Chapter 3 Venous Pathophysiology
Etiology and Natural History of Disease
Primary Venous Disease
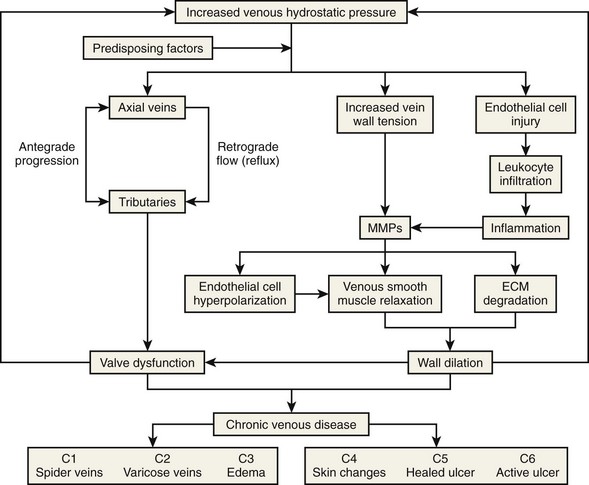
Primary venous disease affects two-thirds of patients with chronic venous disease (CVD). The most accepted theory is based on increased venous hydrostatic pressure transmitted to the vein wall, causing smooth muscle relaxation, endothelial damage, and extracellular matrix degradation with subsequent vein wall weakening and wall dilatation.1 It has also been suggested that valve damage may occur because of local inflammation.2 Leukocyte migration, plasma-granulocyte activation, and increased