Chapter 1 Venous Anatomy
Historical Background
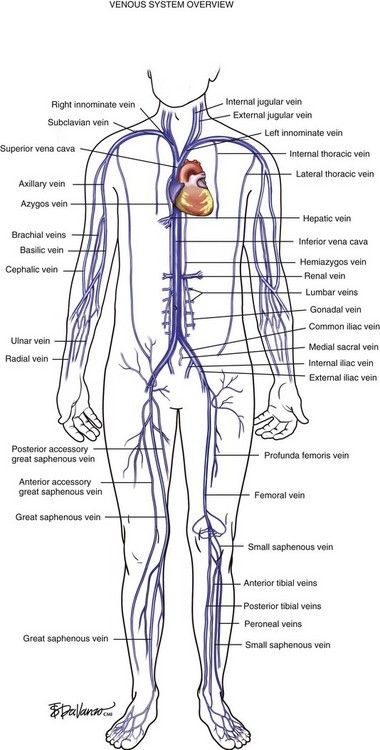
The venous system originates at the capillary level and progressively increases in size as the conduits move proximally toward the heart. The venules are the smallest structures, and the vena cava is the largest. It is critical that all endovascular venous surgeons understand the anatomic relationships between the thoracic, abdominal, and extremity venous systems; especially from the anatomic standpoint (Fig. 1-1). Veins of the lower extremities are the most germane to this book and are divided into three systems: deep, superficial, and perforating. Lower extremity veins are located in two compartments: deep and superficial. The deep compartment is bounded by the muscular fascia. The superficial compartment is bounded below by the muscular fascia and above by the dermis. The term perforating veins is reserved for veins that perforate the muscular fascia and connect superficial veins with deep veins. The term communicating veins is used to describe veins that connect with other veins of the same compartment.
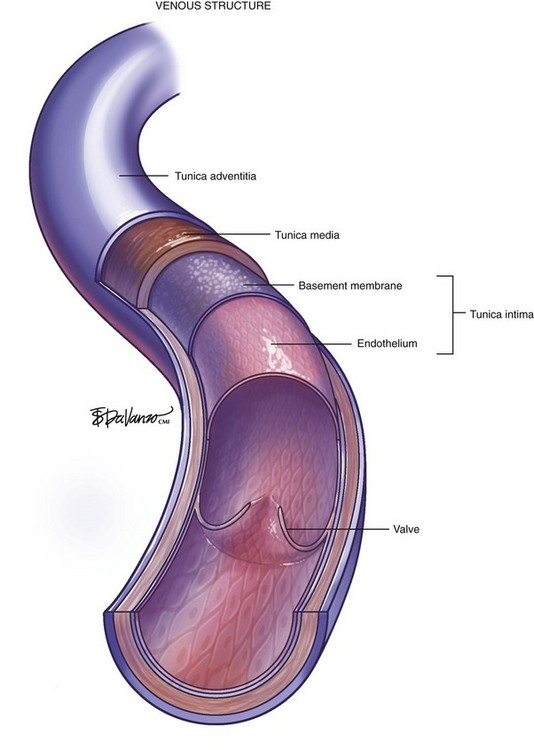
The vein wall is composed of three layers: intima, media, and adventitia. Notably, the muscular tunica media is much thinner than on the pressurized arterial side of the circulation. Venous valves are an extension of the intimal layer, have a bicuspid structure, and support unidirectional flow (Fig. 1-2).
