Chapter 45 Ventricular Tachycardia and Ventricular Fibrillation Without Structural Heart Disease
Idiopathic ventricular tachycardia (IVT) and idiopathic ventricular fibrillation (IVF) have been intermittently recognized and reported since the early 1900s. Since Vakil’s classic description of ventricular aneurysms and their association with ventricular tachycardia (VT), early literature defined VT as being associated with heart disease.1–3 The occurrence of paroxysmal VT in young patients and occasionally those with apparently healthy hearts, emerged from time to time.4,5 The availability of electrocardiographic recordings focused attention on the region of origin of ventricular arrhythmias, including ectopic beats as well as VT.6,7 In 1974, in a careful review of the literature of the twentieth century, investigators from the Chicago School of Electrophysiology noted that “cardiac stimulatory studies suggest the site of origin of ventricular ectopic beats can be identified by QRS configuration.” Ectopic beats were classified according to their bundle branch block–like configuration, with left ventricular origin being ascribed to right bundle branch configurations and right ventricular origin to left bundle branch configurations.8 The latter were credited with a more favorable outcome. However, occasional reports of deaths in young patients existed, disputing a uniform benign prognosis. With advances in the evaluation and management of ventricular arrhythmias, there now exists a far greater body of knowledge regarding these conditions. IVT and IVF have been studied for anatomic substrate, clinical electrophysiology, and outcomes with and without therapy. This chapter summarizes the current understanding of this constellation of conditions.
Definition and Classification
In current-day parlance, IVT refers to VT of unknown cause that occurs in the absence of significant structural heart disease or transient or reversible arrhythmogenic factors (e.g., electrolyte disorders, myocardial ischemia). IVF may include polymorphic VT evolving to VF or may initiate directly as VF. In practice, one cannot categorically exclude patients with evidence of structural heart disease because advances in diagnostic tests demonstrate structural changes in some patients with classic IVT syndromes.9–11 Magnetic resonance imaging (MRI) has shown subtle wall motion disturbances that may represent structural disease.12–15 In addition, a patient could have structural disease caused by a coexisting cardiac disorder. The term idiopathic is sometimes inappropriate given the depth of understanding that now exists for a few of the disorders. For the purposes of this discussion, the syndromes listed in Box 45-1 are assumed to be distinct IVT syndromes. However, it must be acknowledged that these syndromes consist of heterogeneous subtypes, and some share characteristics with other IVT syndromes so that clear distinctions are not always possible. Moreover, reports of “new” IVTs arise frequently.16,17 Some of these may be previously unrecognized syndromes, whereas others may be variants of established forms.
Box 45-1 Idiopathic Ventricular Tachyarrhythmia Syndromes
Epidemiology
Epidemiologic data on IVT syndromes are scarce because of difficulties in recognizing the syndrome and its demographics. The demographics of the clinical syndrome of IVT derived initial impetus from clinical and later electrophysiological studies (EPSs). Pietras et al analyzed 27 patients with chronic recurrent VT classified according to the electrocardiogram (ECG) classification and examined their clinical, ECG, and hemodynamic features.8 They further tabulated 73 reported cases in the then-existing literature. They noted that the mean age of right-sided VT was lower (32 years) than that of left-sided VT (43 years), with a similar trend in the previously reported data (36 vs. 53 years). Females predominated in the case of right-sided VT (52% and 66% in the two series, respectively) compared with left-sided VT (33% and 15% in the two series, respectively). Organic heart disease was almost invariably present with left-sided VT (100% and 85%, respectively) and absent or uncommon in right-sided VT (25% and 48%, respectively). Mortality rates were significantly lower in right-sided VT, with no deaths in their series and a 12% incidence in the reported literature. Chapman et al reported electrical and hemodynamic correlates in a series of patients with “idiopathic VT” in 1975.18 Subsequently, EPSs in this syndrome have defined the arrhythmia mechanisms, subgroups, novel clinical therapies, and their outcomes.9,19
Formal epidemiologic data are lacking, but IVT is considered rare. IVT syndromes arising in the right ventricular outflow tract (RVOT) or the left ventricular outflow tract (LVOT) are the most common forms of IVT. The RVOT origin predominates, accounting for more than 70% of referred patients, although LVOT origin is being increasingly recognized.20–22 Aortic cusp IVT has been reported to account for 17% to 21% of outflow tract IVT.23,24 Analysis of multiple-center experiences suggests that IVT constitutes approximately 10% of all VT referrals to electrophysiologists, but the true incidence in the community remains unclear.23 IVF is believed to represent 5% to 10% of sudden cardiac death (SCD) victims and implantable cardioverter-defibrillator (ICD) recipients.25–27 IVF populations are still being defined. Commonly recognized genetic syndromes include the long QT syndrome (LQTS), Brugada syndrome, and the more uncommon short QT syndrome (SQTS). Information regarding their prevalence is emerging and is discussed below. One important subgroup is associated with an early repolarization abnormality.28 Haissaguerre et al described an early repolarization abnormality in 31% of patients with IVF compared with 5% in control groups in a case-control study. Patients with this finding had a higher 5-year mortality rate. These early repolarization abnormalities have now been classified under the term J-wave syndromes.
Population data exist for some genetically based IVT or IVF syndromes. It has been estimated, for instance, that Brugada syndrome accounts for 4 to 10 cases of SCD per 10,000 inhabitants per year in Thailand and Laos, countries with an apparently high incidence. Nevertheless, the problem may be much larger. Autopsy series suggest that in 5% to 15% of cases of SCD, no evidence of heart disease or other likely cause exists.29–31 These observations raise the possibility that the IVT and IVF syndromes are common but insufficiently recognized. IVF syndromes with early repolarization have been studied in both IVF survivors and population studies. Tikannen et al studied more than 10,684 middle-aged subjects and noted that the early-repolarization pattern of 0.1 mV or more was present in 5.8% (3.5% in inferior leads and 2.4% in lateral leads).32 J-point elevation of at least 0.1 mV in inferior leads was associated with an increased cardiac mortality risk (relative risk [RR], 1.34; P = .03); J-point elevation of more than 0.2 mV in inferior leads occurred in 0.3% of patients, who had a markedly elevated risk of death from cardiac causes (RR, 2.98; P < .001) and from arrhythmia (adjusted RR, 2.92; 95% confidence interval [CI], 1.45 to 5.89; P = .01). Long Q-T interval and left ventricular hypertrophy were actually weaker predictors of this outcome. Juntila and Myerburg noted variations in the frequency of early repolarization ranging from 10% to 42% based on ethnicity in healthy athletes.33 Antzelevitch has proposed three subtypes of J-wave syndrome on the basis of these data.34 Type 1, an early repolarization pattern present predominantly in the lateral precordial leads, is common in healthy male athletes and is rarely seen in IVF survivors; type 2, which has the ECG pattern predominantly in inferior or inferolateral leads, has a higher risk; and type 3, which displays global findings and is associated with the highest IVF risk, is often associated with incessant VF.
A recent retrospective multi-center study evaluated the profile of pediatric patients with IVT. Mean age at first manifestation of the arrhythmia was 5.4 years, with a range of 0.1 to 15 years, and 27% of patients manifested the disorder in infancy.35
Mechanisms and Clinical Presentation
The clinical presentation of patients with IVT is highly variable. IVT arising in the outflow tract most often manifests in the third to sixth decade of life and has a female preponderance.36–38 Palpitations in an otherwise healthy young individual are a classic description in this entity. These can be exercise induced or may occur at rest. Salvos of nonsustained VT (NSVT) are common. Hormonal triggers have been reported, and more frequent arrhythmic events, ventricular ectopic beats, or VT events are seen during exercise or increased activity. These can culminate in near syncope or syncope, dysthymia and, on occasion, cardiac arrest. Cardiac arrest can be the primary presentation in some individuals, particularly if a family history of SCD exists. Others seek medical attention in the asymptomatic state with a family history of SCD or for an electrocardiographic abnormality such as LQTS or Brugada syndrome. Increasing awareness of the familial forms of the arrhythmia and symptomatic high-density ventricular ectopy or NSVT can lead to medical evaluation.