activity of metalloproteinases causing degradation of the valve leaflets support that theory.2,3 Figure 3-1 summarizes the pathophysiologic pathways of CVD.
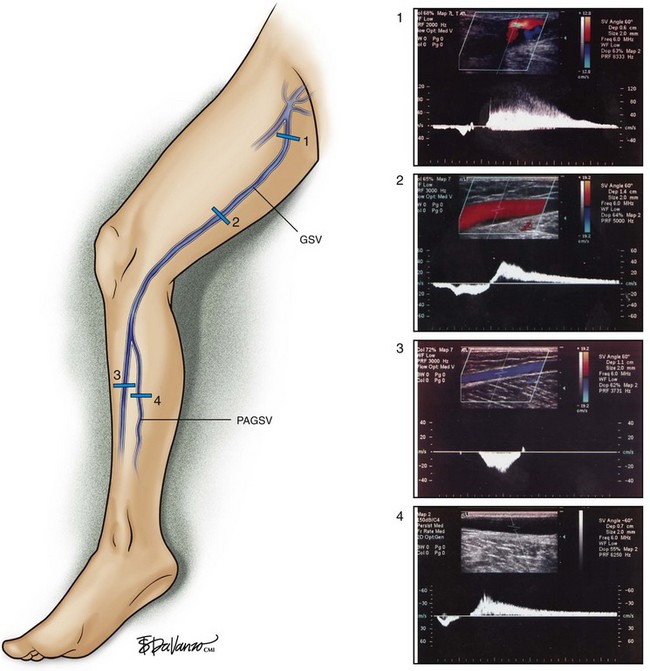
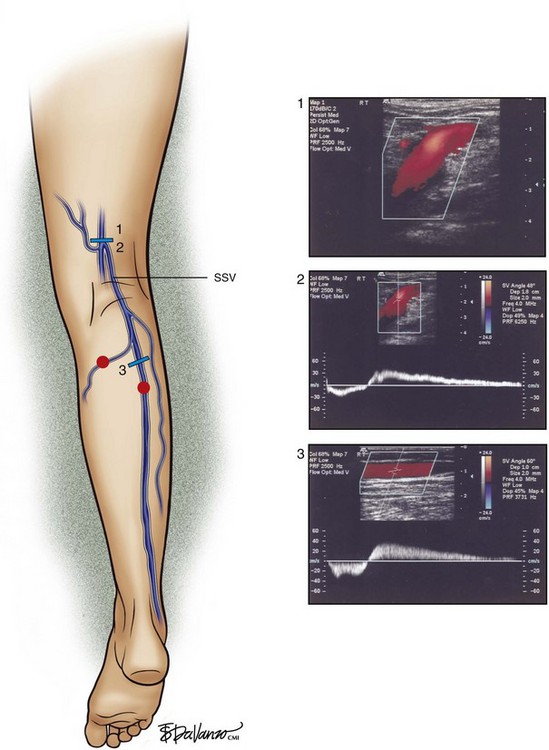
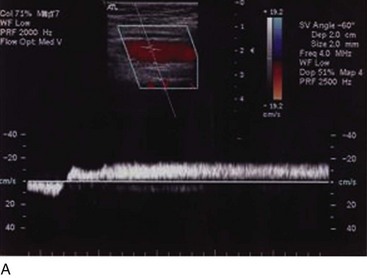
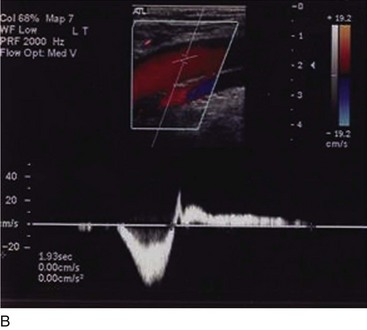
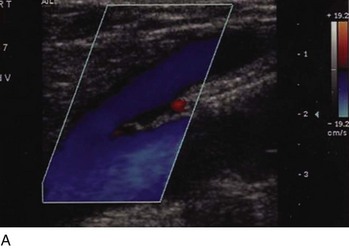
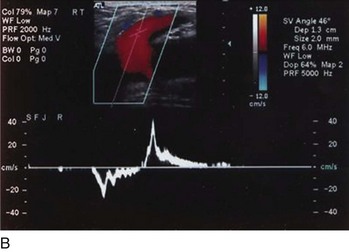
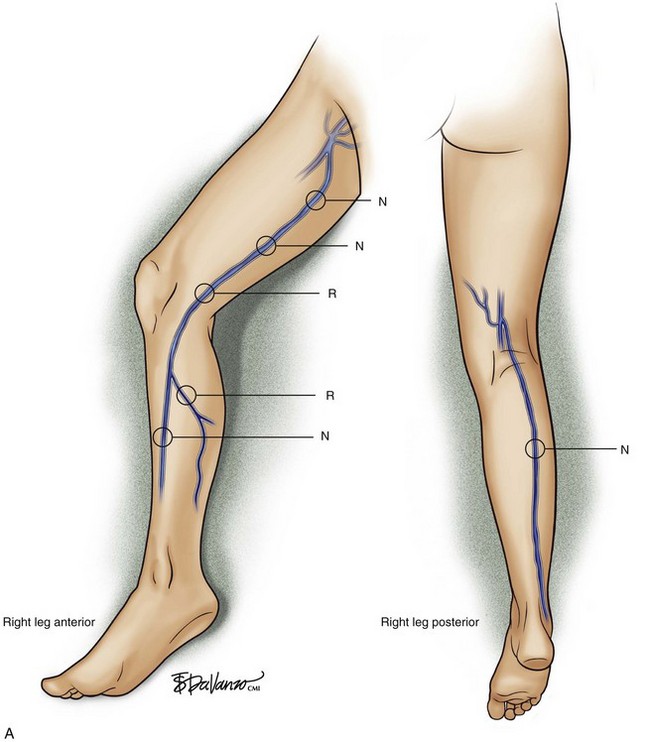
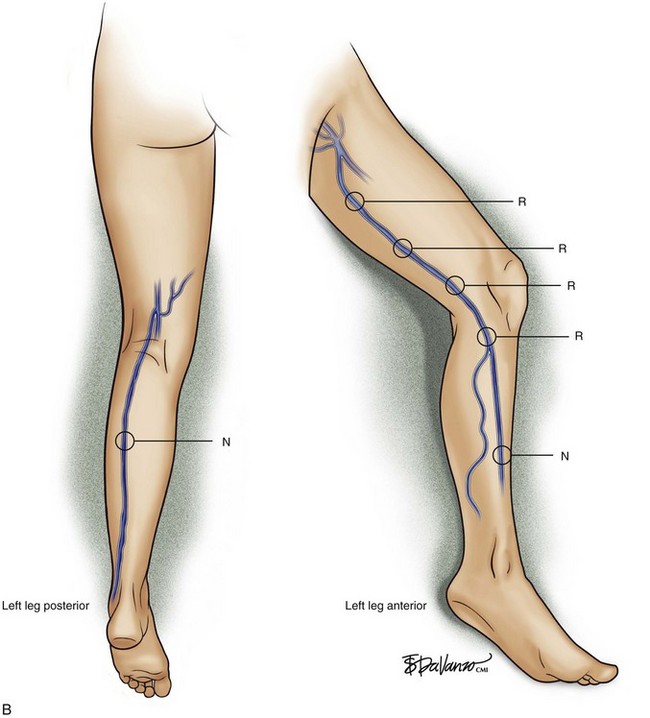
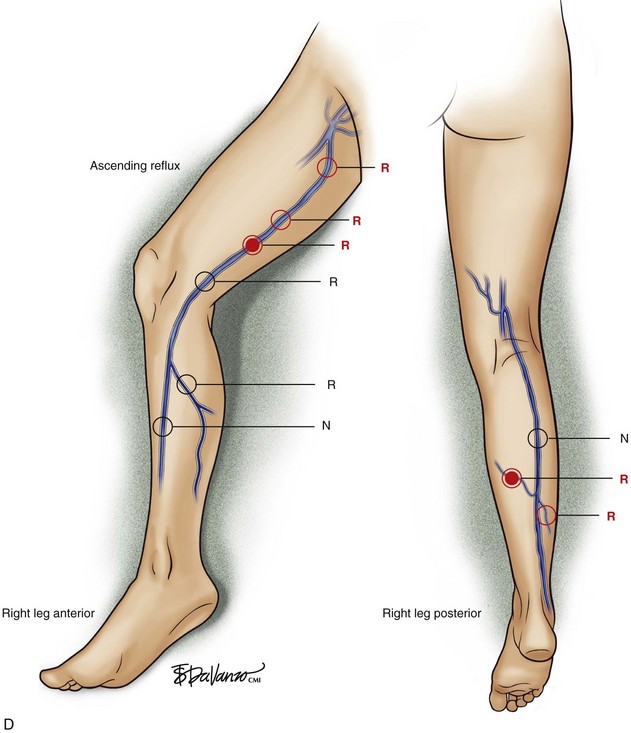
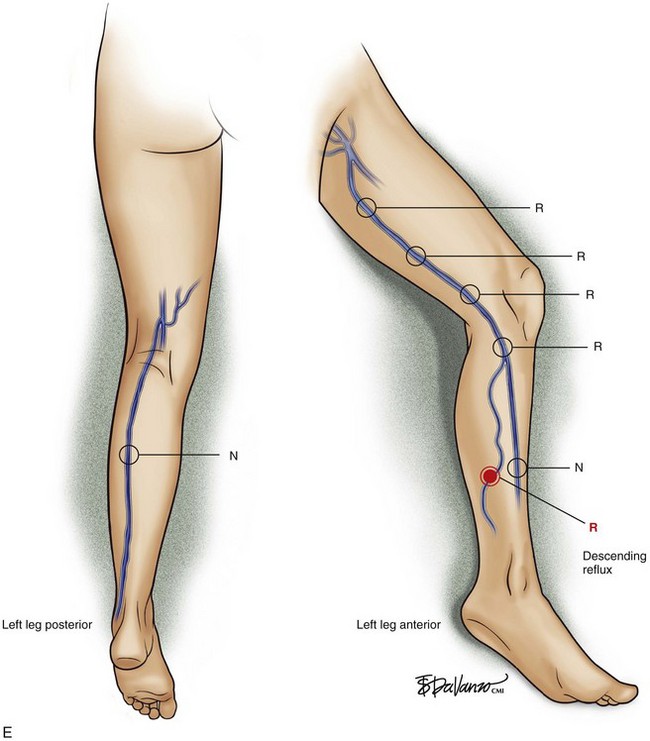
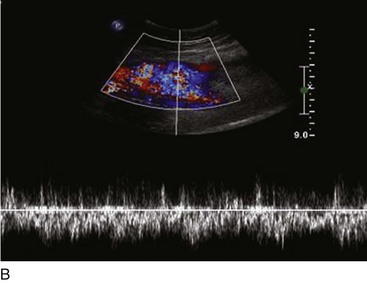
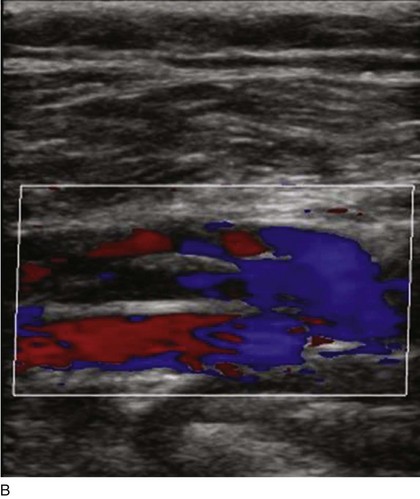
Superficial veins are most commonly involved in primary CVD, followed by perforators and deep veins.4 It has been shown that reflux starts in superficial veins in more than 80% of the patients. In the early stages of CVD, reflux is found in the great saphenous vein (GSV) and its tributaries without almost any junctional involvement (Fig. 3-2). This is followed by reflux in the small saphenous vein (SSV) system (Fig. 3-3) and nonsaphenous veins (Fig. 3-4). Patients with competent saphenous, perforators, and deep veins may also present tributary reflux in 10%, with the GSV tributaries being affected in 65% of the cases.
