Chapter 50 Ventral and Ventrolateral Spine Decompression and Fusion
Ventral spinal decompression was described by Royle1 as early as 1928. This approach remained unused until 1956, when Hodgson and Stock2 reported the use of ventral spinal decompression in the treatment of tuberculous lesions. A progressive increase in the use of ventral and ventrolateral approaches for spinal decompression in treating various spinal lesions such as tuberculosis, pyogenic osteomyelitis, kyphotic deformities, neoplasms (primary and metastatic), and burst fractures3–6 has been observed. The types of ventral approaches used for different spinal levels are summarized in Table 50-1. Ventral and ventrolateral decompression principles for spinal tumors are discussed in depth. This discussion is followed by the management of other types of spinal pathology that may be treated through a ventral decompression.
TABLE 50-1 Classification of Ventral Surgical Approaches
| Spinal Segment | Surgical Approach |
|---|---|
| Cervicothoracic C7-T2 (see Fig. 50-11) | Extended ventral cervical (division of strap muscles) |
| Transsternal | |
| Cervicosternotomy (“trapdoor” approach) | |
| Upper thoracic T2-5 (see Fig. 50-10) | High dorsolateral thoracotomy (third-rib approach with mobilization of scapula) |
| T6-12 | Dorsolateral thoracotomy |
| Thoracolumbar T12-L2 (see Figs. 50-2 to 50-7) | Transthoracic/retroperitoneal with 10th to 12th rib resection; division of diaphragm |
| Lumbar | Retroperitoneal/flank |
| L2-5 | Transabdominal |
| Lumbosacral L5-sacrum | Ventral retroperitoneal (“pelvic brim” approach) |
Spinal Tumors
The surgical management of patients with spinal tumors and associated spinal cord compression has shifted from a laminectomy approach to ventral approaches with ventral decompression.3,7–9 Because most spinal tumors are located ventrally, a laminectomy can limit the degree of ventral resection and can exacerbate, or worsen, existing spinal instability associated with tumors that have destroyed spinal body segments. Several authors10,11 have reported that the results of ventral decompression of spinal tumors with spinal cord compression are significantly better in most cases when compared with radiation therapy (RT) alone or in conjunction with laminectomy. Patchell et al. demonstrated the superiority of combined surgery and radiation in their randomized study.12 Ambulation as their primary end point was significantly better in the combined surgery and radiation cohort. Dorsolateral approaches can also provide some degree of ventral spinal decompression, with the advantage that they allow for both ventral and dorsal decompression and dorsal stabilization with a single exposure. Because of the limited access of the contralateral ventral dural sac, however, this exposure is more suitable in cases with unilateral spinal canal and vertebral involvement. The results of ventral decompression suggest that this is an effective method to preserve and improve neurologic function in patients with neural compromise from primary and metastatic tumors of the thoracic and lumbar spine.3,4,13,14
The radiosensitivity of the tumor plays an important role in overall management. Radiosensitive tumors (e.g., lymphoma, myeloma, Ewing sarcoma, neuroblastoma) can be treated with radiation therapy initially, if the cord compression is the result of epidural tumor alone. Surgical decompression should be the initial treatment when a significant degree of compression can be attributed to bony or ligamentous fragments or spinal deformity, as a result of destruction by tumor. In cases of failed radiation therapy with persistent or recurrent spinal cord compression, surgical intervention is also recommended. The increased complications associated with operating on a previously radiated site also favor surgery before radiation. Surgical decompression can be considered for radiation-resistant tumors such as melanoma or renal cell carcinoma and intermediate radiosensitive tumors such as those of the lung, breast, or prostate. The rate of clinical progression provides the surgeon with valuable information when deciding the optimal timing of intervention. Rapid progression of symptoms is best managed with surgery because the effects of radiation can initially be associated with swelling. The medical status of the patient and ability to tolerate the surgery are also taken into consideration. The issue of radiation sensitivity of tumors is changing. The field of radiation treatment, much like the surgical field, has seen significant advances. Stereotactic delivery of radiation and image modulation are just two examples.
Preoperative Assessment
