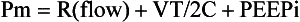Ventilatory Management of Obstructive Airway Disease
Special Challenges of Patients with Severe Airflow Obstruction
Increased Work of Breathing
In this equation R = resistance; VT is tidal volume; C is respiratory system compliance (the inverse of elastance); and PEEPi is auto-PEEP, the positive end-expiratory alveolar pressure in excess of set PEEP because of dynamic hyperinflation (Fig. 10.1).
Increased resistance to airflow is responsible (directly or indirectly) for many of the physiologic disturbances that typify this disease. Flow resistance, an important determinant of Pm, is increased by structural and functional narrowing of the airway. Structural changes include a reduced number of airway channels, as well as narrowed cross-sectional airway caliber. In this already narrowed tapering network of tubes, the additional reduction of airway caliber caused by mucosal edema, functional compression, increased bronchomotor tone, or secretion accumulation noticeably increases the work of breathing because resistance relates linearly to the airway length but inversely to the fourth power of airway radius. For similar reasons, resistance within these critically narrowed airways is highly volume dependent, so that loss of lung volume is accompanied by loss of elastic recoil tension, reduction of cross-sectional area, tendency for expiratory airway collapse, and major increases in the frictional workload.1,2
Dynamic Hyperinflation (Air Trapping)
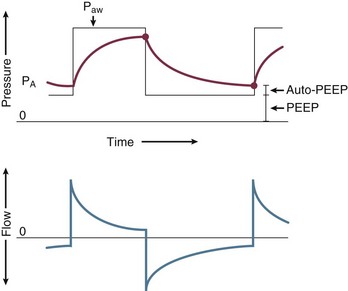
Expiration is normally a passive process that uses elastic energy stored during inflation to drive expiratory airflow. If the energy potential stored during inflation is insufficient to return the system to a relaxed equilibrium before the next inspiration begins, flow continues throughout expiration and alveolar pressure remains positive at end expiration, exceeding the clinician-selected PEEP value (Fig. 10.2).3–9 This positive distending pressure within the alveoli increases the driving pressure for expiratory airflow and increases lung volume, thereby helping to overcome airflow resistance. Unfortunately, such hyperinflation also places the expiratory musculature at a mechanical disadvantage. Furthermore, because the hyperinflating end-expiratory alveolar pressure encourages deflation, it must first be counterbalanced by positive pressure applied to the central airway or by negative pleural pressure before inspiration can begin.10,11 Thus PEEPi adds to the other components of the equation of motion to elevate the mean inflation pressure and inspiratory work of breathing. The process of air trapping contributes to an increase in the respiratory work of breathing in at least two other ways. Hyperinflation drives the respiratory system upward toward the least compliant portion of the pressure-volume relationship, incurring increased elastic work per liter of ventilation (see Fig. 10.1). At these higher volumes the lung approaches its elastic limit as the recoil tension of the distended rib cage becomes expiratory rather than inspiratory in nature. Finally, hyperinflation tends to convert more of the well-perfused (“zone 3”) lung into less-well-perfused tissue, thereby increasing ventilatory deadspace and the minute ventilation requirement.
Generally, the resistance increase in patients with chronic AO and many of those with severe asthma concentrates within small airways.1 Yet for certain asthmatic patients, the central airways and larynx contribute impressively to total resistance, accounting for the helpfulness of helium-oxygen (heliox) mixtures in some (but not all) patients during exacerbated asthma.12 According to some reports, heliox helps to reduce air trapping in patients with COPD as well. Although there is some concern regarding the generalizability and accuracy of such observations, several mechanisms can be invoked, even if the primary site of expiratory obstruction is too peripheral for helium to reduce resistance there. These mechanisms include reduced inspiratory turbulence, faster expiratory flow in non–flow-limited channels with increased wave speed, modestly decreased CO2 production, and perhaps reduced associated gas trapping.
When dynamic collapse occurs during tidal respiration and breathing requirements are high, there is little alternative to hyperinflation, CO2 retention, or both. At the chosen level of minute ventilation, maintaining the lower lung volume may be either too energy costly or physically impossible. For this reason, many patients with severe obstruction do not or cannot decrease their lung volumes when recumbent. Such considerations may help to explain the dyspnea experienced by most patients with severe AO on assuming horizontal positions. For the same recumbent angle, the lateral position allows slightly more decompression than does the supine position because lung volume influences airway caliber, airway resistance, and tendency for collapse as the lung deflates.13 Patients with obesity have a lower resting lung volume and therefore exhibit higher airway resistance and tendency for dependent atelectasis and symptoms when airways are narrowed by bronchoconstriction or disease. Likewise, patients with acute respiratory distress syndrome have higher than normal airway resistance in dependent zones.
