Venous Cutdown
Management of critically ill or injured patients requires immediate and adequate vascular access, especially during trauma resuscitation, when rapid infusion of crystalloid or blood products may be necessary. Venous cutdown, a time-honored surgical technique, has largely been replaced by alternative methods of obtaining venous access, including intraosseous lines, the Seldinger technique, and ultrasound-guided central venous cannulation.1 Nonetheless, venous cutdown still has a role as an emergency method of achieving vascular access when other techniques and equipment are unavailable, particularly in settings outside the United States.
First described by Keeley in 1940 and Kirkham in 1945,2,3 venous cutdown offered an alternative to venipuncture in patients with shock. Though no longer taught as a mandatory procedure in the Advanced Trauma Life Support course, venous cutdown is considered optional and continues to be taught at the discretion of the instructor.4 Realistically, percutaneous vascular access may be infeasible in a pulseless, hypovolemic, or anatomically scarred patient. With a thorough understanding of the anatomy, the procedure, and its potential complications, this mechanically simple procedure can be performed quickly and effectively.5
Indications
Venous cutdown may be used as an alternative to venipuncture for critical patients in need of vascular access when less invasive options have been exhausted or are not available. Patients with severe shock, asystole, or pulseless electrical activity will lack palpable femoral pulses, thus making percutaneous femoral vein catheterization more difficult. Surface landmarks may be obscured and veins may be unusable in intravenous (IV) drug users, the extensively injured, or severely burned patients. Attempts at percutaneous venous cannulation may be complicated or even impossible in such patients. Venous cutdown and interosseous routes (see Chapter 25) are both viable options in such scenarios.
Children
Venipuncture in small children poses a challenge in even the healthiest of patients, let alone those in extremis, whose veins may be poorly visualized. Central vein catheterization, intraosseous line placement, or venous cutdown should be considered as alternative means of emergency vascular access when other peripheral sites have been exhausted. The distal saphenous vein at the ankle is often recommended for venous cutdown in children given its large diameter and anatomic predictability at this location.6,7
Hypovolemic Shock
Initially popularized during the Vietnam War for rapid transfusion, venous cutdown has since been used for resuscitation of patients with profound hypovolemia.8,9 The flow rate of saline through a standard IV extension set cut to a length of 28 cm (12 inches) and inserted directly into the vein is 15% to 30% greater than through a 5-cm, 14-gauge catheter. This difference is larger if pressure is applied to the system. Moreover, the improvement in flow rate through large-bore lines is greater for blood than for crystalloid solutions because the viscous characteristics of blood impede its passage through small-bore tubing.9 A unit of blood can be transfused in as little as 3 minutes through IV extension tubing inserted directly into the vein. Consequently, large-bore lines placed by venous cutdown are an excellent mechanism for the treatment of severe hypovolemia.
Contraindications
Venous cutdown is contraindicated when less invasive alternatives exist and when performing the procedure would cause excessive delay.10 Highly skilled clinicians may perform a cutdown in less than 60 seconds.11 However, multiple studies by Westfall,12 Rhee,13 Iserson,14 and their colleagues have indicated that on average, the procedure takes at least 5 to 6 minutes to complete. Use of the modified Seldinger technique described both by Shockley and Butzier and by Klofas has been shown to decrease that time by 22%.15,16 In general, the use of percutaneously inserted central venous catheters in either the subclavian, internal jugular, or femoral vein is preferable to a cutdown.
Absolute contraindications include major blunt or penetrating trauma involving the extremity on which the procedure is to be performed.17 Relative contraindications include vascular injury proximal to the cutdown site, overlying soft tissue infection, coagulopathies, compromised host defense mechanisms, and impaired wound healing. Other considerations include any previous saphenous vein harvest for coronary artery bypass or other vascular surgery proximal to the anticipated cutdown site.18 The indications for venous cutdown should be weighed against the potential complications.
