Chapter 65
Venous Tumors
Thomas C. Bower
Malignant venous tumors either originate primarily from the vein wall, extrinsically compressing or invading the vein, or grow within it as tumor thrombus.1–5 Most patients with these tumors have such advanced disease that operation cannot be offered. Surgical resection remains the mainstay of treatment, but there is a growing literature on the role of preoperative or postoperative adjuvant therapy.6–9 Secondary tumors of the superior vena cava (SVC) are rarely operable, so SVC obstruction is usually treated with venous stenting.2 Peripheral vein tumors may require concomitant arterial, bone, or adjacent soft tissue resection, with or without axial venous replacement.10–12 Because malignant neoplasms that affect the inferior vena cava (IVC) are most common,13–43 much of this chapter focuses on the diagnosis and treatment of them. The selection of patients, the surgical principles of management, and the techniques used to replace major veins are reviewed.
Definition and Tumor Types
Tumors of the IVC are classified by whether they involve the infrarenal, suprarenal, or suprahepatic segment. The suprarenal IVC has a retrohepatic portion behind the liver and an infrahepatic portion that is located between the caudate lobe and the renal veins.2,3
Intracaval tumor thrombus is defined by level or extent of IVC involvement. Level I thrombus extends to within 2 cm of the renal vein; level II thrombus extends into the suprarenal IVC but below the hepatic veins; level III thrombus is to the hepatic veins but below the diaphragm; and level IV thrombus extends into the right side of the heart.2
The types of primary and secondary tumors are shown in Box 65-1. Primary venous leiomyosarcomas (PVLs) occur more often than arterial sarcomas but are much rarer than retroperitoneal leiomyosarcomas.2 The first venous leiomyosarcoma was described by Perl in 1871.44 Suprarenal involvement occurs in more than 40% of cases,4 and three fourths of these tumors involve retroperitoneal and abdominal veins. The great saphenous vein is the most frequent site of PVL of the lower extremity.5
Venous leiomyosarcomas are polypoid or nodular, are firmly attached to the vein wall, and exhibit less intratumor hemorrhage or necrosis than other sarcomas.2,5 The most common growth pattern is intraluminal, but the tumor may grow through the vein wall and invade adjacent structures, which makes differentiation from other retroperitoneal sarcomas difficult.2,3 Distant metastases to the lung, liver, kidney, bone, pleura, or chest wall occur in half of the patients at the time of diagnosis,4,5 so survival is limited to months if surgery cannot be offered.4
Secondary cancers or sarcomas that involve the IVC are more common than PVL. Venous invasion, luminal obstruction by extrinsic compression, or intraluminal growth is the pathologic process (Fig. 65-1). Retroperitoneal sarcomas are the most common cause of malignant obstruction of the infrarenal segment but may affect higher levels.2,3 Sarcomas displace but rarely invade adjacent structures because of their pseudocapsule. However, the tissue planes between the tumor and IVC may be indistinct with large or irradiated tumors. Visceral or solid organ cancers affect the IVC in anatomic proximity to the site of origin of the neoplasm. For example, cancers of the liver, duodenum, pancreas, kidney, or adrenal glands involve the suprarenal IVC.2,3
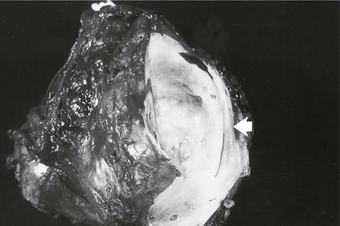
Figure 65-1 Pathology specimen showing both extraluminal and intraluminal growth of a retroperitoneal sarcoma. Invasion of the lumen is shown by the arrow.
A variety of cancers and sarcomas exhibit intraluminal tumor thrombus as part of their biologic behavior, with renal cell carcinoma (RCC) being most common (4%-15% of patients with this cancer).26–43 In fact, RCC is the most common cancer of the IVC that requires operative intervention.2 Tumor thrombus from RCC is found in the renal veins in 15% to 20% of cases21; the right kidney is affected more often than the left one, and tumors with thrombus tend to be larger than 4.5 cm in diameter.2,26,27 However, tumor thrombus can be seen with small renal cell or adrenocortical carcinomas (Fig. 65-2).2,3 In nearly half of patients with RCC and caval involvement, the tumor thrombus extends to within 2 cm of the renal vein–caval confluence (level I). Another 40% of patients have thrombus in the suprarenal IVC below the diaphragm (levels II and III), and in only 10% is thrombus in the right side of the heart (1% of all RCC patients).26,27 Similar to patients with PVL, survival of patients with secondary caval malignant neoplasms is measured in months without treatment.1–3,17
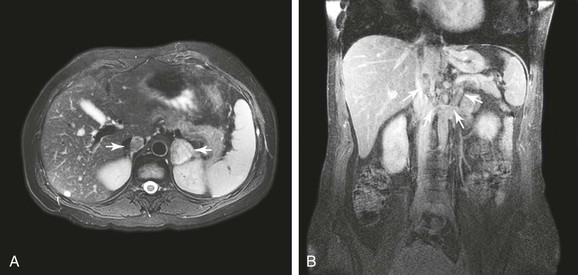
Figure 65-2 Axial (A) and coronal (B) images of a patient with a 3-cm adrenal tumor and associated inferior vena cava (IVC) tumor thrombus. The coronal image shows a 5-mm stalk of tumor thrombus extending from the left adrenal vein, through the left renal vein, and into the IVC (outlined by arrows).
Most extremity venous tumors occur secondary to sarcomas of the bone, cartilage, muscle, or fatty tissues.10–12 Malignant melanoma and fibrohistiocytoma may also cause peripheral vein compression or invasion. PVL of the superficial lower extremity veins occurs as a nodular mobile mass, whereas PVL of the deep veins often invades the adjacent soft tissues.5
Clinical Presentation and Evaluation
Primary leiomyosarcoma of the IVC is more common in women than in men, occurs over a wide age range, and has a mean patient age between 50 and 60 years.5,13–15 More than 80% of patients in the leiomyosarcoma registry compiled by Mingoli and colleagues were women.4 PVL of the peripheral veins affects men and women equally.5 Patients remain asymptomatic for a long time until symptoms or signs occur from metastatic disease or venous obstruction. Early detection is rare. Only 4 of 144 patients with IVC leiomyosarcoma in a review by Mingoli and colleagues had the tumor discovered incidentally.4 Abdominal pain is the most common presentation in 66% to 96% of patients.4,13 A palpable abdominal mass, lower limb edema, weight loss, Budd-Chiari syndrome, and vague symptoms (such as fever, weakness, anorexia, night sweats, and dyspnea) occur less often.4 Consumption coagulopathy is a rare but reported problem.2
Secondary caval tumors are detected in patients between the ages of 40 and 70 years.1–3,13–43 The mean age of patients who underwent IVC resection and replacement for malignant disease in one Mayo Clinic report was 52 years but ranged from 16 to 88 years.1 The majority of patients have symptoms and signs related to the cancer, not to IVC obstruction.1–3,13–25 IVC obstruction is a late sign, so vena cava involvement is identified on imaging studies. Symptoms and signs vary by the segment of IVC obstructed and include pain, arrhythmia, syncope, Budd-Chiari or nephrotic syndrome, and motor or sensory neuropathies. Lower extremity edema and deep venous thrombosis are rare problems with IVC tumors but are seen with primary tumors of the iliac or peripheral veins.1–3,5
A multidisciplinary team is critical to the evaluation and treatment of patients with venous tumors. Medical and surgical oncologists and subspecialists (vascular, hepatobiliary, urologic, orthopedic, neurologic, and cardiothoracic surgeons) are needed to direct the evaluation and to determine treatment. The goals of evaluation include the identification of the type and extent of tumor, a search for metastases, an assessment of the degree of venous obstruction, and the determination of patient risk and performance status.2,3
Computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography, and rarely venography are used alone or in combination to accomplish these anatomic goals.1–3,7,17,18,45–50 CT and MRI are the most common tests used to define the location and extent of the tumor because the combination of axial, coronal, and sagittal images helps to plan the operation (Fig. 65-2). Venous phase imaging provides excellent views of the veins and collaterals. The role of venography is confined to the rare patient in whom histologic diagnosis by intraluminal fine-needle aspiration or true-cut needle biopsy would influence administration of preoperative adjuvant therapy2,3 or when computed tomographic venography or magnetic resonance venography inadequately defines venous occlusions and the collateral pathways.
