CHAPTER 8 Physics of Cardiac Computed Tomography
Computed tomography (CT) was first introduced in 1972 and has since become an indispensable tool for noninvasive diagnostic imaging. It is a robust and reproducible technique and its benefit for noninvasive cardiac imaging was soon envisioned and desired. Technical limitations prevented its applicability until the mid-1980s, but it was not until the introduction of multislice CT scanners with subsecond rotation times and higher volume coverage in the early 2000s1–3 that noninvasive cardiac CT imaging evolved into clinical routine.4–11 Among its various applications, coronary CT angiography (coronary CTA) has emerged as a valuable tool for imaging the coronary arteries, for which it has been shown to quantify coronary artery stenosis with high accuracy and especially to have a high negative predictive value for coronary artery disease (CAD). Imaging the heart and coronary anatomy poses high demands on spatial and temporal resolution. The small coronary arteries, with diameters typically of 2 to 4 mm, require optimal image quality and maximum resolution for reliable visualization and analysis. Because of their location close to the heart muscle and consequent strong movement during the cardiac cycle, their imaging requires almost freezing the heart to avoid motion artifacts. Finally, the scan time for covering the complete heart volume should be within the time of a single breath-hold to avoid breathing artifacts and limit radiation exposure and amount of contrast administered.
First introduced in 2004,12 64-slice CT systems helped establish CT imaging of the heart in clinical routine and were until recently considered state of the art. This section will therefore focus on 64-slice CT because it is still the widest available technology. The most recent developments, such as dual-source CT with high-pitch scanning and volume acquisition CT scanners, are expected to improve cardiac imaging further. These will be discussed at the end of this chapter.
CARDIAC IMAGING REQUIREMENTS
Two goals can generally be defined for cardiac imaging. First, the imaging modality should allow virtual “freezing” of the motion of the heart to avoid motion artifacts. The time interval of the cardiac cycle contributing to an image should be short enough to allow the depiction of at least slow motion heart phases without motion artifacts. Second, the imaging technique should allow the selection of defined heart phases of the cardiac cycle. Because motion intensity varies over the heart cycle, with the slowest motion (usually) present in the diastolic phase, imaging is typically performed during this phase of the cardiac cycle to minimize image blurring caused by motion. A temporal resolution of 250 ms is generally considered sufficient for motion-free imaging for slow to moderate heart rates of up to 65 beats/min, because the length of the diastolic phase shortens with increasing heart rate.13 However, if imaging is desired for phases other than diastole (e.g., for functional information), more stringent requirements are necessary. Posing very rigorous demands on image quality, such as motion-free imaging in all heart phases, would require a temporal resolution shorter than 50 ms.
IMAGE RECONSTRUCTION TECHNIQUES FOR OPTIMIZED TEMPORAL RESOLUTION
The optimal temporal resolution is achieved with algorithms that use the minimum amount of required data for image reconstruction.2,3,14 Generally, CT image reconstruction requires at least a parallel projection data angle range of 180 degrees. As a consequence, the temporal resolution will be on the order of trot/2, where trot is the time required for a full rotation of the scanner. However, the temporal resolution varies with the position in the scan field of view, which is determined by the temporal interval covered by the data contributing to the reconstruction to a particular image point. In a centered region of the scan field of view, the temporal resolution equals half the rotation time. In clinical practice, the heart should be sufficiently centered during data acquisition to maintain stable temporal resolution of half the rotation time. Half-scan reconstruction techniques preserve the spatial resolution compared with standard reconstructions, but because of less data contributing to an image, the noise is usually increased by a factor of 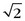 . Special considerations apply to today’s third-generation modern CT scanners, in which data acquisition is performed in fan beam geometry. Data is acquired over a range of 180 degrees plus fan angle and is then transformed to parallel beam geometry using so-called “rebinning techniques.” The previously described half-scan reconstruction algorithm is then applied to achieve the best temporal resolution.
. Special considerations apply to today’s third-generation modern CT scanners, in which data acquisition is performed in fan beam geometry. Data is acquired over a range of 180 degrees plus fan angle and is then transformed to parallel beam geometry using so-called “rebinning techniques.” The previously described half-scan reconstruction algorithm is then applied to achieve the best temporal resolution.
