CHAPTER 138 Ventricular Tumors
Although there were some first descriptions of ventricular neoplasms by Shaw in 1854, it was Walter Dandy who had the most impact on descriptions of and treatment options for ventricular pathologies. During his career, he published more than 30 articles on these tumors and pioneered several surgical approaches to them that are still used today. In 1933, he dedicated a book to this subject, Benign Tumors in the Third Ventricle of the Brain: Diagnosis and Treatment.1
More publications on ventricular lesions followed, and with ongoing technical progress, interest in surgical approaches to the ventricular system was growing. As a result, many studies and books were published on this topic in the following years, including milestones such as Surgery of the 3rd Ventricle by Michael L. J. Apuzzo.2
Pathology
Tumors of the ventricular system account for less than 1% of intracranial lesions,3,4 most of which are benign and slow growing. For this reason, symptoms develop at a late stage in many instances. According to Casotto and coworkers,5 these lesions can be categorized as primary or secondary. Primary, or true, ventricular tumors are those originating from the ventricular wall and extending strictly into the ventricular system. Paraventricular, or secondary, tumors are those originating from structures adjacent to the ventricular system. The major part of such neoplasms is located within the ventricular cavity, but a portion of the tumor is outside the ventricular system.5 Among the most common primary tumors are colloid cysts, choroid plexus papillomas, ependymomas, epidermoid and dermoid cysts, and craniopharyngiomas. Secondary tumors include meningiomas, gliomas, pituitary adenomas, and arachnoid cysts.6 According to Apuzzo,2 only around 10% of these masses are confined to the ventricular system.
Table 138-1 lists the variety of pathologic lesions encountered at our institution in a series of 143 patients who were systematically monitored after surgery. In addition to this patient population, the senior author (H.B.) has surgically treated a considerable number of individuals suffering from various ventricular tumors while working at other institutions. Because this additional patient group has not yet been monitored systematically in the same fashion as the population just mentioned, only a few cases from this second patient group have been included in this chapter.
TABLE 138-1 Histologic Diagnoses of Ventricular Tumors in a Single-Institution Consecutive Series of 143 Patients
| HISTOLOGY | n |
|---|---|
| Colloid cyst | 19 |
| Craniopharyngioma | 16 |
| Pilocytic astrocytoma | 15 |
| Pineocytoma (WHO grade I) | 9 |
| Cavernous malformation | 8 |
| Medulloblastoma | 8 |
| Metastatic tumor | 7 |
| Glioblastoma | 6 |
| Pineal cyst | 6 |
| Subependymoma | 5 |
| Fibrillary astrocytoma | 4 |
| Anaplastic ependymoma | 3 |
| Meningioma | 4 |
| Central neurocytoma | 3 |
| Pineocytoma (WHO grade II) | 3 |
| Anaplastic astrocytoma | 2 |
| Dermoid cyst | 2 |
| Ependymoma | 2 |
| Epidermoid cyst | 2 |
| Gliosis | 2 |
| Hemangioblastoma | 2 |
| Lymphoma | 2 |
| Neurenteric cyst | 2 |
| Pineoblastoma | 2 |
| Pituitary adenoma | 2 |
| Chordoid glioma | 1 |
| Gliosarcoma | 1 |
| Granular cell tumor | 1 |
| Plexus papilloma | 1 |
| Anaplastic plexus papilloma | 1 |
| Germinoma | 1 |
| Teratoma | 1 |
Clinical Features
Because of the deep location of the ventricular system within the brain and its proximity to important neural and vascular structures, ventricular tumors may cause a great variety of clinical symptoms. Grossly, symptoms may be divided into two types: symptoms caused by CSF obstruction and those caused by compression of certain neuronal structures. Because many ventricular tumors are benign and slow growing, they may reach considerable size before causing nonspecific symptoms.7 Common clinical symptoms include headache, vertigo, visual disturbances, difficulty concentrating, changes in personality, cognitive deficits, motor weakness, and epileptic seizures.3 Acute hydrocephalus causes headache, nausea, and vomiting. Additionally, memory deficits and gait disturbances may occur.
Colloid cysts may cause chronic, acute, or intermittent hydrocephalus that becomes clinically manifested as paroxysmal posture with headache, nausea, vomiting, and disturbed consciousness.8 Some authors have described diplopia, psycho-organic syndrome, psychomotor retardation, and short-term memory deficits in patients with these lesions.9 Rarely, colloid cysts may even cause sudden death as a consequence of acute obstructive hydrocephalus.10
Imaging Studies
Ependymomas commonly appear as homogeneous lesions on CT with contrast enhancement. Intratumoral cysts may be apparent, and approximately half of the tumors show calcifications. On MRI, ependymomas may have a variety of appearances. Frequently, they are seen as hypointense or isointense lesions with cystic portions. Intratumorally, necrotic areas, blood vessels, hemorrhage, or hemosiderin may be present. Contrast enhancement is heterogeneous.2 Subependymomas are well-circumscribed lesions that appear isodense or hyperdense on CT. On MRI they appear as lobulated structures, hypointense or isointense on T1-weighted images and hyperintense on T2-weighted ones. Contrast enhancement is weak or absent.11
In up to 75% of cases, choroid plexus papillomas appear as isodense or hyperdense well-circumscribed lesions on CT. Contrast enhancement is high, and calcifications or bleeding can frequently be observed. MRI studies show these lesions to be isointense on T1- and T2-weighted images, and contrast enhancement is homogeneous. MRI does not readily permit differentiation of choroid plexus carcinomas from plexus papillomas.2
In up to 90% of craniopharyngiomas, cystic and solid portions can be seen. On CT, the solid portions show contrast enhancement. On MRI, they appear as partially cystic and partially solid lesions with heterogeneous signal intensity. Contrast enhancement is high.4
Surgical Anatomy
Lateral Ventricle
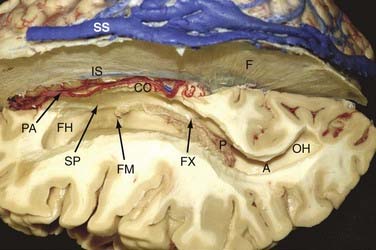
Both lateral ventricles communicate with the third ventricle via the foramina of Monro and are surrounded by the following structures: septum pellucidum, thalamus, caudate nucleus, corpus callosum, and fornix (Fig. 138-1). The largest structure in connection with the lateral ventricle is the corpus callosum. It is the largest commissural fiber tract of the brain and is divided into four different parts: the rostrum, genu (anteriorly), body, and splenium (posteriorly).
Third Ventricle
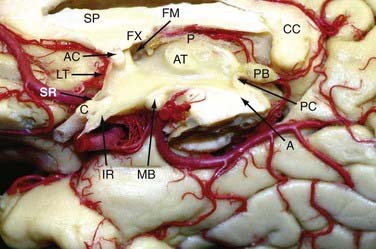
The lateral walls are formed by the thalamus and hypothalamus and separated by the hypothalamic sulcus (Fig. 138-2). In approximately 75% of patients, a connection between the lateral walls can be found in the upper part of the third ventricle and is referred to as the massa intermedia.
The floor of the third ventricle, which extends from the optic chiasm to the aqueduct of Sylvius, includes the infundibulum, the tuber cinereum, the mamillary bodies, and the posterior perforated substance. The roof of the third ventricle extends from the foramen of Monro to the suprapineal recess and is formed by five layers: the fornix (superior layer), the two layers of the tela choroidea (which envelope the vascular layer [velum interpositum]), and the choroid plexus layer. The vascular layer contains the internal cerebral veins and the medial posterior choroidal arteries. Because of its U shape, the junction of the thalamostriate vein with the anterior septal vein to form the internal cerebral vein at the posterior margin of the foramen of Monro is referred to as a venous angle and can be well identified on cerebral angiograms or with CT/MR angiography. In 30% of cases, this junction is located 3 to 7 mm behind the posterior border of the foramen of Monro.12 If such a posteriorly located venous junction is present, posterior enlargement of the foramen of Monro along the choroidal fissure can open a direct trajectory into the third ventricle. The two internal cerebral veins run close to each other up to the pineal recess, where they deviate from the midline and proceed along the superolateral surface of the pineal body to the deepest point of the splenium to form the vein of Galen. The plexus of the third ventricle is attached to the lower layer of the tela choroidea.
Surgical Removal of Lateral Ventricle Tumors
General Aspects
Because of their deep location, the lateral ventricles are not readily accessible for surgery. Either the cerebral hemisphere or the corpus callosum must be traversed to reach the ventricular cavity. To minimize parenchymal traumatization by surgical manipulation, natural pathways such as the cerebral sulci, fissures, and cisterns should be used. It was Yasargil and Abdulrauf who introduced this philosophic shift from transcerebral to transcisternal planning of surgical corridors.13
There are two generally accepted avenues to the lateral ventricle: the transcortical and interhemispheric pathways. The decision to approach transcortically or via an interhemispheric route depends on the location and size of the tumor and varies on a case-by-case basis.13–15 Currently, extirpation of lesions located in the lateral ventricle is in the domain of microsurgery, but an increasing number of studies have reported successful removal with endoscopic techniques.16–20
Depending on the location of the tumor within the lateral ventricle, the lesion can be accessed from either an anterior, anterolateral, or posterior direction.3,13–15,21,22 The surgical approach varies according to the exact location of the tumor within the ventricular cavity, the relationship between the tumor and intraventricular or surrounding anatomic structures, and the tumor’s size and pattern of vascular supply.
The choice between the transcortical and transcallosal routes is controversial.2,3,23,24 Several authors believe that the frequency of postoperative seizures is higher when using the transcortical approach.15,21 More recent studies, however, have shown that this is not necessarily the case.25–27
Approaches to the Lateral Ventricles
The neurosurgical pioneer Walter Dandy was the first to introduce the two elementary concepts of transcortical and interhemispheric approaches for removal of ventricular tumors.1,28 With growing knowledge of neuroanatomy and technical improvements, variations of these trajectories with different entry points have been developed and constantly modified.21–23,29–31 In the beginning, the transcortical approach was preferred for removal of tumors located within the lateral cavities, but recently there has been a shift toward the transcallosal route.
Recently, growing interest in the anatomy of the human white matter with the use of Joseph Klinger’s dissecting technique has improved understanding of the complex fiber system.12,32,33 Moreover, diffusion tensor imaging has made in vivo visualization of association, commissural, and projection fibers possible and has influenced neurosurgical decision making in approaching intraventricular tumors.34–40 Direct visualization of the white matter fiber tracts gives the opportunity to choose the ideal trajectory and passage through the corpus callosum with minimal damage to neuronal structures on an individual patient basis. Preoperative planning can be further enhanced by using the Dextroscope technique, which offers fusion of the imaging studies to form a three-dimensional model. This tool can be used to explore a patient’s individual anatomy and provides the opportunity to digitally simulate various approaches before surgery.41–45
The following is a description of these approaches as used for accessing the lateral cavity. The specific aspects of third ventricular exposure with these approaches are discussed in Chapter 139.
The Anterior Transcallosal Interhemispheric Approach
This approach provides excellent exposure of the frontal horns and bodies of the lateral cavities and even gives access to the anterior part of the third ventricle. The standard position of the patient is supine with elevation and flexion of the head. The incision is made behind the hairline, usually in a bicoronal fashion. For the craniotomy, two bur holes are drilled on the contralateral side close to the sagittal sinus. Preoperative imaging studies with MR or CT venography and intraoperative neuronavigation are recommended for planning placement of the bur holes to avoid damage not only to the sinus but also to irregular bridging veins.46 In general, a craniotomy diameter of 4 to 5 cm is sufficient for tumor removal; however, it may be necessary to vary the size of the craniotomy based on the extent of the tumor and the depth at which it has to be resected. In the best case, the chosen trajectory preserves the bridging veins. Mobilization of the draining veins is important for gaining space, and their sacrifice should be avoided.
Caution needs to be taken regarding the genu of the internal capsule, which reaches the surface of the ventricle lateral to the foramen of Monro where the thalamostriate vein turns medially toward the internal cerebral vein.47–49 Recent publications show that complications related to this approach include mainly seizures, sensory-motor deficits, and visual and cognitive impairment.14,23,50,51
The Anterior Transcortical Approach
The advantage of the transcortical approach is that bridging veins are not a concern.46 The disadvantage of this approach relative to the interhemispheric transcallosal approach is that not only are the commissural fibers dissected but also parts of the projection fibers and the short and long association fibers.
The Superior Frontal Sulcus Approach
As in the transcortical approach, the patient is placed in the supine position with elevation and flexion of the head, depending on the side in which the tumor is located. Instead of entering toward the lateral ventricle by opening the middle frontal gyrus as described earlier, the superior frontal sulcus is chosen for creation of the corridor. Neuronavigation can be used to plan and verify the exact trajectory. The advantages and disadvantages are the same as described for the transcortical approach. One has to be aware that the superior frontal sulcus can be short in length. Therefore, clear identification of it may not be possible in every case.52
The Posterior Interhemispheric Transcingular Approach
The posterior interhemispheric transcingular approach is suitable for exposure of tumors of the atrium and the posterior horn of the lateral ventricle. The patient can be operated on in the prone or sitting position. The craniotomy is placed superior and inferior to the lambdoid suture, including the midline (as in the anterior interhemispheric approach), and can be varied according to the position of the superficial bridging veins. The posterior interhemispheric fissure is then opened widely to minimize retraction, and the precuneus and isthmus of the cingulate gyrus are opened to give access to the ventricle.53 Because the posterior part of the optic radiation courses in the lateral wall of the atrium and posterior horn, one does not interfere with the fibers of the optic radiation when using this approach.54
The Intraparietal Sulcus Approach
The intraparietal sulcus approach has been described for lesions of the medial and lateral portion of the trigone.29,51,54,55 The ventricle is explored by opening the intraparietal sulcus and then dissecting the parietal white matter toward the ventricle. Although this approach leads to direct access to the trigone, one should be aware that it poses a risk for possible neurological complications, such as visual field defects, apraxia, and acalculia.
The Transsylvian Approach to the Temporal Horn
The proximal transsylvian approach was developed by Yasargil in 1967 for the treatment of vascular and neoplastic lesions in the mesial temporal area. Later, this approach was used for selective amygdalohippocampectomy.56 The approach consists of pterional craniotomy and opening of the proximal part of the sylvian fissure with an incision about 3.0 to 5.0 cm long. Through a pial incision lateral to the M1 segment of the middle cerebral artery between the origin of the anterior temporal and temporopolar arteries, one can gain access to the temporal horn. In particular, limbic tumors of the amygdala, hippocampus, and parahippocampal area extending into the temporal horn can be removed with this approach without injuring the adjacent neocortex of the superior, middle, and inferior temporal gyrus and lateral temporo-occipital gyrus.
The Occipitotemporal Sulcus Approach
The occipitotemporal sulcus approach can be used to access lesions in the posterior part of the temporal horn.51 The occipitotemporal sulcus can be opened with a subtemporal craniotomy to allow access to the temporal horn. Placing a lumbar drain before surgery for release of CSF before opening the dura reduces the need for brain retraction. As is the case with other lateral transcortical approaches, there is a risk for postoperative visual field deficits.
Authors’ Surgical Technique
In principle, our technique does not significantly differ from descriptions of the approaches just discussed. Our most important access route to the lateral ventricles is the interhemispheric transcallosal approach. The skin incision is bicoronal, usually at the level of or slightly behind the coronal suture. We place two or three bur holes directly above the superior sagittal sinus. The craniotomy is generally performed on the right side but extends slightly to the contralateral side to completely expose the superior sagittal sinus. Small bur holes are placed obliquely in the skull at the margin of the craniotomy so that the dural flap (with its pedicle toward the sinus) can be sutured to the bone. This allows a straight-line view into the interhemispheric fissure directly at the level of the falx cerebri (Fig. 138-3).
