CHAPTER 42 Ventricular Septal Defect
Ventricular septal defects (VSDs) are among the most common congenital cardiac disorders seen at birth, but are less frequently seen as isolated lesions in adulthood. The reason for this is that a VSD in an infant is usually either large and nonrestrictive, permitting equalization of pressures between the ventricles and leading to heart failure necessitating early surgical repair, or small and restrictive, closing spontaneously without intervention. A VSD can occur as an isolated lesion or in combination with other congenital cardiac anomalies. Blood flow across the defect is typically left to right, and depends on the size of the defect and the pulmonary vascular resistance.1
DEFINITION
Type IV defects also account for 10% of all VSDs, and are called muscular defects. They may be single, but are commonly multiple. Most commonly, multiple defects occur in the apical trabecular septum.1–5
PREVALENCE AND EPIDEMIOLOGY
In the United States, VSD accounts for approximately 20% to 40% of all congenital cardiac malformations—approximately 1 to 2 cases per 1000 live births. Studies have shown that the prevalence of VSD has increased in the United States during the past 30 years. A twofold increase in the prevalence of VSD was reported by the U.S. Centers for Disease Control and Prevention from 1968 to 1980. This increase is primarily attributed to more sensitive detection through echocardiography.6,7
ETIOLOGY AND PATHOPHYSIOLOGY
Alcohol and illicit drug use have been identified as possible risk factors for VSD. There is a twofold increase in the risk of VSD associated with maternal cocaine use during pregnancy. Abnormal blood flow to the heart owing to the vasoconstricting effects of cocaine is a postulated reason for these increases.5,6
VSDs result from a lack of growth or a failure of alignment or fusion of the portions of the ventricular septum. Between weeks 4 and 8 of gestation, the single ventricular chamber is effectively divided into two. This division is accomplished with the fusion of the membranous portion of the ventricular septum, the endocardial cushions, and the bulbous cordis. The muscular portion of the ventricular septum grows cephalad as each ventricular chamber enlarges, eventually meeting with the right and left ridges of the bulbous cordis. The ridges fuse with the tricuspid and mitral valves and the endocardial cushions. The tricuspid valve is separated from the pulmonary valve by the infundibulum, but the mitral valve remains in fibrous continuity with the aortic valve. The endocardial cushions develop concomitantly and finally fuse with the bulbar ridges and the muscular portion of the septum. The fibrous tissue of the membranous portion of the interventricular septum makes the final closure and separates the two ventricles.1–5
The VSD permits a left-to-right shunt to occur at the ventricular level, causing increased pulmonary blood flow, right ventricular volume overload, and compromise of systemic cardiac output. These effects depend on the magnitude of the shunt, which is a function of the size of the VSD and the status of the pulmonary vascular bed. A small VSD with high resistance to flow permits only a small left-to-right shunt. A large interventricular communication allows a large left-to-right shunt. Measuring the ratio of pulmonary-to-systemic circulation (Qp/Qs ratio) is useful for determining the magnitude of the shunt. Over time, as pulmonary vascular resistance increases, right ventricular function is compromised. Eventually, irreversible damage may occur within the pulmonary vascular bed, leading to a reversal of the shunt and the development of increasing cyanosis (Eisenmenger syndrome).1–5
MANIFESTATIONS OF DISEASE
Clinical Presentation
The clinical significance of VSD depends on its size and location, and the level of pulmonary and left ventricular outflow resistance. A restrictive VSD produces a small shunt and does not cause significant hemodynamic derangement. In contrast, a large VSD may progressively lead to higher pulmonary resistance and, finally, to irreversible pulmonary vascular changes, producing Eisenmenger physiology with reversal of the shunt to a right-to-left shunt.1,2
