CHAPTER 190 Ventricular Shunting Procedures
The development of effective shunt procedures represents a landmark accomplishment in neurosurgery. Despite a somewhat notorious failure rate that has spawned a sinister reputation within neurosurgery, the fact remains that no other frequently performed neurosurgical procedure has saved more lives in western society than the placement and revision of ventricular shunts. Although comprehensive epidemiologic studies have never been performed, it is estimated that approximately 25,000 shunts are placed each year in North America and twice as many are revised (total of 75,000 shunt operations).1–5 Shunt operations remain the most frequently performed operation by pediatric neurosurgeons, and they are the most common reason why children undergo any form of brain surgery.3,4 Even though endoscopic third ventriculostomy (ETV) has demonstrated promising and significant clinical utility, its overall role in managing hydrocephalus remains controversial; it appears fair to conclude that less than 50% of patients with hydrocephalus can be successfully managed over the long term with ETV.6–8 Accordingly, it appears that there will continue to be a significant role for ventricular shunts for the foreseeable future.
A wide range of conditions may result in hydrocephalus in adults, including subarachnoid hemorrhage, head trauma, meningitis (or other infections), and normal-pressure hydrocephalus.9,10 Hydrocephalus is more common in children (1 in 2000 births) and most often arises from intraventricular hemorrhage of prematurity, congenital anomalies of the CSF pathways, or spinal dysraphism/myelodysplasia or as a complication of childhood brain tumors, head injuries, or infections. In general, children are more absolutely dependent on their shunts and frequently have a more complicated clinical course than that of adults. This chapter reviews established principles and presents recent developments in ventricular shunt procedures.
Background Physiology and Pathophysiology
Composition of Cerebrospinal Fluid
CSF exists in a fluid continuum with the other important intracranial fluid space, which is the interstitial fluid (ISF). ISF appears to be secreted by vascular endothelial cells, and it moves freely within the perivascular spaces and along white matter tracts to interface with CSF at the ependymal surface. Approximately 10% to 30% of the total CSF volume is contributed in this fashion, which is independent of the CP. Consequently, surgical removal of the CP as a singular intervention is insufficient to control hydrocephalus.11
Shunts—Overview
A simple shunt is one in which a single ventricular catheter connects to a valve and single distal catheter. Approximately 90% of shunts are simple constructs. A complex shunt has more than one ventricular catheter and may have a highly complex layout. Complex shunts are characteristically necessary in situations involving loculated hydrocephalus or posterior fossa cystic CSF collections such as the Dandy-Walker anomaly or Dandy-Walker variant.12 Loculated hydrocephalus most often follows either intraventricular hemorrhage or intraventricular infection. In this situation, dense sheets of thickened fibrotic glia and arachnoid partition the ventricles into multiple CSF-filled cavities. Each may dilate independently unless adequately drained. Before the development of contemporary endoscopic techniques that allow fenestration of the loculated spaces, multiple ventricular catheters were necessary and repeated obstructions were frequently problematic.13
Historical Development of Effective Shunt Systems
Comprehensive reviews of the historical treatment of hydrocephalus have been published, and a detailed discussion is beyond the scope of this chapter.14,15 It is relevant, however, to place the evolution of effective ventricular shunts into a historical context because it is in this context that the critical thought processes that went into developing effective shunt systems are best appreciated.
Broadly, evolution of the understanding and treatment of hydrocephalus can be divided into three periods. The first period extends from antiquity to the end of the 18th century of the common era. This time was a period in which hydrocephalus was recognized and some early anatomic and morphologic observations were made. The earliest observations are attributed to Hippocrates, who recognized the presence of disease, recorded physical findings, and may have tried ventricular puncture as a means of treating it. Other anatomists and naturalists made independent descriptions and observations of the disease, including Galen, Vesalius, and Al-Zahrawi, the great Arab physician of the Middle Ages.15 Pachioni described arachnoid granulations in 1701 but erroneously thought that they were the site of CSF production rather than reabsorption. It was not until 1768 that hydrocephalus was described as a distinct illness by Robert Whytt.15
The second historical period in the development of effective treatment of hydrocephalus occurred between the early 19th century and the midportion of the 20th century. It was during this time that the early anatomic observations were extended and focused on the disease process and that the pathophysiologic principles of hydrocephalus were developed. These insights led to meaningful but insufficient efforts at treatment in the early 20th century. In 1825 Magendie and in 1859 Luschka described the crucial foramina in the fourth ventricle that today bear their names as eponyms and described the circulatory patterns of CSF within the brain. In 1876 the Swedish anatomist Gustaf Retzius published a two-volume encyclopedic anatomic study of the central nervous system that has been claimed to have intellectually set the stage for the explosive developments that were to occur in late 18th and early 19th century neurology and neurosurgery in Europe. The development of radioactive tracers in the early part of the 20th century made studies of the dynamic qualities of CSF circulation possible and led to further insight into hydrocephalus. A steady progression of therapeutic ideas followed that were based on the emerging and evolving ideas of CSF physiology and the pathophysiology of hydrocephalus. Quincke in 1891 first recommended serial lumbar puncture as a treatment of hydrocephalus.15 Keen recommended continuous ventricular drainage, but it was Miculicz in 1896 who first recommended diversion of CSF from the ventricles to another space. He recommended diversion to the subgaleal, subdural, and subarachnoid space, none of which showed long-term effectiveness. Payr in 1908 recommended diversion of CSF into the sagittal sinus and jugular veins with the use of autologous vein grafts. Kausch in 1908 recommended diversion of CSF to the peritoneum but used rubber tubes to accomplish this. Heile similarly advocated diversion of CSF with vein grafts or rubber tubes. He also attempted to treat hydrocephalus by sewing bowel serosa to the dura mater in the hope of increasing CSF absorption.
Several concepts other than diversion of CSF were attempted during this era as well.15 First, medical attempts that included thyroid extracts and diuretics yielded uniform failure. Dandy and Blackfan’s seminal work published in 1914 supported two key ideas in the pathophysiology of hydrocephalus. The first was that the CP was the singular source of CSF production. This observation led Dandy to recommend choroid plexectomy as the preferred treatment of hydrocephalus, and this technique became the preferred method of treating hydrocephalus for years. Endoscopic techniques were added decades later. The second key idea from their early experimental work was that obstruction to outflow of CSF was the central event in hydrocephalus. Consequently, therapeutic efforts were targeted at eliminating the resistance to outflow or providing an alternative pathway for CSF flow. These ideas led to the development of several surgical operations designed to treat hydrocephalus. One such operation was third ventriculostomy, in which the lamina terminalis at the anterior extent of the third ventricle was opened surgically after performing a craniotomy. A similar operation in which the corpus callosum was divided and the ventricle exposed had been proposed earlier in 1908 by Anton and van Brauman and called the “Balkenstich method.” This procedure, like third ventriculostomy, gradually fell out of favor because of high operative morbidity despite success in controlling the hydrocephalus.
The third era in the development of successful shunts began in the midportion of the 20th century.16,17 Although pioneers such as Matson continued making progress with diversion of CSF to such novel target sites as the ureter, the critical event in development occurred not in medicine, surgery, or neurophysiology. Rather, developments in polymer chemistry led to the production of biocompatible products made of silicone that would retain their inert qualities over long periods while implanted. Simultaneously, the development of functional valve systems ushered in the true beginning of the shunt era in the early 1950s. Valves and biocompatible material permitted avoidance of many of the problems that had plagued early efforts toward unrestricted shunting of CSF with either biologic (e.g., vein grafts) or nonbiocompatible (e.g., rubber) shunts.16
From the beginning, great attention has been paid to valves and valve design.18 The story of the development of the first functional VP shunt valve is a classic in neurosurgical history. John Holter was an engineer who had a child born with spina bifida and hydrocephalus. At the time there were no functional valve systems, and shunts were not widely available. Daily ventricular taps through the open fontanelle kept the child alive. Holter worked with the neurosurgeons Nulsen and Spitz and together developed the Spitz-Holter valve in 1952. This valve was implanted into Holter’s child as a ventriculojugular shunt.19 Cardiac problems ensued and resulted in an anoxic brain insult to Holter’s son. Despite these setbacks, the Spitz-Holter valve became the prototype of the more widely used CSF shunt valves. Virtually simultaneously, Pudenz and colleagues created a silicone slit–valve system that was similarly developed and marketed. Since that time there has been a plethora of valve systems devised and developed for the treatment of hydrocephalus.20 Many have been named for the developer (eponymic), but they share conceptual similarities that can be organized according to flow and resistance-to-flow characteristics.
