Chapter 107
Vertebral Artery Disease
Mark D. Morasch
Atherosclerosis and other vasculopathies involving the vertebrobasilar system are known to cause symptoms of posterior circulation ischemia. Approximately 25% of ischemic strokes occur in the vertebrobasilar territory. The vertebrobasilar system supplies blood to the brainstem, cerebellum, and occipital lobes via paired vertebral arteries. Brainstem ischemia, most often the result of atherosclerotic disease involving the vertebrobasilar arteries, remains poorly understood, and misdiagnosis is common. In contradistinction to the clear focal symptoms of anterior circulation ischemia, the symptoms associated with brainstem ischemia can be multiple, varied, and vague. Furthermore, a number of medical conditions may mimic vertebrobasilar ischemia, thus confounding the selection of patients in need of posterior circulation intervention (see Chapter 97). Consequently, clinicians appear to be reluctant to aggressively pursue diagnosis or recommend treatment for many of the surgically correctable lesions that may be responsible for these syndromes.
Vertebrobasilar ischemia is less common than internal carotid artery disease, yet it must be diagnosed appropriately because it is a treatable vasculopathy. Significantly, 50% of patients will initially be evaluated for stroke and 26% for transient ischemic symptoms rapidly followed by stroke.1 For patients who experience vertebrobasilar transient ischemic attacks, disease in the vertebral arteries portends a 22% to 35% risk of stroke over a 5-year period.2–4 The mortality associated with a posterior circulation stroke is 20% to 30%, higher than that for an anterior circulation event.5–7
Pathogenesis
One reason the pathophysiology of vertebrobasilar ischemia is less well understood than that of carotid/anterior hemispheric ischemia is that the peculiar anatomy of the vertebral artery makes it less accessible to surgical reconstruction and postmortem examination. The surgical anatomy of the paired vertebral arteries has traditionally been divided into four segments: V1, the origin of the vertebral artery arising from the subclavian artery to the point at which it enters the C6 transverse process; V2, the segment of the artery buried deep within intertransversarium muscle and the cervical transverse processes of C6 to C2; V3, the extracranial segment between the transverse process of C2 and the base of the skull before it enters the foramen magnum; and V4, the intracranial portion beginning at the atlanto-occipital membrane and terminating as the two vertebral arteries converge to form the basilar artery (Fig. 107-1).
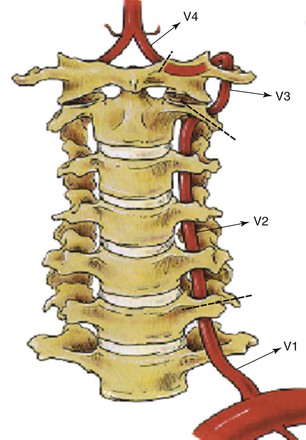
Figure 107-1 The four segments of the vertebral (V) artery. (From Berguer R: Surgical management of the vertebral artery. In Moore WS, editor: Surgery for cerebrovascular disease, New York, NY, 1986, Churchill Livingstone.)
Ischemia affecting the temporo-occipital areas of the cerebral hemispheres or segments of the brainstem and cerebellum characteristically produces bilateral symptoms. The classic symptoms of vertebrobasilar ischemia are dizziness, vertigo, drop attacks, diplopia, perioral numbness, alternating paresthesia, tinnitus, dysphasia, dysarthria, and ataxia. When patients have two or more of these symptoms, the likelihood of vertebrobasilar ischemia is high (Box 107-1). Posterior circulation strokes occur most commonly in relation to large-artery occlusive diseases (Table 107-1). The vertebral artery is the most common large vessel to be involved with an occlusive lesion that leads to stroke (Table 107-2).
Table 107-1
Mechanisms of Posterior Circulation Stroke or Transient Ischemic Attack in 407 Patients
| Mechanism | Number (%) |
| Large-artery occlusive disease | 132 (32) |
| Embolism—cardiac source | 99 (24) |
| Embolism—arterial source | 74 (18) |
| Penetrating artery disease | 58 (14) |
| Vasospasm/migraine | 10 (2) |
| Other causes | 34 (8) |
Adapted from Caplan LR, et al: New England Medical Center Posterior Circulation registry. Ann Neurol 56:389, 2004.
Table 107-2
Frequency of Symptomatic Vascular Occlusive Lesions in 417 Patients
| Lesion | Number |
| Innominate artery | 2 |
| Subclavian artery | 5 |
| Extracranial vertebral artery | 131 |
| Intracranial vertebral artery | 132 |
| Basilar artery | 109 |
| Posterior cerebral artery | 38 |
Adapted from Caplan LR, et al: New England Medical Center Posterior Circulation registry. Ann Neurol 56:389, 2004.
The most common disease affecting the vertebral artery is atherosclerosis. Less common pathologic processes include trauma, fibromuscular dysplasia, Takayasu’s disease, osteophyte compression, dissections, aneurysms, and other arteritides.
Ischemic Mechanisms
In general, the ischemic mechanisms can be broken down into those that are hemodynamic and those that are embolic. The low-flow hemodynamic mechanism of ischemia is better recognized and more frequent than the embolic one.
Low Flow
Patients with low-flow hemodynamic ischemia have transient symptoms in the territory of the basilar artery because they lack appropriate inflow from the vertebral artery and have inadequate compensation from the carotid territory. Hemodynamic symptoms occur as a result of transient end-organ (brainstem, cerebellum, occipital lobes) hypoperfusion and can be precipitated by postural changes or a transient reduction in cardiac output. Ischemia from hemodynamic mechanisms rarely results in infarction. Rather, symptoms are short lived, repetitive, and more of a nuisance than a danger. Some patients may be prone to traumatic injuries from loss of balance. For hemodynamic symptoms to occur in direct relation to the vertebrobasilar arteries, significant occlusive disease must be present in both of the paired vertebral vessels or in the basilar artery. In addition, compensatory contribution from the carotid circulation via the circle of Willis must be incomplete. Alternatively, hemodynamic ischemic symptoms may result from proximal subclavian artery occlusion and the syndrome of subclavian/vertebral artery steal.
Embolic
Embolic causes of vertebrobasilar ischemia may not be as well recognized. Symptoms may be due to microembolization from the heart or aortic arch or, more frequently, from vessels directly leading to the basilar artery. Arterial-to-arterial emboli can arise from atherosclerotic lesions, from intimal defects caused by extrinsic compression or repetitive trauma, and rarely, from fibromuscular dysplasia, aneurysms, or dissections. Although fewer patients suffer from embolic phenomena than from hemodynamic mechanisms, actual infarctions in the vertebrobasilar distribution are most often the result of embolic events. When compared with the hemodynamic mechanisms of ischemia, emboli are much more likely to cause fatal events or dangerous or debilitating infarcts. It is estimated that up to a third of vertebrobasilar ischemic episodes are caused by distal embolization from plaque or mural lesions of the subclavian, vertebral, or basilar arteries (or any combination of these vessels).8 These patients may have transient ischemic attacks or strokes in the territory supplied by the basilar artery. The importance of the embolic mechanism as a cause of vertebrobasilar symptoms has been emphasized in clinical and anatomicopathologic studies. This information has been derived from autopsy studies and magnetic resonance imaging (MRI), which have identified small infarcts in the brainstem and cerebellum and shown their source, via arteriography, to be lesions in the subclavian or vertebral arteries. As opposed to patients with hemodynamic symptomatology, multiple and multifocal infarcts in the brainstem, cerebellum, and occasionally, the posterior cerebral artery territory often develop in patients with embolic ischemia.9,10
Patient Selection for Vertebral Artery Reconstruction
Anatomic Considerations
The minimal anatomic requirement to justify vertebral artery reconstruction for hemodynamic symptoms would be stenosis greater than 60% diameter in both vertebral arteries if both are patent and complete or the same degree of stenosis in the dominant vertebral artery if the opposite vertebral artery is hypoplastic, ends in a posteroinferior cerebellar artery, or is occluded. A single, normal vertebral artery is sufficient to adequately perfuse the basilar artery, regardless of the patency status of the contralateral vertebral artery. Unlike carotid artery disease, the mere presence of vertebral artery stenoses in an asymptomatic patient is rarely an indication for reconstruction because these patients are well compensated from the other vertebral or from the carotid circulation through the posterior communicating vessels.
Etiologic Considerations
Low Flow
For patients with hemodynamic symptoms, it is essential to rule out systemic causes of ischemia before advising evaluation of the vertebrobasilar arteries as the source of symptoms. In the later years of life, vertebral artery stenosis is a frequent arteriographic finding, and dizziness is a common complaint. The presence of both cannot necessarily be assumed to have a cause-effect relationship. The indication for intervention in patients with hemodynamic symptoms depends on the ability to demonstrate insufficient blood flow to the basilar artery. Other common systemic causes of hemodynamic ischemia, aside from vascular occlusion, include orthostatic hypotension, poorly regulated antihypertensive therapy, arrhythmias, heart failure, malfunction of pacemakers, and anemia.