A VSD typically produces a harsh grade IV-VI holosystolic murmur. The murmur is best heard along the left sternal border, is usually louder at the third and fourth intercostal spaces, and is widely transmitted over the precordium with the production of a palpable thrill. The murmur of VSD does not radiate to the left axilla, as with mitral regurgitation, and does not increase in intensity with inspiration, as with tricuspid regurgitation. The smaller and more restrictive the VSD, the more turbulent the flow through the defect, and the louder the murmur. Large defects, with appreciable left-to-right shunts, have wide splitting of S2, which varies with respiration. If pulmonary hypertension develops, the holosystolic murmur diminishes, and the thrill disappears. Cyanosis may become evident, and polycythemia follows. A pulmonary ejection sound may also be noted.1–4
Of patients with congenital VSD, 20% have additional cardiac abnormalities. Most abnormalities are detected at the initial assessment stage; however, aortic valve leaflet prolapse and pulmonary stenosis may also develop subsequently. Aortic regurgitation may result from the high-velocity flow beneath a poorly supported right aortic cusp near the VSD.1
Imaging Technique and Findings
A small VSD usually manifests with no radiographic abnormality. With larger defects, chamber enlargement and pulmonary vascular changes are present, depending on the defect size and volume of the shunt. The typical plain film appearance of a patient with VSD is shunt vascularity associated with right and left ventricular and left atrial enlargement (Fig. 42-1). In young children, and especially in children with larger shunts, the plain film appearance is less specific. There is cardiac enlargement, but interstitial edema is superimposed on shunt vascularity, often giving the appearance of heart failure (Fig. 42-2). With increasing pulmonary resistance, right ventricular and pulmonary artery pressure increase, changing the appearance of the plain film to that of pulmonary hypertension (Fig. 42-3).1
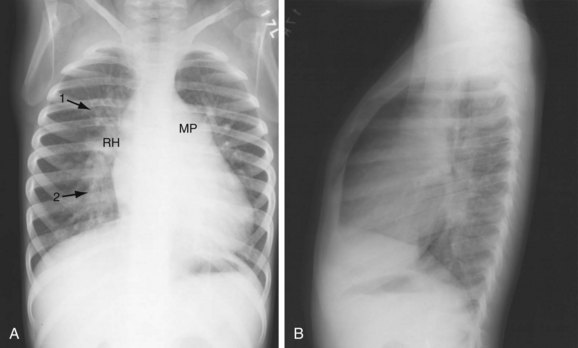
 FIGURE 42-2 Chest examination in a 6-year-old boy with a membranous VSD. A, Posteroanterior examination showing typical signs of shunt vascularity and cardiac enlargement. The main pulmonary artery (MP) and right hilar (RH) and upper lobe (1 arrow) and lower lobe (2 arrow) pulmonary arteries are enlarged. The left pulmonary artery lies posterior to the leftward rotated heart. The pulmonary arterial segments, although dilated, are unsharp (compare with the vessels in Fig. 42-1), typical of shunt vessels in young children. Right heart enlargement is indicated by the leftward displacement of the heart from the midline. B, Lateral examination shows filling of the retrosternal clear space (indicating right heart and pulmonary artery enlargement), and filling of the superior and inferior retrocardiac space, indicating left atrial and left ventricular enlargement.
FIGURE 42-2 Chest examination in a 6-year-old boy with a membranous VSD. A, Posteroanterior examination showing typical signs of shunt vascularity and cardiac enlargement. The main pulmonary artery (MP) and right hilar (RH) and upper lobe (1 arrow) and lower lobe (2 arrow) pulmonary arteries are enlarged. The left pulmonary artery lies posterior to the leftward rotated heart. The pulmonary arterial segments, although dilated, are unsharp (compare with the vessels in Fig. 42-1), typical of shunt vessels in young children. Right heart enlargement is indicated by the leftward displacement of the heart from the midline. B, Lateral examination shows filling of the retrosternal clear space (indicating right heart and pulmonary artery enlargement), and filling of the superior and inferior retrocardiac space, indicating left atrial and left ventricular enlargement.