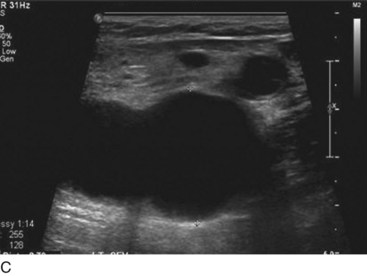
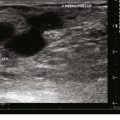
Isolated primary deep vein reflux is rare. It may present as either segmental or axial reflux extending from the femoral vein in the thigh to the below-knee popliteal vein. The most frequent location of primary deep vein reflux is the common femoral vein, followed by the femoral and popliteal veins5 (Fig. 3-5). Because most deep venous reflux is deemed to be caused by superficial venous reflux propagation, both common femoral and femoral vein reflux are associated with GSV incompetence and popliteal vein reflux is associated with SSV and/or gastrocnemial vein incompetence5 (Fig. 3-6). In addition, deep vein reflux has a shorter duration compared with superficial venous reflux. Association between deep and superficial venous reflux ranges from 5% to 38%.5–8
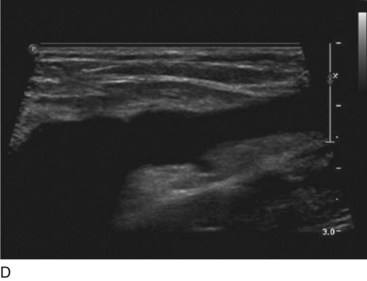
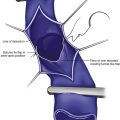
Perforator vein (PV) reflux in primary CVD always occurs in association with superficial vein reflux.9 Essentially, PVs become incompetent secondary to either ascending extension of superficial vein reflux or descending propagation of the reflux in a reentry fashion (Fig. 3-7). Most often, PV reflux originates from the GSV system and renders the deep veins incompetent in 13% of the cases.9 Deep vein reflux secondary to PV reflux is usually segmental and has a short duration.9
Secondary Venous Disease
Secondary venous disease is caused by a thrombotic event or is secondary to trauma. The incidence of arteriovenous fistula (AVFs) as cause of secondary CVD is reduced compared with postthrombotic CVD (Fig. 3-8).
Most frequently, AVFs are created when both common femoral vessels are inadvertently punctured during endovascular procedures or secondary to penetrating or blunt traumatic injuries. Animal models based on AVF creation have been used to explain the findings of chronic venous disease.10 Initially, lower resistance in the distal portion of the artery and pulsatile flow in the vein are noted. Venous hypertension supervenes, distending the veins and causing some degree of edema in the limb but no reflux. Subsequently, the valves are unable to appose what portends the reflux. Continuous venous hypertension and reflux entail valve atrophy and “arterialization” of the venous wall in the long term. In humans, the occurrence of CVD secondary to AVFs is rare and requires a long course to generate clinical impairment that frequently is devastating.