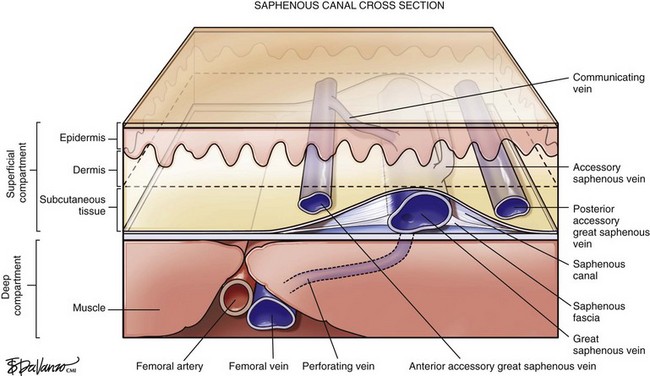
Surgeons interested in performing thermal or chemical ablation therapy of the great saphenous vein (GSV) and its related structures must have a good understanding of the saphenous canal. The importance of the saphenous canal in relation to B-mode ultrasound anatomy is discussed in more detail in Chapter 2. A cross section of the saphenous canal (Fig. 1-3) depicts many of the critical relationships referable to GSV treatment—the most important is how it courses atop the muscular fascia in a quasi-envelope called the saphenous fascia. The saphenous fascia is the portion of the membranous layer of the subcutaneous tissue that overlies the saphenous veins. Veins coursing parallel to the saphenous canal are termed accessory veins, whereas those coursing oblique to the canal are referred to as circumflex veins. Compressible structures superficial to the muscular fascia are potential targets for treatment; however, treating those structures deep to the muscular fascia may lead to a disastrous outcome. Noncompressible structures generally represent major arteries. Perforating veins must pierce the muscular fascia as they drain blood from the superficial to deep systems.
As diagnostic and therapeutic options for venous disorders expanded, the nomenclature proposed in 2002 by the International Interdisciplinary Committee1 required revision. The nomenclature was extended and further refined,2 taking into account recent improvements in ultrasound and clinical surgical anatomy. The term great saphenous vein should be used instead of terms such as long saphenous vein, greater saphenous vein, or internal saphenous vein. The LSV abbreviation, used to describe both the long saphenous vein and lesser saphenous vein, was clearly problematic. For this reason these terms have been eliminated. Similarly, the term small saphenous vein, abbreviated as SSV, should be used instead of the terms short, external, or lesser saphenous vein.
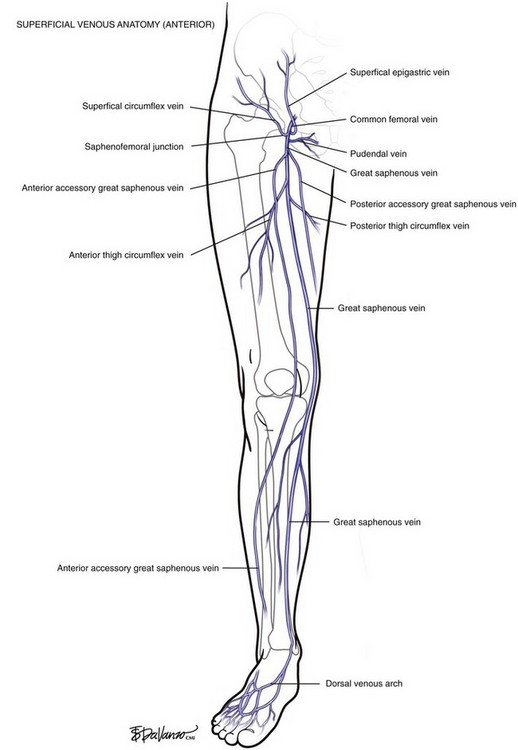
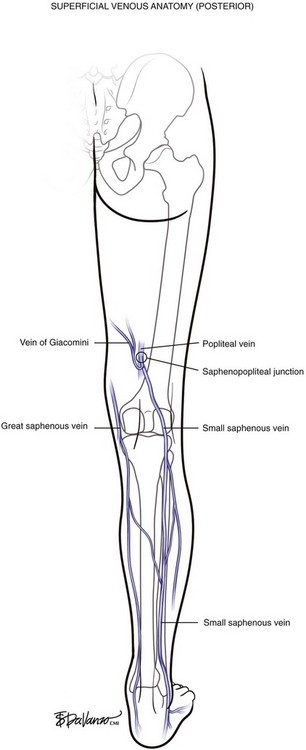
The GSV originates at the medial foot and receives deep pedal tributaries as it courses to the medial malleolus. From the medial ankle, the GSV ascends anteromedially within the calf and continues a medial course to the knee and into the thigh. The termination point of the GSV into the common femoral vein is a confluence called the saphenofemoral junction (SFJ) (Fig. 1-4).
In variant drainage, a standard SPJ may or may not be present. The SSV is truly duplicated in 4% of cases; most often this is segmental and primarily involves the midportion of the vein (Fig. 1-5).