With the introduction of multislice helical CT, almost isotropic three-dimensional resolution became feasible. Dedicated reconstruction algorithms are necessary to optimize temporal resolution, helical artifact reduction, and volume coverage. The basic concepts for phase-correlated imaging for multislice CT have been introduced with four-slice systems1,15 and are used in slightly different forms in today’s scanners.
Modern 64-slice CT scanners all offer rotation times of less than 0.5 second and down to 0.3 second. Thus, it is possible to achieve a temporal resolution of 150 ms, which allows for motion-free imaging in the diastolic phase for moderate to high heart rates. As a consequence, the usage of β blockers, intravenous or oral, to slow down the heart rate is common practice.8 Nevertheless, motion artifacts remain the most important challenge for coronary CTA, even with 64-slice CT systems.
ECG Synchronization
Traditionally, the patient’s electrocardiogram (ECG) has been used to correlate acquisition and reconstruction of CT data with heart motion. Data can be acquired in phases with slow motion to minimize motion artifacts; alternatively, the acquisition and reconstruction of different heart phases are possible for functional studies. Usually, the R wave of the ECG is used as a reference point, with the R wave being the most pronounced peak in the ECG. Alternative approaches to derive the motion signal retrospectively have been proposed, using information from CT raw data directly or from the generated images.16 However, for phase-correlated data acquisition, the ECG signal is used.
The start point of data acquisition or, respectively, data selection for image reconstruction is usually determined relative to the R waves of the ECG signal by a phase parameter. Three phase selection strategies are commonly used (Fig. 8-1)—relative delay (e.g., at 65% of the R-R interval), absolute delay, and absolute reverse (e.g., 400 ms after and before the R wave, respectively). For the relative delay, a temporal delay relative to the start point of the previous R wave is used to determine the start point of the ECG-triggered acquisition or the data reconstruction interval. This delay is determined individually for each heart cycle. For prospective data acquisition, the R-R interval times have to be prospectively estimated based on prior R-R intervals. The absolute delay uses a fixed delay time after onset of the R wave to trigger the data acquisition or start point of the reconstruction. Absolute reverse describes a fixed time prior to the onset of the next R wave for starting data acquisition or the start point of the reconstruction interval. For ECG-triggered data acquisition, the position of the next R wave again has to be estimated from previous R-R interval times.
TECHNICAL PRINCIPLES OF CARDIAC IMAGE ACQUISITION
Prospective Triggering
Prospective triggering uses the ECG signal to start the scan (data acquisition and x-ray on time). This technique is typically used with axial scans, meaning that the patient table is at rest during data acquisition. For imaging, the user specifies the delay time after the R wave; usually, the scan delay time is selected so that the data are acquired during the diastole of the heart. For each position, the scanner is continuously rotating until the ECG trigger signal indicates the desired heart phase, when the x-ray is switched on and data are acquired for 180 degrees plus the fan angle. The number of reconstructed images corresponds to the number of detector rows used. With the x-ray then switched off, the table advances to the next position, which typically corresponds to the total detector collimation (slight overlaps are necessary for contiguous volume coverage). This is repeated until the heart range is covered; thus, the technique is commonly referred to as a “step-and-shoot mode” (Fig. 8-2). Image reconstruction is performed with previously described half-scan algorithms, and so the temporal resolution corresponds to half the rotation time.
However, cardiac CT examinations have recently been subjected to increased scrutiny because of their relatively high radiation dose.17,18 Prospective triggered data acquisition offers the highest dose savings and hence has experienced renewed interest.19–21 In addition, improvements in ECG synchronization, such as so-called adaptive triggering or padding techniques, allow the system to react to ectopic beats and allow image reconstruction in a narrow phase range beyond the specified acquisition phase.
Retrospective Gating
With submillimeter slice thickness, 64-slice CT offers considerable advantages over earlier technology in terms of spatial resolution and volume coverage. Two different scanner concepts were introduced. The volume concept provides a further increase in volume coverage by increasing the number of detector rows to 64. The resolution concept uses 32 physical detector rows in combination with double sampling in the longitudinal z direction. Thus, 64 overlapping slices are simultaneously acquired to increase longitudinal resolution independently of the pitch and reduce helical artifacts.12
With the advent of multislice helical CT scanning, various image reconstruction concepts have been introduced to take full advantage of volumetric ECG-gated data acquisition.1,14 The reconstruction process typically comprises two steps. The first step generally involves multislice helical interpolation algorithms to compensate for the continuous table movement during data acquisition. The second step uses half-scan reconstruction techniques for the generated transverse data segments to optimize temporal resolution.