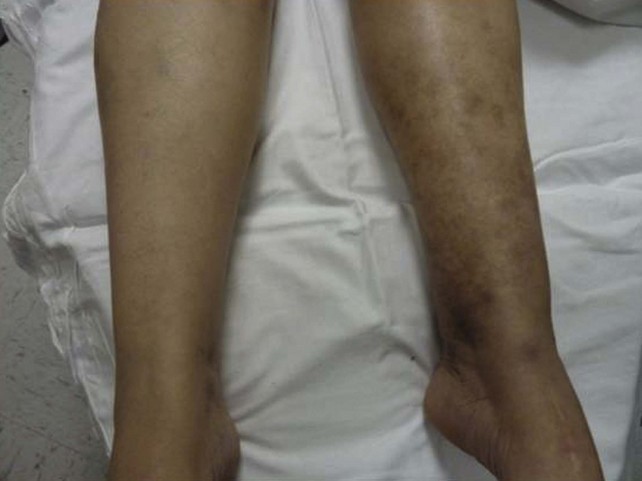
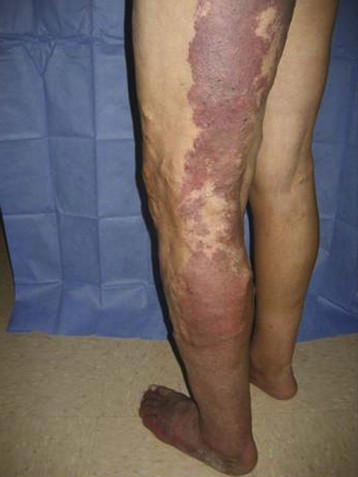
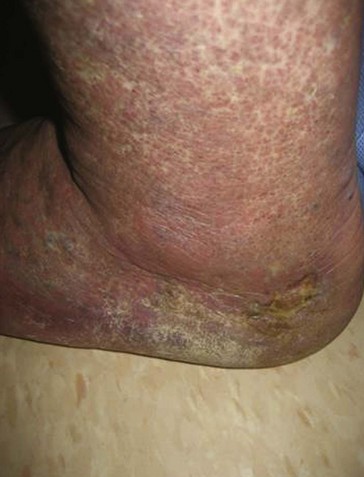
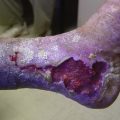
Regardless of the initial thrombi formation, the majority of the limbs evolve with thrombus resolution. Notably, only one-third of the patients with secondary CVD develop postthrombotic syndrome (PTS), which consists of signs and symptoms such as pain, edema, heaviness, and intolerance to efforts that may progress to skin changes and ulcers (Fig. 3-9). Often, patients with PTS have a combination of reflux and obstruction (Fig. 3-10). PTS represents the most severe manifestation of secondary CVD, carrying significant socioeconomic impact, and is discussed in Chapter 15.
The location of the DVT and its extent have been investigated. Involvement of at least one proximal deep vein segment was proposed as a mandatory condition for the development of CVD. Three anatomic and hemodynamic patterns are found following an episode of DVT, reflux, obstruction, or a combination of reflux and obstruction. In that scenario, reflux is caused by destruction of the valves secondary to inflammation. It has been hypothesized that leukocyte infiltration in the venous wall causes amplification and activation of metalloproteinases, leading to venous wall and valve damage.1
Failure in resolving an obstruction by either partial or complete recanalization of the thrombosed segment is found in less than 10% of cases.11,12 Partial recanalization is known to have higher incidence of reflux than complete recanalized segments, and calf veins are more prone to undergo recanalization than proximal segments.11,12 The role of location of the occlusion has been investigated. In a 1-year prospective study of 70 limbs, recanalization rate was lower in femoral veins, while all calf veins had complete recanalization. Finally, patients with reflux or obstruction present with milder changes and symptoms than those with both abnormalities. The presence of reflux and outflow obstruction in the same limb leads to higher rates of skin damage.13,14
Congenital
Congenital vascular malformations contribute to 1% to 3% of CVD. Pure venous malformations are rare and present as an isolated cluster of veins that abuts surrounding tissues, including soft tissue and bone. The most common congenital malformation involving veins is Klippel-Trenaunay, which is characterized by varicose veins, limb hypertrophy, and port-wine stains15 (Fig. 3-11). Agenesis of valves and segments of the deep veins is rare but is known to cause CVD.16
Natural History
CVD slowly evolves over time and is classified based on its clinical (telangiectasias to skin damage), e tiologic (primary, secondary, or congenital), anatomic (superficial, deep, or perforators), and p athophysiologic (reflux, obstruction, or both) patterns, forming the acronym CEAP.17 The clinical portion of the score is extensively used because of its simplicity, being cited as initial stages of CVD, including telangiectasias and varicose veins (C1-C2), and chronic venous insufficiency, including edema, skin changes, and ulcers (C3-C6).