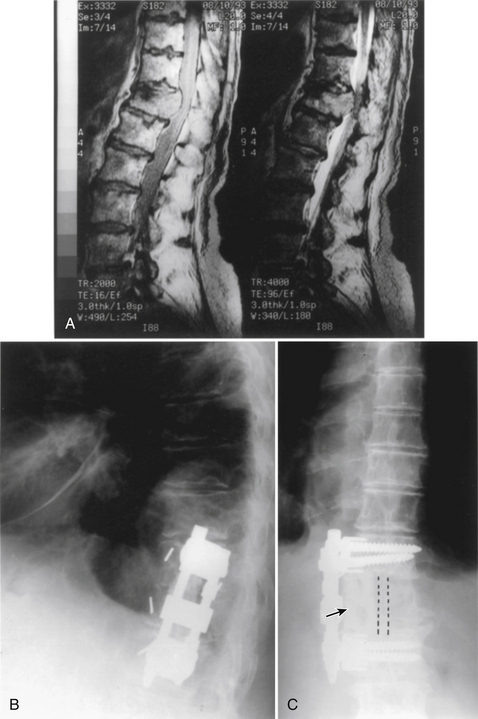
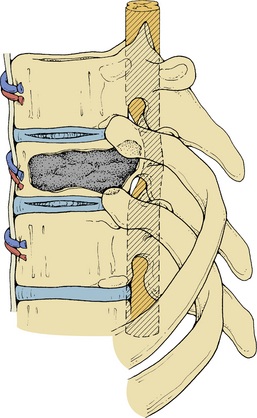
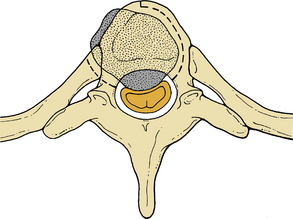
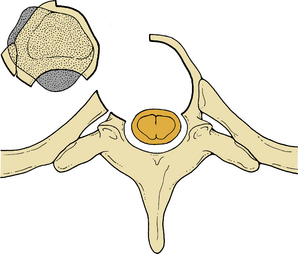
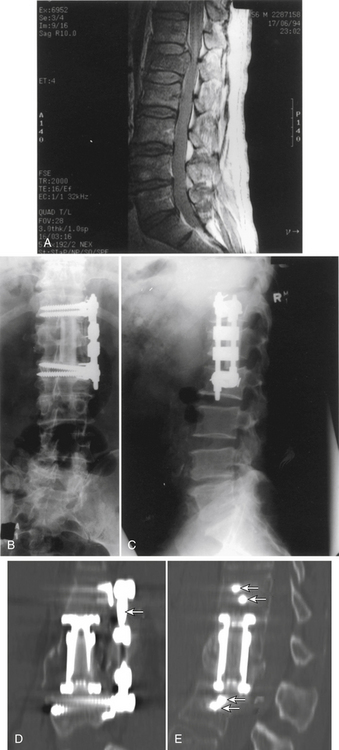
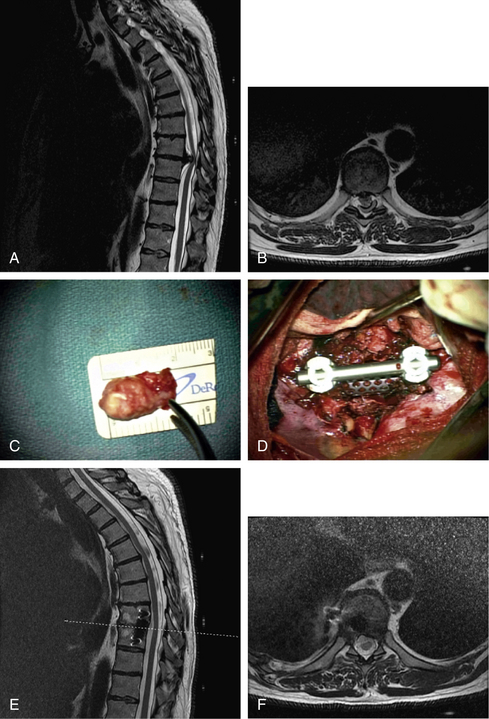
Initially, plain spine radiographs (anteroposterior and lateral) are used to determine the level and extent of tumor involvement. The spinal alignment can also be observed from these films. A CT scan without intravenous contrast at the appropriate spinal levels allows better definition of the degree of bony destruction of the spinal column. Although MRI is less precise than CT in outlining bony destruction, it provides the most precise means for illustrating the site and degree of spinal cord compression by tumor or soft tissues (Fig. 50-1). Myelography and postmyelography CT scan can be used when MRI is contraindicated or unavailable. The use of contrast for determining the degree of vascularity of the tumor with either CT scan or MRI is still at the research level. CT angiographies are becoming more useful in the spine as their resolution is increasing.
To avoid complications from intraoperative and postoperative instability of the spinal column, it is important to assess the spinal stability before performing a vertebral decompression. Stability can be considered in terms of the three-column theory, after the extent of bony destruction produced by tumor has been determined from imaging.15,16 Single-column involvement can be considered relatively stable. The additive destabilizing effects of decompression, however, must factor into the decision-making process.
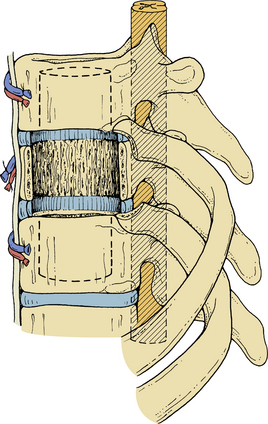
Anterior column and middle column involvement is the most common finding in symptomatic patients with spinal tumors and is frequently associated with some degree of vertebral body collapse and bony retropulsion into the spinal canal (see Fig. 50-1A). If these conditions are treated by corpectomy, with a strut graft used for fusion (see Figs. 50-1B and C), stability of the spinal column can be achieved.
When planning surgery for spinal tumors, an assessment of stability must also take into account angulation and alignment.16 Higher failure rates will be observed with a poorly aligned construct. It is also important to consider the nature of the tumor in terms of its capacity to infiltrate and destroy bony tissue and its response to RT or chemotherapy.
Surgical Management
Intraoperative Monitoring and Anesthetic Management
The authors recommend electrophysiologic monitoring, if available, including somatosensory and motor evoked potentials, at all vertebral levels involving the spinal cord. Electromyography (EMG) monitoring is also useful in the lumbar region, if segmental pedicular fixation is contemplated. Monitoring setup time and cost are definite drawbacks to this type of technology, although its use is generally supported in the literature.12,17–19 In approaches of the upper and middle thoracic spine, a double-lumen endotracheal tube allows the lung in the operative field to be deflated, improving the surgical exposure. In lesions of the lower thoracic and thoracolumbar regions, the lung can be retracted easily. Invasive arterial pressure monitoring and central venous pressure (CVP) monitoring and access are recommended in order to address any blood loss during the surgery.
Positioning
Patients are positioned in the full lateral decubitus position (Fig. 50-2) with an axillary roll placed under the dependent axilla to prevent neurovascular compromise. A mild flexion in the hip will assist mobilization of the psoas muscle should the exposure require it.
Incision and Exposure
Lesions involving the cervicothoracic (C7-T1), upper thoracic (T1-5), lower thoracic (T6-11), thoracolumbar (T12 and L1), and lumbar and sacral (L2 to sacrum) segments require specific approaches (see Table 50-1) and considerations that have been described in other chapters of this text.
Spinal Decompression
Exposure
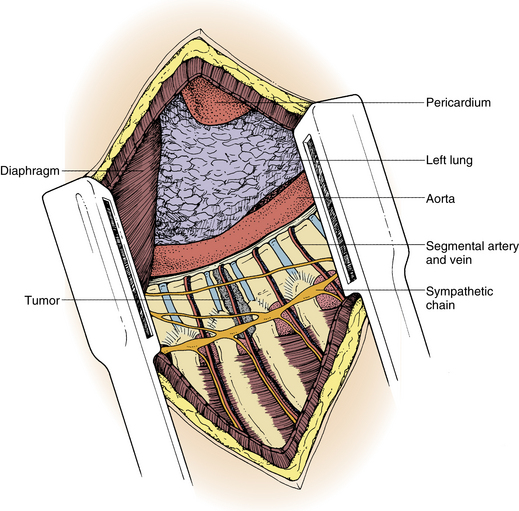
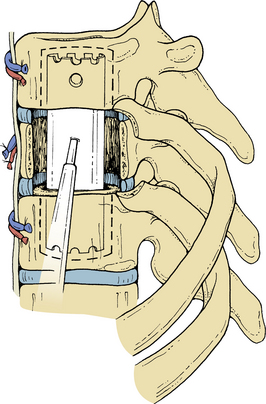
The pleura is sharply incised and reflected. A rib that overlies the level of the pathology in the midaxillary level is resected. The appropriate rib is identified using intraoperative imaging. The segmental vessels at the level of the pathology and of the vertebral bodies above and below the lesion (Fig. 50-3) are ligated and divided. Division of segmental vessels over the vertebral body in the middle of the body reduces the risk of vascular compromise of the spinal cord by taking advantage of collateral vessels from adjacent levels. The periosteum is reflected medially, and the anterior longitudinal ligament is identified.