Anatomy and Selection of the Site
The Great Saphenous Vein
The great saphenous vein is the longest vein in the body, and it runs subcutaneously throughout much of its course (Fig. 23-1). It is most easily accessible at the ankle but may also be cannulated below the knee and below the femoral triangle. The great saphenous vein begins just anterior to the medial malleolus, where it is a continuation of the medial marginal vein of the foot. The vein crosses 1 cm anterior to the medial malleolus and, together with the saphenous nerve, ascends along the anteromedial aspect of the leg.19,20 The saphenous nerve at this level is of relatively little clinical significance in that, if transected, it causes sensory loss in only a small area along the medial aspect of the foot. At the ankle, the vessel can be exposed with minimal blunt dissection. The vein’s superficial, predictable, and isolated location has made the distal saphenous vein the traditional pediatric cutdown site.19
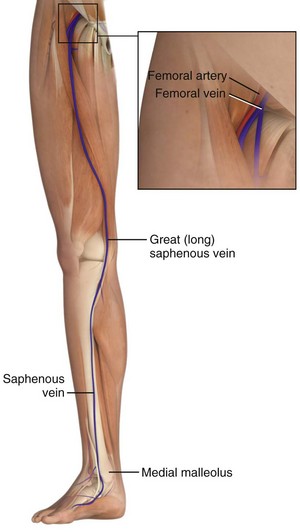
Figure 23-1 Superficial veins of the lower limb.
At the knee, the saphenous vein lies superficially and medially. A cutdown performed 1 to 4 cm below the knee and immediately posterior to the tibia has been described in the pediatric literature.6 This site is seldom used, however, because of its many disadvantages, including kinking of the line as the knee is flexed and risk for injury to the saphenous branch of the genicular artery and the saphenous nerve.21 Of note, the great saphenous vein is duplicated in the calf in 25% of the population and may be present on exploration.22
In the thigh, the saphenous vein begins on the medial aspect of the knee and crosses anterolaterally as it ascends toward the femoral triangle. Approximately 4 cm below the inguinal ligament and 3 cm lateral to the pubic tubercle, the saphenous vein dips through the fossa ovalis, where it penetrates the femoral sheath and joins the femoral vein. The saphenous vein is easily isolated from the surrounding fat at this site because of its large caliber (4 to 5 mm in outside diameter) and superficial relationship to the femoral sheath (Fig. 23-2). Also lying anteromedially in the thigh is the lateral femoral cutaneous vein, which has a smaller diameter and lies lateral to the great saphenous vein.20 The saphenous vein at the thigh is a preferred site for cutdown given its large diameter and ease of accessibility. An 8.5-Fr catheter is easily introduced at this level and is ideal for rapid infusion of crystalloid or blood during resuscitation.17
The Basilic Vein
Veins of the dorsal venous network of the hand unite to form the cephalic and basilic veins, which travel along the radial and ulnar sides of the forearm, respectively (Fig. 23-3). At the midforearm level, the basilic vein crosses anterolaterally and then courses ventrally at the medial epicondyle. The medial cubital vein crosses over from the radial side of the arm to join the basilic vein just above the medial epicondyle. The basilic vein then continues proximally, where it occupies a superficial position between the biceps and pronator teres muscles. In this segment it lies in close proximity to the medial cutaneous nerve, which supplies sensation to the ulnar side of the forearm. At approximately midway in the upper part of the arm, the basilic vein perforates the deep fascia, where it joins the brachial vein and continues on into the axillary vein.20
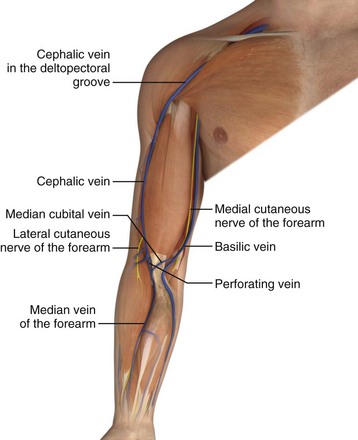
Figure 23-3 Veins of the upper limb.