Every shunt system consists of three central components—a ventricular catheter, a valve, and a distal catheter. Additional components such as tapping reservoirs, on-off devices, pressure transducers, and antisiphon devices may also be present, and there may be more than one ventricular catheter. Simple shunts have only a ventricular catheter, valve, and distal catheter, whereas complex shunts have more elaborate arrangements. Most commonly this consists of multiple ventricular catheters, but many different arrangements are possible. Loculated hydrocephalus occurs when fibrotic adhesions develop within the ventricles and cause isolated pools of CSF to arise that maintain independent capability of dilating and expanding. Before the development of contemporary endoscopic fenestration techniques, each of these loculated spaces required a ventricular catheter to ensure adequate drainage. Multiple ventricular catheters are still occasionally required for complex multiloculated hydrocephalus and are commonly required for some well-established congenital anomalies such as the Dandy-Walker variant or anomaly. Some commercially available systems have fused one or more of the aforementioned elements together to form a unishunt (to prevent potential disconnection21), but these systems still contain the same three conceptual components as all others.
Ventricular Catheter
The ventricular catheter is the component of the shunt that traverses the space from the ventricle to the valve. A variety of designs of ventricular catheters have been used over the years, but they share similar design principles. The rostral end of the catheter has a rounded tip and multiple holes along the proximal shaft of the catheter. Different design models have featured holes of different size. Proximal ventricular catheter obstruction is the most common cause of mechanical shunt failure, and the tissue implicated in such failure is an ingrowth of CP and gliotic neural tissue.22 Initially, it was thought that larger holes in the ventricular catheter would result in fewer shunt obstructions; however, experience with catheters with large holes showed that proximal obstruction still occurred. More importantly, the larger holes provided an opportunity for more robust ingrowth of CP and a secondary risk for hemorrhage when the catheters needed to be removed for revision. Other types of ventricular catheters were developed with mechanical adjuncts to keep CP away from the holes.23 The Portnoy catheter had a series of Silastic ribs that gave it an appearance similar to a honey dipper and were designed to deflect the CP. Similarly, the Carrea cage had a series of four rib-like Silastic structures that laid in parallel with the shaft of the catheter and were designed to deflect CP away. Both systems were associated with higher rates of hemorrhage and revisions and did not prevent proximal catheter obstruction. As a result, most current systems have multiple rows of very small holes arranged concentrically along the axis of the ventricular catheter. The exact number of holes or volume necessary to ensure adequate flow has never been firmly established, and it appears to be an area of modest investigation. Ginsberg and colleagues demonstrated that a single 500-µm-diameter hole permits flow and relieves the associated hydrostatic pressure.24 However, these investigators were seeking to understand the minimum volume that needed to be opened to ultrasonically clean an obstructed catheter rather than determine the necessary or optimum criteria for shunt flow. Lin and associates demonstrated that approximately 60% of flow occurs through the first row of holes in a shunt catheter and that more than 80% flows through the first two rows. Further understanding of the mechanisms contributing to proximal catheter function may substantially reduce morbidity from proximal shunt malfunction.25
Another important recent technologic advance in ventricular catheter design is the antimicrobial impregnation of ventricular catheters with antibiotics (antimicrobial-impregnated shunts). Two systems are commercially available. In the Codman Bactiseal system, the catheter is impregnated with clindamycin and rifampin as part of the manufacturing process. This patented process results in the gradual release of antimicrobials over a period of approximately 4 weeks after implantation of the device. Clindamycin and rifampicin are the preferred antimicrobials because of technical issues involving incorporation into the polymer, even though other antibiotics have greater effectiveness against organisms that commonly cause shunt infection. Results have varied widely, with some groups reporting nearly complete elimination of shunt infection after incorporation of antimicrobial-impregnated shunts. Other series have demonstrated a modest or nil impact of antimicrobial impregnation. The second commercially available system is the Medtronic BioGlide system, in which the ventricular catheter is impregnated with a polymer that gradually absorbs antibiotic when the device is soaked in a solution of antibiotic. The advantage of the system is that it affords the operating surgeon the choice of antibiotics to use. The disadvantages are that the catheter becomes very slippery and can readily be dropped or disconnected. Furthermore, the antibiotic migrates off the device more rapidly, and there is markedly less published experience with this system.26
Shunt Valves
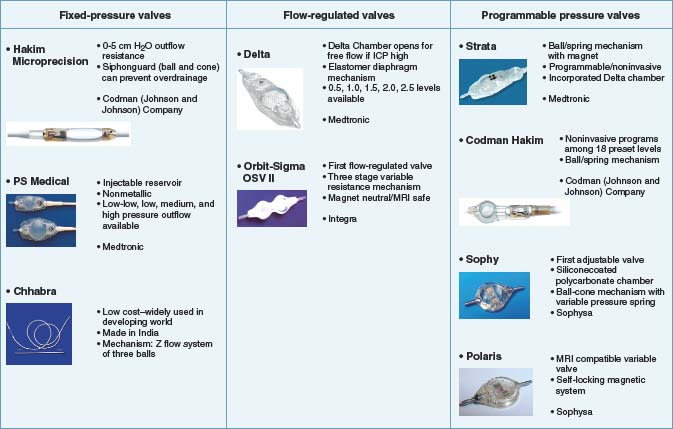
Since development of the first Spitz-Holter and Pudenz valves, valve design has received the greatest attention in the overall development of effective shunt systems (Table 190-1).27,28 Although this empirically appears logical because the valve is mechanically the most complex (and hence potentially most vulnerable) part of the shunt, several important studies have shown that valve mechanics play a limited role in the overall rate of shunt failure or success. The first of these landmark studies was the Shunt Design Trial, a multicenter prospective survey of the effectiveness of different widely used shunt systems in the 1990s.29,30 In this trial the three most common types of commercially available shunts were compared with regard to shunt function. No difference was noted in the initial report, nor with longer term follow-up.30 Other subsequent studies have repeated this finding.31
Fixed-pressure valves were the first valves developed for ventricular CSF shunts and overcame a significant barrier to successful CSF shunting, which was the development of low-pressure headaches. There are four different design styles of fixed-pressure valves: silicone rubber slit valves, silicon rubber diaphragm valves, silicon rubber miter valves, and metallic spring and ball valves. Each of these designs shares a common technical goal, which is to provide fixed resistance to outflow of CSF from the ventricle. If such resistance is not provided, the majority of patients will suffer from overdrainage, low-pressure chronic severe headaches. The resistance values are manufactured to three broad levels (low, medium, and high) that reflect different ranges within the normal spectrum of intracranial pressure. Examples of fixed-pressure valves that were widely used include the (first-generation) Hakim valves, the PS Medical (differential pressure) valve, the Denver valve, and the Chhabra valve, which is manufactured in India and still used widely throughout the world.32,33 Generally, these valves functioned well, but over time it became evident that debilitating overdrainage headaches still developed in up to 10% to 12% of patients with fixed-pressure valves. Clinically, this was manifested as an unyielding and life-altering headache that was accompanied by radiographic evidence of successful and sufficient drainage (typically small or nonvisible ventricles on computed tomography [CT]).34–36 This led to the development of flow-regulated valves, which regulated the amount of flow through the system when they were functioning within the normal physiologic range. The two most widely used systems of this design are the PS Medical Delta valve and the Orbis Sigma valve.31,37
Programmable valves can be adjusted noninvasively to vary resistance to outflow across a physiologically normal range. Two clinical observations drove the development of variable-pressure programmable valves. The first was that a significant (although smaller than with fixed-pressure valves) subset of patients treated with flow-regulated valves suffered from chronic headache. The second was the belief among selected groups of neurosurgeons that the ideal outflow resistance for an individual patient was a dynamic rather than a static system. Thus, the ideal outflow pressure for a patient varies with time, and periodic manipulation of outflow pressure ensures optimal function and the fewest symptoms of discomfort. For a persistently symptomatic patient plagued with endless headaches, outflow resistance can be varied to minimize or eliminate symptoms. Controversy still surrounds these ideas, and there are broad variations in clinical practice with regard to the utility of externally programmable valves. Proponents of the less costly flow-regulated valve systems contend (1) that the vast majority of pediatric patients do not require variable outflow resistance and the additional cost is unnecessary and (2) that manipulation of outflow resistance can distract one from the far more common problem of proximal catheter obstruction and can lead to delays in diagnosis of shunt failure. An additional vulnerability of programmable valves is the requirement that they be retested and reset after magnetic resonance imaging (MRI) because the magnetic field of the scanner characteristically affects settings of the programmable valve. Proponents of programmable valves consider the capability of varying outflow resistance of the shunt an important and nearly essential tool in the armamentarium used in treating hydrocephalus.38–41 The only prospective trial that compared programmable (Strata) and nonprogrammable valves showed similar survival across groups.42 Another important prospective study compared conventional flow-regulated valves with the markedly less expensive Chhabra system that is produced in India and found no difference in shunt survival in groups of shunted children in sub-Saharan Africa.33