Vertebral Body Decompression
The intervertebral discs above and below the involved vertebral body are identified and resected initially by sharp dissection (Fig. 50-4). Disc material is cleared with curettes and pituitary rongeurs. Removing the rib head will allow identification of the ipsilateral pedicle and its continuation into the vertebral body. The pedicle is an important marker for the orientation and position of the spinal canal. Using sharp curettes, rongeurs, and a high-speed drill, the vertebral body is resected ventrally to dorsally, except for a rim of the ventral portion of the vertebral body. This rim protects the aorta and inferior vena cava from accidental trauma. Resection of the vertebral body can progress as far as the opposite pedicle (Figs. 50-5 and 50-6), and the entire dorsal aspect of the vertebral body can be removed. Sufficient bone needs to be removed to clear the posterior longitudinal ligament of any compression of the dura. The dissection can also be continued dorsolaterally to allow decompression of the spinal nerve roots. The tumor involvement and the quality of the residual bone for instrumentation will determine the extent of bony removal. Techniques to augment the strength of the purchase into the bone are discussed in subsequent paragraphs.
Rostrocaudal Dissection
Special care is afforded to the cartilaginous end plates and the central regions of cancellous bone of vertebral bodies adjacent to the corpectomy site. Removal is performed using a small high-speed bur or curette (Fig. 50-7) or osteotomes and rongeurs, depending on the bone consistency. This allows troughs to be created in the vertebral bodies above and below the corpectomy site to allow subsequent reconstruction with a bone graft, an implant, or an acrylic graft. Preparing the end plates to accommodate the construct requires special attention. When the construct involves bone, either autograft or allograft, an eventual fusion will be desired. In this circumstance the end plates require adequate vascular supply for achieving fusion. The structural integrity of the graft can also fail if it is suboptimal or radiated. Methylmethacrylate (MMA), on the other hand, will not fuse, and the overall construct strength can be weakened by aggressive removal of the end plates. The risk of telescoping and the graph imploding into the vertebral body can also occur when the end plates are destroyed. MMA, however, will tolerate radiation.
Intraspinal Decompression
After adequate bony resection, decompression, and removal of all devitalized bone and tumor tissue, the posterior longitudinal ligament is resected to expose the dura mater that encloses the spinal cord and segmental nerve roots. Any tumor or bone impinging on the dural sac or nerve root is carefully removed to allow decompression of these structures. The goal of surgery should be radical tumor resection and decompression. In patients who have previously received RT, the posterior longitudinal ligament is frequently adherent to the dura mater and may be difficult to separate. In these cases, it may be advisable to leave it in situ. In most virgin cases the dissection plane between tumor and dura is easily exploited.
Avoiding Complications during Spinal Decompression
Complications Related to the Approach
A thoracotomy carries pulmonary risks such as atelectasis and pneumonia. The retroperitoneal exposure may injure the spleen, kidney, or ureter, and a prolonged postoperative ileus may occur. Any unrepaired defect in the abdominal wall or diaphragm may be the site of visceral herniation. Using vertebral body screws with manual confirmation of bicortical penetration requires considerable dissection of the contralateral aspect of the vertebral body, placing the aorta, inferior vena cava, and iliac vessels at risk if this is not done meticulously. Injury to the lumbar hypogastric plexus at L5 in males may be complicated by retrograde ejaculation. Chyle leak from damage to the thoracic duct is best managed with immediate repair.
Neurologic Injury
A carefully staged approach to spinal decompression with adequate exposure and identification of segmental vessels, nerve roots, and the dural tube markedly reduces the risk of nerve root and spinal cord injury. Nuwer20 concluded that intraoperative neurophysiologic monitoring is a cost-effective way of reducing the potential for a neurologic deficit. Intraoperative information is valuable not only to the prognosis but also by altering intraoperative and postoperative management. Hardware revision and removal during the operation may be performed on the basis of intraoperative neurophysiologic changes.
Spinal Reconstruction
Graft Material
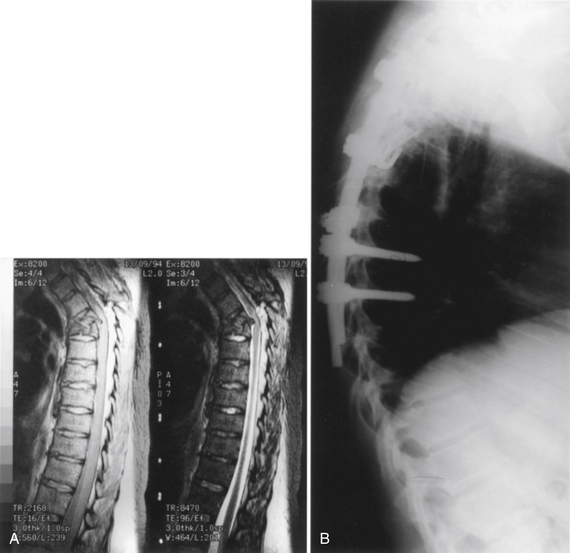
The appropriate type of material to use for spinal column reconstruction depends on the nature of the lesion and the patient’s life expectancy. In cases of trauma, for benign lesions, or for patients with malignant tumors who have a relatively long life expectancy (>2 years), reconstruction is best when using autogenous bone from the iliac crest or rib for single vertebral body defects. If two or more vertebral levels are involved with the neoplasm, an allograft (e.g., from the fibula or humerus) can be used. It is often useful to supplement the allograft strut with local autograft bone (Fig. 50-8).
For patients with an expected survival of 18 months or less, a synthetic construct using PMMA with or without titanium cages can be used. Expandable cages with only titanium construct have also been used to provide ventral support and reconstruction in difficult cases (see Fig. 50-8D and E). Biomechanical studies looking at intervertebral fixation have shown resistance of the implants to cyclical fatigue within typical normal physiologic loading and superior reduction of intervertebral motion and increased spinal stiffness.11,21–27 The use of expandable vertebral protheses or cages is gaining popularity. An example of such a device can be found with the X-mesh cage (DePuy Spine, Raynham, MA).
Reconstruction Technique
Reconstruction techniques aim to provide solid fixation of adjacent spinal segments. Failure of these constructs is usually the result of reconstruction material dislodging at proximal and/or distal ends where the material fits into adjacent spinal segments. Early spinal changes in the cancellous bone of adjacent vertebral segments, seen on postoperative MRI scans, can be an indication of potential failure of these regions to anchor the construct. Another important situation is one in which adjacent vertebral segments are involved with disease but are not collapsed and are not causing spinal cord compression. PMMA can be used to strengthen the adjacent bone. Alternatively, supplemental dorsal instrumentation may be required. The technique of vertebroplasty has grown significantly in popularity for osteopenic fractures in the elderly.28–32 This technique can complement and add strength to a ventral or even dorsal construct.