A more proximal cutdown site had previously been recommended to avoid the network of interconnecting veins at the level of the antecubital fossa.23 However, a closer association between the basilic vein and the medial cutaneous nerve in this segment may result in transection of the nerve and subsequent sensory loss on the ulnar side of the forearm.
The Cephalic Vein
This cephalic vein begins on the radial aspect of the wrist, crosses anteromedially, and ascends toward the antecubital fossa. In the forearm, it lies in close association with the lateral cutaneous nerve, which supplies sensory innervation to the radial aspect of the forearm (see Fig. 23-3). In the antecubital fossa, it lies subcutaneously and just lateral to the midline. It is at this level where the median cubital vein connects to the cephalic and basilic veins. The cephalic vein then ascends in the upper part of the arm over the lateral aspect of the biceps muscle and through the deltopectoral groove. Just below the clavicle, it pierces the clavipectoral fascia, becomes a deep structure, and enters the axillary vein.20
Venous cutdown is easily performed on the cephalic vein because of its large diameter and superficial location. In the forearm, it is important to avoid the lateral cutaneous nerve. A preferred location is in the antecubital fossa at the distal flexor crease. Cutdown on the cephalic vein at the wrist has also been reported, but the thin skin overlying the vein at this level usually permits simple percutaneous cannulation when the vein is available for cannulation.24 The cephalic vein may also be entered in the deltopectoral groove. However, the slightly deeper position and possible interference with the performance of other procedures make this approach more difficult.
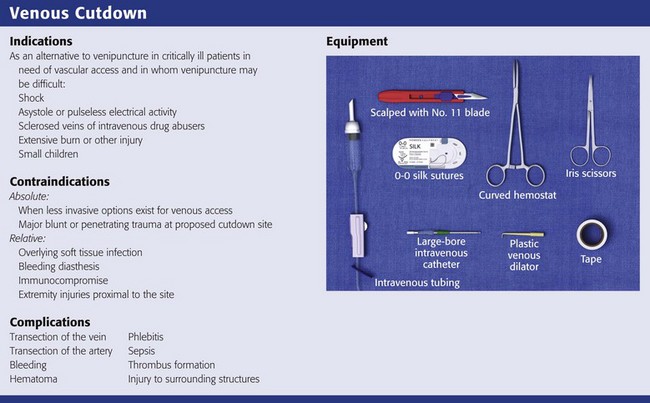
Equipment
Tables 23-1, 23-2, and 23-3 list the flow rates of various fluids through some commonly used catheter systems. It is essential to know the relative flow rates if maximal benefit is to be obtained from the time spent performing the cutdown. Excellent flow rates can be achieved by threading IV tubing directly into the vein or by using a 5-cm, 10-gauge IV catheter. Cut sterile tubing to the appropriate length and leave a slight bevel on the end to facilitate cannulation of the opened vein.11,25
TABLE 23-1
Comparative Average Flow Rates (mL/min) for Tap Water*
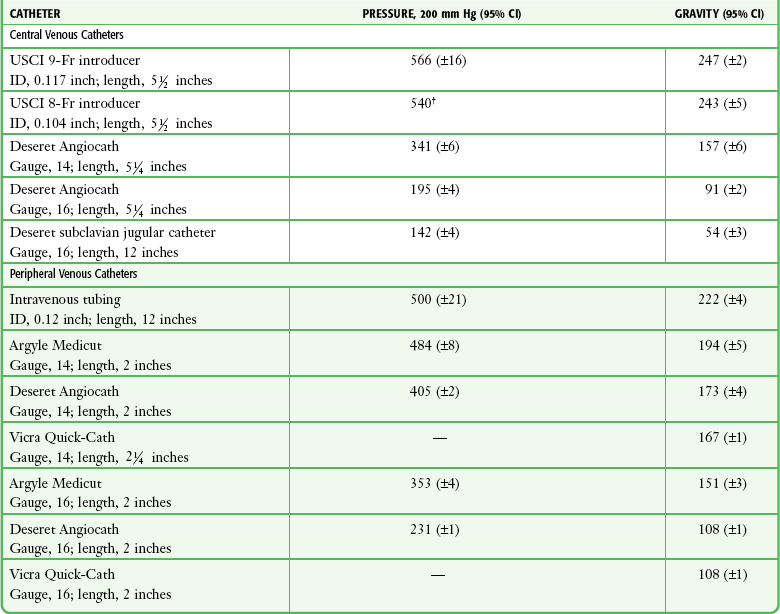
CI, confidence interval; ID, internal diameter.