Electrocardiographic recordings and exercise testing frequently assist in the classification of IVT and should be routinely performed. These are discussed in the next section and more extensively in Chapters 53 and 70.
Electrocardiographic Features of Idiopathic Ventricular Tachyarrhythmias
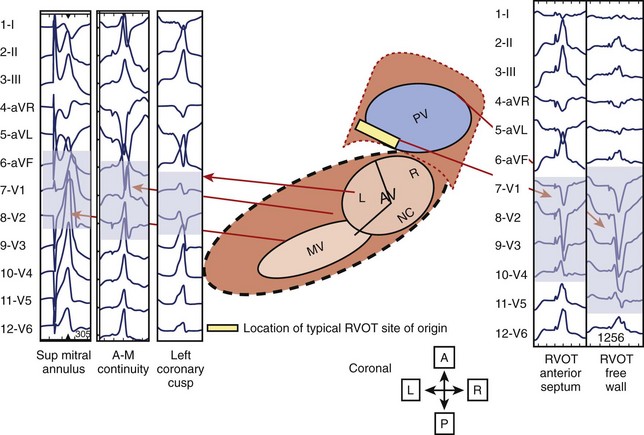
Analysis of the surface ECG morphology is an essential aspect of localization of the origin of IVT. The ECG during sinus rhythm is often normal, although a right bundle branch block (RBBB) morphology has been described with outflow tract VT in 10% of patients.20 The primary forms of IVT include arrhythmias arising from the RVOT or LVOT, the aortomitral continuity, and intrafascicular re-entry. These four types of IVTs can be distinguished by characteristic ECG patterns that reflect the origin or mechanism of the arrhythmias. The electrocardiographic features of the RVOT and LVOT reflect the anatomic proximity of these structures. As shown in Figure 45-1, the RVOT is anterior to the right and left aortic cusps. Accurate ECG lead placement is essential to distinguish electrocardiographic features from these areas. Because of the proximity of these anatomic sites, small changes in lead position may have a substantial impact on the ECG morphology of arrhythmias arising from this region. In addition, the close anatomic relationships of the outflow tracts and the great arteries and coronary vasculature make exact localization potentially problematic. Other factors include the exact position of the aortic cusps relative to the RVOT, the axis of the heart, and the precise connections of muscle fibers connecting the tissue above the aortic cusps to the left ventricle.
Right Ventricular Outflow Tract
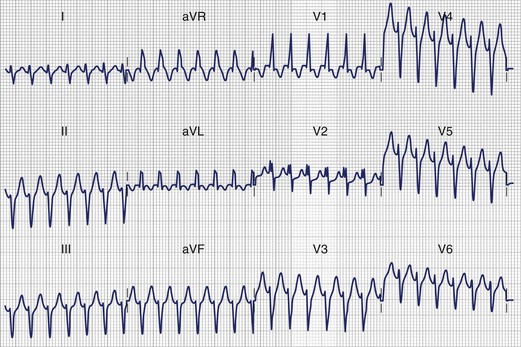
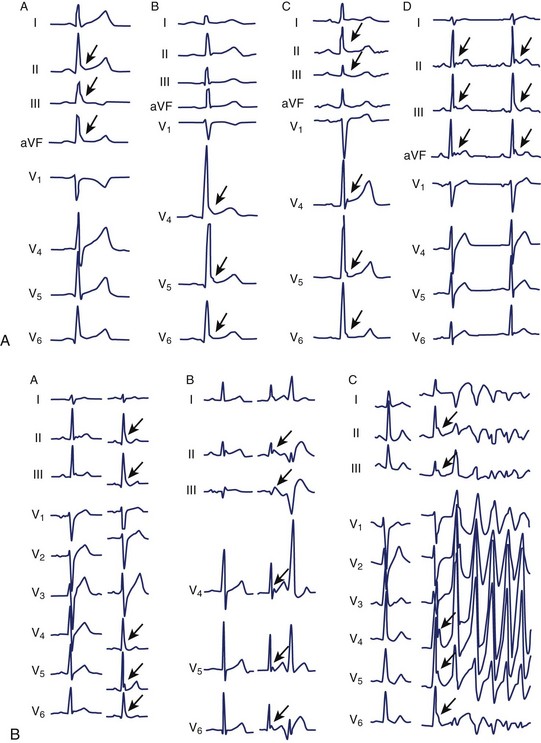
The clinical features and the general ECG characteristics of VT arising from the RVOT were described by Buxton in 1983.19 The ECG exhibits a morphology resembling left bundle branch block (LBBB) with an inferior axis. These arrhythmias are associated with exercise, and in many patients the arrhythmias are nonsustained and repetitive. Others may simply have frequent monomorphic ventricular ectopy. Figure 45-2 was recorded from a patient with repetitive VT arising from the RVOT. Jadonath determined the usefulness of the 12-lead ECG in localizing the origin of RVOT tachycardia in patients who underwent ablation of arrhythmias.39 They divided the RVOT into nine segments and assessed the QRS morphology recorded by pacing at each segment. A QS pattern in lead aVR and monophasic R waves in leads II, II, and aVF were noted in all patients at all nine sites. Pacing at anterior sites produced either a dominant Q wave or qR pattern in lead I, but a monophasic R or Rs complex was never observed. Conversely, a dominant R wave was observed in lead I during pacing from posterior sites. The QRS exhibited a QS pattern in lead aVL during pacing from anterior sites, but the R wave became progressively larger in lead aVL with more posterior sites. Early R-wave transition in the precordial leads was likely to be observed during pacing from the posterosuperior aspect of the septum. Similar results were reported by Yoshida and colleagues.40 Coggins observed that VT arising from the septal side of the RVOT had a negative QRS complex in aVL, whereas VT arising from the lateral aspect of the RVOT was associated with a positive QRS in aVL.21
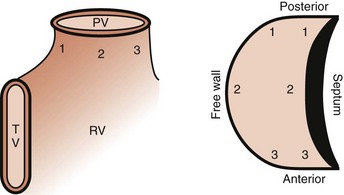
Electroanatomic mapping shows border regions as a low-voltage region in the RVOT at the breakout point or origin of the VT.41 An analysis by Dixit categorized RVOT arrhythmias as arising anterior and leftward on the septal side, as opposed to posterior and more rightward, and distinguished the electrocardiographic features of these arrhythmias from those arising on the free wall. Figure 45-3 displays the scheme used in this analysis.42
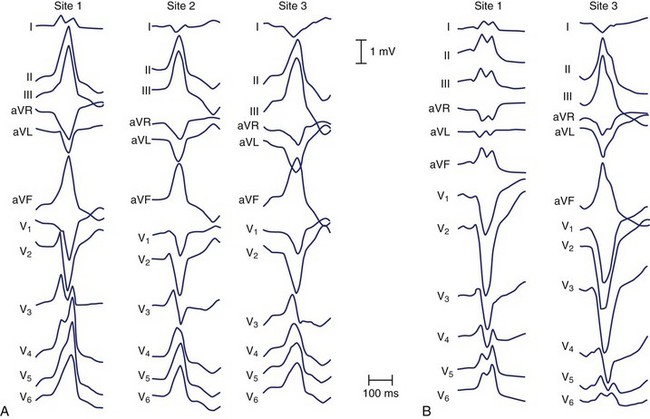
The electrocardiographic features corresponding to these sites are shown in Figure 45-4. As noted by other authors, the amplitude of the R wave in limb lead I depends on the origin being anterior or posterior. They observed that arrhythmias arising from the free wall of the RVOT show more delayed R-wave transition in the precordial leads and notching in the inferior leads.
