CHAPTER 118 Venous Sonography of the Upper Extremities and Thoracic Outlet
Upper extremity deep venous thrombosis (DVT) was historically considered an uncommon, benign, and self-limited condition.1 As such, thrombi involving the upper extremities were thought to be of little clinical significance and often undertreated because the risk of propagation was also believed to be low. More recent studies have found this not to be the case. The incidence of upper extremity DVT is far more common than previously thought, especially with the increased use of central venous catheters and placement of cardiac pacemakers, and in patients with cancer and hypercoagulable states. Upper extremity DVT is also seen to be associated with significant complications, including pulmonary embolism, superior vena cava syndrome, postthrombotic venous insufficiency, and loss of venous access.
VENOUS ANATOMY
The relevant venous anatomy of the upper extremities and thoracic inlet includes the deep venous system composed of the internal jugular, brachiocephalic (or innominate), subclavian, axillary, and paired brachial veins. The superficial basilic and cephalic veins are also usually included in the examination (Fig. 118-1).
The axillary vein lies medial and inferior to the axillary artery. The subclavian vein is anterior and inferior to the subclavian artery (Fig. 118-2). Knowing these relationships will aid in identification of these vessels and help in distinguishing possible large collaterals from the native vessels.
PREVALENCE, ETIOLOGY, AND RISK FACTORS
The exact prevalence of symptomatic upper extremity DVT in the general population is unknown but is estimated to be approximately 0.2%.2 In patients with deep venous thrombosis, approximately 90% involve the lower extremity and the remaining 10% involve the upper extremity.3
With the increasing use of central venous catheters, the prevalence of upper extremity DVT has increased (Fig. 118-3). Earlier studies documented thrombosis in 2% to 12% of patients with central venous catheters.4 In more recent studies, upper extremity DVT has been documented in 50% to 60% of patients with central venous catheters. The most powerful independent predictor of upper extremity DVT was the presence of a catheter, with the risk factor increasing sevenfold in these patients.5,6 The position of the catheter tip has been found to correlate with the incidence of upper extremity DVT. Catheters at the junction of the right atrium and the superior vena cava and those in the mid superior vena cava (SVC) have the lowest incidence of associated thrombosis and those with the catheter tip in the brachiocephalic vein have a higher incidence.4 The ideal position for the catheter tip is at the cavoatrial junction. Catheter material and diameter have also been found to affect the incidence of thrombus. The lowest rates have been for polyurethane and silicone catheters and for those with an external diameter less than 2.8 mm.7,8 In the pediatric population, two thirds of DVT cases occur in the upper extremity, in contrast to adults, and are usually secondary to catheter placement.6
Cancer is a significant risk factor for upper extremity DVT secondary to alterations in coagulability factors, low-grade disseminated intravascular coagulation, and stasis secondary to tumor compression.9 Bilateral upper extremity DVT was found to be more common in patients with malignancy. The risk of thrombosis increases significantly in patients with both cancer and central venous catheters.11
Hypercoagulability (e.g., antithrombin, protein C, and protein S deficiencies; presence of antiphospholipid antibodies) is found to be prevalent in idiopathic upper extremity DVT in which no obvious associated disease or triggering factor is present. In patients with idiopathic upper extremity DVT, 42% to 56% of patients have been found to have clotting abnormalities in recent studies.6,13,14 Transient causes of hypercoagulability such as estrogen use, pregnancy, and ovarian hyperstimulation have also been observed in women with idiopathic upper extremity DVT.11,12
Additional predisposing factors for upper extremity DVT include venous stasis, trauma, surgery, sepsis, and thoracic outlet obstruction secondary to anatomic anomalies. Interestingly, conventional risk factors associated with lower extremity DVT, including obesity, advanced age, and surgery were not significant risk factors for patients with non–catheter-related upper extremity DVT.3 Patients with upper extremity DVT were found to be more often male, younger, leaner, and more likely to smoke than those with lower extremity DVT. Recent immobility and prior venous thromboembolism also play less of role in patients with upper extremity DVT; however, cancer was more common.13