Ultrasonography
Echocardiographic evaluation of VSD is a noninvasive modality that accurately delineates the morphology and associated defects (Figs. 42-4 through 42-6). Hemodynamic evaluation of the defect and the presence of elevated pulmonary artery pressure, insufficiency of the aortic valve, and distortion of the valve apparatus all are accurately evaluated by echocardiography. When limitations in image quality of TTE prevent adequate evaluation, TEE can be performed.8–10
Doppler echocardiography allows for the calculation of the Qp/Qs ratio, for estimation of gradients across the VSD, and for prediction of right-sided heart pressures. Combined color flow and pulsed Doppler assessments can provide substantial reliable information about the magnitude of left-to-right VSD flow and resistance, in most cases obviating an invasive catheter study. Echocardiography with color flow mapping is a more effective technique for detecting VSD than is cardiac catheterization. Contrast echocardiography also has been used to enhance the sensitivity and amplify the assessment of VSD by M-mode and two-dimensional techniques.9
A complete segmental echocardiographic study can provide accurate information about the size, location, and number of septal defects, and associated lesions. Perimembranous VSD is identified by septal dropout in the area adjacent to the septal leaflet of the tricuspid valve and below the right border of the aortic annulus. The subaortic VSD (type II) appears just below the posterior semilunar valve cusps, entirely superior to the tricuspid valve. The subpulmonary VSD (type I) appears as echo dropout within the outflow septum and extending to the pulmonary annulus. The inlet AV septal type of VSD (type III) extends from the fibrous annulus of the tricuspid valve into the muscular septum, and is often entirely beneath the septal tricuspid leaflet. Muscular VSD (type IV) is identified by echocardiography by echo-free septal areas located in the inlet, trabecular, or outlet portions.8–11
Computed Tomography
Dilated pulmonary arteries should alert the observer to the possibility of an intracardiac shunt (Fig. 42-7). When measured in the same axial section, the main pulmonary artery should be no greater in caliber than the ascending aorta. Dilated pulmonary arteries are a sign of increased pulmonary artery pressure and left-to-right shunting. Right-sided volume loading from the shunt is reflected in leftward cardiac rotation bringing the right ventricular free wall toward the left chest wall.12 Right ventricular hypertension is reflected in varying degrees of free wall hypertrophy and septal flattening. In contrast to the left-to-right shunt in ASD, the right ventricle in VSD is volume and pressure loaded, resulting in flattening and reversal of normal septal curvature throughout the cardiac cycle (Fig. 42-8). The defect of a VSD is a gap in a portion of the interventricular septum. Type II defects are found in the posterior portion of the septum, just inferior to the aortic valve (Fig. 42-9). Partial closure and closure by formation of a membranous septal aneurysm are common. Muscular defects appear as a break in the continuity of the interventricular septum, allowing communication between left and right ventricles (Fig. 42-10).13,14
Magnetic Resonance Imaging
ECG gated MRI provides excellent anatomic delineation of the size and location of the VSD, and the presence and character of associated congenital malformations. Cine MRI acquisitions provide functional assessment of chamber function and shunt flow. MRI has a sensitivity and specificity of greater than 90% in delineating VSD. Black blood imaging (e.g., double inversion recovery fast spin-echo imaging) provides high-resolution visualization of defects (Figs. 42-11 through 42-13). Use of gradient-echo acquisition takes advantage of the high intrinsic contrast between the blood pool and shunt flow, and the three-dimensional capability of MRI for explicit demonstration of an intracardiac shunt in any anatomic plane (Figs. 42-14 and 42-15).15 Myocardial mass and ventricular volumes and function can be obtained by planimetric analysis of gradient-echo acquisitions. Shunt volume, valvular function, pressure gradients, and blood velocity and flow across valves and conduits can be estimated using velocity encoded cine phase contrast imaging (Fig. 42-16).16,17
Nuclear Medicine and Positron Emission Tomography
Nuclear imaging is generally less frequently used in the assessment of VSDs, but may be employed in some patients for assessing cardiac function. Equilibrium radionuclide angiography uses ECG gating to define the temporal relationship between the nuclear data and the components of the cardiac cycle. The left anterior oblique view is used for qualitative analysis of global left ventricular ejection fractions and stroke volumes using a radioactivity count-based approach throughout the cardiac cycle.18–20