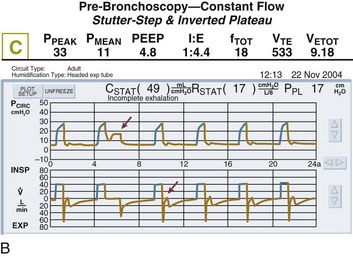
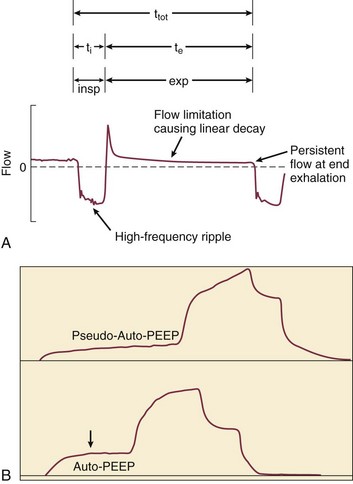
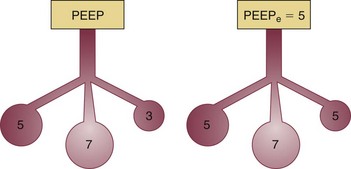
In fact, the distribution of gas trapping varies regionally throughout any diseased lung, depending on the local mechanical properties of the airways. Therefore, at the end of the expiratory cycle some zones remain patent, and some have sealed much earlier in the deflation cycle (see Fig. 10.2). Consequently, the end-expiratory value of auto-PEEP detected at the airway opening may not reflect the magnitude of gas trapping.14 Clues to the presence of regional closure are often seen when the airway is occluded at end expiration; the auto-PEEP value shows an atypically slow rise to its final value as quasi-occluded small airways decompress into the common airway. In such cases, PEEP often eliminates this characteristic. During volume-controlled ventilation, plateau pressure tracks hyperinflation more reliably than direct measurements of PEEPi.
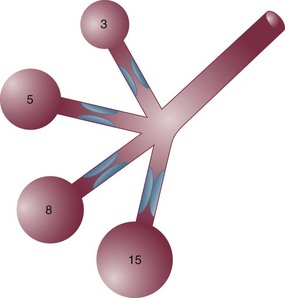
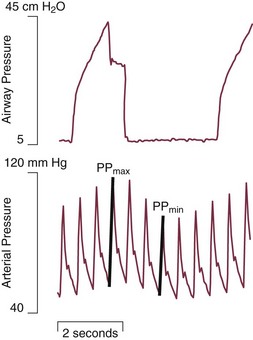
In some patients, especially those who passively receive ventilatory support, the problems presented by air trapping and dynamic hyperinflation are as much cardiovascular as pulmonary in nature. A relatively high fraction of the resulting positive alveolar pressure is transmitted to the pleural space, where it impedes venous return and confuses interpretation of hemodynamic pressure measurements made with pulmonary artery catheters (Fig. 10.3). Lung distention also adds to pulmonary vascular resistance, exacerbating the tendencies of patients with cor pulmonale toward low cardiac output and hypotension. Marked respiratory variation of systolic and pulse pressures during passive inflation indicates phasically adverse cardiac loading and strongly implies the possibility of dynamic hyperinflation (Fig. 10.4).
Increased Minute Ventilation Requirement
Ventilation-perfusion (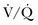 ) mismatching is widespread in patients with severe AO, reducing the efficiency of carbon dioxide elimination.1 It is not uncommon for the resting minute ventilation requirement to exceed 12 L per minute (twice the normal value) in patients with exacerbated asthma or extensive emphysema and strong chemical drives to breathe. Not only do such increases in ventilation requirement act as a linear cofactor in the work of the breathing equation already discussed, but the high minute ventilation requirement itself increases most components of inspiratory pressure: flow, elastance (the reciprocal of compliance), tidal volume, and auto-PEEP. It is not surprising, therefore, that enormous increases in the oxygen consumption rate of the ventilatory muscles have been observed in patients with obstructive lung disease. During exacerbations, the oxygen consumed by ventilation and the metabolic demands associated with heightened vigilance, agitation, or anxiety may double the total body oxygen consumption observed during fully supported breathing. The prevalent combination of impaired
) mismatching is widespread in patients with severe AO, reducing the efficiency of carbon dioxide elimination.1 It is not uncommon for the resting minute ventilation requirement to exceed 12 L per minute (twice the normal value) in patients with exacerbated asthma or extensive emphysema and strong chemical drives to breathe. Not only do such increases in ventilation requirement act as a linear cofactor in the work of the breathing equation already discussed, but the high minute ventilation requirement itself increases most components of inspiratory pressure: flow, elastance (the reciprocal of compliance), tidal volume, and auto-PEEP. It is not surprising, therefore, that enormous increases in the oxygen consumption rate of the ventilatory muscles have been observed in patients with obstructive lung disease. During exacerbations, the oxygen consumed by ventilation and the metabolic demands associated with heightened vigilance, agitation, or anxiety may double the total body oxygen consumption observed during fully supported breathing. The prevalent combination of impaired 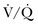 matching, hypoventilation, and diffusion impairment result in arterial oxygen desaturation that generally responds well to modest supplementation of inspired oxygen.
matching, hypoventilation, and diffusion impairment result in arterial oxygen desaturation that generally responds well to modest supplementation of inspired oxygen.
Reduced Mechanical Efficiency
In patients with exacerbated COPD or decompensated asthma, the oxygen consumed in the ventilatory task is disproportionate to the amount of mechanical work actually performed. The muscles of the hyperinflated ventilatory system are inefficiently aligned, force-length relationships of the shortened end-inspiratory fibers are suboptimal, and normally efficient coordination among the various muscles of the ventilatory group is often disrupted.14–16 The energy cost of breathing, therefore, is greatly increased for the pressure and mechanical work actually generated by the breathing effort.