*Mean of three trials with a hydrostatic pressure head of 1 m.
†Ninety-five percent confidence interval not calculated because all three trials resulted in 11.1 seconds for 100-mL flow.
From Mateer JR, Thompson BM, Aprahamian C, et al. Rapid fluid resuscitation with central venous catheters. Ann Emerg Med. 1983;12:150. Reproduced by permission.
TABLE 23-2
Comparative Average Flow Rates (mL/min, 200–mm Hg Pressure) for Red Blood Cells
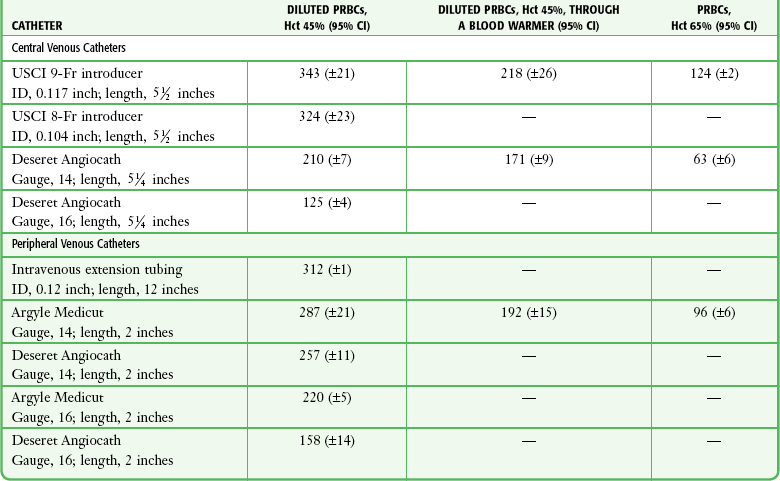
CI, confidence interval; Hct, hematocrit; ID, internal diameter; PRBCs, packed red blood cells.
From Mateer JR, Thompson BM, Aprahamian C, et al. Rapid fluid resuscitation with central venous catheters. Ann Emerg Med. 1983;12:151. Reproduced by permission.
TABLE 23-3
Comparative Average Flow Rates (mL/min)
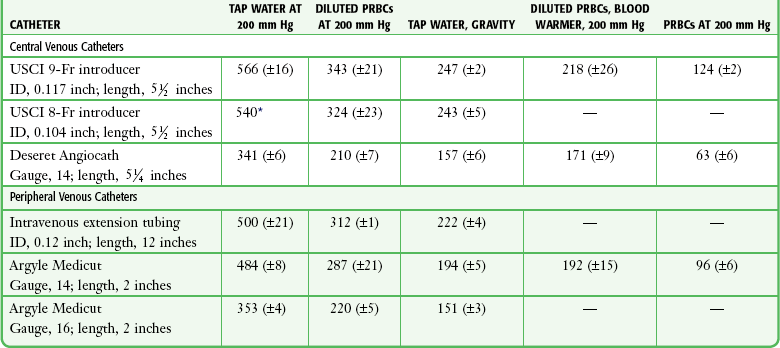
ID, internal diameter; PRBCs, packed red blood cells.
*Ninety-five percent confidence interval not calculated because all three trials resulted in 11.1 seconds for 100-mL flow.
From Mateer JR, Thompson BM, Aprahamian C, et al. Rapid fluid resuscitation with central venous catheters. Ann Emerg Med. 1983;12:151. Reproduced by permission.