Common Tumors of the Lateral Ventricles
Ependymomas
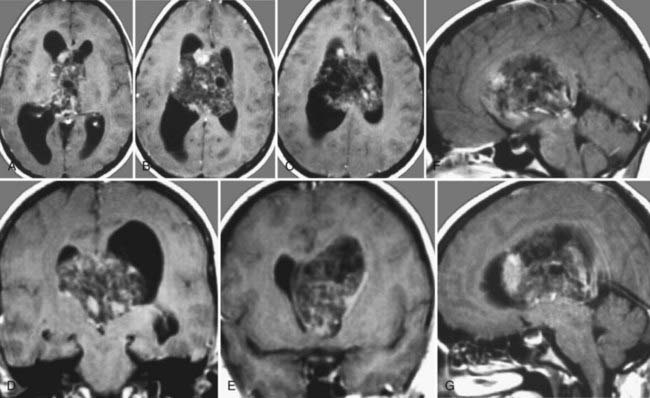
The first description of ependymomas was made by Bailey and Cushing in 1926.57 Ependymomas account for about 2% to 9% of intracranial tumors, and they are more frequent in the pediatric population, with an incidence of 6% to 12% and a peak in the first 3 years of life. They are equally likely to occur in boys and girls.58 Ependymomas arise from ependymal cells, and thus they are found in the entire ventricular system, especially in the infratentorial region.59–63 These tumors usually tend to grow slowly and show perivascular pseudorosettes and ependymal rosettes as key features on histologic examination. Most ependymomas are classified as grade II tumors according to the World Health Organization (WHO) classification of 2007, but there also exists an anaplastic variant, and progression to it from lower grades has been described.58,63,64 Therefore, histopathologic examination is recommended in any case. CT images of ependymomas appear isodense, with heterogeneous contrast enhancement. On MRI, they are well circumscribed, isointense to hypointense, and enhance heterogeneously.
Clinical symptoms arise from obstructive hydrocephalus, which leads to intracranial hypertension. In addition, cognitive changes are seen frequently, especially memory and attention deficits.65
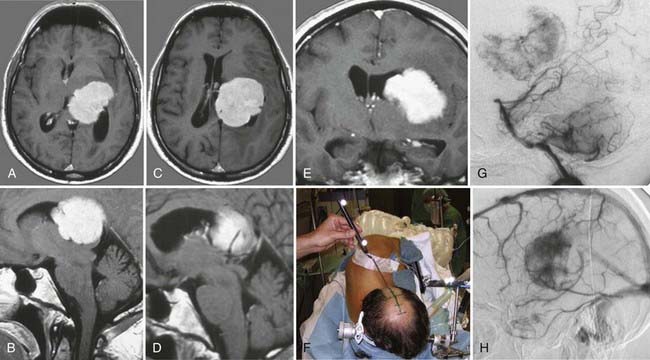
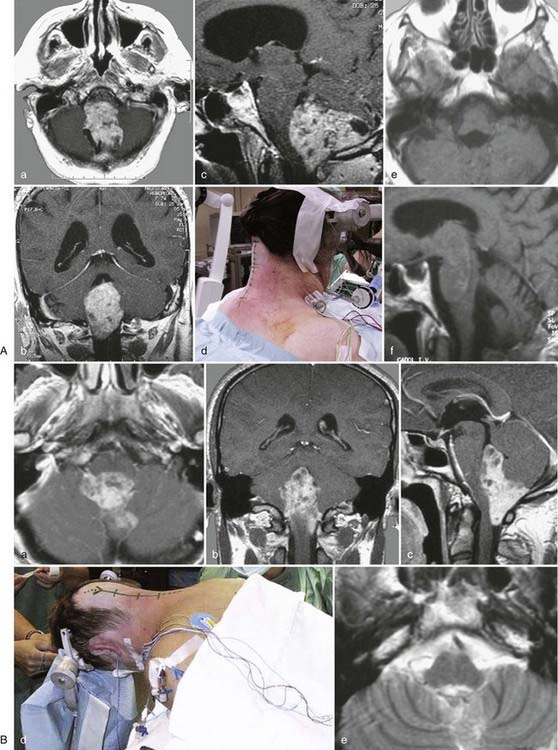
Surgery plays a key role in the treatment of ependymomas because gross-total resection (Fig. 138-4) is associated with improved prognosis.66 If pathologic examination confirms malignant features and MRI studies show tumor remnants, radiotherapy should be discussed (Fig. 138-5).67
Subependymomas
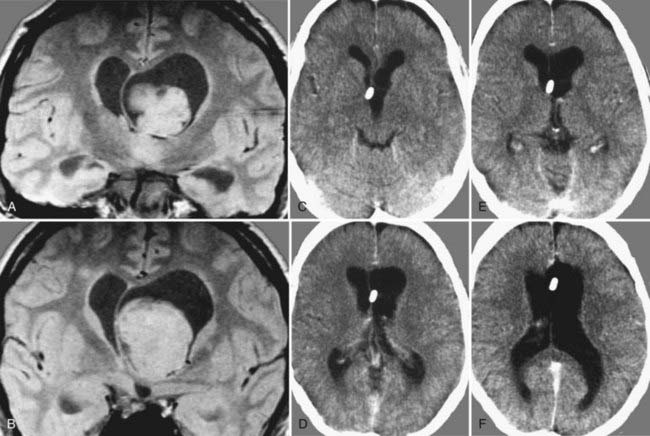
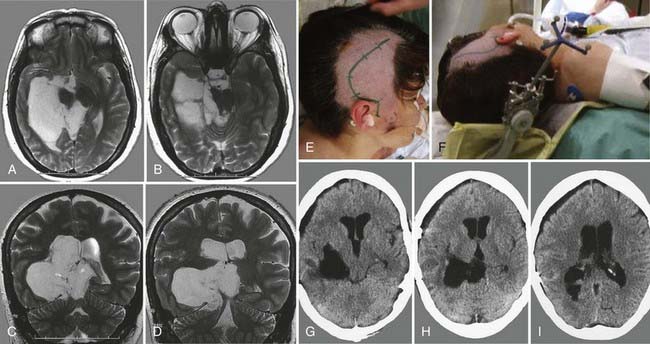
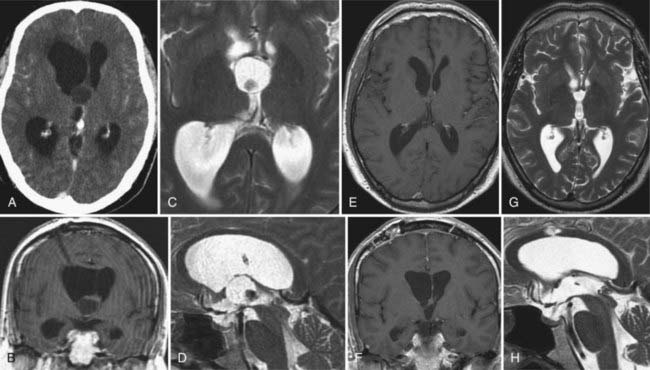
Subependymomas were first described by Scheinker in 1945.68 Subependymomas are rare and account for just 0.2% to 0.7% of intracerebral tumors.69,70 They are benign, slow-growing tumors, often attached to the ventricular wall, but they do not invade surrounding structures. Therefore, this pathology is classified as WHO grade I. Although they most commonly arise within the fourth ventricle, they are located within the lateral ventricles in up to 40% of patients.58,71,72 Subependymomas show a male preponderance, with a male-to-female ratio of 2.3 : 1. Histologically, this tumor shows isomorphic nuclei in clustered patterns, a fibrillary matrix, and small cysts.58 On CT these lesions appear as hypodense to isodense lobular masses with cystic components that do not usually show contrast enhancement. On MRI, this pathology appears as a solid to cystic tumor.
Depending on location and progression, subependymomas become clinically evident by obstruction of CSF pathways, with signs of increased intracranial pressure typically causing headache, nausea and vomiting, gait disturbances, or vertigo (Fig. 138-6). Patients most commonly become symptomatic between the ages of 40 and 60. Because of the benign natural history of these tumors, they remain clinically silent in many cases and are often detected only during autopsy studies.69,72 At present, with easy access to cerebral imaging studies, a growing number of subependymomas are diagnosed incidentally.70,72–74
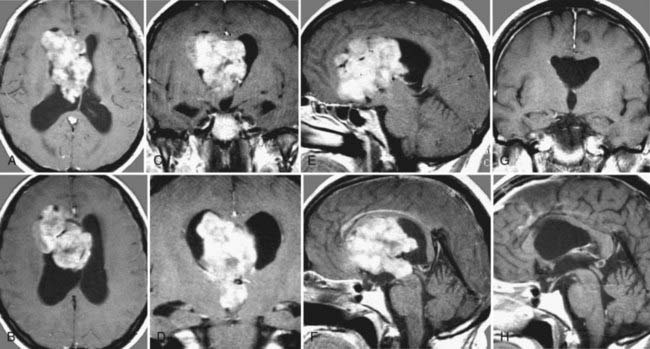
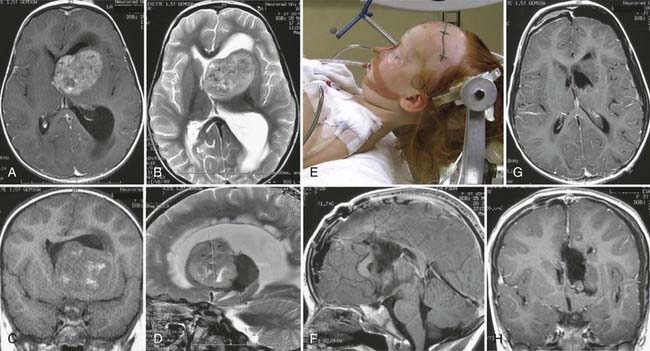
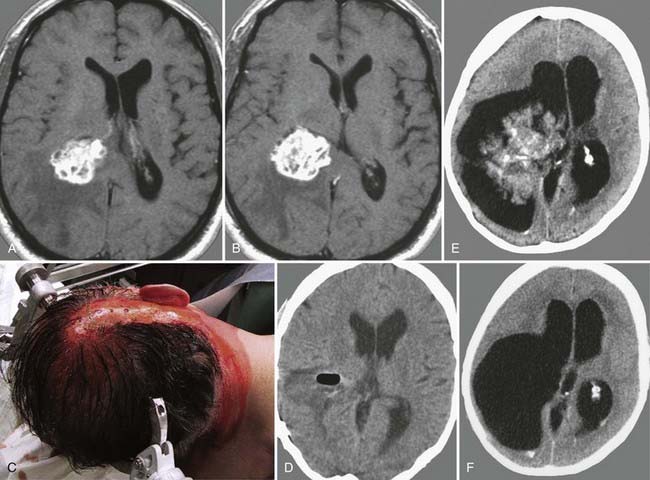
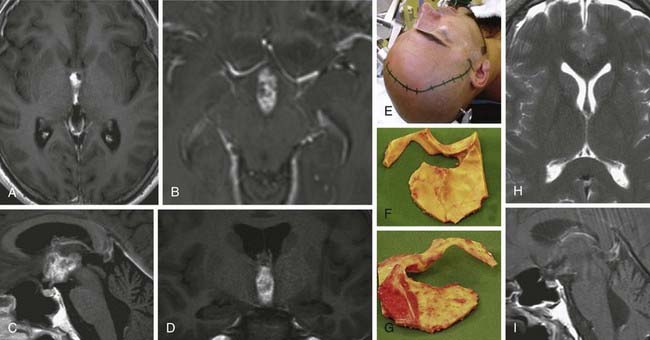
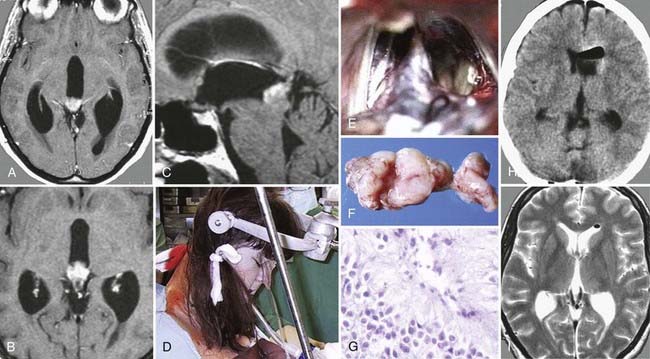
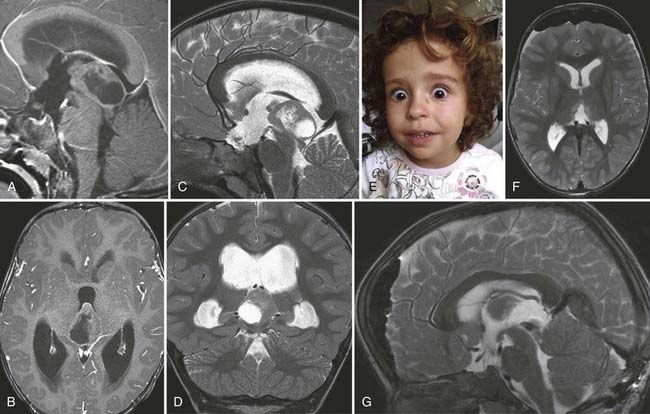
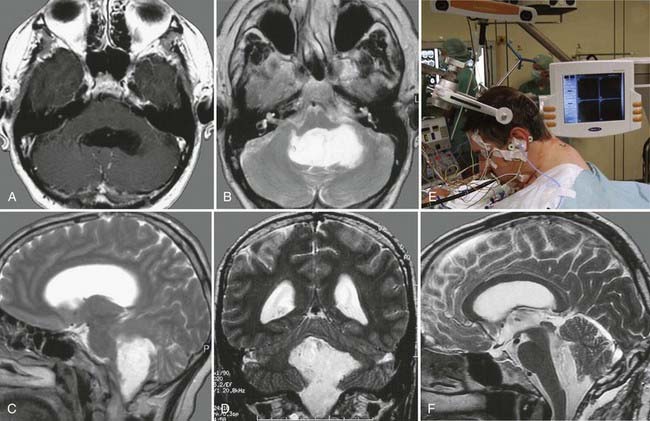
FIGURE 138-6 Preoperative axial (A and B), coronal (C and D), and sagittal (E and F) magnetic resonance images (MRIs) of a 32-year-old woman (the patient shown in Fig. 138-3). The patient had severe headache, vomiting, and memory deficit. The solid, highly vascularized tumor, a subependymoma, was completely removed as shown on postoperative coronal (G) and sagittal (H) MRIs. There were no neurological or other deficits after the microsurgical procedure.