COMPLICATIONS
The most serious complication of upper extremity deep venous thrombosis is pulmonary embolism (PE). Once thought to be uncommon, PE is now reported to have a prevalence of 7% to 36% in patients with upper extremity DVT.2,13,14 Clinically, the prevalence of symptomatic PE at presentation has been reported to be fourfold less common in patients with upper extremity DVT when compared with patients with lower extremity DVT. However, after 3-month follow-up, the incidence of major or fatal bleeding, recurrent DVT, recurrent PE, or fatal PE was the same. Not surprisingly, patients with cancer were found to have the worst prognosis. Mortality from PE ranged from 11% to 34%.13
CLINICAL PRESENTATION
Clinically, the most common presentation of upper extremity DVT is upper extremity and face swelling and pain. The edema is typically nonpitting. Less common signs and symptoms include skin discoloration, a sense of coldness in the hand and forearm, tenderness over the affected vein, paresthesia, and numbness. Malfunction of a central venous catheter may also be an indication of thrombosis. Collateral veins can develop over the shoulder and chest wall. A tender cord may be palpable, especially in the axillary region. These signs and symptoms, however, are nonspecific and confirmation of the diagnosis by objective testing is necessary.14 In addition, many cases of upper extremity DVT are asymptomatic, especially when related to catheter placement.4
IMAGING TECHNIQUE
Color Doppler duplex ultrasonography with compression technique has become the imaging modality of choice for the diagnosis of upper extremity DVT and is highly accurate for making this diagnosis.15 Sonography has the advantage of being noninvasive, requiring no venipuncture, ionizing radiation, or contrast agent. It is a potentially portable examination and can be performed at the bedside for critically ill patients. It can be performed regardless of renal function and serial follow-up examinations are easily done. Unlike venography, the internal jugular and peripheral brachiocephalic veins can be evaluated, despite the presence of thrombus in the more peripheral vessels. Limitations of duplex sonography include inability to visualize the superior vena cava and more central portions of the brachiocephalic veins. In addition, small nonocclusive thrombus may be missed in the subclavian vein secondary to the inability to compress this vessel because of the overlying clavicle.16 Furthermore, differentiation of a large collateral from the native vein may be difficult in patients with chronic deep venous thrombosis.
The reported sensitivity of color Doppler sonography for the diagnosis of upper extremity DVT has ranged from 78% to 100%, with a specificity of 82% to 100%.17,18 False-positive examination results are thought to be rare. False-negative results can occur secondary to limitations in the ability to compress vessels (see earlier).
ULTRASOUND FINDINGS
Ultrasound imaging of normal venous structures demonstrates complete apposition of the vessel walls with compression (Fig. 118-4). The vessel walls are thin and the lumens are generally anechoic, unless slow flow is present. Normal venous valves are easily visualized as thin leaf-like mobile structures and should not be mistaken for wall-adherent thrombus or sequelae from prior thrombosis.
Diagnostic criteria for upper extremity venous thrombosis by ultrasound are similar to those described for lower extremity deep venous thrombosis.19 The principal criterion for the diagnosis of thrombus is noncompressibility of the vessel lumen. Acute thrombus usually fills and distends the involved vessel and is typically anechoic to hypoechoic (Fig. 118-5). Color Doppler flow imaging makes visualization of anechoic and nonocclusive thrombus easier because it permits direct visualization of blood flow dynamics.20 Spectral and color Doppler flow imaging demonstrate absence of flow in the presence of occlusive thrombus (Fig. 118-6). Nonocclusive thrombus will generally be outlined by color and demonstrate some evidence of flow in the vessel (Fig. 118-7).