There appears to be greater consensus that programmable valves play a valuable role in treating adults with hydrocephalus. This is particularly so for older patients, who are at risk for the development of normal-pressure hydrocephalus and thereby at increased risk for the development of subdural hematomas.43,44 In this patient group, the programmable valve allows outflow resistance to be initiated at a higher level to prevent sudden ventricular decompression with concomitant ventricular collapse, cortical infolding, and tearing of the draining veins. Once an initial period of drainage is completed and a period of adjustment to ventricular shunting has occurred, outflow resistance can be decreased or adjusted as needed to optimize the clinical impact of ventricular drainage.45
Distal Catheter
The distal catheter is by far the largest and longest component of a ventricular shunt, but it generally functions the best and has the fewest problems of all the shunt components. The most common problem related to distal catheter function is fracture of the catheter.46 Fracture characteristically occurs at points where movement across the shunt is maximized. In practical terms, this means that most ventricular shunts that fracture do so between the mastoid and the clavicle. This is the region of greatest movement because the shunt is secured to and moves with the skull above and the chest wall and abdomen below. The distal catheter may also pull out of the atrium or peritoneum with growth of the patient, or an infected pseudocyst may develop around the distal catheter tip, but these are not problems in which the catheter itself can be implicated as being causative.47 Hernia or varicoceles with migration of the distal catheter into the scrotum of a male patient have also been repeatedly observed and reported.48,49 Infection of the shunt may result in the development of an abdominal pseudocyst around the distal catheter. This was originally thought to be focal ascites, but focal distal CSF accumulation is now thought to strongly suggest infection.50,51 Tunneling for placement of the distal catheter can cause traumatic injury to adjacent structures.52 Very rarely, perforation of a hollow viscus may occur.53–55 Each of these complications has spurred innovative approaches in design that have resulted in a number of structural variations in distal catheter construction.56
The proximal end of the distal catheter is always open to enable coupling with the shunt valve, but the distal end of the shunt catheter may be either open or closed with a distal slit.57,58 Other distal tip designs have incorporated basket-like projections around the distal tip to prevent obstruction to outflow from adjacent tissues (e.g., omentum) within the peritoneum. One system that was initially popular in the 1970s incorporated a spring into the distal catheter tip. The intent of the spring was to prevent distal kinking and obstruction, but an unfortunate side effect (which led to U.S. Food and Drug Administration [FDA] notification and virtual elimination from widespread use) was a pronounced increase in the incidence of viscus perforation.
The distal catheter may be homogeneously impregnated with barium or may have only a single stripe of barium within its wall. Barium impregnation allows the catheter to be visualized radiographically and fractures or placement problems (e.g., tight coiling within the abdomen, which may suggest preperitoneal placement) to be detected. Some authors contend that solid barium catheters tend to leach barium salts over the extended life span of the shunt. The barium in turn is thought to precipitate locally within tissues as barium salts, which increases the focal tethering effect and risk for catheter fracture.59 Catheters with single barium stripes leach less barium, are associated with a reduction in local tethering from the deposition of barium salts, and consequently appear to have a lower risk for fracture. The practical tradeoff is that catheters with barium stripes may be more difficult to see on routine radiographs, particularly in large or obese patients.
Commercially Available Shunt Systems
There are dozens of commercially available shunts on the market in the western world. The best choice for any given patient must be made on an individualized and admittedly subjective basis that takes into account the neurosurgeon’s experience and confidence in a particular system, as well as circumstances unique to the cause and clinical history of the patient’s hydrocephalus. Although many individual studies have claimed superior performance for a given system, there is no compelling concordance of findings that indicates the superiority of any given system or combination of components. There has been a general trend toward the use of flow-regulated valves, and the proponents and critics of programmable and antimicrobial-impregnated systems embrace and support their views fervently. However, no shunt system has been convincingly shown to be superior overall. Warf and colleagues33 made this point most strikingly in a report that retrospectively compared overall shunt performance between groups of children in Uganda in whom the Codman-Hakim Micro-Precision shunt system from North America or the Chhabra shunt system manufactured in India was implanted. Despite the fact that the Chhabra shunt system costs a small fraction of the cost of the other shunt system, there was no statistically significant difference in overall performance. These findings, however, cannot be directly extrapolated to Western practice for several reasons. First, the groups were not identical and the review was retrospective and suffered from the inherent limitations of this design. Furthermore, the Chhabra system has not been subjected to evaluation by the FDA, which since 1976 has been granted authority by Congress to issue regulations requiring reporting of adverse events for marketed medical devices. Whether the progressively complex and expensive shunt systems that are being designed and released should be tested against existing equipment in head-to-head trials before release to the public appears to be a fair one in a progressively austere medical economic climate.
Ventriculosubgaleal Shunts
Rationale and Indications
Ventriculosubgaleal (VSG) shunts have limited application and inherently limited duration of function. However, in appropriate circumstances (e.g., premature infants with very low birth weight), VSG shunts are extremely useful and safe.60,61 With this shunt, CSF is diverted from the lateral ventricle to a small pocket that is gently dissected beneath the galea. From this location, CSF enters the cervical lymphatics and can be absorbed gradually for several weeks or months. These shunts are typically used as temporizing devices in very small premature infants in whom intracerebral hemorrhages of prematurity and posthemorrhagic hydrocephalus develop. These infants are often fragile and very ill and are uniquely sensitive to temperature and blood loss.62–64 Accordingly, the rapid and relatively avascular way in which these devices can be placed offers a decided advantage. Other temporizing uses of VSG shunts have been proposed.65,66
Infection and complication rates with VSG shunts are low.67 Infection is typically manifested as shunt failure with rapid retraction of the pocket. More classic signs of infection such as erythema and fever are uncommon. Wound failure is a common problem with these procedures because of the delicate quality and inherent fragility of neonatal skin. In practical terms, VSG shunts take about 10 to 20 minutes to insert and optimally last about 8 to 12 weeks. Consequently, they do not represent an effective long-term treatment of hydrocephalus but may play a very valuable role in temporizing until more definitive treatment with a VP shunt can be accomplished when the child is a little older (and has more acceptable anesthetic risks and less risk for hydrocephalus).68
Signs and Symptoms of Failure
Subgaleal shunts typically demonstrate an abbreviated duration of function.69 Characteristically, the subgaleal fluid collection will become smaller and tighter as the scalp galeal space shrinks. Occasionally, the fluid collection will be firm enough to inwardly deflect the malleable infant skull. Typically, a shrinking, progressively firm pocket indicates the end of useful utility of a subgaleal shunt. Conversion to a more permanent type of shunt is often necessary, but on rare occasion the subgaleal space can be reopened (lifting up the skin). The other alternative by which VSG shunt failure can occur is with a pocket that never forms well. This cause of failure may be variable because good function is sometimes achieved with a small pocket. However, more often a VSG shunt in which a pocket never forms indicates impaired function and a need for revision or promotion to a more permanent type of shunt. If a subcutaneous collection of CSF does not form, the clinical course must be observed closely and a low threshold be given to re-exploration of the VSG shunt. If progress is good (fontanelle soft and flat, head growth arrested, sunsetting resolved, ventricles small or stable on CT), the shunt need not be revised even in the absence of a significant subgaleal pocket.
Ventricular Reservoirs
Rationale and Indications
A ventricular reservoir is typically used in two clinical situations. The first is for posthemorrhagic hydrocephalus of the newborn. Serial percutaneous taps are performed to control the hydrocephalus. This simple system is similarly resistant to obstruction from clots as the VSG shunt. Percutaneous taps are labor intensive, however, and introduce some possibility of infecting the device.70 They are best performed by maintaining strict diligence to the skin over the reservoir. The skin must be gently but carefully prepared before puncture, and it must not be repeatedly punctured in exactly the same location. One technique that has proved useful is to designate an imaginary clock face on the skin outline of the reservoir. By designating a specific pattern for taps (2-, 4-, and 6-o’clock taps) on different days, the hazards of repeated taps are minimized. Many neurosurgeons insist that the devices be tapped by members of the neurosurgery team, whereas others have trained neonatologists and house officers to perform taps, which are then performed serially.