Management of patients in whom an incidental finding is diagnosed is still under debate. If clinical signs are absent, routine follow-up with imaging studies is recommended. If dynamic growth during follow-up or the development of clinical symptoms occurs, microsurgical removal of the mass is the treatment of choice. Because of the benign origin of this entity, radical extirpation defines cure. If imaging studies reveal residual tumor, further treatment options depend on the patient’s condition. If no further deficits have occurred, surveillance with MRI is justified. In patients with existing neurological deficits caused by the remnant mass, operative resection (or eventually radiotherapy) should be discussed.70,72,74
Central Neurocytomas
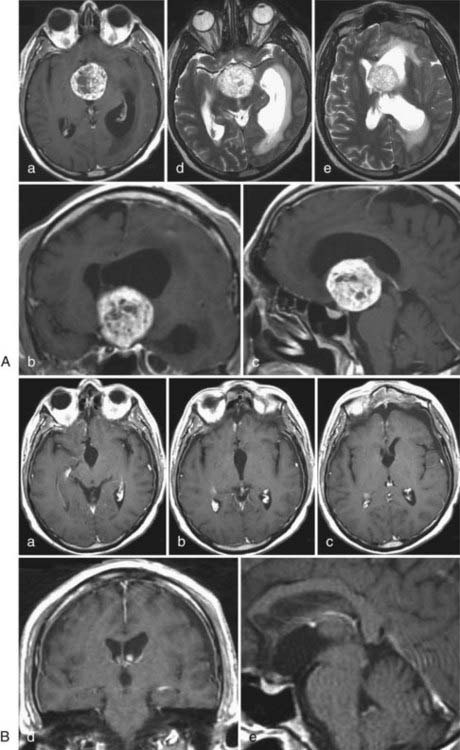
These rare tumors were first described by Hassoun and colleagues in 1982.75 This emphasizes the fact that this lesion was just recently determined to be distinct from other intraventricular pathologies and therefore has often been mistaken for another entity.75 Overall, central neurocytomas represent only 0.1% to 0.5% of all intracranial tumors.76,77 They generally occur in the lateral ventricles, with a predilection for the left frontal horn. Typically, they are detected between the second and fourth decades of life, with equal distribution between the sexes.77–79 Histologic examination of central neurocytomas shows uniform round cells, perivascular pseudorosettes, and a honeycomb appearance, thus indicating their tendency to mimic the architectural patterns of intracerebral gliomas.77,80 They are classified as WHO grade II tumors.58
On CT and MRI, central neurocytomas appear hyperdense and hyperintense (with moderate contrast enhancement), respectively. The presence of necrosis, cysts, and calcifications in these tumors sometimes causes a heterogeneous appearance.77,81 Differential diagnosis includes oligodendroglioma, ependymoma, and papilloma arising from the choroid plexus.77,82
In most cases, central neurocytomas are slow growing and become clinically evident as a result of increased intracranial pressure from obstructive hydrocephalus that causes symptoms of headache, nausea, vomiting, gait disturbance, and cognitive deficits. Surgical removal remains the treatment of choice and defines cure (Fig. 138-7). Nevertheless, although central neurocytomas are generally considered to be benign, malignant courses with progression of remnant tumor and cerebral and spinal dissemination have been reported and associated with unfavorable clinical outcomes.83–86 If radical removal cannot be achieved, adjuvant therapies such as radiation therapy and chemotherapy are options, but their benefits are still under debate. Reduction of tumor mass has been reported with both therapies.58,84,85,87,88
Low-Grade Gliomas
Fibrillary astrocytomas and subependymal giant cell astrocytomas (SEGAs) can occur frequently within the lateral ventricles. The fibrillary component is the most frequent variant of common astrocytomas. On histopathologic examination, nuclear atypia is present, including enlarged, irregular hyperchromatic nuclei. Additionally, mucoid cysts can occur within the matrix. This tumor shows diffuse and infiltrative growth; giant cell astrocytomas are found in patients suffering from tuberous sclerosis. This coexistence is well documented in the literature, and the neoplasm is typically located in the vicinity of the foramen of Monro and can extend to the cavity of the lateral ventricle.89–92 Unfortunately, astrocytomas are closely associated with morbidity and mortality in this particular setting.91,93,94 SEGA is considered a benign and thus slow-growing tumor originating from the wall of the lateral ventricles. The lesion is structurally based on spindle and gemistocyte-like cells. In some cases, additional ganglion-like cells can be found. Clusters, perivascular pseudopalisades, and calcification are typical histologic characteristics. Consequently, SEGA was categorized as WHO grade I in 2007.58,89 The tumor typically occurs in patients younger than 20 years.
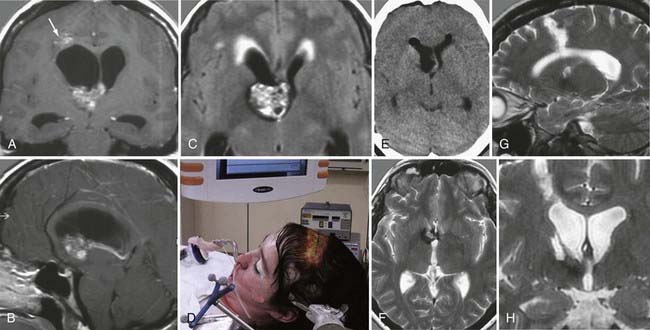
On CT, this lesion appears hypodense and well circumscribed and shows contrast enhancement. On T1- and T2-weighted MRI, the tumor appears as a hypointense mass but in some cases can have high signal intensity on T2-weighted images (Fig. 138-8A). In all cases the tumor shows enhancement when contrast material is administered (Fig. 138-8B), with a somewhat heterogeneous appearance caused by the irregular occurrence of calcification.91,92
Neurological symptoms are caused mainly by obstruction of CSF pathways, which leads to typical clinical symptoms related to the increased intracranial pressure; however, astrocytomas can bleed, which can result in intraventricular hemorrhage, depending on their location, and lead to sudden deterioration. If symptomatic lesions are diagnosed, surgical resection is the treatment of choice. With regard to incidental findings of ventricular gliomas, treatment is still controversial. In patients with small nonsymptomatic tumors, clinical follow-up with regular outpatient consultations and imaging studies is justified. If the lesion displays dynamic growth and can potentially displace CSF pathways, surgical removal in the absence of actual neurological deficits should be considered.89,91,92,95,96
High-Grade Gliomas
The tendency toward malignant change in astrocytomas correlates with the patient’s age. High-grade gliomas of the lateral ventricle may frequently arise from the corpus callosum, the septum pellucidum, or the thalamus and show a predilection for the anterior horn of the lateral ventricle.97
Anaplastic astrocytomas are malignant lesions (WHO grade III) with typical diffuse and infiltrating growth patterns (Fig. 138-9). The mean age at diagnosis peaks between 45 and 51 years. There is a slight male preponderance, with a male-to-female ratio that varies from 1.1 : 1 to 1.6 : 1.98,99 Histologic features include increased cellularity, nuclear atypia, and mitotic activity. When anaplastic transformation is in progress, the nuclei vary in number, size, and shape. Additionally, abnormal mitoses can be detected.
Glioblastoma is the most malignant tumor known in the central nervous system. This tumor accounts for 12% to 15% of all intracranial tumors and shows astrocytic differentiation in 60% to 75% of cases. The mean age at diagnosis is 45 to 75 years, with a male-to-female ratio of 1.28 : 1. Histopathologic study reveals the typical malignant characteristics, such as nuclear atypia, cellular pleomorphism, a high mitotic rate, and necrosis. These are WHO grade IV tumors.58,100
Choroid Plexus Papillomas
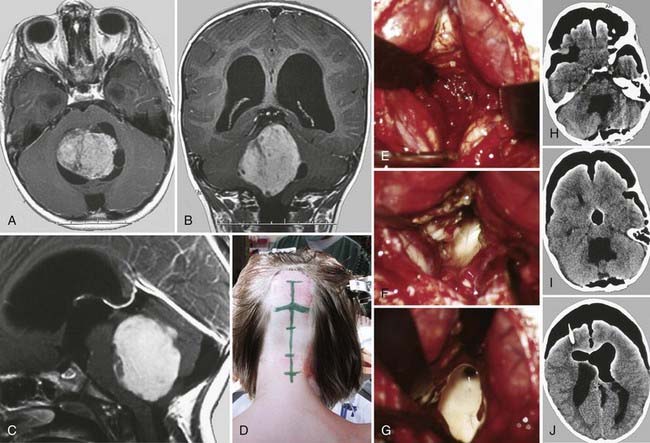
Choroid plexus papillomas account for 0.5% to 0.6% of intracranial lesions in adults and for 2% to 5% of intracranial neoplastic lesions in children. They are among the most common tumors in children younger than 2 years. When these lesions are located in the lateral ventricle, the median age at diagnosis is 18 months, and there is no predilection to occur in either sex.58 In around 20% of pediatric cases, these tumors show malignant transformation; approximately half of them are located within the atrium. They are similar in aspect to the glomus of the choroid plexus, being formed by a single cell layer of cuboid or cylindrical cells surrounded by a thin fibrovascular structure. In the latest WHO classification, choroid plexus papillomas are classified as grade I. In imaging studies, these tumors frequently contain calcifications, hemorrhagic elements, or cysts. In some cases, choroid plexus papillomas may fill the ventricular cavity, whereas in others, the ventricle may be markedly dilated ipsilaterally.
Surgical removal is the treatment of choice (Fig. 138-10). It is helpful to expose and interrupt the proximal arterial supply from the choroidal artery to devascularize the tumor at an early stage of the procedure. Debulking is then continued with the aid of an ultrasonic aspirator. At the end of the procedure, the remaining portions of the apparently healthy choroid plexus are resected as well to ensure complete tumor removal.101–104
Meningiomas
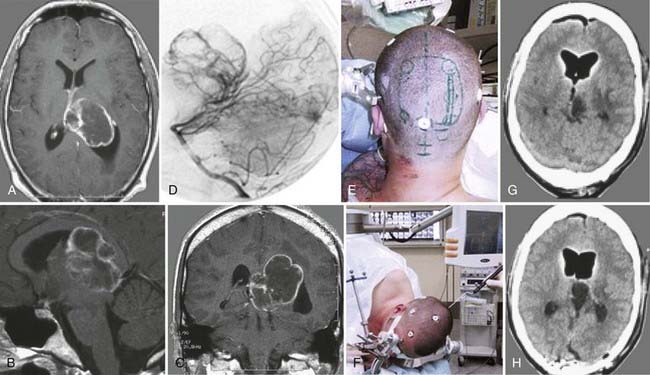
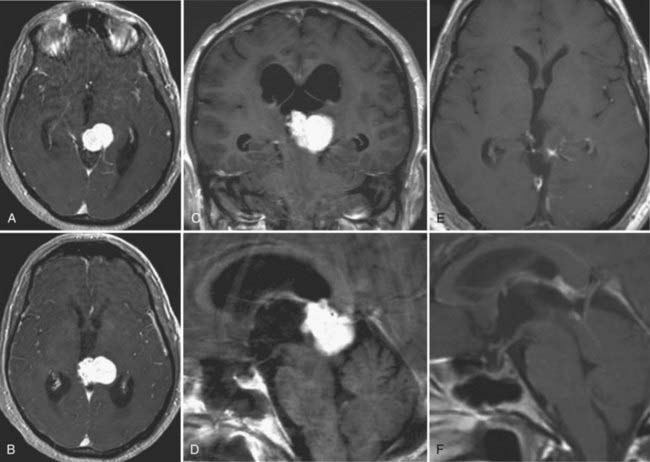
Meningiomas of the lateral ventricle originate from the stroma of the choroid plexus and arise at the tela choroidea. Ventricular meningiomas account only for 1% to 5% of all ventricular tumors and are located within the atrium in most cases.105,106 They may show calcifications and, occasionally, cystic degeneration.107 Usually, these tumors are highly vascularized (Fig. 138-11). The arterial supply stems from branches of the choroidal arteries, and they drain into the deep ventricular veins. Their histopathologic features and natural behavior are the same as those in any other location in the neural axis. Therefore, most intraventricular meningiomas are benign, slow-growing lesions (WHO grade I). Reports exist of a malignant course in some meningiomas, with recurrence and progression to atypical (WHO grade II) or anaplastic (WHO grade III) variants with the occurrence of metastases.108–110
On radiologic images, they typically appear as a solid enhancing mass with cystic components. Meningiomas usually become clinically evident as a result of obstruction of CSF pathways, which consequently leads to obstructive hydrocephalus. In most cases, typical symptoms of elevated intracranial pressure do not occur, but rather, cognitive deficits affecting attention and memory dominate the clinical findings. Patients with these symptoms have a good prognosis overall after removal of the cause.65 As a result of their location, these tumors can gain mass volume before causing neurological symptoms.106,111
Because of their typically benign nature, radical microsurgical removal of these tumors defines cure, but postoperative follow-up with regular imaging studies is recommended. Asymptomatic lesions can be monitored by imaging studies, whereas symptomatic lesions and meningiomas with documented growth should be resected microsurgically before they can cause obstructive hydrocephalus and result in rapid neurological deterioration.112
Epidermoid Cysts
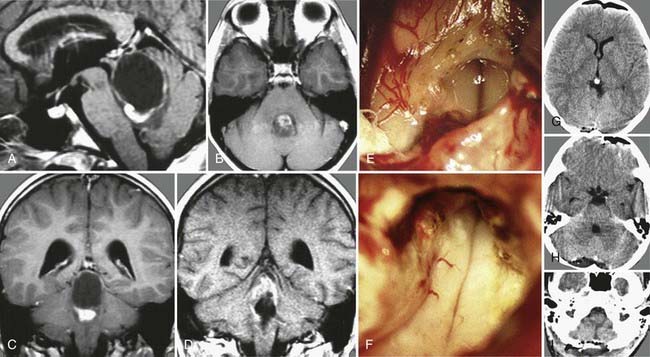
Epidermoid cysts only occur rarely within the ventricular system, and if so, they show a predilection for the fourth ventricle, from which they may extend into the third ventricle. In our follow-up series, we have encountered two patients who harbored an epidermoid cyst within the lateral cavity: one was a huge paraventricular lesion that extended widely into the lateral ventricle, and the other was a primary lateral ventricle epidermoid cyst (Fig. 138-12).
Approaches to the Third Ventricle
The Transnasal Transsphenoidal Endoscopic Approach to the Third Ventricle
During the past decade, use of the endoscope for transsphenoidal surgery has gained increased popularity. Although it was an adjunct in the beginning, pure endoscopic removal of pituitary adenomas has now become routine at many centers.113–115 The endoscopic transsphenoidal approach to the third ventricle is used primarily for tumors that originate in the sellar area and for those showing suprasellar extension, such as pituitary adenomas, Rathke’s cleft cysts, or cystic craniopharyngiomas. The main limitation of this approach is difficulty in performing fine dissection if the tumor has a firm consistency and is adherent to the optic pathway and perforators.
The Subfrontal Trans–Lamina Terminalis Approach
The subfrontal interhemispheric trans–lamina terminalis approach is most suitable for lesions located in the anterior part of the third ventricle, especially for those that develop anterior to a line joining the anterior ridge of the foramen of Monro and the cerebral aqueduct.116 Before surgery, a lumbar drain is inserted for early CSF release and relaxation of the brain. The patient is placed in the supine position with slight extension of the head. A bicoronal incision is made with preservation of a large pericranial flap for closure of the frontal sinus. A unilateral or bilateral craniotomy is performed, depending on the precise location and extension of the tumor. The dura is opened parallel to the orbital roof. The olfactory bulbs are sharply dissected from the olfactory sulcus. All basal arachnoid membranes are opened to allow the brain to fall backward and thus increase the working space. The optic nerves, the chiasm, and the vascular complex of the anterior cerebral arteries have to be well identified. The lamina terminalis is then opened for further tumor resection.
The advantage of this approach is good visualization of the optic nerves and chiasm (behind or above it), the anterior communicating artery, the lamina terminalis, both A2 segments (and below the chiasm), both internal carotid arteries, the posterior communicating arteries, their perforating branches, and the pituitary stalk. With careful dissection, the cerebral cortex can be kept intact.117–119
The Anterior Interhemispheric Transcallosal Approach
This approach was also described earlier in the discussion on lateral ventricular tumors. Lesions that extend from the third ventricle within the lateral ventricles with a wide foramen of Monro are especially suitable for this approach. Depending on the venous anatomy, the foramen of Monro can be enlarged by opening the choroidal fissure posteriorly as far as the junction of the anterior septal vein with the internal cerebral vein.12 The exact anatomic variations can be studied preoperatively with CT or MR venography.
Authors’ Surgical Technique
The Interhemispheric Subfrontal Trans–Lamina Terminalis Approach
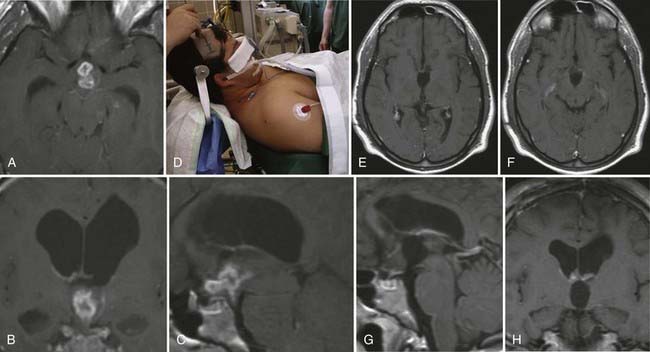
This approach provides straight-line access to the anterior portion of the third ventricle, which is most suitable, for instance, for craniopharyngiomas (Fig. 138-13A and B). The patient is placed in the supine position. A bicoronal skin incision is made, and a midline frontobasal craniotomy is performed. After opening the dura mater on both sides of the frontal poles, the initial portion of the superior sagittal sinus is ligated, and the insertion of the falx is completely resected from the crista galli. To gently elevate both frontal lobes, the olfactory nerves are freed from their arachnoid sleeve on both sides. Arachnoid dissection is carried out interhemispherically, and the lamina terminalis is exposed by gently mobilizing the anterior cerebral arteries. Opening the lamina terminalis up to the genu of the corpus callosum provides wide access into the third ventricle (![]() see Video 138-1).
see Video 138-1).