Technique
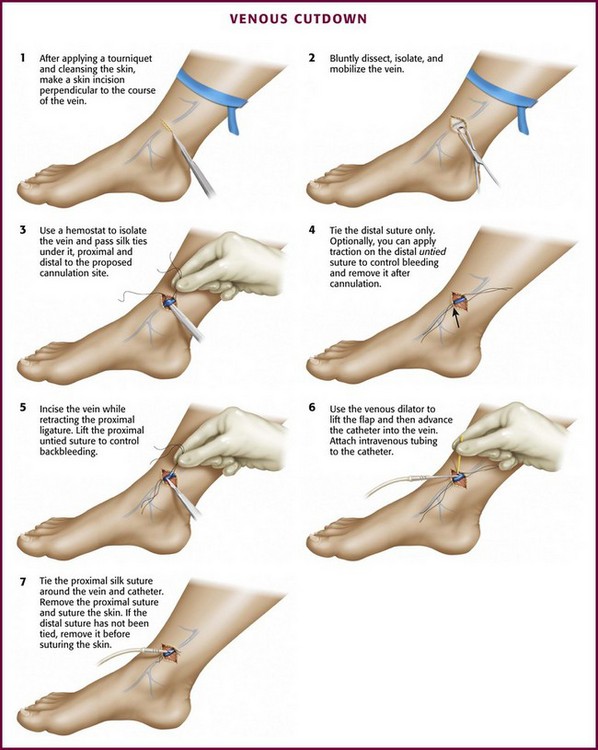
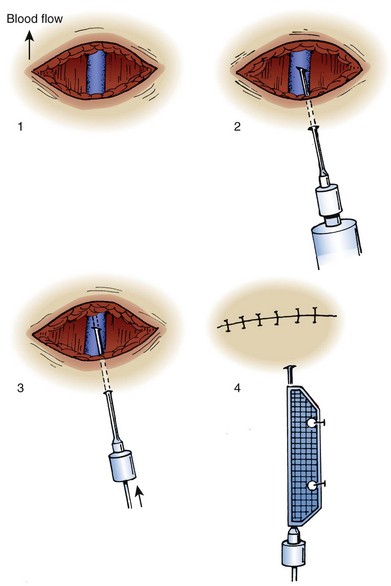
The technique of venous cutdown is essentially the same regardless of the vessel cannulated (Fig. 23-4). Prepare the skin around the incisional area with an antiseptic solution and then cover it with sterile drapes. Place a tourniquet proximal to the cutdown site to help visualize the vein. For children, immobilize the lower part of the leg or elbow (depending on the cutdown site) on a padded board before beginning the procedure.
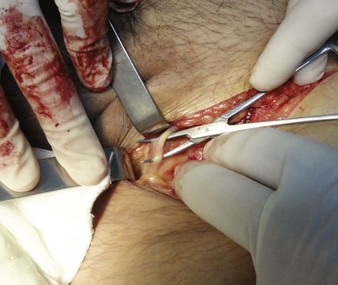
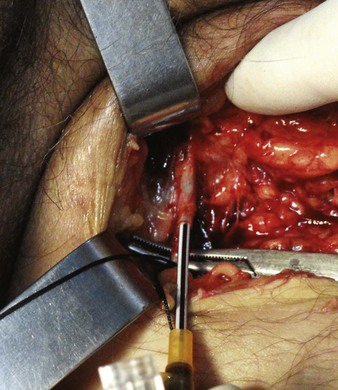
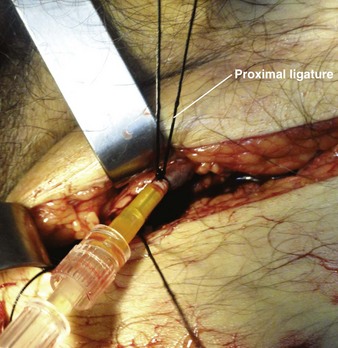
For the standard venous cutdown technique, after mobilizing the vein, use a hemostat to pass proximal and distal silk ties under the vein for stabilization (Fig. 23-5).