Ventriculoperitoneal Shunts
Rationale and Indications
Much has been discussed in the early parts of this chapter about the rationale for ventricular shunts, and this material applies most directly to VP shunts. VP shunts simply represent the best available treatment for patients with hydrocephalus who are not candidates for ETV. The choice of candidacy for ETV varies among surgeons and centers, and it should be actively considered an important treatment option for all children with hydrocephalus.6 Reported experience differs widely, but it appears fair to conclude that the incidence of acute complications is higher and more serious with ETV, yet successful ETV eliminates the need for long-term shunting and the attendant morbidity, cost, and suffering that come with shunt failures.5 Late failures with ETV are rare but definitely occur and must be watched for.71,72 Half of shunts fail within a year, and life with a shunt is typically one requiring multiple surgeries that arise at unpredictable times.73–75 There is concordance of data that babies have the highest incidence of failure of ETV and that patients with congenital aqueductal stenosis, tectal gliomas, or other “pure” obstructive causes of hydrocephalus are preferred candidates for ETV.75 Patients with myelodysplasia, infections, and posthemorrhagic hydrocephalus have been reported in multiple series to have a higher incidence of complications with ETV. In the end, no single factor determines whether a patient is best served by a shunt or ETV, but rather the decision must be individualized by objective assessment of the patient’s unique clinical situation, background, anatomy, social circumstances, and disease process in conjunction with the operating surgeon’s experience with ETV and shunt placement.76
Technique (See Video 190-1) 
VIDEO 190-1 Insertion of a ventriculoperitoneal shunt.
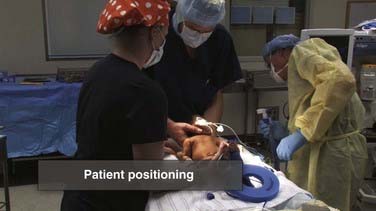
VP shunts are inserted and revised under general anesthesia. Typically, the patient is placed in the supine position, but occasionally a lateral position may be useful (Fig. 190-1). A lateral position with an occipital ventricular catheter and tunneling posteriorly over the scapulae to gain access to the peritoneum at the costal margin can be a particularly useful variation for children with birth prematurity and posthemorrhagic hydrocephalus. Such children often have percutaneous gastrostomy (PEG) tubes that exit in the subxiphoid region. The proximity of these incisions to shunt incisions at the time of shunt placement or if PEG revision or manipulation is required can create additional risk for infection.77,78 The supine position is otherwise preferred. The patient is positioned and bolstered to make the mastoid, clavicle, and xiphoid coplanar. This makes tunneling easier and safer regardless of whether it is performed rostrally (bottom up) or caudally (top down). All pressure points are carefully padded.
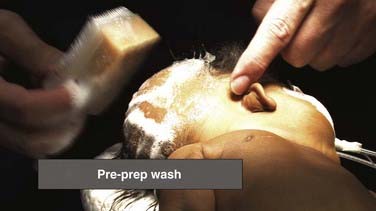
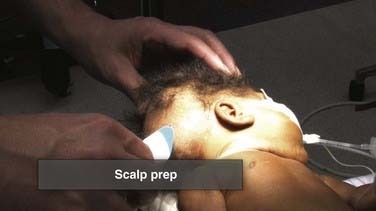
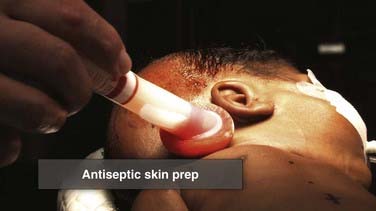
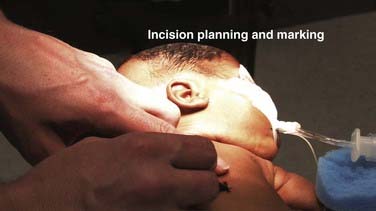
Appropriate prophylaxis against infection is critical. Virtually all shunt infections occur as a result of microbial inoculation of the shunt at the time of insertion.79–82 Consequently, meticulous attention to the detail of prophylaxis is essential. Protocols differ among institutions, and there is some emerging evidence suggesting that adherence to a strict protocol may be more important than the specific components of the protocol (i.e., which antibiotic is chosen). Preparation of the scalp and the skin is the first step of infection prophylaxis. Selection of the site for insertion of the ventricular catheter must take into account the condition of the scalp and the potential for impaired healing (e.g., in situations of multiple previous shunt operations), in addition to considerations dictated by the ventricular anatomy. The scalp at the incision site must be carefully cleansed of any dander or eschar from previous wounds (Figs. 190-2 and 190-3). Removal of hair is another topic around which opinions and reported results vary, but most neurosurgeons placing ventricular shunts remove the hair in at least the region immediately around the shunt incision. Removal of hair from around the field also reduces the likelihood of hair that is unprepared or prepared only at its base and then pulled back across an operative field that is sterile. It is established that all surgical fields contain some small number of bacteria and that no field is absolutely sterile. Prophylactic measures are designed to minimize the number of bacteria in the field to as absolutely low a number as possible so that the immune system of the patient may eliminate them. Experience with shunt surgery would dictate that only a small number of bacteria need to be present in the field for shunt infection to result, and thus it would seem prudent to take all reasonable steps to reduce inoculation of bacteria into the field as much as possible. It is preferable to remove the hair in a gentle fashion with clippers rather than with a razor because small nicks and cuts in unprepared skin can increase the risk for infection. The scalp is then prepared with an antimicrobial solution (Fig. 190-4), and great care is taken to ensure sufficient contact time before the placement of drapes or wiping of residual cleanser away so that maximum antimicrobial killing impact is realized. For iodine-based preparations, the solution must sit on the skin for several minutes after the agent has completely dried. Chlorhexidine-based preparations similarly require several minutes’ contact time for optimal impact. Great care must be taken with these agents to avoid inadvertent contact with mucous membranes or sensory organs such as the eyes or ears because devastating toxicity with loss of end-organ function (e.g., blindness, deafness) has been reported. We generally prefer a meticulous but not overly vigorous scrub as a preparatory wash followed by careful and comprehensive multistep preparation (scrub and paint). The hair is clipped, the preparatory wash is performed, and the incisions are marked (Fig. 190-5). A preoperative time-out may then be taken in which all members of the operating team (surgeon, anesthesia, operating room nurses/technicians) cease their activities and all conversation, confirm patient identity, and sequentially review the planned procedure, including side and site, necessary equipment, and potential for additional items such as implants, blood for transfusion, or special tools needed for the procedure. When there is agreement, the remainder of the preparation ensues.
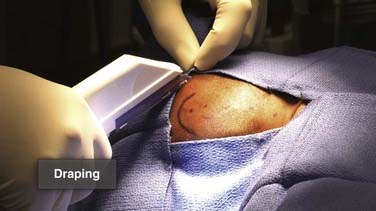
Additional measures for barrier protection include the use of iodine-impregnated adherent drapes directly over the skin to be incised and routine surgical drapes to isolate a sterile surgical field (Fig. 190-6). It has been demonstrated that wearing two pairs of gloves by all surgical team members provides important protection against loss of glove integrity, which has been demonstrated to exceed 30% in at least one study.
Administration of a weight-appropriate dose of preoperative antibiotic has also been demonstrated to reduce infection rates.83 The drug must be infused before any incision for optimum benefit. No single antibiotic has been shown to be superior, so many surgeons use a second-generation cephalosporin because of its low toxicity, broad coverage, and low cost. Many surgeons prefer to continue antibiotics postoperatively for one dose or 24 hours, but this has not been shown to lower infection rates.
