the polymer.53,54 Additionally, PMMA cement is poorly radiopaque, so opacifiers such as barium sulphate, zirconium dioxide, or powdered tantalum are added to the cement mixture.54 These materials do not undergo polymerization and can act as stress risers within the cured cement.53 It should be noted, however, that current PMMA-based cements are highly effective despite these drawbacks.
Cortoss is a new cement based on bisphenol-a-glycidyl-dimethacrylate (bis-GMA) resin. Unlike PMMA, it does not require premixing and is supplied as a two-part paste that is mixed upon release from the delivery gun. This allows the filler to be dispensed as needed during the procedure. After it has been mixed, hardening occurs in 5 to 8 minutes and can support weight-bearing loads immediately after setting.55 Cortoss has a higher compressive strength, bending modulus, and shear strength compared with PMMA53 and does not appear to develop the interposed fibrous layer between the interface of the composite and extant bone.56 Last, Cortoss is inherently radiopaque, so no additional opacifying agents are required.55
Despite these advantages, Cortoss has been shown to have comparable but not superior efficacy to typical PV PMMA cements. In a study by Middleton et al,54 34 patients underwent PV at a total of 42 levels. Cortoss was injected using a unipedicular approach with a mean injectate volume of 2.2 mL. Approximately 82% of patients reported improvement in symptoms after PV, and 79% required less analgesia. There was one incident each of pulmonary embolism (PE), generalized rash, transient radicular leg pain, and retropulsion of cement 1 year later secondary to progression of metastatic disease, with each incident representing 2.9% of the study population; however, 88% of patients had no complications.54 Previously reported rates of complication of PE are anywhere from 0% to 7% of PMMA vertebroplasties, and for transient radicular pain, there is a reported range of 1% to 8%.54
Conclusion
Recent reviews and editorials have called for a more critical evaluation of these procedures. Watts et al57 reviewed the literature and concluded that controlled multicenter trials are needed to determine the short- and long-term safety. Garfin et al20 concluded that there is a 95% improvement in pain and significant improvement in function after these procedures. They emphasized that the procedure is technically demanding with the potential for significant complications. They recommended further efficacy and safety studies. Jarvik and Deyo58 called for RCTs or some type of control cohort to compare long-term outcomes carefully. Einhorn59 calls for careful monitoring of outcomes and minimal training standards. Birkmeyer60 calls for randomized clinical trials, citing insufficient evidence from case series to prove safety, efficacy, and cost-effectiveness. The prospective RCTs described above seem to confuse rather than clarify our understanding of the efficacy of PV. Ideally, a prospective RCT with a larger sample size and appropriate control group can be performed in the future.
1 Stallmeyer MJB, Zoarski G. Patient evaluation and selection. In: Mathis JM, Deramond H, Belkoff SM, editors. Percutaneous vertebroplasty and kyphoplasty. ed 2. New York: Springer; 2006:60-61.
2 Huang C, Ross PD, Wasnich RD. Vertebral fracture and other predictors of physical impairment and health care utilization. Arch Intern Med. 1996;156:2469-2475.
3 Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. Pharmacoeconomics. 2002;20:289-303.
4 Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
5 Charnley J. The reaction of bone to self-curing acrylic cement. A long-term histological study in man. J Bone Joint Surg Br. 1970;52:340-353.
6 Galibert P, Deramond H. Percutaneous acrylic vertebroplasty as a treatment of vertebral angioma as well as painful and debilitating diseases. Chirurgie. 1990;116:326-334. discussion 335
7 Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166-168.
8 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897-1904.
9 Kaemmerlen P, Thiesse P, Jonas P, et al. Percutaneous injection of orthopedic cement in metastatic vertebral lesions. N Engl J Med. 1989;321:121.
10 Lieberman IH, Dudeney S, Reinhardt MK, et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631-1638.
11 Maynard AS, Jensen ME, Schweickert PA, et al. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol. 2000;21:1807-1812.
12 Karam M, Lavelle WF, Cheney R. The role of bone scintigraphy in treatment planning, and predicting pain relief after kyphoplasty. Nucl Med Comm. 2008;29:247-253.
13 McGraw JK, Cardella J, Barr JD, et al. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14:827-831.
14 Gangi A, Sabharwal T, Irani FG, et al. Quality assurance guidelines for percutaneous vertebroplasty. Cardiovasc Intervent Radiol. 2006;29:173-178.
15 Gaughen JRJr, Jensen ME, Schweickert PA, et al. Relevance of antecedent venography in percutaneous vertebroplasty for the treatment of osteoporotic compression fractures. AJNR Am J Neuroradiol. 2002;23:594-600.
16 Vasconcelos C, Gailloud P, Beauchamp NJ, et al. Is percutaneous vertebroplasty without pretreatment venography safe? Evaluation of 205 consecutives procedures. AJNR Am J Neuroradiol. 2002;23:913-917.
17 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(suppl 1):21-30.
18 Laredo JD, Hamze B. Complications of percutaneous vertebroplasty and their prevention. Semin Ultrasound CT MR. 2005;26:65-80.
19 Burton AW, Rhines LD, Mendel E. Vertebroplasty and kyphoplasty: a comprehensive review. Neurosurg Focus. 2005;18:e1.
20 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
21 Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Eng J Med. 2009;361:6.
22 Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Eng J Med. 2009;361:6.