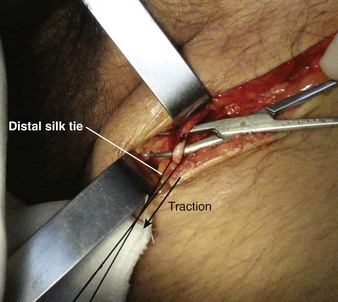
Tie the distal ligature after initial placement, but leave the ends long for maneuvering the vein. As an option, use traction on the distal suture to control the vein, but do not tie it off. If the distal suture is tied, the vein will be sacrificed for future use. Leave the proximal ligature untied to maneuver the vein for insertion of the catheter or tubing and control of backbleeding (by lifting the sutures). Elevate the vein with a hemostat and stretch it flat. This provides good visualization, controls the vessel, and limits bleeding when the vessel is incised. Alternatively, place gentle traction on the proximal tie to control oozing around the puncture site. Using a No. 11 scalpel blade or a pair of iris scissors, incise through one third to one half the diameter of the vein at a 45-degree angle. If the incision is too small, the catheter may pass into a false channel in the adventitia. Conversely, if the incision is too large, the vein may tear completely and retract from the field buried within tissue.26 If desired, make a longitudinal incision in the vein to avoid transecting the vessel, but realize that this technique makes it more difficult to identify the lumen. Also be aware that some bleeding will normally occur after the vein has merely been nicked on the surface. To perform a mini-cutdown at this point, puncture the vein with an IV catheter and introducer needle and do not make an incision in the vein.
Use of a plastic venous dilator can help identify and elevate the vessel lumen. Thread the small, pointed tip of the dilator into the vein to expose the lumen before advancing the tip of the catheter. Alternatively, bend a sterile 20-gauge needle at a 90-degree angle to serve as a venous dilator or elevator. A vein dilator is useful for very small veins, such as in pediatric cutdowns, but is generally unnecessary in adults. To thread large catheters in adults, grasp the proximal edge of the vessel with small forceps or a mosquito hemostat. Apply countertraction and advance the catheter. Never force a catheter that will not advance easily (Fig. 23-6).
Once the catheter is advanced into the lumen, backbleed any air from the cannula and connect it to IV tubing. Tie the proximal ligature around both the vessel and the cannula (Fig. 23-7). If the distal suture has not been tied, remove it. If it has been tied, cut the ends of the suture near the knot. Remove the tourniquet, affix the catheter to the skin, and close the incision. Apply antibiotic ointment at the point where the catheter passes through the skin, and dress the wound. In an emergency situation one may delay skin closure if necessary and simply wrap the wound with sterile dressing. Loop the IV tubing under the outer layers of the dressing to minimize the risk of pulling the cannula out if the external IV line is inadvertently tugged.
Mini-Cutdown
The mini-cutdown is an alternative method designed to preserve the vein and bypass the time-consuming step of inserting a catheter into the vein.27 It is preferred if time is limited. Basically, a deep vein is cannulated through an incision under direct vision with a standard peripheral IV catheter. Use a skin incision and blunt dissection to locate the vessel. Once identified, puncture the vein under direct vision with a percutaneous venous catheter. Introduce the needle through either the skin incision or a separate stab incision. If an over-the-needle device (e.g., Angiocath, Medicut) is used, withdraw the needle and discard it. With a through-the-needle device, thread the cannula into the vein and withdraw the needle to the surface of the skin (Fig. 23-8). Place a guard on the tip of the needle, fix the catheter device to the skin, and close the incision. This method eliminates the need for tying or cutting the vein, thereby permitting repeated catheterization. Venipuncture is easier and uses the same equipment as percutaneous venous cannulation. A simple skin incision may also permit direct visualization of veins in an obese patient and facilitate standard percutaneous venipuncture.
