Chapter 11 Treatment of Spider Telangiectasias
Historical Background
Although the treatment of varicose veins was in the phase of further refinement, the treatment of telangiectasias was not seriously attempted until the 1930s. It was Biegeleisen who is credited with initially attempting injection into the perivascular space around telangiectatic areas. Later, he implemented intravascular injections using homemade microneedles.1 These early efforts led to disappointing results, primarily because the sclerosing solutions, such as sodium morrhuate, were very caustic. It was not until the 1970s that others attempted to treat spider telangiectasias with intravascular injection using less caustic solutions such as sodium tetradecyl sulfate (STS) (Sotradecol) and hypertonic saline. It was these agents that propelled the treatment of telangiectasias forward. The enthusiasm for these treatments increased steadily as Foley’s publication, relating to this new technique, gained momentum.2
Etiology
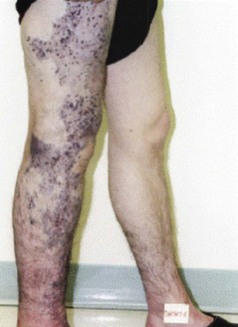
Although research continues to be done in this area, there is consensus today that telangiectasias result from a number of causes, alone or more likely in combination with other etiologic factors. Telangiectatic leg veins, according to the contemporary research, arise as a result of venous hypertension secondary to a number of different causes and conditions. The etiology of varicose veins and telangiectasias, for the most part, is similar. The pathophysiology of telangiectasias is usually broadly categorized as genetic/congenital, acquired and iatrogenic. Some of the genetic causes of telangiectasias include nevus flammeus (port- wine stains), nevus araneus (spider telangiectasia, which can also result from acquired diseases), and Klippel-Trenaunay syndrome. Congenital conditions associated with telangiectasias include Maffucci syndrome and Rothmund-Thomson syndrome (poikiloderma). Acquired causes of telangiectasias can arise from a primary cutaneous disorder, such as varicose veins and keratosis lichenoides chronica, or the result of a disorder with a secondary cutaneous component, such as lupus erythematosus, a collagen disorder, and mastocytosis (telangiectasia macularis eruptiva perstans). Hormonal influences (estrogen and progesterone) also play a role in the pathogenesis of telangiectasia. Pregnancy places the person at risk for the development of telangiectasia as early as a couple of weeks after conception. Birth control pills, menses, and the time just before ovulation are also associated with the development or worsening of telangiectasia, and increased venous dispensability. Topical steroids, particularly at high doses, have also been identified as a possible causative factor. Last, physical insults, like trauma (contusions) and infection, have also been implicated as causal forces. See Box 11-1 for a comprehensive listing of the many causes of lower leg cutaneous telangiectasia.
Box 11–1 Causes of Cutaneous Telangiectasia of the Lower Extremities
Congenital Neuroangiopathies
Congenital poikiloderma (Rothmund–Thomson syndrome)
Essential progressive telangiectasia
Cutis marmorata telangiectatica congenita
Other Acquired/Primary Cutaneous Diseases
Necrobiosis lipoidica diabeticorum
Infection
Generalized essential telangiectasia
Progressive ascending telangiectasia
Modified from Goldman MP, Bennett RG. J Am Acad Dermatol 1987;17:167.
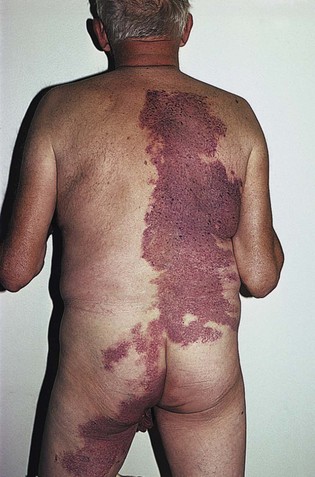
Fig 11–4 Nevus flammeus.
(From Weiss RA, Goldman MP, Bergan JJ, et al. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. St Louis: Elsevier, 2007, Fig. 4.6, p. 76.)