Left Ventricular Outflow Tract
Ventricular arrhythmias arising from the LVOT are distinguished from RVOT arrhythmias by several differences in the QRS morphology. Earliest ventricular activation may arise in the sinus of Valsalva, below the aortic valve, or on the epicardium. Figure 45-5 was recorded from a patient with repetitive VT whose arrhythmia origin was just below the anterolateral aspect of the aortic valve. Callans et al described four patients with monomorphic VT mapped to the LVOT.43 Two patients, whose arrhythmias originated above the mediosuperior aspect of the mitral annulus, had VT that exhibited a right bundle, inferior-axis VT with a dominant R wave in lead V1. The ECGs of these patients resembled those recorded from the five patients studied by Lamberti; in these patients, intracardiac echocardiography had been used to demonstrate that in all of them, VT arose close to the left coronary cusp in the area of atriomitral continuity.44 The other two patients described by Callans had VT that arose from the basal aspect of the superior left ventricular septum. The ECGs recorded from these patients had a left bundle, inferior-axis QRS mapped to the LVOT. The ECGs recorded from these patients were distinguished from the ECGs of those with arrhythmias arising from the RVOT by an earlier precordial R-wave transition (median lead V3 vs. lead V5), more rightward axes, taller R waves inferiorly, and small R waves in lead V1. Absence of an R wave in lead V1 and late precordial transition suggested an origin in the RVOT. These results are similar to those reported by Kamakura and Krebs, who found that if the R/S ratio in lead V3 was 1 or more, the origin was likely to be in the LVOT.45,46 Kanagaratnam described a variant of LVOT VT in 12 patients whose arrhythmias arose from the aortic sinus of Valsalva.47 Two characteristic QRS morphologies were observed. ECGs recorded from all patients had (1) a left-bundle, inferior-axis, tall monophasic R waves inferiorly and (2) early precordial lead transition with rS or RS in lead V1 and Rs pattern in leads V2 or V3. Those in whom the tachycardia was ablated from the noncoronary sinus were distinguished by a notched R in lead I.
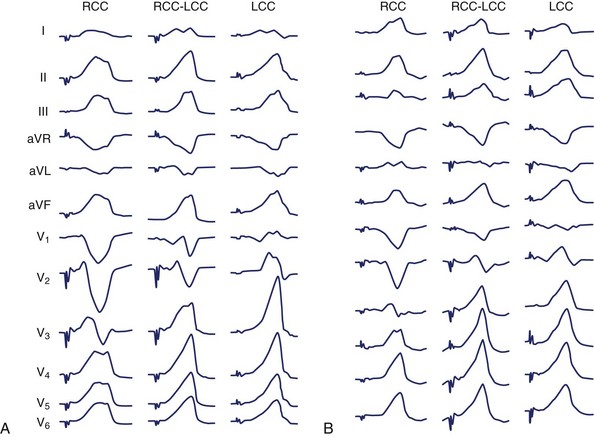
The use of intracardiac echocardiography and radiographic imaging has helped refine the origin of LVOT arrhythmias and their relationship to the coronary cusps and aortomitral continuity.48,49 Pacemapping from the aortic cusps can be difficult because of catheter stability and high thresholds; Figure 45-6 shows characteristic ECG patterns associated with this region. Recordings from the right coronary cusp typically exhibit notching in the downstroke of lead V1 and an R in limb lead I. A W pattern in lead V1 is observed near the commissure of the right and left coronary cusps. Arrhythmias arising from the left coronary cusp exhibit a qrS pattern and either a QS or rS in limb lead I. Each of these locations is associated with a prominent R wave in the inferior leads and an R-wave transition in leads V2 or V3.
Ventricular arrhythmias arising from the aortomitral continuity are characterized by a qR complex in lead V1 and an RS or rs complex in limb lead I. The relationship between aortic and mitral valves is shown in Figure 45-7. The R wave in lead V1 becomes more monophasic as the origin moves more leftward across the superior aspect of the mitral annulus. Figure 45-8 illustrates the differences among the paced or spontaneous beats arising from the RVOT, the left coronary cusp, the aortomitral continuity, and the superior aspect of the mitral annulus.
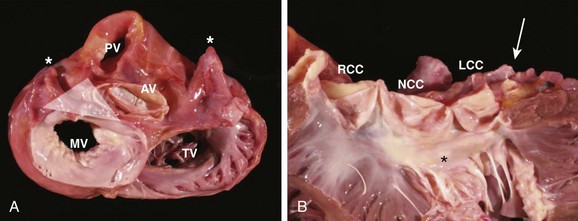
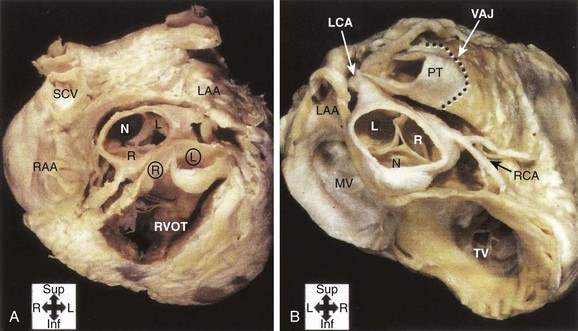
FIGURE 45-7 Gross anatomy of the heart. A, View from the atria toward the valvular apparatus showing the anatomic relationship between the aortic valve (AV), mitral valve (MV), pulmonary valve (PV), and tricuspid valve (TV). Asterisks indicate the left and the right atrial appendages, respectively. The white triangle represents the area of the aortomitral continuity corresponding to Figure 45-2. B, View of the anterior leaflet of the mitral valve (asterisk) after opening of the left ventricle and the aortic valve, showing the close relationship of the aortic valve and the anterior leaflet of the mitral valve. The arrow indicates the left main artery. LCC, Left coronary cusp; NCC, noncoronary cusp; RCC, right coronary cusp.
(From Steven D: Ventricular tachycardia arising from the aortomitral continuity in structural heart disease: Characteristics and therapeutic considerations for an anatomically challenging area of origin, Circ Arrhythm Electrophysiol 2:660–666, 2009.)
Idiopathic Verapamil-Sensitive Left Ventricular Tachycardia
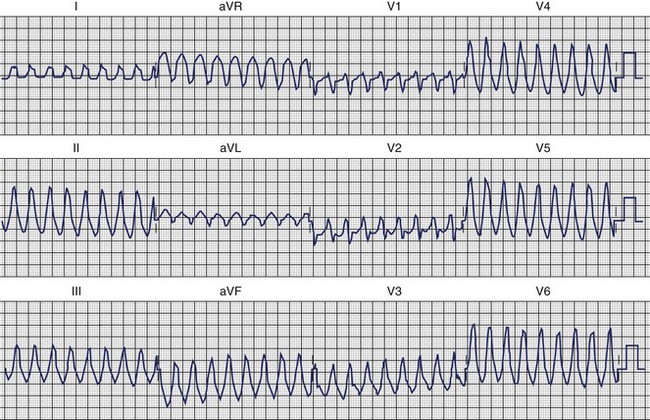
Idiopathic verapamil-sensitive VT that has a QRS pattern of RBBB and left-axis deviation was described by Lin in 1983.50 This is typically seen in adolescents and young adults up to the age of 40 years, with a male preponderence.23 ECG morphologies can include a small r–deep S configuration in some precordial leads. Subsequent studies, which focused on the electrophysiological basis of the arrhythmia and ablation techniques, demonstrated that the QRS axis is usually leftward and superior, although it could be indeterminate or rightward. Electrophysiological data demonstrate that re-entry either is intrafascicular or involves abnormal Purkinje tissue with decremental conduction properties and is sensitive to verapamil.51–58 The exact QRS morphology depends on which branches of the fascicles provide the exit points, and unsuccessful attempts to ablate the arrhythmia have shown changes in QRS morphology that suggest the potential for multiple exit points. Re-entry in and exit points from the left bundle branches predominate, with more than 80% involving the left posterior fascicle as an exit. Anterior left fascicular exit has also been observed. Slowly rising “ rounded” ventricular potentials have been reported during VT, suggesting verapamil-sensitive regions. Sharp potentials have also been seen at exit points, possibly from Purkinje network cells.23 These have been targeted for catheter ablation. Figure 45-9 demonstrates idiopathic left-sided VT caused by fascicular re-entry that was ablated by applying radiofrequency (RF) energy at the site of a presystolic “fascicular” potential on the inferior aspect of the septum approximately 2 to 3 cm from the apex.