Problems and Hazards of Ventilation with Positive Pressure
The hemodynamic sensitivity to positive-pressure ventilation of patients with AO arises for several reasons. The overexpanded lungs press outward on the chest wall, raising intrapleural pressure. When breathing efforts are silenced, as they are immediately after sedation and intubation, mean intrathoracic pressure abruptly changes from modestly negative to markedly positive. Increased pleural pressure raises the right atrial back-pressure to venous return. Simultaneously, increased peripheral vascular capacitance (caused in part by drug effects) and reduced peripheral vascular tone limit the rise in mean systemic pressure, the upstream driver of venous return. Blood pressure routinely falls, and cardiac output falls disproportionately to oxygen demand. Absolute values of measured central vascular pressures (central venous and wedge pressures) may therefore be misleadingly high and do not reflect intravascular filling and preload adequacy.7 Marked respiratory variation of systolic and pulse pressures with the ventilatory cycle (“paradox”) is a hemodynamic marker of relative hypovolemia caused by such mechanisms (see Fig. 10.4). Depending on choice of tidal volume, backup frequency, and set (and auto) PEEP, the afterload to right ventricular ejection may rise with any further lung expansion, whereas the tendency for alveolar deadspace creation is accentuated. Consequently, great care must be taken not to ventilate excessively and to provide adequate intravenous fluid support during this period. This advice pertains especially to patients with AO who require cardiac resuscitation.17
Interactions of Pressure-Targeted Modes with Auto-PEEP
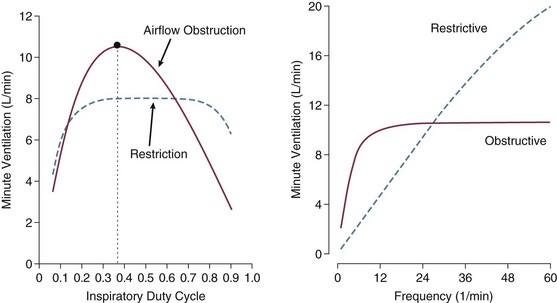
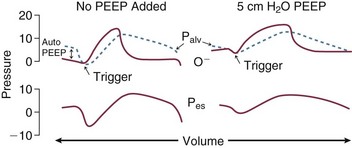
Pressure-targeted modes of ventilation, exemplified by pressure control, airway pressure release ventilation (APRV), and pressure support, have become increasingly popular to employ in the care of intubated patients, as well as in those receiving noninvasive ventilation by facemask. Because the development of auto-PEEP reduces the pressure difference between airway opening and alveolus that drives inspiration, it has a powerful influence on ventilation efficacy (Fig. 10.5). As already described, auto-PEEP varies not only with airway mechanics but also with the pattern of breathing and minute ventilation. For a fixed value of applied airway pressure, inspiratory tidal volume in patients with AO will be more sensitive to the frequency and the inspiratory time fraction (an expression of the inspiratory-to-expiratory [I : E] ratio) than are normal subjects or those with restrictive disease18 (Fig. 10.6). Faster breathing frequencies, for example, allow auto-PEEP to build, and this auto-PEEP must first be counterbalanced for inspiratory airflow to begin. If the patient is passive or the amount of inspiratory muscle force remains constant, delivered tidal volume falls as the auto-PEEP builds.
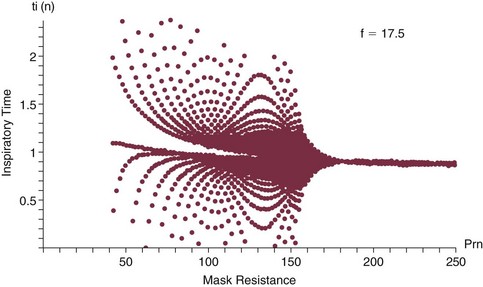
This auto-PEEP/driving pressure interaction may result in an intriguing phenomenon resembling chaotic respiration during noninvasive ventilation with a leaky mask interface.19 The coupled PEEPi and tidal volume form a “feed-forward” system in which a building auto-PEEP of one cycle adversely influences the tidal volume of the next one. But this smaller tidal volume also reduces the auto-PEEP that follows that restricted cycle, which in turn allows the subsequent breath—the third in the cycle—to have a larger effective driving pressure and tidal volume, and the cycling variation continues. This may account for some of the wide variability in breathing rhythm often observed in these patients.20 If the mask leak volume is a function of the I : E ratio, it can be shown mathematically and experimentally that fractal and chaotic tidal volume delivery may occur, even when the patient’s effort and mechanics remain unchanged (Fig. 10.7).19 The consequences for comfort and sleep efficiency are likely to be significant, but clinical data are lacking on these issues at this time.
Principles of Managing the Ventilated Patient with Severe Airflow Obstruction
Most patients hospitalized with exacerbations of asthma or COPD can be managed effectively by regimens that incorporate aggressive secretion clearance techniques, antibiotics, corticosteroids, intensified bronchodilators, hydration, cardiovascular support, secretion lubricants (e.g., guaifenesin), and supplemental oxygen. Noninvasive ventilation often helps as a temporizing measure for those with disease of mild-moderate severity, especially when cough is adequate to clear airway secretions and the patient is fully alert and accepting of a full facemask.21–26 Only a minority of such patients treated in this way need translaryngeal intubation and institution of mechanical ventilatory support unless the problem is complicated by coexisting cardiovascular, infectious, or neuromuscular problems. When mechanical ventilation is required, however, the rationale underlying certain key management principles can easily be understood against a background of the physiologic derangements already described. These principles are as follows: (1) provide adequate support for muscle rest at adequate PaO2 and pH; (2) do not overventilate; (3) minimize the minute ventilation requirement; (4) minimize risk of barotrauma; (5) maintain adequate bronchial hygiene; (6) prevent panic reactions; (7) establish appropriate nutrition. Each principle will be discussed in more detail:
Principle 1: Provide adequate support to rest the ventilatory muscles, while avoiding hypoxemia and profound acidemia.
