Chapter 30 Ventricular Arrhythmias in Congenital Heart Disease
Pathophysiology
Most information concerning patients with ventricular tachycardia (VT) and congenital heart disease pertains to tetralogy of Fallot, as compared with other forms of congenital heart disease. Tetralogy of Fallot is the most common cyanotic congenital cardiac malformation. The core lesion is an underdeveloped subpulmonary infundibulum, which is superiorly and anteriorly displaced, resulting in the well-known tetrad of pulmonary stenosis, ventricular septal defect, aortic override, and right ventricular (RV) hypertrophy. Correction of the defect involves patch closure of the ventricular septal defect and relief of RV outflow tract (RVOT) obstruction, which typically requires resection of a large amount of RV muscle. When the procedure was first performed, it was not done through the tricuspid valve but required a ventriculotomy. The pulmonic valve annulus is usually small, and repair with a transannular patch leads to chronic pulmonic insufficiency, which can be severe if associated with downstream obstruction caused by significant pulmonary arterial stenosis. It has been hypothesized that ventricular arrhythmias in these patients are of the result of years of chronic cyanosis, followed by the placement of a ventriculotomy, increased RV pressures caused by inadequate relief of obstruction, and severe pulmonic regurgitation with RV dysfunction. Such factors can lead to myocardial fibrosis and result in the substrate for reentrant ventricular arrhythmias.1
Using intraoperative mapping, VT after surgical correction of tetralogy of Fallot has been classified into two types: VT originating from the RVOT, which is considered to be related to prior right ventriculotomy or reconstruction of the RVOT (Fig. 30-1); and VT originating from the RV inflow tract septum, which is thought to be related to closure of the ventricular septal defect.
The electrophysiological (EP) mechanism responsible for VT after surgical correction of congenital heart disease is typically a macroreentrant circuit within the RV around a scar or prosthetic materials used during surgical repair. Reentry circuit isthmuses are located within anatomically defined pathways bordered by unexcitable tissue. Four discrete anatomical isthmuses that often support VT have been identified. The most common isthmus is between the superior aspect of the tricuspid annulus and unexcitable scar/patch in the free wall of the RVOT. The other isthmuses exist between the pulmonic valve and RV free wall scar (in the absence of a transannular patch), between the septal patch and tricuspid annulus through the region of the ventriculo-infundibular fold, and between the septal patch and pulmonic valve.2,3
The sites of the diastolic activation and delayed conduction along the reentrant circuit have been shown to have significant abnormalities such as fibrosis, adiposis, and degeneration of the myocardium. The scattered surviving myocyte islets embedded in the extensive adiposis and/or fibrosis can form an electrical maze around the surgical suture area, resembling the histological findings in the border zone of infarcted myocardium. Furthermore, RV remodeling induced by pressure or volume load promotes hypertrophy and fibrosis, which can potentially result in slow conduction, providing the link between impaired hemodynamics and VT.3
Clinical Considerations
Epidemiology
Congenital heart disease is the most common form of birth defect, with an estimated 1% to 2% of live newborns afflicted by moderate or severe types. Ventricular arrhythmias late after repair of congenital heart disease are a common finding, predominantly in those with tetralogy of Fallot, and potentially contribute to sudden cardiac death (SCD) in this population. The incidence of late SCD has been debated but ranges from 1% to 5% during a follow-up period of 7 to 20 years after surgery. The incidence of arrhythmias generally increases as the patient with congenital heart disease ages. Patients with surgical correction of ventricular septal defect or pulmonary stenosis also have a higher than normal risk of serious ventricular arrhythmias and SCD.1 SCD is the most common cause of death in patients after repair of congenital heart disease, with a 25- to 100-fold increased risk compared with the general population.4
In patients with tetralogy of Fallot, serious ventricular arrhythmias are rare during the first 10 to 15 years following corrective surgery. This is followed by a steady increase in the incidence of ventricular arrhythmias, primarily VT, which are prevalent in 15% of adult patients late after surgery. The incidence of VT in this population is 11.9%, with an 8.3% risk of SCD by 35 years of follow-up.3–7
Although tetralogy of Fallot is typically cited as the archetypal lesion when VT in the adult congenital heart disease patient population is discussed, serious ventricular arrhythmias can also develop in other types of congenital heart malformations, even in the absence of direct surgical scarring to ventricular muscle, including congenital aortic stenosis, transposition of the great arteries when the RV supports the systemic circulation, severe Ebstein anomaly, certain forms of single ventricle, and ventricular septal defect with pulmonary arterial hypertension. The appearance of ventricular arrhythmias in these cases commonly coincides with deterioration in overall hemodynamic status.8 In the past 20 years, many patients with tetralogy of Fallot have undergone transatrial repair, operating on the RV through the tricuspid valve after right atriotomy. In these cases, there is no incision in the RV free wall around which reentry can occur, although reentry around the ventricular septal defect patch can still take place.