Polymorphic Ventricular Tachycardia and Idiopathic Ventricular Fibrillation
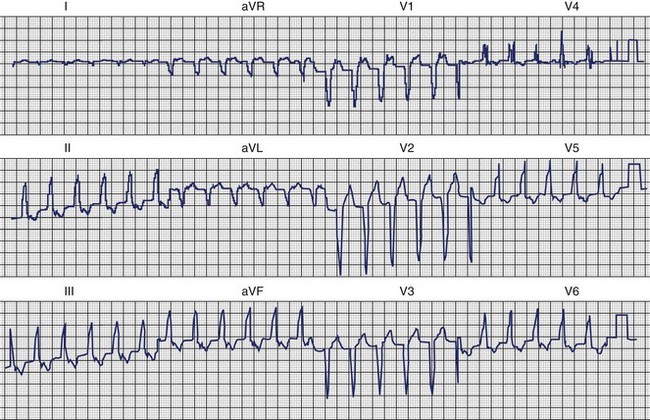
Polymorphic VT (PMVT) has been observed in clinical conditions in patients without structural heart disease. Genetically based disorders have been associated with PMVT and are detailed in Chapters 62 to 65. Initially described by Belhassen, IVF has been the focus of increased investigation in the last decade.26,59 Abnormalities in the early phase of repolarization have been reported in survivors of IVF. Haissaguerre et al observed notching of the S wave, QRS slurring, and ST-segment elevation with T-wave inversion, especially in inferior and lateral leads (Figure 45-10). Early repolarization was more frequent in patients with IVF than in control subjects (31% vs. 5%; P < .001). Among these patients, those with early repolarization were more likely to be male and have a history of syncope or sudden cardiac arrest during sleep than those without early repolarization. Ventricular ectopics that initiated ventricular arrhythmias could be mapped to sites concordant with the localization of repolarization abnormalities. The incidence of recurrent VF was greater in those with a repolarization abnormality than in those without such an abnormality (hazard ratio [HR], 2.1; P = .008). Triggering premature beats arising from the Purkinje network have been incriminated as the initiators of PMVT or IVF in some patients. In IVF, these triggers may arise in the right ventricle (RV) or the left ventricle (LV), with the former having longer QRS durations than the latter and prominent S waves rather than R waves in lead V1.23 Triggering premature beats are often prominent after resuscitation from an episode of IVF (see Figure 45-10). J-point elevation, especially in inferior leads, has been associated with increased risk.60 QRS slurring may also be associated with increased risk.61
Clinical Electrophysiology
Remarkable advances have been made in understanding congenital LQTS and Brugada syndrome. These disorders are described in detail elsewhere in this text, but because they may provide insights into other IVT syndromes, certain aspects are discussed here. The mechanism of VT (usually torsades de pointes) in patients with LQTS is not known with certainty. However, one theory is that the mechanism is re-entry resulting from increased dispersion of repolarization caused by differential prolongation of action potential duration (APD) in myocardial cells with particular lengthening in M cells.62,63 Re-entry may be triggered by EADs caused by abnormal calcium kinetics that result from delayed repolarization. This could explain why different mutations producing action potential prolongation by distinct mechanisms result in similar VTs. However, differences exist between the circumstances under which arrhythmias arise. In a model of LQTS, sympathetic activity had different effects in the three subtypes. In LQT1, sympathetic activity was necessary for increased dispersion of repolarization, and torsades de pointes did not develop without it. In LQT2, dispersion of repolarization and tendency for torsades de pointes varied with the level of sympathetic activity, whereas in LQT3, enhanced sympathetic activity reduced dispersion of repolarization and was protective against torsades de pointes.63
The mechanism of VT in Brugada syndrome has also been hypothesized to be re-entry as a consequence of increased dispersion of repolarization. In this case, the increased dispersion of repolarization is thought to result from differential action potential abbreviation, with the greatest effect occurring in epicardial cells.64 Re-entry could be triggered by phase 2 re-entry. Several mutations may produce the Brugada syndrome phenotype. This may account for the differences in clinical characteristics seen in some nocturnal death disorders encountered in Southeast Asia. Little is known about the short-coupled variant of torsades de pointes and catecholaminergic polymorphic VT (CPVT) syndrome.65–69 On the basis of the appearance of the tachyarrhythmias, re-entry caused by dispersion of repolarization is a possible mechanism. However, the mechanism by which this occurs and the factors that produce it are unknown.
The usefulness of clinical EPS in IVT syndromes associated with PMVT or VF is limited. First, ventricular tachyarrhythmias cannot be induced in all IVT syndromes, notably LQTS. Second, when induced, it is difficult to confirm that the induced VT is the same as the spontaneous arrhythmia. Third, the induced VTs are difficult to study in a systematic fashion because they are rapid, polymorphic, poorly tolerated, and often require countershock. In contrast to LQTS, VT or VF is often inducible in patients with Brugada syndrome. In addition, a provocative test is available. The characteristic ECG pattern of RBBB and ST elevation may not be present at rest, but it may be provoked by administration of ajmaline, flecainide, pilscainide, or procainamide. VT cannot usually be induced in patients with the short-coupled variant of torsades de pointes. Isoproterenol infusion can be used to provoke VT in patients with CPVT syndrome. Adenosine can suppress IVT in patients with RVOT VT.68 IVF has been studied in the electrophysiology laboratory and is described in detail in Chapter 95. Triggering premature ventricular beats can have a uniform morphology, and localization by mapping and catheter ablation may be effective in selected patients.
The clinical electrophysiology of the monomorphic forms of IVT differ considerably from the forms associated with VF and PMVT. The most common form of monomorphic IVT is RVOT VT. It has been shown to result from cyclic adenosine monophosphate–mediated triggered activity, which depends on DADs.9 The underlying pathophysiological basis for this abnormality remains unclear, particularly why it arises from localized areas of the heart. A genetic abnormality for a variant form of RVOT VT has been reported, but a familial distribution is not usually observed.69 EPS has been very useful in the investigation of this syndrome. RVOT VT is often induced by rapid pacing or isoproterenol infusion. In some cases, infusions of aminophylline, atropine, epinephrine, or very high-dose isoproterenol (up to 14 µg/min) may be required.70 RVOT VT usually presents with a single QRS morphology, typically a LBBB pattern and inferior axis consistent with an RVOT origin. It cannot be entrained. It usually terminates in response to adenosine, verapamil, β-blockers, or vagal maneuvers. Multiple configurations in individual patients have been reported.71
Mapping of RVOT VT can localize this tachycardia to virtually all regions of the outflow tract. Figure 45-10 is a three-dimensional map of the propagation of a single VT wavefront. Note that the site of origin in the outflow tract is focal in nature and well defined by the mapping technique. Noncontact mapping can define the focus in a single cycle, but sequential mapping with magnetic electroanatomic techniques may be used in stable, sustained VT. Tachycardias arising from the LVOT and epicardial sites have many features in common with RVOT VT, including a response to adenosine,42,72 which suggests a common mechanism and justifies the designation “adenosine-sensitive VTs.” Sites of origin can often be predicted by careful examination of the configuration in the 12-lead ECG.42,44
Left ventricular fascicular tachycardia is another important monomorphic IVT syndrome. It is less common than RVOT VT, although it is more prevalent in series arising from Asian countries than from Western countries. This arrhythmia is believed to result from re-entry. Although previously thought to result from micro–re-entry, recent investigations suggest a macro–re-entrant circuit.73–79 Characteristic features include induction and termination with programmed stimulation, a relatively narrow QRS, the presence of a Purkinje system potential, and entrainment. Left ventricular fascicular tachycardia is more commonly associated with the left posterior fascicle and has an RBBB–left axis configuration. The RBBB–right axis configuration of this VT is associated with the anterior fascicle.80–82 Termination with verapamil is usual. Rarely adenosine may terminate this VT if catecholamines were required for its induction.83 Incessant forms of left ventricular fascicular VT can result in a tachycardia-induced cardiomyopathy.84
Adrenergic monomorphic VT can present with a left or right bundle branch QRS pattern. Clinical and electrophysiological characteristics of this VT are poorly defined. This form of VT has electrophysiological properties that would be expected with enhanced automaticity as its basis. It is often initiated by catecholamine infusion and exercise, but it cannot be induced, entrained, or terminated by programmed stimulation. Verapamil has no effect on this form of VT, whereas adenosine has been reported to result in transient suppression.82,83 It is terminated and suppressed by β-blockers.