Right ventricular function is best evaluated using first-pass radionuclide angiography techniques. Analyzing right and left ventricular function independently during this brief transit is possible. Regional function also can be assessed from generated outlines of ventricular silhouettes. Tc 99m radiopharmaceuticals are also used for first-pass studies. The finding of abnormal right ventricular ejection fraction in the absence of intrinsic right ventricular disease is evidence of acquired pulmonary hypertension in the presence of a VSD.18–20
Angiography
Although echocardiography and MRI generally obviate the need for invasive catheterization for the evaluation of VSD, catheterization can give accurate measurements of pulmonary vascular resistance, pulmonary reactivity, and volume of shunting. Response to pulmonary vasodilators can be determined and can guide therapy especially in patients in whom there is suspicion for the development of shunt reversal (Eisenmenger syndrome). Angiography can provide information on the location of a defect, number of defects, and degree of aortic insufficiency. The left ventriculogram (Fig. 42-17) in the long-axis view is the most useful projection for angiographic identification of a perimembranous VSD. The defect can be seen as a discontinuity of the chamber immediately beneath the anterior contour of the aortic valve. A muscular VSD (Fig. 42-18) is observed as a discontinuity in the septal wall away from the semilunar and AV valves.1–4
In patients with left-to-right shunts, oxygen saturation is increased in the right ventricle compared with the right atrium because of shunting of highly oxygenated blood from the left ventricle into the right ventricle causing the so-called step-up. Cardiac catheterization also provides precise information about the magnitude of the left-to-right shunt, and estimates pulmonary artery pressures and resistance. A pulmonary vascular resistance of greater than 8 Wood units with pulmonary vasodilation testing during catheterization indicates inoperability.1–4
SYNOPSIS OF TREATMENT OPTIONS
Medical
The natural history of VSD encompasses a wide spectrum, ranging from spontaneous closure to congestive cardiac failure and death in early infancy. VSD is generally considered a surgical disorder, and no specific medical therapy is available. Patients with VSD-associated symptoms of heart failure may require heart failure therapy, however, including but not limited to diuretics, digitalis, and angiotensin-converting enzyme inhibitors. Patients with arrhythmias may require antiarrhythmic therapy. Medical management further includes endocarditis antibiotic prophylaxis for all patients.1
The medical treatment of infants with a VSD depends on the size of the VSD. A tiny defect (Roger disease) does not require any intervention. Medium and larger defects require various degrees of medical management and eventual surgical closure. The goals of therapy are to relieve symptoms, to minimize frequency and severity of respiratory infections, and to facilitate normal growth. Restricting the activities of a child with an isolated VSD is rarely necessary. Children with a VSD should be followed-up at least once or twice yearly to detect changes in the clinical picture that suggest the development of pulmonary vascular changes.1,2
Patients in whom the VSD is small do not require treatment because approximately 80% of such lesions get smaller or heal spontaneously. A VSD that either decreases in size or closes completely during the first year of life presents no future problems to the patient. Patients with VSD and pulmonary vascular disease who are deemed inoperable because of irreversibly elevated pulmonary vascular resistance require more intensive support and symptomatic therapy as cyanosis progresses and activity becomes more limited. Improvement in the symptoms associated with the polycythemia of Eisenmenger syndrome may be provided by partial exchange transfusion for red blood cell volume reduction.1–3
Surgical and Interventional
In patients with small and even some moderate VSDs with normal pulmonary artery pressure, there is a tendency for the VSD to become smaller and eventually close. Surgery is not indicated in these patients. For symptomatic patients, or patients with larger VSDs and elevated pulmonary resistance, surgical closure is indicated. The timing of surgery is very important, however. The ideal time to intervene is when the likelihood of spontaneous VSD closure is lowest, and the risk of irreversible pulmonary vascular disease and ventricular dysfunction is minimized. This is a narrow time frame, which is sometimes difficult to ascertain unless close follow-up is exercised.1