Because the venous pressure increases in the lower extremity veins for hydrostatic reasons in during standing, it has been believed that reflux develops in a retrograde fashion. However, multiple studies have demonstrated that this is not true.18–21 Predominantly, reflux starts from the saphenous veins and their tributaries and progresses proximally, distally, or in both directions.22 In a longitudinal study of 116 limbs, progression of reflux occurred in 31. GSV and tributaries were the most common anatomic sites affected by reflux progression, followed by perforator veins. Seventeen limbs had extension of preexisting reflux in a proximal or distal direction, or both, and 14 limbs had reflux in a new segment that was independent of the preexisting site. Among patients with new signs or symptoms, documented reflux progression by duplex ultrasound was found in 53.8%, which was significantly higher than the 23.3% of patients without new symptoms (p = .04). Bernardini et al.23 corroborated the findings that the reflux most frequently starts in the GSV and its tributaries, reporting progression of venous disease in 94% of the patients in a mean period of 4 years. Further evidence is provided by a recent study on wall characteristics24 and interventional studies in which reflux in the saphenous vein was corrected after eliminating reflux in the saphenous tributaries.25–27
Secondary CVD was found to progress faster than primary CVD,12 for reasons that are likely to be multivariate. The presence of reflux and obstruction aggravates the clinical status of patients, as shown in a study by Johnson et al.13 In a cohort of 64 patients, overall progression of the CEAP clinical class occurred in 31.5%. Notably, secondary CVD CEAP class 4 to 6 was noted in 4% of the limbs at a 1-year period, increasing to 25% at 5-year follow-up.14
Prandoni et al. analyzed the data of 1626 consecutive patients and found that residual thrombosis, unknown origin, and thrombophilia are risk factors for recurrent DVT over a period of 10 years.28 Long-term follow-up with duplex ultrasound in a prospective cohort of 153 patients with recurrent DVT showed increased risk of skin damage (C4-C6) in patients with previous recurrent ipsilateral DVT.29
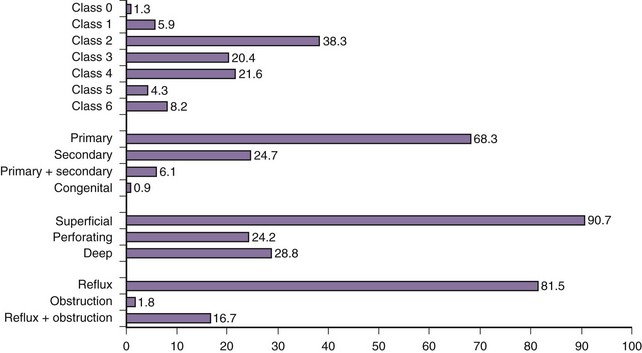
An overview of clinical distribution (reflux, obstruction, or both), classification, and pathophysiology of CVD in consecutive patients attending a vascular clinic is shown in Figure 3-12. Most patients have primary vein reflux, which is most often found in the superficial veins and varicose veins. Skin damage is present in about one-third of the patients, while isolated deep vein reflux and obstruction are uncommon.30
Pearls and Pitfalls
Obesity
An association between obesity and CVD has been identified. The role of obesity as a causative versus an aggravating factor remains debatable. It is known that obese patients have higher intraabdominal pressure compared with nonobese patients and have reduced distensibility of the veins that could explain the higher incidence of CVD. Van Rij and associates31 reported an increased incidence of more severe CVD (CEAP 4-6) in obese patients than in nonobese patients. Interestingly, the authors concluded that obese patients have better venous calf muscle pump than nonobese patients. Sedentary behavior may explain the reduced effect of the pump to compensate reflux.