Perforating Veins
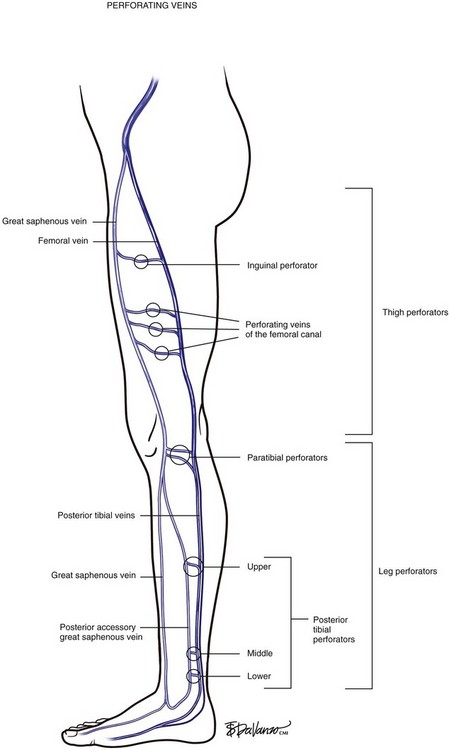
Identifying perforating veins based on the original descriptions of investigators (i.e., Cockett, Sherman, Dodd) is falling into disfavor. Descriptive terms based on topography, which designate the anatomic location, have become the contemporary approach. Perforating veins pass through defects in the deep fascia to connect deep and superficial veins of the calf or thigh. Venous valves prevent reflux of blood from the deep veins into the superficial system. Perforating veins may connect the GSV to the deep system at the femoral, posterior tibial, gastrocnemius, and soleal vein levels. Located between the ankle and the knee are perforating veins formerly known as Cockett’s perforators that connect the posterior tibial venous system with the PAGSV of the calf (a.k.a., posterior arch vein) (Fig. 1-6).
Deep Veins
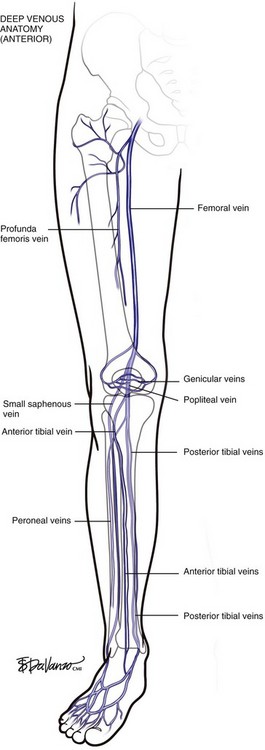
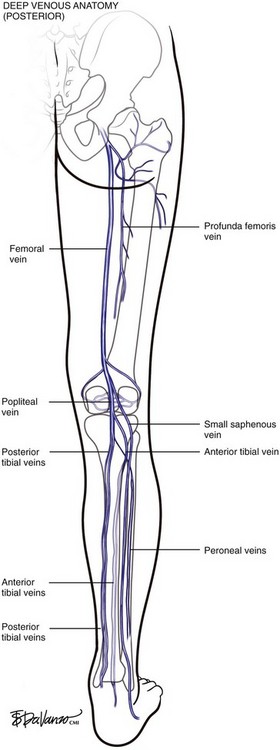
At the upper margin of the popliteal fossa, above the adductor canal, the femoral vein originates from the popliteal vein. The “superficial femoral vein” terminology was clearly problematic and has been abandoned since the femoral vein is a deep structure. The deep femoral vein (profunda femoris) drains the deep muscles of the lateral thigh, communicates with the popliteal vein, and serves as a critical collateral vessel in cases where the femoral vein occludes with thrombus. The common femoral vein runs from the confluence of the femoral vein and the deep femoral vein to the external iliac vein at the level of the inguinal ligament (Figs. 1-7 and 1-8).