Embolic
In contrast to individuals with low-flow states among patients with posterior circulation ischemia secondary to microembolism and appropriate lesions in a vertebral or subclavian artery, the potential source of the embolus needs to be eliminated regardless of the status of the contralateral vertebral artery. Patients with symptomatic vertebrobasilar ischemia secondary to emboli are candidates for surgical correction of the lesion irrespective of the condition of the contralateral vertebral artery. However, surgical intervention is not indicated in asymptomatic patients who harbor suspicious radiographic findings.
Global Ischemia
Another indication for vertebral artery reconstruction pertains to patients who have extensive extracranial disease with one or both internal carotid arteries occluded and global manifestations of cerebrovascular ischemia. In these patients the carotid arteries may be occluded or involved with severe siphon stenosis, thus making direct revascularization via the internal carotid arteries impossible or inadvisable, and reconstruction of the vertebral artery may offer the best option for reestablishment of adequate cerebral blood flow. In patients with global ischemia, the vertebral arteries are important pathways for cerebral revascularization when they are critically stenosed or occluded. Demonstration of normal-sized posterior communicating arteries increases the likelihood of successfully correcting global ischemic symptoms by vertebral artery reconstruction. Once the diagnosis of vertebrobasilar ischemia (or global ischemia requiring vertebral revascularization) has been confirmed and significant pathology identified radiographically, endovascular or surgical correction may be considered.
Differential Diagnosis
Identifying patients who can benefit from vertebral artery reconstruction begins with an accurate assessment of the symptom complex, followed by efforts to exclude other causes of the symptoms (Box 107-2). These other medical conditions include inappropriate use of antihypertensive medications, cardiac arrhythmias, anemia, brain tumors, and benign vertiginous states. A thorough investigation consists of ruling out (1) inner ear pathology, (2) cardiac arrhythmias, (3) internal carotid artery stenosis/occlusion, and (4) inappropriate use of medications (Box 107-3).
Evaluation of patients with posterior circulation ischemia should include assessment of the precise circumstances associated with the development of symptoms. Symptoms often appear on standing in older individuals with poor sympathetic control of their venous tone, which causes excessive pooling of blood in the veins of the leg. This is particularly common in patients with diabetes who have diminished sympathetic venoconstrictor reflexes. A 20-mm Hg drop in systolic pressure on rapid standing is a criterion for a diagnosis of orthostatic hypotension causing low flow in the vertebrobasilar system. In these cases the drop in pressure triggers the symptoms of posterior circulation ischemia.
Any systemic mechanism that decreases the mean pressure of the basilar artery may be responsible for hemodynamic symptomatology, and affected individuals may or may not have concomitant vertebral artery stenosis or occlusion. Because certain prescription medications can mimic vertebrobasilar ischemia, patient medications require thorough review. In fact, excessive use of antihypertensive medications is the most common etiology of posterior circulation symptoms and can also cause hemodynamic posterior circulation ischemia by decreasing perfusion pressure and inducing severe orthostatic hypotension.
A cardiac source is the second-most common cause of brainstem ischemia, especially in older adults, and thorough evaluation should include 24-hour Holter monitoring for arrhythmias and echocardiography to assess heart valve function. Arrhythmias are another common cause of symptoms as a result of decreased cardiac output. Patients with ischemia secondary to arrhythmias often report the association of palpitations with the appearance of symptoms. In addition, transesophageal echocardiography may be useful in patients with a suspected embolic mechanism of ischemia to rule out a cardiac source.
In some patients, the cause of the drop in mean arterial pressure can be corrected simply by readjusting their antihypertensive regimen, by the administration of antiarrhythmic drugs, or by inserting a cardiac pacemaker. In patients with orthostatic hypotension, the problem may not respond to medical treatment, and only reconstruction of a diseased or occluded vertebral artery will render the patient asymptomatic in the presence of persistent oscillations in blood pressure.
Investigation must also be undertaken to exclude inner ear pathology, including rare cerebellopontine-angle tumors. In addition, neurologic evaluation to rule out benign vertiginous states should be considered.
Because patients are often initially seen with a combination of anterior and posterior hemispheric symptoms, investigation of the great vessels and the carotid circulation is usually warranted. An important aspect of the history is identifying triggering events such as positional or postural changes. This is followed by a thorough physical examination, which includes palpation, auscultation, pulse examination, and comparative arm blood pressures (recumbent and standing).
Physical examination can alert the physician to the possibility of subclavian steal in patients with differences in brachial blood pressure greater than 25 mm Hg or with diminished or absent pulses in one arm. The diagnosis of reversal of vertebral artery flow can be made accurately by noninvasive indirect methods and demonstrated directly by duplex imaging of the reversal of flow in the vertebral artery (see Chapter 16 and Chapter 98).
Patients may relate their symptoms to turning or extending their head. These dynamic symptoms usually appear when turning the head to one side. Symptoms are caused by extrinsic compression of the vertebral artery, usually the dominant or the only one, by arthritic bone spurs.11 To differentiate this mechanism from dizziness or vertigo secondary to labyrinthine disorders that appear with head or body rotation, the patient should attempt to reproduce the symptoms by first turning the head slowly and then repeating the maneuver briskly, as when shaking the head from side to side. In labyrinthine disease, the sudden inertial changes caused by the latter (brisk) maneuver result in immediate symptoms and nystagmus. Conversely, with extrinsic vertebral artery compression, a short delay occurs before imbalance develops.
Diagnostic Tests
Once a suspicion of vertebrobasilar ischemia has been entertained, only a few diagnostic tests are available to clearly ascertain the vertebral anatomy.
Duplex Ultrasonography
Although duplex ultrasound is an excellent tool for detecting lesions in the carotid artery, it has significant limitations when used to detect vertebral artery pathology. Direct visualization of the second portion of the vessel is difficult because of its intraosseous course through the transverse processes of C2 to C6. The usefulness of duplex ultrasound lies in its ability to confirm reversal of flow within the vertebral arteries and detect changes in flow velocity consistent with a proximal stenosis.12 In addition, ultrasound imaging can diagnose great-vessel pathology and confirm subclavian steal.
Computed Tomography and Magnetic Resonance Imaging
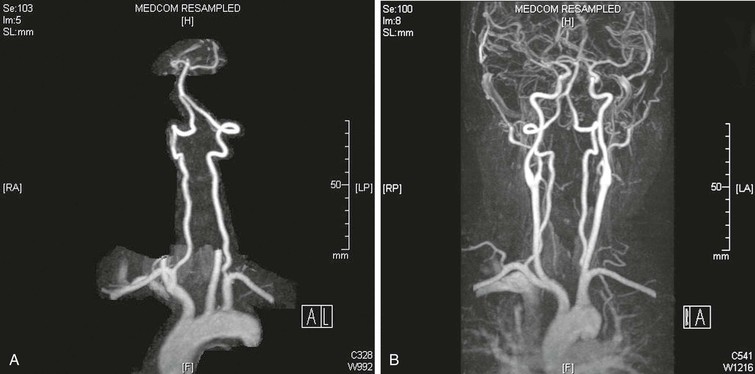
MRI is another modality that provides a safe, noninvasive, detailed evaluation of both the extracranial and intracranial vasculature, as well as structures within the posterior fossa. Contrast-enhanced magnetic resonance angiography (MRA) with three-dimensional reconstruction and maximum image intensity techniques provides full imaging of the vessels, including the supra-aortic trunks and the carotid and vertebral arteries (Fig. 107-2).
Arteriography
Despite the technologic advances in MRI and CT evaluation, selective subclavian and vertebral angiography remains the “gold standard” for preoperative evaluation of patients with vertebrobasilar ischemia. Some surgeons still consider angiography mandatory before endovascular intervention or surgical reconstruction. The most common site of disease, the vertebral artery origin, may not be well imaged with ultrasound or magnetic resonance angiography and, in fact, can often be displayed only with oblique projections that are not part of the standard aortic arch evaluation. Patients with suspected vertebral artery compression should undergo dynamic angiography, which incorporates provocative positioning. Finally, delayed imaging should be performed to demonstrate reconstitution of the extracranial vertebral arteries through cervical collaterals.