MRI is the study of choice to define the upper extent of intracaval tumor thrombus and to differentiate bland from tumor thrombus in the infrarenal cava.43 Because not every patient can tolerate MRI, improvements in multidetector CT imaging have been welcomed. Guzzo and associates50 studied 41 patients after operation who had multidetector CT imaging before surgery. CT findings concurred with the intraoperative pathologic findings in 84% of this group, and the level of tumor thrombus was accurately depicted in 96% of patients. Few studies have compared MRI and CT in these circumstances. Two small studies show accuracies between 75% and 100% for CT and between 75% and 88% for MRI.48,49 MRI has been used to predict IVC wall invasion in patients with tumor thrombus. Those with an abnormal signal on either side of the caval wall on gadolinium imaging, an IVC diameter of 40 mm or more, a level III or level IV thrombus, or an IVC diameter of 18 mm together with a renal vein ostium diameter of 14 mm are at high risk of tumor adherence to the vein wall (90% sensitivity).46
Ultrasonography provides an accurate assessment of peripheral vein obstruction, but its ability to image the iliac system and IVC is hampered by bowel gas or when the tumor distorts the veins.2,3 Preoperative or intraoperative transesophageal echocardiography is sometimes used to corroborate the proximal extent of tumor thrombus if it is near the right atrium.26
If the tumor is localized and there are no metastases, a preoperative medical risk assessment is done, including a detailed cardiopulmonary evaluation. Of equal importance is an assessment of patient performance status.2 Patients in excellent physical condition with no or minimal limitation in daily activities (scores 0 and 1) have the best chance to maintain a similar quality of life after operation.1,17 Those who are either bedridden or need assistance in performing self-care (scores 3 and 4) are not offered operation at the author’s institution.
Treatment Approach and Type of Reconstructions
At the time of diagnosis, most patients with IVC tumors have advanced disease, which precludes operation. Those with diffuse metastases, poor cardiopulmonary function, or physical debility should not undergo surgical resection, in my opinion. In contrast, patients with localized tumors, a good performance status, and few medical comorbidities are candidates for surgical resection.1–3,13–25
Operative treatment and approach depends on the tumor type and its extent, the segment of IVC involved by the tumor, the degree of caval obstruction, and the status of collateral veins. The choice of incision is based on the patient’s body habitus, the segment of IVC affected, and whether major liver resection or cardiopulmonary bypass is needed. A midline abdominal incision works well to approach the infrarenal or infrahepatic segment for patients with narrow costal margins and for patients with enlarged subcutaneous abdominal wall venous collaterals (Fig. 65-3). A bilateral subcostal incision is useful for patients with wide costal margins who need infrahepatic or retrohepatic IVC replacement and concomitant liver resection (Fig. 65-4). This incision can be extended by a median sternotomy in patients with large RCCs and level III or level IV tumor thrombus in whom cardiopulmonary bypass may be needed. Last, some patients who need major liver resection and retrohepatic IVC replacement are best approached by a right thoracoabdominal incision through the eighth or ninth interspace.2
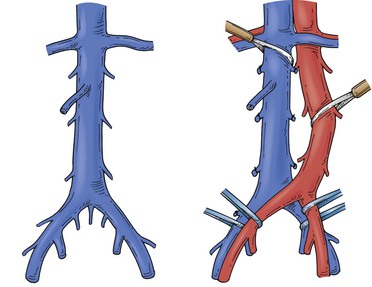
Figure 65-3 Tumors of the infrarenal segment can be approached through a midline abdominal incision. The infrarenal aorta and common iliac arteries often require partial mobilization to allow access to the lower vena cava and the proximal common iliac veins. Several lumbar veins may require division, and the surgeon must be wary of one to three small, middle, or lateral sacral veins that can cause troublesome bleeding if they are torn.
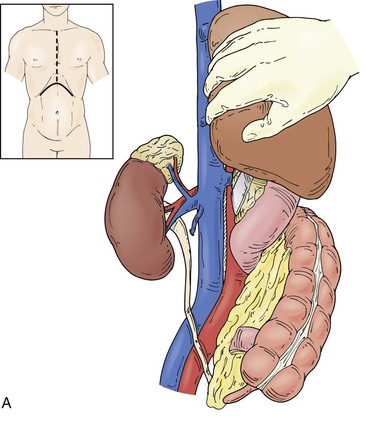
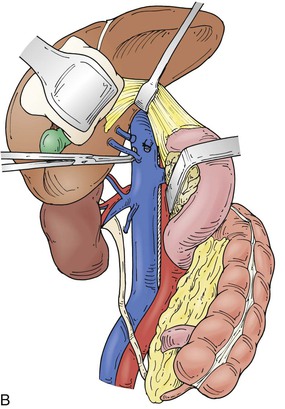
Figure 65-4 The suprarenal segment can be approached through a midline abdominal, bilateral subcostal, or a right thoracoabdominal incision. The abdominal incision can be extended through the sternum if access is needed to the right heart, as in some patients with tumor thrombus. A right medial visceral rotation is used to expose either the retrohepatic (A) or infrahepatic (B) segment. Further mobilization of the infrahepatic segment requires ligation and division of between one and four caudate lobe veins.
Surgical treatment of secondary tumors of the iliac and peripheral veins is also dependent on the extent of tumor, but adjacent arterial and nerve involvement must be anticipated.10–12 Long-segment chronic occlusions of the iliac veins rarely require replacement unless collateral veins are sacrificed in the course of resection. Axial lower extremity autogenous venous reconstruction is needed when preoperative imaging shows a paucity of collateral veins draining into the ipsilateral saphenous, deep femoral, or iliac systems. Contralateral saphenous vein is the first choice as a conduit and can be used as a straight, panel, or spiral graft. Venous reconstruction should follow arterial reconstruction when both vessels are resected. Anticipated patency rates of autogenous reconstructions in small clinical series have approached 80% at 2 and 5 years.12 A prosthetic graft has been used in the infrainguinal position but carries a lower patency rate than that of autogenous reconstructions.11 The precept I use for treatment of patients with lower extremity malignant neoplasms is that in-line autogenous or prosthetic reconstruction should be done in those who have patent axial veins at the time of tumor resection. This is particularly important for the common femoral or popliteal vein.