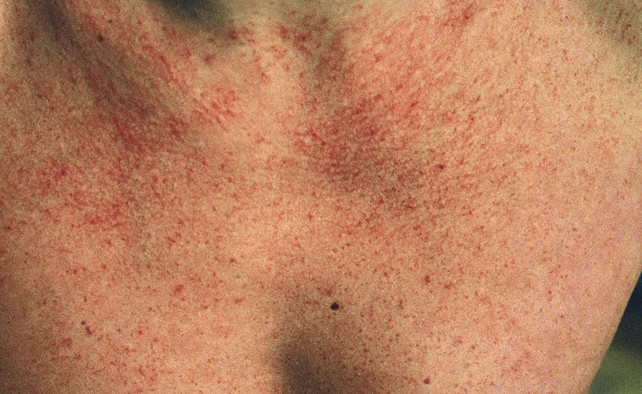
Fig 11–6 Extensive fine red telangiectasia on the chest of a severely sun-damaged 50-year-old woman.
(From Weiss RA, Goldman MP, Bergan JJ, et al. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. St Louis: Elsevier, 2007, Fig. 4.19, p. 85.)
Endovascular Instrumentation
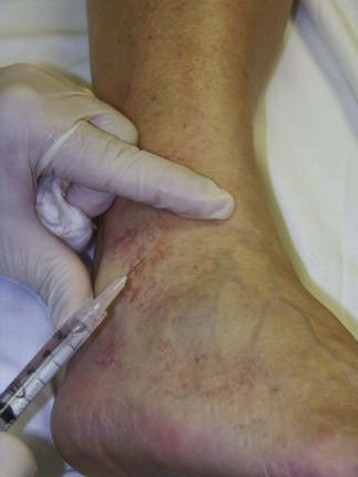
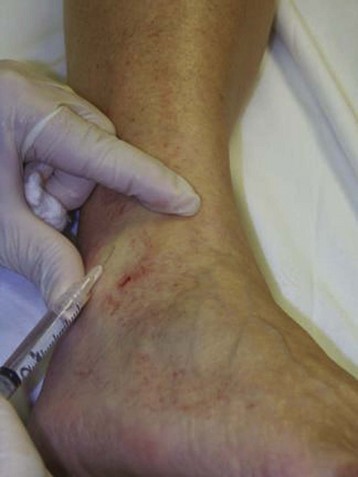
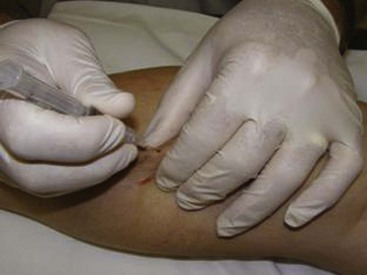
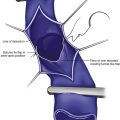
The basic endovascular instrumentation used for the treatment of spider telangiectasias includes needles for access and syringes to deliver the sclerosant to the affected areas. The needles used for telangiectasia are typically 30 gauge, although a 27-gauge butterfly needle may be used sometimes for larger reticular veins. Smaller needles, as small as a 33 gauge, can also be used but they tend to bend too easily when they are penetrating the skin (Fig. 11-9).
The environment of care for sclerotherapy should include a comfortable table for the patient, a comfortable room temperature, and ample lighting. The treatment table height should permit the physician to sit comfortably on a stool with his or her legs under the table without having to lean over the table and the patient for access. Environmental lighting should be bright and capable of providing adequate indirect illumination without any glare on the patient’s skin (Fig. 11-10).
General supplies would include alcohol swabs, cotton balls, tape, and compression supplies such as Ace wraps or Coban. Patients can supply their own stockings or they can be provided by the practice, which would require keeping a fairly large inventory (Figs. 11-11 and 11-12).
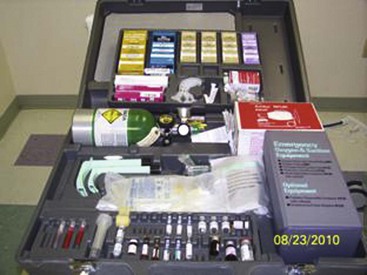
Last, but also most important, is emergency equipment. Fortunately, life-threatening complications are very rare; however, they are always possible. Basic emergency equipment must minimally include oxygen, airway equipment, epinephrine, steroids, and antihistamines (Fig. 11-13).
Imaging
First and foremost are magnifying glasses or loupes. These aids help to visualize the insertion of the needle into the smaller veins, particularly those that are less than 1 mm in diameter. Because loupes typically have 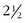 times the magnification, they facilitate good visualization while the needle pierces the skin and enters the vein (Fig. 11-14).
times the magnification, they facilitate good visualization while the needle pierces the skin and enters the vein (Fig. 11-14).
A number of lighting systems are used for visualization of veins to be treated. Vein lights provide visualization of vessels just under the skin that are sometimes too deep for normal visualization. These lights create a “shadow” from the absorption of the blood in the vein. Polarized lights are also used to provide better visualization through the skin (Fig. 11-15).