The appearance and echogenicity of thrombus will evolve over time (Fig. 118-8). Generally, as thrombus becomes more chronic, its echogenicity increases and the clot retracts, with resultant decrease in the distention of the vessel. The visualized thrombus may be more eccentric and focal in location within the vessel lumen, with skip areas present. The wall may thicken and be incompletely compressible (Fig. 118-9). Although some veins may regain a normal appearance and compressibility over time, other vessels may demonstrate sequelae of chronic venous disease, such as frozen valve leaflets, synechia, and partial recanalization of the vessel. The affected native vessel may collapse and fibrose, with resulting collateral formation. In vessels in which thrombus has resolved, venous insufficiency or reflux may later be present.
The diagnosis of recurrent thrombosis or acute thrombosis superimposed on chronic thrombosis is problematic by ultrasound. Demonstration of new areas of thrombus not identified on the initial examination and/or considerable enlargement (>2 mm) of the compressed vein diameter between the two examinations have been used as criteria in clinical studies for recurrent lower extremity DVT.21 Traditional x-ray or contrast-enhanced MR venography may be necessary for patients with clinically suspected recurrent DVT.
The normal spectral Doppler waveform of the upper extremity and neck veins are characterized by two phasic variations. Right atrial contraction results in a sawtooth pattern to the waveform, which is synchronized to the pulse rate. Superimposed on this cardiac pulsatility is a phasic change in amplitude caused by the respiratory cycle.22 An increase in amplitude and venous return is seen during inspiration and a decrease is noted during expiration (Fig. 118-10). Luminal diameter will also vary with respiration. Sniffing can cause momentary collapse of a normal vessel; a Valsalva maneuver will cause the vessel to distend and velocity of venous flow to decrease.15
Although direct visualization of thrombus in the brachiocephalic veins and superior vena cava is difficult, if not impossible, it has been shown that loss or dampening of respiratory and cardiac phasicity in the subclavian and internal jugular veins is a very sensitive predictor of more centrally located thrombus in the brachiocephalic veins or SVC (Fig. 118-11). Loss of cardiac pulsatility has been reported as a more sensitive predictor than loss of respiratory phasicity alone. Conversely, the demonstration of normal cardiac and respiratory phasicity is highly predictive of the absence of thrombus in these vessels.22 Comparison should be made to the waveform in the contralateral subclavian and internal jugular veins to confirm that the abnormal waveform is only present on the affected symptomatic side. Bilateral dampening, however, can occur with thrombosis of the superior vena cava.
PITFALLS
There are limitations in duplex ultrasound imaging of the upper extremity and thoracic inlet venous structures. As noted, considerable constraint is placed by the normal anatomy of the region, with the clavicle, manubrium, and sternum limiting visualization and compressibility of the veins, especially centrally. Compression may also be limited by body habitus, overlying bandages, or indwelling catheters. Nonocclusive thrombus in regions that cannot be compressed may be missed. Color Doppler imaging may aid in the detection of nonocclusive thrombus, but care must be taken not to oversaturate the images with color, which can obscure thrombus (Fig. 118-12).
Slow-flowing blood can appear echogenic and mimic thrombus (Fig. 118-13). One should look carefully for swirling or slow-moving particles on real-time imaging, the presence of which will distinguish slow-flowing blood from true thrombus. Compressibility of the vessel will also confirm absence of thrombus.
SYNDROMES
Thoracic Outlet Syndrome
The anatomy of the thoracic outlet region can functionally be thought of as composed of three areas of narrowing: (1) the interscalene triangle; (2) the costoclavicular space; and (3) the subcoracoid or retropectoralis minor space (Fig. 118-14). The interscalene triangle is bounded by the anterior and middle scalene muscles and the first rib to which they are attached. The three trunks of the brachial plexus and subclavian artery pass through this region. The subclavian vein passes anterior to the anterior scalene muscle. The divisions of the brachial plexus and subclavian artery and vein then travel under the clavicle into the costoclavicular space, which lies between the clavicle and underlying first rib and is bounded anteriorly by the subclavius muscle. These structures enter the axilla through the subcoracoid (retropectoralis minor) space, bounded by the coracoid process and insertion of the pectoralis minor muscle superiorly and anteriorly and by the ribs posteriorly.