Inferior Vena Cava Resection without Replacement
The decision to replace the IVC during tumor resection is controversial but depends on whether the patient has problems from the caval obstruction, such as lower extremity edema or renal insufficiency.1–4,51 Patients with chronic IVC occlusion and well-developed venous collaterals that are not interrupted by operation can have the vena cava resected en bloc with the tumor with little venous morbidity.2 Patients with rapid occlusion of the IVC, few venous collaterals, and lower extremity edema are best treated with graft replacement.1–3
Although resection of the suprarenal IVC without replacement has been described, my preference always has been to reconstruct this segment because of the potential for acute kidney failure and lower extremity edema.1–3,18 The ability to predict which patients will tolerate resection without renal failure is difficult even if the paravertebral, lumbar, epigastric, adrenal, and gonadal venous pathways are patent.2,3 I reconstruct the remnant left or right renal vein if tumor resection involves a nephrectomy. Preservation of outflow through the remaining renal vein is necessary if the patient develops intraoperative anuria or an acute reduction in urine flow.1,16
Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus
The most common malignant neoplasm to involve the IVC with intraluminal tumor thrombus is RCC. In most cases, except with very large cancers and bulky thrombus extending well into the retrohepatic vena cava, the thrombus can be removed en bloc with the cancer by transection of the renal vein at its confluence with the IVC.2,3,26,27 Early ligation of the renal artery may shrink the thrombus and “simplify” operation when the proximal extent of tumor is near the hepatic veins or right atrium.26,32 Most patients have tumor thrombus confined to the infrahepatic IVC. The tumor can be removed by clamping this segment after the caudate lobe veins of the liver have been divided,26 which causes little hemodynamic change (Fig. 65-4A). Patients with thrombus in the retrohepatic IVC extending to the hepatic veins may benefit from total vascular isolation of the liver to remove the tumor and to minimize blood loss. Dilated lumbar veins may cause troublesome backbleeding, even with caval clamping and inflow occlusion to the liver. Mobilization of the retrohepatic IVC on its right lateral side allows ligation of these rare veins. Another cause of backbleeding with total vascular isolation is a replaced left hepatic artery. Some patients with level III thrombus require venovenous bypass during total vascular isolation to support hemodynamics (total vascular isolation and venovenous bypass techniques are discussed under retrohepatic IVC replacement with major liver resection).26
Resection of a large RCC that involves the liver and has retrohepatic IVC tumor thrombus is challenging (Fig. 65-5). Choice of incision and exposure are key first steps. Nesbitt and colleagues suggest nephrectomy first, followed by ligation and amputation of the renal vein rather than en bloc resection.27 This maneuver facilitates access to and exposure of the vena cava and simplifies removal of the tumor thrombus. Renal artery embolization has been used to reduce tumor vascularity with large cancers and to shrink the tumor thrombus. However, few data support its routine use. The Cleveland Clinic group compared the results of 135 patients who had preoperative embolization, radical nephrectomy, and IVC tumor thrombectomy with those of 90 patients who did not have embolization.40 There was no benefit from this treatment. In fact, the authors found a higher mortality rate in the embolized group (13% vs 3%) and a fivefold higher risk of perioperative death by multivariable analysis. Moreover, such treated individuals had greater transfusion requirements and a higher postoperative complication rate (43% vs 29%). The level of tumor thrombus was not significantly reduced with this therapy. At the Mayo Clinic, renal artery embolization is reserved for palliation of symptomatic and inoperable RCC. Most blood loss during radical nephrectomy comes from disruption of dilated perirenal and retroperitoneal venous collaterals due to caval obstruction that are not decompressed with renal artery embolization. Early ligation of the renal artery, removal of the intracaval tumor thrombus, and restoration of normal IVC blood flow before the radical nephrectomy is completed helps decompress these veins.
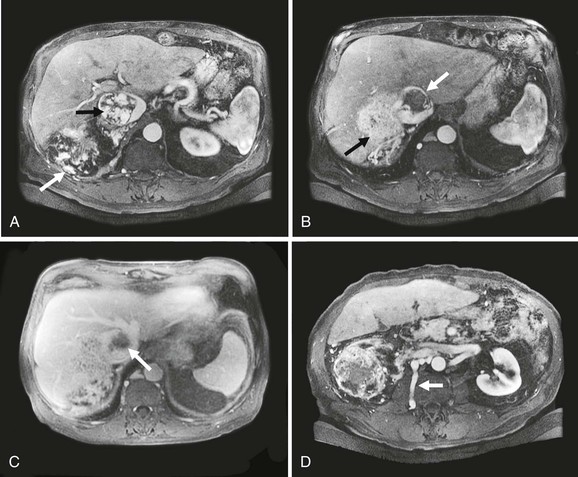
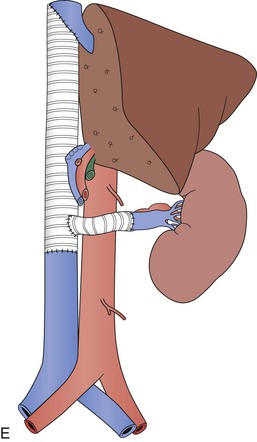
Figure 65-5 Axial CT images of a patient with a large vascular renal cell cancer, right liver lobe hepatic metastasis, and vascular thrombus extending both from the right hepatic vein and from the renal vein into the IVC, which is obstructed (A-C, arrows). Note the large lumbar vein collateral in D. The renal cell cancer was resected, the right lobe of the liver was removed, and because of caval wall invasion, the IVC was replaced from the suprahepatic segment to the infrarenal vena cava with a short graft to reconstruct the left renal vein (E). Note that the rings of the graft are kept intact at the anastomoses to avoid compression.
Intraoperative transesophageal echocardiography is used to guide the position of the upper caval clamp for those with level III tumor thrombus and to document clearance of tumor from the IVC.26 When thrombus clearly involves the right side of the heart, cardiopulmonary bypass, with or without circulatory arrest, may be needed.2,3,26 Concomitant deep hypothermia may be required in some patients, but this increases the risk of coagulopathy.2,3,26
The IVC is closed primarily unless tumor adherence or wall invasion requires partial or circumferential resection. Patch angioplasty or graft replacement is necessary in these circumstances.2,3,26 I patch the cava if 50% of the wall circumference requires resection (Fig. 65-6) and prefer this technique to interposition graft replacement, which becomes necessary in patients with extensive tumor remnants adherent to the IVC wall. Invasion of the renal vein ostium or the IVC by tumor thrombus significantly lessens survival unless these areas are resected. Zini and colleagues found a nearly sixfold increased risk of death from RCC on multivariable analysis controlled for tumor size, stage, and thrombus level in 32 patients who did not have complete removal of the tumor thrombus.46
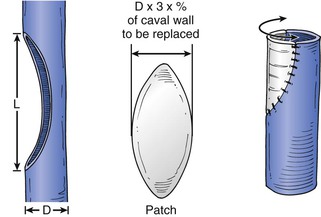
Figure 65-6 If closure of a caval defect will narrow the vein, a bovine pericardial patch is used. The diameter of the patch is estimated from the percentage circumference resected from the normal vena cava. See diagram for calculation. In most cases, the patch assumes a wide elliptical or circular configuration.