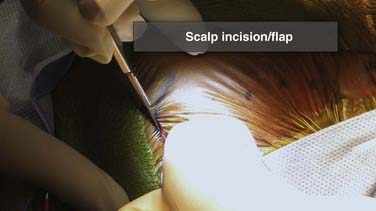
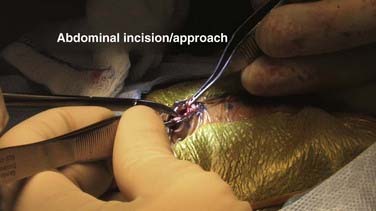
The cranial incision is chosen so that the ventricle is approached from either a coronal or an occipital trajectory. Individual contradictory papers have suggested that one approach or the other is associated with longer shunt survival, but this has not been supported over time and repeated studies.84 The choice of approach is based on subjective, individual factors related to ventricular size and configuration, condition of the scalp, presence of any other drainage catheters such as external ventricular drains, and surgeon preference and experience. In general, a coronal approach is preferred when the ventricles are small. A suitable entry point for a coronal catheter in an infant is at the far lateral corner of the anterior fontanelle. For a larger child and adult, the ideal coronal entry point is 11 cm above the nasion and 4 cm off midline. A trajectory that targets the medial canthus of the ipsilateral eye will puncture the anterior horn of the lateral ventricle in a location that allows the foramen of Monroe to be visualized and traversed if endoscopy is performed and generally ensures that the catheter is anterior to the bulk of the CP.85,86 The potential to keep the ventricular catheter clear of CP is another potential theoretical advantage of coronal catheters. An occipital approach is often used for large ventricles in a young child, in whom it is advantageous to reduce both the length of tunneling and the number of incisions that need to heal for successful placement of the shunt. The occipital entry point is placed on the flat portion of the occiput above the superior part of the pinna and approximately 4 cm from the midline (Fig. 190-7). The catheter should encounter the ventricle at a depth of 4 to 5 cm when passed along a trajectory that aims at the medial canthus of the ipsilateral eye. Occipital entry can also be measured from the inion. A suitable and safe corridor is obtained with an entry point that is 7 cm above the inion and 4 cm off midline. If loculated hydrocephalus is present, multiple catheters may be needed. After the cranial incision is made and a small flap turned, a small bur hole is made. In very young infants this can be accomplished effectively by using a knife with a No. 15 blade on a long handle and gently rotating the blade on the skull surface so that the edge of the blade scrapes the skull away (see Fig. 190-7). Care is taken so that downward pressure on the blade is minimized and active scraping of the side of the blade against the skull can be palpated by the surgeon’s fingers. In this manner a small penetration will be made at the level of the tip of the blade into the epidural space. Once this occurs, a small surgical clamp can be used like a Kerrison rongeur to gently yet effectively remove a small window of bone and gain exposure to the dura. In older children (older than 6 months) and adults, a high-speed pneumatic drill or craniotome is used to make a small bur hole. The intended trajectory of the ventricular catheter must be carefully and constantly considered when making the bur hole. This is particularly important for patients with thick skulls because a small, improperly directed bur hole may limit or impair a proper trajectory and prevent proper ventricular puncture.
Placement of the peritoneal catheter may be performed either endoscopically or through a small open laparotomy.87,88 Again, the choice is based on individual patient characteristics, including a history of abdominal surgery, injury, or illness such as necrotizing enterocolitis of the newborn, which may have an impact on intraperitoneal anatomy and the consequent risk for complications. The assistance of a general surgeon may be useful if the abdominal history is significant. If a laparotomy is to be performed, a subxiphoid incision is the easiest and most straightforward approach because only the skin, the transverse rectus fascia, and the peritoneum need to be identified and opened (Fig. 190-8). A paramedian subcostal incision may be used, and the surgeon must be attentive to whether the rectus abdominis is traversed before the oblique muscles are encountered. Once encountered, the oblique muscles are gently dissected along their fibers to minimize bleeding and the peritoneum is grasped with a small forceps or curved clamp. Each layer is identified and tagged during the opening so that a layered meticulous closure may be performed reliably. A ventricular shunt procedure is only as good as its weakest step, and it is entirely possible for an imperfectly closed abdominal wound to compromise or doom an otherwise successful operation.
Trochar exposure of the peritoneum is achieved by sharply opening the transverse abdominal fascia and drawing it upward between two surgical clamps. In so doing, the anterior abdominal wall is lifted away from the underlying omentum and bowel and secured. In a single brisk, yet smooth motion the trocar is advanced downward and toward the umbilicus while upward tension is maintained on the anterior abdominal wall. The trocar punctures the peritoneum, and the sheath provides protection to structures within it. The laparoscope may be useful to confirm intraperitoneal localization.87,89–91
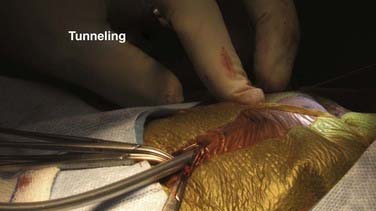
Tunneling may be performed in either direction, but most neurosurgeons always tunnel in the same direction. Advantages of tunneling rostrally include a low risk of injuring the skin at the tunneling site, a reduced risk for chest or lung puncture, and a fixed target (skull) that can be cautiously used to backstop tunneling efforts. Advantages of tunneling caudally include a larger distal incision that can be manipulated to capture the tunneler as it descends and no risk of intracranial penetration in young children. Regardless of the direction of tunneling, great care must be taken to avoid a trajectory that allows the tunneler to pass under the ribs, under the clavicle, deep in the neck, or across the skull base. Meticulous attention to positioning so that the mastoid, the clavicle, and the xiphoid are coplanar will facilitate tunneling and make it safer (Fig. 190-9). The tunneler is a surgical steel rod that either is covered with a clear plastic sheath or has a robust silk thread tied through its tip. A slight bend similar to the shape of a hockey stick can be placed in the distal end to impart greater maneuverability and control. The skin at the site where tunneling commences is gently held up with forceps, and the tunneler is introduced into the fatty subcutaneous tissue. It is gently advanced with back-and-forth small rotatory movements of the tip that allow the tip to be visualized and palpated as it traverses the subcutaneous tissue. There is often significant resistance at the posterior nuchal line at the junction of the cervical and cranial skin. Great care needs to be exercised when traversing this region so that the chest or the skull base is not inadvertently punctured from a rebound phenomenon. Once the two incisions have been connected, the steel rod is removed and the peritoneal catheter is advanced through the sheath to the distal incision.
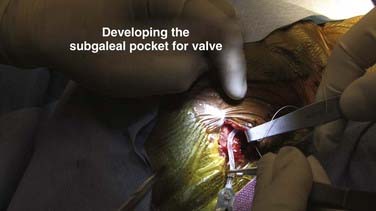
A small pocket is dissected at the proximal incision to allow the valve to sit neatly beneath the skin (Fig. 190-10). It is common for this pocket to initially be dissected insufficiently, but rarely does it happen that the pocket is not wide enough. Rather, inexperienced shunt surgeons will fail to recognize a web of galea at the distal end of the pocket that is restricting the size of the pocket as it enters the track through which the peritoneal catheter passes. Repeated robust dissection that massively widens the pocket but does little to extend it is a common error. Instead, it is preferable to use a headlight and look down the track, identify the offending band, and open the distal end of the pocket to allow the valve to sit neatly within the pocket.
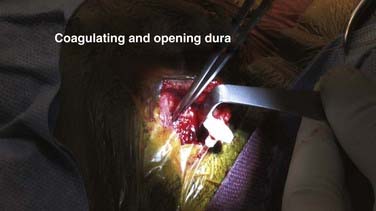
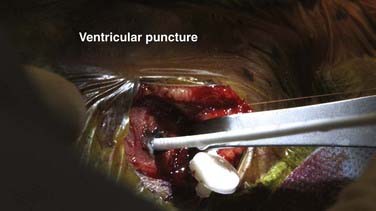
Once the assembled shunt system is in the subcutaneous space and easy free flow through the system has been demonstrated, the ventricle is punctured. The dura is coagulated with bipolar cautery and opened as a pinhole just large enough to admit the bipolar tips (Fig. 190-11). The pia is then coagulated and opened sharply with a No. 15 blade or needle. The ventricular catheter is advanced deliberately approximately 5 cm. This distance will reach the ventricle from either trajectory if the ventricles are enlarged, and it is unlikely to injure deep subcortical structures if the trajectory is mistaken (Fig. 190-12). With experience, ventricular puncture becomes straightforward for all cases except small ventricles.
Adjuncts that can be used for the positioning of ventricular catheters include endoscopy, ultrasound, and frameless navigation.92,93 Small-diameter (1.2 mm) endoscopes have been developed by several manufacturers and are widely used by many neurosurgeons for the placement of VP shunts. The Shunt Placement Study was a multicenter prospective trial that examined whether shunts placed under endoscopic guidance exhibited longer survival than did those placed freehand.94 The results showed that survival of endoscope-placed shunts was no better and that the infection rate for endoscopically placed shunts was higher. There were significant limitations in this study, however, and many neurosurgeons continue to widely use endoscopy to ensure that the shunt is placed properly within the ventricle.