Principle 2: Do not overventilate.
Profound respiratory acidosis and hypoxemia accentuate pulmonary hypertension, impair the right ventricle, and should be reversed. Nevertheless, although it is important to provide adequate ventilation and to reverse hypoxemia, overventilation is detrimental on several counts. Rapid reduction in the alveolar CO2 tension tends to cause bronchoconstriction and impair neuromuscular and cardiovascular function. Furthermore, excess ventilation exacerbates dynamic hyperinflation and auto-PEEP, whereas moderate PaCO2 elevations are generally well tolerated.27 Generally, it is a mistake to depress the PaCO2 below the level that the patient chronically maintains. Such a strategy may temporarily reset chemical drives, effectively increasing respiratory workload intensity once spontaneous breathing resumes. If PaCO2 falls sufficiently, the patient will not maintain unassisted breathing without intolerable effort, potentially delaying discontinuation of mechanical breathing assistance.
Principle 3: Minimize minute ventilation requirement.
Principle 4: Minimize the risk of barotrauma.
Principle 5: Maintain effective bronchial hygiene.
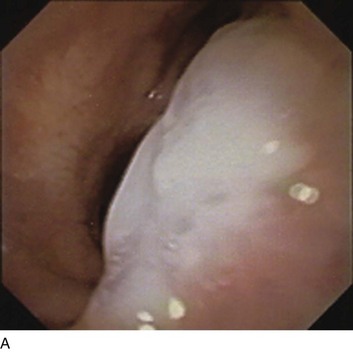
Secretion retention may dramatically increase airflow resistance and effectively seal off entire banks of functional alveoli, preventing their participation in ventilation. Thickened central airway secretions are a particular risk during mechanical ventilation, whether invasive or noninvasive (Fig. 10.8).28 Apart from raising the end-inspiratory pressure, the resulting dynamic hyperinflation can detrimentally affect cardiovascular function, work of breathing, and ventilatory capability. In addition to effective suctioning, bronchodilators, adequate hydration, corticosteroids, mucolytics, mucolubricants, and infection control, frequent repositioning, mobilization, and physiotherapy are fundamental to secretion management. Percussive ventilation or vibro-percussive vest treatments often complement mobilization effectively when tolerated. Tracheotomies not only reduce resistance and provide improved access to the lower airway but also eliminate the direct connection between the pharynx and trachea established by tracheal intubation.
Principle 6: Prevent panic reactions.
Principle 7: Maintain appropriate nutrition, assure adequate hemoglobin concentration, and prevent obstipation.
Practical Management of the Ventilated Patient
Intubation
An understandable reluctance to initiate mechanical ventilation in patients with COPD or perennial asthma exists because ventilatory assistance may be needed for prolonged periods and because many such individuals are so chronically disabled as to be miserable or despondent at home—even when everything is going as well as possible from a physiologic viewpoint. The development of comfortable noninvasive systems, coupled with supportive trials and clinical experience, has given rise to the initial use of mask ventilation in those who are alert and can tolerate it. However, the most severely affected patients, especially those with copious, thick, and retained secretions, claustrophobia, anxiety, cardiovascular decompensation, or irreversible somnolence, continue to require intubation to stabilize their deteriorating conditions.29
Initial Support
PostIntubation Problems
One reason for the agitation that some patients experience is a sudden buildup of positive intrathoracic pressure through the process of dynamic hyperinflation. When these intubated patients are deeply sedated and paralyzed, respiratory efforts cease and vasodilation occurs related to hypercapnia and sedation. The consequent rise of intrathoracic pressure, coupled with a fall in mean systemic vascular pressure, almost routinely depresses venous return and cardiac output (see Fig. 10.3). Therefore, the physician is well advised to remain alert to the predictable development of cardiovascular depression and hypotension following intubation or sedation and be prepared to intervene to reduce ventilation or to support the circulation at the initially selected level, or both. A catastrophic error is to misinterpret the development of sudden hypotension as the uncloaking of tension pneumothorax and then to undertake needle puncture of the chest wall. In such individuals it is also wise to remember the potential contribution of auto-PEEP to hypotension during cardiopulmonary resuscitation attempts.
Machine Settings
The triggering threshold of the ventilator is set to be as sensitive as possible, and the auto-PEEP level is estimated when feasible to do so. (Measurement typically requires passive inflation to allow predictable occlusion of the circuit at end-expiration.) If auto-PEEP exceeds 5 cm of water and expiratory flow is limited during tidal breathing (which is almost invariably the case during the initial phase), an uncomfortable patient who makes spontaneous breathing efforts may benefit from the addition of a low level of end-expiratory pressure to counterbalance auto-PEEP and reduce the breathing workload (Fig. 10.9). PEEP levels in excess of 15 cm H2O may be necessary in some instances to reestablish patency of some air channels.
The plateau pressure is a better guide to the degree of hyperinflation than is the measured level of auto-PEEP, for reasons already given. First, most machines do not allow estimation of auto-PEEP in a patient who is spontaneously triggering the ventilator and varies the length of the respiratory cycle. On the other hand, a plateau pressure estimate is usually recordable during triggered, as well as during controlled, volume-cycled breathing. Just as importantly, the auto-PEEP estimated by central airway occlusion is simply the volume-weighted average of those airways that remain open at end expiration, which generally have shorter time constants and better mechanical properties than those that seal earlier in the expiratory period at higher pressure (see Fig. 10.2).