Some patients need interruption of the infrarenal IVC because of chronic occlusive bland thrombus. Blute and colleagues reported 40 such caval interruptions among 160 cases treated for level II, III, or IV tumor thrombus during a 24-year period.41 The infrarenal IVC was either ligated or resected, or a Greenfield filter was placed inside of it. This group assessed postoperative disability according to the American Venous Forum International Consensus Committee. Of the 40 patients, 16 (40%) showed no disability, 12 had class I disability, 12 others had class II disability, and no one had class III disability. These authors recommend interruption of the infrarenal IVC in select patients with chronic occlusion or tumor involvement to minimize the chance of perioperative pulmonary embolism.
The aforementioned operative techniques for the management of patients with RCC tumor thrombus also apply to other cancers that exhibit intracaval tumor extension.
Inferior Vena Cava Replacement
At the Mayo Clinic, we believe that the vena cava at any level should be replaced if it is only partially obstructed and a majority of its circumference requires resection to provide clear tumor margins.1–3,17 We prefer large-diameter, externally supported polytetrafluoroethylene grafts (20 mm) and have found their patency rates to be greater than 95% in more than 90 patients now operated on at my institution. The UCLA group favors smaller diameter grafts because they believe that these generate higher blood flow velocities to maintain patency.16 Others use aortic or vena cava homografts with good success.2,25 I used a femoral vein panel graft to reconstruct the IVC in one patient who had resection of a recurrent tumor during which intestinal resection was needed.1 A stenosis occurred at the upper caval anastomosis, which required balloon angioplasty. Perhaps a spiral vein or homograft would have provided better radial force to resist visceral compression. Whereas an arteriovenous fistula has been used to enhance patency of IVC reconstructions in some series, this adjunct is rarely used in our current practice.
Retrohepatic Inferior Vena Cava Replacement in Conjunction with Major Liver Resection
Resection of liver cancers or sarcomas with perihepatic IVC involvement has been reported from a number of centers, including our own.1,13–25 These procedures may be done with in situ or ex situ techniques. It is our opinion that in situ resection with IVC replacement has broader applicability, versatility, and similar efficacy to those done ex situ.17
Our technique of combined liver resection and retrohepatic IVC replacement has evolved over the years (Fig. 65-7).17 Total vascular isolation, selective use of venovenous bypass to maintain hemodynamics, choice of a secure position for the upper caval cross-clamp, and ligation of the afferent and efferent lobar vasculature before parenchymal division are important steps. After exclusion of intra-abdominal spread of tumor beyond the confines of safe resection, intraoperative ultrasonography is used to exclude occult intraparenchymal metastases and to determine the proximity of tumor to major vascular structures, including the planned remnant hepatic vein. The infrahepatic or pararenal IVC and the vessels in the gastrohepatic ligament are isolated before the liver is mobilized and the suprahepatic IVC is dissected free. Patients with large tumors that preclude complete mobilization with an abdominal incision may need extension of that incision into the right side of the chest or else an incision of the diaphragm. Hepatic resection is performed with the CUSA device (Valleylab, Boulder, Colo) after a 5-minute period of inflow vascular occlusion to the liver. Additional ischemic preconditioning by inflow occlusion is used for patients at risk of abnormal parenchymal bleeding, as occurs in some repeated liver operations or with polycystic liver disease. Total vascular isolation is initiated when the surgeon is ready to transect the IVC to complete en bloc tumor resection. The sequence of IVC clamping with total vascular isolation is infrahepatic, hepatic artery and portal vein in the gastrohepatic ligament, and then suprahepatic. Extracorporeal venovenous bypass from the infrarenal IVC to the jugular vein is used if systolic blood pressure cannot be kept above 100 mm Hg with intravenous fluid. The need for venovenous bypass seems to be higher in patients with preexisting cardiopulmonary dysfunction or those older than 60 years.1 Low-dose intravenous heparin (1000-2000 units) is administered before the IVC is cross-clamped, unless there has been significant blood loss during the liver resection from which the patient may be auto-anticoagulated. The upper caval anastomosis is performed during total vascular isolation and often includes the remnant hepatic veins. The anastomosis is tested and flushed with the patient in head-down position and the lungs inflated to 30 mm Hg to avoid air embolism. This allows washout of acid metabolites from the liver before transfer of the suprahepatic caval clamp onto the graft. The lower anastomosis is fashioned end to end to the IVC. The position of the graft is marked and its length checked during maximum inspiration and expiration before the graft is cut to fit. This maneuver avoids torsion or buckling of the graft, which can obstruct the hepatic veins. The rings are kept close to the anastomosis to avoid graft compression (Fig. 65-8). The graft is circumferentially wrapped with omentum to keep it from contact with the bowel.
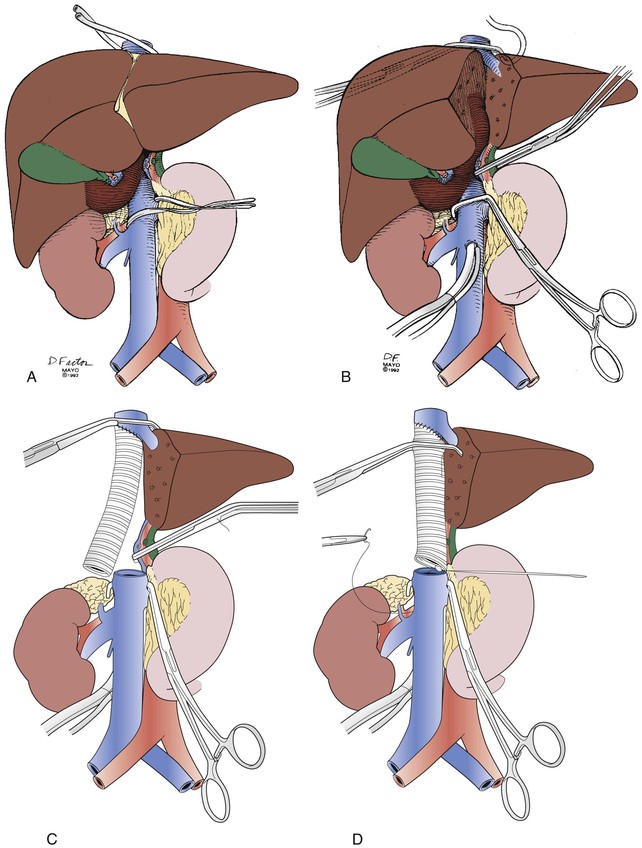
Figure 65-7 Operative technique for replacement of the retrohepatic inferior vena cava (IVC) in conjunction with major liver resection. A, Isolation of the IVC above and below the level of the tumor as well as isolation and division of the hepatic artery and portal vein branches. B, Hepatic vascular exclusion is used to complete resection of the liver, tumor, and retrohepatic IVC. If necessary, venovenous bypass via a cannula is performed. C, The upper caval anastomosis is performed first. D, The suprahepatic caval clamp is transferred across the graft after acid metabolites have been flushed from the liver. The lower caval anastomosis is completed. (From Bower TC, et al: Vena cava replacement for malignant disease: is there a role? Ann Vasc Surg 7:51-62, 1993, with permission.)