23 Chung SK, Lee SH, Kim DY, et al. Treatment of lower lumbar radiculopathy caused by osteoporotic compression fracture: the role of vertebroplasty. J Spinal Disord Tech. 2002;15:461-468.
24 Nakano M, Hirano N, Matsuura K, et al. Percutaneous transpedicular vertebroplasty with calcium phosphate cement in the treatment of osteoporotic vertebral compression and burst fractures. J Neurosurg. 2002;97(suppl 3):287-293.
25 Peh WC, Gilula LA, Peck DD. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223:121-126.
26 Wetzel SG, Martin JB, Radu EW, et al. Percutaneous vertebroplasty: a minimal-invasive procedure for pain treatment. Schweiz Rundsch Med Prax. 2005;94:595-598.
27 Hentschel SJ, Burton AW, Fourney DR, et al. Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: refuting proposed contraindications. J Neurosurg Spine. 2005;2:436-440.
28 Burton AW, Reddy SK, Shah HN, et al. Percutaneous vertebroplasty—a technique to treat refractory spinal pain in the setting of advanced metastatic cancer: a case series. J Pain Symptom Manage. 2005;30:87-95.
29 Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29:2270-2276. discussion 2277
30 Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27:217-223.
31 Tseng Y, Yang T, Tu P, et al. Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vertebroplasty. Spine. 2009;34(18):1917-1922.
32 Kim SH, Kang HS, Choi JA, et al. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiol. 2004;45:440-445.
33 Trout AT, Kallmes DF, Lane JI, et al. Subsequent vertebral fractures after vertebroplasty: association with intraosseous clefts. AJNR Am J Neuroradiol. 2006;27:1586-1591.
34 Ahn Y, Lee J, Le H, et al. Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J Neurosurg Spine. 2008;9:129-136.
35 Deen HG, Aranda-Michel J, Reimer R, et al. Balloon kyphoplasty for vertebral compression fractures in solid organ transplant recipients: results of treatment and comparison with primary osteoporotic vertebral compression fractures. Spine J. 2006;6:494-499.
36 Silverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(suppl 2):S27-S31.
37 McKiernan F, Faciszewski T, Jensen R. Quality of life following vertebroplasty. J Bone Joint Surg Am. 2004;86-A:2600-2606.
38 Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine. 2002;27:E419-E422.
39 Ratliff J, Nguyen T, Heiss J. Root and spinal cord compression from methylmethacrylate vertebroplasty. Spine. 2001;26:E300-E302.
40 Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine. 2002;27:E416-E418.
41 Tozzi P, Abdelmoumene Y, Corno AF, et al. Management of pulmonary embolism during acrylic vertebroplasty. Ann Thorac Surg. 2002;74:1706-1708.
42 Chen HL, Wong CS, Ho ST, et al. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg. 2002;95:1060-1062. table of contents
43 Scroop R, Eskridge J, Britz GW. Paradoxical cerebral arterial embolization of cement during intraoperative vertebroplasty: case report. AJNR Am J Neuroradiol. 2002;23:868-870.
44 Phillips FM, Todd Wetzel F, Lieberman I, et al. An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine. 2002;27:2173-2178. discussion 2178-2179
45 Walker DH, Mummaneni P, Rodts GEJr. Infected vertebroplasty. Report of two cases and review of the literature. Neurosurg Focus. 2004;17:E6.
46 Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol. 2004;15:1185-1192.
47 Liu JT, Liao WJ, Tan WC, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2010;21:359-364.
48 Deramond H, Saliou G, Aveillan M, et al. Respective contributions of vertebroplasty and kyphoplasty to the management of osteoporotic vertebral fractures. Joint Bone Spine. 2006;73:610-613.
49 Gerszten PC, Monaco EA3rd. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation. Neurosurg Focus. 2009;27(6):E9.
50 Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Science. 2002;30:1376-1383.
51 Shimony JS, Gilula LA, Zeller AJ, et al. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 2004;232:846-853.
52 Georgy BA, Wong W. Plasma-mediated radiofrequency ablation assisted percutaneous cement injection for treating advanced malignant vertebral compression fractures. AJNR Am J Neuroradiol. 2007;28:700-705.
53 Gheduzzi S, Webb JJ, Miles AW. Mechanical characteristics of three percutaneous vertebroplasty biomaterials. J Mater Sci Mater Med. 2006;17:421-426.
54 Middleton ET, Rajaraman CJ, O’Brien DP, et al. The safety and efficacy of vertebroplasty using Cortoss cement in a newly established vertebroplasty service. Br J Neurosurg. 2008;22(2):252-256.
55 Palussière J, Berge J, Gangi A, et al. Clinical results of an open prospective study of a bis-GMA composite in percutaneous vertebral augmentation. Eur Spine J. 2005;14(10):982-991.
56 Erbe EM, Clineff TD, Gualtieri G. Comparison of a new bisphenol-a-glycidal dimethacrylate-based cortical bone void filler with polymethyl methacrylate. Eur Spine J. 2001;10(suppl 2):S147-S152.
57 Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429-437.
58 Jarvik JG, Deyo RA. Cementing the evidence: time for a randomized trial of vertebroplasty. AJNR Am J Neuroradiol. 2000;21:1373-1374.
59 Einhorn TA. Vertebroplasty: an opportunity to do something really good for patients. Spine. 2000;25:1051-1052.