Retrospective ECG-gated reconstruction allows reconstruction of the images at an arbitrary phase of the cardiac cycle. The start position for image reconstruction is shifted relative to the R peak and a stack of images can be reconstructed at adjacent z positions, depending on the volume coverage of the scanner. Figure 8-3A shows successive stacks reconstructed in consecutive heart cycles covering the cardiac volume. The entire volume can be reconstructed without gaps in the illustrated heart phases, as well as any other user-selected heart phases. This is a prerequisite for true functional volume imaging of the heart.
or, in symbols,
where d is the table feed, trot is the rotation time, T is the total collimation, and fH is the heart rate. The maximum pitch (pmax) consequently has to fulfill the following condition:
This implies that for helical CT of the heart, the pitch is limited to relatively small values. For example, a heart rate of 60 beats/min and a rotation time of 0.33 second results in a maximum pitch of 0.33. As shown in Figure 8-3A, the slope of the detector position is proportional to the pitch that is correctly chosen so as to ensure continuous volume coverage. If the pitch is too high, volume gaps will occur between image stacks reconstructed at different heart cycles (see Fig. 8-3B). Typically, the pitch is set prior to the scan and stays constant throughout the scan, even if the heart rate changes. Therefore, the pitch should be chosen according to the minimum expected heart rate for an individual patient.
Multisegment Image Reconstruction
Dedicated reconstruction techniques can improve the temporal resolution of an image by using data from multiple subsequent heart cycles. Using so-called multisegment image reconstruction, the temporal resolution can be improved up to trot/(2N) by using data of N subsequent heart cycles.1 However, for an increasing number of segments used, the achieved improvement in temporal resolution comes at the expense of slower volume coverage because the pitch is no longer only limited by heart rate but also by the number of heart cycles used for reconstruction.
Finally, with multisegment reconstruction with N > 1 segments, the optimal temporal resolution can only be achieved if the patient’s heart rate and gantry rotation time of the scanner are properly desynchronized (Fig. 8-4A). Only if the start and end of the segments fit together and form a complete half-scan can the half-scan be subdivided into N segments of equal size and the temporal resolution be equal to trot/(2N). If the heart rate and rotation time are (partially) synchronized, the segments cover different temporal data intervals and the temporal resolution is determined by the segment with the longest duration (see Fig. 8-4B). Thus, the benefits of multisegment reconstruction are only available for a few “sweet spots,” determined by rotation time and heart rate (Fig. 8-5).
Optimal Reconstruction Phase
An important advantage of retrospective ECG gating is the ability to analyze the ECG trace after the scan for possible modification of the synchronization of the ECG signal and data reconstruction. Particularly for irregular heart rates or inappropriately detected R peaks (e.g., extrasystoles), the editing of the time interval and R peak positions has a beneficial effect.22
The intensity of the heart motion varies substantially during the heart cycle, with strong motion during the contraction in systole and slow motion during relaxation in diastole. Generally, the diastolic phase is the most suitable phase for motion-free imaging of the heart, but with increasing heart rate and reduced duration of the diastolic phase, imaging in the systolic phase more frequently yields superior image quality. Additionally, the course of the ECG trace shows variations for the individual patient in addition to its dependency on heart rate. Thus, the prediction of the optimal heart phase for imaging is generally not feasible. It has been common practice to determine the optimal phase adaptively by reconstructing multiple heart phases and analyzing them individually. More recently, automatic algorithms have been introduced to determine the optimal reconstruction phase in a single reconstruction step.23
ECG Tube Current Modulation
Whereas retrospective ECG-gated data acquisition is the more robust acquisition mode compared with ECG-triggered data acquisition, it is also associated with high radiation exposure because of the continuous data acquisition at relatively low pitch. The low pitch is a consequence of the required phase-consistent coverage of the heart; however, coverage of all heart phases is not necessary if only a single heart phase—for example, in diastole—is targeted for image reconstruction. In this case, a significant portion of the acquired data is redundant. ECG-controlled tube current modulation, in which the tube current is reduced during phases of less importance, has been shown to reduce the radiation dose effectively during retrospective ECG-gated cardiac helical CT.3,18 Algorithms have been developed that restrict the nominal tube current to a predefined time window and reduce the tube current during the remaining time to about 20% of its nominal value (Fig. 8-6). The benefit of not switching off x-ray radiation completely lies in the preserved ability of continuous volume reconstruction in all phases of the cardiac cycle; specifically, functional imaging is still feasible. For images acquired at low dose and used for functional analysis, the image quality in terms of signal-to-noise ratio can be improved by using thick slices in image reconstruction.