The flow tracing gives some indication of the underlying presence of gas trapping but does not indicate its severity. For example, a severely obstructed airway may be totally occluded and therefore unable to transmit its high pressure to the pressure sensor located within the machine circuitry. Similarly, a narrow airway may give rise to an almost imperceptible flow at end expiration. Several features of the flow tracing are of value: High-frequency variations of the flow tracing suggest the presence of retained airway secretions or water in the external tubing. An abrupt transition between the earliest part of expiration and what follows (“hockey sticking”) indicates a flow limitation during tidal breathing and the potential value of added PEEP if flow persists to the onset of the next breathing cycle (Fig. 10.10). Several signs appearing in the monitored airway pressure and flow signals have been reported that appear to be signatures of partial central airway occlusion, as by mucus plugging (see Fig. 10.8).28
Support Phase Management
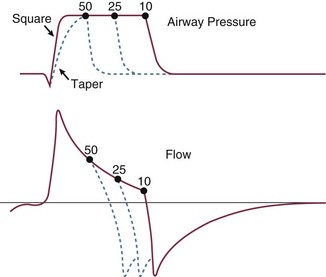
Apart from improving expiratory resistance and reducing the minute ventilation requirement, another intervention aimed at reducing mean alveolar pressure and auto-PEEP is to modestly lengthen the available expiratory time (e.g., by increasing inspiratory flow rate). The flow rate should initially be set at approximately four times the minute ventilation requirement when using a constant inspiratory flow profile. Extending the expiratory time further is usually fruitless, unless minute ventilation is simultaneously reduced. Decelerating flow profiles are often poorly tolerated by the patient with severe AO who makes spontaneous efforts because the latter half of the inspiratory period may require higher flows than imposed by the tightly regulated and stereotyped flow waveform of the ventilator. Adjustments to the flow criterion off-switch that triggers expiration can help manage this problem during pressure-supported ventilation (Fig. 10.11).
PEEP and CPAP in Severe Airflow Obstruction
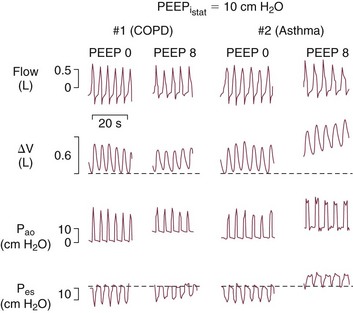
The deliberate use of PEEP in patients with AO has historically been considered undesirable, but there is now ample reason to believe that most of these patients benefit from the application of low levels of PEEP or continuous positive airway pressure (CPAP).5,10,11 When applied downstream of airways that collapse dynamically during the exhalation phase of tidal breathing, PEEP helps to improve the effective triggering responsiveness of the machine without significantly increasing the alveolar pressure or hyperinflation (Fig. 10.12). Most benefit is provided to patients receiving volume-controlled ventilation who do not experience proportionate increases of peak static or peak dynamic cycling pressures after PEEP application.10,11,30 When using a fixed level of targeted pressure (pressure control or pressure support), tidal volume may increase after PEEP is applied. This occurs because the added PEEP counterbalances auto-PEEP to allow the applied inspiratory pressure to more effectively drive inspiratory flow. In effect, PEEP improves the pressure gradient that drives inspiratory flow and delivers tidal volume. (Should minute ventilation increase with PEEP application, total PEEP may also rise.) Applied PEEP may also help to keep airways more widely patent, and thereby improve secretion clearance. Finally, the application of the external PEEP may help to even the distribution of ventilation among multiple units with heterogeneous time constants (Fig. 10.13).
Newer Modes of Ventilation in Airflow Obstruction
Currently, the majority of ventilatory support of AO is still provided with modes of ventilation that are now decades old—flow-controlled, volume-cycled ventilation (“assist-control”); pressure assist control (pressure control, PCV); pressure support (PSV); and SIMV. When combined with PEEP/CPAP and an attentive provider, these time-tested options suffice for the majority of patients. Increasingly, however, practitioners have recognized the need to offload responsibility for minute-by-minute and even intrabreath adjustment of settings for flow and pressure delivery in response to changing conditions of mechanics or ventilatory demand. Patients with dyspnea generally need faster rise of pressure and flows to their target values, unimpeded inspiratory flow, and precise termination of the ventilator’s inspiratory phase so as to avoid collisions between the patient’s and the ventilator’s cycling rhythms. Once set, however, a specified flow pattern regulates the ventilator’s gas delivery, whereas pressure control caps airway pressure at the targeted value. Just as importantly, these time-cycled assist control modes (whether flow or pressure regulated) disregard the duty cycle rhythm variations of the patient’s own drive center; Therefore, asynchrony occurs commonly in these modes, and asynchrony may result not only in dyspnea but also in adverse outcomes.31 In either case the relative power contribution of the machine declines as effort increases and rises as patient effort declines. Appropriate moment-by-moment intracycle adjustment of flow or pressure is not an option with these “traditional” modes of ventilation. Logic dictates that better synchrony between patient and machine would require continuously monitored feedback and flexibility to adjust to the vagaries of patient need.