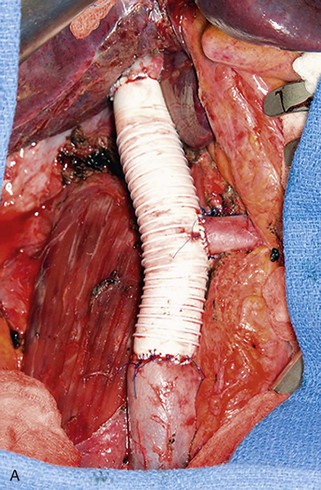
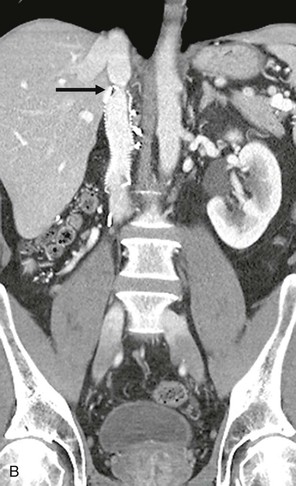
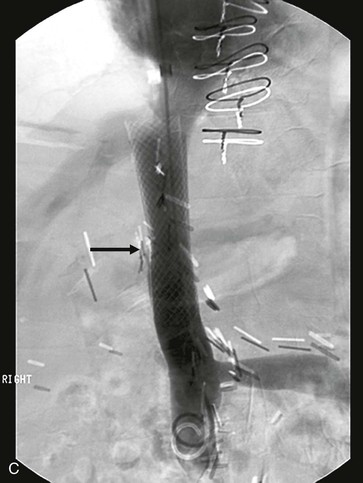
Figure 65-8 Intraoperative photograph (A) and CT scan (B) of a patient who had suprarenal inferior vena cava (IVC) resection with reimplantation of the left renal vein. Note the stenosis at the upper graft–IVC anastomosis where the rings have been removed from the graft (arrow). This area was successfully stented (C).
The major limitation to in situ liver resection and caval reconstruction is warm hepatic ischemia time. Even though our mean liver ischemia time has been 18 minutes it can be unpredictable.17 In addition to the 5 to 10 minutes of hepatic vascular inflow occlusion for ischemic preconditioning, we have used perioperative allopurinol to potentially enhance ischemic tolerance in a few patients, but not as a routine.17 We have used cardiopulmonary bypass and hypothermic circulatory arrest to reconstruct one patient with PVL of the retrohepatic IVC (Fig. 65-9). Others prefer ex situ hepatic resection and autotransplantation of the hepatic remnant because of the unpredictability of hepatic ischemic time.23,24 An advantage of this technique is isolated hypothermic resection with the protective effects of liver perfusion, which allows time to perform difficult vascular reconstructions.17,23,24 However, ex situ resection increases operative time and the number of vascular anastomoses, carries higher perioperative mortality and liver failure rates, and has a real but low risk for salvage orthotopic liver transplantation.18,48,49

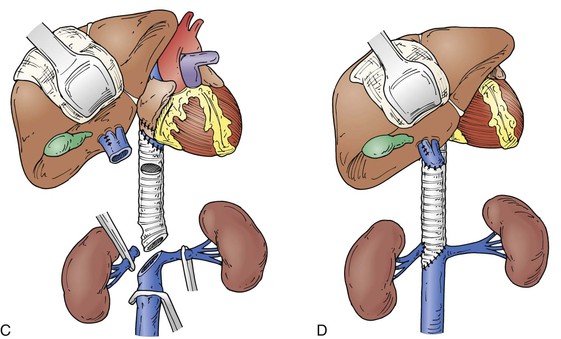
Figure 65-9 Patient with an infiltrative retrohepatic IVC leiomyosarcoma extending from the right renal vein to the cavoatrial junction. The tumor infiltrated the origin of the right hepatic vein (A, B). Reconstruction was accomplished with use of cardiopulmonary bypass and hypothermic circulatory arrest for organ protection. The IVC was replaced with a 20-mm externally supported PTFE graft from the atrium to the left renal vein–caval confluence. The hepatic orifices were recreated and reimplanted onto the graft as a patch. The right renal vein also was reimplanted (C, D).
The accrued data from a number of centers have now firmly established the technical feasibility of these operations.1,5,13–25 The addition of other ischemic protective factors to preserve hepatic function, detailed selection factors, and better neoadjuvant therapies may enhance the utility of these operations in the future.17
Most patients have elevation in their liver function test results and are mildly auto-anticoagulated during the first several days, depending on the length of warm ischemia time. Liver function test results return to baseline within 7 to 10 days.1–3,17 I obtain ultrasound or CT imaging of the IVC graft and the remnant hepatic and portal veins to assess patency if the liver function test results remain elevated more than what is expected for a particular operation. Subcutaneous heparin is begun once the surgeon thinks the risk of hemorrhage is low and as long as the platelet counts are in normal range. Patients are started on oral warfarin before hospital dismissal, with a goal international normalized ratio of 2 to 3. Patients remain anticoagulated for at least 6 months, after which they are prescribed aspirin only, as long as imaging studies show the caval graft to be widely patent.1–3,17 Most patients with infrarenal grafts are anticoagulated lifelong.
Management of the Iliac Veins during Tumor Resection
Management of the iliac veins during sacral resection or hemipelvectomies can be challenging. For patients undergoing sacral resection, the anterior stage of the operation often is done first. If the resection will extend to the L5 vertebral body, the lower aorta and IVC require mobilization. Patients may have between one and three small vein branches at the iliac vein–caval confluence that can be thin walled or broad based. Simple ligation of these branches is ineffective, and suture ligation is needed. Isolation of the common and external iliac arteries and veins is done before dissection of the internal iliac artery and vein branches. Individual ligation of as many internal iliac artery and vein branches as possible reduces blood loss during the bone resection. The internal iliac artery branches are first ligated, followed by those of the internal iliac veins. In some patients with high sacral resection, an effort is made to preserve the posterior division branches of the internal iliac artery. I prefer to control but not ligate the main internal iliac vein trunks; the more caudal and posterior branches are isolated and ligated. This prevents distention of the distal branches, many of which are thin walled and can cause troublesome bleeding if they are inadvertently injured. Several techniques can be used to control and to ligate or oversew short, broad-based internal iliac vein branches as shown in Figure 65-10. Patch angioplasty of the external or common iliac vein is preferred to graft replacement whenever possible. I use 12- or 14-mm-diameter PTFE grafts if segmental resection is done. However, replacement of both common iliac veins is rarely done if they are encased by tumors. If prosthetic graft is used, it is covered by omentum, adjacent soft tissue, or bovine pericardium.