Syris polarized lights with magnification (Syris, Gray, ME) also provide better visualization through the skin (Fig. 11-16).
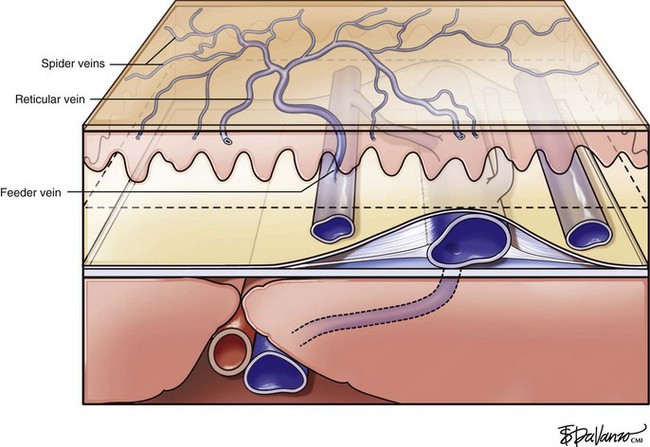
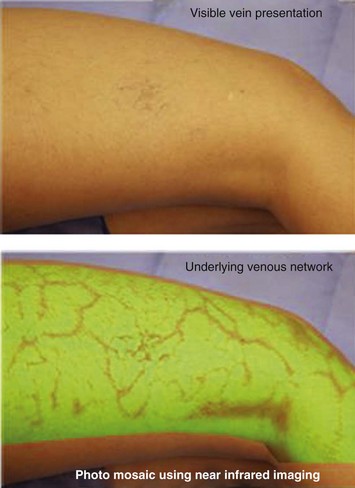
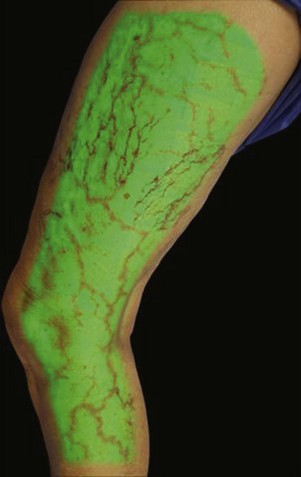
Infrared visualization is also done. Infrared lights allow the practitioner to see veins a few millimeters under the skin because these lights provide infrared images of the hemoglobin contained within the red cells circulating in the vessel that is then projected back onto the skin using the VeinViewer or a camera. This is particularly helpful for identifying “feeding” reticular veins that could be causing the telangiectasias (Fig. 11-17).
Operative Steps
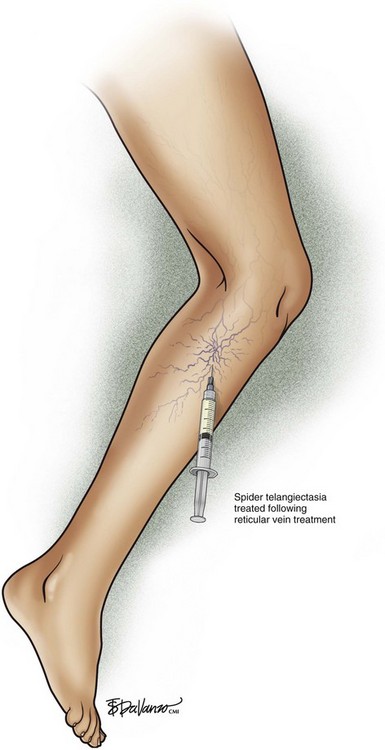
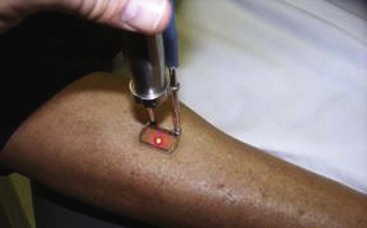
Treatment should begin at the source of reflux, if the source has been determined, or proximal to it if the precise location is not known. In the latter case, the larger veins are treated prior to the treatment of the smaller ones. It can be assumed that the source of reflux is the perigeniculate perforators, located usually just above the knee, for the lateral venous plexus area (Fig. 11-19).