Compression and irritation of neural and vascular structures by repetitive movement and/or arm elevation can occur in these regions of narrowing. The interscalene triangle is the most common site of entrapment. The subclavian vein and artery are most often compressed at the costoclavicular space.23 Anatomic variants such as cervical ribs, hypertrophy of the C7 transverse processes, and cervical bands can also result in entrapment.24
Color Doppler sonography has been found to be a useful aid for the diagnosis of TOS because it allows direct visualization of the vessels in conjunction with spectral Doppler waveforms during maneuvers designed to result in vascular compression.24 Longley and colleagues23 have found that in symptomatic patients with thrombosis or significant compression of the subclavian vein during arm abduction (90 to180 degrees), color Doppler sonography has a sensitivity of 92% and a specificity of 95% for the detection of thoracic outlet syndrome. Criteria used in this study for a positive result were detection of thrombus or change in the Doppler waveform, from a normal subclavian waveform to one showing complete loss of cardiac and respiratory pulsatility or cessation of flow. Evaluation of the subclavian artery yielded less helpful results, with 20% of asymptomatic individuals demonstrating changes in Doppler waveform on hyperabduction.
Venous imaging using CT angiography (CTA) or MR angiography (MRA) of TOS may also be useful, with imaging performed with the patient’s arms alongside the body and then elevated over the head. Comparison of vascular patency using multiplanar and three-dimensional reformations of CTA or MRA can provide evidence for TOS. With CT or MRI, evidence of bony or soft tissue impingement on the brachial plexus can also be assessed. This technique has been found to be useful in evaluating the arterial structures but is less helpful in evaluating venous compression secondary to overlap of findings with asymptomatic individuals.25
Ultrasound offers the advantage of allowing visualization of the vessels during dynamically induced symptoms. Ultrasound also allows for imaging the patient in an upright or seated position, similar to a clinical examination, as opposed to CT or MRI, which have to be performed with the patient supine. Disadvantages include limited evaluation of the surrounding soft tissue and bony structures, especially in the region of the pulmonary apex.25
Paget-Schroetter Syndrome
James Paget and Leopold von Schroetter, independently, first described Paget-Schroetter syndrome, a primary form of upper extremity DVT—also known as effort-induced thrombosis—in the mid to late 19th century. It is usually seen in young, otherwise healthy, individuals. Development of thrombus is usually seen in the dominant arm after strenuous activities such as weight lifting, pitching, or rowing. Damage to the intima is believed to occur from heavy exertion, resulting in activation of the coagulation cascade.26 These patients are usually treated more aggressively with thrombolytic therapy because the risk for developing long-term sequelae such as post-thrombotic syndrome is higher.27
Lemierre’s Syndrome
Lemierre’s syndrome is a septic thrombophlebitis of the internal jugular vein usually caused by the anaerobic gram-negative rod Fusobacterium necrophorum, although other strains of fusobacterium have been implicated.28 F. necrophorum makes up part of the normal flora of the mouth. Lemierre’s syndrome is an uncommon sequela of acute pharyngotonsillitis that can be potentially life-threatening. Infection from the oropharynx may spread by direct extension to the parapharyngeal space or through venous or lymphatic dissemination. Thrombophlebitis of the internal jugular vein results from the surrounding inflammation and acts as a nidus for further septic embolization and septicemia. The lungs and joints are common locations for septic emboli and abscesses.28 The first series of cases was described by Lemierre in 1936. In the preantibiotic era, the mortality rate for the condition was 90%.29
Generally more common in young healthy adults, clinical signs and symptoms can be nonspecific. Patients typically present with pharyngitis but this may be absent in some cases. Fever, rigors, cervical adenopathy, and malaise are common accompanying symptoms. Because of their proximity to the carotid sheath, cranial nerves IX to XII may be affected, with associated neurologic signs and symptoms. In later stages, symptomatology is related to the location of the septic emboli.28,30
Ultrasound or CT imaging demonstrates thrombus within the internal jugular vein (Fig. 118-15). Surrounding inflammatory changes in the parapharyngeal region are best demonstrated by CT, as are septic emboli in the chest.