Risk Stratification
Noninvasive Risk Stratification
Although controversy still exits, considerable progress has been made toward identifying noninvasive risk factors for VT and SCD in congenital heart disease patients. Tetralogy of Fallot is perhaps the one condition for which such data are fairly extensive. Independent predictors of clinical VT include QRS duration equal to or greater than 180 milliseconds, late and rapid increase in QRS duration after surgery, dispersion of QRS duration on the surface electrocardiogram (ECG), increased QT interval dispersion, high-grade ventricular ectopy on Holter monitoring, complete heart block, older age at surgery (especially older than 10 years), presence of a transannular RVOT patch, increased RV systolic pressures, RVOT aneurysm, pulmonic and tricuspid regurgitation, and left ventricular diastolic dysfunction.1,6,7
Electrophysiological Testing
Despite advances in noninvasive risk stratification, identification of high-risk subgroups has not been sufficiently accurate to guide management decisions reliably. More recently, in patients with repaired tetralogy of Fallot, inducible monomorphic or polymorphic sustained VT by programmed ventricular stimulation was found to be a significant independent predictor of subsequent clinical sustained VT or SCD in patients with and without antiarrhythmic drug therapy, VT ablation, or implantable cardioverter-defibrillator (ICD). In this patient population, the rate of inducible sustained VT is approximately 35%, which is similar to that reported in patients with prior myocardial infarction (MI) and left ventricular ejection fractions less than 40% and spontaneous nonsustained VT. Additionally, the diagnostic value of EP testing (sensitivity, 77%; specificity, 80%; diagnostic accuracy, 79%) and prognostic significance (relative ratio, 4.7 for subsequent clinical VT or SCD) compares favorably with programmed ventricular stimulation in post-MI patients. Independent risk factors for inducible sustained VT were age greater than 18 years at the time of testing, palpitations, prior palliative surgery, frequent or complex ventricular ectopy, or nonsustained VT, and a cardiothoracic ratio of 0.6 or more on chest radiograph.7
Nonetheless, EP testing yield remains too imperfect and too impractical to be recommended as a general screening tool and is usually reserved for selected patients with concerning symptoms (e.g., palpitations, dizziness, or unexplained syncope) or Holter findings, when VT is suspected but not yet proven. Additionally, the subpopulation of patients deemed at intermediate risk of SCD based on a combination of other parameters may benefit most from risk stratification with an EP study.7,8
Principles of Management
Implantable Cardioverter-Defibrillator
Secondary prevention
ICD implantation is recommended in patients who have survived cardiac arrest or an episode of sustained VT with hemodynamic compromise.9 The most common anatomical diagnosis among device recipients is tetralogy of Fallot, followed by transposition of the great arteries and aortic stenosis. The rate of appropriate therapies for VT or ventricular fibrillation (VF) events in this patient population averages 9.8% per year and about 35% over a follow-up period of 5 years, with the median time to first shock less than 1 year.5,6,9,10
Primary prevention
Some subgroups of patients with congenital heart disease are recognized as being at risk for life-threatening ventricular arrhythmias and SCD, including those with large scars from a prior ventriculotomy (e.g., tetralogy of Fallot repair), and those who develop advanced degrees of ventricular dysfunction from long-standing hemodynamic burdens (e.g., congenital aortic stenosis, single ventricle). Recent data support the benefit of ICD implantation for primary prevention in patients deemed to be at high risk for SCD. Up to 44% of patients with tetralogy of Fallot repair who received prophylactic ICDs experience sustained ventricular tachyarrhythmias, and appropriate therapies occur in 7.7% of patients per year. These annual rates are comparable to those of other high-risk populations, including patients with primary prevention ICDs for ischemic, dilated, or hypertrophic cardiomyopathy. A combination of surgical, hemodynamic, ECG, and EP factors modulates the risk of appropriate shocks.5,6