Monomorphic IVT with both LBBB and RBBB morphologies is observed in infants and children.84 These arrhythmias may be incessant, which can result in a tachycardia-induced cardiomyopathy. More often, they have a good prognosis and may also resolve spontaneously. The extent to which these arrhythmias have similar mechanisms to RVOT VT, left ventricular fascicular VT, and adrenergic monomorphic VT is uncertain. Other forms of monomorphic VT may be encountered in patients without significant structural heart disease in clinical practice. Often, these cannot be classified as one of the common forms detailed earlier.
Principles of Practice
Two classes of conditions associated with VT must be excluded before a presumptive diagnosis of IVT is made (Box 45-2). Extrinsic proarrhythmic factors (e.g., conditions that cause electrolyte disturbances or drugs that prolong repolarization) may be associated with VT in the presence of normal cardiac structure and function and are discussed elsewhere in this text. In addition, VT may exist with undetected cardiac disease and can mimic IVT syndromes. A comprehensive evaluation that attempts to exclude all potential conditions that could contribute to arrhythmogenesis requires invasive procedures for definitive diagnosis. This is essential in the patient who presents with cardiac arrest or a hemodynamically compromising VT, but the extent to which it should be applied to patients with less symptomatic presentations or suspected IVT must be judged individually.
Box 45-2 Disorders that Mimic Nonstructural Ventricular Tachyarrhythmia Syndromes
The third function of EPS in patients with IVT is therapeutic. EPS has been reported to be useful for guiding antiarrhythmic drug therapy in patients with IVF, although this approach is now not widely recommended. RF catheter ablation is widely used in the monomorphic IVT syndromes after mapping of the IVT origin at EPS. Figure 45-10 is a noncontact three-dimensional map of catheter ablation of the RVOT focus. Note that individual lesions are marked at the site of application relative to the focal origin and propagation of the arrhythmia. Catheter ablation may be the therapy of choice for many patients with IVT syndromes.
Evidence-Based Therapy
No clinical trials have addressed therapeutic options in patients with IVT and IVF syndromes. Retrospective and prospective registries have produced data that are used to guide therapy. The reliability of the results and their applicability to future patients are uncertain because databases are small and biases created by the referral process may exist. Nevertheless, they provide the best available evidence. Consensus statements from professional societies have leaned heavily on these resources.23,25,35,59 The largest registry exists for patients with LQTS. Results from this database support the use of β-blockers as a mainstay of therapy but report a persistently high risk despite β-blocker therapy in patients with a history of cardiac arrest or syncope.85 A report from an international registry suggests that medical therapy with β-blockers, sodium channel blockers, and amiodarone are ineffective for IVF. This contradicts a prior study from Belhassen et al, which reported success in EPS-guided treatment with certain sodium channel blockers, especially quinidine.86
Management of Idiopathic Ventricular Tachycardia and Ventricular Fibrillation
Prevention of SCD is the first priority in patients with IVT and IVF syndromes. Affected individuals are often young and have normal cardiovascular function. They have few competing risks, and long-term survival may depend on the risk of arrhythmic death and complications resulting from therapy. Unfortunately, precise arrhythmic risk assessment for IVT syndromes is difficult. First, data for reliable outcome prediction are scarce. Second, heterogeneity exists within the IVT population. Arrhythmic risk may be affected by a particular genotype and by variations among a particular genotype. Penetrance for a given genotype has been found to vary unpredictably.87 Inadequate diagnoses in families pose another challenge that is often encountered during the evaluation of living members of a family in which multiple SCDs have occurred. Affected living family members are assumed to carry the “malignant” genotype, and prophylactic therapy is often considered. However, it is often not possible to determine the genotype if it is an ambiguous phenotype and because genetic testing is often not available. In such instances, the individual is either at very high risk or very low risk. Fortunately, risk assessment should continue to improve as IVT registries collect more data, diagnostic methods improve, and genetic testing becomes a widespread clinical tool.
The potential for SCD, uncertainty of arrhythmic risk, and limitations of existing therapeutic options complicate the management of IVT and IVF syndromes. It is widely assumed that the ICD is an acceptable therapy for IVF and malignant forms of IVT. Results from clinical trials support the use of ICDs over amiodarone for reduction of all-cause mortality in survivors of cardiac arrest and patients with certain forms of VT.88 However, available clinical trial evidence has been largely obtained from cardiac arrest or VT populations with heart disease and multiple competing mortality risks. These trials may be poor guides to selection of therapy in patients with IVT syndromes. Long-term data on ICD efficacy are now available from some registries and observational studies.89–92 These data suggest that long-term prevention of SCD is achieved with ICD therapy, with an acceptable complication rate. Recently, catheter ablation of triggering VPBs has been performed in patients with IVF and the Brugada syndrome at specialized centers. Evidence to support reduction in arrhythmic event rates exists, but no large clinical study or comparative evaluation of catheter ablation versus the ICD is available. As such, this therapy must be considered adjunctive to ICD implantation at this time. This is discussed further in Chapters 94 and 95.
Patients with IVT are often children or adolescents. ICDs may have a significant advantage in these patients in that they ensure therapeutic compliance. In this group of patients, compliance with medications is often difficult, especially in the absence of symptoms. Devices also offer female patients protection during pregnancy without the need for antiarrhythmic drug therapy. However, young patients face numerous replacements of the ICD generator and leads and a long period of exposure to the complications of ICD therapy. Furthermore, quality of life may be adversely affected by concern about body image, isolation and ridicule from peers, and dependence on medical services.93 ICD shocks, whether appropriate or inappropriate, may diminish quality of life still further.
Despite reasons for considerable concern with regard to the effects of ICD therapy on quality of life in the young population and the risks of long-term ICD therapy, no alternatives are likely to provide more reliable protection against arrhythmic death. ICDs are therefore recommended if the risk of arrhythmic death is high. Survivors of cardiac arrest or rapid ventricular tachyarrhythmias are usually assumed to be at high risk. This fact is borne out by results from the Unexplained Cardiac Arrest Registry of Europe (UCARE), which estimated the rate of recurrence of cardiac arrest or syncope in patients with idiopathic VF to be about 30%.25
LQTS may be a prototype for IVT syndromes in that it demonstrates how progress improves care but also creates new areas of uncertainty. Only approximately 70% of LQTS carriers have unequivocal QT prolongation, and approximately 12% have a normal Q-T interval. In addition, SCD is the first manifestation of the syndrome in at least 10% of affected persons. Several genetic defects and sporadic mutations that cause this disorder are probably still undiscovered. Moreover, the types of provocative factors, the risk of sustained VT, and the response to therapy vary with the specific genetic defect. Despite the effectiveness of the therapy itself, failure of medical therapy may result from several sources, including incomplete compliance, inadequate drug dose, or exposure to proarrhythmic drugs. Patients who present with VT or syncope, those with symptoms despite β-blockers, and other individuals at high risk are candidates for ICD therapy. Left cervico-thoracic ganglionectomy and permanent pacing probably prevent SCD in some individuals, but failures have been reported. They may be useful adjuncts or alternatives in patients who are intolerant of β-blockers or in those who are not able to comply with drug therapy. Experimental therapies include administration of supplemental potassium chloride for HERG mutations, potassium channel openers for LQT1 and LQT2, and mexiletine or flecainide for LQT subtypes 3, 2, and possibly 1.92,93
In contrast to LQTS, widely accepted treatment alternatives to the ICD for preventing arrhythmic death in other IVT syndromes associated with PMVT or VF are not yet available. In Brugada syndrome, sodium channel blockers are used to provoke characteristic ECG abnormalities and may provoke VT.92 Other drugs such as β-blockers and amiodarone have not provided adequate protection. Likewise, as noted earlier, the UCARE database results indicate failure of treatment with amiodarone, β-blockers, and sodium channel blockers in patients with IVF.25 In contrast, treatment with class I antiarrhythmic drugs, mostly quinidine, guided by EPS was reported to be effective in one small series.86 It is possible that the distinctive effects of quinidine on the transient outward current were responsible for the excellent protective effect that was observed. However, this approach has not been widely embraced, although certain subgroups of LQTS and short QT syndrome have been shown to respond to type 1 drugs. Verapamil and amiodarone may inhibit VT in the short-coupled variant of torsades de pointes but have not been shown to prevent SCD.94 β-Blockers are reportedly effective in patients with CPVT, but the experience may be too limited to adopt this approach with confidence. As yet, no well-defined role exists for surgical intervention (other than left cervicothoracic sympathectomy in LQTS) for polymorphic IVT and IVF syndromes.