CONCLUSION
KEY POINTS
 Upper extremity deep venous thrombosis (DVT) has a much higher prevalence than originally thought, especially in the patient population with central venous catheters, underlying malignancy, and hypercoagulability states.
Upper extremity deep venous thrombosis (DVT) has a much higher prevalence than originally thought, especially in the patient population with central venous catheters, underlying malignancy, and hypercoagulability states. Pulmonary embolism is the most serious complication of upper extremity DVT and has been reported in up to 35% of patients with upper extremity DVT.
Pulmonary embolism is the most serious complication of upper extremity DVT and has been reported in up to 35% of patients with upper extremity DVT. Color Doppler duplex sonography with compression technique is the imaging modality of choice for the diagnosis of upper extremity DVT.
Color Doppler duplex sonography with compression technique is the imaging modality of choice for the diagnosis of upper extremity DVT. Loss of phasicity in the spectral Doppler waveforms in the internal jugular or subclavian veins can be indicative of more central thrombosis.
Loss of phasicity in the spectral Doppler waveforms in the internal jugular or subclavian veins can be indicative of more central thrombosis.Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med. 2005;24:829-838.
Demondion X, Herbinet P, et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735-1750.
Fraser JD, Anderson DR. Deep venous thrombosis: Recent advances and optimal investigation with US. Radiology. 1999;211:9-24.
Nazarian GK, Foshager MC. Color Doppler sonography of the thoracic inlet veins. Radiographics. 1995;15:1357-1371.
1 Tilney NL, Griffiths HJG, Edwards EA. Natural history of major venous thrombosis of the upper extremity. Arch Surg. 1970;101:792-796.
2 Hingorani A, Ascher E. Upper extremity deep venous thrombosis. Perspect Vasc Surg Endovasc Ther. 1999;110:47-57.
3 Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper-extremity deep venous thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605-1611.
4 Luciani A, Clement O, Halimi P, et al. Catheter-related upper extremity deep venous thrombosis in cancer patients: a prospective study based on Doppler US. Radiology. 2001;220:655-660.
5 Mustafa S, Stein PD, Patel KC, et al. Upper extremity deep venous thrombosis. Chest. 2003;123:1953-1956.
6 Gaitini D, Beck-Razi N, Haim N, Brenner B. Prevalence of upper extremity deep venous thrombosis diagnosed by color Doppler duplex sonography in cancer patients with central venous catheters. J Ultrasound Med. 2006;25:1297-1303.
7 Boswald M, Lugauer S, Bechert T, et al. Thrombogenicity testing of central venous catheters in vitro. Infection. 1999;27(Suppl 1):S30-S33.
8 Lokich JJ, Becker B. Subclavian vein thrombosis in patients treated with infusion chemotherapy for advanced malignancy. Cancer. 1983;52:1586-1589.
9 Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med. 2005;24:829-838.
10 Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21:3665-3675.
11 Héron E, Lozinguez O, Alhenc-Gelas M, et al. Hypercoagulable states in primary upper extremity deep venous thrombosis. Arch Intern Med. 2000;160:382-386.
12 Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
13 Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with upper-extremity deep vein thrombosis. Results from the REITE registry. Chest. 2008;133:143-148.
14 Longley DG, Finlay DE, Letourneau JG. Sonography of the upper extremity and jugular veins. AJR Am J Roentgenol. 1993;160:957-962.