As noted, multiple risk factors for VT and SCD in congenital heart disease patients have been identified in an effort to better define which are most likely to benefit from a primary prevention ICD. Recently, a risk score to predict appropriate ICD shocks in tetralogy patients with ICDs for primary prevention indications was derived from six clinical variables (surgical, hemodynamic, electrocardiographic, and EP factors) identified by multivariate analyses (Table 30-1). Patients with fewer than 3 points (low risk) experienced no appropriate shocks. In patients with 3 to 5 points (intermediate risk) and more than 5 points (high risk), appropriate shocks were received by 3.8% and 17.5% of patients per year, respectively.5 Although these risk-stratification schemes can provide reasonable sensitivity, they have suboptimal specificity for patients at highest risk because of the small population size and the relatively low incidence of SCD in congenital heart disease patients (approximately 2% per decade of follow-up in patients with tetralogy of Fallot repair).9 EP testing can potentially help to identify patients at risk for malignant ventricular arrhythmias; however, patient selection for screening with EP testing and the timing and frequency of testing remain to be elucidated. At present, there is no generally accepted scheme for rhythm surveillance in asymptomatic tetralogy patients. 5,7,8
TABLE 30-1 Risk Score for Appropriate Defibrillator Shocks in Primary Prevention in Patients with Tetralogy of Fallot
| Variable | Exponential Values of Beta Coefficients | Points Attributed |
|---|---|---|
| Prior palliative shunt | 3.2 | 2 |
| Inducible sustained ventricular tachycardia | 2.6 | 2 |
| QRS duration ≥180 msec | 1.4 | 1 |
| Ventriculotomy incision | 3.4 | 2 |
| Nonsustained ventricular tachycardia | 3.7 | 2 |
| Left ventricular end-diastolic pressure ≥12 mm Hg | 4.9 | 3 |
| Total points | 0–12 |
From Khairy P, Harris L, Landzberg MJ, et al: Implantable cardioverter-defibrillators in tetralogy of Fallot, Circulation 117:363-370, 2008.
Even in patients considered at risk for malignant ventricular arrhythmias, the benefit of ICD implantation should be carefully weighed against the risks of such a procedure in this unique group of patients. Transvenous implantation of the ICD may not be feasible in patients with the more complex varieties of congenital heart disease, necessitating epicardial insertion of the ICD leads with the added morbidity of a sternotomy or thoracotomy for epicardial electrodes. Even when transvenous implantation procedures are feasible, they can be very challenging in patients with distorted anatomy, requiring that the implanting physician be well acquainted with the details of congenital heart lesions and the types of surgical repairs. Because of the considerable variation in surgical techniques and individual anatomy, careful review of detailed operative reports is essential in these cases. Additionally, the ICD lead failure rate from both insulation breaches and conductor breaks is high in this patient population, exceeding 20% over 5 years (likely due to the young age and high activity level of this patient group), which results in added morbidity of corrective procedures. Moreover, the negative psychological impact of an implanted device and inappropriate shocks (occurring in up to 47%, predominantly caused by supraventricular tachycardias and lead failures) in a relatively young patient should not be underestimated.5,9,10
For all these reasons, ICD decisions in congenital heart disease patients should be individualized, taking into account the various risk factors, all of which must then be viewed in the context of the individual patient’s history and general hemodynamic status to refine the selection process.8–10
Antiarrhythmic Drug Therapy
Similar to patients with post-MI VT, antiarrhythmic agents are generally not considered a stand-alone therapy in congenital heart disease patients with sustained ventricular arrhythmias or cardiac arrest, but can be considered in two main settings: as adjunctive therapy in patients with an ICD and as preventive therapy in patients who do not want or are not candidates for an ICD. ICD patients who experience frequent symptoms or device discharges triggered by ventricular arrhythmias may benefit from adjunctive drug therapy. When antiarrhythmic drug therapy is required, beta blockers and sotalol are commonly used. Amiodarone can carry significant long-term risk of adverse events given the young age of the patient population. The efficacy of dofetilide and dronedarone has not been adequately evaluated.7,8
Surgical Repair
Surgical interventions to improve cardiac status and alleviate residual hemodynamic problems (such as septal defect or valvular regurgitation) may play a role in reducing arrhythmia risk in certain carefully selected congenital heart disease patients, especially those with new-onset or worsening arrhythmias. Furthermore, VT mapping and ablation can be performed intraoperatively at the time of cardiac surgery. Nonetheless, the impact of surgery on modifying the risk for SCD remains controversial.5,7,8