Synthetic Constructs
The technique of Errico and Cooper,7 in which PMMA is pressure injected into a Silastic tube that is fitted against the vertebral bodies above and below, provides an ideally suited construct for patients with metastatic lesions (Fig. 50-9). Silastic tubing of varying diameters (typically 15–20 mm) is cut to a measured length (from the outer edge of the upper and lower troughs of adjacent vertebral segments to the corpectomy site). One 6-mm-diameter hole is made in the center of the tubing with a rongeur, and three small holes are made laterally, two at the rostral end and one at the caudal end. Small bites are also made at the ends of the tubing to allow extrusion of cement overflow. The three smaller lateral holes allow air bubbles and excess cement to flow out easily. The side of the Silastic tubing facing the spinal cord is free of the central and lateral holes to avoid cement extrusion into the spinal canal. The Silastic tubing is passed into the space between two adjacent vertebral bodies at the corpectomy site and positioned so that there is no bending of the tubing that could obstruct cement flow. Low-viscosity, slow-curing PMMA is prepared and is kept in a large 50-mL syringe. When it has become semiliquid, the PMMA is injected through the center hold of the Silastic tubing, filling the tubing until PMMA can be seen passing out from the ends of the tube (see Fig. 50-9). Certain PMMA mixtures are now available, allowing for more controlled timing of the reaction and more reliable handling characteristics. The tube must be observed carefully to avoid spilling the PMMA into the spinal canal. Curved Penfield dissectors can be used to protect the dural tube. As the PMMA in the Silastic tubing becomes harder, more PMMA is prepared and placed ventral and lateral to the Silastic tube until it is continuous with the borders of the upper and lower vertebrae. During polymerization and hardening of the PMMA, copious saline irrigation is used to help dissipate the heat. Hemostasis is attained with bipolar coagulation.
Bony Graft Fusion in Malignant and Nonmalignant Disease
In patients with nonmalignant disease, or with malignant disease with a relatively longer survival period (usually >2 years), a bone graft is used to supplement the synthetic construct described earlier (see Fig. 50-1).
Fusion
Grafting
A tricortical iliac crest bone graft can be used up to a two-level corpectomy. More extensive decompression may necessitate a humeral or fibular allograft. Such an allograft strut has greater biomechanical strength than an iliac crest, with an acceptable fusion rate. The high cortical bone content, however, means that it may take up to 1 year for the graft to incorporate.33 A supplemental local autograft (e.g., from rib or vertebral body) enhances the rate of fusion when using allografts (see Fig. 50-8). If grafts are taken from the iliac crest, the osteotomies should be perpendicular to the surface to the iliac crest and parallel to each other. A double-bladed oscillatory saw is useful in obtaining parallel surfaces. When these grafts are to be used for subtotal or total vertebral body replacement, several extra millimeters should be taken to allow for further reshaping. In the midthoracic and upper thoracic spine, rib strut grafts taken at the time of the thoracotomy are usually adequate if combined with cages.
Avoiding Complications Related to Fusion
Hemorrhage
1. Careful positioning of the patient on the operating table will avoid unnecessary pressure on the abdomen. This is particularly relevant in the lateral and prone positions. Obstruction of the vena cava and collateral flow from the lower extremities into the paravertebral plexus will have an impact on the venous congestion of the epidural venous plexus and contribute to blood loss.
2. Timing of the end-plate preparation and decortication may be associated with additional blood loss; this should only be done after the bony exposure has been completed, soft tissue has been excised, and bone graft has been harvested. Bleeding from decortication sites should not be treated with bone wax, because this reduces the capacity for osteogenesis.34 Excessive bleeding usually slows after the graft is inserted and may be controlled with Gelfoam.
3. Avoiding inadvertent injury and excessive bleeding, the segmental vessels should be clearly identified and dissected so that they may be suture ligated and divided in a controlled, safe manner. In the lower thoracic and upper lumbar region, the artery of Adamkiewicz and other radicular arteries supplying the anterior and posterior spinal arteries should not be sacrificed. Preoperative angiogram of selected spinal levels is recommended in instances in which there is a concern that these vessels may be at risk during the approach. The authors usually reserve preoperative spinal angiography for cases with a long-standing fixed kyphotic deformity in which the spinal cord blood supply may be tenuous or for cases in which preoperative embolization is desired.
Pseudarthrosis
Pseudarthrosis refers to a lack of bony union and may account for a poor clinical result. It is worth noting, however, that fibrous pseudarthrosis may limit spinal movement and allow a good clinical outcome with symptomatic relief. Moreover, even when bony fusion has occurred, patients can remain symptomatic. Meticulous attention to graft site preparation and use of autograft, where possible, enhances fusion rates. In cases of traumatic lesions, the supplementation of the fusion with a local vertebral body autograft, where possible, enhances fusion rates. In cases of traumatic lesions, the supplementation of the fusion with a local vertebral body autograft that is osteoinductive may be appropriate.
Harvesting Autogenous Iliac Crest Bone
The iliac crest is the most common site from which bone grafts are taken. Consideration of the following complications during this procedure may help reduce the donor site morbidity associated with this procedure.35–38 Donor site pain is common and can continue to be a problem in one out of five patients, lasting up to 2 years postsurgery.39
Cosmetic deformity can be a problem when full-thickness grafts are taken from the iliac crest. When larger grafts are taken and cosmetic deformity becomes a concern, three techniques are useful in preventing crest deformities: (1) The trapdoor method uses the crest as a hinge; (2) the subcrestal window avoids resection of the rostral margin of the crest;40 and (3) oblique sectioning of the crest allows the crest to be reconstituted.39,41 Reconstruction of the crest, using different techniques, has been described: rib,42–44 bioactive apatite and wollastonite-containing glass ceramic,45 or methylmethacrylate.46 Although infection is not a major concern, it does occur occasionally. A deep wound infection at the iliac donor site is treated like other wound infections adjacent to bone. It will require drainage, irrigation, and appropriate antibiotic coverage. Hematoma is common at the wound site. Gelfoam or bone wax can be used, but microcrystalline collagen is best for reducing bleeding from cancellous bone.47 Suction drainage, although not a proven method, may be used to reduce the incidence of wound hematomas. Gait disturbance, with a limp or abductor lurch as a result of considerable stripping of the outer table muscles, can cause hip abductor weakness. With bone graft taken from the dorsal crest, patients may have difficulty with hip extension, which is evident when climbing stairs or rising from a chair.
Stress fractures can occur after full-thickness grafts are taken from the ventral iliac crest.48 Stress fractures, as a result of the pull from the sartorius and rectus femoris muscles, can be avoided by harvesting the graft well away from the anterior superior iliac spine.49 Moreover, taking long strips of bone along the iliac crest increases the risk of ilium fracture.