In type I VSD, the risk of irreversible aortic valve damage caused by cusp prolapse leads to earlier repair. With perimembranous and muscular defects, surgery may be delayed 1 year or more if the infant is thriving, and the pulmonary artery pressure is not elevated. Multiple VSDs present a different problem. If a large shunt is present and persists longer than 6 to 8 weeks, pulmonary artery banding and removal after age 2 years with an attempt at septation is reasonable. Banding is also a reasonable repair for a VSD complicated by straddling or overriding of the AV valves.1
Besides surgical repair, percutaneous transcatheter device occlusion of perimembranous and, in some instances, muscular VSD can be performed. This technique may be associated with further complications, including conduction anomalies and valve dysfunction. Treatment of patients with VSD and aortic regurgitation is controversial. In patients with a large, hemodynamically significant left-to-right shunt, repair of the VSD is indicated, but aortic regurgitation is repaired only if it is at least moderate in severity. If a type I VSD without aortic regurgitation is identified in early childhood, an argument for prophylactic closure of the VSD can be made to prevent the future potential complication of aortic valve incompetence. In the presence of moderate or severe aortic regurgitation, valve surgery would be required (valvuloplasty is preferred to valve replacement).8
INFORMATION FOR THE REFERRING PHYSICIAN
KEY POINTS
 VSDs are among the most common congenital cardiac disorders seen at birth, but are less frequently seen as isolated lesions in adulthood.
VSDs are among the most common congenital cardiac disorders seen at birth, but are less frequently seen as isolated lesions in adulthood. Blood flow across the defect is typically left to right, and depends on the size of the defect and the pulmonary vascular resistance.
Blood flow across the defect is typically left to right, and depends on the size of the defect and the pulmonary vascular resistance. The clinical significance of the VSD depends on its size and location, the level of pulmonary pressure, and the left ventricular outflow resistance.
The clinical significance of the VSD depends on its size and location, the level of pulmonary pressure, and the left ventricular outflow resistance. The initial imaging modality should generally be a chest radiograph, which gives certain clues for the presence or absence of a VSD.
The initial imaging modality should generally be a chest radiograph, which gives certain clues for the presence or absence of a VSD.Ammash NM, Warnes CA. Ventricular septal defects in adults. Ann Intern Med. 2001;135:812-824.
Boxt LM. MR imaging of congenital heart disease. Magn Reson Imaging Clin North Am. 1996;4:327-359.
Boxt LM. Magnetic resonance and computed tomographic evaluation of congenital heart disease. J Magn Reson Imaging. 2004;18:827-847.
Boxt LM, Rozenshtein AR. MR imaging of congenital heart disease. Magn Reson Imaging Clin North Am. 2003;11:27-48.
Fayad LM, Boxt LM. Chest film diagnosis of congenital heart disease. Semin Roentgenol. 1999;34:228-248.
Hagler DJ, Edwards WD, Seward JB, et al. Standardized nomenclature of the ventricular septum and ventricular septal defects, with applications for two-dimensional echocardiography. Mayo Clin Proc. 1985;60:741-752.
Minette MS, Sahn DJ. Ventricular septal defects. Circulation. 2006;114:2190-2197.
Moodie DS. Diagnosis and management of congenital heart disease in the adult. Cardiol Rev. 2001;9:276-281.
Soto B, Becker AE, Moulaert AJ, et al. Classification of ventricular septal defects. Br Heart J. 1980;43:332-343.
Wang ZJ, Reddy GP, Gotway MB, et al. Cardiovascular shunts: MR imaging evaluation. RadioGraphics. 2003;23(Spec No):S181-S194.
1 Minette MS, Sahn DJ. Ventricular septal defects. Circulation. 2006;114:2190-2197.
2 Kanter KR. Management of infants with coarctation and ventricular septal defect. Semin Thorac Cardiovasc Surg. 2007;19:264-268.
3 Soto B, Bargeron LMJr, Diethelm E. Ventricular septal defect. Semin Roentgenol. 1985;20:200-213.
4 Moodie DS. Diagnosis and management of congenital heart disease in the adult. Cardiol Rev. 2001;9:276-281.
5 Ammash NM, Warnes CA. Ventricular septal defects in adults. Ann Intern Med. 2001;135:812-824.
6 Ferencz C, Correa Villasenor A. Epidemiology of cardiovascular malformations: the state of the art. Cardiol Young. 1991;1:264-284.