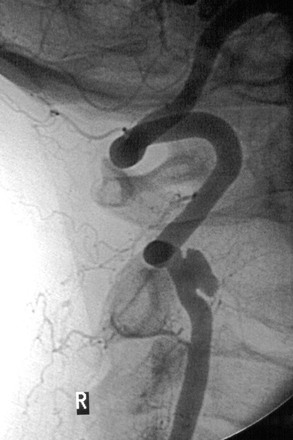
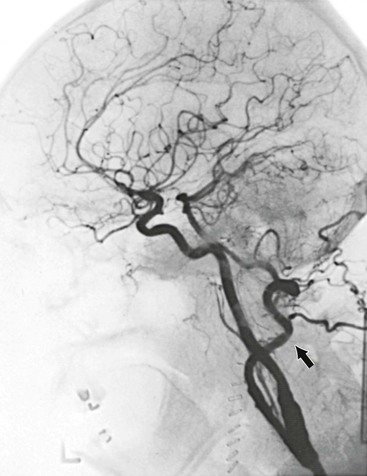
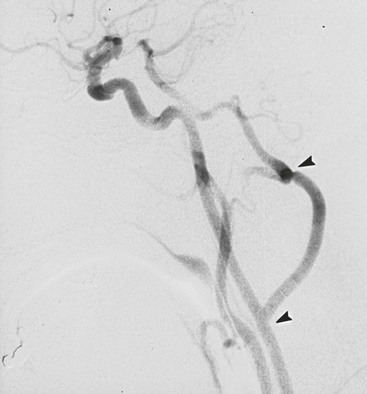
Arteriographic investigation of vertebral artery pathology necessitates systematic positions and projections to evaluate the vertebrobasilar system from its origin to the top of the basilar artery. Arteriographic evaluation begins with an arch view, which determines the presence or absence of a vertebral artery on each side. Angiography shows whether one vertebral artery is dominant (generally the left) and can clearly define aberrant vessel anatomy. The most common anomalous origin involves the left vertebral artery, which can originate from the aortic arch in 6% of patients. A much rarer variant involves the right vertebral artery, which can be seen to arise from the innominate or right common carotid artery (Fig. 107-3). This anatomy usually occurs in patients with an aberrant retroesophageal right subclavian artery. Arch views must be obtained in at least two projections: right and left posterior oblique views. Usually, these two projections will sufficiently display the first segment (V1) of the vessel from its subclavian origin up to the transverse process of C6.
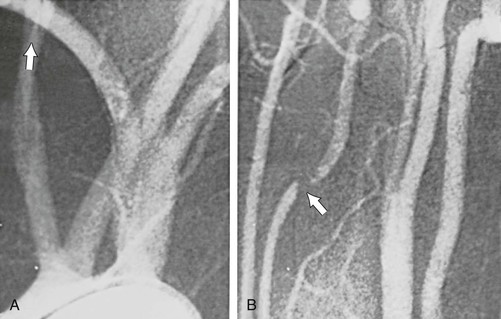
Figure 107-3 Arch injection in a 33-year-old woman with vertebrobasilar ischemia. A, The right vertebral artery and the right external carotid artery arise from a long common right carotid trunk (arrow). The right internal carotid artery is congenitally absent. The right subclavian arises as the last branch of the aorta. B, The right vertebral artery, which has an anomalous origin, enters the spine at a high level (C4) and is severely compressed (arrow) at this level with head rotation.
Disease Distribution
V1 Segment
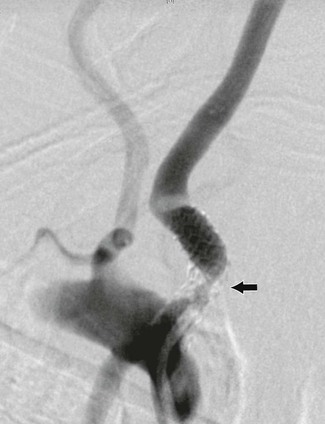
The most common atherosclerotic lesion of the vertebral artery is stenosis of its origin. The prevalence of such lesions is approximately 20% to 40% in patients with cerebrovascular disease.8 This lesion may be missed in standard arch views because of superimposition of the subclavian artery over the first segment of the vertebral artery. Additional oblique projections may be needed to “throw off” the subclavian artery to obtain a clear view of the origin of the vertebral artery (Fig. 107-4). The presence of a poststenotic dilatation in the first centimeter of the vertebral artery suggests that there may be a significant stenosis at its origin hidden by an overlying subclavian artery. Redundancy and kinks are common, but only very severe kinks associated with poststenotic dilatations can be responsible for hemodynamic symptoms.
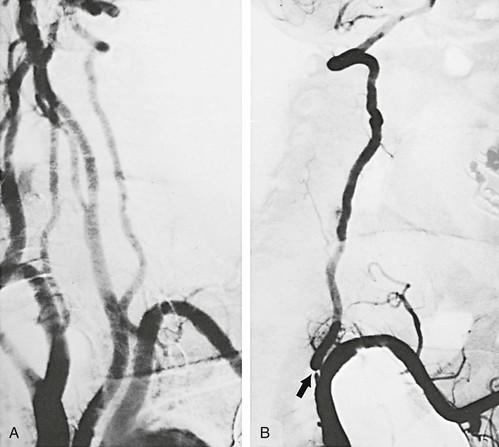
Figure 107-4 A, Arch arteriogram on anteroposterior projection. B, Severe stenosis (arrow) of a dominant left vertebral artery seen only after additional oblique rotation of the patient (right). (From Berguer R: Role of vertebral artery surgery after carotid endarterectomy. In Bergan JJ, et al, editors: Reoperative arterial surgery, Orlando, Fla, 1986, Grune & Stratton, pp 555-564.)
V2 Segment
Visualization of the second segment (V2) of the vertebral artery, from C6 to the top of the transverse process of C2, is accomplished in the oblique arch views in conjunction with selective subclavian injections. A point should be made to attempt to angiographically identify the point of entry of the vertebral arteries into the transverse processes of the spine. Whether a patient has an abnormally low entry at the level of C7 instead of C6 should be noted. This finding is associated with a short V1 segment of the artery. In this circumstance, the short extraosseous length can create challenges for reconstruction at the V1 level. In addition, extrinsic compression by musculotendinous structures is common in vertebral arteries with an abnormally high level of entry into the spine, usually C4 or C5 (see Fig. 107-2). Such compression is due to the sharp angulation resulting from the abnormal level of entry. The level of entry into the spine is best determined on unsubtracted views.
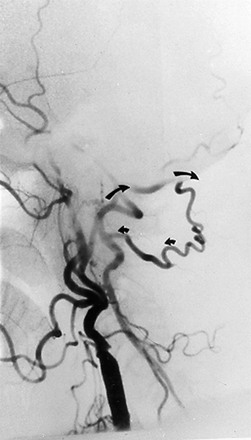
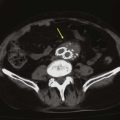
The most common pathology of the V2 segment is extrinsic compression of the vertebral artery by osteophytes,11 but compression can also be caused by the edges of the transverse foramina (Fig. 107-5) or the intervertebral joints. Positional changes or rotation or extension of the neck usually triggers compression of the vertebral artery in this segment. Arteriography is required to demonstrate extrinsic dynamic compression of the vertebral artery. This is performed either with the patient sitting up, by means of bilateral brachial injections, or if the transfemoral approach is used, with the patient supine in the Trendelenburg position with the head resting against a block. The reason to position the patient either sitting up or in a 25-degree Trendelenburg position is to mimic the effects of the weight of the head on the spine. With the patient standing, the weight of the head acting on the cervical spine changes its curvature and decreases the distance between C1 and C7. This longitudinal compression of the spine often enhances the extrinsic compression effects caused by osteophytic spurs. In these positions, intended to exert axial compression on the cervical vertebrae, the angiographer should obtain the specific rotation or extension of the head that provokes the symptoms. When the patient is rendered symptomatic, arteriographic injection should demonstrate the extrinsic compression that developed with the head rotation or extension.13 The vertebral artery may be normal in one projection and occluded by extrinsic compression in the other (Fig. 107-6). The agent of compression may be bone (an osteophyte) or tendon (longus colli).
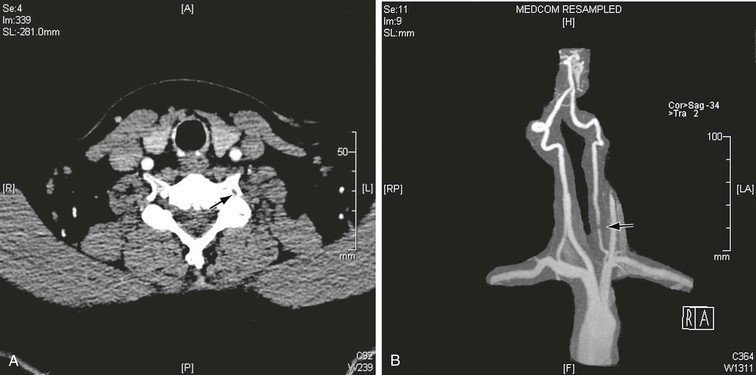
Figure 107-5 CT (A) and MRA (B) images demonstrating large osteophytes occluding the vertebral artery at C5.
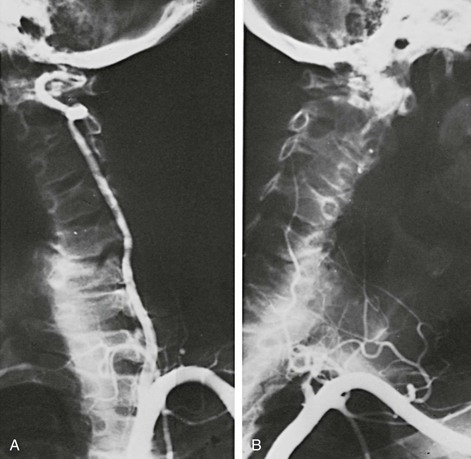
Figure 107-6 A patient with a single vertebral artery showing minimal extrinsic compression (A) when the neck is rotated to the right and occlusion (B) when the neck is rotated to the left. (From Berguer R: Surgical management of the vertebral artery. In Moore WS, editor: Surgery for cerebrovascular disease, New York, NY, 1986, Churchill Livingstone.)