Perforation of the peritoneum can occur with a ventral approach to the inner table of the iliac crest because the peritoneum is closely related to the inner surface of the abdominal wall and iliacus muscles.50 Herniation of abdominal contents can occur after removal of full-thickness grafts that include the iliac crest.51
Placing the skin incision behind the anterior superior iliac spine can minimize injury to the lateral femoral cutaneous nerve. Anatomic variation of the lateral femoral cutaneous nerve places this nerve at risk in less than 10% when dissection is 3 cm dorsal to the anterior iliac spine.52
Instrumentation
T1 to T9
When the corpectomy involves only the thoracic spine, supplementary instrumentation is generally unnecessary because the thoracic spine, unlike the lumbar spine, is supported by the rib cage. However, if there is three-column involvement, additional instrumentation will be necessary (Fig. 50-10).3 For the upper thoracic spine (T1-3), ventral cervical plates can be used (Fig. 50-11).
T10 to L5
The T12 vertebral body and lumbar spine receive little or no additional support from the rib cage, and in this region there is a greater degree of extension of the spine with spinal motion. Ventral instrumentation is necessary to supplement the reconstruction and to prevent excessive extension that can lead to extrusion of the graft or synthetic construct.13,53,54
Supplemental Ventral Instrumentation
The rationale for using ventral instrumentation can be understood best by considering the biomechanics of the ventral fixation device. Shono et al.55 and Gertzbein56 have described the biomechanics of thoracolumbar ventral fixation devices when loss of anterior and middle column integrity is present. The Kaneda device,53 which has two cross-fixed rods linked to four vertebral body screws (see Figs. 50-1 and 50-8), allows rigid stabilization against forces of axial compression, flexion, extension, and rotation. The quadrangular construct created by the two independent rods linked by the two cross-fixed bars provides greater resistance to flexion-extension and rotation than a single-rod system such as the Zielke system. The insertion of the vertebral body screws in nonparallel (triangular) alignment controls ventral and downward displacement. In the ventrally destabilized spine, the Kaneda construct provides superior fixation compared with dorsal instrumentation (such as a laminar hook or pedicle screw system), especially against flexion and axial compression forces. The vertebral body screws should be placed in a triangular fashion to avoid pullout and the need to engage the contralateral cortical bone of the vertebral body (see Fig. 50-11). If disruption of dorsal elements is present, ventral instrumentation alone is insufficient to provide stability (see Fig. 50-10). Most important, regardless of the rigidity of instrumentation, the spinal construct will eventually fail unless solid bony fusion occurs. One of the key concepts of ventral fusion is that the bone graft should be placed under compression to allow greater graft stability and fusion to adjacent vertebral bodies.41
Instrumentation Technique
The basic principle of this technique entails inserting screws into the midpoint of the vertebral bodies above and below the corpectomy site and connecting these by a rod or plate. Initially, Kostuik-Harrington instrumentation was used. This has been supplanted by the Kaneda device, CD Horizon Antares Spinal System, and Z-plate system. Multiple instrumentation systems are now available from most spinal implant companies. Above T10, the small size of the vertebral bodies may make screw placement difficult, although screws can usually be placed as high as T6 in select cases. Below L4, the iliac veins and origin of the inferior vena cava tend to impede the safe placement of some of these systems. The Z-plate system, which has a lower profile, has recently been modified to allow ventral instrumentation of the midthoracic and lower thoracic spine. Most systems now use the fixed head screws, which can be placed in the vertebral body through a staple or perforated plate, allowing greater stability of the screw-vertebral body interface. By having screws in place, the surgeon can then distract the corpectomy and resection cavity in order to accommodate the vertebral body reconstruction. Rods are then placed in position, and compression can be applied to the anterior column support, which eventually leads to a solid construct with optimal alignment. The plate systems, by nature, do not allow the flexibility and finer degree of adjustments required for a complex reconstruction.
Avoiding Complications Related to Instrumentation
The role of instrumentation in the context of any spinal pathology is to provide stability either transiently, while fusion occurs, or permanently. Placement of instrumentation, however, carries risks and can cause complications. Direct trauma related to poorly positioned instruments is typically noted immediately. Erosion of screws into soft tissues can present in a delayed fashion. Poorly placed instruments can also fail to achieve the primary goal of providing stability. Patients will often complain of persistent mechanical pain. Assessment of the bone quality is often limited, and bone density studies can only provide limited information preoperatively. The interface between the instrumentation and the bone, also described as the purchase, can be improved by injecting PMMA into the vertebral body. The technique of vertebroplasty can be performed intraoperatively,57–59 using radiopaque PMMA and injecting it while in liquid form under fluoroscopy to ensure safe distribution in the vertebral body and avoid the spinal canal (see Figs. 50-11D and E).
Other Spinal Pathology
Osteomyelitis of the Spine
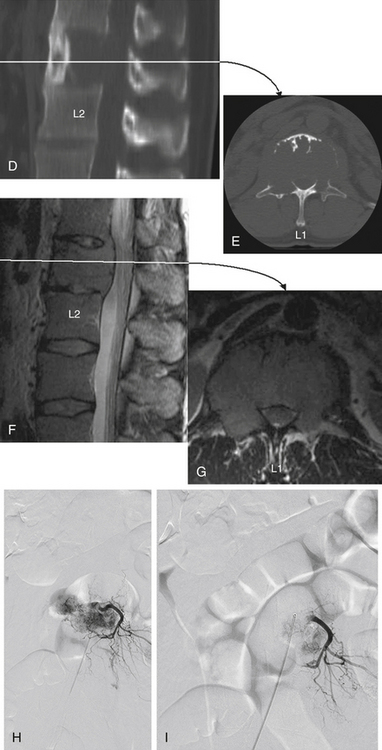
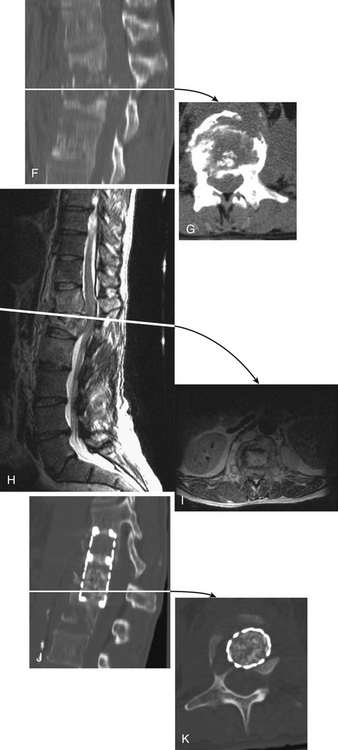
The most common infections of the spinal column are (1) infections caused by pyogenic organisms (Staphylococcus aureus and coliform bacilli are the most common pyogenic bacteria found), (2) infections caused by fungi (actinomycetes and blastomycetes are the most common organisms found), and (3) tuberculosis (Pott disease) (see Fig. 50-11).