Hansbrough and associates described a mini-cutdown procedure with a 10-gauge IV catheter (Deseret 10-gauge Angiocath).25 Flow rates of blood and saline with this catheter are equal to those obtained when IV extension tubing is placed in a vein via the more time-consuming standard venous cutdown technique. This catheter allows one to infuse a unit of whole blood in 2 to 3 minutes if high pressure and oversized IV tubing are used.
Modified Cutdown Technique
Shockley and Butzier described a further modification in which a guidewire, dilator, and sheath system is inserted after standard cutdown and venotomy.15 To perform this modification, set up the guidewire, dilator, and sheath system before making the skin incision. Once the vein has been incised, insert the end of the guidewire followed by the dilator and sheath. Remove the wire and dilator while leaving the sheath. Ligatures are not usually needed with this technique. These authors found that when performed by novices, this technique saved more than 2 minutes in comparison to the standard technique. Moreover, in the event of a transection, there was an increased vein salvage rate. Klofas used a similar technique at the distal saphenous vein.16 He also developed a model for teaching the modified technique with wood, gauze, cast padding, and tape.
Complications
Complications of venous cutdown include local hematoma, infection, sepsis, phlebitis, embolization, wound dehiscence, and injury to associated structures. An indirect but significant complication is deterioration of an unstable patient during a time-consuming attempt at cutdown. Documentation of complications and their frequency in the literature has been sparse. Bogen reported a 15% complication rate in 234 cases.28 Infection and phlebitis each occurred at a rate of 4%. Infectious complications may result from the introduction of pathogens during placement of the line, transcutaneous invasion along the course of the cannula, or deposition of blood-borne organisms on the tip of the catheter.29 A clear correlation exists between the incidence of infectious complications and the length of time that a catheter is left in place. Moran and colleagues found that the infection rate rose from 50% to 78% when a catheter was left in place for more than 48 hours.30 Druskin and Siegel,29 studying a mixed population of patients who had undergone cutdown and others who had catheters inserted percutaneously, found that the incidence of culture-positive catheter tips rose from 0% to 52% after 48 hours.29 In the study by Moran and coworkers,30 Staphylococcus albus was the predominant organism that was isolated, but organisms more commonly thought of as pathogenic (Staphylococcus aureus, Enterococcus spp., and Proteus spp.) were isolated with greater frequency from cutdowns that had been in place for long periods. Rhee and coauthors reported a 1.4% infection rate and one episode of cellulitis after 73 cutdown attempts.13 All catheters were removed within 24 hours.
Some evidence indicates that the rate of infectious complications decreases when a broad-spectrum topical antibiotic ointment is applied to the cutdown site. Moran and colleagues found an infectious complication rate of 18% when topical polymyxin B–neomycin-bacitracin (Neosporin) was used as opposed to a 78% rate in a placebo-treated group.30 In this study, topical antibiotic use resulted in only a moderate decrease (from 53% to 37%) in the incidence of phlebitis but a significant decrease (from 86% to 14%) in the incidence of phlebitis associated with positive cultures. This suggests that phlebitis is often a chemical or an irritative process rather than the result of infection. Whatever the cause, the incidence of phlebitis is clearly related to the duration of catheterization.8,28,31 Early catheter removal is a key factor in the prevention of both phlebitis and the infectious complications of venous cutdown. This is especially true of lines inserted during emergency resuscitative treatment. Such lines should be removed as soon as the patient’s condition permits and alternative access routes are in place.9,10
References
1. Balls, A, LoVecchio, F, Kroeger, A, et al. Ultrasound guidance for central venous catheter placement: results from the Central Line Emergency Access Registry Database. Am J Emerg Med. 2010;28:561.
2. Keeley, JL. Intravenous injections and infusions. Am J Surg. 1940;50:485.
3. Kirkham, JH. Infusion into the internal saphenous vein at the ankle. Lancet. 1945;2:815.
4. Committee on Trauma, American College of Surgeons. Advanced Trauma Life Support Instructor Manual. Chicago: American College of Surgeons; 2008.