Padberg and colleagues also reported a correlation between higher body mass index (BMI) and severity of the CVD32 in a cohort of morbidly obese patients (BMI >40 kg/m2). Obese patients had longer mean ulcer healing time, up to 7 months. Notably, 62% of those patients had no anatomic evidence of reflux despite severe CVD changes.32 Perhaps the disease in that subset of patients may be secondary to microcirculatory changes or even segmental venous hypertension associated with lymphatic drainage impairment. Therefore, a high level of suspicion should be raised when evaluating such patients because venous reflux may not be the cause of skin damage.
Effects of Superficial Vein Reflux on the Deep Veins
The role of superficial venous reflux as a causative factor for deep venous reflux has been investigated. Over the past three decades, selected patients with CVD who presented with deep venous reflux, advanced venous stasis, and skin damage have been subject to a multiple techniques to improve venous return, including valve transposition, repair, or even axillary vein transfer.33
Several authors have advocated treatment of superficial venous reflux instead of direct procedures to correct deep venous reflux. Walsh et al.6 performed GSV stripping in 29 limbs with primary CVD, including only CEAP classes 1 through 3, and achieved success abolishing femoral vein reflux in 93% of the cases. In a similar subset of patients including only CEAP classes 1 through 3, Sales et al.8 achieved hemodynamic normalization of the deep veins in 94% of the patients. However, at the other extreme of the disease presentation, Padberg et al.34 treated 11 limbs with active ulcers and obtained hemodynamic success in only 27%. All perforators were ligated and GSV stripping was performed. Nonetheless, the patients did improve, and the ulcers healed with no recurrence at mean 16-month follow-up. Ciostek et al. analyzed photoplethysmography and duplex ultrasound reflux parameters following GSV ablation in 11 patients with secondary CVI and found no statistically significant improvement in deep venous reflux.35
The rationale for treating superficial venous reflux before any deep venous intervention is based on clinical and hemodynamic findings. Patients with superficial reflux associated with deep venous reflux present with milder deep vein impairment than do patients with isolated axial deep venous reflux, suggesting propagation and therefore response to a secondary mechanism as seen in study of 152 limbs.5 In addition, deep vein reflux is abolished in patients with initial disease after saphenous vein interruption, corroborating the former mechanism of superficial venous reflux propagation.6,8 Hemodynamic results may be compromised in patients with skin damage or secondary CVD due to prolonged inflammatory changes and more severe dilation of the deep veins.
Labropoulos N, Gasparis AP, Tassiopoulos AK. Prospective evaluation of the clinical deterioration in post-thrombotic limbs. J Vasc Surg. 2009;50:826-830.
Labropoulos N, Jen J, Jen H, et al. Recurrent deep vein thrombosis: long-term incidence and natural history. Ann Surg. 2010;251:749-753.
Labropoulos N, Leon L, Kwon S, et al. Study of the venous reflux progression. J Vasc Surg. 2005;41:291-295.
Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85-98.
1 Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85-98.
2 Coleridge Smith PD, Thomas P, et al. Causes of venous ulceration: a new hypothesis. Br Med J. 1988;296:1726-1727.
3 Raffetto JD, Qiao X, Koledova VV, et al. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: potential implications in varicose veins. J Vasc Surg. 2008;48:447-456.
4 Labropoulos N, Leon L, Kwon S, et al. Study of the venous reflux progression. J Vasc Surg. 2005;41:291-295.
5 Labropoulos N, Tassiopoulos AK, Kang SS, et al. Prevalence of deep venous reflux in patients with primary superficial vein incompetence. J Vasc Surg. 2000;32:663-668.
6 Walsh JC, Bergan JJ, Beeman S, et al. Femoral venous reflux abolished by greater saphenous vein stripping. Ann Vasc Surg. 1994;8:566-570.
7 Puggioni A, Lurie F, Kistner RL, et al. How often is deep venous reflux eliminated after saphenous vein ablation? J Vasc Surg. 2003;38:517-521.
8 Sales CM, Bilof ML, Petrillo KA, et al. Correction of lower extremity deep venous incompetence by ablation of superficial venous reflux. Ann Vasc Surg. 1996;10:186-189.
9 Labropoulos N, Tassiopoulos AK, Bhatti AF, et al. Development of reflux in the perforator veins in limbs with primary venous disease. J Vasc Surg. 2006;43:558-562.