These organisms usually reach the spinal column by hematogenous spread. The seeding of these infections is typically localized to the disc space because this avascular compartment is harder for the patient’s own immune system to control. This “disc”-centric pathology is important when reviewing the images and trying to distinguish infectious from neoplastic pathology. Infection becomes symptomatic as a result of neural compromise from an associated extradural abscess or bony deformity from vertebral collapse with adjacent bone overgrowth that compromises the spinal canal (gibbus formation).
Identification of predisposing factors for infection following spinal surgery has been described by Wimmer et al.60 Medical conditions such as diabetes, obesity, corticosteroid therapy, chronic infection, and smoking were identified in patients with a higher rate of infection. Surgical variables such as previous spinal surgery, extended preoperative hospitalization (P < .001), and high blood loss (P < .01) were identified as risk factors. The role of prophylactic antibiotic administration has been demonstrated by Horwitz and Curtin61 to reduce wound infection with spinal surgery. Some institutions have adopted a prolonged duration of antibiotic doses for patients at higher risk, using the factors mentioned previously.
In these infective cases, it is important to assess, using axial CT images, the degree of spinal canal narrowing from vertebral collapse or gibbus formation (see Fig. 50-11F and G). In the absence of spinal canal narrowing, an extradural abscess or granuloma is the likely cause of spinal symptoms. MRI or myelography to delineate neural (spinal cord or nerve root) compromise is also indicated for these patients before surgical decompression (see Figs. 50-11H to K).
Surgical decompression is indicated in patients with progressive symptoms of spinal cord compression. The thoracic region is the most common site of osteomyelitis, and dorsolateral spinal approaches (e.g., costotransversectomy) usually allow adequate spinal decompression. Occasionally, ventral decompressive procedures are necessary when there is (1) progressive spinal compression; (2) osteomyelitis of the cervical spine; (3) spinal infections with kyphotic angulation in the lower lumbar spine, in which a retroperitoneal approach with corpectomy can be performed; and (4) extensive involvement of the vertebral body that cannot be adequately decompressed by the dorsolateral approach (see Fig. 50-11).
Resection of Hemivertebra
A hemivertebra may become symptomatic and cause a severe, progressive deformity of the spine with neurologic compromise.9 This usually occurs when the anomaly lies low in the lumbar spine and results in congenital scoliosis with the hemivertebra as the apical part of the curve. The hemivertebra is resected ventrally to dorsally, back to the level of the epidural space, with the base of the pedicle also resected. An autologous bony strut graft with ventral instrumentation can be used for stability. Either during this procedure or during a second operation, a dorsal approach is used to resect the dorsal elements of the hemivertebra.
Thoracic and Thoracolumbar Fractures
These fractures can be approached by a ventral, ventrolateral (see Figs. 50-8 and 50-10), dorsolateral, or dorsal approach depending on certain features.20,62–64 When lesions are ventral in the thoracic spine, available options for surgical exposure that allow decompression and stabilization include costotransversectomy, a lateral extracavitary approach, a transthoracic extrapleural approach, or a transthoracic transpleural approach. The authors favor the latter approach when neural decompression is an important goal, as in the patient with incomplete spinal cord injury (see Figs. 50-8 and 50-10). For burst fractures at the thoracolumbar junction, a transthoracic/retroperitoneal (10th rib approach) exposure is used to achieve ventral decompression and reconstruction (see Fig. 50-8). For midlumbar fractures (L2 or L3), a retroperitoneal approach is used. Low lumbar fractures (L4 or L5) are approached via a dorsolateral approach because the nerve roots may be retracted with greater facility, allowing easier decompression.
The transthoracic and retroperitoneal approaches allow a single-stage procedure with decompression and removal of pathologic material ventral to the dura mater over several vertebral segments and reconstruction with bone graft and instrumentation. Three-column injuries necessitating the use of ventral decompression require supplemental dorsal instrumentation (see Fig. 50-10).
Decompression and Stabilization
Surgical exposures to other thoracic and lumbar levels (T1-3 and T3-L2) are described in other chapters in this text. The fractured or retropulsed bone segment is identified under the microscope and removed, using curettes and a high-speed pneumatic drill. The intervertebral discs and end plates of adjacent vertebral bodies are removed to allow adequate fusion of the bony graft inserted between the intact adjacent vertebral bodies above and below the decompression. Important principles of direct visualization of neural elements should be observed in spinal decompression associated with spinal trauma.
Transthoracic Discectomy
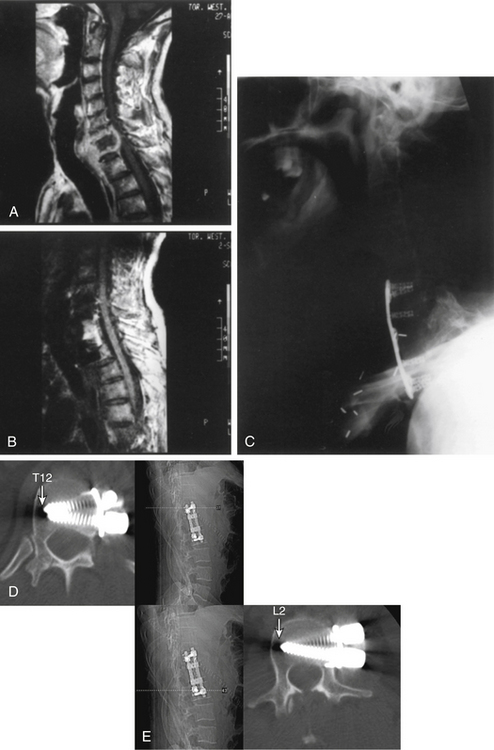
Symptomatic thoracic intervertebral disc herniations are relatively uncommon, with an incidence of 1 per million,65 constituting approximately 0.25% to 0.75% of all symptomatic disc lesions. They usually occur between T4 and T12. Patients may appear with radicular symptoms or spinal cord compression, depending on whether the disc has herniated laterally or centrally. MRI is the imaging technique of choice for the diagnosis (Fig. 50-12). Initially, thoracic disc herniations were approached by laminectomy with poor results.26,27,44,46,59,66–69 Although patients with lateral disc herniation had slightly better outcomes compared with central disc herniation, in both cases a number of patients failed to improve, continued to deteriorate, or had postoperative paraparesis as a complication.26,27,44,46,59,66–69 In 1960, a lateral approach via a costotransversectomy was used with encouraging results.70 Recently, a more direct ventral transpleural approach has provided further reduction in neurologic morbidity.59,68
Thoracic Endoscopic Surgery
Thoracic endoscopic surgery was first described for treatment of Pott disease in 1951.71 The expansion of video technology in the early 1990s affected endoscopic capabilities, providing a better-quality image with small equipment.69,72 Thoracoscopic approaches to the thoracic spine for sympathectomy, discectomy, and paraspinal neurogenic tumor with low morbidity and mortality have been described.8 Thoracoscopic-assisted treatment of thoracic and lumbar fractures has been carried out on more than 371 patients to date.73 Expansion of these new minimally invasive techniques seems appealing when reported by a small number of authors.8,13,74 Caution should be exercised when adopting these new surgical techniques because there is a significant learning curve. Certain pathologic entities still require open procedures to achieve adequate decompression and control bleeding.