Proportional Assist Ventilation
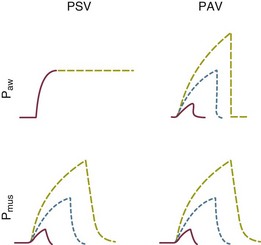
Proportional assist ventilation (PAV), a mode based on the equation of motion of the respiratory system that regulates delivered pressure in proportion to externally sensed inspiratory flow and volume, effectively mimics the actions of an auxiliary muscle in patients without gas trapping.32–34 Quite unlike pressure support, which targets the same pressure for every breath, PAV takes resistance and compliance into account and is meant to provide help in proportion to effort (Fig. 10.14). Unfortunately, ventilatory impedance varies considerably in patients with AO, and a considerable fraction of inspiratory muscle effort is spent in counterbalancing variable auto-PEEP, an event that precedes the onset of inspiratory flow. Given the strong dependence of dynamic hyperinflation on minute volume (VE) and the expiratory time constant, PAV cannot easily fulfill its intended function in patients with severe OA and changing mechanics or minute ventilation needs. Despite these theoretical disadvantages, modern adaptations of PAV—which monitor respiratory mechanics on an ongoing basis—have shown at least equivalent comfort in patient trials, as well as no greater incidence of missed triggering events and greater tidal volume variability than pressure support, both in invasive and noninvasive settings.35 Although reassuring, no outcome advantage over PSV has yet been convincingly demonstrated in any setting.
Neurally Adjusted Ventilatory Assist
Neurally adjusted ventilatory assist (NAVA) is similar in concept to PAV in that it attempts to regulate the machine’s power support moment by moment by sensing inspiratory effort from the patient.34,36 The difference is that the diaphragmatic electromyogram (EMG) provides the signal, so the electrical timing and intensity of phrenic nerve traffic regulate the amplitude of the pressure delivered. NAVA depends on drive to breathe, not the lung’s mechanical response. A thin, multielectrode esophageal catheter is used to pick up the strength and contour of the EMG signal, and failure to detect it has not been a major problem. The dynamic hyperinflation drawbacks of PAV in patients with AO are theoretically nullified by placing the “effort detector” closer to the respiratory drive controller—inspiratory efforts related to auto-PEEP are tracked and supported well before inspiratory flow actually begins. Inherent protective reflexes are believed to help the patient avoid overdistention, even if the machine’s power boost factor is set inappropriately high. Despite its intuitive appeal, NAVA is still too new to the clinical arena to confirm its theoretical advantage.
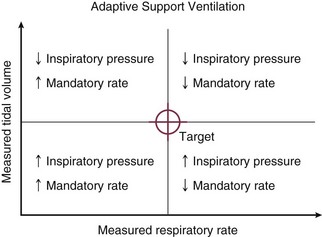
Adaptive Support Ventilation
Adaptive support ventilation (ASV) is a mode that regulates machine output with pressure-targeted breaths delivered at a variable frequency and with variable pressure in accordance with breathing pattern feedback from the patient (Fig. 10.15). Its intent is to minimize the work of breathing and auto-PEEP, and it does this by optimizing the tidal volume and frequency combination that make up minute ventilation. As such, ASV is one step closer to closed-loop ventilation, in which clinical goals are set, monitored, and accomplished automatically. Once the clinician has determined the PEEP, FIO2, and cycling triggers for pressure support, he or she must input the patient’s ideal body weight (from which the series deadspace is estimated) and the fraction of estimated “normal” minute ventilation the machine should provide. It then varies the mandatory breath number and the pressure targets of a pressure-regulated SIMV algorithm. All the while, the machine tries to nudge the patient toward the ventilatory pattern optimum that should minimize deadspace, work of breathing, and auto-PEEP.
Weaning (“Liberation”) Phase
Patients may fail to wean from mechanical ventilation for a wide variety of reasons. Among these are hypoxemia, cardiac arrhythmias, cardiovascular instability, and psychological dependence. However, imbalance between ventilatory capability and demand is perhaps the most common reason for failure to wean in patients with ventilatory failure either of itself or because it provokes one or more of the other factors just mentioned.37,38
Predicting Readiness for Spontaneous Breathing
In clinical practice a panel of indices has long been used to predict the outcome of the weaning trial. Most individual elements of these demand or capability panels can be classified as indicators of either one or the other, but not both. Some capability indicators depend on patient effort as well. Thus, a maximal inspiratory pressure measurement exceeding 30 cm H2O and a minute ventilation requirement of less than 10 L per minute are standard components of the traditional predictive battery. Although minute ventilation has been criticized as unreliable when used alone, it is still a highly useful observation, particularly when referenced to blood gas measurements and integrated into an evaluation of other panel elements. When the patient is calm, a high degree of variation of minute ventilation suggests some degree of ventilatory reserve.39 Because the product of minute ventilation and the average inspiratory pressure per breath are the main components of the breathing workload, minute ventilation must not be disregarded, even when more integrative indices are in use, such as the frequency-to–tidal volume ratio (rapid shallow breathing index, RSBI).40 For example, a rising RSBI does not necessarily portend failure if minute ventilation rises as well. Published criteria for RSBI lose reliability in the presence of neuromuscular weakness, severe restriction, or chronic illness requiring ventilator support.38
Numerous other weaning outcome indices have been suggested over the years, but none stands alone as infallible, including the RSBI. The most successful of these indicators reliably relate power requirement to the ability of the patient to sustain it. Certain physiologic measurements such as the P0.1 (a measurable indicator of ventilatory drive now offered on some of the newest ventilators) have predictive appeal. These are not universally available, however, and cannot be relied on for definitive judgment in every case. Because many factors may limit the patient’s ability to be removed from the ventilator, more than one single indicator is usually necessary to observe. Alertness, degree of cardiovascular compensation, clinical trajectory over the preceding days, oxygenation status, secretion load, upper airway patency, coughing efficiency, and psychological well-being are as important as any single predictive measure based on mechanics and muscle strength. Successful weaning protocols take all such factors into account.37,38,40
Weaning Approaches
Preparations
Preparations for ventilator withdrawal should include ensuring adequate nocturnal rest with fully supported breathing, adequate nutrition, good circulatory reserve, avoidance of excessive intravascular volume and edema, treatment of infection, appropriate body positioning, and judicious sedation.37,40 Obstipation, urinary retention, pleural effusions, gastric distention, musculoskeletal pain, severe anemia, and chemical imbalances must be avoided or reversed. During the full support phase of ventilation, care must be taken not to allow sedatives to accumulate or secretions to collect within the airways. Heat-moisture exchangers do not hydrate the airway secretions reliably, especially in low humidity environments or when high inspired concentrations of oxygen have been in use. Active humidification often proves the better choice when secretion load or clearance is problematic. Inspection of the central airway prior to attempted extubation may be rational when the patient has been ventilated for lengthy periods, as inspissated secretions may not have been adequately suctioned. Withdrawal of sedatives should be attempted on a daily basis in an effort to prevent oversedation, especially when the sedating drug is infused continuously. The patient must not depend on high levels of PEEP for either oxygenation or ventilatory comfort. It must be remembered that PEEP and CPAP aid ventilation in patients with flow limitation and auto-PEEP.