Ultrasound has been embraced by some neurosurgeons as being a very useful adjunct for the placement of ventricular catheters.95 The development of bur hole ultrasound probes of high quality was a significant step in increasing the utility of ultrasound. Previously, significant enlargement of the bur hole was needed to gain access to the epidural space for placement of the ultrasound probe. In infants, the fontanelle could be used. There is definitely a learning curve in the use of intracranial ultrasound, but advocates claim that the technique is highly useful in providing real-time anatomic feedback that ensures proper ventricular placement with a fraction of the cost or potential risk for infection associated with endoscopy. Further prospective studies are under way that will continue to investigate the role of ultrasound in the placement of ventricular catheters.
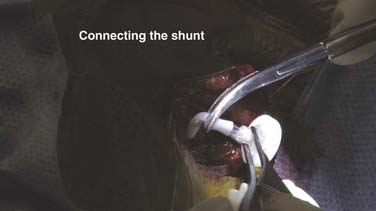
Once ventricular puncture has been accomplished successfully, the ventricular catheter is cut off and connected to the valve or reservoir (Fig. 190-13). CSF flow should be vigorous and pulsatile and, with experience, can be recognized as ventricular. The catheter should not need to be longer than 7 cm from a coronal approach or 11 cm from an occipital approach. Need for a longer ventricular catheter may suggest placement that crosses midline or is either too deep or anatomically suboptimal.
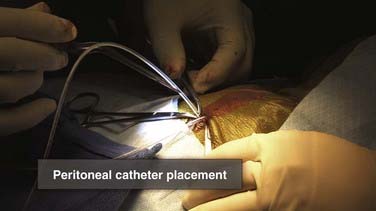
Once connected, distal flow is ensured and the peritoneal catheter is inserted directly into the peritoneum. The edges of the peritoneum are held up and the distal catheter is introduced. Resistance or failure of the peritoneal catheter to advance is suggestive of either preperitoneal placement or intraperitoneal adhesions causing loculations. Either event should prompt careful examination of the peritoneal exposure and consideration of an expanded exposure to ensure accurate and sufficient peritoneal placement (Fig. 190-14).
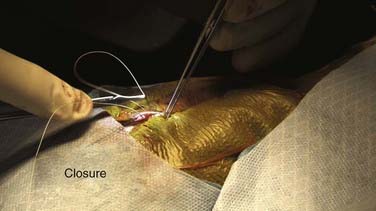
Meticulous closure and placement of sterile dressings complete the operative procedure (Fig. 190-15). Some have advocated that dressings are unnecessary, but the majority of neurosurgeons performing shunt operations still prefer to place a dressing for at least 24 to 48 hours postoperatively.
Ventriculoatrial or Ventriculocardiac Shunts
Rationale and Indications
VA shunts preceded VP shunts historically but have evolved to be infrequently placed because of the potentially higher morbidity and mortality with them.96,97 Complications of VA shunts may include endocarditis, cor pulmonale, shunt nephritis, and the potential for propagation into the heart or pulmonary artery of a blood clot on the distal catheter (pulmonary embolus) or the distal catheter itself in the event of a disconnection.98–100 Another significant liability of VA shunts is the predictable failure that accompanies natural growth of the child as the fixed-length distal (atrial) catheter is gradually pulled out of the heart.
Technique
An alternative technique involves direct puncture of the internal jugular or subclavian vein percutaneously in the same manner in which a central venous catheter is placed.101–103 In children it is useful to have the assistance of an experienced pediatric surgeon because the incidence and severity of complications with central venous access are significantly elevated in children. Once the jugular vein is cannulated, the Seldinger technique is used to secure access to the vein and allow dilation of the tract and venotomy to permit entry of the distal (atrial) catheter. A J-wire is introduced and the track is dilated. The distal catheter is manipulated and measured under C-arm fluoroscopy to ensure that its tip resides in the right atrium (T7-8). Another technique that is selectively performed for confirmation of atrial positioning of the distal catheter is to use the tip of the catheter as an electrocardiographic lead and observe for a change in polarity of the P wave on the electrocardiogram. Catheter length is adjusted and cut appropriately before attachment at the distal end of the valve.
Ventriculopleural Shunts
Rationale and Indications
VPl shunts are never used as first-line procedures to control hydrocephalus. This has largely resulted from the limited and variable capacity of the pleural cavity to reabsorb fluid, particularly in infants and small children. However, a VPl shunt can be a useful temporizing intervention if intra-abdominal infection or peritoneal insufficiency prevents distal catheter placement in the peritoneum, and several series have reported long-term success in a significant percentage of patients.104 Contraindications to implantation of the catheter in the chest include active chest infection, compromised pulmonary reserve, and a history of thoracic surgery, which would increase the risk for adhesions. A preoperative chest radiograph is important in ruling out pneumonia, pleural effusion, or congenital anomalies, which may have an impact on patient risk and hence candidacy for VPl shunt implantation.
Alternative Distal Shunt Sites
Historically, placement of shunts in many alternative distal sites has been described.105,106 Some remain enthusiastically supported by limited numbers of surgeons and authors to the present time; however, broad utility is sufficiently lacking to discuss these systems at length in a general text of this nature. Interested readers are referred to primary papers describing ventricular-gallbladder and other novel distal site shunts.107
Treatment of hydrocephalus with ventricular shunting procedures represents a paradox in neurosurgery. Technically, ventricular shunting procedures remain among the most challenging operations in neurosurgery. No other procedure has a progressive failure rate of 50% 2 years after the operation.108 Indeed, few if any other neurosurgical operations can be completely ruined at any point up to and including the last stitch. Yet ventricular shunting procedures are relegated by the less experienced to be “easy” operations that lack the glamor or respect conferred to complex intracranial or spinal procedures.
Ventricular shunting procedures are similarly paradoxical from an outcomes perspective. On the one hand, ventricular shunts represent a great success that offers a life of hope and promise to literally thousands of patients who could not have been treated two generations ago. Patients who are treated successfully and maintained with ventricular shunts can achieve developmental outcomes that allow highly functional living and normal independence and prosperity.109 Without treatment, patients may die or suffer profound developmental delay.
Furthermore, little substantive progress has been made in extending overall shunt survival in the last 35 years despite extensive clinical experience and a sizable literature. Complications from ventricular shunting procedures are unequivocally multifactorial.74,110,111 Recently, cooperative multicenter investigations have been initiated that are using properly designed trials with validated instruments to measure outcome. Such studies will have greater power and should yield far more powerful evidence to address the difficult challenges that surround the management of patients with ventricular shunts.
Age at the time of shunt placement and time since previous revision are important predictors of shunt survival.75
Aronyk KE. The history and classification of hydrocephalus. Neurosurg Clin N Am. 1993;4:599.
Bayston R. Ventriculoperitoneal shunt–associated infection. J Infect. 1991;23:343.
Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38:295.
Cochrane DD, Kestle J. Ventricular shunting for hydrocephalus in children: patients, procedures, surgeons and institutions in English Canada, 1989-2001. Eur J Pediatr Surg. 2002;12(Suppl 1):S6.
Del Bigio MR. Epidemiology and direct economic impact of hydrocephalus: a community based study. Can J Neurol Sci. 1998;25:123.
Drake JM. Shunt infections. J Neurosurg. 1996;85:985.
Drake JM, Kulkarni AV, Kestle J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv Syst. 2009;25:467.
Fulmer BB, Grabb PA, Oakes WJ, et al. Neonatal ventriculosubgaleal shunts. Neurosurgery. 2000;47:80.
Kestle JR, Drake JM, Cochrane DD, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284.
Kestle J, Drake J, Milner R, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230.
Kestle J, Milner R, Drake J. The Shunt Design Trial: variation in surgical experience did not influence shunt survival. Pediatr Neurosurg. 1999;30:283.
Patwardhan RV, Nanda A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139.
Tubbs RS, Smyth MD, Wellons JC3rd, et al. Life expectancy of ventriculosubgaleal shunt revisions. Pediatr Neurosurg. 2003;38:244.
Tuli S, Drake J, Lawless J, et al. Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg. 2000;92:31.
Warf BC. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. J Neurosurg. 2005;102:358.
1 Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg. 1995;23:254.
2 Cochrane D, Kestle J, Steinbok P, et al. Model for the cost analysis of shunted hydrocephalic children. Pediatr Neurosurg. 1995;23:14.
3 Del Bigio MR. Epidemiology and direct economic impact of hydrocephalus: a community based study. Can J Neurol Sci. 1998;25:123.
4 Cochrane DD, Kestle J. Ventricular shunting for hydrocephalus in children: patients, procedures, surgeons and institutions in English Canada, 1989-2001. Eur J Pediatr Surg. 2002;12(Suppl 1):S6.