The Lateral Subfrontal Trans–Lamina Terminalis Approach
This approach requires a combined pterional cranio-orbital craniotomy. Extradural resection of the anterior clinoid process and incision of the falciform ligament allow mobilization of the optic nerve. The patient is placed in the supine position with the head rotated 45 degrees to the left side and extended dorsally. A typical pterional craniotomy is performed that includes the right orbital rim and removal of the orbital roof. In some instances, a cranio-orbitozygomatic approach can be performed to allow even more retraction and provide a good inferior to superior viewing trajectory (Fig. 138-14).
The anterior transcortical approach can be used to first enter the right lateral ventricle via a transsulcal transfrontal parenchymal approach. Use of neuronavigation is helpful for establishing the appropriate trajectory. After opening the ventricular ependyma, CSF is released completely from the ventricle, and the foramen of Monro is visualized. With this approach, contrary to the midline interhemispheric transcallosal approach, a slightly oblique view is obtained. Incision of the tela choroidea lateral to the fornix and dissection of the internal cerebral veins and the medial posterior choroidal artery provide access to the third ventricle. This approach has been used, for instance, in patients with cavernous malformations, as shown in Figure 138-15 and ![]() Video 138-3.
Video 138-3.
The Anterior Interhemispheric Transcallosal Paraforniceal Approach
After adequate exposure and reflection of the dura mater, the interhemispheric space on the right side is dissected down to the anterior portion of the corpus callosum. The corpus callosum is incised medial to the pericallosal artery, and the right lateral ventricle is opened. In lesions that have already enlarged the foramen of Monro, no further action may be necessary. The tumor can be debulked and gradually removed. However, in many instances a paraforniceal transchoroidal approach is necessary. This approach is suitable for gaining access to the anterior two thirds of the third ventricle from the roof to the bottom (Fig. 138-16). Dissection starts lateral to the right fornix at the posterior margin of the foramen of Monro, and the tela choroidea is gradually incised. The plexus choroideus and the right internal vein are left laterally. Dissection is carried out between the two internal cerebral veins while being careful to avoid the medial posterior choroidal artery. After incising the inferior membrane of the tela choroidea and mobilizing the choroid plexus of the third ventricle, the right fornix is gently mobilized toward the opposite side, and the right thalamus (including the thalamostriate vein) can be visualized posteriorly up to the posterior commissure and superiorly to the splenium of the corpus callosum (see ![]() Video 138-4).
Video 138-4).
The Occipital Transtentorial Approach to the Pineal Region
This approach is most suitable for patients with a tight infratentorial space or large tumors of the pineal region with significant caudal extension that requires an adequate superior to inferior viewing trajectory (Fig. 138-17).
The Infratentorial Supracerebellar Approach
We use this approach for accessing small tumors of the pineal region, such as pineocytomas (or fibrillary astrocytomas) (Fig. 138-18) or lesions involving the midbrain tectum or posterior thalamus (e.g., cavernous malformations, various gliomas). Great attention is paid to the superficial cerebellar veins, which are always preserved. Usually, these lesions are located more or less in the midline; however, they can be accessed unilaterally via the infratentorial supracerebellar space. The side of access depends on the local venous pattern. In all cases, the arachnoid that surrounds the tectal plate and the veins that drain into the vein of Galen are dissected meticulously to clearly identify all anatomic structures in the region.
Common Tumors of the Third Ventricle
Colloid Cysts
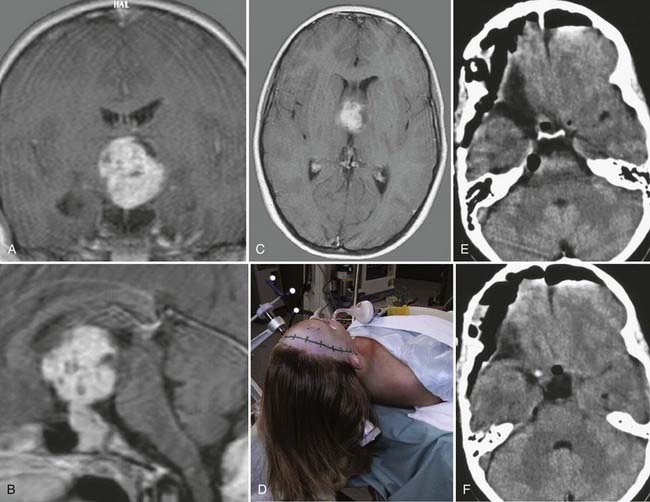
Colloid cysts are benign tumors with an incidence of 0.5% to 1% that are most commonly located in the roof of the third ventricle.120 Most contain gelatinous viscous material. This pathology was first described by Wallmann in 1858 as an autopsy finding, and Walter Dandy was the first to successfully remove this kind of tumor in 1921. The tumor can remain clinically silent for a long time and is therefore often detected only at autopsy.121 Colloid cysts can be found in patients of any age, with a peak between the second and fourth decades and no differences in distribution according to sex.
Colloid cysts can attain a large volume that potentially occludes the foramen of Monro and leads to acute, obstructive hydrocephalus. Mechanisms of occlusion include shifting as a result of changes in CSF flow patterns after lumbar puncture, shunt dysfunction, or hemorrhage. In 75% of patients, the initial symptom is headache with changing intensity. The episodic appearance of symptoms with irregular symptom-free intervals makes the diagnosis somewhat challenging based purely on clinical examination. Other symptoms arising from elevated intracranial pressure include nausea, vomiting, dizziness, and fatigue. A sudden increase in intracranial pressure from rapid-onset hydrocephalus can lead to sudden death.122,123 By compression of surrounding anatomic structures (e.g., thalamus, pituitary gland, and optic chiasm), the cyst can cause thermal deregulation, electrolyte and hormone imbalances, endocrine dysfunction, altered personality, memory loss, or visual changes.
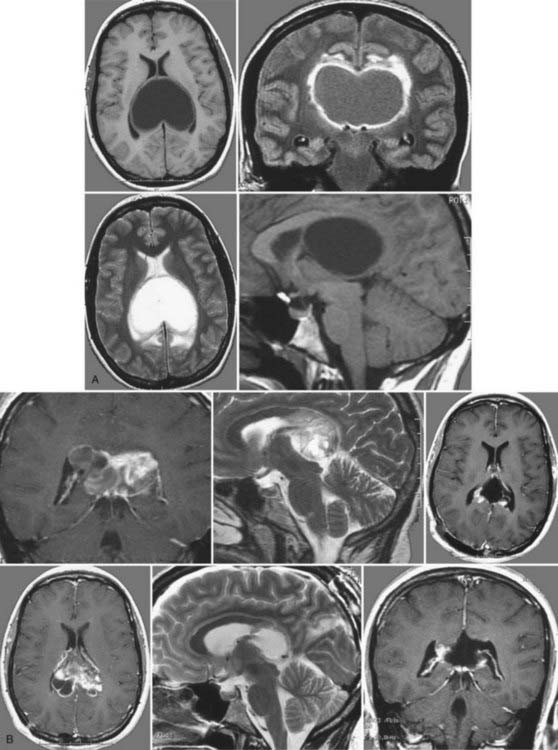
On plain CT, the lesion appears as a hyperdense mass but also has isodense and hypodense parts. This lesion does not usually show contrast enhancement and rarely demonstrates rim enhancement. On MRI, the tumor is either isointense to hyperintense on T1-weighted images or hypointense to hyperintense on T2 sequences. The radiologic appearance depends on the amount of gelatinous material within the tumor (Fig. 138-19).
Treatment options include conservative, microsurgical, or endoscopic therapy. An asymptomatic patient may be managed with follow-up imaging studies. If neurological changes are seen, prompt imaging studies should be obtained. The only definite treatment of this pathology is resection of the cyst by microsurgical or endoscopic techniques. Neuronavigation serves as an important tool to further optimize the surgical trajectory and improves the safety and efficiency of operative interventions. In any case, rupture of the cyst must be avoided because this will lead to ventriculitis and meningitis.13,120,124–136
Ependymomas
As described earlier in the section “Common Tumors of the Lateral Ventricles,” ependymomas are generally considered to be slow-growing, noninvasive tumors that arise from the walls of the ventricles. Clinical evidence of third ventricular ependymomas results mainly from obstructive hydrocephalus with a consequent increase in intracranial pressure and cognitive deficits. On MRI and CT, ependymomas appear as well-circumscribed lesions with heterogeneous contrast enhancement (see Fig. 138-14). Removal is still in the domain of microsurgery, but there are reports of successful removal purely by endoscopic technique.58,65,67,137–139
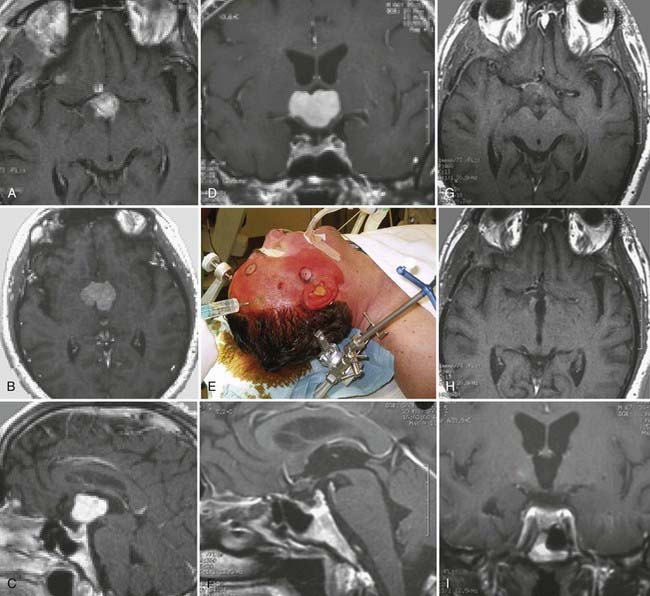
Craniopharyngiomas
Craniopharyngiomas originate from layers of Rathke’s pouch within the sellar region. They are considered benign and slow growing. With increasing volume, they tend to displace surrounding neuronal and vascular structures and can potentially invade the third ventricle.140 The most common histologic pattern is that of an adamantinomatous craniopharyngioma. Its microscopic features include squamous epithelium, lobules, palisades, and cysts filled with debris. Structures consisting of keratin and cholesterol can be detected. This entity is classified as WHO grade I.58
CT reveals a mixed-appearing solid mass with calcification and heterogeneous contrast enhancement caused by the presence of nodules and capsules. Cysts appear hyperintense on T1-weighted MRI, whereas nodules and solid portions of the tumor are isointense. When contrast material is administered, these components show enhancement.141
Microsurgical resection is the treatment of choice. The lesion can be accessed either by the anterior interhemispheric subfrontal approach (see Fig. 138-13A and B) or by the combined pterional cranio-orbital transsylvian approach (Fig. 138-20). In recent years, successful treatment by endoscopic technique has also been reported; cystic intrasellar tumors are especially suitable for this treatment. Neuroendoscopy can be used for both diagnosis and treatment of these tumors. Moreover, tumor resection and additional resolution of tumor-related hydrocephalus can be achieved at the same time.135,142 If radical resection is not accomplished, tumor recurrence is expected.58,116,143–145
Gliomas
On CT and MRI, pilocytic astrocytomas appear well circumscribed and show contrast enhancement. In anaplastic astrocytomas, MRI shows the typical feature of high-grade glioma, a central necrotic area surrounded by ring contrast enhancement.58
Therapeutic options depend on the patient’s clinical condition, histologic findings, and tumor size and degree of expansion. Therefore, interventions range from performing radical resection and diagnostic biopsy to the administration of adjuvant therapies, or a combined approach. Although microsurgical removal has been the only operative means for decades, neuroendoscopy has brought a new treatment option to the field.13,20,146–149
Chordoid gliomas originate within the third ventricle (Fig. 138-21). They are noninvasive and show slow progress. Choroid gliomas were defined as an independent entity in 1998, and they correspond histologically to WHO grade II tumors.58,150 These tumors are rare, and knowledge of their incidence is still based mainly on a few small series and case reports with short follow-up periods.151 Analyses show that this tumor is diagnosed at a mean age of 46 years, with a male-to-female ratio of 1 : 2.
Chordoid gliomas are located in the midline, mostly in the anterior part of the third ventricle. With increasing volume, this tumor displaces surrounding neuronal structures and causes obliteration of the CSF pathway, consequently leading to elevated intracranial pressure. Depending on the location of the anatomic displacements, endocrine disturbances, impaired vision, and cognitive deficits have been reported.152 In terms of histopathologic findings, this neoplasm consists of epithelioid cells in clusters and row formations.153,154
On CT, the tumor appears ovoid shaped and well circumscribed, with homogeneous contrast enhancement. T1-weighted MRI reveals the mass as isointense to hypointense, and T2-weighted images display the surrounding edema.155 The use of dynamic contrast medium has been reported to further help differentiate this lesion radiologically from meningiomas and malignant gliomas.155 Microsurgical resection is the only treatment option available, and thus far, adjuvant therapies have failed. If radical removal of the tumor is not achieved, it can recur.156,157
Pineal Region Tumors
In 1913, the first attempts to remove tumors located in the pineal region resulted in significant morbidity and mortality. As neuroanatomic knowledge and technical expertise improved, Oppenheim, Krause, and Dandy were able to report successful removal of pineal tumors. Since then, there has been steady improvement in treatment modalities and surgical approaches for these lesions.158
In addition to lesions arising primarily from pineal parenchyma, secondary neoplasms are commonly detected in the pineal region, such as astrocytomas, meningiomas, ependymomas, and metastases to the brain.159–162 Primary lesions originate from pineal and germ cells and range histologically from benign to malignant entities. They include pineocytomas, pineoblastomas, and papillary lesions. Although pineocytomas are considered benign (WHO grade I), papillary tumors vary in their behavior and are graded WHO II to III. Histopathologically, pineoblastomas are malignant lesions and are thus classified as WHO grade IV.
Pineocytomas are considered benign and rare neoplasms that typically show slow progress, but progression toward malignancy has been reported.163 They originate from pineal cells and account for less than 1% of intracranial lesions. The mean age of patients at diagnosis is 38 years. Because of their benign behavior, they displace the surrounding anatomy and can expand into the third ventricle. Clinical evidence of pineocytomas results from compromise of the affected neuronal structures and includes Parinaud’s syndrome, endocrine imbalances, cranial nerve palsies, diminished mental state, or symptoms of intracranial hypertension.
Imaging-wise, pineocytomas appear as well-demarcated, hypodense or slightly hyperdense masses that show homogeneous contrast enhancement on CT. On MRI, they look lobulated and isointense on T1-weighted images and hyperintense on T2-weighted images, and they again show homogeneous enhancement when contrast material is administered. Their appearance is well circumscribed, and they displace surrounding structures without invasion. In addition, calcification can be detected.164
Pineoblastomas are ranked WHO grade IV and arise from the pineal parenchyma. Although their incidence overall is very low, they represent 40% of all primary pineal neoplasms. Extraneural dissemination through an implanted ventriculoperitoneal shunting system has been reported. In this case, radiotherapy was administered.165 These lesions occur predominantly in children and affect both sexes equally.
CT reveals a mass that is less well demarcated than noted with pineocytomas. This lesion also shows heterogeneous contrast enhancement, and signs of calcification are common. Pineoblastomas appear hypointense to isointense on T1-weighted images. Defined margins with surrounding neuronal structures are poorly discriminated. On T2-weighted sequences these lesions display hyperintensity, and radiologic features of invasion of the surrounding anatomy are seen.166,167
Clinical symptoms are caused by increased intracranial pressure as a result of obstruction of the CSF pathway. On imaging studies, they appear hypodense with heterogeneous contrast enhancement on CT and hyperintense on contrast-enhanced T1- and T2-weighted MRI sequences. The absence of fat, hemorrhage, and calcification helps differentiate this lesion from other entities found in the pineal region.168
Radical surgical resection is considered the treatment of choice, followed by radiotherapy. If tumor remnants are present, recurrence is possible. Craniospinal dissemination may occur, and thus imaging studies of the whole neuraxis should be included in staging and follow-up.169
Depending on the histopathology of pineal region tumors, the patient’s neurological condition, and characteristics of the tumor (location, size, and expansion rate), the following should be discussed with patients on an individual basis: microsurgical or endoscopic removal, biopsy, and adjuvant treatments.166,170–175
Tectal Tumors Involving the Third Ventricle
Focal tectal gliomas typically cause signs of elevated intracranial pressure as a result of obstructive hydrocephalus (Fig. 138-22). Their histopathologic features are consistent with those of other low-grade gliomas.