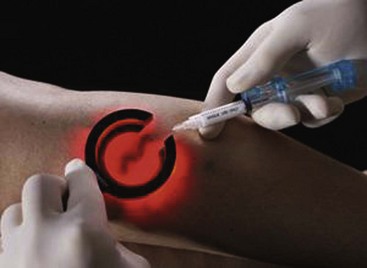
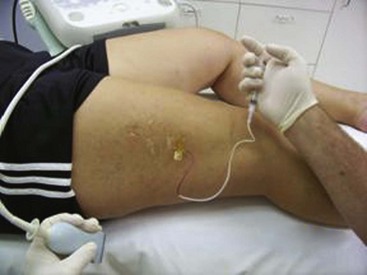
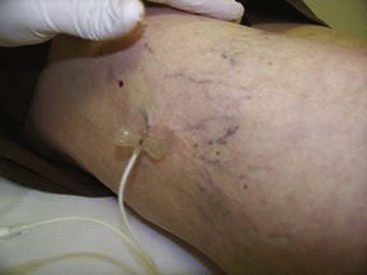
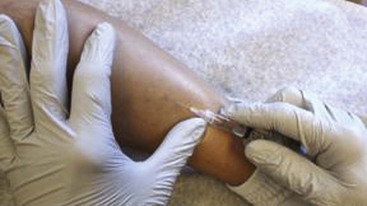
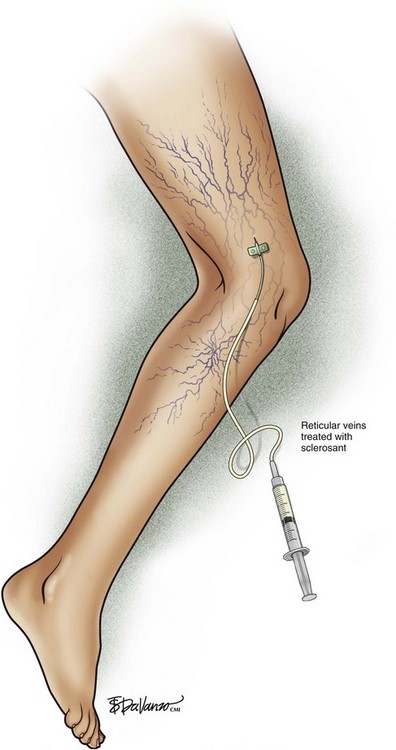
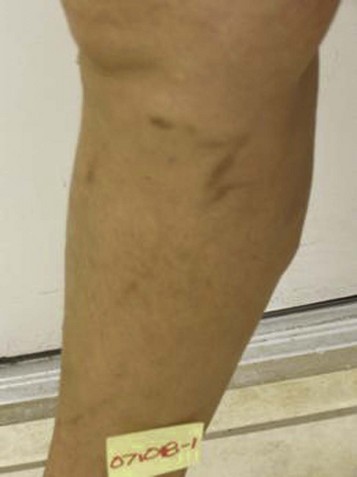
Complete treatment of the reticular feeding veins is performed in a given area before moving to the treatment of the smaller spider telangiectasias in the same area. Sclerosant injected into the feeder vein often travels into the spiders, thus effectively treating both the feeding veins and the spider veins. Access with the needle, as described earlier, is done with aspiration to confirm placement into the larger reticular feeder veins. The method of injection should be smooth and with very little pressure on the plunger. The volume of the injection solution depends on the size of the reticular vein. By definition, reticular veins range from 1 mm to 3 mm in diameter. Using 2 mm as an average, the volume of a 5-cm segment is 0.16 mL. Therefore a 15-cm segment would require 0.5 mL of sclerosant. Imaging with vein lights, a vein viewer, and polarized lights is sometimes very helpful (Figs. 11-20 through 11-22).
After the injection is stopped, the needle is then held in position for several seconds, up to 30 seconds. This increases the contact time of the sclerosant with the vein wall. The choice and concentration of sclerosant for spider veins are based on the size of the spider vein and practitioner preference, with ranges from 0.05% to 0.25% for STS and from 0.25% to 0.5% for POL, 11.7% HS, and 48% glycerin diluted with lidocaine. The total volume of the injection depends on which type and concentration of sclerosant are being used. For example, the maximum dose for STS is 10 mL of 3%, so if one is injecting a 0.25% solution, the total volume would be 120 mL (Figs. 11-23 and 11-24).