The V2 segment is a frequent site for the development of traumatic or spontaneous arteriovenous fistulae, which should easily be identified with angiography. Fistulae occur commonly in the V2 segment because fixation of the adventitia of the vertebral artery to the periosteum of the foramina makes the former vulnerable to luxation/subluxation of the vertebrae or to fractures of their lateral mass. The close proximity of the artery to its surrounding venous plexus results in an arteriovenous fistula whenever the artery and the vein are damaged in continuity. The vertebral artery may tear completely or incompletely (dissection) as a consequence of stretch injury after brisk rotation or hyperextension of the neck.
Although most vertebral artery aneurysms are found intracranially, the V2 segment is the most common site of extracranial aneurysmal degeneration (Fig. 107-7). Two thirds of all extracranial vertebral artery aneurysms involve the V2 segment, and most form as pseudoaneurysms secondary to trauma or dissection.13a True extracranial vertebral artery aneurysms are rare, accounting for less than 1% of all vertebral artery lesions.13b In one review, all patients with a true extracranial vertebral artery aneurysm also had some form of connective tissue disorder.13a Most extracranial vertebral aneurysms are asymptomatic and found incidentally. Many, however, do present with symptoms, which can include a palpable neck mass, dizziness, headaches, and/or neurologic deficit from cerebral ischemia or nerve compression. Rupture is rare. Because the natural history is unknown, vertebral ligation, with or without reconstruction, is most often recommended for large extracranial vertebral aneurysms.13a
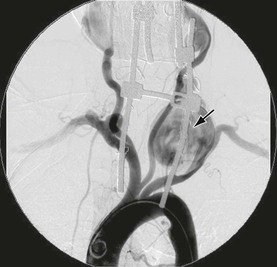
Figure 107-7 V2 segment true aneurysm in a patient with connective tissue disorder. Also note the left common right subclavian and right external carotid aneurysms.
In addition to aneurysms and fistulae, dissection, fibromuscular disease, and embolizing atherosclerotic plaque can be identified in the V2 segment. Disease in the second segment of the vertebral artery can result in extracranial occlusion or stenotic lesions in the intraforaminal segment, which commonly give rise to emboli.
V3 Segment
The third segment of the vertebral artery (V3) extends from the top of the transverse process of C2 to the atlanto-occipital membrane. After crossing this membrane, the artery enters the foramen magnum at the base of the skull and becomes intradural. The first two cervical vertebrae are the most mobile of the spine. About 50% of neck rotation occurs between C1 and C2. The vertebral artery is redundant at this level to allow the arc of displacement of the transverse process of the atlas (80 degrees) to which the vertebral artery is attached. Imaging of V3 is not complete without stressed views in rotation when this segment is fully patent in a neutral position.
The most common problems at the V3 level are related to trauma and arterial dissection and include occlusive lesions, arteriovenous fistulae, and pseudoaneurysms. Extracranial vertebral artery dissection occurs most commonly in the V3 segment because here the artery is most mobile and most vulnerable to mechanical injury. Clinically, extracranial vertebral dissection may present with severe occipitocervical neck pain with associated dizziness, vertigo, double vision, ataxia, and dysarthria.13c Dissection may be associated with fibromuscular dysplasia or occur in a normal artery after seemingly trivial trauma (Fig. 107-8). Vertebral artery dissection follows hyperextension or rotation of the neck and has been associated with, amongst other activities, practicing yoga, painting a ceiling, coughing, vomiting, sneezing, and cervical chiropractic manipulation.13d,13e Vertebral dissection is rare, with an overall incidence of 1 per 100,000 but it is a significant cause for stroke in a young and otherwise healthy population.13f In one study, 62% of dissections observed serially with angiography went on to heal with complete resolution.13g The remaining dissections went on to vessel occlusion, remained narrowed or developed pseudoaneurysmal dilatation and/or AV fistulae (Fig. 107-9). One half of patients diagnosed with extracranial vertebral artery dissection will develop no neurologic symptoms, whereas 21% will experience mild neurologic sequelae, 25% will suffer moderate to severe deficits, and 4% will die.13h Notably, there appears to be no relationship between recanalization rates and neurologic outcome.13i

Figure 107-8 Intramural dissection of the vertebral artery at the V3 segment in a 40-year-old woman with Klippel-Feil syndrome and subluxation of the atlantoaxial joint.
An arteriovenous fistula results from rupture of the wall of the vertebral artery into its surrounding venous plexus. In long-standing fistulae, the pulsatile mass formed by the fistula and its dilated venous channels is called an arteriovenous aneurysm. The vertebral artery may be compressed in the pars atlantica of the third segment between the occipital ridge and the arch of the atlas. In these patients the symptoms of low-flow posterior circulation systems are usually precipitated by head extension or rotation.
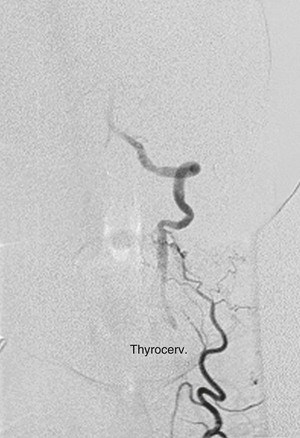
When the vertebral artery is occluded proximally, it usually reconstitutes at the V3 segment by collateral blood vessels from the external carotid via the occipital artery (Fig. 107-10) or by collaterals from the ipsilateral subclavian artery via branches of the thyrocervical trunk (Fig. 107-11).14 Because of this collateral network, the distal vertebral and basilar arteries usually remain patent despite a proximal vertebral artery occlusion. This anatomic finding is crucial for developing surgical strategy. Delayed angiographic views are of the utmost importance in identifying a patent V3 segment that can be exploited as a distal target for reconstruction.
V4 Segment
The fourth segment (V4) is infrequently affected by atherosclerosis. At this level the vertebral artery is vulnerable to direct trauma and stretch injuries that can lead to intimal damage, thrombosis, embolization, and dissection. This segment is also prone to arteriovenous fistula formation and aneurysmal degeneration. Finally, the basilar artery should be seen clearly in a lateral projection. Subtracted views are needed to eliminate the temporal bone density in the lateral projection. In the Towne anteroposterior view, routinely used in neuroradiology, the basilar artery is foreshortened and therefore the resolution is poor. Advanced atherosclerotic disease of the basilar artery is a contraindication to reconstruction of vertebral artery lesions.
Vertebral Artery Reconstructive Procedures
Accumulated experience has shown that with appropriate surgical intervention, predictable resolution of hemodynamic symptoms and cessation of embolic events can occur.
Disease Location
The location of disease will dictate the type of surgical reconstruction that is required. With rare exceptions, most reconstructions of the vertebral artery are performed to relieve an orificial stenosis (V1 segment) or stenoses, dissection, or occlusion of its intraspinal component (V2 and V3 segments).15
V1 Segment
A number of operations have been described for treating stenosing ostial lesions in V1.16,17 Transposition of the proximal vertebral artery onto the adjacent carotid artery is the most common reconstruction. Less commonly, a bypass using either saphenous vein or expanded polytetrafluoroethylene can be performed from either the common carotid artery or the adjacent subclavian artery.18,19 Alternatively, a subclavian-vertebral artery endarterectomy can be performed, but this operation is fraught with technical challenges.
V2 Segment
The second segment of the vertebral artery, the portion that ascends within the foramina of the cervical vertebrae, is the site of a wide variety of pathologic conditions. However, because of its mostly interosseous position, the V2 segment is rarely accessed surgically. The most common indication for exposure of the V2 segment is hemorrhage, which when untreatable endoluminally, is best relieved by proximal and distal ligation of the artery. This has not been associated with worsening neurologic sequelae. After complete V2 occlusion, patency of the distal extracranial segment is often maintained by collaterals from the external carotid or subclavian artery. Ligation (at the C1-C2 level) and bypass to the distal (V3 segment) vertebral artery may be indicated for embolizing V2 pathology.
V3 Segment
Reconstruction of the distal (V3 segment) vertebral artery is usually performed at the C1-C2 level. The technique most often used to reconstruct the distal vertebral artery includes great or small saphenous vein bypass from the common carotid, subclavian, or proximal vertebral artery.14,16 Alternatively, the radial artery can be used as a conduit in the absence of suitable vein. Transposition of the external carotid or hypertrophied occipital artery into the distal vertebral artery, as well as transposition of the distal vertebral artery into the side of the internal carotid artery, has also been described.
Suboccipital Segment
For more distal pathology, the vertebral artery can also be accessed surgically above the level of the transverse process of C1. Surgical exposure at the suboccipital segment requires resection of the C1 transverse process and part of its posterior arch. Reconstruction at this level is limited to saphenous vein bypass from the distal internal carotid artery. Bypasses above the level of C1 (suboccipital) are technically demanding and have been required in only 4% of cases of distal vertebral artery reconstruction.