5. Custalow, CB, Kline, JA, Marx, JA, et al. Emergency department resuscitative procedures: animal laboratory training improves procedural competency and speed. Acad Emerg Med. 2002;9:6.
6. Aldeman, S. An emergency intravenous route for the pediatric population. JACEP. 1976;5:596.
7. Gauderer, MW. Vascular access techniques and devices in the pediatric patient. Surg Clin North Am. 1992;72:1267.
8. Dudley HAF, ed. Hamilton Bailey’s Emergency Surgery, 10th ed, Bristol, England, John Wright & Sons, 1977:28.
9. Dronen, SC, Yee, AS, Tomlanovich, MC. Proximal saphenous vein cutdown. Ann Emerg Med. 1981;10:328.
10. Knopp, R. Venous cutdowns in the emergency department. JACEP. 1978;7:429.
11. Posner, M, Moore, EE. Distal saphenous vein cutdown—technique of choice for rapid volume resuscitation. J Emerg Med. 1985;3:395.
12. Westfall, MD, Price, KR, Lambert, M, et al. Intravenous access in the critically ill trauma patient: a multicentered, prospective, randomized trial of saphenous cutdown and percutaneous femoral access. Ann Emerg Med. 1994;23:541.
13. Rhee, KJ, Derlet, RW, Beal, SL. Rapid venous access using saphenous vein cutdown at the ankle. Am J Emerg Med. 1989;7:263.
14. Iserson, KV, Criss, EA. Pediatric venous cutdowns: utility in emergency situations. Pediatr Emerg Care. 1986;2:231.
15. Shockley, LW, Butzier, DJ. A modified wire-guided technique for venous cutdown access. Ann Emerg Med. 1990;19:393.
16. Klofas, E. A quicker saphenous vein cutdown and a better way to teach it. J Trauma. 1997;43:985.
17. Chappell, S, Vilke, GM, Chan, TC, et al. Peripheral venous cutdown. J Emerg Med. 2006;31:411.
18. Wilson SE, ed. Vascular Access, 5th ed, Philadelphia: Lippincott: Williams & Wilkins, 2010.
19. Hollinshead WH, ed. Textbook of Anatomy, 2nd ed, New York, Harper & Row, 1967:442.
20. Gray, H. The veins. In: Clemente CD, ed. Anatomy of the Human Body. 30th ed. Philadelphia: Lea & Febiger; 1985:820.
21. Anderson JE, ed. Grant’s Atlas of Anatomy, 7th ed, Baltimore, Williams & Wilkins, 1978:4.6.
22. Bergen, JJ. The Vein Book. Burlington, VT: Academic Press; 2007.
23. Simon, RR, Hoffman, JR, Smith, M. Modified new approaches for rapid intravenous access. Ann Emerg Med. 1987;16:44.
24. Talan, DA, Simon, RR, Hoffman, JR. Cephalic vein cutdown at the wrist: comparison to the standard saphenous vein ankle cutdown. Ann Emerg Med. 1988;17:38.
25. Hansbrough, JF, Cain, TL, Millikan, JS. Placement of 10-gauge catheter by cutdown for rapid fluid replacement. J Trauma. 1983;23:231.
26. Stanley-Brown, EG. The venous cutdown. Arch Pediatr. 1958;75:480.
27. Shiu, MH. A method for conservation of veins in the surgical cutdown. Surg Gynecol Obstet. 1972;134:315.
28. Bogen, JE. Local complications in 167 patients with indwelling venous catheters. Surg Gynecol Obstet. 1960;110:112.
29. Druskin, MS, Siegel, PD. Bacterial contamination of indwelling intravenous polyethylene catheters. JAMA. 1963;185:966.
30. Moran, JM, Atwood, RP, Rowe, M. A clinical and bacteriologic study of infections associated with venous cutdown. N Engl J Med. 1963;272:554.
31. Collins, RN, Braun, PA, Zinner, SH, et al. Risk of local and systemic infection with polyethylene intravenous catheters. N Engl J Med. 1968;279:34.