The relatively high radiation exposure associated with ECG-gated helical data acquisition compared with prospective ECG trigger techniques has resulted in improved modulation techniques; these allow further reduction of the tube current outside the specified heart phase, typically to 4% of its nominal value. Although functional imaging might no longer be possible, it still maintains the advantages of volumetric data acquisition and flexibility to react to heart rate irregularities.20
For prospectively gated sequential scanning techniques, the duration of the x-ray is usually set to a predetermined time window based on the R-R interval length of the heart rate prior to beginning the scan. Recently introduced algorithms, referred to as padding or adaptive triggering, use a new technique that includes continuous analysis of the individual heart rate, which thus can vary the location and duration of the scanning window.19,24
NEW DEVELOPMENTS IN CARDIAC CT IMAGING
Dual-Source CT
Motion artifacts remain the most important challenge for coronary CTA, even with 64-slice CT systems. Thus, the use of β blockers prior to scanning is an important step of the cardiac CT examination to control the heart rate to values generally below 60 beats/min. If stable imaging at all heart rates is desired, the target temporal resolution is less than 100 ms, independent of the heart rate. For conventional third-generation CT systems, this would require an increased gantry rotation speed to ensure a clinically robust improvement of the temporal resolution.25 An alternative scanner concept that provides enhanced temporal resolution without the need for a faster rotation time is dual-source CT (DSCT), with two tubes and two corresponding detectors.26,27 In the first clinical realization of this type of system, two acquisition systems are mounted onto the rotating gantry, with an angular offset of 90 degrees (SOMATOM Definition, Siemens Healthcare, Forchheim, Germany).28 In the axial plane, one detector covers the entire field of view (50 cm in diameter), whereas the other detector is restricted to a smaller, central field of view (26 cm). The two detectors are equivalent in the longitudinal direction, using the manufacturer’s high-resolution concept of providing simultaneous acquisition of 64 overlapping 0.6-mm slices by means of z-flying focal spot technology.
An important consequence of the high stable temporal resolution is the optional use of β blockers. Studies have shown that high diagnostic accuracy is achieved in patients with high heart rates without the administration of β blockers.29,30
Efforts to reduce radiation dose to the patient are continued in dual-source systems. For retrospective ECG-gated examinations, the table feed can be optimally adapted to the heart rate as a consequence of the single-segment reconstruction approach. Thus, the pitch is significantly increased at elevated heart rates, thereby not only reducing the examination time but also the radiation dose to the patient. For low heart rates, radiation dose values for ECG-gated helical cardiac examinations with DSCT are similar to those obtained with comparable conventional CT systems, and even lower values are achieved for higher heart rates.31 Finally, ECG-triggered prospective scanning modes are available for patients with sufficiently stable heart rates.24
The newest generation of DSCT technology introduces a high-pitch scan mode to acquire a helical CT data set while covering the entire volume of the heart in one cardiac cycle.32 Prerequisites for the feasibility of such a scan mode were technical improvements, such as a faster rotation time (0.28 second), a larger detector coverage (two 4-cm detectors that acquire 128 slices each), and pitch values up to 3.2 in dual-source scanning. The scan mode is an implementation of the prospective triggered ECG mode; however, with the fast helical scan, the heart is covered within a heartbeat. Images are reconstructed with a temporal resolution that corresponds to 25% of the rotation time (75 ms). With a total acquisition time on the order of 250 ms, it is interesting to note that throughout the data set, subsequent images are reconstructed at later points of time in the cardiac cycle. The immediate advantage of this scan mode, however, is the elimination of the additional radiation exposure that typically occurs during low-pitch gated scanning. Thus, it might be possible to achieve effective dose values of 1 mSv or lower.33
CT with Volume Detectors
Technologic advances since the establishment of 64-slice cardiac CT have diverged, with one approach focusing on temporal resolution (DSCT; see earlier) and the second focusing on increasing volume coverage.34–36 With area detector technology and related cone beam reconstruction techniques, the aim is to allow coverage of the entire cardiac anatomy in a single heartbeat without movement of the table. The first commercially available system was introduced in 2007 (Aquilion ONE, Toshiba Medical Systems, Tustin, Calif). It uses a 320-row detector and 0.5-mm detector elements. Thus, single heartbeat cardiac imaging becomes feasible with prospective ECG-triggered image acquisition. The theoretical advantages of this system is the elimination of stair-step artifacts between slabs of images from different heart cycles, which are inherent to techniques that require multiple gantry rotations to cover the whole heart volume. The diagnostic quality of this one shot that covers the entire heart, however, depends on the available temporal resolution. With a rotation time of 0.35 second, it is necessary to use multisegment reconstruction techniques to achieve temporal resolution beyond 175 ms.37
SUMMARY AND OUTLOOK
KEY POINTS
 The goal of cardiac CT imaging is to image the heart free of motion artifacts in a specified phase of the cardiac cycle. This requires high temporal and spatial resolution combined with fast volume coverage and minimal radiation dose.