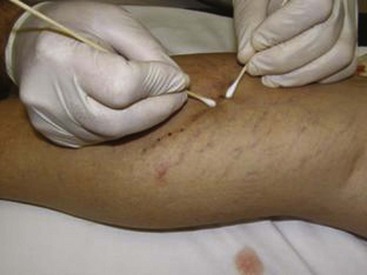
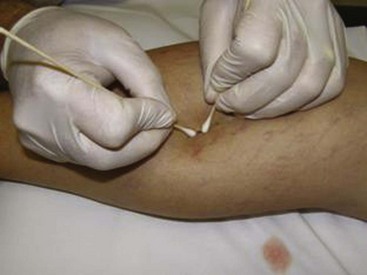
Many recommend placing a cotton ball over the injection site immediately after each injection to achieve hemostasis, to compress the vein walls together, and to prevent the filling of the vein with blood, which would lead to a clot. Atraumatic tape, such as paper tape, is used to hold the cotton ball in place when it is used. Further compression may be accomplished with foam pads, Ace wraps, or compression stockings. There is some data to suggest that compression stockings worn for several weeks after treatment can decrease adverse events, such as staining, but there are no data to suggest that compression stockings enhance the effectiveness of the treatment.3
Pearls and Pitfalls
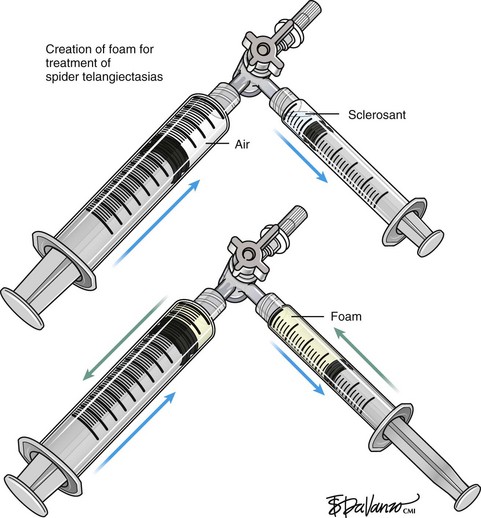
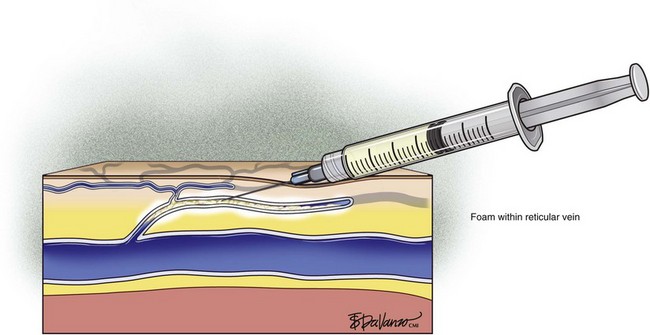
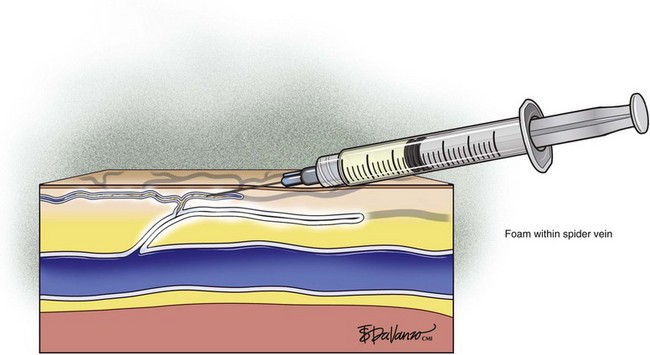
Foam sclerotherapy has greatly improved the outcome of varicose vein sclerotherapy.4 However, its merits in terms of the treatment of spider telangiectasias are less clear. The foam actually increases the surface area of contact by displacing the blood as opposed to injecting the sclerosing agent only, which is diluted by the blood. The foam then thickens the blood vessel wall, causing less blood to pass through. The same effect of foaming can be achieved with the use of only the sclerosing agent by keeping the needle within the vein after injection and maintaining slight pressure on the plunger. The reticular feeding veins may benefit from foam because they are larger and more difficult to visualize.