Depending on the patient’s neurological status and the histologic patterns, size, and expansion of the lesion, treatment options include surgical removal by microsurgical or endoscopic means, diagnostic biopsy, radiotherapy, or a combined approach (see ![]() Video 138-6).58,176,177
Video 138-6).58,176,177
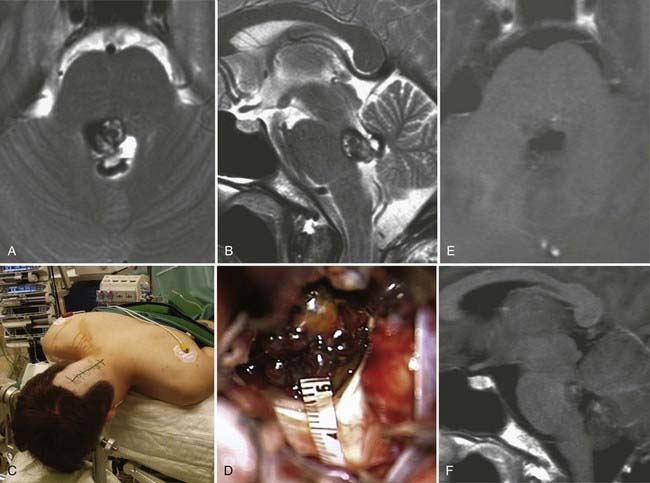
Cavernous Malformation
Even when located within the ventricular system, the tumor usually remains clinically silent but potentially becomes symptomatic by obstruction of CSF pathways, which produces clinical signs of elevated intracranial pressure (see Fig. 138-15). However, when bleeding within the third ventricle occurs, a dynamic course of neurological deterioration is possible.
Depending on the patient’s condition and the location, size, and degree of expansion of this pathology, radiation therapy is regarded as an alternative option.178–183
Surgical Removal of Fourth Ventricle Tumors
Approaches to the Fourth Ventricle
For a long time, either splitting the vermis or resecting parts of the cerebellar hemispheres was used to approach tumors of the fourth ventricle, as advocated by Dandy.184–186 Although he stated that there should be no disturbance in function, we know today that incision of the vermis can produce a so-called vermal split syndrome characterized by disturbed coordination of tandem gait along with neurobehavioral changes.187,188 This procedure has also led to the observation of apraxia of the oral and pharyngeal musculature and thereby to cerebellar mutism, especially in children.189 Although the exact pathophysiologic mechanisms responsible for these symptoms are not clear thus far, such symptoms are frequently seen after removal of midline tumors involving the vermis.
The Telovelar or Transcerebellomedullary Fissure Approach
The telovelar or transcerebellomedullary fissure approach consists of a standard median suboccipital craniotomy extending from the transverse sinus to the foramen magnum. It is performed with the patient either in the sitting or in the prone position. Depending on the size and caudal extension of the lesion, the C1 lamina must sometimes also be removed. The dura is opened in a Y-shaped fashion. The arachnoid membranes are then dissected around the tonsils, the uvula, and the medulla oblongata while taking care to protect the PICA and its branches. By gentle retraction of the tonsils laterally and superiorly, the cerebellomedullary fissure (consisting of the tonsillouvular and tonsillomedullary spaces) can then be opened widely. To further enhance exposure of the area of the aqueduct and lateral recess, the inferior medullary velum should be dissected and incised sharply.190–192
Authors’ Surgical Technique
Operating within the fourth ventricle and achieving wide exposure up to the aqueduct require a wide opening of the ventricle in its inferior portion. This access route is provided by the telovelar approach. If possible, the patient should be placed in the sitting position with the head flexed and firmly fixed in the Mayfield holder (Fig. 138-23A). An alternative to the sitting position is the so-called Concorde position, which allows the surgeon to approach the fourth ventricle similar to having the patient in the sitting position. The patient is placed in the prone position, the head and body are elevated, and the head is fixed in anteflexion and slightly rotated toward the left side (Fig. 138-23B). The surgeon stands on the left side of the patient and obtains almost the same view as with the patient placed in the sitting position. The skin incision extends from above the occipital protuberance down to the level of the C2 spinous process.
Common Tumors of the Fourth Ventricle
Ependymomas
Ependymomas are the most common tumors of the fourth ventricle in adults. In most instances, they originate from the lower floor of the fourth ventricle and expand in all directions (see Fig. 138-23A and B). Invasion of the neuraxis may be very limited, but occasionally, the tumor may infiltrate deeply into the lower medulla. Such infiltration of the neuraxis can usually be anticipated from preoperative MRI sequences. Further expansion of the tumor may be seen in the superior, inferior, dorsal, and lateral directions, with or without invasion of the surrounding neural structures. In some instances, even large ependymomas may have a rather small site of attachment (vascular pedicle) located at the lower floor of the fourth ventricle, whereas the remaining portion of tumor may not be firmly attached to the neuraxis.2,58
When a tumor extends into the lateral recess or even into the cerebellopontine angle, these lateral portions are dealt with separately. Small supplying arteries from the lower medulla are coagulated at low current intensity and cut sharply with microscissors. Continuous electrophysiologic monitoring during dissection is mandatory. At the end of resection, meticulous hemostasis is obtained at the site of tumor attachment. This not only controls bleeding efficiently but may also eliminate microscopic tumor residue. As a general rule, it is important to remove the tumor completely because total resection is associated with a lower risk for recurrence193 (see ![]() Video 138-7).
Video 138-7).
Medulloblastomas
Medulloblastomas are the most common tumors of the fourth ventricle in childhood. In many instances, medulloblastomas do not invade the floor of the fourth ventricle but rather the middle cerebellar peduncles; nevertheless, the fourth ventricle may be completely filled with tumor up to the aqueduct, and the neuraxis and cerebellum may be severely compressed. Moreover, these neoplasms are highly vascularized, and at their site of attachment (usually the roof of the fourth ventricle) there is no clear-cut boundary with the cerebellum. Exposure is obtained with the telovelar approach (Fig. 138-24). It is important to reduce the volume of the mass as rapidly as possible to avoid intralesional bleeding and reduce the duration of surgery and thus the amount of blood loss.
Many tumors will have an insertion point at the level of the brachium pontis and lateral recess but rarely in the midline. The tumor insertion site should be inspected carefully. Occasionally, hemorrhagic tumor residues are found here that can easily be separated from the cerebellum. Concomitantly, hemostasis is obtained by gentle bipolar coagulation. This aids in avoiding significant blood loss, maintaining a bloodless surgical field, and recognizing all pertinent anatomic structures. Finally, the dura is sutured in watertight fashion.8,58,192,194
Hemangioblastomas
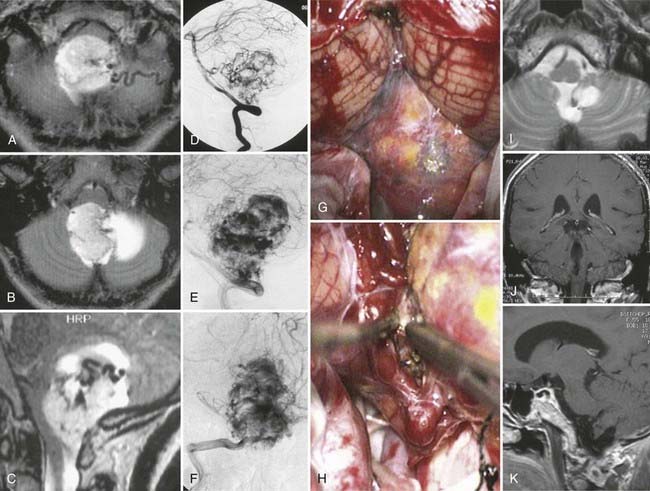
Hemangioblastomas of the fourth ventricle are highly vascularized lesions (Fig. 138-25). Resection of hemangioblastomas requires a special technique that differs completely from removal of an ependymoma or medulloblastoma. These lesions have a significant arterial supply, mainly from branches of the distal PICA and from numerous small arteries emerging from the cerebellum and neuraxis, in addition to one or several large draining veins. Moreover, branches of the choroid plexus may also supply the lesion. Precise study of preoperative digital subtraction angiograms is mandatory to understand where the main feeding arteries and draining veins are located. Hemangioblastomas cannot be debulked because of their typical morphology. If a hemangioblastoma is penetrated before it is completely separated from its arterial supply, hemostasis can be very difficult or impossible to achieve. Therefore, these lesions must be gradually separated from their arterial supply and removed in one piece while preserving the main draining veins intact until the entire lesion has been isolated from the cerebellum and neuraxis.
The exposure is similar to that for resection of other tumors of the fourth ventricle. Arachnoid dissection to separate the cerebellar tonsils and caudal PICA loops from the lower vermis can be carried out in a fashion similar to that for ependymomas or other tumors. However, the first important step should be to identify the main arterial supply with the aid of an intraoperative ultrasound probe. The supplying arteries can easily be coagulated and separated from the lesion such that in many instances, an initial view of the interior of the superior fourth ventricle is possible. Dissection and devascularization of the lesion are then performed in a circular fashion. Use of small cottonoids and gentle compression of the lesion allow further dissection between the hemangioblastoma and neuraxis for gradual exposure of the surface of the lesion. Care is taken to maintain patency of the draining vein until the very end of this dissection. One should bear in mind that even if most of the visible supplying arteries have been interrupted, a significant amount of residual arterial supply from small transparenchymal arteries of the neuraxis may still be present. Only after the lesion has been completely separated from the cerebellum and neuraxis can the draining veins also be coagulated and divided. With this technique, the surgical field is continuously kept bloodless so that all anatomic details can be seen clearly throughout the procedure, and even large lesions can be removed safely (see Fig. 138-25). Frequent use of the Doppler microprobe is helpful in verifying local hemodynamics and assessing the direction of flow in lesion-supplying vessels. Furthermore, the Doppler signal obtained from the draining vein provides useful information about the amount of residual arterial supply and progress in devascularizing the lesion. Continuous electrophysiologic monitoring reassures the surgeon that function of the neuraxis is intact during these maneuvers.190,191,195–199
Epidermoid Cysts
Epidermoid cysts can sometimes fill the fourth ventricle completely and expand into the lateral recess (Fig. 138-26). Because the lesion itself is not vascularized, debulking appears readily possible. However, epidermoid cysts may be separated by firm arachnoid membranes that divide the lesion into multiple compartments. Moreover, on the outer surface of the neuraxis, the lesion may involve pial vessels that must be sharply dissected and preserved. With a normal suction tube of large caliber or an ultrasonic aspirator, the portion located within the fourth ventricle can easily be debulked. Gentle use of a dissector also helps in separating remote portions of epidermoid cysts from the cerebellum or neuraxis. Within the lateral recess and in patients in whom the cerebellopontine angle has also been invaded, meticulous dissection around the rootlets of the caudal cranial nerves and the PICA and its branches is necessary. In most instances, complete removal of the avascular lesion is possible, and the arachnoid membranes that form compartments of the epidermoid cysts can also be resected. At the end of resection, we always irrigate the fourth ventricle, the lateral recess, and the perimedullary space with saline solution to eliminate small lesion residues that may serve as foci for regrowth of the cyst.201–202
Pilocytic Astrocytoma
The best exposure is the telovelar approach, and tumor debulking is performed with an ultrasonic aspirator. When a cyst is present, it is punctured and the cyst fluid is evacuated. The initially expanded lesion then collapses, and the surrounding wall of solid tumor is gradually resected. Visualization of the roof of the fourth ventricle requires a certain retraction of the uvula and nodulus of the vermis, which can be the site of tumor origin. Great effort should be applied to remove the tumor completely because cure can be achieved by radical resection of a benign glioma. For this purpose, combining the telovelar and supracerebellar approaches allows optimal visualization of all portions of the tumor (Fig. 138-27).
When using the supracerebellar approach, care is taken to avoid damage to draining veins on the superior surface of the cerebellum. The thick arachnoid of the tectal cistern must be incised gradually while paying attention to the medial cerebellar draining veins. Once the tectal plate has been exposed, gentle retraction of the anterior lobulus quadrangularis, the lobulus centralis, and the culmen of the cerebellar vermis provides access to the superior medullary velum invaded by the tumor. Meticulous hemostasis in the most superior portion of the fourth ventricle is also achieved with use of the supracerebellar route.58,203–205
Exophytic Cavernous Malformations
Although cavernous malformations involving the fourth ventricle originate mainly from the neuraxis, intralesional hemorrhage and exophytic growth may lead to significant expansion into the fourth ventricle (Fig. 138-28). As for other lesions, optimal exposure is obtained with the telovelar approach. Similar to resection of ependymomas, dissection is carried out on the floor of the fourth ventricle because most of these lesions are not directly connected to the cerebellum. If a hematoma cavity is present, the cavity is opened and the hematoma is evacuated by aspiration. By using small cottonoid pledgets and bipolar coagulation at low current intensity, the vascular lesion is gently separated from the neuraxis, and the caverns are coagulated to shrink the entire lesion. Dissection is carried out in a circular fashion. Separated portions of the malformation are either removed directly or isolated with additional cottonoid pledgets. Electrophysiologic mapping of the rhomboid fossa and establishment of the exact site of the facial colliculus play a crucial role in preserving the function of the facial nerve (see ![]() Video 138-8). With this technique, total removal is achieved without leaving residual portions of cavernoma behind. We always perform meticulous bipolar coagulation of the parenchymal cavernoma bed for local hemostasis and to avoid recurrence at this site.179,192,206–209
Video 138-8). With this technique, total removal is achieved without leaving residual portions of cavernoma behind. We always perform meticulous bipolar coagulation of the parenchymal cavernoma bed for local hemostasis and to avoid recurrence at this site.179,192,206–209
Neuroendoscopy for Intraventricular Tumors
History and General Aspects
Treatment of hydrocephalus was the first indication for use of an endoscope in surgery for ventricular pathologies. In 1922, Walter Dandy used an endoscope to treat two children with hydrocephalus and coined the term ventriculoscopy.28,210 During the following years, endoscopic treatment of hydrocephalus consisted of coagulation of the choroid plexus or creation of an opening from the ventricular system to the subarachnoid space at the floor of the third ventricle, a procedure well known today as ventriculocisternostomy.16 In 1963, the endoscope was used for the first time for visualization of intraventricular tumors.211,212 Endoscopic surgery on intraventricular tumors represented a challenge because of the lack of appropriate instruments and difficulty of maintaining hemostasis. With the image quality of the endoscopes at that time, the smallest amount of blood made surgery impossible. For this reason, most of the early procedures consisted of biopsy.213–217 Intraventricular or paraventricular cysts also represented a good target for use of the endoscope in an effort to avoid the eventual need for a shunt or to minimize the length of inserted catheter by creating a communication between different encapsulated compartments.218–220
With technical advances in optical systems, better image quality, and the availability of improved instrumentation, more tumors were operated on endoscopically. In particular, colloid cysts represented an ideal pathology for endoscopic surgery because of their low vascularity, consistency, and location.10,125,221–227 Even today, colloid cysts are among the intraventricular lesions most often treated endoscopically.16 Neuroendoscopic biopsy of intraventricular neoplasms and tumors of the pineal and tectal region, especially with respect to treatment of coexisting obstructive hydrocephalus, is an established procedure today.17,177,227–232 Complete tumor removal by endoscopic means is still restricted to small, well-circumscribed lesions, cystic tumors, or colloid cysts because of the aforementioned advantages.17,135,227,233–235 Tumor types removed endoscopically include SEGAs, exophytic low-grade gliomas, central neurocytomas, small choroid plexus tumors, and purely intraventricular craniopharyngiomas.16 With larger tumors, microsurgical technique is still the gold standard for removal, but the additional use of an endoscope, so-called endoscope-assisted microneurosurgery, can be an advantage to gain a better overview within hidden corners of the ventricular system.125,236
Endoscopic Resection of Colloid Cysts
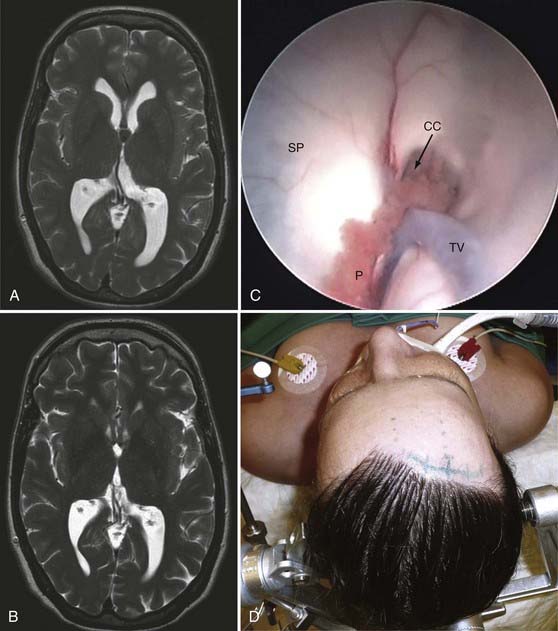
Endoscopic resection of colloid cysts has proved to be a safe method with good long-term results.124,127,131,225 When compared with microsurgical resection, the endoscopic technique showed similar neurological outcomes and the same percentage of recurrence. However, hospitalization and the duration of the surgical procedure are significantly shorter when using the endoscopic approach.129,237 A good indication for open microsurgical resection is a very firm consistency of the colloid cyst, because with the endoscope, neither piecemeal removal nor suction of its contents is possible. Unfortunately, reliable information concerning the cyst’s consistency is not available from preoperative imaging (Fig. 138-29).