15 Sheikh MA, Topoulos AP, Deitcher SR. Isolated internal jugular vein thrombosis: risk factors and natural history. Vasc Med. 2002;7:177-179.
16 Falk RL, Smith DF. Thrombosis of upper extremity thoracic inlet veins: diagnosis with Duplex sonography. AJR Am J Roentgenol. 1987;149:677-682.
17 Mustafa BO, Rathbun SW, Whitsett TL, Raskob GE. Sensitivity and specificity of ultrasonography in the diagnosis of upper extremity deep vein thrombosis. Arch Intern Med. 2002;162:401-404.
18 Baarslag HJ, van Beek EJR, et al. Prospective study of color duplex ultrasonography compared with contrast venography in patients suspected of having deep venous thrombosis of the upper extremities. Ann Intern Med. 2002;136:865-872.
19 Fraser JD, Anderson DR. Deep venous thrombosis: recent advances and optimal investigation with US. Radiology. 1999;211:9-24.
20 Knudson GJ, Wiedmeyer DA, et al. Color Doppler sonographic imaging in the assessment of upper-extremity deep venous thrombosis. AJR Am J Roentgenol. 1990;154:399-403.
21 Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N Engl J Med. 1997;337:663-669.
22 Patel MC, Berman LH, Moss HA, McPherson SJ. Subclavian and internal jugular veins at Doppler US: abnormal cardiac pulsatility and respiratory phasicity as a predictor of complete central occlusion. Radiology. 1999;211:579-583.
23 Longley DG, Yedlicka JW, Molina EJ, et al. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158:623-630.
24 Dubuisson AS. The thoracic outlet syndrome. http://www.medschool.lsuhsc.edu/Neurosurgery/nervecenter/TOS.html, 2008. Available at Accessed October 6, 2009
25 Demondion X, Herbinet P, Van Sint Jan S, et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735-1750.
26 Vijaysadan V, Zimmerman AM, Pajaro RE. Paget-Schroetter syndrome in the young and active. J Am Board Fam Pract. 2005;18:314-319.
27 Urchel HC, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69:1663-1669.
28 Screaton NJ, Ravenel JG, et al. Lemierre syndrome: forgotten but not extinct—report of four cases. Radiology. 1999;213:369-374.
29 Lemierre A. On certain septicemias due to anaerobic organisms. Lancet. 1936;1:701-703.