The risk of arrhythmic death is believed to be low in monomorphic IVT syndromes such as RVOT VT and its variants. Unless features are present to suggest a malignant potential, such as syncope or very rapid tachycardia, ICD therapy is not indicated. Instead, the goal is to relieve symptoms or prevent the detrimental effects of incessant tachycardia.59,91 If symptoms are absent or minimal and tachycardia is infrequent, no treatment may be necessary. Pharmacologic therapy with β-blockers or calcium antagonists, alone or in combination, is usually safe and often effective.59 Amiodarone, sotalol, encainide, and flecainide may be effective. Nicorandil, a potassium channel opener, has been reported to terminate and suppress adenosine-sensitive VT.93 If adequate control is obtained with well-tolerated and presumably safe pharmacologic therapy (e.g., with β-blockers or calcium channel blockers), then this therapy may be preferred in many individuals. If symptoms are intermittent, then drugs could also be used on an “as needed” basis.
Nonpharmacologic therapy should be considered for patients with disease refractory to drug therapy or who are intolerant of drug therapy, those who prefer it to a lifelong need for drugs, and those who wish to become pregnant. Many patients prefer to discontinue all medications when pregnant, a time when therapy should not be withdrawn because changes in cardiovascular function due to pregnancy could exacerbate some forms of VT or cause more severe symptoms. Catheter ablation offers the possibility of permanent correction, with a high probability of success (Figure 45-11). The techniques of mapping and ablation are described in Chapters 94 and 95. In brief, contact catheter mapping or noncontact mapping is used to identify the site of earliest ventricular activation. Presystolic potentials are usually present at this site, and repetitive firing can usually be demonstrated at this location, often after isoproterenol administration.94 RF contact catheter ablation lesions are delivered at this site and in adjoining areas. Occasionally, more than one site may be seen. Efficacy rates can exceed 80% to 90% in RVOT VT, with a 5% VT recurrence rate. Predictors of failure with catheter ablation include failure to induce and map the arrhythmia at EPS, multiple VT morphologies, a δ wave–like beginning of the QRS, and a poor match between the 12-lead ECG during VT and during pacing at the ablation site.95 Left heart catheterization is not required for most cases of RVOT VT, which minimizes the risk of complications estimated at less than 2%. Cardiac perforation and tamponade have been reported, and ablation of foci near the bundle of His region can result in heart block. High-power irrigated ablation could injure the posterior regions of the RVOT artery trunk and the coronary ostia and pulmonary arterial trunk. Risks may also be greater if lesions are delivered in the LVOT VT or in the epicardial regions. Thromboembolism, coronary arterial injury, and aortic valve injury may occur during LVOT VT ablation. Heart block, aortic valve injury, and coronary artery injury are potential risks with ablation of aortic cusp VT and mitral annulus VT. Specialized technical approaches and multi-modality imaging are often needed to avoid complications with these arrhythmias. See Chapter 94 for more detailed technical considerations.
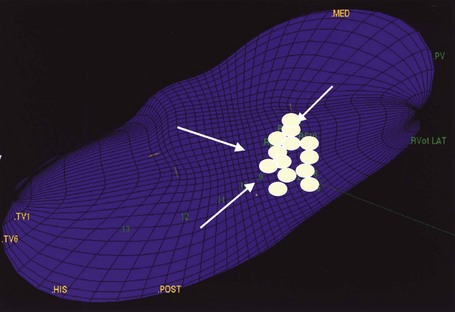
FIGURE 45-11 Catheter ablation guided by three-dimensional noncontact mapping in the same patient with ventricular tachycardia arising from the right ventricular outflow tract shown in Figure 45-4. Ablation lesions (circular markers) are shown individually at the focal origin and in the surrounding region. Successful ablation was achieved.
Idiopathic fascicular VT is also believed to have a benign long-term outlook, although the extent of experience is less than that for RVOT VT.59 Treatment is indicated for the prevention or control of symptoms and for protection against the potential deleterious effects of incessant tachycardia. Calcium antagonists have been shown to be effective in many patients, as have β-blockers. Drug therapy or catheter ablation are both reasonable approaches. Probably, many types of monomorphic IVT do not have characteristics of left ventricular fascicular VT or RVOT VT. The prognosis of such arrhythmias cannot be assumed to be as benign as these IVTs. Clinical judgment must be used individually to assess the risk-to-benefit ratio of ICD implantation, catheter ablation, or drug trials in these patients.
Key References
Aiba T, Suyama K, Aihara N, et al. The role of Purkinje and pre-Purkinje potentials in the reentrant circuit of verapamil-sensitive idiopathic LV tachycardia. Pacing Clin Electrophysiol. 2001;24:333-344.
Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias. Europace. 2009;11:771-817.
Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7(4):549-558.
Buxton AE, Waxman HL, Marshlinski FE, et al. Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation. 1983;68:917-927.
Calkins H. Role of invasive electrophysiologic testing in the evaluation and management of right ventricular outflow tract tachycardias. Card Electrophysiol Rev. 2000;4:71-75.
Cappato R, Furlanello F, Giovinazzo V, et al. J wave, QRS slurring, and ST elevation in athletes with cardiac arrest in the absence of heart disease: Marker of risk or innocent bystander? Circ Arrhythm Electrophysiol. 2010;3(4):305-311.
Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649-654.
Coggins DL, Lee RJ, Sweeney J, et al. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994;23:1333-1341.
Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: Distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2008;14:1-7.
Garratt CJ, Elliott P, Behr E, et al. Heart Rhythm UK position statement on clinical indications for implantable cardioverter defibrillators in adult patients with familial sudden cardiac death syndromes. Europace. 2010;12(8):1156-1175.
Junttila J, Sager SJ, Freiser M, McGonagle S, Castellanos A, Myerburg RJ. Inferolateral early repolarization in athletes. J Intervent Card Electrophysiol. 2011;31(1):33-38.
Kanagaratnam L, Tomassoni G, Schweiker R, et al. Ventricular tachycardias arising from the aortic sinus of Valsalva: An under-recognized variant of left outflow tract ventricular tachycardia. J Am Coll Cardiol. 2001;37:1408-1414.
Lerman BB, Stein KM, Markowitz SM, et al. Recent advances in right ventricular outflow tract tachycardia. Card Electrophysiol Rev. 1999;3:210-214.
Markowitz SM, Litvak BL, Ramirez de Arellano EA, et al. Adenosine-sensitive ventricular tachycardia: Right ventricular abnormalities delineated by magnetic resonance imaging. Circulation. 1997;96:1192-1200.
Rosso R, Kogan E, Belhassen B, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol. 2008;52(15):1231-1238.
Sacher F, Probst V, Iesaka Y, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: A multicenter study. Circulation. 2006;114(22):2317-2324.
Toivonen L, Nieminen M. Persistent ventricular tachycardia resulting in left ventricular dilatation treated with verapamil. Int J Cardiol. 1986;13:361-365.
Tandri H, Bluemke DA, Ferrari VA, et al. Findings on magnetic resonance imaging of idiopathic right ventricular outflow tachycardia. Am J Cardiol. 2004;94:1441-1445.
Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361(26):2529-2537.
Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 48, 2006. e247–e234
1 Vakil RJ. Repetitive paroxysmal ventricular tachycardia with fusion beats. Br Heart J. 1957;19:293-295.
2 Thind US, Blakemore WS, Zinsser HF. Ventricular aneurysmectomy for the treatment of recurrent ventricular tachyarrhythmia. Am J Cardiol. 1971;27:690-694.