5 Patwardhan RV, Nanda A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139.
6 Jones RF, Stening WA, Brydon M. Endoscopic third ventriculostomy. Neurosurgery. 1990;26:86.
7 Tuli S, Alshail E, Drake J. Third ventriculostomy versus cerebrospinal fluid shunt as a first procedure in pediatric hydrocephalus. Pediatr Neurosurg. 1999;30:11.
8 Drake JM, Kulkarni AV, Kestle J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv Syst. 2009;25:467.
9 Amin NM. Normal-pressure hydrocephalus. Am Fam Physician. 1983;28:147.
10 Vassilouthis J. The syndrome of normal-pressure hydrocephalus. J Neurosurg. 1984;61:501.
11 Milhorat TH, Hammock MK, Chien T, et al. Normal rate of cerebrospinal fluid formation five years after bilateral choroid plexectomy. Case report. J Neurosurg. 1976;44:735.
12 Udvarhelyi GB, Epstein MH. The so-called Dandy-Walker syndrome: analysis of 12 operated cases. Childs Brain. 1975;1:158.
13 Ciricillo SF, Cogen PH, Harsh GR, et al. Intracranial arachnoid cysts in children. A comparison of the effects of fenestration and shunting. J Neurosurg. 1991;74:230.
14 Aronyk KE. The history and classification of hydrocephalus. Neurosurg Clin N Am. 1993;4:599.
15 Aschoff A, Kremer P, Hashemi B, et al. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22:67.
16 Weiss SR, Raskind R. Twenty-two cases of hydrocephalus treated with a Silastic ventriculoperitoneal shunt. Int Surg. 1969;51:13.
17 Weiss SR, Raskind R. Further experience with the ventriculoperitoneal shunt. Prophylactic antibiotics. Int Surg. 1970;53:300.
18 Portnoy HD, Amirjamshidi A, Hoffman HJ, et al. Shunts: which one, and why? Surg Neurol. 1998;49:8.
19 Keen J. Casey Holter and the Spitz-Holter valve. Eur J Pediatr Surg. 1992;2(suppl 1):5.
20 Borgbjerg BM, Gjerris F, Albeck MJ, et al. Frequency and causes of shunt revisions in different cerebrospinal fluid shunt types. Acta Neurochir (Wien). 1995;136:189.
21 Aldrich EF, Harmann P. Disconnection as a cause of ventriculoperitoneal shunt malfunction in multicomponent shunt systems. Pediatr Neurosurg. 1990;16:309.
22 Gower DJ, Lewis JC, Kelly DLJr. Sterile shunt malfunction. A scanning electron microscopic perspective. J Neurosurg. 1984;61:1079.
23 Steinbok P, Cochrane DD. Shunt removal by choroid plexus coagulation. J Neurosurg. 1996;85:981.
24 Ginsberg HJ, Sum A, Drake JM, et al. Ventriculoperitoneal shunt flow dependency on the number of patent holes in a ventricular catheter. Pediatr Neurosurg. 2000;33:7.
25 Sekhar LN, Moossy J, Guthkelch AN. Malfunctioning ventriculoperitoneal shunts. Clinical and pathological features. J Neurosurg. 1982;56:411.
26 Gower DJ, Gower VC, Richardson SH, et al. Reduced bacterial adherence to silicone plastic neurosurgical prosthesis. Pediatr Neurosci. 1985;12:127.
27 Serlo W, von Wendt L, Heikkinen ES, et al. Ball and spring or slit and core valve for hydrocephalus shunting? Ann Clin Res. 1986;18(suppl 47):103.
28 Miethke C, Affeld K. A new valve for the treatment of hydrocephalus. Biomed Tech (Berl). 1994;39:181.
29 Kestle J, Milner R, Drake J. The Shunt Design Trial: variation in surgical experience did not influence shunt survival. Pediatr Neurosurg. 1999;30:283.
30 Kestle J, Drake J, Milner R, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230.
31 Davis SE, Levy ML, McComb JG, et al. The delta valve: how does its clinical performance compare with two other pressure differential valves without antisiphon control? Pediatr Neurosurg. 2000;33:58.
32 Chhabra DK, Agrawal GD, Mittal P. “Z” flow hydrocephalus shunt, a new approach to the problem of hydrocephalus, the rationale behind its design and the initial results of pressure monitoring after “Z” flow shunt implantation. Acta Neurochir (Wien). 1993;121:43.
33 Warf BC. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. J Neurosurg. 2005;102:358.
34 Gruber R. The relationship of ventricular shunt complications to the chronic overdrainage syndrome: a follow-up study. Z Kinderchir. 1981;34:346.
35 Oi S, Matsumoto S. Slit ventricles as a cause of isolated ventricles after shunting. Childs Nerv Syst. 1985;1:189.
36 Serlo W, Heikkinen E, Saukkonen AL, et al. Classification and management of the slit ventricle syndrome. Childs Nerv Syst. 1985;1:194.
37 Sainte-Rose C. Shunt obstruction: a preventable complication? Pediatr Neurosurg. 1993;19:156.
38 Rohde V, Mayfrank L, Ramakers VT, et al. Four-year experience with the routine use of the programmable Hakim valve in the management of children with hydrocephalus. Acta Neurochir (Wien). 1998;140:1127.
39 Yamashita N, Kamiya K, Yamada K. Experience with a programmable valve shunt system. J Neurosurg. 1999;91:26.
40 Kay AD, Fisher AJ, O’Kane C, et al. A clinical audit of the Hakim programmable valve in patients with complex hydrocephalus. Br J Neurosurg. 2000;14:535.
41 Reinprecht A, Dietrich W, Berger A, et al. Posthemorrhagic hydrocephalus in preterm infants: long-term follow-up and shunt-related complications. Childs Nerv Syst. 2001;17:663.
42 Kestle JR, Walker ML. A multicenter prospective cohort study of the Strata valve for the management of hydrocephalus in pediatric patients. J Neurosurg. 2005;102:141.
43 Boon AJ, Tans JT, Delwel EJ, et al. Dutch normal-pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg. 1997;87:687.
44 Caruso R, Cervoni L, Vitale AM, et al. Idiopathic normal-pressure hydrocephalus in adults: result of shunting correlated with clinical findings in 18 patients and review of the literature. Neurosurg Rev. 1997;20:104.
45 Kamano S, Nakano Y, Imanishi T, et al. Management with a programmable pressure valve of subdural hematomas caused by a ventriculoperitoneal shunt: case report. Surg Neurol. 1991;35:381.
46 Cuka GM, Hellbusch LC. Fractures of the peritoneal catheter of cerebrospinal fluid shunts. Pediatr Neurosurg. 1995;22:101.
47 Gifford RR, Plaut MR. Abdominal catheter retraction in a ventriculoperitoneal shunt. Clin Pediatr (Phila). 1974;13:84.
48 Ramani PS. Extrusion of abdominal catheter of ventriculoperitoneal shunt into the scrotum. Case report. J Neurosurg. 1974;40:772.
49 Levey SH, Cooper P, Schiffman D. Simulated testicular torsion in a neonate: complication of ventriculoperitoneal shunt. Urology. 1977;9:174.
50 Gutierrez FA, Raimondi AJ. Peritoneal cysts: a complication of ventriculoperitoneal shunts. Surgery. 1976;79:188.
51 Frykberg T, Olden L. Infection as a cause of peritoneal catheter dysfunction in ventriculo-peritoneal shunting in children. Z Kinderchir. 1983;38(suppl 2):84.
52 Grosfeld JL, Cooney DR, Smith J, et al. Intra-abdominal complications following ventriculoperitoneal shunt procedures. Pediatrics. 1974;54:791.
53 Sells CJ, Loeser JD. Peritonitis following perforation of the bowel: a rare complication of a ventriculoperitoneal shunt. J Pediatr. 1973;83:823.
54 Nishijima M, Endoh S, Ohyama H, et al. Gastric perforation by a ventriculoperitoneal shunt. Neurosurgery. 1982;10:754.
55 Christoph CL, Poole CA, Kochan PS. Operative gastric perforation: a rare complication of ventriculoperitoneal shunt. Pediatr Radiol. 1995;25(suppl 1):S173.
56 Agha FP, Amendola MA, Shirazi KK, et al. Unusual abdominal complications of ventriculo-peritoneal shunts. Radiology. 1983;146:323.
57 Sparrow OC. Laboratory performance of single-piece ventriculoperitoneal shunts with distal slit-valve control. J Neurosurg. 1989;70:946.