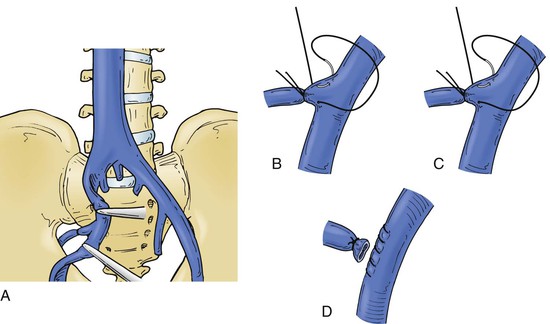
Figure 65-10 The internal iliac vein anatomy is variable, and some branches may be short and broad, which makes their control difficult (A). Methods of control are illustrated. The vein branches can be ligated or suture ligated as shown in B and C. The common and external iliac vein can be clamped, the distal branch suture ligated, and the internal iliac vein divided and oversewn as shown in D.
Outcomes and Survival
Patient outcome is dictated by the same factors that affect operative risk, namely, tumor stage, segment and length of vein that requires resection or replacement, and patient performance status and comorbidities.1–3,13–43
Comparison of outcomes between studies is difficult because they include a variety of malignant neoplasms and lump together patients who have circumferential IVC resection and graft replacement with those who have primary or patch closure of the IVC. These are important distinctions because the hemodynamic and physiologic stresses of partial resection of the IVC wall with patch angioplasty or removal of the infrarenal segment vary considerably in those who require retrohepatic IVC replacement in conjunction with major liver resection or need cardiopulmonary bypass and circulatory arrest to remove tumor thrombus. Table 65-1 lists the larger, more contemporary series as they relate to tumor type, complexity of the operation, segment of IVC treated, and tumor size. For example, in the series by Kieffer’s group, the mortality rate was 20%, but the tumors were very large, and 14 of the 20 patients had involvement of the suprarenal retrohepatic or suprahepatic IVC, of which 5 involved the hepatic veins or had cardiac extension. Thirteen of their patients had IVC graft replacement.13 In contrast, the Brigham and Women’s group had no mortality among 20 patients treated for PVL, but only 5 patients required prosthetic graft replacement of the IVC, one patient had tumor involvement above the hepatic vein, and another needed partial liver resection (one patient each required resection of a portion of the aorta or an iliac artery).14 The majority of the 11 patients in the Illuminati report from the University of Rome, Italy, had involvement of the infrarenal IVC.15 Mortality rates from resection of secondary IVC tumors, in which a majority of patients needed major liver resection, range from 0% to 6.9%.
Table 65-1
Contemporary Series of Primary and Secondary Inferior Vena Cava Malignant Neoplasms
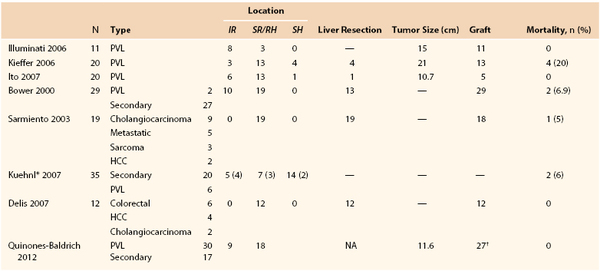
* Nine superior vena cava and 26 inferior vena cava tumors. The location of the nine patients with grafts is shown in parentheses.
† The entire inferior vena cava was replaced with hepatic and renal vein reimplantation in eight patients; six patients had the suprarenal and pararenal inferior vena cava replaced, with seven renal vein reimplantations; and four patients had the infrarenal and pararenal inferior vena cava replaced, with four renal vein reimplantations.
HCC, Hepatocellular carcinoma; IR, infrarenal segment; PVL, primary venous leiomyosarcoma; SH, suprahepatic segment; SR/RH, suprarenal-retrohepatic segment.
A recent report from Quinones-Baldrich and the UCLA group included 47 patients who underwent IVC resection and en bloc tumor excision between 1990 and 2011.16 The majority of tumors were sarcomas (77%), of which 30 originated primarily within the IVC. Of the 47 patients, 11 had primary repair of the IVC, 9 required patch angioplasty, but 27 had circumferential resection and cava replacement with a PTFE ringed graft. Of these 27 patients, 18 had replacement of more than one caval segment. Of these 18 patients, 8 had replacement of all caval segments with renal and hepatic vein reimplantation; 6 others had replacement of the pararenal and suprarenal IVC, with 7 renal veins reimplanted; and 4 had the infrarenal and pararenal segment replaced, which included reimplantation of 4 renal veins. The remaining 9 patients had infrarenal IVC grafts. Impressively, there was no mortality in this series. Major morbidity was 10.6% and included bowel obstruction, temporary acute renal failure, reoperation for bleeding, chylous ascites, and graft thrombosis in one each.
Major morbidity with these operations ranges between 11% and 33%.1,13–19 Only one graft infection was reported among the series listed in Table 65-1.1 Prosthetic graft patency is excellent, ranging from 85% to 100% during a follow-up ranging from 18 months to nearly 4 years.1,13–19 One of my patients has a patent graft 18 years after combined infrarenal aorta and IVC replacement. We now have replaced the IVC in 91 patients, with 3 known graft occlusions, 2 of which occurred as a consequence of late sepsis after additional adjuvant therapies for recurrent disease. The UCLA group reported a graft thrombosis from similar problems.16
The operative mortality and morbidity rates for patients with RCC and intracaval tumor thrombus are the lowest at high-volume centers and have improved over time. A Mayo Clinic report by Blute and coworkers showed the mortality rate to be 8.1% for 86 patients with RCC and tumor thrombus operated on between1970 and 1989 but only 3.8% among 105 patients treated between 1990 and 2000.26 Complication rates were higher in those with level III and level IV thrombus. If venovenous bypass was used instead of cardiopulmonary bypass for these patients, the complication rate decreased from 31% to 17%.