3 Nitter-Hauge S, Storstein O. Surgical treatment of recurrent ventricular tachycardia. Br Heart J. 1973;35:1132-1135.
4 Rally CR, Walters MB. Paroxysmal ventricular tachycardia without evident heart disease. J Can Med Assoc. 1962;86:268-273.
5 Hair TEJr., Eagen JT, Orgain ES. Paroxysmal ventricular tachycardia in the absence of demonstrable heart disease. Am J Cardiol. 1962;9:209-214.
6 Bisteni A, Sodi-Pallares D, Medrano GA, et al. A new approach for the recognition of ventricular premature beats. Am J Cardiol. 1960;5:358-369.
7 Rosenbaum MB. Classification of ventricular extrasystoles according to form. J Electrocardiol. 1969;2:289-298.
8 Pietras RJ, Mautner R, Denes P, et al. Chronic recurrent right and left ventricular tachycardia: Comparison of clinical hemodynamic and angiographic findings. Am J Cardiol. 1977;40:32-37.
9 Lerman BB, Stein KM, Markowitz SM, et al. Recent advances in right ventricular outflow tract tachycardia. Card Electrophysiol Rev. 1999;3:210-214.
10 Carlson MD, White RD, Trohman RG, et al. Right ventricular outflow tract ventricular tachycardia: Detection of previously unrecognized anatomic abnormalities using cine magnetic resonance imaging. J Am Coll Cardiol. 1994;24:720-727.
11 Markowitz SM, Litvak BL, Ramirez de Arellano EA, et al. Adenosine-sensitive ventricular tachycardia: Right ventricular abnormalities delineated by magnetic resonance imaging. Circulation. 1997;96:1192-1200.
12 Tandri H, Bluemke DA, Ferrari VA, et al. Findings on magnetic resonance imaging of idiopathic right ventricular outflow tachycardia. Am J Cardiol. 2004;94:1441-1445.
13 Globits S, Kreiner G, Frank H, et al. Significance of morphological abnormalities detected by MRI in patients undergoing successful ablation of right ventricular outflow tract tachycardia. Circulation. 1997;96:2633-2640.
14 Proclemer A, Basadonna PT, Slavich GA, Miani D, Fresco C, Fioretti PM. Cardiac magnetic resonance imaging findings in patients with right ventricular outflow tract premature contractions. Eur Heart J. 1997;18:2002-2010.
15 Sinha S, Calkins H. Idiopathic right ventricular outflow tract tachycardia: No longer idiopathic? J Cardiovasc Electrophysiol. 2006;17:776.
16 Swan H, Piippo K, Viitasalo M, et al. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035-2042.
17 Chiladakis J, Vassilikos V, Maounis T, et al. Unusual features of right and left idiopathic ventricular tachycardia abolished by radiofrequency catheter ablation. Pacing Clin Electrophysiol. 1998;21:1831-1834.
18 Chapman JH, Schrank JP, Crampton RS. Idiopathic ventricular tachycardia: An intracardiac electrical hemodynamic and angiographic assessment of six patients. Am J Med. 1975;59:470-480.
19 Buxton AE, Waxman HL, Marshlinski FE, et al. Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation. 1983;68:917-927.
20 Movsowitz C, Schwartzman D, Callans DJ, et al. Idiopathic right ventricular outflow tract tachycardia: Narrowing the anatomic location for successful ablation. Am Heart J. 1996;131:930-936.
21 Coggins DL, Lee RJ, Sweeney J, et al. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994;23:1333-1341.
22 Hachiya H, Aonuma K, Yamauchi Y, et al. Electrocardiographic characteristics of left ventricular outflow tract tachycardia. Pacing Clin Electrophysiol. 2000;23(Pt 2):1930-1934.
23 Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias. Europace. 2009;11:771-817.
24 Yamada T, Yoshida N, Murakami Y, et al. Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of Valsalva in the aorta: The activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm. 2008;5:184-192.
25 Survivors of out-of-hospital cardiac arrest with apparently normal heart. Need for definition and standardized clinical evaluation. Consensus Statement of the Joint Steering Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United States. Circulation. 1997;95:265-272.
26 Belhassen B, Viskin S. Idiopathic ventricular tachycardia and fibrillation. J Cardiovasc Electrophysiol. 1993;4:356-368.
27 Meissner MD, Lehmann MH, Steinman RT, et al. Ventricular fibrillation in patients without significant structural heart disease: A multicenter experience with implantable cardioverter-defibrillator therapy. J Am Coll Cardiol. 1993;21:1406-1412.
28 Haïssaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
29 Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649-654.
30 Loire R, Tabib A. Unexpected sudden cardiac death: An evaluation of 1000 autopsies. Arch Mal Coeur Vaiss. 1996;89:13-18.
31 Bacci M, Giusti G. Diagnostic possibilities and limitations of the necropsy examination on cadavers exhumed because of sudden death. Pathologica. 1996;88:25-28.
32 Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361(26):2529-2537.
33 Junttila J, Sager SJ, Freiser M, McGonagle S, Castellanos A, Myerburg RJ. Inferolateral early repolarization in athletes. J Intervent Card Electrophysiol. 2011;31(1):33-38.
34 Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7(4):549-558.
35 Pfammatter JP, Paul T. Idiopathic ventricular tachycardia in infancy and childhood: A multicenter study on clinical profile and outcome. Working Group on Dysrhythmias and Electrophysiology of the Association for European Pediatric Cardiology. J Am Coll Cardiol. 1999;33:2067-2072.
36 Nakagawa M, Takahashi N, Nobe S, et al. Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:633-638.
37 Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J. 2000;139:1009-1013.
38 Joshi S, Wilber DJ. Ablation of idiopathic right ventricular outflow tract tachycardia: Current perspectives. J Cardiovasc Electrophysiol. 2005;16(Suppl 1):S52-S58.
39 Jadonath RL, Schwartzman DS, Preminger MW, et al. Utility of the 12-lead electrocardiogram in localizing the origin of right ventricular outflow tract tachycardia. Am Heart J. 1995;130:1107-1113.
40 Yoshida Y, Hirai M, Murakami Y, et al. Localization of precise origin of idiopathic ventricular tachycardia from the right ventricular outflow tract by a 12-lead ECG: A study of pace mapping using a multielectrode “basket” catheter. Pacing Clin Electrophysiol. 1999;22:1760-1768.
41 Furushima H, Chinushi M, Iijima K, et al. Relationship between electroanatomical voltage mapping characteristics and breakout site of ventricular activation in idiopathic ventricular tachyarrhythmia originating from the right ventricular outflow tract septum. J Interv Card Electrophysiol. 2011. In press
42 Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: Distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2008;14:1-7.
43 Callans DJ, Volker M, Schwartzman D, et al. Repetitive monomorphic tachycardia from the left ventricular outflow tract: Electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol. 1997;29:1023-1027.
44 Lamberti F, Calo L, Pandozi C, et al. Radiofrequency catheter ablation of idiopathic left ventricular outflow tract tachycardia: Utility of intracardiac echocardiography. J Cardiovasc Electrophysiol. 2001;12:529-535.
45 Kamakura S, Shimizu W, Matsuo K, et al. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation. 1998;98:1525-1533.
46 Krebs ME, Krause PC, Engelstein ED, et al. Ventricular tachycardias mimicking those arising from the right ventricular outflow tract. J Cardiovasc Electrophysiol. 2000;11:45-51.
47 Kanagaratnam L, Tomassoni G, Schweiker R, et al. Ventricular tachycardias arising from the aortic sinus of Valsalva: An under-recognized variant of left outflow tract ventricular tachycardia. J Am Coll Cardiol. 2001;37:1408-1414.
48 Ouyang F, Fotuhi P, Ho SY, et al. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol. 2002;39:500-508.
49 Bala R, Garcia FC, Hutchinson MD, et al. Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm. 2010;7:312-322.
50 Lin FC, Finley CD, Rahimtoola SH, Wu D. Idiopathic paroxysmal ventricular tachycardia with a QRS pattern of right bundle branch block and left axis deviation: A unique clinical entity with specific properties. Am J Cardiol. 1983;52:95-100.