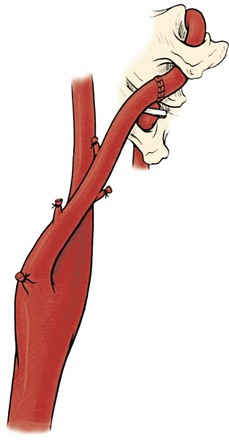
Transposition of the Proximal Vertebral Artery into the Common Carotid Artery
The approach to the proximal vertebral artery is the same as the approach for a subclavian-to-carotid transposition. The incision is placed transversely about a fingerbreadth above the clavicle and directly over the two heads of the sternocleidomastoid muscle (Fig. 107-12). Subplatysmal skin flaps are created to provide adequate exposure. Dissection is carried down directly between the two bellies of the sternocleidomastoid, and the omohyoid muscle is divided. The internal jugular vein and vagus nerve are retracted laterally and the carotid sheath entered. The carotid artery should be exposed proximally as far as possible, which is facilitated if the surgeon temporarily stands at the head of the patient and looks down into the mediastinum.
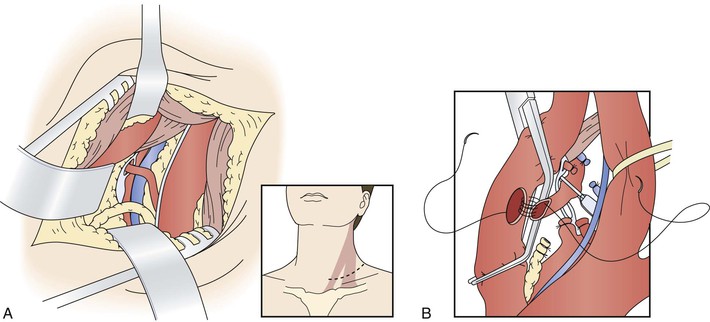
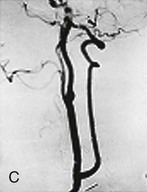
Figure 107-12 A, Access to the proximal vertebral artery between the sternocleidomastoid muscle bellies. B, Transposition of the proximal vertebral artery to the posterior wall of the common carotid artery. C, Proximal vertebral–to–common carotid transposition. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
After the carotid artery is mobilized, the sympathetic chain is identified running behind and parallel to it. On the left side, the thoracic duct is divided between ligatures while avoiding transfixion sutures, which will result in lymph leaks. The proximal end of the thoracic duct is doubly ligated. Accessory lymph ducts—often seen on the right side—are identified, ligated, and divided. The entire dissection is confined medial to the prescalene fat pad that covers the scalenus anticus muscle and phrenic nerve. These latter structures are left unexposed lateral to the field. The inferior thyroid artery runs transversely across the field, and it is ligated and divided.
The vertebral vein is next identified emerging from the angle formed by the longus colli and scalenus anticus and overlying the vertebral artery and, at the bottom of the field, the subclavian artery. Unlike its sister artery, the vertebral vein has branches. It is ligated in continuity and divided. Below the vertebral vein lies the vertebral artery. It is important to identify and avoid injury to the adjacent sympathetic chain. The vertebral artery is dissected superiorly to the tendon of the longus colli and inferiorly to its origin in the subclavian artery. The vertebral artery is freed from the sympathetic trunk resting on its anterior surface without damaging the trunk or the ganglionic rami. Preserving the sympathetic trunks and the stellate or intermediate ganglia resting on the artery usually requires freeing the vertebral artery from these structures, and after dividing its origin, the latter is transposed anterior to the sympathetics.
Once the artery is fully exposed, an appropriate site for reimplantation in the common carotid artery is selected. The patient is given heparin systemically. The distal portion of the V1 segment of the vertebral artery is clamped below the edge of the longus colli with a microclip placed vertically to indicate the orientation of the artery and to avoid axial twisting during its transposition. The proximal vertebral artery is closed by transfixion with 5-0 polypropylene suture immediately above the stenosis at its origin. The artery is divided at this level, and its proximal stump is further secured with a hemoclip. The artery is then brought to the common carotid artery, and its free end is spatulated for anastomosis.
The carotid artery is then cross-clamped. An elliptical 5- to 7-mm arteriotomy is created in the posterolateral wall of the common carotid artery with an aortic punch. The anastomosis is performed in open fashion with continuous 6-0 or 7-0 polypropylene suture while avoiding any tension on the vertebral artery, which tears easily. Before completion of the anastomosis, any slack in the suture is tightened appropriately with a nerve hook, standard flushing maneuvers are performed, and the suture is tied to reestablish flow (see Fig. 107-12C).
When simultaneous carotid endarterectomy is planned, the vertebral artery is approached through the standard carotid incision extended inferiorly to the head of the clavicle. With this approach, the sternocleidomastoid muscle is lateral and the field is a bit narrower than when the vertebral artery is approached between the heads of the sternocleidomastoid. The remaining steps of the operation are performed as described previously.20
Distal Vertebral Artery Reconstruction
Common Carotid–Vertebral Artery Bypass
Reconstruction of the distal vertebral artery is generally done at the C1-C2 level. Rarely, the reconstruction is done between C1 and the base of the skull via a posterior approach. Although various techniques can be applied to revascularize the vertebral artery in its V3 segment (between the transverse processes of C1 and C2), the approach to the vertebral artery at this level is the same for all procedures.14,21
The incision is made anterior to the sternocleidomastoid muscle, the same as for a carotid operation, and is carried superiorly to immediately below the earlobe (Fig. 107-13). The dissection proceeds between the internal jugular vein and the anterior edge of the sternocleidomastoid to expose the spinal accessory nerve. The nerve is followed distally as it joins the jugular vein and crosses in front of the transverse process of C1, which can easily be felt by the operator’s finger. The next step involves identification of the levator scapulae muscle by removal of the fibrofatty tissue overlying it. With the anterior edge of the levator scapulae identified, the surgeon searches for the anterior ramus of C2. With the ramus as a guide, a right-angle clamp is slid over the ramus to elevate the levator scapulae, which is transected from its origin (Fig. 107-14). The proximal stump of the levator is excised up to its insertion on the C1 transverse process. The C2 ramus divides into three branches after crossing the vertebral artery. The artery runs below, in contact with the nerve and perpendicular to it. The surgeon cuts the ramus (Fig. 107-15) before its branching; underneath it, the vertebral artery can be identified. A small minority of patients may develop noticeable but self-limiting posterior scalp numbness as a result of cutting the ramus.
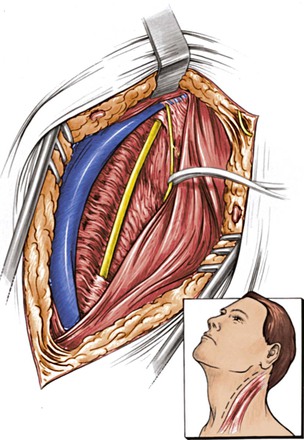
Figure 107-13 Retrojugular approach and isolation of the spinal accessory nerve. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
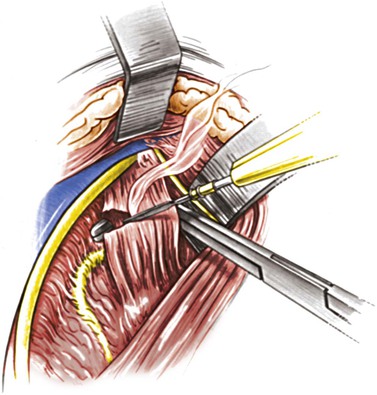
Figure 107-14 Dividing the levator scapulae over the C2 ramus. The vagus, internal jugular vein, and internal carotid artery are anterior to the muscle. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
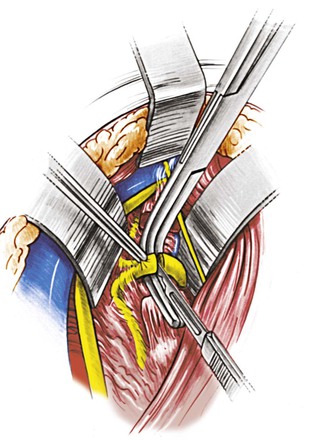
Figure 107-15 Dividing the anterior ramus of C2 to expose the underlying vertebral artery running perpendicular to the anterior ramus. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
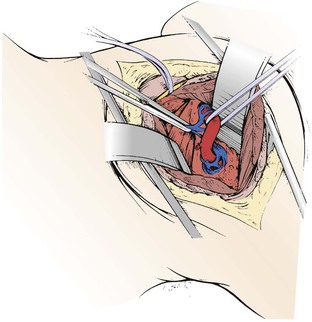
Dissection of the artery at this level is best accomplished with loupe magnification, advisable for all vertebral artery reconstructions. The artery is freed from the surrounding veins with extreme care because hemorrhage is difficult to control at this level. Before encircling the artery with fine silicone vessel loops (Fig. 107-16), one must ensure that a branch from the occipital collateral artery does not enter the posterior aspect of the vertebral artery at the location at which the surgeon is dissecting. Tearing of this important collateral vessel complicates preparation of the distal vertebral artery unnecessarily. Once the vertebral artery is encircled, the distal common carotid artery is dissected and prepared to receive a saphenous vein graft. There is no need to dissect the carotid bifurcation. The location selected for the proximal anastomosis of the saphenous vein graft on the common carotid artery should not be too close to the bifurcation because cross-clamping at this level may fracture any underlying atheroma.