7 Lewis DA, Loffredo CA, Correa-Villasenor A, et al. Descriptive epidemiology of membranous and muscular ventricular septal defects in the Baltimore-Washington Infant Study. Cardiol Young. 1996;6:281-290.
8 Tweddell JS, Pelech AN, Frommelt PC. Ventricular septal defect and aortic valve regurgitation: pathophysiology and indications for surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:147-152.
9 Helmcke F, de Souza A, Nanda NC, et al. Two-dimensional and color Doppler assessment of ventricular septal defect of congenital origin. Am J Cardiol. 1989;63:1112-1116.
10 Miller-Hance WC, Silverman NH. Transesophageal echocardiography (TEE) in congenital heart disease with focus on the adult. Cardiol Clin. 2000;18:861-892.
11 Sutherland GR, Godman MJ, Smallhorn JF, et al. Ventricular septal defects: two dimensional echocardiographic and morphological correlations. Br Heart J. 1982;47:316-328.
12 Schad N, Stark P. The radiologic features of cardiac rotation: a pictorial essay. J Thorac Imaging. 1992;3:81-87.
13 Berthaux XA, Brenot P, Angel C, et al. Multirow detector computed tomography assessment of intraseptal dissection and ventricular pseudoaneurysm in postinfarction ventricular septal defect. Circulation. 2001;104:497-498.
14 Romaguera R, Paya R, Ridocci F, et al. Ventricular septal defect as casual finding in non-invasive CT-angiography. Eur Heart J. 2008;29:1438.
15 Wang ZJ, Reddy GP, Gotway MB, et al. Cardiovascular shunts: MR imaging evaluation. RadioGraphics. 2003;23(Spec No):S181-S194.
16 Didier D, Higgins CB. Identification and localization of ventricular septal defect by gated magnetic resonance imaging. Am J Cardiol. 1986;57:1363-1368.
17 Brenner LD, Caputo GR, Mostbeck G, et al. Quantification of left to right atrial shunts with velocity-encoded cine nuclear magnetic resonance imaging. J Am Coll Cardiol. 1992;20:1246-1250.
18 Jimenez-Angeles L, Medina-Banuelos V, Valdes-Cristerna R, et al. Analysis of ventricular contraction by factorial phase imaging with equilibrium radionuclide angiography. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1081-1084.
19 Chin BB, Metzler SD, Lemaire A, et al. Left ventricular functional assessment in mice: feasibility of high spatial and temporal resolution ECG-gated blood pool SPECT. Radiology. 2007;245:440-448.
20 Sharir T. Gated myocardial perfusion imaging for the assessment of left ventricular function and volume: from SPECT to PET. J Nucl Cardiol. 2007;14:631-633.