Foam sclerotherapy is also associated with greater risk in terms of potential complications because air is added to the vascular system. Several reports of neurologic events have been noted. These risks may be reduced by using CO2 as the gas rather than air. CO2 rapidly dissolves in blood, whereas the nitrogen in air does not.5
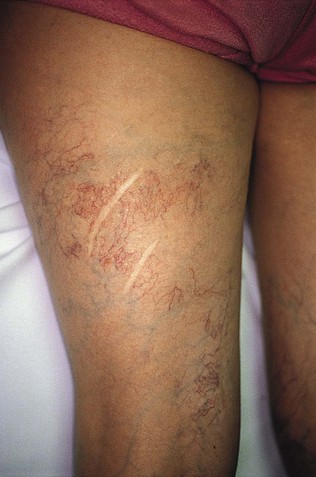
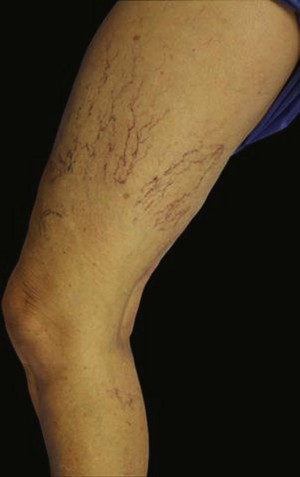
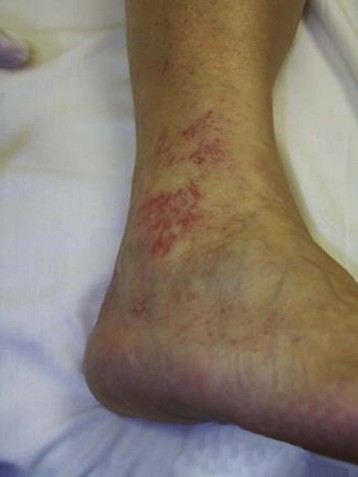
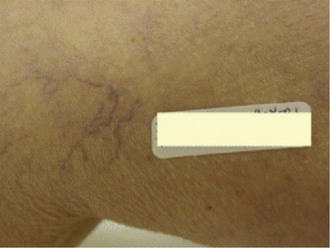
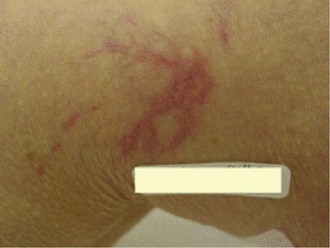
Sclerotherapy therapy may not improve spider telangiectasias if matting develops in the vasculature. Matting is the formation of new, fine spider veins in an area previously injected. This area may improve with additional treatments or may need further evaluation. Various imaging devices may be used to assess for venous hypertension caused by an incompetent saphenous or perforator vein. Ultrasound may show an underlying refluxing vein that should be treated. Vein lights and infrared imaging, such as the vein viewer and polarized light, may help to identify the reticular vein feeding the spider complex (Fig. 11-25).
Complications and Adverse Sequelae
Blisters and Folliculitis
Hyperpigmentation
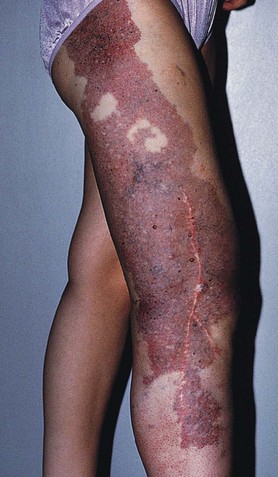
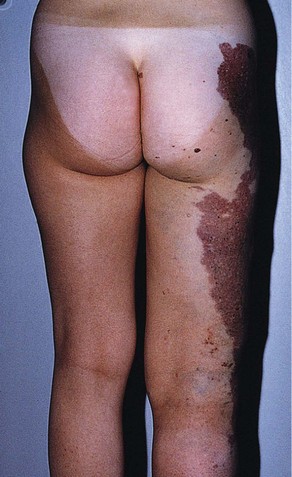
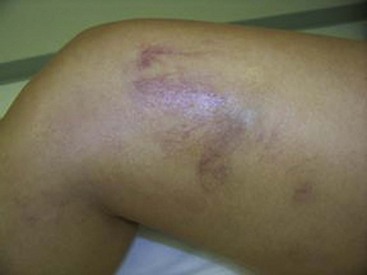
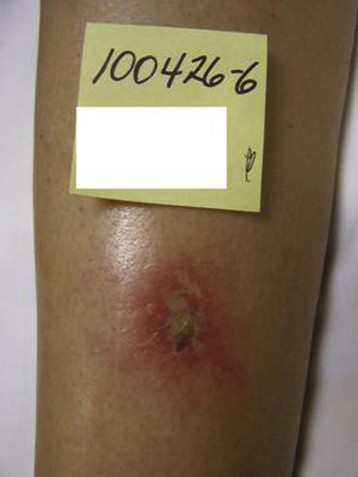
Hyperpigmentation, or cutaneous pigmentation, is a relatively common occurrence after treatment, regardless of the sclerosing agent that is used (Fig. 11-26). Hyperpigmentation is often very temporary in nature; however, rare persistence can occur for a small number of patients 1-year posttreatment. Most areas of pigmentation occur along the linear aspect of the treated vein. These areas indicate that the vein is no longer functioning. Less commonly encountered occurrences, such as those found at the injection site(s), can also occur as the result of the sclerosing agent’s endothelial damage, inflammation, and red blood cell extravasation into the area. Areas below the knee and vessels from 0.6 to 1.2 mm in diameter are at greatest risk. Affected areas usually resolve within 6 to 12 months after sclerotherapy.