Closure and Postoperative Care
Routine closure with approximation of all muscle layers in surgery involving the thoracic cavity is performed with one or two thoracostomy tubes, one passing to the apex and/or one to the dependent region of the chest cavity, connected to an underwater suction seal allowing drainage of air and blood. The drains are removed once the drainage is less than 100 mL over a 12-hour period, which usually occurs by postoperative day 2 or 3. A major complication of both the transpleural and the ventrolateral approaches to the thoracolumbar spine is blood loss, which occurs during both the decompressive procedure and the fusion. The blood loss occurs from the cancellous surfaces of the bone graft and vertebral body sites. The blood lost should be estimated and replaced intraoperatively, and the hematocrit should be followed closely postoperatively. Pulmonary complications are low if relatively young, healthy patients are selected for these procedures.75 Daily chest radiographs will help the physician monitor for pneumothorax and pleural effusions.
Patients who have undergone transthoracic surgery begin ambulation soon after removal of throacostomy tubes. Sufficient analgesic must be given at all stages of postoperative care to reduce pain associated with this type of surgery. Intercostal nerve blocks can be used before closure of the thoracostomy. Intrathoracic catheters for administration of narcotic analgesics are also helpful. Depending on neurologic recovery and capability for independence and support at home, the patient may return home or require further rehabilitation at an appropriate facility.
Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984;189:65-76.
Errrico T.J., Cooper P.R. A new method of thoracic and lumbar body replacement for spinal tumors: technical note. Neurosurgery. 1993;32:678-680.
Nuwer M.R. Spinal cord monitoring. Muscle Nerve. 1999;22:1620-1630.
Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366:643-648.
White A.A.III, Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: Lippincott-Raven; 1978.
1. Royle N. The operative removal of an accessory vertebra. Med J Aust. 1928;1:467.
2. Hodgson A., Stock F. Anterior spinal fusion: a preliminary communication on radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266.
3. Cooper P.R., Errico T.J., Martin R., et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
4. Harrington K.D. Anterior cord decompression and spinal stabilization for patients with metastatic lesions of the spine. J Neurosurg. 1984;61:107-117.
5. Sacks S. Anterior interbody fusion of the lumbar spine. J Bone Joint Surg [Br]. 1965;47:211-223.
6. Sacks S. Anterior interbody fusion of the lumbar spine: indications and results in 200 cases. Clin Orthop. 1966;44:163-170.
7. Errrico T.J., Cooper P.R. A new method of thoracic and lumbar body replacement for spinal tumors: technical note. Neurosurgery. 1993;32:678-680.
8. Han P.P., Kenny K., Dickman C.A. Thoracoscopic approaches to the thoracic spine: experience with 241 surgical procedures. Neurosurgery. 2002;51:88-95.
9. Sundaresan N., Galicich J.H., Bains M.S., et al. Vertebral body resection in the treatment of cancer involving the spine. Cancer. 1984;53:1393-1396.
10. Hall A.J., Mackay N.N. The results of laminectomy for compression of the cord or cauda equine by extradural malignant tumour. J Bone Joint Surg [Br]. 1973;55:497-505.
11. Young R.F., Post E.M., King G.A. Treatment of spinal epidural metastases: randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741-748.
12. Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366:643-648.
13. Kostuik J.P., Errico T.J., Gleason T.F., et al. Spinal stabilization of vertebral column tumors. Spine. 1988;13:250-256.
14. Sundarsesan N., Galicich J.H., Lane J.M., et al. Treatment of neoplastic epidural cord compression by vertebral body resection and stabilization. J Neurosurg. 1985;63:676-684.
15. Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984;189:65-76.
16. White A.A.III, Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: Lippincott-Raven; 1978.
17. Deletis V., Sala F. The role of intra-operative neurophysiology in the protection of documentation of surgically induced injury to the spinal cord. Ann N Y Acad Sci. 2001;939:137-144.
18. Kothbauer K., Deletis V., Epstein F.J. Intra-operative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg. 1997;26:247-254.
19. Luk K.D., Hu Y., Wong Y.W., et al. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26:1772-1777.
20. Nuwer M.R. Spinal cord monitoring. Muscle Nerve. 1999;22:1620-1630.
21. Burkus J.K. Intervertebral fixation: clinical results with anterior cages. Orthop Clin North Am. 2002;33:349-357.
22. Kanayama M., Cunningham B.W., Haggerty C.J., et al. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg. 2000;9(Suppl 2):259-265.
23. Maciejczak A., Radek A. [Lumbar interbody fusion: biomechanical significance for the spine. Neurol Neurochir Pol. 1998;32:1247-1259.
24. Matge G. Cervical cage fusion with 5 different implants: 250 cases. Acta Neurochir (Wien). 2002;144:539-549.
25. Matge G., Leclercq T.A. Rationale for interbody fusion with threaded titanium cages at cervical and lumbar levels: results on 357 cases. Acta Neurochir (Wein). 2000;142:425-433.
26. Murakami H., Horton W.C., Kawahara N., et al. Anterior lumbar interbody fusion using two standard cylindrical threaded cages, a single mega-cage, or dual nested cages: a biomechanical comparison. J Orthop Sci. 2001;6:343-348.
27. Tsantrizos A., Baramki H.G., Zeidman S., et al. Segmental stability and compressive strength of posterior lumbar interbody fusion implants. Spine. 2000;25:1899-1907.
28. Eck J.C., Hodges S.D., Humphreys S.C. Vertebroplasty: a new treatment strategy for osteoporotic compression fractures. Spine. 1977;2:143-153.
29. Jensen M.E., Dion J.E. Percutaneous vertebroplasty in the treatment of osteoporotic compression fractures. Neuroimaging Clin North Am. 2000;10:547-568.
30. Linville D.A. Vertebroplasty and kyphoplasty. South Med J. 2002;95:583-587.
31. Predey T.A., Sewall L.E., Smith S.J. Percutaneous vertebroplasty: new treatment for vertebral compression fractures. Am Fam Physician. 2002;66:611-615.