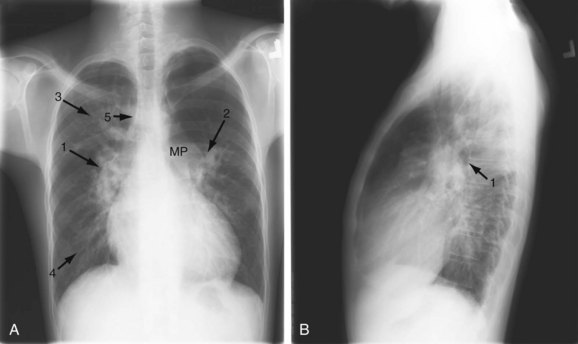
 FIGURE 42-1
FIGURE 42-1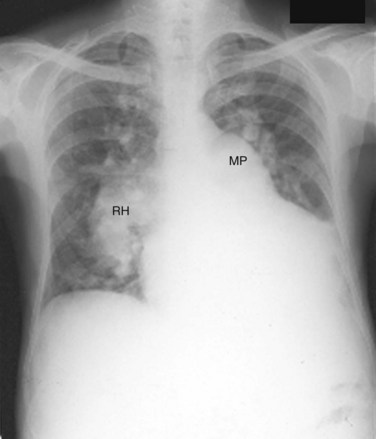
 FIGURE 42-3
FIGURE 42-3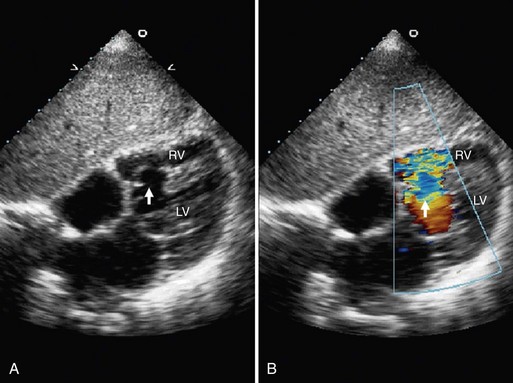
 FIGURE 42-4
FIGURE 42-4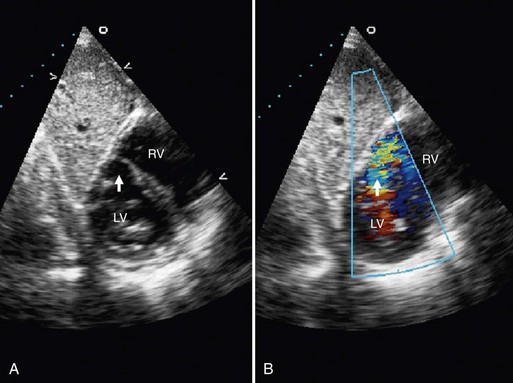
 FIGURE 42-5
FIGURE 42-5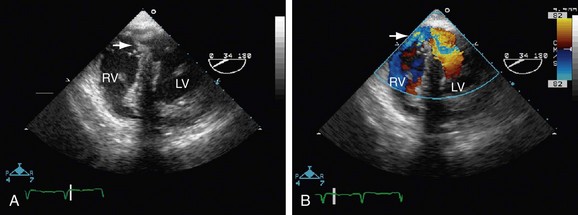
 FIGURE 42-6
FIGURE 42-6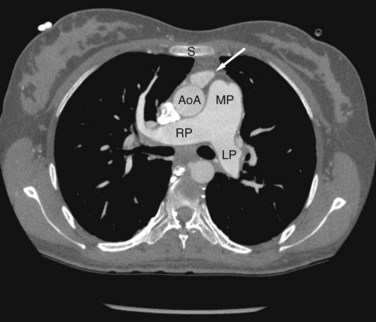
 FIGURE 42-7
FIGURE 42-7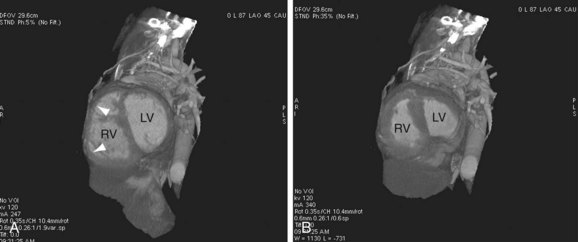
 FIGURE 42-8
FIGURE 42-8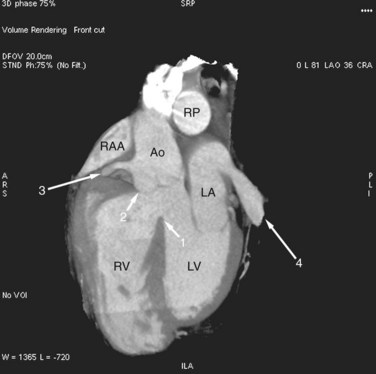
 FIGURE 42-9
FIGURE 42-9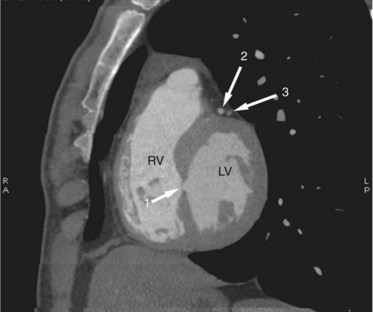