The use of frequent wakeups, intermittent dosing, and short-acting sedatives (especially in the hours prior to spontaneous breathing trials and weaning attempts) has helped to avoid the common problem of benzodiazepine metabolite hangover. When benzodiazepines are given for lengthy periods, lingering sedative effects may persist for up to a week after the last dose is given. Dexmedetomidine (Precedex), a sedative agent with relatively little hypnotic action, has proved helpful in some cases in which calm alertness is desired but difficult to otherwise achieve.41 In well-selected cases, alertness-enhancing drugs such as modafinil (Provigil) have been helpful. Delirium that interferes with weaning occurs commonly and may benefit from such agents as quetiapine and olanzepine.42
Specific Modes
Considerable effort has gone into the delineation of the optimal weaning technique. It is generally true that the majority of patients do not need a lengthy period of gradual machine withdrawal once the primary problems that brought the patient to medical attention have been treated and the adverse effects of fluids and drugs given during their acute problems have been addressed.43 It is also true, however, that a distinct subset of these patients with underlying AO or other chronic impairments not amenable to therapy cannot tolerate abrupt transitions to spontaneous breathing. More graded reloading is sometimes necessary because of fragile cardiovascular status, neuromuscular weakness, or psychological factors. Pressure support ventilation is generally to be preferred to SIMV, as the muscle and cardiovascular reloading process tends to be less sudden and more predictable. Intermittent T-piece weaning makes little sense to employ in these patients; each transition to fully spontaneous breathing abruptly imposes a full stress workload. All patients, however, should be tested with low-level pressure support or T-piece breathing before any gradual withdrawal of support is undertaken, as the latter may not be necessary.44 Once the patient is breathing on low-level pressure support or from an oxygenated T-piece, observation should be continued at least 30 minutes, but generally less than 2 hours before decannulation of the airway is attempted. During the attempt at spontaneous breathing, the patient must be watched carefully and not allowed to fatigue because recovery from that condition may require more than a day to restore energy reserve.45
Periextubation Phase
The immediate postextubation phase should be as carefully managed as the ventilated one. The first 24 hours off the ventilator are often difficult and tenuous, but in successful cases there should be progressive improvement. Coughing, deep breathing, adequate oxygenation, avoidance of arrhythmias, adequate bronchodilation and airstream hydration, maintenance of a clear central airway, and a mechanically efficient posture are crucial. Oral refeeding must be undertaken with extreme caution because swallowing difficulty in chronic dysfunction can persist days to weeks after extubation in patients who have been ventilated for lengthy periods. CPAP and intermittent noninvasive ventilation may be especially helpful in selected patients,46 especially in the nighttime hours. Noninvasive ventilation maintains patency of an edematous upper airway and provides ventilation support during sleep. This is often important in patients who have received sedating medications or are sleep deprived. It must be used judiciously, however, and carefully applied. Ventilation by pressurized mask impedes secretion clearance, and if a humidifier is not used, mouth breathing during bilevel positive airway pressure (BiPAP) dries secretions and encourages displacement of oral material into the central airway. Therefore, it is common for patients who receive mask ventilation after extubation to require reintubation for clearance of thickened airway mucus.
References
1. Hogg, JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004; 364:709–721.
2. Rossi, A, Poggi, R, Roca, J. Physiologic factors predisposing to chronic respiratory failure. Respir Care Clin North Am. 2002; 8:379–404.
3. Kimball, WR, Leith, DE, Robins, AG. Dynamic hyperinflation and ventilator dependence in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1982; 126:991–995.
4. Laghi, F, Goyal, A. Auto-PEEP in respiratory failure. Minerva Anestesiol. 2012; 78(2):201–221.
5. Marini, JJ. Dynamic hyperinflation and auto-positive end-expiratory pressure: Lessons learned over 30 years. Am J Respir Crit Care Med. 2011; 184(7):756–762.
6. Calverley, PM, Koulouris, NG. Flow limitation and dynamic hyperinflation: Key concepts in modern respiratory physiology. Eur Respir J. 2005; 25:186–199.