Upper Extremity Veins
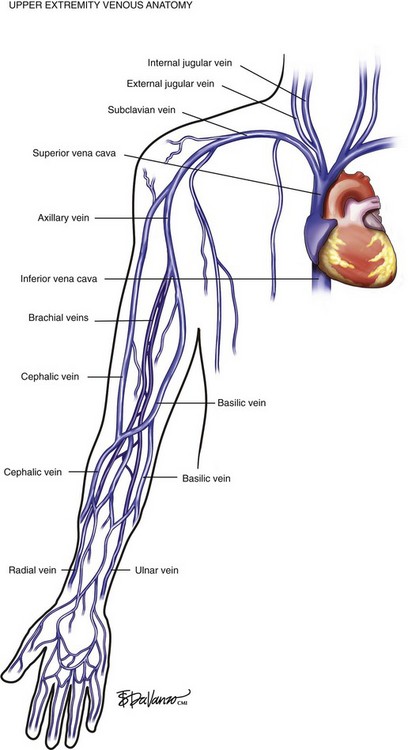
Venous blood from the hand drains into the forearm via the deep radial and ulnar veins and the superficial cephalic and basilic veins. In the upper arm, deep drainage from the paired brachial veins enters the axillary vein at the shoulder. The axillary vein also drains the superficial tissues via the cephalic vein—which enters the deltopectoral groove—and the basilic veins of the medial arm. The subclavian vein is protected by the clavicle as it carries upper extremity blood from the axillary vein. The subclavian vein then picks up drainage from the head and neck via the jugular veins and ultimately empties into the innominate veins of the thoracic cavity. Right and left innominate veins drain into the SVC and enter the right atrium of the heart. The SVC also receives venous blood from the azygos system, which drains the thoracic cage via intercostal veins and ultimately enters the SVC (Fig. 1-9).
Lower Extremity Nerves
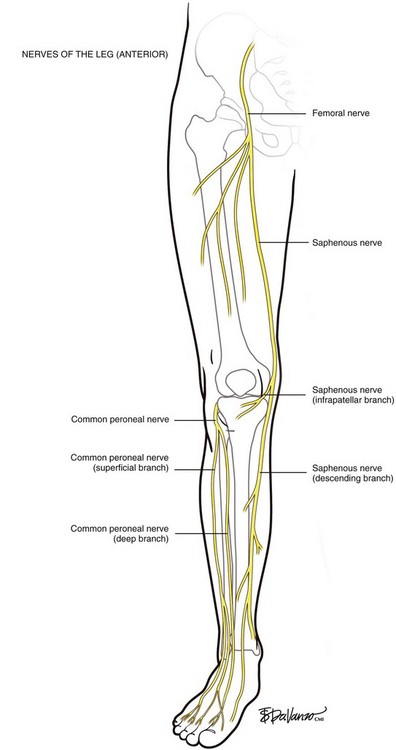
The posterior division of the femoral nerve provides sensory fibers to the inner surface of the leg (saphenous nerve), to the quadriceps muscles (muscular branches), and to the hip and knee joints. The saphenous nerve descends beneath the sartorius muscle, winding around its posterior edge and exiting at the adductor canal. The infrapatellar branch pierces the sartorius muscle and courses anteriorly to the infrapatellar region. The descending branch passes down the medial aspect of the leg juxtaposed to the great saphenous vein and here is at highest risk for injury from thermal ablation procedures. At the lower third of the leg, it divides into two branches: one of the branches of the descending portion of the saphenous nerve courses along the medial border of the tibia and ends at the ankle, whereas the other branch passes anterior to the ankle and is distributed to the medial aspect of the foot, sometimes reaching as far as the metatarsophalangeal joint of the great toe (Fig. 1-10).
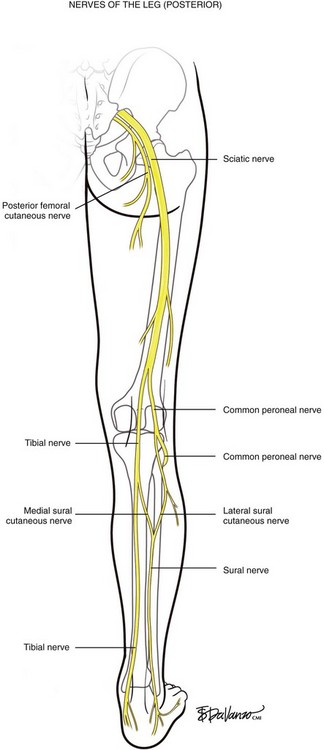
The most interesting issue referable to surgical work in the popliteal space is clearly the neuroanatomy. The sciatic nerve descends the posterior thigh and divides into the tibial and common peroneal nerves in the popliteal area (Fig. 1-11). The exact location of this division can range several centimeters proximally or several centimeters distally. The tibial nerve continues its descent to the ankle, and its innervation mostly affects motor function. The common peroneal nerve, however, divides near the zone of the head of the fibula, into deep and superficial branches. The common peroneal nerve courses anteriorly around the fibula, taking a sharp turn as it rounds the fibular neck to enter the anterior compartment. Because of the sharp turn, the nerve is more tethered than the superficial branch; immediately below the fibular head, the deep peroneal nerve lies on the anterior cortex of the fibula for a distance of 3 to 4 cm. The deep peroneal nerve innervates the dorsiflexors of the leg and, when injured, results in the dramatic foot drop. The tissues innervated by the superficial peroneal nerve provide only sensory information for interpretation in the brain.