Survival for patients with IVC leiomyosarcoma seems to be best with curative resection. A study of 120 patients from the International Registry of Inferior Vena Cava Leiomyosarcomas by Mingoli and colleagues compared outcomes in 67 patients who had extensive tumor and IVC resection with those in 53 patients who had limited resection of the IVC wall.51 A variety of factors were analyzed, but multivariable analysis was unable to show a significant difference in survival or disease recurrence between the two groups. The other surgical series of IVC leiomyosarcoma show improved survival with curative resection. The report by Kieffer and associates had mean 3- and 5-year actuarial survival rates of 52% and 34.8%, respectively.13 The Ito paper showed a mean disease-free survival of 21 months but a median overall survival of 71 months for the 19 patients who had complete resection.14 The cumulative disease-free survival rate in the report from Illuminati and associates was 44% at 5 years.15
Meaningful impact of these operations on survival rates for patients with secondary IVC malignant neoplasms has improved during the past decade. Overall survival in the study from the Mayo Clinic was 89.3% at 1 year, 80.3% at 2 years, and 75% at 3 years.1 The mean survival among those who had infrarenal IVC resection and replacement was 3.1 years, but it decreased to 2.88 years in those who had the entire IVC replaced and 2.26 years in those with suprarenal caval replacement. In another report from this group that analyzed retrohepatic IVC replacement with major liver resection, overall survival of patients who had partial or segmental liver resection and replacement of the retrohepatic IVC was 21% at 5 years; the median overall survival was 38 months, but the median recurrence-free survival was 11.5 months. Survival was best for patients with intrahepatic cholangiocarcinoma, which was 69% at 3 years.17 The 1-, 3-, and 5-year survival rates reported by the group from Munich, Germany, were 76%, 32%, and 21%, respectively, with a median survival of 29 months. This group found incomplete resection and cardiopulmonary risk to have a significant negative impact on survival.18 The median follow-up reported by the University of Miami group was 24 months, at which point 4 of the 12 patients in the surgical group had died of recurrent disease.
The greatest survival benefit is for patients with RCC and IVC tumor thrombus.26,35,36,52 Five-year survival rates range from 40% to 65% for patients with venous involvement and no metastases but fall significantly to 6% to 28% in the presence of metastases.35 The report by Blute and coworkers had a 5-year cancer-specific survival rate of 59% in those who had no nodal or metastatic disease at the time of operation.26 Survival was found to be negatively affected by performance status, positive lymph nodes or distant metastases, and sarcomatoid features on the basis of multivariate analysis in the study by Klatte and associates.36
There is a growing literature on the role of adjuvant therapy, particularly for patients with retroperitoneal sarcomas or RCC. Local relapse is common if the sarcoma is large, high grade or an R0 resection is not achieved. Radiation therapy combined with wide local tumor excision has improved local rates of recurrence. Select individuals in whom an R0 resection is anticipated may benefit from a combination of external beam radiation therapy to a dose of 45 to 50 Gy in combination with an intraoperative boost of 10 to 20 Gy. Intraoperative focused radiation therapy, in which the bowel is retracted away from the field of treatment, seems to provide a more favorable therapeutic ratio between local control and complications to other tissues in the field, such as nerve, blood vessels, bone, and soft tissue.7 Targeted molecular therapies using tyrosine kinase inhibitors such as sunitinib may reduce RCC size to enhance resection but do not seem to reduce the extent of tumor thrombus.6
Few studies have assessed quality of life after operation. In the reports from the author’s group, careful patient selection has allowed more than 80% of patients operated on for malignant disease of the IVC to maintain an excellent status performance after operation, even if they later develop regional or distant metastases.1,17
Venous Tumors in the Chest
Lung cancer with mediastinal adenopathy is the most common cause of SVC obstruction. Lymphoma, follicular or medullary thyroid cancer, teratoma, and thymoma are the most common primary mediastinal malignant neoplasms and together with lung cancer account for 60% to 85% of cases with malignant SVC obstruction.2 Angiosarcoma and synovial cell sarcoma less frequently cause this problem. Many patients with malignant SVC obstruction have unresectable disease, similar to secondary IVC malignant neoplasms, so the venous occlusion is treated with stenting. Surgical resection of the tumor is offered to patients with localized disease and without involvement of the aortic arch, great vessels, or heart. In our practice, SVC resection with replacement is rarely performed, and when it is, we use a prosthetic graft. Venous reconstruction of the innominate or subclavian veins is feasible and is done with a bovine patch or prosthetic or autogenous vein replacement. Kuehnl and associates described nine patients who underwent SVC resection and reconstruction for malignant disease.18 Four patients had non–small cell carcinoma of the lung; two had malignant thymoma; and leiomyosarcoma, germ cell metastases, and cancer of unknown origin accounted for the remaining three. Prosthetic grafts were used to replace the SVC in four patients, all of whom had lung cancer. The others had a wedge excision of the vein with primary closure.
Primary vascular smooth muscle tumors are rare. A report by Butany and the Toronto, Ontario, Canada, group included 230 malignant smooth muscle neoplasms of vascular origin during the years 1987 through 2005.53 Pulmonary vein leiomyosarcoma was the most common in the chest, found in 18 patients. There was female predominance, and heart symptoms were most common, including palpitations, dizziness, syncope, dyspnea, and right-sided heart failure. Mean survival in the 18 patients was 26 months. Among all sites of vascular smooth muscle tumors during this time frame, the overwhelming majority involved the IVC, followed in order by the peripheral arteries, the pulmonary vein and artery, and the veins of retroperitoneal or uterine origin.
Selected Key References
Bower TC, Nagorney DM, Cherry KJ Jr, Toomey BJ, Hallett JW, Panneton JM, Gloviczki P. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000;31:270–281.
Klatte T, Pantuck AJ, Riggs SB, Kleid MD, Shuch B, Zomorodian N, Kabbinavar FF, Belldegrun AS. Prognostic factors for renal cell carcinoma with tumor thrombus extension. J Urol. 2007;178:1189–1195.
Mingoli A, Feldhaus RJ, Cavallaro A, Stipa S. Leiomyosarcoma of the inferior vena cava: analysis and search of world literature on 141 patients and report of the three new cases. J Vasc Surg. 1991;14:688–699.
This is a large, collective review on IVC leiomyosarcomas..
Pouliot F, Shuch B, LaRochelle JC, Pantuck A, Belldegrunt AS. Contemporary management of renal tumors with venous tumor thrombus. J Urol. 2010;184:833–841.
Sarmiento JM, Bower TC, Cherry KJ, Farnell MB, Nagorney DM. Is combined partial hepatectomy with segmental resection of the inferior vena cava justified for malignancy? Arch Surg. 2003;138:624–630.
This paper analyzes outcomes of patients undergoing resection of the retrohepatic IVC in conjunction with major liver resection. There is a detailed outline of the surgical technique and an analysis of outcomes for various types of tumors. The paper by Delis and colleagues19 also provides an analysis of patients undergoing similar tumor and IVC resection..
Subramanian VS, Stephenson AJ, Goldfarb DA, Fergany AF, Novick AC, Krishnamurthi V. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology. 2009;74:154–159.
Zini L, Destrieux-Garnier L, Leroy X, Villers A, Haulon S, Lemaitre L, Koussa M. Renal vein ostium wall invasion of renal cell carcinoma with inferior vena cava tumor thrombus: prediction by renal and vena caval vein diameters and prognostic significance. J Urol. 2008;179:450–454.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Bower TC, et al. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000;31:270–281.
2. Bower TC. Evaluation and management of malignant tumors of the inferior vena cava. Rutherford RB. Rutherford’s vascular surgery. ed 7. Elsevier Saunders: Philadelphia; 2010:983–995.
3. Bower TC. Primary and secondary tumors of the vena cava and iliac veins. Gloviczki P. Handbook of venous disorders. ed 3. Hodder Arnold: London; 2009:574–582.