32. Watts N.B., Harris S.T., Genant H.K. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429-437.
33. Bernard T.N.Jr., Whitecloud T.S.III. Cervical spondylotic myelopathy and myeloradiculopathy: anterior decompression and stabilization with autogenous fibula strut graft. Clin Orthop Relat Res. 1987;221:149-160.
34. Geary J., Frantz V. New absorbable bone wax: experimental and clinical studies. Ann Surg. 1947;132:1128.
35. Arrington E.D., Smith H.G., Chambers H.G., et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300-309.
36. Fowler B.L., Dall B.E., Rowe D.E. Complications associated with harvesting autogenous iliac bone graft. Am J Orthop. 1995;24:895-903.
37. Kurz L.T., Garfin S.R., Booth R.E.Jr. Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine. 1989;14:1324-1331.
38. Seiler J.G.III, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9:91-97.
39. Mirovsky Y., Neuwirth M.G. Comparison between the outer table and intracortical methods of obtaining autogenous bone graft from the iliac crest. Spine. 2000;25:1722-1725.
40. Behairy Y.M. Al Sebai W: A modified technique for harvesting full-thickness iliac crest bone graft. Spine. 2001;25:695-697.
41. Wolfe S.A., Kawamoto H.K. Taking the iliac-bone graft. J Bone Joint Surg [Am]. 1978;60:411.
42. Defino H.L., Rodriguez-Fuentes A.E. Reconstruction of anterior iliac crest bone graft donor sites: presentation of a surgical technique. Eur Spine J. 1999;8:491-494.
43. Harris M.B., Davis J., Gertzbein S.D. Iliac crest reconstruction after tricortical graft harvesting. J Spinal Disord Tech. 1994;7:216-221.
44. Torode I.P. Pelvic reconstruction after bone-graft harvesting. J Pediatr Orthop. 1994;14:381-382.
45. Asano S., Kaneda K., Satoh S., et al. Reconstruction of an iliac crest defect with a bioactive ceramic prothesis. Eur Spine J. 1994;3:39-44.
46. Lubicky J.P., DeWald R.L. Methylmethacrylate reconstruction of large iliac crest bone graft donor sites. Clin Orthop Relat Res. 1982;164:252-256.
47. Cobden R.H., Thrasher E.L., Harris W.H. Topical emostatic agents to reduce bleeding from cancellous bone: a comparison of microcrystalline collagen, thrombin, and thrombin-soaked gelatin foam. J Bone Joint Surg [Am]. 1976;58:70-73.
48. Porchet F., Jaques B. Unusual complications at iliac crest bone graft donor site: experience with two cases. Neurosurgery. 1996;39:856-859.
49. Ebraheim N.A., Yang H., Lu J., et al. Anterior iliac crest bone graft: anatomic considerations. Spine. 1997;22:847-849.
50. Dosoglu M., Orakdogen M., Tevruz M., et al. Enterocutaneous fistula: a complication of posterior iliac bone graft harvesting not previously described. Acta Neurochir (Wien). 1998;140:1089-1092.
51. Ousmane M.L., Herbecq P., Jasatis L., Kerreneur J.M. [Colon hernia through a defect in the iliac crest after bone graft harvesting. Ann Fr Anesth Reanim. 2000;19:749-750.
52. Murata Y., Takahashi K., Yamagata M., et al. Injury to the lateral femoral cutaneous nerve during harvest of iliac bone graft, with reference to the size of the graft. J Bone Joint Surg [Br]. 2002;84:798-801.
53. Kaneda K. The textbook of spinal surgery. Phiadelphia: Lippincott-Raven; 1991. 959
54. Manabe S., Tateishi A., Abe M., et al. Surgical treatment of metastatic tumors of the spine. Spine. 1989;14:41-47.
55. Shono Y., Kaneda K., Yamamoto I. A biomechanical analysis of Zielke, Kaneda, and Cotrel-Dubousset instrumentations in thoracolumbar scoliosis: a calf spine model. Spine. 1991;16:1305-1311.
56. Gertzbein S.D., Offierski C. Complete fracture-dislocation of the thoracic spine without spinal cord injury: a case report. J Bone Joint Surg [Am]. 1979;61:449-451.
57. Evans A.J., Jensen M.E., Kip K.E., et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty: retrospective report of 245 cases. Radiology. 2003;226:366-372.
58. Fourney D.R., Schomer D.F., Nader R., et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21-30.
59. Peters K.R., Guiot B.H., Martin P.A., et al. Vertebroplasty for osteoporotic compression fractures: current practice and evolving techniques. Neurosurgery. 2002;51:96-103.
60. Wimmer C., Gluch H., Franzreb M., et al. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord Tech. 1998;11:124-128.
61. Horwitz N., Curtin J. Prophylactic antibiotics and wound infections following laminectomy for lumbar disc herniation. J Neurosurg. 1975;43:727-731.
62. Cook W.A. Transthoracic vertebral surgery. Ann Thorac Surg. 1971;12:54-68.
63. Erickson D.L., Leider L.L., Browne W. One-stage decompression-stabilization for thoracolumbar fractures. Spine. 1977;2:143-153.
64. Flesch J.F., Leider L.L., Erickson D.L., et al. Harrington instrumentation and spine fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine. J Bone Joint Surg [Am]. 1977;59:143-153.
65. Wood K.B., Blair J.M., Aepple D.M., et al. The natural history of asymptomatic thoracic disc herniations. Spine. 1997;22:525-529.
66. Arseni C., Nash F. Protrusion of thoracic intervertebral discs. Acta Neurochir (Wien). 1963;11:418-430.
67. Love J., Schorn V. Thoracic disc protrusion. JAMA. 1965;191:627.
68. Reeves D.L., Brown H.A. Thoracic intervetebral disc protrusion with spinal cord compression. J Neurosurg. 1969;28:24-28.
69. Robertson D.P., Simpson R.K., Rose J.E., et al. Video-assisted endoscopic thoracic ganglionectomy. J Neurosurg. 1993;70:238-240.
70. Hulme A. The surgical approach to thoracic intervertebral disc protrusions. J Neurol Neurosurg Psychiatry. 1960;23:133.
71. Kux E. The endoscopic approach to the vegetative nervous system and its therapeutic possibilities. Chest. 1951;20:139-147.
72. Friedel G., Toomes H., Linder A. Selective video-assisted thoracoscopic sympathectomy. Thorac Cariovasc Surg. 1993;41:245-248.
73. Khoo L.T., Beisse R., Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurgery. 2002;51:104-117.
74. Fessler R.G. Minimally invasive spine surgery. Neurosurgery. 51, 2002. iii-iv
75. Dales R.E., Dionne G., Leech J.A., et al. Preoperative prediction of pulmonary complications following thoracic surgery. Chest. 1993;104:155-159.