7. Pepe, PE, Marini, JJ. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: The auto-PEEP effect. Am Rev Respir Dis. 1982; 126:166–170.
8. Rossi, A, Polese, G, Brandi, G, Conti, G. Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med. 1995; 21:522–536.
9. Rossi, A, Gottfried, SB, Zocchi, L, et al. Measurement of static compliance of the total respiratory system in patients with acute respiratory failure during mechanical ventilation. The effect of intrinsic positive end-expiratory pressure. Am Rev Respir Dis. 1985; 131:672–677.
10. Marini, JJ. Should PEEP be used in airflow obstruction? Am Rev Respir Dis. 1989; 140:1–3.
11. Smith, TC, Marini, JJ. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol. 1988; 65:1488–1499.
12. Marini, JJ. Heliox in chronic obstructive pulmonary disease … time to lighten up? Crit Care Med. 2000; 28:3086–3087.
13. Marini, JJ, Tyler, ML, Hudson, LD, et al. Influence of head-dependent positions on lung volume and oxygen saturation in chronic airflow obstruction. Am Rev Respir Dis. 1984; 129:101–105.
14. Leatherman, JW. Mechanical ventilation in obstructive lung disease. Clin Chest Med. 1996; 17:577–590.
15. Similowski, T, Yan, S, Gauthier, AP, et al. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991; 325:917–923.
16. Orozco-Levi, M. Structure and function of the respiratory muscles in patients with COPD: Impairment or adaptation? Eur Respir J. 2003; 22(46):41s–51s.
17. Lapinsky SE Leung, RS. Auto-PEEP and electromechanical dissociation. N Engl J Med. 1996; 335:674–675.
18. Marini, JJ, Crooke, PS, Truwit, JD. Determinants and limits of pressure preset ventilation: A mathematical model of pressure control. J Appl Physiol. 1989; 67:1081–1092.
19. Hotchkiss, JR, Adams, AB, Dries, DJ, et al. Dynamic behavior during noninvasive ventilation. Chaotic support? Am J Resp. Crit Care Med. 2001; 163:374–378.
20. Jubran, A, Van de Graaff, WB, Tobin, MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995; 152:129–136.
21. Brochard, L, Mancebo, J, Wysocki, M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995; 333:817–822.
22. Brochard, L, Isabey, D, Piquet, J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990; 323:1523–1530.
23. Lightowler, JV, Wedzicha, JA, Elliott, MW, Ram, FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane Systematic Review and Meta-analysis. BMJ. 2003; 326:185.
24. British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002; 57:192–211.
25. Nava, S, Ceriana, P. Causes of failure of noninvasive mechanical ventilation. Respir Care. 2004; 49:295–303.
26. Ambrosino, N, Foglio, K, Rubini, F, et al. Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: Correlates for success. Thorax. 1995; 50:755–757.
27. Feihl, F, Perret, C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med. 1994; 150:1722–1737.
28. Zamanian, M, Marini, JJ. Pressure-flow signatures of central-airway mucus plugging. Crit Care Med. 2006; 34:223–226.
29. Celli, BR, MacNee, W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004; 23:932–946.
30. Ranieri, VM, Giuliani, R, Cinnella, G, et al. Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis. 1993; 147:5–13.
31. Thille, AW, Rodriguez, P, Cabello, B, et al. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006; 32(10):1515–1522.
32. Younes, M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis. 1992; 145:114–120.
33. Giannouli, E, Webster, K, Roberts, D, Younes, M. Response of ventilator-dependent patients to different levels of pressure support and proportional assist. Am J Respir Crit Care Med. 1999; 159:1716–1725.
34. Kacmarek, RM. Proportional assist ventilation and neurally adjusted ventilatory assist. Respir Care. 2011; 56(2):140–152.
35. Navalesi, P, Hernandez, P, Wongsa, A, et al. Proportional assist ventilation in acute respiratory failure: Effects on breathing pattern and inspiratory effort. Am J Respir Crit Care Med. 1996; 154:1330–1338.
36. Sinderby, C, Navalesi, P, Beck, J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999; 5:1433–1436.
37. Marini, JJ. Weaning from mechanical ventilation. N Engl J Med. 1991; 324:1496–1498.
38. Epstein, SK. Weaning from ventilatory support. Curr Opin Crit Care. 2009; 15(1):36–43.
39. Wysocki, M, Cracco, C, Teixeira, A, et al. Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit Care Med. 2006; 34:2076–2083.
40. Hill, NS. Following protocol: Weaning difficult-to-wean patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164:186–187.
41. Hipp, DM, Ely, EW. Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurotherapeutics. 2012; 9(1):158–175.
42. Girard, TD, Kress, JP, Fuchs, BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled Trial): A randomised controlled trial. Lancet.. 2008; 371(9607):126–134.
43. Esteban, A, Frutos, F, Tobin, MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995; 332:345–350.
44. Hooper, MH, Girard, TD. Sedation and weaning from mechanical ventilation: Linking spontaneous awakening trials and spontaneous breathing trials to improve patient outcomes. Crit Care Clin. 2009; 25(3):515–525.
45. Laghi, F, D’Alfonso, N, Tobin, MJ. Pattern of recovery from diaphragmatic fatigue over 24 hours. J Appl Physiol. 1995; 79:539–546.
46. Esteban, A, Frutos-Vivar, F, Ferguson, ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004; 350:2452–2460.