4. Mingoli A, et al. Leiomyosarcoma of the inferior vena cava: analysis and search of world literature on 141 patients and report of three new cases. J Vasc Surg. 1991;14:688–699.
5. Dzsinich C, et al. Primary venous leiomyosarcoma: a rare but lethal disease. J Vasc Surg. 1992;15:595–603.
6. Cost NG, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59:912–918.
7. Petersen IA, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52:469–475.
8. Bartlett E, et al. Current treatment for the local control of retroperitoneal sarcomas. J Am Coll Surg. 2011;213:436–446.
9. Zisman A, et al. Renal cell carcinoma with tumor thrombus extension. Biology, role of nephrectomy and response to immunotherapy. J Urol. 2003;169:909–916.
10. Matsushita M, et al. Sequelae after limb-sparing surgery with major vascular resection for tumor of the lower extremity. J Vasc Surg. 2001;33:694–699.
11. Schwarzbach MHM, et al. Results of limb-sparing surgery with vascular replacement for soft tissue sarcoma in the lower extremity. J Vasc Surg. 2005;42:88–97.
12. Nishinari K, et al. Venous reconstructions in lower limbs associated with resection of malignancies. J Vasc Surg. 2006;44:1046–1050.
13. Kieffer E, et al. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg. 2006;244:289–295.
14. Ito H, et al. Leiomyosarcoma of the inferior vena cava: survival after aggressive management. Ann Surg Oncol. 2007;14:3534–3542.
15. Illuminati G, et al. Prosthetic replacement of the infrahepatic inferior vena cava for leiomyosarcoma. Arch Surg. 2006;141:919–924.
16. Quinones-Baldrich W, et al. Inferior vena cava resection and reconstruction for retroperitoneal tumor excision. J Vasc Surg. 2012;55:1386–1393.
17. Sarmiento JM, et al. Is combined partial hepatectomy with segmental resection of the inferior vena cava justified for malignancy? Arch Surg. 2003;138:624–630.
18. Kuehnl A, et al. Resection of malignant tumors invading the vena cava: perioperative complications and long-term follow-up. J Vasc Surg. 2007;46:533–540.
19. Delis S, et al. Combined liver and inferior vena cava resection for hepatic malignancy. J Surg Oncol. 2007;96:258–264.
20. Arii S, et al. Significance of hepatic resection combined with inferior vena cava resection and its reconstruction with expanded polytetrafluoroethylene for treatment of liver tumors. J Am Coll Surg. 2004;239:712–721.
21. Hemming AW, et al. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712–721.
22. Yoshidome H, et al. Should the inferior vena cava be reconstructed after resection for malignant tumors? Am J Surg. 2005;189:419–424.
23. Oldehafer KJ, et al. Long-term experience after ex situ liver surgery. J Surg. 2000;127:520–527.
24. Lodge JPA, et al. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471–479.
25. Praseedom RJ, et al. Leiomyosarcoma of the retrohepatic vena cava treated by excision and reconstruction with an aortic homograft: a case report and review of the literature. Surg Innov. 2007;14:287–291.
26. Blute ML, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94:33–41.
27. Nesbitt JC, et al. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Ann Thorac Surg. 1997;63:1592–1600.
28. Gettman MT, et al. Charlson co-morbidity index as a predictor of outcome after surgery for renal cell carcinoma with renal vein, vena cava or right atrium extension. J Urol. 2003;169:1282–1286.
29. Frank I, et al. An outcome prediction model for patients with clear cell renal carcinoma treated with radical nephrectomy based on tumor stage, size, grade, and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400.
30. Leibovich BC, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005;174:1759–1763.
31. Haferkamp A, et al. Renal cell carcinoma with tumor thrombus extension into the vena cava: prospective long-term follow-up. J Urol. 2007;177:1703–1708.
32. Ciancio G, et al. Surgical management of renal cell carcinoma with tumor thrombus in the renal and inferior vena cava: the University of Miami experience in using liver transplantation techniques. Eur Urol. 2007;51:988–995.
33. Terakawa T, et al. Clinical outcome of surgical management for patients with renal cell carcinoma involving the inferior vena cava. Int J Urol. 2007;14:781–784.
34. Lambert EH, et al. Prognostic risk stratification and clinical outcomes in patients undergoing surgical treatment for renal cell carcinoma with vascular tumor thrombus. Urol. 2007;69:1054–1058.
35. Pouliot F, et al. Contemporary management of renal tumors with venous tumor thrombus. J Urol. 2010;184:833–841.
36. Klatte T, et al. Prognostic factors for renal cell carcinoma with tumor thrombus extension. J Urol. 2007;178:1189–1195.
37. Goetzel MA, et al. A contemporary evaluation of cytoreductive nephrectomy with tumor thrombus: morbidity and long-term survival. Urol Oncol. 2004;22:182.
38. Sweeney P, et al. Surgical management of renal cell carcinoma associated with complex inferior vena caval thrombi. Urol Oncol. 2003;21:327.
39. Karnes RJ, et al. Surgery insight: management of renal cell carcinoma with associated inferior vena cava thrombus. Nat Clin Pract Urol. 2008;5:329.
40. Subramanian VS, et al. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology. 2009;74:154–159.
41. Blute ML, et al. Results of inferior vena caval interruption by Greenfield filter, ligation or resection during radical nephrectomy and tumor thrombectomy. J Urol. 2007;178:440–445.
42. Chiche L, et al. Adrenocortical carcinoma extending into the inferior vena cava: presentation of a 15-patient series and review of the literature. Surgery. 2006;139:15–27.
43. Boorjian SA, et al. Surgery for vena caval tumor extension in renal cancer. Curr Opin Urol. 2009;19:473–477.
44. Perl L. Ein Fall von Sarkom der Vena cava inferior. Virchows Arch Pathol Anat. 1871;53:378–383.
45. Padovan RS, et al. Venous spread of renal cell carcinoma: MDCT. Abdom Imaging. 2007;32:530–537.
46. Zini L, et al. Renal vein ostium wall invasion of renal cell carcinoma with inferior vena cava tumor thrombus: prediction by renal and vena caval vein diameters and prognostic significance. J Urol. 2008;179:450–454.
47. Laissey JP, et al. Renal carcinoma: diagnosis of venous invasion with GD-enhanced MR venography. Eur Radiol. 2000;10:1138.
48. Hallscheidt PJ, et al. Preoperative staging of renal cell carcinoma with inferior vena cava thrombus using multidetector CT and MRI: prospective study with histopathological correlation. J Comput Assist Tomogr. 2005;29:64–68.
50. Guzzo TJ, et al. The accuracy of multidetector computerized tomography for evaluating tumor thrombus in patients with renal cell carcinoma. J Urol. 2009;181:486–490.
51. Mingoli A, et al. The effect of extend of caval resection in the treatment of inferior vena cava leiomyosarcoma. Anticancer Res. 1997;17:3877–3882.
52. Wagner B, et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol. 2009;55:452.
53. Butany J, et al. Vascular smooth muscle tumors: 13 cases and a review of the literature. Int J Angiol. 2006;15:43–50.