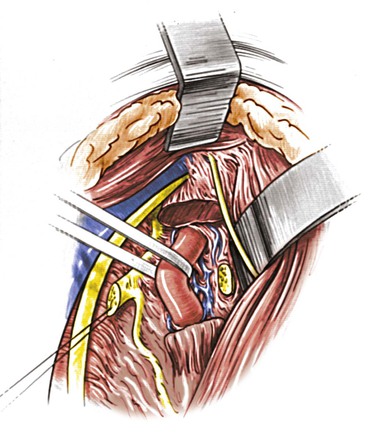
Figure 107-16 After the vertebral venous plexus is dissected away, the vertebral artery is slung with a polymeric silicone (Silastic) loop for clamping and anastomosis. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
A saphenous vein graft or other suitable conduit of appropriate length is harvested from the thigh and prepared. A valveless segment facilitates back-bleeding of the vertebral artery after completion of the distal anastomosis. The patient is given intravenous heparin. The vertebral artery is elevated by gently pulling the loop and is occluded with a small J-clamp to isolate this segment for an end-to-side anastomosis. The vertebral artery is opened longitudinally with a coronary knife for a length adequate to accommodate the spatulated end of the vein graft. The end-to-side anastomosis is performed with continuous 7-0 polypropylene and fine needles. The distal anastomosis is assessed for backflow, and if satisfactory, a Heifetz clip is placed in the vein graft proximal to the anastomosis to restore flow through the vertebral artery.
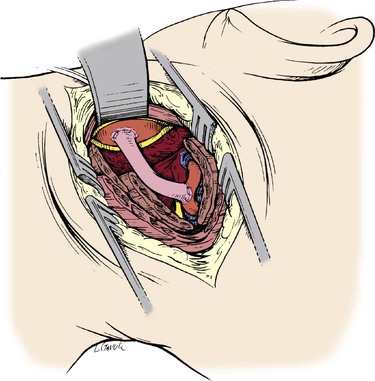
The proximal end of the graft is passed beneath the jugular vein and in proximity to the side of the common carotid artery. The common carotid artery is then cross-clamped, an elliptical arteriotomy is made in its posterior wall with an aortic punch, and the proximal vein graft is anastomosed end-to-side to the common carotid artery with continuous 6-0 polypropylene (Figs. 107-17 and 107-18). Before the anastomosis is completed, standard flushing maneuvers are performed, the suture is tied, and flow is reestablished. Next, the vertebral artery is occluded with a clip immediately below the anastomosis so that competitive flow or the potential for recurrent emboli is avoided. In the absence of suitable common carotid artery, the ipsilateral subclavian artery can be used as a donor vessel.
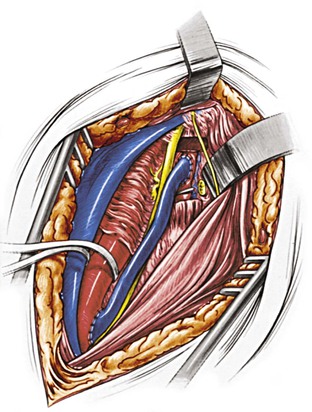
Figure 107-17 A completed common carotid artery–to–distal vertebral artery bypass using the saphenous vein. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
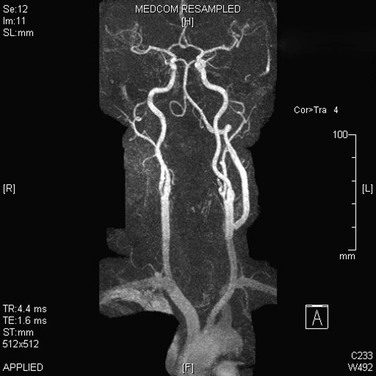
Figure 107-18 Follow-up MRA showing a patent distal vertebral bypass.
External Carotid–Vertebral Artery Transposition
The distal vertebral artery may also be revascularized via the external carotid artery (ECA) either directly by means of transposition of the ECA to the distal vertebral artery or by anastomosis of the proximal end of a graft to the ECA. Transposition of the ECA to the distal vertebral artery (Figs. 107-19 and 107-20) requires a carotid bifurcation free of disease and a long ECA trunk. ECAs that divide early are often too small to match the caliber of the vertebral artery. If the trunk of the ECA is of adequate size and length to reach the vertebral artery, the ECA is skeletonized by dividing all its branches. The ECA is then rotated over the internal carotid artery and beneath the internal jugular vein to construct an end-to-side anastomosis to the distal vertebral artery at the C1-C2 level. After completion of this anastomosis, the vertebral artery is permanently occluded with a clip immediately below the anastomosis.
Vertebral Artery–Internal Carotid Artery Transposition
Another method of revascularization of the distal vertebral artery is transposition of this vessel into the distal cervical internal carotid artery below the transverse process of C1 (Fig. 107-21). This technique is particularly applicable for patients with inadequate saphenous vein or in whom the ECA cannot be used because of unsuitable anatomy or disease of the carotid bifurcation. This is a straightforward end-to-side anastomosis between the distal vertebral artery and the distal cervical internal carotid artery. This procedure, however, is contraindicated in patients with contralateral internal carotid artery occlusion, in whom the risk of cerebral ischemia during the anastomosis would be prohibitive.
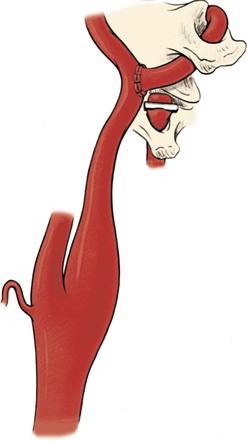
Figure 107-21 Transposition of the distal vertebral artery into the cervical internal carotid artery. (From Berguer R, et al: Surgery of the arteries to the head, New York, NY, 1992, Springer-Verlag.)
A small number of patients have disease that extends up to the level of C1 and require revascularization in the most distal segment of the extracranial vertebral artery.22,23 To accomplish this, the vertebral artery must be exposed in its pars atlantica, where the artery runs parallel to the lamina of the atlas before entering the foramen magnum.
Posterior Suboccipital Vertebral Artery Bypass
The approach to the suboccipital segment of the distal vertebral artery is posterior.24 The patient lies prone in the “park bench” position. The incision is racket shaped, with a horizontal segment below the occipital bone from the midline laterally to the level of the sternocleidomastoid. There, the incision becomes oblique and follows the posterior belly of the sternocleidomastoid muscle. The superficial nuchal muscle layer (splenius capitis and semispinalis) is transected (Fig. 107-22). The accessory spinal nerve is isolated laterally below the sternocleidomastoid muscle. The transverse process of C1 is located by palpation. The short posterior muscles between the atlas and occipital bone (obliquus capitis and rectus capitis posterior major) are divided (Fig. 107-23). The artery can now be seen enveloped by a dense venous plexus (Fig. 107-24), from which it is extricated by bipolar coagulation and microligature of these veins. The artery can be exposed from its emergence at the top of C1 to the dura mater (Fig. 107-25). Through the same posterior approach, the internal carotid artery can be isolated posterior and medial to the sternocleidomastoid after mobilization and retraction of the hypoglossal and vagus nerves, which at this level cover its posterior wall. The distal anastomosis of the vein graft to the vertebral artery is performed first. The vein is allowed to distend over the lamina of the atlas and into its site of anastomosis at the posterior wall of the internal carotid artery (Figs. 107-26 and 107-27).
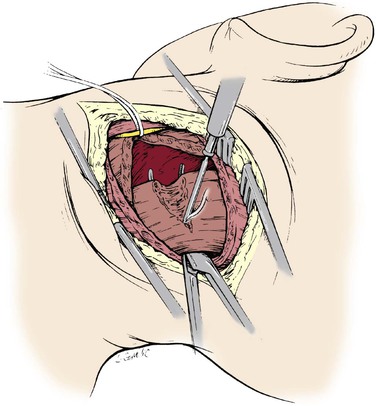
Figure 107-22 Posterior approach to the suboccipital segment of the distal vertebral artery: division of the splenius capitis and semispinalis.
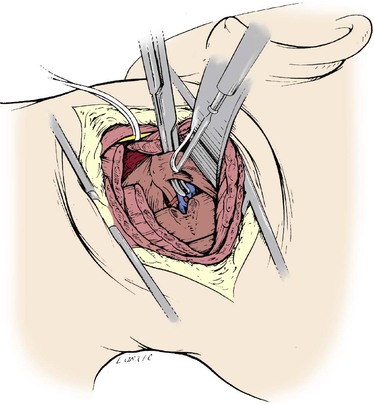
Figure 107-23 Posterior approach to the suboccipital vertebral artery: division of the obliquus and rectus capitis.
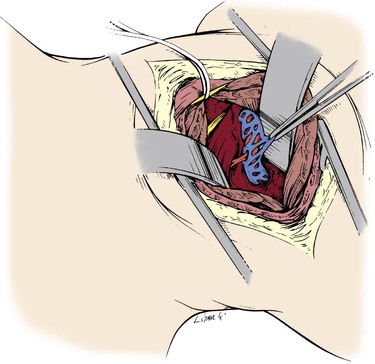
Figure 107-24 The pars atlantica of the distal vertebral artery exposed and surrounded by a dense venous plexus.