Air Embolism
An inadvertent injection into an artery is best prevented with arterial visualization using duplex imagery and having the patient in an upright position when the challenging malleolar area is injected (Fig. 11-28).
Allergic Reactions
Some of the sclerosing agents that are most often associated with an allergic reaction include:
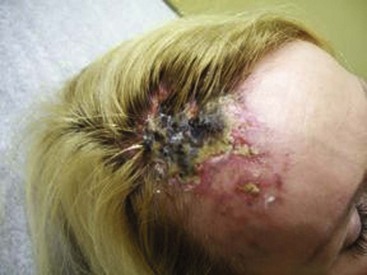
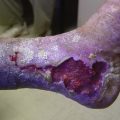
Cutaneous Necrosis
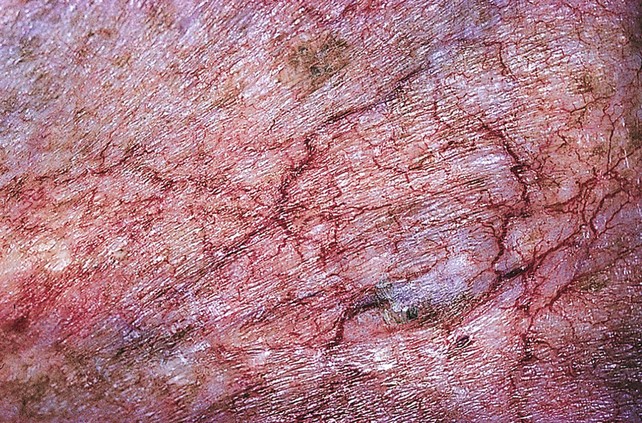
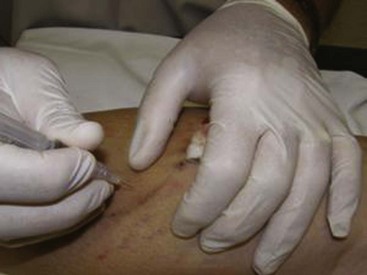
Cutaneous necrosis can be prevented with the dilution of sclerosing solutions and the injection of hyaluronidase into multiple sites around the extravasation within 60 minutes of the episode, should extravasation occur (Fig. 11-29).
Comparative Effectiveness
Comparative research studies have been conducted to determine the relative effectiveness of the various sclerosants that are used for spider telangiectasias. Curlin and Ratz compared the effectiveness of STS 0.5%, POL 0.25%, and HS 20% with heparin. The results of this research indicated that POL was best tolerated while HS and STS showed fastest clearing. In terms of effectiveness, there were no statistically significant differences among STS 0.5%, POL 0.25%, and HS 20% with heparin.6
Goldman7 compared STS 0.25% and POL 0.5% and found little difference in terms of effectiveness. Another study, comparing 100% CG, POL 0.25% solution, and POL 0.25% foam, indicated that CG was associated with higher degrees of pain, better clearance, and no staining or matting, while foam POL caused the most staining and matting.8 Another study, by Leach and Goldman, compared 72% glycerin diluted 2 : 1 with 1% lidocaine with epinephrine and STS 0.25%. This study showed significantly better and more rapid clearance with the glycerin and less staining than the STS.9
Laser Treatment of Spider Telangiectasias
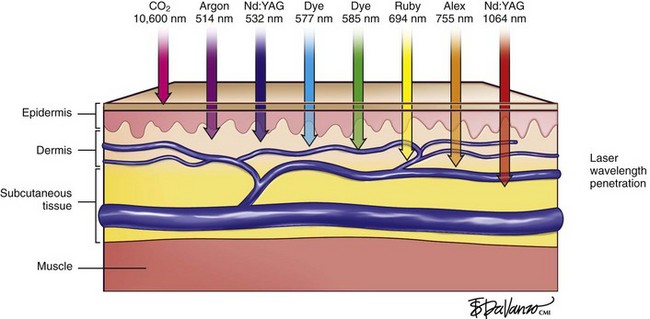
Types of Lasers
Regardless of the laser equipment used, however, there are some pitfalls and concerns that are common to all; therefore, the physician should resist the temptation of overtreating the vessels by delivering unnecessary passes and/or greater than necessary fluences. These actions may lead to blanching, hypopigmentation, and/or necrosis with hyperpigmentation (Fig. 11-30; see Fig. 6-1).