Catheter Ablation
Additionally, catheter ablation can be considered as isolated VT therapy for those patients with hemodynamically stable monomorphic and slow VT, and even then, follow-up EP studies are necessary to ensure that the same or different circuits cannot be induced before dismissing the need for an ICD.8
Furthermore, patients after tetralogy repair with a history of syncope and induced monomorphic VT during EP testing may be considered for either ICD placement or an attempt at catheter ablation of the VT with or without backup ICD implantation.2,7
Electrocardiographic Features
During normal sinus rhythm (NSR), QRS widening after tetralogy of Fallot repair is probably the result of a combined effect of the surgical injury to the myocardium and the right bundle branch, and from the RV enlargement. Therefore, a prolonged QRS duration cannot be considered the specific expression of delayed intraventricular conduction from an arrhythmogenic substrate, but a nonspecific marker of electrical instability.11
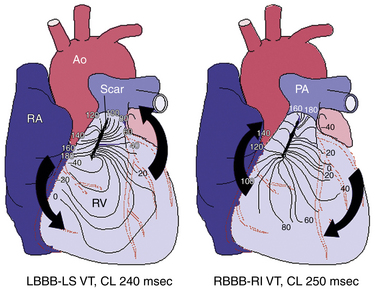
The VT is most commonly monomorphic and macroreentrant, rotating clockwise or counterclockwise around myotomy scars or surgical patches. QRS morphology during VT is determined by the pattern of ventricular activation around the scar. Most commonly, left or right bundle branch block with right inferior axis morphology is seen during clockwise rotation around the scar. Less commonly, left bundle branch block with left axis morphology is observed (Fig. 30-2).12 Right bundle branch block–like morphology can be present if the tachycardia exits on the septal aspect of the RV free wall.
Mapping
For VTs that are not mappable because of hemodynamic instability or termination during catheter manipulation or entrainment mapping, pace mapping during sinus rhythm is used to identify exit sites of the reentrant circuit. Reentry circuit isthmuses can be approximated by pace mapping at sites where the QRS morphology matches that of the VT with an S-QRS interval of at least 40 milliseconds. If the VT can be briefly tolerated, the catheter is moved to the presumed isthmus site during NSR, and the VT is reinduced to confirm the position within the circuit either by entrainment mapping or by termination during radiofrequency (RF) energy application.3
Electroanatomical mapping can be of value because the presence of multiple circuits and the complexity of anatomical alteration, particularly in the vicinity of surgical scar, can pose significant challenges to mapping and ablation procedures. Additionally, voltage mapping of the area of interest can help identify the area of scar and border zone and guide conventional mapping techniques. Furthermore, most reentrant circuit isthmuses are located within anatomically defined isthmuses bordered by unexcitable tissue. These boundaries can be identified by three-dimensional mapping during normal NSR. Transecting anatomical isthmuses by linear RF lesions during NSR can potentially eliminate the tachycardia, even when conventional mapping is not feasible because of poorly tolerated or noninducible tachycardia.1,3,11
When VT is short-lived, hemodynamically unstable, or cannot be reproducibly initiated, simultaneous multisite data acquisition using a noncontact mapping system (EnSite 3000; St. Jude Medical, St. Paul, Minn.) can help localize the VT site of origin. Propagation of activation within the RV can be traced throughout the whole tachycardia cycle by analyzing color-coded isopotential maps to identify the protected zone of the reentrant circuit between surgical barriers, anatomical barriers, or both. Additionally, dynamic substrate mapping allows the creation of voltage maps from a single cardiac cycle and provides the ability to identify low-voltage areas, as well as fixed and functional block, on the virtual endocardium through noncontact methodology.13
Ablation
The critical isthmus of the VT circuit is the usual target of ablation. A good pace map and entrainment with concealed QRS fusion and a prolonged S-QRS interval indicate more precisely an adequate site for ablation. Linear ablation lesions can also be performed using data obtained from substrate mapping to target anatomical isthmuses between surgical and structural lines of block. In tetralogy of Fallot patients, most VT circuits use the anatomical isthmus between the RVOT free wall scar or patch and the tricuspid annulus or pulmonic valve, and/or the septal scar or patch and either the tricuspid annulus or the pulmonic valve. Ablation lines transecting these anatomical isthmuses eliminate the VT in most patients. In one report, all of the ablation lines that needed to be created extended from 10 to 40 mm.3,14