51 Ohe T, Shimomura K, Aihara N, et al. Idiopathic sustained left ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation. 1988;77:560-568.
52 Nakagawa H, Beckma KJ, McClelland JH, et al. Radiofrequency catheter ablation of idiopathic left ventricular tachycardia guided by a Purkinje potential. Circulation. 1993;88:2607-2617.
53 Wen MS, Yeh SJ, Wang CC, et al. Radiofrequency ablation therapy in idiopathic left ventricular tachycardia with no obvious structural heart disease. Circulation. 1994;89:1690-1696.
54 Chen YJ, Chen SA, Tai CT, et al. Radiofrequency ablation of idiopathic left ventricular tachycardia with changing ECG morphology. Pacing Clin Electrophysiol. 1998;21:1668-1671.
55 Tsuchiya T, Okumura K, Honda T, et al. Significance of late diastolic potential preceding Purkinje potential in verapamil sensitive idiopathic left ventricular tachycardia. Circulation. 1999;99:2408-2413.
56 Aiba T, Suyama K, Aihara N, et al. The role of Purkinje and pre-Purkinje potentials in the reentrant circuit of verapamil-sensitive idiopathic LV tachycardia. Pacing Clin Electrophysiol. 2001;24:333-344.
57 Nogami A, Naito S, Tada H, et al. Demonstration of diastolic and presystolic Purkinje potentials as critical potentials in a macroreentry circuit of verapamil-sensitive idiopathic left ventricular tachycardia. J Am Coll Cardiol. 2000;36:811-823.
58 Miyauchi Y, Kobayashi Y, Ino T, Atarashi H. Identification of the slow conduction zone in idiopathic left ventricular tachycardia. Pacing Clin Electrophysiol. 2000;23:481-487.
59 Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 48, 2006. e247–e234
60 Rosso R, Kogan E, Belhassen B, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol. 2008;52(15):1231-1238.
61 Cappato R, Furlanello F, Giovinazzo V, et al. J wave, QRS slurring, and ST elevation in athletes with cardiac arrest in the absence of heart disease: Marker of risk or innocent bystander? Circ Arrhythm Electrophysiol. 2010;3(4):305-311.
62 Vincent GM, Timothy K, Zhang L. Congenital long QT syndrome. Card Electrophysiol Rev. 1999;1:207-209.
63 Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778-786.
64 Gussak I, Antzelevitch C, Bjerregaard P, et al. The Brugada syndrome: Clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol. 1999;33:5-15.
65 Leenhardt A, Glaser E, Burguera M, et al. Short-coupled variant of torsades de pointes: A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206-215.
66 Von Bernuth G, Bernsau U, Hoffman W, et al. Tachyarrhythmic syncope in children with structurally normal hearts with and without QT prolongation in the electrocardiogram. Eur J Pediatr. 1982;138:206-210.
67 Leenhardt A, Lucet V, Denjoy I, et al. Catecholaminergic polymorphic ventricular tachycardia in children: A 7-year follow-up of 21 patients. Circulation. 1995;91:1512-1519.
68 Wilber DJ, Baerman J, Olshansky B, Kall J, Kopp D. Adenosine-sensitive ventricular tachycardia. Clinical characteristics and response to catheter ablation. Circulation. 1993;87:126-134.
69 Lerman BB, Dong B, Stein KM, et al. Right ventricular outflow tract tachycardia due to a somatic cell mutation in G protein subunit12. J Clin Invest. 1998;101:2862-2868.
70 Calkins H. Role of invasive electrophysiologic testing in the evaluation and management of right ventricular outflow tract tachycardias. Card Electrophysiol Rev. 2000;4:71-75.
71 Lokhandwala Y, Vora A, Naik A, et al. Dual morphology of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 1999;10:1326-1334.
72 Yeh S, Wen M, Wang C, et al. Adenosine-sensitive ventricular tachycardia from the anterobasal left ventricle. J Am Coll Cardiol. 1997;30:1339-1345.
73 Stellbrink C, Diem B, Schauerte P, et al. Transcoronary venous radiofrequency catheter ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 1997;8:916-921.
74 Goy JJ, Tauxe F, Fromer M, et al. Ten-years’ follow-up of 20 patients with idiopathic ventricular tachycardia. Pacing Clin Electrophysiol. 1990;13:1142-1147.
75 Kottkamp H, Chen X, Hindricks G, et al. Radiofrequency catheter ablation of idiopathic left ventricular tachycardia: Further evidence for microreentry as the underlying mechanism. J Cardiovasc Electrophysiol. 1994;5:268-273.
76 Han J, Sung RJ. Evaluation and management of idiopathic ventricular tachyarrhythmias—update. Card Electrophysiol Rev. 2000;4:61-65.
77 Tsuchiya T, Okumura K, Honda T, et al. Significance of late diastolic potential preceding Purkinje potential in verapamil-sensitive idiopathic left ventricular tachycardia. Circulation. 1999:2408-2413.
78 Friedman PA, Beinborn DA, Schultz J, Hammil SC. Ablation of noninducible idiopathic left ventricular tachycardia using a noncontact map acquired from a premature complex with tachycardia morphology. Pacing Clin Electrophysiol. 2000;23:1311-1314.
79 Lai L, Lin J, Hwang J, Huang S. Entrance site of the slow conduction zone of verapamil-sensitive idiopathic left ventricular tachycardia: Evidence supporting macroreentry in the Purkinje system. J Cardiovasc Electrophysiol. 1998;9:184-190.
80 Aiba T, Suyama K, Matsuo K, et al. Mid-diastolic potential is related to the reentrant circuit in a patient with verapamil-sensitive idiopathic left ventricular tachycardia. J Cardiovasc Electrophysiol. 1998;9:1004-1007.
81 Lerman BB. Response of nonreentrant catecholamine-mediated ventricular tachycardia to endogenous adenosine and acetylcholine: Evidence for myocardial receptor-mediated effects. Circulation. 1993;87:382-390.
82 Nogami A, Naito S, Tada H, et al. Verapamil-sensitive left anterior fascicular ventricular tachycardia: Results of radiofrequency ablation in six patients. J Cardiovasc Electrophysiol. 1998;9:1269-1278.
83 Sung RJ, Shapiro WA, Shen EN, et al. Effects of verapamil on ventricular tachycardias possibly caused by reentry, automaticity, and triggered activity. J Clin Invest. 1983;81:688-699.
84 Toivonen L, Nieminen M. Persistent ventricular tachycardia resulting in left ventricular dilatation treated with verapamil. Int J Cardiol. 1986;13:361-365.
85 Moss AJ, Zareba W, Hall WJ, et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616-623.
86 Belhassen B, Viskin S, Fish R, et al. Effects of electrophysiologic-guided therapy with class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:1301-1312.
87 Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome—clinical impact. Circulation. 1999;99:529-533.
88 Domanski MJ, Saksena S, Epstein AE, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. J Am Coll Cardiol. 1999;34:1090-1095.
89 Sacher F, Probst V, Iesaka Y, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: A multicenter study. Circulation. 2006;114(22):2317-2324.
90 Garratt CJ, Elliott P, Behr E, et al. Heart Rhythm UK position statement on clinical indications for implantable cardioverter defibrillators in adult patients with familial sudden cardiac death syndromes. Europace. 2010;12(8):1156-1175.
91 Vardas PE, Auricchio A, Blanc JJ, et al. ESC/EHRA Guidelines for cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2007;28(18):2256-2295.
92 Brugada R, Brugada J, Antzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510-515.
93 Kobayashi Y, Miyata A, Tanno K, et al. Effects of nicorandil, a potassium channel opener, on idiopathic ventricular tachycardia. J Am Coll Cardiol. 1998;32:1377-1383.
94 Lee SH, Tai CT, Chiang CE, et al. Determinants of successful ablation of idiopathic ventricular tachycardias with left bundle branch block morphology from the right ventricular outflow tract. Pacing Clin Electrophysiol. 2002;25:1346-1351.
95 Rodriguez LM, Smeets JLRM, Timmermans C, Wellens HJJ. Predictors for successful ablation of right- and left-sided idiopathic ventricular tachycardia. Am J Cardiol. 1997;79:309-314.