The goal of cardiac CT imaging is to image the heart free of motion artifacts in a specified phase of the cardiac cycle. This requires high temporal and spatial resolution combined with fast volume coverage and minimal radiation dose. Data acquisition and image reconstruction are synchronized to the heart motion using the patient’s ECG signal.
Data acquisition and image reconstruction are synchronized to the heart motion using the patient’s ECG signal.Achenbach S, Ropers D, Kuettner A, et al. Contrast-enhanced coronary artery visualization by dual-source computed tomography—initial experience. Eur J Radiol. 2006;57:331-335.
Kopp A, Schröder S, Küttner A, et al. Coronary arteries: retrospectively ECG-gated multidetector row ct angiography with selective optimization of the image reconstruction window. Radiology. 2001;221:683-688.
Kuettner A, Beck T, Drosch T, et al. Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol. 2005;45:123-127.
Mollet N R, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318-2323.
Nieman K, Cademartiri F, Lemos PA, et al. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002;106:2051-2054.
Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664-666.
1 Kachelriess M, Ulzheimer S, Kalender W. ECG-correlated image reconstruction from subsecond multi-slice spiral CT scans of the heart. Med Phys. 2000;27:1881-1902.
2 Taguchi K, Anno H. High temporal resolution for multi-slice helical computed tomography. Med Phys. 2000;27:861-872.
3 Ohnesorge B, Flohr T, Becker C, et al. Cardiac imaging by means of electrocardiographically gated multisection spiral CT—initial experience. Radiology. 2000;217:564-571.
4 Achenbach S, Ulzheimer S, Baum U, et al. Noninvasive coronary angiography by retrospectively ECG-gated multi-slice spiral CT. Circulation. 2000;102:2823-2828.
5 Becker C, Knez A, Ohnesorge B, et al. Imaging of noncalcified coronary plaques using helical CT with retrospective EKG gating. AJR Am J Roentgenol. 2000;175:423-424.
6 Knez A, Becker C, Leber A, et al. Non-invasive assessment of coronary artery stenoses with multidetector helical computed tomography. Circulation. 2000;101:e221-e222.
7 Nieman K, Oudkerk M, Rensing B, et al. Coronary angiography with multi-slice computed tomography. Lancet. 2001;357:599-603.
8 Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147-154.
9 Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552-557.
10 Hendel RC, Patel MR, Kramer CM, et al. American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology. ACCF/ACR/SCCT/SCMR/ASNC/ NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497.
11 Hoffmann U, Nagurney JT, Moselewski F, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251-2260.
12 Flohr T G, Stierstorfer K, Ulzheimer S, et al. Image reconstruction and image quality evaluation for a 64-slice CT scanner with z-flying focal spot. Med. Phys. 2005;32:2536-2547.
13 Hong C, Becker CR, Huber A, et al. ECG-gated reconstructed multi-detector row ct coronary angiography: effect of varying trigger delay on image quality. Radiology. 2001;220:712-717.
14 Flohr T, Ohnesorge B, Bruder H, et al. Image reconstruction and performance evaluation for ECG-gated spiral scanning with a 16-slice CT system. Med Phys. 2003;30:2650-2662.
15 Kachelriess M, Kalender W. Electrocardiogram-correlated image reconstruction from subsecond spiral computed tomography scans of the heart. Med Phys. 1998;25:2417-2431.
16 Kachelriess M, Sennst DA, Maxlmoser W, Kalender WA. Kymogram detection and kymogram-correlated image reconstruction from subsecond spiral computed tomography scans of the heart. Med Phys. 2002;29:1489-1503.