 FIGURE 42-10
FIGURE 42-10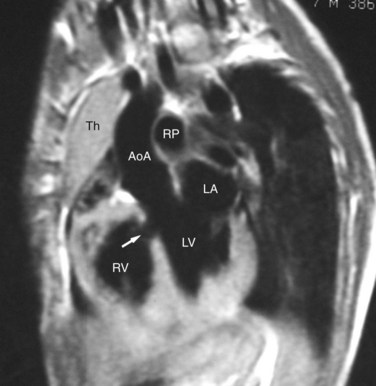
 FIGURE 42-11
FIGURE 42-11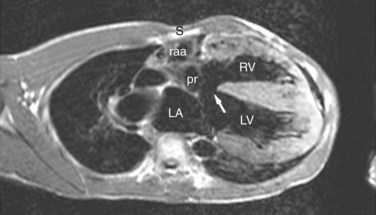
 FIGURE 42-12
FIGURE 42-12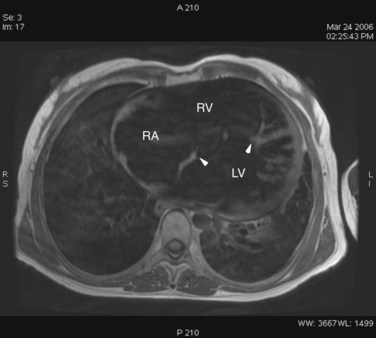
 FIGURE 42-13
FIGURE 42-13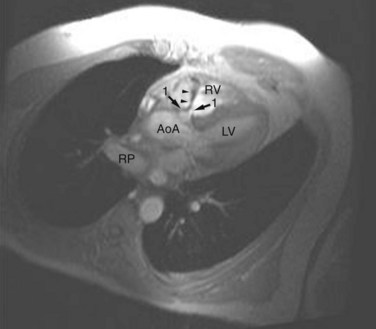
 FIGURE 42-14
FIGURE 42-14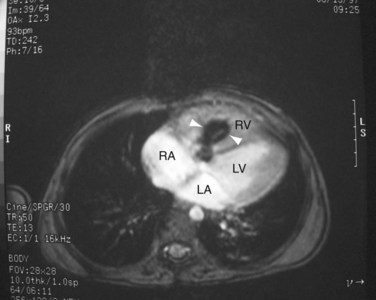
 FIGURE 42-15
FIGURE 42-15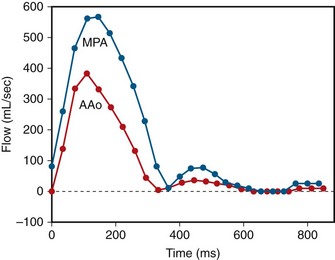
 FIGURE 42-16
FIGURE 42-16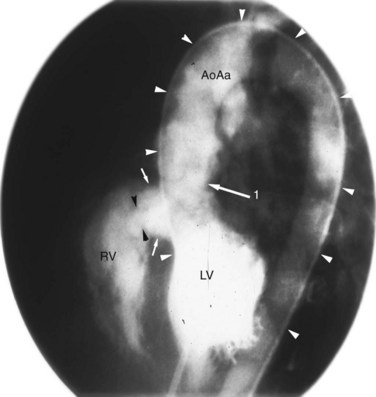
 FIGURE 42-17
FIGURE 42-17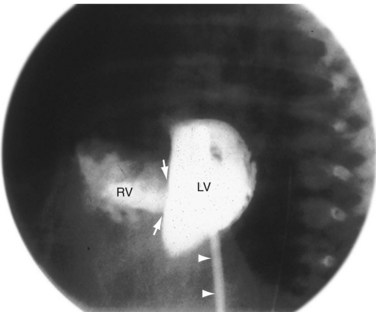
 FIGURE 42-18
FIGURE 42-18