10 Bergan JJ, Pascarella L, Schmid-Schonbein GW. Pathogenesis of primary chronic venous disease: Insights from animal models of venous hypertension. J Vasc Surg. 2008;47:183-192.
11 Yamaki T, Nozaki M. Patterns of venous insufficiency after an acute deep vein thrombosis. J Am Coll Surg. 2005;201:231-238.
12 Labropoulos N, Gasparis AP, Pefanis D, et al. Secondary chronic venous disease progresses faster than primary. J Vasc Surg. 2009;49:704-710.
13 Johnson BF, Manzo RA, Bergelin RO, et al. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995;21:307-312. discussion 313
14 Labropoulos N, Gasparis AP, Tassiopoulos AK. Prospective evaluation of the clinical deterioration in post-thrombotic limbs. J Vasc Surg. 2009;50:826-830.
15 Lee BB, Bergan J, Gloviczki P, et al. Diagnosis and treatment of venous malformations Consensus Document of the International Union of Phlebology (IUP)-2009. Int Angiol. 2009;28:434-451.
16 Gloviczki P, Duncan A, Kalra M, et al. Vascular malformations: an update. Perspect Vasc Surg Endovasc Ther. 2009;21:133-148.
17 Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248-1252.
18 Labropoulos N, Delis K, Nicolaides AN, et al. The role of the distribution and anatomic extent of reflux in the development of signs and symptoms in chronic venous insufficiency. J Vasc Surg. 1996;23:504-510.
19 Caggiati A, Rosi C, Heyn R, et al. Age-related variations of varicose veins anatomy. J Vasc Surg. 2006;44:1291-1295.
20 Pittaluga P, Chastane S, Rea B, et al. Classification of saphenous refluxes: implications for treatment. Phlebology. 2008;23:2-9.
21 Garcia-Gimeno M, Rodriguez-Camarero S, Tagarro-Villalba S, et al. Duplex mapping of 2036 primary varicose veins. J Vasc Surg. 2009;49:681-689.
22 Labropoulos N, Giannoukas AD, Delis K, et al. Where does venous reflux start? J Vasc Surg. 1997;26:736-742.
23 Bernardini E, De Rango P, Piccioli R, et al. Development of primary superficial venous insufficiency: the ascending theory. Observational and hemodynamic data from a 9-year experience. Ann Vasc Surg. 2010;24:709-720.
24 Labropoulos N, Kokkosis AA, Spentzouris G, et al. The distribution and significance of varicosities in the saphenous trunks. J Vasc Surg. 2010;51:96-103.
25 Labropoulos N, Leon L, Engelhorn CA, et al. Sapheno-femoral junction reflux in patients with a normal saphenous trunk. Eur J Vasc Endovasc Surg. 2004;28:595-599.
26 Pittaluga P, Chastanet S, Rea B, et al. Midterm results of the surgical treatment of varices by phlebectomy with conservation of a refluxing saphenous vein. J Vasc Surg. 2009;50:107-118.
27 Pittaluga P, Chastanet S, Locret T, et al. The effect of isolated phlebectomy on reflux and diameter of the great saphenous vein: a prospective study. Eur J Vasc Endovasc Surg. 2010;40:122-128.
28 Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
29 Labropoulos N, Jen J, Jen H, et al. Recurrent deep vein thrombosis: long-term incidence and natural history. Ann Surg. 2010;251:749-753.
30 Labropoulos N. Hemodynamic changes according to the CEAP classification. Phlebolymphology. 2003;40:125-129.
31 van Rij AM, De Alwis CS, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35:739-744.
32 Padberg FJr, Cerveira JJ, Lal BK, et al. Does severe venous insufficiency have a different etiology in the morbidly obese? Is it venous? J Vasc Surg. 2003;37:79-85.
33 Raju S. Venous insufficiency of the lower limb and stasis ulceration. Changing concepts and management. Ann Surg. 1983;197:688-697.
34 Padberg FTJr, Pappas PJ, Araki CT, et al. Hemodynamic and clinical improvement after superficial vein ablation in primary combined venous insufficiency with ulceration. J Vasc Surg. 1996;24:711-718.
35 Ciostek P, Michalak J, Noszczyk W. Improvement in deep vein haemodynamics following surgery for varicose veins. Eur J Vasc Endovasc Surg. 2004;28:473-478.