Operative Results
Perioperative
Perioperative complication rates differ for proximal versus distal vertebral artery repairs. Perioperative complications that can follow any reconstruction include stroke, bleeding, thrombosis, and nerve injury. Intraoperative completion imaging with digital angiography is useful and should be considered for all types of vertebral artery reconstruction. A significant number of reparable technical flaws may be identified, and repair can prevent reconstruction failure.
Proximal Reconstructions
The technically easier proximal operations have been reported to have a combined stroke and death rate of 0.9%.12 Among patients undergoing proximal operations in one report, no deaths or strokes occurred in those who underwent only a vertebral reconstruction. When the proximal vertebral artery reconstruction was combined with a carotid operation, the observed stroke and death rates increased to 5.7% in that same report.12 Berguer et al12 reported four instances of immediate postoperative thrombosis (1.4%). Three of the four patients had vein grafts interposed between the vertebral artery and the common carotid because of a short V1 segment. The grafts kinked and thrombosed. Other complications that are particular to proximal reconstruction include vagus and recurrent laryngeal nerve palsy (2%), Horner’s syndrome (8.4% to 28%), lymphocele (4%), and chylothorax (0.5%).
Distal Reconstructions
Operations on the distal vertebral artery carry higher stroke and death rates than do operations on the proximal vertebral artery. Distal reconstructions have a combined stroke and death rate of 3%.25 The immediate graft thrombosis rate has been reported at 8%, and spinal accessory nerve injury occurs in 2% of patients.26
Long-Term Outcomes
Results after both proximal and distal vertebral artery reconstruction are generally equal to or better than those reported in series reviewing other forms of extracranial cerebrovascular reconstruction.25,27 Proximal vertebral artery reconstruction has long-term results equal to or better than those after carotid surgery. After proximal vertebral–to–common carotid transposition, patency rates at 5 and 10 years equal or exceed 95% and 91%, respectively. When selected appropriately, more than 80% of patients will have relief of their symptoms after proximal surgical reconstruction.27
For distal bypass reconstruction, patency rates of 87% and 82% should be expected at 5 and 10 years.25,27 Seventy percent of patients undergoing distal vertebral artery reconstruction are dead at 5 years of follow-up, mostly from cardiac disease, whereas 97% of survivors are free of stroke at 5 years. Symptoms are expected to be cured in 71% of patients and improved in 16%.25,27
Predictably, in patients treated for severe hemodynamic symptoms who also suffer from compensatory high blood pressure, appropriate reconstruction and reperfusion of the brainstem results in significant improvement in their hypertension.
Endovascular Therapy
Endovascular Treatment
In the last decade, endovascular treatment of vertebral artery disease has gained favor as an alternative to surgery. A growing number of case reports and small nonrandomized case series suggest that endovascular intervention in the posterior circulation may be safe and technically feasible.28–39 Endovascular access to the vertebral artery is relatively straightforward. Patients are pretreated with dual-therapy aspirin and clopidogrel. The procedure can be performed under local anesthesia, enabling continuous neurologic monitoring of the patient. Most cases are performed from a femoral approach (93%), although transbrachial (3%) and transradial (5%) access has also been used, as noted in one recent review.39a The stenotic lesions are crossed and treated with 0.014- or 0.018-inch guide wires and small coronary-diameter balloons and stents. Procedures can be performed with or without the assistance of embolic protection, although vertebral arteries are usually too small to accommodate most distal protection devices. One large review found distal protection used in only 2% of cases.39a
Periprocedural risks include access complications, distal embolization and stroke, arterial rupture, stent malposition and vessel thrombosis or dissection. Later, restenosis and stent fracture are not uncommon.
Overall, retrospective reviews suggest that endoluminal vertebral artery intervention is reasonably safe, although a selection bias exists. A 2005 Cochrane review identified 313 interventions for vertebral artery stenosis, with just over half using stent placement as part of the treatment. The technical success rate was 95%, and the 30-day stroke and death rate was 6.4%.40 The Cochrane group concluded that although angioplasty with stenting for vertebral artery stenosis is technically feasible, evidence is currently insufficient to support its routine application.
A subset of 16 patients treated within the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS 2001) represents the only report of a randomized controlled trial comparing endoluminal therapy with best medical care for symptomatic vertebral stenosis. No 30-day strokes or deaths occurred in either group, although two of eight patients who underwent endoluminal therapy experienced transient ischemic symptoms. Furthermore, with a mean follow-up of 4.5 years, no posterior circulation strokes were noted in either group.
Despite high technical success rates, vertebral artery angioplasty alone, especially when used for the treatment of disease at the origin of the vessel, appears to have an unacceptably high rate of restenosis. Adjuvant stent placement seems to add to the clinical durability, but this is certainly not a panacea; plus stents carry inherent morbidity of their own, such as malposition and potential stent fracture. In their series of 105 patients who underwent endovascular stenting for symptomatic vertebral artery disease, Jenkins et al achieved 100% radiographic improvement (residual stenosis ≤30%). The authors reported immediate (30-day) periprocedural risk of death of 1% and periprocedural complication rate of 4.8%. Complications included transient ischemic attack, flow-limiting dissection, hematoma, and catheter-access–site problems. At 1 year of follow-up, six patients had died and five had experienced a vertebrobasilar stroke; at approximately 2.5 years of follow-up 70% of patients remained symptom free, but 13% of patients had restenosis requiring retreatment.41
In the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) trial, 18 patients with extracranial vertebral artery disease underwent angioplasty and stenting. Technical success (determined as less than 50% residual stenosis following treatment) was achieved in 17 (94%) of the 18 patients. No periprocedural neurologic complications were observed. The investigators, however, reported 6-month restenosis rates of 50%. These recurrences were symptomatic in 39% of cases.42
A recent systematic review of the available literature noted a weighted mean technical success rate of 97%. The authors estimated mean periprocedural stroke and death rate from combined angioplasty and stenting to be around 1.1%. Transient ischemic events occurred in 1.5% of patients. Recurrent symptoms occurred in 8% of patients within a reported follow-up range of 6 to 54 months, and greater than 50% restenosis developed in 23% of the subset of patients who underwent follow-up imaging.39a
Late stent fracture with concomitant in-stent restenosis may be a problem plaguing endoluminal therapies that target lesions at the vertebral artery origin. Anatomically, the vertebral artery takes origin from the subclavian artery at a near right angle. In addition, the first portion of the subclavian artery has relative mobility, whereas the vertebral artery becomes fixed as it passes into the transverse foramen of C6. This particular anatomy may create unique mechanical forces that make stent fracture more likely than it is for other parts of the body (Fig. 107-28).
The use of drug-eluting stents to impede neointimal hyperplasia and prevent restenosis has been well established in the coronary arteries and may be beneficial in the treatment of vertebral disease.43 Ogilvy et al reported a series of patients with the longest follow-up thus far (21 months); for these patients, drug-eluting stents were used in vertebral artery origin stenoses. The authors found that the incidence of in-stent restenosis (>50% diameter) decreased from 38% in patients who received non–drug-eluting stents to 17% in those who received drug-eluting stents.44 Other reports also suggest decreased restenosis rates with drug-eluting stents; however, the majority of those studies have mean patient follow-up times of less than 1 year.45,46 Treatment with drug-eluting stents requires long-term dual antiplatelet therapy. It remains unclear whether differing stent makeup will have a significant impact in the outcomes of patients who undergo interventions of the vertebral artery.
As with open surgical techniques, with the exception of the small subset of patients from CAVATAS 2001, only retrospective case series exist for endoluminal therapies for the treatment of vertebral artery disease. Presently, no level I data support the routine application of angioplasty and stenting of the vertebral artery over best medical therapy. Currently underway is the single, multicenter, randomized Vertebral Artery Stenting Trial (VAST), designed to prospectively analyze the impact of percutaneous vertebral interventions over medical therapy for stenting of intracranial or extracranial vertebral artery stenosis.47
Although vertebral artery angioplasty and stenting may be a relatively safe and effective approach that avoids the morbidity associated with major surgery, most available data on the efficacy of this therapy are limited to single-center retrospective reports that carry inherent selection bias. The only randomized data available are underpowered, and definitive conclusions on the effectiveness of endovascular therapy for vertebral disease cannot be drawn. At present, the technique should be reserved for select cases and should be performed in centers with high volume experience and established acceptable outcomes in both clinical success and safety until indications for routine application become more clear.
Conclusion
Atherosclerotic vertebral artery disease is an underdiagnosed cause of posterior circulation ischemia. Revascularization of the vertebral artery is often a viable option and should be considered in symptomatic patients in whom medical therapy has failed. Both surgical and endoluminal approaches to treating vertebral artery pathology may be considered; the choice between the two is often determined by the anatomic location of the lesion requiring intervention. Such consideration requires a complete understanding of the vertebrobasilar anatomy using appropriate imaging studies. Open techniques for revascularization of the vertebral artery have proven clinical durability and acceptable surgical morbidity in experienced hands. Endoluminal techniques, which have gained momentum over the past decade, have shown clinical feasibility but have yet to deliver on durability benchmarks set by open surgical revascularization. For each individual patient who suffers from medically refractive vertebrobasilar ischemia, practitioners must carefully balance the risks of surgery versus the limitations of endoluminal intervention before recommending intervention.