17 Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056-1065.
18 Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500-507.
19 Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008;246:742-753.
20 Stolzmann P, Scheffel H, Schertler T, et al. Radiation dose estimates in dual-source computed tomography coronary angiography. Eur Radiol.. 2008;18:592-599.
21 Steigner ML, Otero HJ, Cai T, et al. Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 2009;25:85-90.
22 Cademartiri F, Mollet NR, Runza G, et al. Improving diagnostic accuracy of MDCT coronary angiography in patients with mild heart rhythm irregularities using ECG editing. AJR Am J Roentgenol. 2006;186:634-638.
23 Ruzsics B, Gebregziabher M, Lee H, et al. Coronary CT angiography: automatic cardiac-phase selection for image reconstruction. Eur Radiol. 2009;19:1906-1913.
24 Stolzmann P, Leschka S, Scheffel H, et al. Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology. 2008;249:71-80.
25 Halliburton SS, Stillman AE, Flohr T, et al. Do segmented reconstruction algorithms for cardiac multi-slice computed tomography improve image quality? Herz. 2003;28:20-31.
26 Robb R, Ritman E. High-speed synchronous volume computed tomography of the heart. Radiology. 1979;133:655-661.
27 Ritman E, Kinsey J, Robb R, et al. Three-dimensional imaging of heart, lungs, and circulation. Science. 1980;210:273-280.
28 Flohr TG, McCollough CH, Bruder H, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256-268.
29 Scheffel H, Alkadhi H, Plass A, et al. Accuracy of dual-source CT coronary angiography: First experience in a high pre-test probability population without heart rate control. Eur Radiol. 2006;16:2739-2747.