The sural nerve is formed by the union of the medial sural cutaneous nerve (MSCN) and the lateral sural cutaneous nerve (LSCN). When viewed from the posterior position, the sural nerve is arranged like the letter “Y” in most persons. The MSCN is a branch of the tibial nerve, and the LSCN originates from the common peroneal nerve. The site of union is usually in the lower third of the leg or just below the ankle.3 The SSV travels in proximity to the sural nerve in the lower leg, where the nerve is joined at the midline. Where the gastrocnemius muscle bellies become prominent in the upper calf, the sural nerve is separated into the MSCN and LSCN. Therefore the SSV in the upper calf is distanced from the sural nerve, making this segment safer for thermal ablation
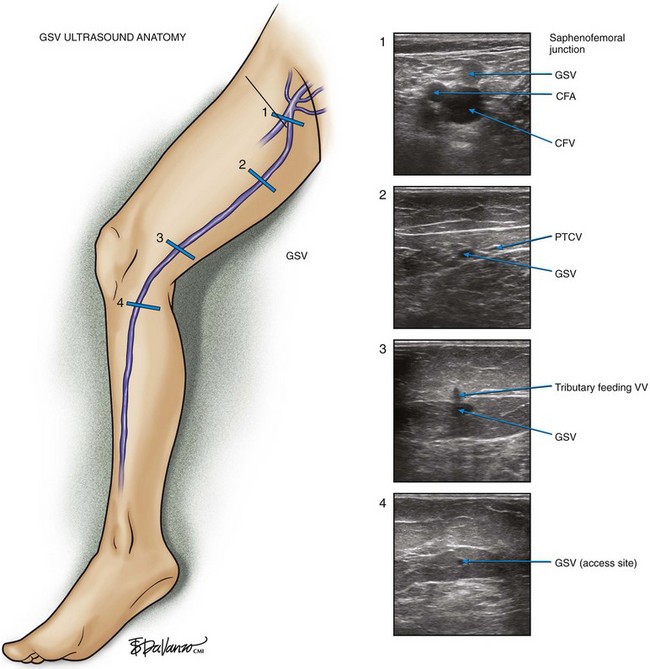
As stated earlier, B-mode ultrasound has made it possible to develop an accurate diagnosis and treatment plan for patients with venous disease. The main reference point of the ultrasound examination is at the inguinal crease where the GSV empties into the CFV. In the first cross section of Figure 1-12, one can study the saphenofemoral junction as it looks using B-mode (gray scale) ultrasound.
Etiology and Natural History of Disease
Early theories were based on the belief that varicose veins resulted from the effects of venous hypertension secondary to valvular incompetence at the saphenofemoral or saphenopopliteal junction resulting in retrograde flow of blood down a hydrostatic pressure gradient. Unfortunately, there is little evidence of a constitutive valvular abnormality in primary venous disease. The theories do not explain why truncal varicosities are often found below competent valves, why normal valves are often seen between variceal segments, or why venous dilation often precedes valvular incompetence.4,5 Rather than being initiated at the saphenofemoral junction, both detailed studies of surgical specimens and ultrasound observation suggest that primary valvular incompetence is a multicentric process that develops simultaneously in discontinuous venous segments.6
Histologic and ultrastructural studies of varicose saphenous veins have found hypertrophy of the vein wall with increased collagen content,7 together with disruption of the orderly arrangements of smooth muscle cells and elastin fibers.8,9 Cultures of smooth muscle cells from varicose saphenous veins have demonstrated disturbed collagen synthesis, overproduction of collagen type I, and reduced synthesis of collagen type III.10 Because collagen type I is thought to confer rigidity and collagen type III to confer distensibility to tissues, such changes could contribute to the weakness and reduced elasticity of varicose veins. A complicating factor is the heterogeneity of the varicose vein wall; hypertrophic segments can alternate with thinner atrophic segments with fewer smooth muscle cells and reduced extracellular matrix.