Operative Steps
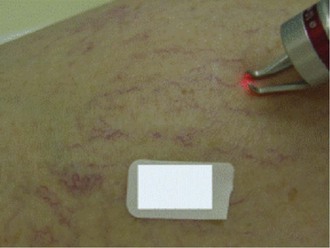
Eye injury, for both the patient and the practitioner, is also a potential complication of laser treatment. It is essential, therefore, that adequate protective eyewear be worn in order to block the laser wavelength. Lead shields are also used, especially if the laser is used near the eyes (Figs. 11-31 to 11-33)
Comparative Effectiveness
To date, there have been only a few studies comparing cutaneous laser treatment of spider telangiectasia and sclerotherapy. In 2002, research supported the fact that lasers were more effective for sclerotherapy than for spider telangiectasia.10 A second study led to similar results, but a greater degree of patient satisfaction was observed with sclerotherapy.11 In 2004, a sequential study comparing Nd:YAG 1064-nm laser treatment followed by sclerotherapy and sclerotherapy followed by laser treatment. The results of this research suggested that the best results were achieved with sclerotherapy followed by laser treatment.12
The current consensus is that sclerotherapy remains the primary treatment of choice for leg telangiectasias, but some are convinced that a combination of sclerotherapy and laser may have synergistic effects. This belief was challenged, however, in 1990 by Golman and Fitzpatrick.13 This research indicated that there were no statistically significant improvements in terms of outcome with this combination. Furthermore, increased complications were observed when compared with the complications encountered with sclerotherapy alone.
1 Biegeleisen HI. Telangiectasia associated with varicose veins: treatment by microinjection technique. JAMA. 1934;102:2092.
2 Foley WT. The eradication of venous blemishes. Cutis. 1975;15:665.
3 Nootheti PK, Cadag KM, Magpantay A, et al. Efficacy of graduated compression stockings for an additional 3 weeks after sclerotherapy treatment for reticular and telangiectatic leg veins. Dermatol Surg. 2009;35:53-57.
4 Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and telangiectatic leg veins. Dermatol Surg. 2005;31:631-635.
5 Morrison N, Neuhardt DL, Rogers CR, et al. Comparison of side effects using air and carbon dioxide foam for endovenous chemical ablation. J Vasc Surg. 2008;47:830-836.
6 Curlin MC, Ratz JL. Treatment of telangiectasia: comparison of sclerosing agents. J Dermatologic. Surg Oncol. 1987;13:1181.
7 Goldman MP. Treatment of varicose and telangiectatic leg veins: double-blind prospective trial between aethoxysclerol and sotradecol. Derm Surg. 2002;28:52.
8 Kern P, Ramelet AA, Wutschert R, et al. Single-blind, randomized study comparing chromated glycerin, polidocanol solution and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30:367-372.
9 Leach B, Goldman MP. Comparative trial between sodium tetradecyl sulfate and glycerin in the treatment of telangiectatic leg veins. Dermatol Surg. 2003;29:612.
10 Lupton JR, Alster TS, Romero P. Clinical comparison of sclerotherapy vs long pulsed Nd:YAG laser treatment of lower extremity telangiectasia. Derm Surg. 2002;28:694-697.
11 Coles MC, Werner RS, Zelickson BD. Comparative pilot study evaluating the treatment of leg veins with a long pulse Nd:YAG laser and sclerotherapy. Lasers Surg Med. 2002;30:154-159.
12 Levy J, Elbahr C, Jouve E, Mordon S. Comparison and sequential study of long pulsed Nd:YAG 1064 nm laser and sclerotherapy in leg telangiectasia treatment. Lasers Surg Med. 2004;34:273.
13 Goldman MP, Fitzpatrick RE. Pulsed-dye laser treatment of leg telangiectasia with and without simultaneous sclerotherapy. J Derm Surg Oncol. 1990;16:338-344.