Authors’ Surgical Technique
The patient is placed in the supine position, and the head is fixed in the Mayfield holder, turned slightly to the side opposite that of the tumor site, and elevated approximately 30 degrees (see Fig. 138-1). A frameless neuronavigation system is used for precise preoperative planning of the trajectory of the endoscope. The trajectory depends on the location of the cyst in relation to the foramen of Monro. If the cyst is located posteriorly, the bur hole has to be placed more anteriorly. After a slightly curved skin incision, part of the pericranium is excised for later dura replacement. Placement of the bur hole and opening of the dura are performed in standard fashion. Because of the diameter of the endoscope (6 mm), the lateral ventricle is first punctured with a Cushing needle to confirm the correct trajectory. Once the ventricle is reached with the endoscope, anatomic landmarks such as the choroid plexus, thalamostriate vein, head of the caudate nucleus, fornix, and foramen of Monro are identified. In most cases, a colloid cyst filling the foramen of Monro can be distinguished by its greenish gray membrane. If the cyst is hidden by the choroid plexus, the latter can be coagulated to get better visualization. This also helps reduce hemorrhage from the plexus during manipulation of the cyst. The resection technique for the cyst depends on its consistency. If the content is liquid, the cyst can be punctured and the contents aspirated with an appropriate suction tube. Sometimes one has to open the cyst with microscissors and remove the contents in piecemeal fashion with the micro–grasping forceps. The capsule can be coagulated and cut with microscissors. Bleeding during removal of the capsule can be controlled by irrigation with warm, lactated Ringer’s solution. After removal of the endoscope, a small piece of Gelfoam is introduced into the trajectory channel, and the periosteum is placed onto the coagulated dura. The bur hole is covered with a titanium miniplate for cosmetic reasons (see ![]() Video 138-9).
Video 138-9).
Outcome and Complications
A review of the literature shows that outcomes after surgery for ventricular tumors are generally quite satisfactory, even after removal of complex ventricular tumors. In our single-institution series of 143 patients (see Table 138-1), 78% had a postoperative Karnofsky Performance Scale (KPS) score of 80% to 100%, and 92% had a score higher than 70%. A KPS score of 20% to 40% was assessed in only 5% of patients. There were no surgery-related deaths in this series. Except for minor problems such as postoperative wound infection or CSF leakage, there were no major or devastating complications. The most prominent postoperative neurological disorder was a cranial nerve deficit in 27% of the patients, mostly involving the oculomotor system. Seventeen percent of the patients had speech problems or cognitive dysfunction that gradually improved with time. In another 17%, impaired coordination with gait ataxia and disturbed balance was noted. Reduced psychomotor performance during the early postoperative period or slightly disturbed consciousness was seen in 12% of the patients, 8% had motor deficits, 3% complained of sensory problems, and 7.5% showed hormonal dysregulation. Nearly half of the patient population was free of new or any postoperative focal neurological deficits. Eighteen percent of the patients required postoperative external ventricular drainage, but usually only during the first 24 hours after surgery. Seven percent required permanent postoperative shunt placement. Tumor recurrence was noted in 14%, mainly in patients harboring a craniopharyngioma, a colloid cyst, a glioblastoma, a metastatic tumor, an anaplastic glioma, or a pineoblastoma.
Amin-Hanjani S, Charbel FT. Flow-assisted surgical technique in cerebrovascular surgery. Surg Neurol. 2007;68(suppl 1):S4-S11.
Anderson RC, Ghatan S, Feldstein NA. Surgical approaches to tumors of the lateral ventricle. Neurosurg Clin N Am. 2003;14:509-525.
Apuzzo MLJ. Surgery of the 3rd Ventricle, 2nd ed. Baltimore: Williams & Wilkins; 1998.
Bertalanffy H, Benes L, Miyazawa T, et al. Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002;25:1-53.
Cappabianca P, Cinalli G, Gangemi M, et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(suppl 2):575-597.
Charalampaki P, Filippi R, Welschehold S, et al. Tumors of the lateral and third ventricle: removal under endoscope-assisted keyhole conditions. Neurosurgery. 2005;57(4 suppl):302-311.
D’Angelo VA, Galarza M, Catapano D, et al. Lateral ventricle tumors: surgical strategies according to tumor origin and development—a series of 72 cases. Neurosurgery. 2005;56(1 suppl):36-45.
Ellenbogen RG. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001;10(6):E2.
Fernandez-Miranda JC, Rhoton ALJr, Alvarez-Linera J, et al. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008;62(6 suppl 3):989-1026.
Fronda C, Miller D, Kappus C, et al. The benefit of image guidance for the contralateral interhemispheric approach to the lateral ventricle. Clin Neurol Neurosurg. 2008;110:580-586.
Hellwig D, Bauer BL, Schulte M, et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52:525-533.
Hernesniemi J, Romani R, Dashti R, et al. Microsurgical treatment of third ventricular colloid cysts by interhemispheric far lateral transcallosal approach—experience of 134 patients. Surg Neurol. 2008;69:447-453.
Horn EM, Feiz-Erfan I, Bristol RE, et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60:613-618.
Kawashima M, Li X, Rhoton ALJr, et al. Surgical approaches to the atrium of the lateral ventricle: microsurgical anatomy. Surg Neurol. 2006;65:436-445.
Kim DG, Paek SH, Kim IH, et al. Central neurocytoma: the role of radiation therapy and long term outcome. Cancer. 1997;79:1995-2002.
Kockro RA, Stadie A, Schwandt E, et al. A collaborative virtual reality environment for neurosurgical planning and training. Neurosurgery. 2007;61(5 suppl 2):379-391.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
Ono M, Kubik S. Atlas of Cerebral Sulci. Stuttgart, Germany: Thieme; 1990.
Osztie E, Hanzely Z, Afra D. Lateral ventricle gliomas and central neurocytomas in adults: diagnosis and perspectives. Eur J Radiol. 2009;69:67-73.
Rhoton ALJr. Cranial Anatomy and Surgical Approaches. Schaumburg, IL: Congress of Neurological Surgeons; 2003.
Rhoton ALJr. The lateral and third ventricles. Neurosurgery. 2002;51(4 suppl):S207-S271.
Souweidane MM, Hoffman CE, Schwartz TH. Transcavum interforniceal endoscopic surgery of the third ventricle. J Neurosurg Pediatr. 2008;2:231-236.
Staempfli P, Reischauer C, Jaermann T, et al. Combining fMRI and DTI: a framework for exploring the limits of fMRI-guided DTI fiber tracking and for verifying DTI-based fiber tractography results. Neuroimage. 2008;39:119-126.
Tew JM, van Loveren HR, Keller JT. Brain tumors. In: Tew JM, editor. Atlas of Operative Microneurosurgery. Philadelphia: WB Saunders, 2001.
Tirakotai W, Hellwig D, Bertalanffy H, et al. The role of neuroendoscopy in the management of solid or solid-cystic intra- and periventricular tumours. Childs Nerv Syst. 2007;23:653-658.
Yasargil MG. Microneurosurgery: Microneurosurgery of CNS Tumors. Stuttgart, Germany: Thieme; 1996.
Yasargil MG. Microneurosurgery. Zurich: Thieme; 1988.
Yasargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008;62(6 suppl 3):1029-1040.
Yasargil MG, Krayenbuhl N, Roth P, et al. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2009.
1 Dandy W. Benign Tumors in the Third Ventricle of the Brain: Diagnosis and Treatment. Baltimore: Charles C Thomas; 1933.
2 Apuzzo MLJ. Surgery of the 3rd Ventricle, 2nd ed. Baltimore: Williams & Wilkins; 1998.
3 Ellenbogen RG. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001;10(6):E2.
4 Suh DY, Mapstone T. Pediatric supratentorial intraventricular tumors. Neurosurg Focus. 2001;10(6):E4.
5 Casotto A, Buoncristiani P, Signorini E, et al. Third ventricular gliomas. Report of 7 cases with benign clinical behaviour. Acta Neurochir (Wien). 1985;74:43-48.
6 Carmel PW. Tumours of the third ventricle. Acta Neurochir (Wien). 1985;75:136-146.
7 Piepmeier JM. Tumors and approaches to the lateral ventricles. Introduction and overview. J Neurooncol. 1996;30:267-274.
8 Tew JM, van Loveren HR, Keller JT. Brain tumors. In: Tew JM, editor. Atlas of Operative Microneurosurgery. Philadelphia: WB Saunders, 2001.
9 Cetinalp E, Ildan F, Boyar B, et al. Colloid cysts of the third ventricle. Neurosurg Rev. 1994;17:135-139.
10 Decq P, Le Guerinel C, Brugieres P, et al. Endoscopic management of colloid cysts. Neurosurgery. 1998;42:1288-1294.
11 Ragel BT, Osborn AG, Whang K, et al. Subependymomas: an analysis of clinical and imaging features. Neurosurgery. 2006;58:881-890.
12 Ture U, Yasargil MG, Pait TG. Is there a superior occipitofrontal fasciculus? A microsurgical anatomic study. Neurosurgery. 1997;40:1226-1232.
13 Yasargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008;62(6 suppl 3):1029-1040.
14 Anderson RC, Ghatan S, Feldstein NA. Surgical approaches to tumors of the lateral ventricle. Neurosurg Clin N Am. 2003;14:509-525.
15 D’Angelo VA, Galarza M, Catapano D, et al. Lateral ventricle tumors: surgical strategies according to tumor origin and development—a series of 72 cases. Neurosurgery. 2005;56(1 suppl):36-45.
16 Cappabianca P, Cinalli G, Gangemi M, et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(suppl 2):575-597.
17 Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998;88:496-505.
18 Macarthur DC, Buxton N, Vloeberghs M, et al. The effectiveness of neuroendoscopic interventions in children with brain tumours. Childs Nerv Syst. 2001;17:589-594.
19 Souweidane MM. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2005;57(4 suppl):312-318.
20 Charalampaki P, Filippi R, Welschehold S, et al. Tumors of the lateral and third ventricle: removal under endoscope-assisted keyhole conditions. Neurosurgery. 2005;57(4 suppl):302-311.
21 Asgari S, Engelhorn T, Brondics A, et al. Transcortical or transcallosal approach to ventricle-associated lesions: a clinical study on the prognostic role of surgical approach. Neurosurg Rev. 2003;26:192-197.
22 Gokalp HZ, Yuceer N, Arasil E, et al. Tumours of the lateral ventricle. A retrospective review of 112 cases operated upon 1970-1997. Neurosurg Rev. 1998;21:126-137.
23 Bellotti C, Pappada G, Sani R, et al. The transcallosal approach for lesions affecting the lateral and third ventricles. Surgical considerations and results in a series of 42 cases. Acta Neurochir (Wien). 1991;111:103-107.
24 Kasowski H, Piepmeier JM. Transcallosal approach for tumors of the lateral and third ventricles. Neurosurg Focus. 2001;10(6):E3.
25 Aryan HE, Ozgur BM, Jandial R, et al. Complications of interhemispheric transcallosal approach in children: review of 15 years experience. Clin Neurol Neurosurg. 2006;108:790-793.
26 Aicardi J. Epilepsy syndromes, 2nd ed. Engel J, Pedley TA, editors. Epilepsy: A Comprehensive Textbook, Vol 3. New York: Lippincott Williams & Wilkins. 2008:2283-2695.
27 Engel J, Pedley TA, 2nd ed. Epilepsy: A Comprehensive Textbook, Vol 3. New York: Lippincott Williams & Wilkins. 2008.
28 Dandy W. Diagnosis, localization and removal of tumors of the third ventricle. Bull Johns Hopkins Hosp. 1922;33:188-189.
29 Pendl G, Ozturk E, Haselsberger K. Surgery of tumours of the lateral ventricle. Acta Neurochir (Wien). 1992;116:128-136.
30 Spencer DD, Collins WF. Surgical management of the lateral intraventricular tumors. New York. 2nd ed. Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques: Indications, Methods, and Results, Vol 1. 1988:583-596.
31 Yasargil MG. Microneurosurgery: Microneurosurgery of CNS Tumors. Stuttgart, Germany: Thieme; 1996. 313-318
32 Ture U, Yasargil MG, Friedman AH, et al. Fiber dissection technique: lateral aspect of the brain. Neurosurgery. 2000;47:417-426.
33 Yasargil MG, Ture U, Yasargil DC. Surgical anatomy of supratentorial midline lesions. Neurosurg Focus. 2005;18(6B):E1.
34 Abe O, Masutani Y, Aoki S, et al. Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr. 2004;28:533-539.
35 Chao YP, Cho KH, Yeh CH, et al. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172-3187.
36 Fernandez-Miranda JC, Rhoton ALJr, Alvarez-Linera J, et al. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008;62(6 suppl 3):989-1026.
37 Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989-994.
38 Huang H, Zhang J, Jiang H, et al. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195-205.
39 Staempfli P, Jaermann T, Crelier GR, et al. Resolving fiber crossing using advanced fast marching tractography based on diffusion tensor imaging. Neuroimage. 2006;30:110-120.
40 Staempfli P, Reischauer C, Jaermann T, et al. Combining fMRI and DTI: a framework for exploring the limits of fMRI-guided DTI fiber tracking and for verifying DTI-based fiber tractography results. Neuroimage. 2008;39:119-126.
41 Kockro RA, Serra L, Tseng-Tsai Y, et al. Planning and simulation of neurosurgery in a virtual reality environment. Neurosurgery. 2000;46:118-135.
42 Kockro RA, Stadie A, Schwandt E, et al. A collaborative virtual reality environment for neurosurgical planning and training. Neurosurgery. 2007;61(5 suppl 2):379-391.
43 Kockro RA, Hwang PY. Virtual temporal bone: an interactive 3-dimensional learning aid for cranial base surgery. Neurosurgery. 2009;64(5 suppl 2):216-229.
44 Ng I, Hwang PY, Kumar D, et al. Surgical planning for microsurgical excision of cerebral arterio-venous malformations using virtual reality technology. Acta Neurochir (Wien). 2009;151:453-463.
45 Stadie AT, Kockro RA, Reisch R, et al. Virtual reality system for planning minimally invasive neurosurgery. Technical note. J Neurosurg. 2008;108:382-394.
46 Fronda C, Miller D, Kappus C, et al. The benefit of image guidance for the contralateral interhemispheric approach to the lateral ventricle. Clin Neurol Neurosurg. 2008;110:580-586.