Despite advances in our understanding of varicose veins, the underlying etiology remains elusive. Varicose veins demonstrate diverse histologic abnormalities, including irregular thickening of the intima, fibrosis between the intima and adventitia, atrophy and disruption of elastic fibers, thickening of individual collagen fibers, and disorganization of the muscular layers in varicosed tributaries.11–15 Varicose veins have increased collagen with a decrease in smooth muscle and elastin content.16,17 Most recent evidence suggests that such changes in the vein wall precede the development of reflux.18,19 The exact cause of primary valvular incompetence in superficial veins remains unknown. However, valvular incompetence is thought to result as a phenomenon secondary to dilatation of weakened vein walls, with enlargement of the valve ring preventing adaptation of the leaflets.17 Interestingly, studies suggest the strength of the valves is far greater than the strength of the venous wall.19
1 Caggiati A, Bergan JJ, Gloviczki P, et al. International Interdisciplinary Consensus Committee on Venous Anatomical Terminology. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg. 2002;36:416-422.
2 Caggiati A, Bergan JJ, Gloviczki P, et al. International Interdisciplinary Consensus Committee on Venous Anatomical Terminology. Nomenclature of the veins of the lower limb: extensions, refinements, and clinical application. J Vasc Surg. 2005;41:719-724.
3 Mahakkanukrauh P, Chomsung R. Anatomical variations of the sural nerve. Clin Anat. 2002;15:263-266.
4 Alexander CJ. The theoretical basis of varicose vein formation. Med J Aust. 1972;1:258-261.
5 Cotton LT. Varicose veins. Gross anatomy and development. Br J Surg. 1961;48:589-597.
6 Labropoulos N, Giannoukas AD, Delis K, et al. Where does venous reflux start? J Vasc Surg. 1997;26:736-742.
7 Travers JP, Brookes CE, Evans J, et al. Assessment of wall structure and composition of varicose veins with reference to collagen, elastin and smooth muscle content. Eur J Vasc Endovasc Surg. 1996;11:230-237.
8 Porto LC, Ferreira MA, Costa AM, et al. Immunolabeling of type IV collagen, laminin, and alpha-smooth muscle actin cells in the intima of normal and varicose saphenous veins. Angiology. 1998;49:391-398.
9 Wali MA, Eid RA. Changes of elastic and collagen fibers in varicose veins. Int Angiol. 2002;21:337-343.
10 Sansilvestri-Morel P, Rupin A, Badier-Commander C, et al. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001;38:560-568.
11 Ascher E, Jacob T, Hingorani A, et al. Expression of molecular mediators of apoptosis and their role in the pathogenesis of lower-extremity varicose veins. J Vasc Surg. 2001;33:1080-1086.
12 Bouissou H, Julian M, Pieraggi MT, et al. Vein morphology. Phlebology. 1988;3(suppl 1):1-11.
13 Jones GT, Solomon C, Moaveni A, et al. Venous morphology predicts class of chronic venous insufficiency. Eur J Vasc Endovasc Surg. 1999;18:349-354.
14 Lowell RC, Gloviczki P, Miller VM. In vitro evaluation of endothelial and smooth muscle function of primary varicose veins. J Vasc Surg. 1992;16:679-686.
15 Porto LC, Azizi MA, Pelajo-Machado M, et al. Elastic fibers in saphenous varicose veins. Angiology. 2002;53:131-140.
16 Travers JP, Brookes CE, Evans J, et al. Assessment of wall structure and composition of varicose veins with reference to collagen, elastin and smooth muscle content. Eur J Vasc Endovasc Surg. 1996;11:230-237.
17 Gandhi RH, Irizarry E, Nackman GB, et al. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J Vasc Surg. 1993;18:814-820.
18 Rose SS, Ahmed A. Some thoughts on the aetiology of varicose veins. J Cardiovasc Surg. 1986;27:534-543.
19 Cotton LT. Varicose veins. Gross anatomy and development. Br J Surg. 1961;48:589-597.