58 Hahn YS. Use of the distal double-slit valve system in children with hydrocephalus. Childs Nerv Syst. 1994;10:99.
59 Shimotake K, Kondo A, Aoyama I, et al. Calcification of a ventriculoperitoneal shunt tube. Case report. Surg Neurol. 1988;30:156.
60 Fulmer BB, Grabb PA, Oakes WJ, et al. Neonatal ventriculosubgaleal shunts. Neurosurgery. 2000;47:80.
61 Willis BK, Kumar CR, Wylen EL, et al. Ventriculosubgaleal shunts for posthemorrhagic hydrocephalus in premature infants. Pediatr Neurosurg. 2005;41:178.
62 James HE, Bejar R, Gluck L, et al. Ventriculoperitoneal shunts in high risk newborns weighing under 2000 grams: a clinical report. Neurosurgery. 1984;15:198.
63 Boynton BR, Boynton CA, Merritt TA, et al. Ventriculoperitoneal shunts in low birth weight infants with intracranial hemorrhage: neurodevelopmental outcome. Neurosurgery. 1986;18:141.
64 Pezzotta S, Locatelli D, Bonfanti N, et al. Shunt in high-risk newborns. Childs Nerv Syst. 1987;3:114.
65 Steinbok P, Cochrane DD. Ventriculosubgaleal shunt in the management of recurrent ventriculoperitoneal shunt infection. Childs Nerv Syst. 1994;10:536.
66 Kariyattil R, Mariswamappa K, Panikar D. Ventriculosubgaleal shunts in the management of infective hydrocephalus. Childs Nerv Syst. 2008;24:1033.
67 Tubbs RS, Banks JT, Soleau S, et al. Complications of ventriculosubgaleal shunts in infants and children. Childs Nerv Syst. 2005;21:48.
68 Taylor AG, Peter JC. Advantages of delayed VP shunting in post-haemorrhagic hydrocephalus seen in low-birth-weight infants. Childs Nerv Syst. 2001;17:328.
69 Tubbs RS, Smyth MD, Wellons JC3rd, et al. Life expectancy of ventriculosubgaleal shunt revisions. Pediatr Neurosurg. 2003;38:244.
70 Martinez-Lage JF, Poza M, Esteban JA. Mechanical complications of the reservoirs and flushing devices in ventricular shunt systems. Br J Neurosurg. 1992;6:321.
71 Hader WJ, Drake J, Cochrane D, et al. Death after late failure of third ventriculostomy in children. Report of three cases. J Neurosurg. 2002;97:211.
72 Mohanty A, Vasudev MK, Sampath S, et al. Failed endoscopic third ventriculostomy in children: management options. Pediatr Neurosurg. 2002;37:304.
73 Liptak GS, McDonald JV. Ventriculoperitoneal shunts in children: factors affecting shunt survival. Pediatr Neurosci. 1985;12:289.
74 Drake JM. Shunt infections. J Neurosurg. 1996;85:985.
75 Tuli S, Drake J, Lawless J, et al. Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg. 2000;92:31.
76 Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38:295.
77 Graham SM, Flowers JL, Scott TR, et al. Safety of percutaneous endoscopic gastrostomy in patients with a ventriculoperitoneal shunt. Neurosurgery. 1993;32:932.
78 Rahmin M, Roston A, Miskovitz P. PEG placement in patients with ventriculoperitoneal shunts. Gastrointest Endosc. 1994;40:395.
79 Venes JL. Control of shunt infection. Report of 150 consecutive cases. J Neurosurg. 1976;45:311.
80 George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979;51:804.
81 Bayston R. Ventriculoperitoneal shunt–associated infection. J Infect. 1991;23:343.
82 Borgbjerg BM, Gjerris F, Albeck MJ, et al. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien). 1995;136:1.
83 Fan-Havard P, Nahata MC. Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm. 1987;6:866.
84 Dickerman RD, McConathy WJ, Morgan J, et al. Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: revisited. J Clin Neurosci. 2005;12:781.
85 Yamamoto M, Oka K, Nagasaka S, et al. Ventriculoscope-guided ventriculoperitoneal shunt and shunt revision. Technical note. Acta Neurochir (Wien). 1994;129:85.
86 Walker ML, Carey L, Brockmeyer DL. The NeuroNavigational 1.2-mm Neuroview Neuroendoscope. Neurosurgery. 1995;36:617.
87 Box JC, Young D, Mason E, et al. A retrospective analysis of laparoscopically assisted ventriculoperitoneal shunts. Surg Endosc. 1996;10:311.
88 Goitein D, Papasavas P, Gagne D, et al. Single trocar laparoscopically assisted placement of central nervous system–peritoneal shunts. J Laparoendosc Adv Surg Tech A. 2006;16:1.
89 Fanelli RD, Mellinger DN, Crowell RM, et al. Laparoscopic ventriculoperitoneal shunt placement: a single-trocar technique. Surg Endosc. 2000;14:641.
90 Reardon PR, Scarborough TK, Matthews BD, et al. Laparoscopically assisted ventriculoperitoneal shunt placement using 2-mm instrumentation. Surg Endosc. 2000;14:585.
91 Handler MH, Callahan B. Laparoscopic placement of distal ventriculoperitoneal shunt catheters. J Neurosurg Pediatr. 2008;2:282.
92 Gil Z, Siomin V, Beni-Adani L, et al. Ventricular catheter placement in children with hydrocephalus and small ventricles: the use of a frameless neuronavigation system. Childs Nerv Syst. 2002;18:26.
93 Woerdeman PA, Willems PW, Han KS, et al. Frameless stereotactic placement of ventriculoperitoneal shunts in undersized ventricles: a simple modification to free-hand procedures. Br J Neurosurg. 2005;19:484.
94 Kestle JR, Drake JM, Cochrane DD, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284.
95 Shkolnik A, McLone DG. Intra-operative real-time ultrasonic guidance of intracranial shunt tube placement in infants. Radiology. 1982;144:573.
96 Ignelzi RJ, Kirsch WM. Follow-up analysis of ventriculoperitoneal and ventriculoatrial shunts for hydrocephalus. J Neurosurg. 1975;42:679.
97 Vernet O, Campiche R, de Tribolet N. Long-term results after ventriculoatrial shunting in children. Childs Nerv Syst. 1993;9:253.
98 Piatt JHJr, Hoffman HJ. Cor pulmonale: a lethal complication of ventriculoatrial CSF diversion. Childs Nerv Syst. 1989;5:29.
99 Noe HN, Roy S3rd. Shunt nephritis. J Urol. 1981;125:731.
100 Olsen L, Frykberg T. Complications in the treatment of hydrocephalus in children. A comparison of ventriculoatrial and ventriculoperitoneal shunts in a 20-year material. Acta Paediatr Scand. 1983;72:385.
101 Lehman RA, Hart JC. Subclavian catheterization for the placement of a ventriculovenous shunt. Neurosurgery. 1983;12:346.
102 Matsuoka Y, Kawajiri K, Hayazaki K, et al. A simplified atrial catheterization technique for ventriculo-atrial shunt: puncture of the subclavian vein through the infraclavicular approach. Neurol Med Chir (Tokyo). 1993;33:444.
103 Harrison MJ, Welling BG, DuBois JJ. A new method for inserting the atrial end of a ventriculoatrial shunt. Technical note. J Neurosurg. 1996;84:705.
104 Martinez-Lage JF, Torres J, Campillo H, et al. Ventriculopleural shunting with new technology valves. Childs Nerv Syst. 2000;16:867.
105 West KW, Turner MK, Vane DW, et al. Ventricular gallbladder shunts: an alternative procedure in hydrocephalus. J Pediatr Surg. 1987;22:609.
106 Irby PB3rd, Wolf JSJr, Schaeffer CS, et al. Long-term follow-up of ventriculoureteral shunts for treatment of hydrocephalus. Urology. 1993;42:193.
107 Aldana PR, James HE, Postlethwait RA. Ventriculogallbladder shunts in pediatric patients. J Neurosurg Pediatr. 2008;1:284.
108 Borgbjerg BM, Gjerris F, Albeck MJ, et al. A comparison between ventriculo-peritoneal and ventriculo-atrial cerebrospinal fluid shunts in relation to rate of revision and durability. Acta Neurochir (Wien). 1998;140:459.
109 Casey AT, Kimmings EJ, Kleinlugtebeld AD, et al. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27:63.
110 Choudhury AR. Avoidable factors that contribute to the complications of ventriculoperitoneal shunt in childhood hydrocephalus. Childs Nerv Syst. 1990;6:346.
111 Piatt JHJr, Carlson CV. A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg. 1993;19:233.