30 Hope A, Bleach N, Ghiacy S. Lemierre’s syndrome as a consequence of acute supraglottitis. J Laryngol Otol. 2002;116:216-218.

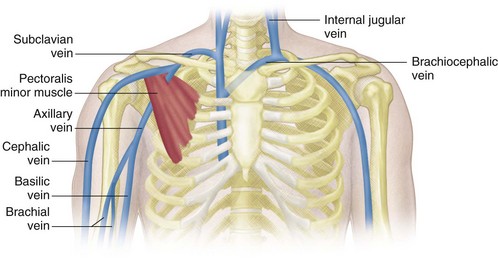
 FIGURE 118-1
FIGURE 118-1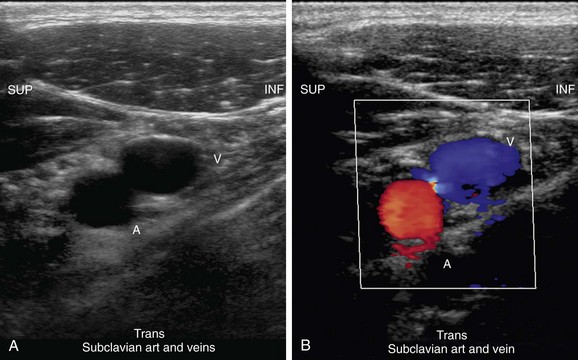
 FIGURE 118-2
FIGURE 118-2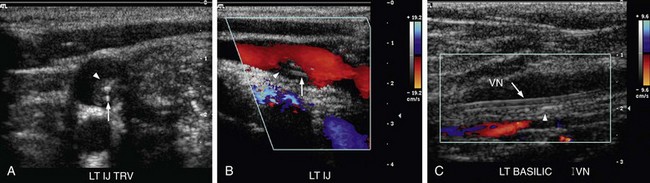
 FIGURE 118-3
FIGURE 118-3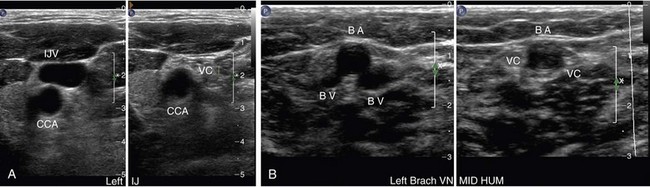
 FIGURE 118-4
FIGURE 118-4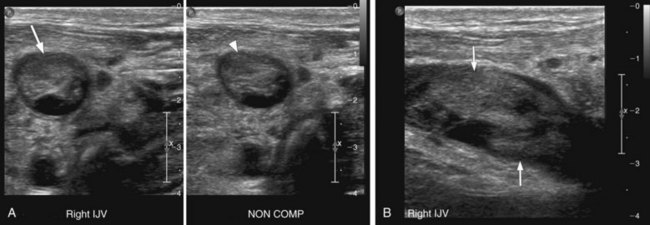
 FIGURE 118-5
FIGURE 118-5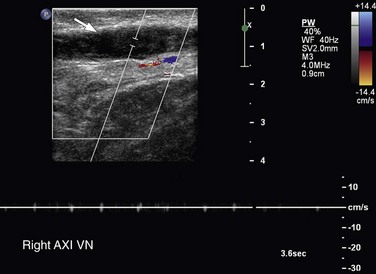
 FIGURE 118-6
FIGURE 118-6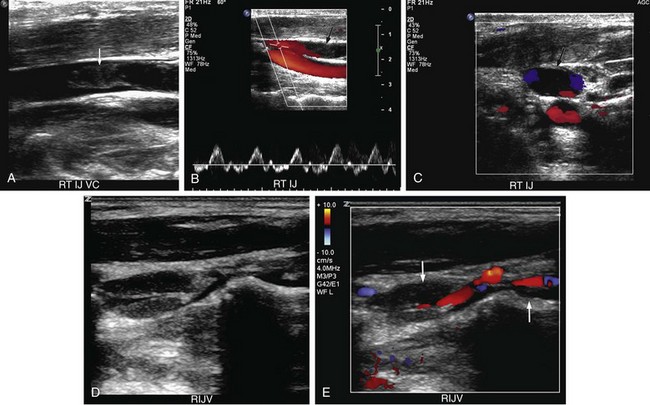
 FIGURE 118-7
FIGURE 118-7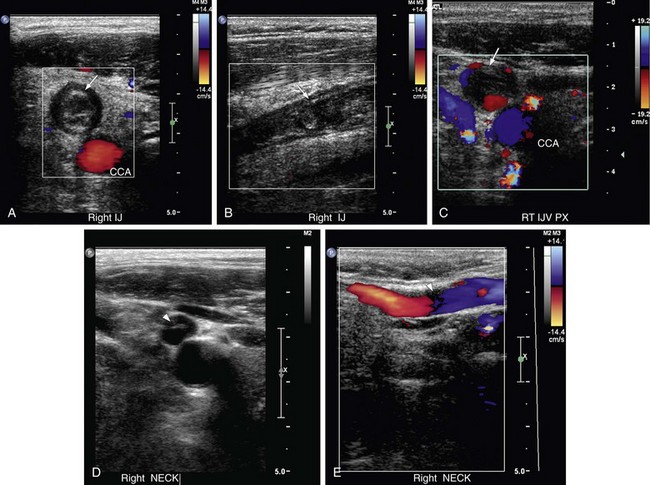
 FIGURE 118-8
FIGURE 118-8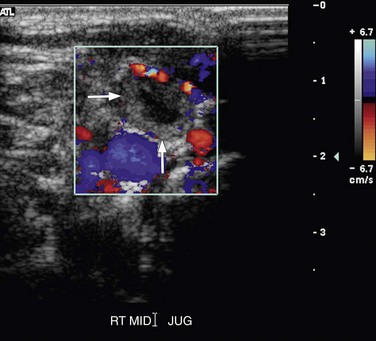
 FIGURE 118-9
FIGURE 118-9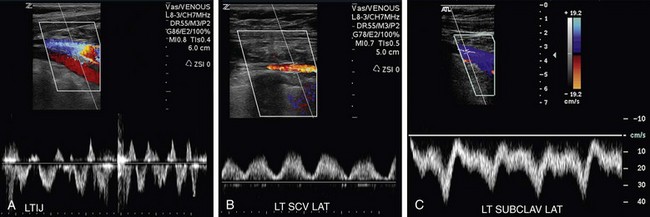
 FIGURE 118-10
FIGURE 118-10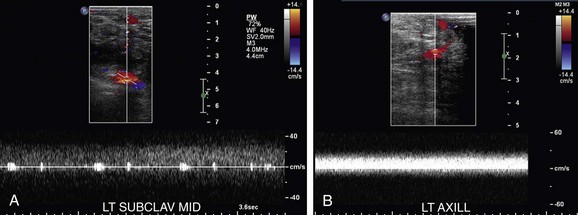
 FIGURE 118-11
FIGURE 118-11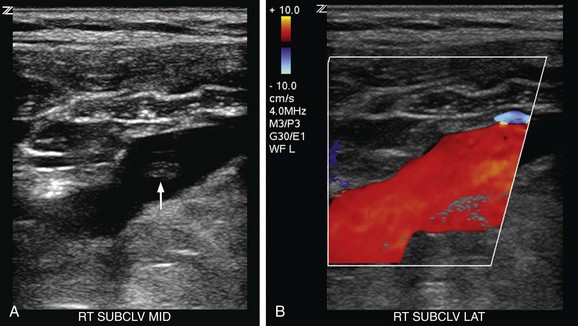
 FIGURE 118-12
FIGURE 118-12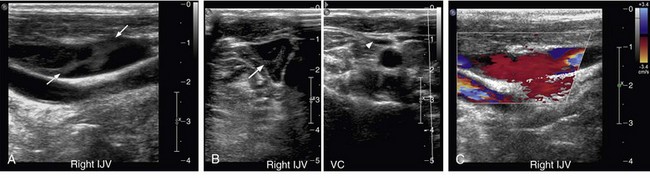
 FIGURE 118-13
FIGURE 118-13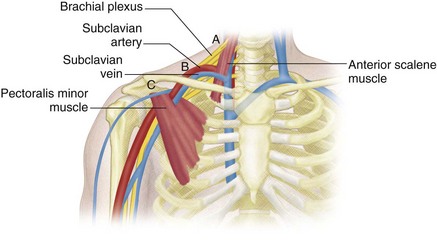
 FIGURE 118-14
FIGURE 118-14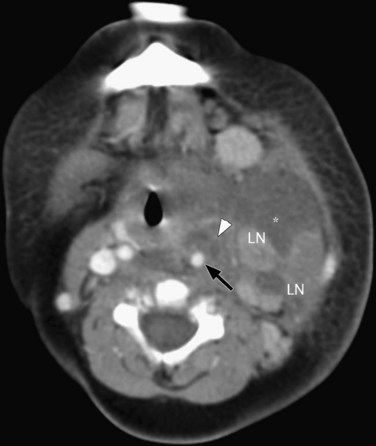
 FIGURE 118-15
FIGURE 118-15