30 Achenbach S, Ropers U, Kuettner A, et al. Randomized comparison of 64-slice single- and dual-source computed tomography coronary angiography for the detection of coronary artery disease. JACC Cardiovasc Imaging. 2008;1:177-186.
31 McCollough CH, Primak AN, Saba O, et al. Dose performance of a 64-channel dual-source CT scanner. Radiology.. 2007;243:775-784.
32 Achenbach S, Marwan M, Schepis T, et al. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3:117-121.
33 Hausleiter J, Bischoff B, Hein F. Feasibility of dual-source cardiac CT angiography with high-pitch scan protocols. J Cardiovasc Comput Tomogr. 2009;3:236-242.
34 Kondo C, Mori S, Endo M, et al. Real-time volumetric imaging of human heart without electrocardiographic gating by 256-detector row computed tomography: initial experience. J Comput Assist Tomogr. 2005;29:694-698.
35 Mori S, Endo M, Tsunoo T, et al. Physical performance evaluation of a 256-slice CT-scanner for four-dimensional imaging. Med Phys. 2004;31:1348-1356.
36 Mori S, Endo M, Obata T, et al. Properties of the prototype 256-row (cone beam) CT scanner. Eur Radiol. 2006;16:2100-2108.
37 Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535-546.

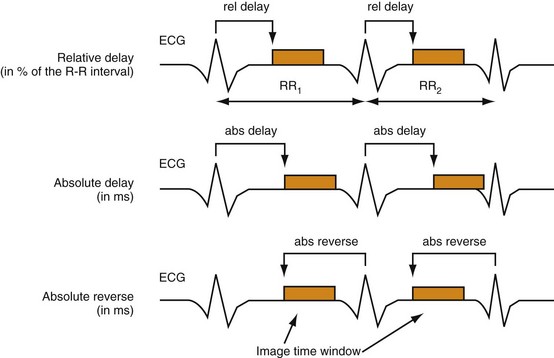
 FIGURE 8-1
FIGURE 8-1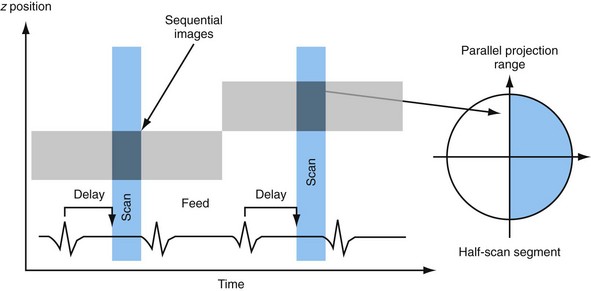
 FIGURE 8-2
FIGURE 8-2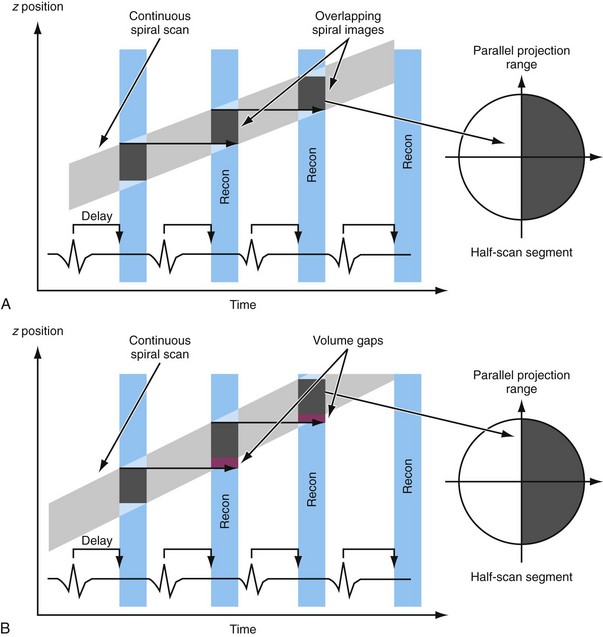
 FIGURE 8-3
FIGURE 8-3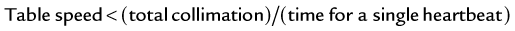
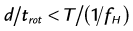
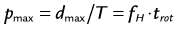
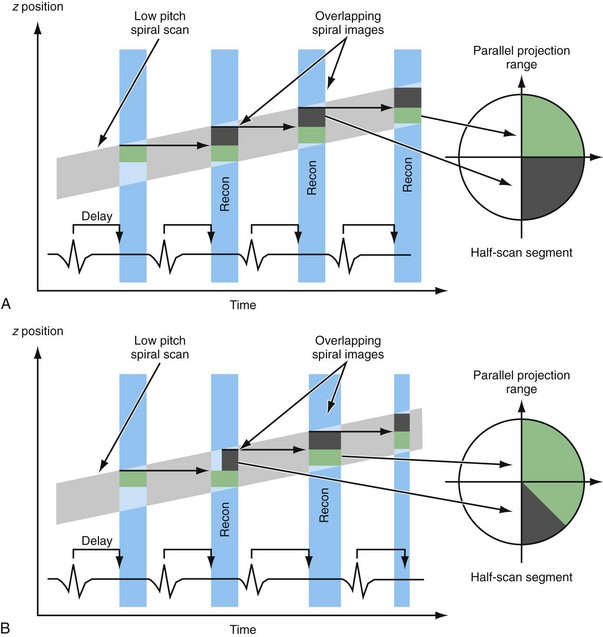
 FIGURE 8-4
FIGURE 8-4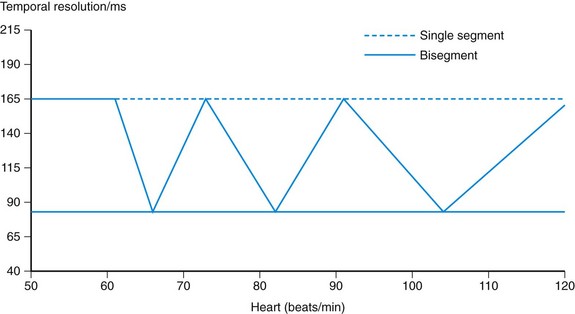
 FIGURE 8-5
FIGURE 8-5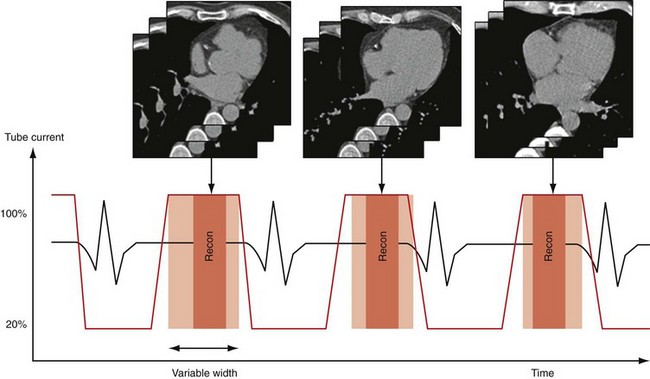
 FIGURE 8-6
FIGURE 8-6