47 Kawashima M, Li X, Rhoton ALJr, et al. Surgical approaches to the atrium of the lateral ventricle: microsurgical anatomy. Surg Neurol. 2006;65:436-445.
48 Natori Y, Fukui M, Rhoton ALJr. [Anterior interhemispheric transcallosal approach for lateral ventricle lesions.]. No Shinkei Geka. 1997;25:777-784.
49 Rhoton ALJr. The lateral and third ventricles. Neurosurgery. 2002;51(4 suppl):S207-S271.
50 Rosenfeld JV, Freeman JL, Harvey AS. Operative technique: the anterior transcallosal transseptal interforniceal approach to the third ventricle and resection of hypothalamic hamartomas. J Clin Neurosci. 2004;11:738-744.
51 D’Angelo VA, Galarza M, Catapano D, et al. Lateral ventricle tumors: surgical strategies according to tumor origin and development—a series of 72 cases. Neurosurgery. 2005;56(1 suppl):36-45.
52 Ono M, Kubik S. Atlas of Cerebral Sulci. Stuttgart, Germany: Thieme; 1990.
53 Yasargil MG. Microneurosurgery. Zurich: Thieme; 1988.
54 Rhoton ALJr. Cranial Anatomy and Surgical Approaches. Schaumburg, IL: Congress of Neurological Surgeons; 2003.
55 Fornari M, Savoiardo M, Morello G, et al. Meningiomas of the lateral ventricles. Neuroradiological and surgical considerations in 18 cases. J Neurosurg. 1981;54:64-74.
56 Yasargil MG, Krayenbuhl N, Roth P, et al. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112:168-185.
57 Cushing H, Bailey A. Classification of the Tumours of the Glioma Group on a Histogenetic Basis with a Correlated Study of Prognosis. Philadelphia: JB Lippincott; 1926.
58 Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
59 Schwartz TH, Kim S, Glick RS, et al. Supratentorial ependymomas in adult patients. Neurosurgery. 1999;44:721-731.
60 Daneyemez M, Can C, Izci Y, et al. The tanycytic ependymoma of the lateral ventricle: case report. Minim Invasive Neurosurg. 1999;42:201-203.
61 Kaplan A. Ependymoma of lateral ventricle. Report of a case of more than twelve years’ survival. AMA Am J Dis Child. 1950;80:963-969.
62 Matyja E, Naganska E, Zabek M, et al. Myxopapillary ependymoma of the lateral ventricle with local recurrences: histopathological and ultrastructural analysis of a case. Folia Neuropathol. 2003;41:51-57.
63 Zhang S, Wang X, Zhang Z, et al. Tanycytic ependymoma arising from the right lateral ventricle: a case report and review of the literature. Neuropathology. 2008;28:427-432.
64 Metellus P, Figarella-Branger D, Guyotat J, et al. Supratentorial ependymomas: prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer. 2008;113:175-185.
65 Buhl R, Huang H, Gottwald B, et al. Neuropsychological findings in patients with intraventricular tumors. Surg Neurol. 2005;64:500-503.
66 Nazar GB, Hoffman HJ, Becker LE, et al. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72:408-417.
67 Ragel BT, Townsend JJ, Arthur AS, et al. Intraventricular tanycytic ependymoma: case report and review of the literature. J Neurooncol. 2005;71:189-193.
68 Scheinker IM. Subependymoma: a newly recognized tumor of subependymal derivation. J Neurosurg. 1945;2:232-240.
69 Lombardi D, Scheithauer BW, Meyer FB, et al. Symptomatic subependymoma: a clinicopathological and flow cytometric study. J Neurosurg. 1991;75:583-588.
70 Maiuri F, Gangemi M, Iaconetta G, et al. Symptomatic subependymomas of the lateral ventricles. Report of eight cases. Clin Neurol Neurosurg. 1997;99:17-22.
71 Lobato RD, Cabello A, Carmena JJ, et al. Subependymoma of the lateral ventricle. Surg Neurol. 1981;15:144-147.
72 Nishio S, Morioka T, Mihara F, et al. Subependymoma of the lateral ventricles. Neurosurg Rev. 2000;23:98-103.
73 Kawaguchi T, Kumabe T, Shimizu H, et al. 201Tl-SPECT and 1H-MRS study of benign lateral ventricle tumors: differential diagnosis of subependymoma. Neurosurg Rev. 2005;28:96-103.
74 Koutourousiou M, Georgakoulias N, Kontogeorgos G, et al. Subependymomas of the lateral ventricle: tumor recurrence correlated with increased Ki-67 labeling index. Neurol India. 2009;57:191-193.
75 Hassoun J, Gambarelli D, Grisoli F, et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151-156.
76 Sharma MC, Rathore A, Karak AK, et al. A study of proliferative markers in central neurocytoma. Pathology. 1998;30:355-359.
77 Zhang D, Wen L, Henning TD, et al. Central neurocytoma: clinical, pathological and neuroradiological findings. Clin Radiol. 2006;61:348-357.
78 Hassoun J, Soylemezoglu F, Gambarelli D, et al. Central neurocytoma: a synopsis of clinical and histological features. Brain Pathol. 1993;3:297-306.
79 Maiuri F, Spaziante R, De Caro ML, et al. Central neurocytoma: clinico-pathological study of 5 cases and review of the literature. Clin Neurol Neurosurg. 1995;97:219-228.
80 Valdueza JM, Westphal M, Vortmeyer A, et al. Central neurocytoma: clinical, immunohistologic, and biologic findings of a human neuroglial progenitor tumor. Surg Neurol. 1996;45:49-56.
81 Wichmann W, Schubiger O, von Deimling A, et al. Neuroradiology of central neurocytoma. Neuroradiology. 1991;33:143-148.
82 Osztie E, Hanzely Z, Afra D. Lateral ventricle gliomas and central neurocytomas in adults diagnosis and perspectives. Eur J Radiol. 2009;69:67-73.
83 Eng DY, DeMonte F, Ginsberg L, et al. Craniospinal dissemination of central neurocytoma. Report of two cases. J Neurosurg. 1997;86:547-552.
84 Kim DG, Paek SH, Kim IH, et al. Central neurocytoma: the role of radiation therapy and long term outcome. Cancer. 1997;79:1995-2002.
85 Sgouros S, Carey M, Aluwihare N, et al. Central neurocytoma: a correlative clinicopathologic and radiologic analysis. Surg Neurol. 1998;49:197-204.
86 Yasargil MG, von Ammon K, von Deimling A, et al. Central neurocytoma: histopathological variants and therapeutic approaches. J Neurosurg. 1992;76:32-37.
87 Javedan SP, Manwaring K, Smith KA. Treatment of posterior third ventricular central neurocytoma with endoscopic biopsy, endoscopic third ventriculostomy and stereotactic radiosurgery. Minim Invasive Neurosurg. 2003;46:165-168.
88 Kim IY, Kondziolka D, Niranjan A, et al. Gamma knife radiosurgery for intraventricular meningiomas. Acta Neurochir (Wien). 2009;151:447-452.
89 Buccoliero AM, Franchi A, Castiglione F, et al. Subependymal giant cell astrocytoma (SEGA): is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology. 2009;29:25-30.
90 Fujiwara S, Takaki T, Hikita T, et al. Subependymal giant-cell astrocytoma associated with tuberous sclerosis. Do subependymal nodules grow? Childs Nerv Syst. 1989;5:43-44.
91 Nishio S, Morioka T, Suzuki S, et al. Subependymal giant cell astrocytoma: clinical and neuroimaging features of four cases. J Clin Neurosci. 2001;8:31-34.
92 Nishizaki T, Orita T, Abiko S, et al. Subependymal giant cell astrocytoma associated with tuberous sclerosis: with special reference to cell kinetic studies—case report. Neurol Med Chir (Tokyo). 1990;30:695-697.
93 Shepherd CW, Gomez MR, Lie JT, et al. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991;66:792-796.
94 Webb DW, Fryer AE, Osborne JP. Morbidity associated with tuberous sclerosis: a population study. Dev Med Child Neurol. 1996;38:146-155.
95 Kawasaki S, Yamamoto Y, Sunami N, et al. [Subependymal giant cell astrocytoma associated with tuberous sclerosis exhibiting rapid regrowth after 17 years: a case report.]. No Shinkei Geka. 1999;27:550-556.
96 Lunsford LD, Niranjan A. The rationale for rational surgery for fibrillary astrocytomas. Clin Neurosurg. 2001;48:20-36.
97 Muragaki Y, Chernov M, Tajika Y, et al. Coincidence of central neurocytoma and multiple glioblastomas: a rare case report. J Neurooncol. 2009;93:431-435.
98 Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479-489.
99 Central Brain Tumor Registry of the United States. Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004-2006. Hinsdale, IL: Brain Tumor Registry of the United States; 2006.
100 Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93-108.
101 Di Rocco F, Caldarelli M, Sabatino G, et al. Lateral ventricle choroid plexus papilloma extending into the third ventricle. Pediatr Neurosurg. 2004;40:314-316.
102 Erman T, Gocer AI, Erdogan S, et al. Choroid plexus papilloma of bilateral lateral ventricle. Acta Neurochir (Wien). 2003;145:139-143.
103 Nagib MG, O’Fallon MT. Lateral ventricle choroid plexus papilloma in childhood: management and complications. Surg Neurol. 2000;54:366-372.
104 Turcotte JF, Copty M, Bedard F, et al. Lateral ventricle plexus papilloma and communicating hydrocephalus. Surg Neurol. 1980;13:143-146.
105 Bertalanffy A, Roessler K, Koperek O, et al. Intraventricular meningiomas: a report of 16 cases. Neurosurg Rev. 2006;29:30-35.
106 Liu M, Wei Y, Liu Y, et al. Intraventricular meningiomas: a report of 25 cases. Neurosurg Rev. 2006;29:36-40.
107 Majos C, Coll S, Aguilera C, et al. Intraventricular mass lesions of the brain. Eur Radiol. 2000;10:951-961.
108 Eom KS, Kim DW, Kim TY. Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression: a case report. Clin Neurol Neurosurg. 2009;111:619-623.
109 Erman T, Gocer AI, Tuna M, et al. Malignant meningioma of the lateral ventricle. Case report. Neurosurg Focus. 2003;15(4):ECP2.
110 Kim EY, Kim ST, Kim HJ, et al. Intraventricular meningiomas: radiological findings and clinical features in 12 patients. Clin Imaging. 2009;33:175-180.
111 Bhatoe HS, Singh P, Dutta V. Intraventricular meningiomas: a clinicopathological study and review. Neurosurg Focus. 2006;20(3):E9.
112 Nakamura M, Roser F, Bundschuh O, et al. Intraventricular meningiomas: a review of 16 cases with reference to the literature. Surg Neurol. 2003;59:491-503.
113 Dehdashti AR, Ganna A, Karabatsou K, et al. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 2008;62:1006-1015.
114 Zhang X, Fei Z, Zhang W, et al. Endoscopic endonasal transsphenoidal surgery for invasive pituitary adenoma. J Clin Neurosci. 2008;15:241-245.
115 Zhang Y, Wang Z, Liu Y, et al. Endoscopic transsphenoidal treatment of pituitary adenomas. Neurol Res. 2008;30:581-586.
116 Dehdashti AR, de Tribolet N. Frontobasal interhemispheric trans–lamina terminalis approach for suprasellar lesions. Neurosurgery. 2005;56(2 suppl):418-424.
117 Maira G, Anile C, Colosimo C, et al. Craniopharyngiomas of the third ventricle: trans–lamina terminalis approach. Neurosurgery. 2000;47:857-863.
118 Shibuya M, Takayasu M, Suzuki Y, et al. Bifrontal basal interhemispheric approach to craniopharyngioma resection with or without division of the anterior communicating artery. J Neurosurg. 1996;84:951-956.
119 Shirane R, Ching-Chan S, Kusaka Y, et al. Surgical outcomes in 31 patients with craniopharyngiomas extending outside the suprasellar cistern: an evaluation of the frontobasal interhemispheric approach. J Neurosurg. 2002;96:704-712.
120 El Ghandour NM. Endoscopic treatment of third ventricular colloid cysts: a review including ten personal cases. Neurosurg Rev. 2009;32:395-402.
121 Demirci S, Dogan KH, Erkol Z, et al. Sudden death due to a colloid cyst of the third ventricle: report of three cases with a special sign at autopsy. Forensic Sci Int. 2009;189:e33-e36.
122 Humphries RL, Stone CK, Bowers RC. Colloid cyst: a case report and literature review of a rare but deadly condition. J Emerg Med. Sep 22, 2008. [Epub ahead of print]
123 Le Gars D, Lejeune J, Desenclos C. [Tumors of the third ventricle: review of the literature.]. Neurochirurgie. 2000;46:296-319.
124 Acerbi F, Rampini P, Egidi M, et al. Endoscopic treatment of colloid cysts of the third ventricle: long-term results in a series of 6 consecutive cases. J Neurosurg Sci. 2007;51:53-60.
125 Charalampaki P, Filippi R, Welschehold S, et al. Endoscope-assisted removal of colloid cysts of the third ventricle. Neurosurg Rev. 2006;29:72-79.
126 Choudhari KA. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2008;62:E1384.
127 Greenlee JD, Teo C, Ghahreman A, et al. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008;62(3 suppl 1):51-55.
128 Hernesniemi J, Romani R, Dashti R, et al. Microsurgical treatment of third ventricular colloid cysts by interhemispheric far lateral transcallosal approach—experience of 134 patients. Surg Neurol. 2008;69:447-453.
129 Horn EM, Feiz-Erfan I, Bristol RE, et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60:613-618.
130 Idris Z, Hallaert G, Vanhauwaert D, et al. Frameless neuronavigation–guided endoscopic total en-bloc removal of a third ventricular colloid cyst: a case report on surgical technique. Minim Invasive Neurosurg. 2008;51:173-177.
131 Levine NB, Miller MN, Crone KR. Endoscopic resection of colloid cysts: indications, technique, and results during a 13-year period. Minim Invasive Neurosurg. 2007;50:313-317.
132 Longatti P, Godano U, Gangemi M, et al. Cooperative study by the Italian Neuroendoscopy Group on the treatment of 61 colloid cysts. Childs Nerv Syst. 2006;22:1263-1267.
133 Paleologos TS, Wadley JP, Kitchen ND, et al. Interactive image-guided transcallosal microsurgery for anterior third ventricular cysts. Minim Invasive Neurosurg. 2001;44:157-162.
134 Shapiro S, Rodgers R, Shah M, et al. Interhemispheric transcallosal subchoroidal fornix-sparing craniotomy for total resection of colloid cysts of the third ventricle. J Neurosurg. 2009;110:112-115.
135 Tirakotai W, Hellwig D, Bertalanffy H, et al. The role of neuroendoscopy in the management of solid or solid-cystic intra- and periventricular tumours. Childs Nerv Syst. 2007;23:653-658.
136 Zohdi A, El Kheshin S. Endoscopic approach to colloid cysts. Minim Invasive Neurosurg. 2006;49:263-268.
137 Luther N, Souweidane MM. Neuroendoscopic resection of posterior third ventricular ependymoma. Case report. Neurosurg Focus. 2005;18(6A):E3.
138 Courville CB, Broussalian SL. Plastic ependymomas of the lateral recess. Report of eight verified cases. J Neurosurg. 1961;18:792-799.
139 Waldron JS, Tihan T. Epidemiology and pathology of intraventricular tumors. Neurosurg Clin N Am. 2003;14:469-482.
140 Wang KC, Kim SK, Choe G, et al. Growth patterns of craniopharyngioma in children: role of the diaphragm sellae and its surgical implication. Surg Neurol. 2002;57:25-33.
141 Choi SH, Kwon BJ, Na DG, et al. Pituitary adenoma, craniopharyngioma, and Rathke cleft cyst involving both intrasellar and suprasellar regions: differentiation using MRI. Clin Radiol. 2007;62:453-462.
142 Nakamizo A, Inamura T, Nishio S, et al. Neuroendoscopic treatment of cystic craniopharyngioma in the third ventricle. Minim Invasive Neurosurg. 2001;44:85-87.