Selected Key References
Berguer R, Flynn LM, Kline RA, Caplan L. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg. 2000;31:9–18.
Retrospective review of a large series of all types of surgical vertebral reconstruction..
Berguer R, Morasch MD, Kline RA. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 1998;27:852–859.
Retrospective review of the largest series of distal vertebral reconstructions to date..
Caplan LR, Wityk RJ, Glass TA, Tapi AJ, Pazdera L, Chang HM, Teal P, Dashe JF, Chaves CJ, Breen JC, Vemmos K, Amarenco P, Tettenborn B, Leary M, Estol C, Dewitt LD, Pessin MS. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389–398.
Comprehensive review of the natural history of posterior circulation lesions..
Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane Database Syst Rev. 2005;(2).
Cochrane meta-analysis of the literature on endoluminal therapy for vertebral disease..
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Wityk RJ, et al. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation registry. Arch Neurol. 1998;55:470–478.
2. Cartlidge NE, et al. Carotid and vertebral-basilar transient cerebral ischemic attacks. A community study, Rochester, Minn. Mayo Clin Proc. 1977;52:117–120.
3. Heyman A, et al. Clinical and epidemiologic aspects of vertebrobasilar and nonfocal cerebral ischemia. Berguer R, et al. Vertebrobasilar arterial occlusive disease. Medical and surgical management. Raven Press: New York, NY; 1984:27–36.
4. Whisnant JP, et al. Carotid and vertebral-basilar transient ischemic attacks: effect of anticoagulants, hypertension, and cardiac disorders on survival and stroke occurrence—a population study. Ann Neurol. 1978;3:107–115.
5. Jones HR Jr, et al. Temporal profile (clinical course) of acute vertebrobasilar system cerebral infarction. Stroke. 1980;11:173–177.
6. McDowell FH, et al. The natural history of internal carotid and vertebral-basilar artery occlusion. Neurology. 1961;11:153–157.
7. Patrick BK, et al. Temporal profile of vertebrobasilar territory infarction. Prognostic implications. Stroke. 1980;11:643–648.
8. Caplan LR, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389–398.
9. Caplan L, et al. Embolism in the posterior circulation. Berguer R, et al. Vertebrobasilar arterial disease. Quality Medical: St. Louis, Mo; 1992:52–65.
10. Pessin M. Posterior cerebral artery disease and occipital ischemia. Berguer R, et al. Vertebrobasilar arterial disease. Quality Medical: St. Louis, Mo; 1992:66–75.
11. Bauer R. Mechanical compression of the vertebral arteries. Berguer R, et al. Vertebrobasilar arterial occlusive disease: medical and surgical management. Raven: New York, NY; 1984:45–71.
12. Berguer R, et al. Noninvasive diagnosis of reversal of vertebral-artery blood flow. N Engl J Med. 1980;302:1349–1351.
13. Ruotolo C, et al. Dynamic arteriography. Berguer R, et al. Vertebrobasilar arterial disease. Quality Medical: St. Louis, Mo; 1992:116–123.
13a. Morasch MD, et al. Primary extracranial vertebral artery aneurysms. Ann Vasc Surg. 2013;27:418–423 [May] .
13b. Sultan S, et al. Operative and endovascular management of extracranial vertebral artery aneurysm in Ehlers-Danlos syndrome: a clinical dilemma—case report and literature review. Vasc Endovas Surg. 2002;5:389–392 [36] .
13c. Park KW, et al. Vertebral artery dissection: natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. 2008;44(3):109–115.
13d. Fisher CM, et al. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci. 1978;5:9–19.
13e. Hufnagel A, et al. Stroke following chiropractic manipulation of the cervical spine. J Neurol. 1999;246:683–688.
13f. Bogousslavsky J, et al. Ischemic stroke in adults younger than 30 years of age. Cause and prognosis. Arch Neurol. 1987;44:479–482.
13g. Mokri B, et al. Spontaneous dissections of the vertebral arteries. Neurology. 1988;38:880–885.
13h. Thanvi B, et al. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383–388.
13i. Caso V, et al. Recanalization of cervical artery dissection: influencing factors and role in neurological outcome. Cerebrovasc Dis. 2004;17:93–97.
14. Berguer R. Distal vertebral artery bypass: technique, the “occipital connection,” and potential uses. J Vasc Surg. 1985;2:621–626.
15. Berguer R, et al. Surgery of the arteries to the head. Springer-Verlag: New York, NY; 1992.
16. Edwards WH, et al. The surgical approach to significant stenosis of vertebral and subclavian arteries. Surgery. 1980;87:20–28.
17. Roon AJ, et al. Vertebral artery reconstruction. Am J Surg. 1979;138:29–36.
18. Berguer R, et al. Vertebral artery bypass. Arch Surg. 1976;111:976–979.
19. Berguer R, et al. Vertebral artery reconstruction. A successful technique in selected patients. Ann Surg. 1981;193:441–447.
20. McNamara MF, et al. Simultaneous carotid-vertebral reconstruction. J Cardiovasc Surg (Torino). 1989;30:161–164.
21. Kieffer E, et al. Reconstruction of the distal cervical vertebral artery. Bergeron P, et al. Vertebrobasilar arterial occlusive disease: medical and surgical management. Raven: New York, NY; 1984:265–289.
22. Berguer R. Revascularization of the vertebral arteries. Nyhus L, et al. Mastery of surgery. Little, Brown: Boston, Mass; 1997:1937.
23. Berguer R. Complex carotid and vertebral revascularizations. Pearce WH, et al. Vascular surgery in the endovascular era. Greenwood Academic: Evanston, Ill; 2008:344–352.
24. Berguer R. Suboccipital approach to the distal vertebral artery. J Vasc Surg. 1999;30:344–349.
25. Berguer R, et al. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 1998;27:852–859.
26. Thevenet A, et al. Surgical repair of vertebral artery stenoses. J Cardiovasc Surg (Torino). 1984;25:101–110.
27. Berguer R, et al. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg. 2000;31:9–18.
28. Cloud GC, et al. Vertebral artery origin angioplasty and primary stenting: safety and restenosis rates in a prospective series. J Neurol Neurosurg Psychiatry. 2003;74:586–590.
29. Dabus G, et al. Endovascular treatment of the vertebral artery origin in patients with symptoms of vertebrobasilar ischemia. Neuroradiology. 2006;48:917–923.
30. Hauth EA, et al. Angioplasty or stenting of extra- and intracranial vertebral artery stenoses. Cardiovasc Intervent Radiol. 2004;27:51–57.
31. Henry M, et al. Angioplasty and stenting of extracranial verterbral artery stenosis. Int Angiol. 2005;311–324.
32. Janssens E, et al. Percutaneous transluminal angioplasty of proximal vertebral artery stenosis: long-term clinical follow-up of 16 consecutive patients. Neuroradiology. 2004;46:81–84.
33. Jenkins JS, et al. Vertebral artery stenting. Catheter Cardiovasc Interv. 2001;54:1–5.
34. Kim HY, et al. Transient global amnesia following vertebral artery angioplasty and stenting. Eur Neurol. 2006;56:133–135.
35. Levy EI, et al. Transluminal stent-assisted angioplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results. Neurosurgery. 2001;48:1215–1221.
36. Lin YH, et al. The impact of lesion length on angiographic restenosis after vertebral artery origin stenting. Eur J Vasc Endovasc Surg. 2006;32:379–385.
37. Mukherjee D, et al. Percutaneous intervention for symptomatic vertebral artery stenosis using coronary stents. J Invasive Cardiol. 2001;13:363–366.
38. Piotin M, et al. Percutaneous transluminal angioplasty and stenting of the proximal vertebral artery for symptomatic stenosis. AJNR Am J Neuroradiol. 2000;21:727–731.
39. Wehman JC, et al. Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J Interv Cardiol. 2004;17:219–232.
39a. Antoniou GA, et al. Percutaneous transluminal angioplasty and stenting in patients with proximal vertebral artery stenosis. J Vasc Surg. 2012;55:1167–1177.
40. Coward LJ, et al. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane Database Syst Rev. 2005;(2).
41. Jenkins JS, et al. Endovascular stenting for vertebral artery stenosis. J Am Coll Cardiol. 2010;55(6):538–542.
42. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35(6):1388–1392.
44. Ogilvy CS, et al. Restenosis rates following vertebral artery origin stenting: does stent type make a difference? J Invasive Cardiol. 2010;22(3):119–124.
45. Steinfort B, et al. Midterm outcomes of paclitaxel-eluting stents for the treatment of intracranial posterior circulation stenoses. J Neurosurg. 2007;106(2):222–225.
46. Vajda Z, et al. Treatment of stenoses of vertebral artery origin using short drug-eluting coronary stents: improved follow-up results. AJNR Am J Neuroradiol. 2009;30(9):1653–1656.
47. Compter A, et al. VAST: Vertebral Artery Stenting Trial. Trials. 2008;9:65.