RF ablation is usually performed with an open saline-irrigated catheter (power limit, 50 W), a closed irrigation catheter, or an 8-mm-tip catheter (power limit, 70 W). Ablation is performed during VT when possible. If VT is terminated or slowed during RF application, additional lesions are placed to connect the adjoining anatomical boundaries across the VT circuit isthmus, as defined by voltage mapping and pace mapping techniques, and RF lesions are placed during NSR until unipolar pacing fails to capture. After completion of the lesions, programmed stimulation is repeated to reassess tachycardia inducibility.3
Ablation success for any particular VT is defined by lack of reinducibility. Completeness of linear RF lesion lines can be validated by demonstrating absence of capture during pacing along the line, double potentials, and activation mapping during pacing from above or below the line.14
The experience with catheter ablation of VT in congenital heart disease is limited. Several reports have described single-center experiences spanning several eras of technological advances. In a report of 20 patients with VT and congenital heart disease, 50% of patients were unable to undergo ablation because of VT noninducibility, hemodynamic instability, access or anatomical problems, or proximity of the ablation target to the His bundle. The acute success rate for mappable VTs was 83%, with a long-term recurrence rate of 40%. In another report, immediate noninducibility of sustained or nonsustained VT was achieved in 15 of 16 patients (94%) after the ablation procedure. Repeat ventricular stimulation 5 to 7 days later revealed noninducibility in 14 patients (88%). At successful ablation sites, a good pace map during sinus rhythm could be found in 15 of the 16 patients (94%). However, an area of slow conduction, defined as mid-diastolic low-amplitude endocardial potential, could be found in only 3 patients (19%). The mean activation time of the endocardial electrogram preceding the QRS complex on the surface ECG at the ablation site was –69 ± 16 milliseconds. In 9 of 11 patients with inducible sustained VT (82%), entrainment with concealed fusion and a prolonged S-QRS interval (10 to 120 milliseconds) could be demonstrated. A recent report of 11 patients with VT after repair of congenital heart disease used electroanatomical substrate mapping during NSR in addition to conventional mapping, when feasible. RF ablation eliminated VT in all patients, with 91% remaining free of VT at a follow-up time of 30 ± 29 months. In another report, noncontact mapping facilitated successful ablation in 8 of 10 patients (80%).1,3,14
1. Walsh E.P. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007;115:3224-3234.
2. Khairy P., Stevenson W.G. Catheter ablation in tetralogy of Fallot. Heart Rhythm. 2009;6:1069-1074.
3. Zeppenfeld K., Schalij M.J., Bartelings M.M., et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241-2252.
4. Roos-Hesselink J.W., Karamermer Y. Significance of postoperative arrhythmias in congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S2-S6.
5. Khairy P., Harris L., Landzberg M.J., et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363-370.
6. Khairy P., Aboulhosn J., Gurvitz M.Z., et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868-875.
7. Le Gloan L., Khairy P. Management of arrhythmias in patients with tetralogy of Fallot. Curr Opin Cardiol. 2010;26:60-65.
8. Warnes C.A., Williams R.G., Bashore T.M., et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118:e714-e833.
9. Walsh E.P. Practical aspects of implantable defibrillator therapy in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S38-S40.
10. Tomaske M., Bauersfeld U. Experience with implantable cardioverter-defibrillator therapy in grown-ups with congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S35-S37.
11. Folino A.F., Daliento L. Arrhythmias after tetralogy of Fallot repair. Indian Pacing Electrophysiol J. 2005;5:312-324.
12. Josephson M.E. Catheter and surgical ablation in the therapy of arrhythmias. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia, Lippincott: Williams & Wilkins; 2008:746-888.
13. Pratola C., Baldo E., Toselli T., et al. Contact versus noncontact mapping for ablation of ventricular tachycardia in patients with previous myocardial infarction. Pacing Clin Electrophysiol. 2009;32:842-850.
14. Kriebel T., Saul J.P., Schneider H., et al. Noncontact mapping and radiofrequency catheter ablation of fast and hemodynamically unstable ventricular tachycardia after surgical repair of tetralogy of Fallot. J Am Coll Cardiol. 2007;50:2162-2168.