143 Shi XE, Wu B, Fan T, et al. Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg. 2008;110:151-159.
144 Zhang YQ, Ma ZY, Wu ZB, et al. Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg. 2008;44:435-443.
145 Fahlbusch R, Hofmann BM. Surgical management of giant craniopharyngiomas. Acta Neurochir (Wien). 2008;150:1213-1226.
146 Forsyth PA, Shaw EG, Scheithauer BW, et al. Supratentorial pilocytic astrocytomas. A clinicopathologic, prognostic, and flow cytometric study of 51 patients. Cancer. 1993;72:1335-1342.
147 Cohen-Gadol AA, Geryk B, Binder DK, et al. Conquering the third ventricular chamber. J Neurosurg. 2009;111:590-599.
148 Kasowski HJ, Nahed BV, Piepmeier JM. Transcallosal transchoroidal approach to tumors of the third ventricle. Neurosurgery. 2005;57(4 suppl):361-366.
149 Souweidane MM, Hoffman CE, Schwartz TH. Transcavum interforniceal endoscopic surgery of the third ventricle. J Neurosurg Pediatr. 2008;2:231-236.
150 Brat DJ, Scheithauer BW, Staugaitis SM, et al. Third ventricular chordoid glioma: a distinct clinicopathologic entity. J Neuropathol Exp Neurol. 1998;57:283-290.
151 Galloway M, Afshar F, Geddes JF. Chordoid glioma: an uncommon tumour of the third ventricle. Br J Neurosurg. 2001;15:147-150.
152 Pomper MG, Passe TJ, Burger PC, et al. Chordoid glioma: a neoplasm unique to the hypothalamus and anterior third ventricle. AJNR Am J Neuroradiol. 2001;22:464-469.
153 Cenacchi G, Roncaroli F, Cerasoli S, et al. Chordoid glioma of the third ventricle: an ultrastructural study of three cases with a histogenetic hypothesis. Am J Surg Pathol. 2001;25:401-405.
154 Kawasaki K, Kohno M, Inenaga C, et al. Chordoid glioma of the third ventricle: a report of two cases, one with ultrastructural findings. Neuropathology. 2009;29:85-90.
155 Grand S, Pasquier B, Gay E, et al. Chordoid glioma of the third ventricle: CT and MRI, including perfusion data. Neuroradiology. 2002;44:842-846.
156 Carrasco R, Pascual JM, Reina T, et al. Chordoid glioma of the third ventricle attached to the optic chiasm. Successful removal through a trans–lamina terminalis approach. Clin Neurol Neurosurg. 2008;110:828-833.
157 Vanhauwaert DJ, Clement F, Van Dorpe J, et al. Chordoid glioma of the third ventricle. Acta Neurochir (Wien). 2008;150:1183-1191.
158 Krause F. Operative Freilegung der Vierhügelplatte, nebst Beobachtungen über Hirndruck und Dekompression. Zentralbl Chir. 1926;53:2812-2819.
159 Ahn JY, Chung YS, Kwon SO, et al. Isolated pineal region metastasis of small cell lung cancer. J Clin Neurosci. 2005;12:691-693.
160 Amini A, Schmidt RH, Salzman KL, et al. Glioblastoma multiforme of the pineal region. J Neurooncol. 2006;79:307-314.
161 Constantine C, Miller DC, Gardner S, et al. Osseous metastasis of pineoblastoma: a case report and review of the literature. J Neurooncol. 2005;74:53-57.
162 Senft C, Raabe A, Hattingen E, et al. Pineal parenchymal tumor of intermediate differentiation: diagnostic pitfalls and discussion of treatment options of a rare tumor entity. Neurosurg Rev. 2008;31:231-236.
163 Howard BM, Hofstetter C, Wagner PL, et al. Transformation of a low-grade pineal parenchymal tumour to secondary pineoblastoma. Neuropathol Appl Neurobiol. 2009;35:214-217.
164 Dario A, Cerati M, Taborelli M, et al. Cytogenetic and ultrastructural study of a pineocytoma case report. J Neurooncol. 2000;48:131-134.
165 Fraser G, Rampling R, Smith C, et al. Long-term survival following extra-neural metastasis from a pineoblastoma. J Neurooncol. 2000;48:141-144.
166 Barlas O, Bayindir C, Imer M, et al. Non-resective management of pineoblastoma. Minim Invasive Neurosurg. 2000;43:163-170.
167 Nakamura M, Saeki N, Iwadate Y, et al. Neuroradiological characteristics of pineocytoma and pineoblastoma. Neuroradiology. 2000;42:509-514.
168 Chang AH, Fuller GN, Debnam JM, et al. MR imaging of papillary tumor of the pineal region. AJNR Am J Neuroradiol. 2008;29:187-189.
169 Lekovic GP, Gonzalez LF, Shetter AG, et al. Role of Gamma Knife surgery in the management of pineal region tumors. Neurosurg Focus. 2007;23(6):E12.
170 Boscherini D, Pintucci M, Mazzucchelli L, et al. Neuroendoscopic management of a solitary pineal region tumor. Case report of an adenocarcinoma metastasis. Minim Invasive Neurosurg. 2006;49:247-250.
171 Hernesniemi J, Romani R, Albayrak BS, et al. Microsurgical management of pineal region lesions: personal experience with 119 patients. Surg Neurol. 2008;70:576-583.
172 Kim IY, Jung S, Moon KS, et al. Neuronavigation-guided endoscopic surgery for pineal tumors with hydrocephalus. Minim Invasive Neurosurg. 2004;47:365-368.
173 Mori Y, Kobayashi T, Hasegawa T, et al. Stereotactic radiosurgery for pineal and related tumors. Prog Neurol Surg. 2009;23:106-118.
174 Tirakotai W, Riegel T, Stiegel A, et al. Peri-operative quality of life assessment in endoscopically treated patients with pineal region tumours. Childs Nerv Syst. 2007;23:659-663.
175 Tsumanuma I, Tanaka R, Fujii Y. Occipital transtentorial approach and combined treatments for pineal parenchymal tumors. Prog Neurol Surg. 2009;23:26-43.
176 Guillamo JS, Monjour A, Taillandier L, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528-2539.
177 Oka K, Kin Y, Go Y, et al. Neuroendoscopic approach to tectal tumors: a consecutive series. J Neurosurg. 1999;91:964-970.
178 Johnson PC, Wascher T, Golfinos J, et al. Definition and pathological features. In: Awad IA, Barrow DL, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:1-11.
179 Bertalanffy H, Benes L, Miyazawa T, et al. Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002;25:1-53.
180 Krayenbuehl H, Siebenmann R. Small vascular malformations as a cause of primary intracerebral hemorrhage. J Neurosurg. 1965;22:7-20.
181 Kupersmith MJ, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery. 2001;48:47-53.
182 Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol. 2005;63:319-328.
183 Voigt K, Yasargil MG. Cerebral cavernous haemangiomas or cavernomas. Incidence, pathology, localization, diagnosis, clinical features and treatment. Review of the literature and report of an unusual case. Neurochirurgia (Stuttg). 1976;19:59-68.
184 Dandy W. The Brain. Hagerstown, MD: WF Prior; 1966.
185 Kempe LG. Operative Neurosurgery. New York: Springer; 1970. 1-3
186 Kempe LG. Operative Neurosurgery. New York: Springer; 1970. 14-33
187 Bastian AJ, Mink JW, Kaufman BA, et al. Posterior vermal split syndrome. Ann Neurol. 1998;44:601-610.
188 Dailey AT, McKhann GM, Berger MS. The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg. 1995;83:467-475.
189 De Smet HJ, Baillieux H, Catsman-Berrevoets C, et al. Postoperative motor speech production in children with the syndrome of ‘cerebellar’ mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neurol. 2007;11:193-207.
190 Gok A, Alptekin M, Erkutlu I. Surgical approach to the fourth ventricle cavity through the cerebellomedullary fissure. Neurosurg Rev. 2004;27:50-54.
191 Matsushima T, Inoue T, Inamura T, et al. Transcerebellomedullary fissure approach with special reference to methods of dissecting the fissure. J Neurosurg. 2001;94:257-264.
192 Mussi AC, Rhoton ALJr. Telovelar approach to the fourth ventricle: microsurgical anatomy. J Neurosurg. 2000;92:812-823.
193 Guyotat J, Signorelli F, Desme S, et al. Intracranial ependymomas in adult patients: analyses of prognostic factors. J Neurooncol. 2002;60:255-268.
194 Souweidane MM, Morgenstern PF, Christos PJ, et al. Intraoperative arachnoid and cerebrospinal fluid sampling in children with posterior fossa brain tumors. Neurosurgery. 2009;65:72-78.
195 Amin-Hanjani S, Charbel FT. Flow-assisted surgical technique in cerebrovascular surgery. Surg Neurol. 2007;68(suppl 1):S4-11.
196 Amin-Hanjani S, Meglio G, Gatto R, et al. The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery. 2008;62(6 suppl 3):1346-1353.
197 Hu WW, Zheng XJ, Shen G, et al. [Diagnosis and micro-neurosurgery for the fourth cerebral ventricle tumors.]. Zhonghua Zhong Liu Za Zhi. 2007;29:144-146.
198 Kamitani H, Hirano N, Takigawa H, et al. Attenuation of vascularity by preoperative radiosurgery facilitates total removal of a hypervascular hemangioblastoma at the cerebello-pontine angle: case report. Surg Neurol. 2004;62:238-243.
199 Nishimoto A, Kawakami Y. Surgical removal of hemangioblastoma in the fourth ventricle. Surg Neurol. 1980;13:423-427.
200 Gelabert-Gonzalez M, Allut AG, Bandin-Dieguez J, et al. [Epidermoid cyst of the IV ventricle.]. Rev Neurol. 2007;44:764-765.
201 Hila H, Bouhaouala MH, Darmoul M, et al. [Vermian epidermoid cyst revealed by head injury.]. Neurochirurgie. 2006;52:63-66.
202 Lauvin-Gaillard MA, Legeais M, Velut S, et al. [Epidermoid cyst of the fourth ventricle.]. J Radiol. 2009;90:618-621.
203 Garg A, Chugh M, Gaikwad SB, et al. Juvenile pilocytic astrocytoma presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004;100(5 suppl Pediatrics):525-529.
204 Preusser M, Dietrich W, Czech T, et al. Rosette-forming glioneuronal tumor of the fourth ventricle. Acta Neuropathol. 2003;106:506-508.
205 Rajesh BJ, Rao BR, Menon G, et al. Telovelar approach: technical issues for large fourth ventricle tumors. Childs Nerv Syst. 2007;23:555-558.
206 Gross BA, Batjer HH, Awad IA, et al. Brainstem cavernous malformations. Neurosurgery. 2009;64:E805-E818.
207 Sindou M, Yada J, Salord F. Functional results after microsurgical resection of brain stem cavernous malformations (retrospective study of a 12 patient series and review of the recent literature). Acta Neurochir (Wien). 2000;142:843-852.
208 Zausinger S, Yousry I, Brueckmann H, et al. Cavernous malformations of the brainstem: three-dimensional-constructive interference in steady-state magnetic resonance imaging for improvement of surgical approach and clinical results. Neurosurgery. 2006;58:322-330.
209 Quinones-Hinojosa A, Lyon R, Du R, et al. Intraoperative motor mapping of the cerebral peduncle during resection of a midbrain cavernous malformation: technical case report. Neurosurgery. 2005;56(2 suppl):E439.
210 Hsu W, Li KW, Bookland M, et al. Keyhole to the brain: Walter Dandy and neuroendoscopy. J Neurosurg Pediatr. 2009;3:439-442.
211 Guiot G, Comoy C. Ventriculoscopy. Rev Prat. 1963;13:3655-3656.
212 Guiot J, Rougerie J, Fourestier M, et al. Intracranial endoscopic explorations. Presse Med. 1963;71:1225-1228.
213 Apuzzo ML, Heifetz MD, Weiss MH, et al. Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg. 1977;46:398-400.
214 Auer LM, Holzer P, Ascher PW, et al. Endoscopic neurosurgery. Acta Neurochir (Wien). 1988;90:1-14.
215 Fukushima T, Ishijima B, Hirakawa K, et al. Ventriculofiberscope: a new technique for endoscopic diagnosis and operation. Technical note. J Neurosurg. 1973;38:251-256.
216 Fukushima T. Endoscopic biopsy of intraventricular tumors with the use of a ventriculofiberscope. Neurosurgery. 1978;2:110-113.
217 Powell MP, Torrens MJ, Thomson JL, et al. Isodense colloid cysts of the third ventricle: a diagnostic and therapeutic problem resolved by ventriculoscopy. Neurosurgery. 1983;13:234-237.
218 Heilman CB, Cohen AR. Endoscopic ventricular fenestration using a “saline torch.”. J Neurosurg. 1991;74:224-229.
219 Powers SK. Fenestration of intraventricular cysts using a flexible, steerable endoscope and the argon laser. Neurosurgery. 1986;18:637-641.
220 Powers SK. Fenestration of intraventricular cysts using a flexible, steerable endoscope. Acta Neurochir Suppl. 1992;54:42-46.
221 Abdou MS, Cohen AR. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89:1062-1068.
222 Levine NB, Miller MN, Crone KR. Endoscopic resection of colloid cysts: indications, technique, and results during a 13-year period. Minim Invasive Neurosurg. 2007;50:313-317.
223 Lewis AI, Crone KR, Taha J, et al. Surgical resection of third ventricle colloid cysts. Preliminary results comparing transcallosal microsurgery with endoscopy. J Neurosurg. 1994;81:174-178.
224 Mathiesen T, Grane P, Lindquist C, et al. High recurrence rate following aspiration of colloid cysts in the third ventricle. J Neurosurg. 1993;78:748-752.
225 Hellwig D, Bauer BL, Schulte M, et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52:525-533.
226 Rodziewicz GS, Smith MV, Hodge CJJr. Endoscopic colloid cyst surgery. Neurosurgery. 2000;46:655-660.
227 Schroeder HW, Wagner W, Tschiltschke W, et al. Frameless neuronavigation in intracranial endoscopic neurosurgery. J Neurosurg. 2001;94:72-79.
228 Chernov MF, Kamikawa S, Yamane F, et al. Neurofiberscopic biopsy of tumors of the pineal region and posterior third ventricle: indications, technique, complications, and results. Neurosurgery. 2006;59:267-277.
229 Fiorindi A, Longatti P. A restricted neuroendoscopic approach for pathological diagnosis of intraventricular and paraventricular tumours. Acta Neurochir (Wien). 2008;150:1235-1239.
230 Gangemi M, Maiuri F, Colella G, et al. Endoscopic surgery for pineal region tumors. Minim Invasive Neurosurg. 2001;44:70-73.
231 O’Brien DF, Hayhurst C, Pizer B, et al. Outcomes in patients undergoing single-trajectory endoscopic third ventriculostomy and endoscopic biopsy for midline tumors presenting with obstructive hydrocephalus. J Neurosurg. 2006;105(3 suppl):219-226.
232 Prat R, Galeano I. Endoscopic biopsy of foramen of Monro and third ventricle lesions guided by frameless neuronavigation: usefulness and limitations. Clin Neurol Neurosurg. 2009;111:579-582.
233 Abdullah J, Caemaert J. Endoscopic management of craniopharyngiomas: a review of 3 cases. Minim Invasive Neurosurg. 1995;38:79-84.
234 Cinalli G, Spennato P, Cianciulli E, et al. The role of transventricular neuroendoscopy in the management of craniopharyngiomas: three patient reports and review of the literature. J Pediatr Endocrinol Metab. 2006;19(suppl 1):341-354.
235 Teo C, Nakaji P. Neuro-oncologic applications of endoscopy. Neurosurg Clin N Am. 2004;15:89-103.
236 Charalampaki P, Filippi R, Welschehold S, et al. Endoscopic and endoscope-assisted neurosurgical treatment of suprasellar arachnoidal cysts (Mickey Mouse cysts). Minim Invasive Neurosurg. 2005;48:283-288.
237 Grondin RT, Hader W, MacRae ME, et al. Endoscopic versus microsurgical resection of third ventricle colloid cysts. Can J Neurol Sci. 2007;34:197-207.