139 Venomous Snakebites in North America
• Mortality following venomous snakebites in the United States is less than 1%, especially when patients are treated with antivenom.
• Permanent local sequelae following pit viper bites occur in at least 10% of patients.
• No field management (first aid) measure has been proved to be effective for pit viper bites other than expeditious transport to definitive medical care.
• The key to management of a venomous snakebite is judicious and timely use of antivenom.
• Hypotensive victims of snakebite are treated with antivenom and intravenous fluids; vasopressors are a last resort.
• All patients with a suspected venomous snakebite who have any signs or symptoms of envenomation are admitted to the hospital.
• Fasciotomy is rarely needed following pit viper bites and is performed only in the setting of objectively documented compartment syndrome.
Perspective
The two families of dangerously venomous snakes in North America are the pit vipers (family Crotalidae, subfamily Crotalinae) and the elapids (family Elapidae). Pit vipers found in the United States are the rattlesnakes (genera Crotalus and Sistrurus), cottonmouth water moccasins (Agkistrodon piscivorus), and copperheads (Agkistrodon contortrix). Mexico has a number of species of rattlesnakes and other genera of pit vipers (e.g., Bothrops, Bothriechis, Porthidium). The only indigenous elapids in North America are the coral snakes, which are found in the southern and southwestern part of the United States and Mexico. The coral snakes of the United States belong to two different genera—Micrurus (the eastern coral snake Micrurus fulvius and the Texas coral snake Micrurus tener) and Micruroides (the Sonoran coral snake Micruroides euryxanthus). Sixteen species of coral snakes are found in Mexico.1
Epidemiology
Pit vipers inflict approximately 99% of the 7000 to 8000 venomous snakebites that occur in the United States each year.2,3 The incidence of venomous snakebite is lower in Canada and higher in Mexico, although precise numbers are hard to determine.
Pathophysiology
Snake venom, particularly pit viper venom, is a complex mixture of enzymes, low-molecular-weight (nonenzymatic) proteins, metallic ions, and other constituents.4,5 The venom is highly variable, depending on the species of snake; its geographic origin; its age, health, and diet; and the time of year.6 Among the most important components found in pit viper venom are phospholipase A2 enzymes, which cause cellular disruption and tissue damage; hyaluronidase, which facilitates the distribution of venom within tissues; and thrombin-like enzymes, which affect various aspects of the coagulation cascade and lead to coagulation abnormalities.
Also of major importance in pit viper venom–induced coagulopathy are metalloproteinases known as hemorrhagins, which increase vascular permeability and damage endothelial cells.7,8 Some rattlesnakes, such as certain specimens of Mohave rattlesnakes (Crotalus scutulatus), possess neurotoxic components in their venom. These presynaptically acting toxins prevent the release of acetylcholine at neuromuscular junctions. Depending on their geographic location, Mohave rattlesnakes may possess this component and are termed venom A–producing rattlesnakes.9,10
Other rattlesnakes that may, based on their geographic origin, possess neurotoxic components closely related to Mohave toxin include the eastern diamondback rattlesnake (Crotalus adamanteus), the timber rattlesnake (Crotalus horridus), the southern Pacific rattlesnake (Crotalus oreganus helleri), and the tiger rattlesnake (Crotalus tigris).11–15
Coral snake venom is less complex than pit viper venom but is among the most toxic venom in North America. The primary effects of coral snake venom are neurotoxic and are due to a component in the venom that blocks the postsynaptic end plates at neuromuscular junctions.16,17
Anatomy
Pit vipers get their name from the sensitive heat receptors (foveal organs) located on the anterior of their heads, slightly below and between the nostril and eye (Fig. 139.1). These organs aid the snake in finding prey, aiming its strike, and determining the volume of venom to be injected. Other characteristics typical of a pit viper include a triangular-shaped head (also found in many nonvenomous snakes), elliptical (catlike) pupils, and in the United States, the presence of a single row of scales that spans the underside of its tail (Fig. 139.2). Nonvenomous snakes in the United States generally have a double row of scales crossing the ventral aspect of the tail. Rattlesnakes can also usually be identified by the keratin plates that make up their unique caudal rattle.
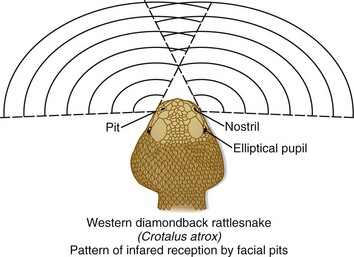
Fig. 139.1 The heat-sensing pits or foveal organs of pit vipers.
(Adapted from original drawing by Marlin Sawyer. With permission.)
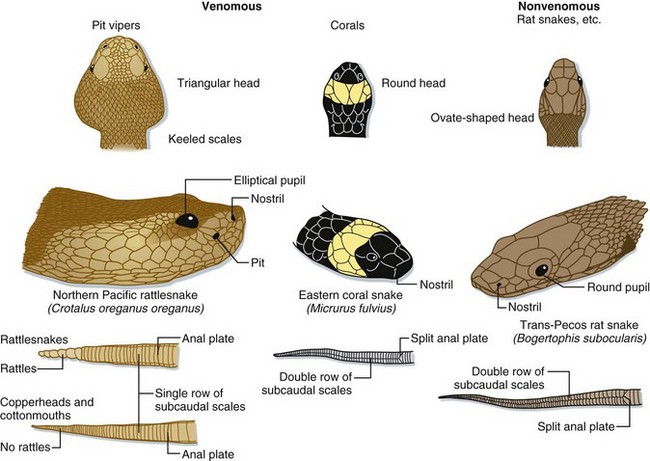
Fig. 139.2 Anatomic comparison of pit vipers, coral snakes, and nonvenomous snakes of the United States.
Pit vipers have a triangular-shaped head, elliptical pupils, heat-sensing pits, and a single row of subcaudal scales on the ventral aspect of the tail. Coral snakes are identified by their color pattern (see Fig. 139.4) because they have round pupils and a single row of subcaudal scales, as do most harmless snakes in the United States.
(Adapted from original drawing by Marlin Sawyer. With permission.)
Pit vipers range in size from the diminutive pygmy rattlesnake (Sistrurus miliarius), typically 40 to 50 cm in length, to the massive eastern diamondback rattlesnake (C. adamanteus), which can attain lengths of greater than 1.5 m (Fig. 139.3).18
Coral snakes are identified in the United States by their characteristic color pattern—red, yellow, and black bands that completely encircle the body, with the red and yellow bands being contiguous. In harmless coral snake mimics, such as milk snakes (Lampropeltis spp.), the red and yellow bands are separated by a band of black (Fig. 139.4). Color patterns cannot, however, be used outside the United States to reliably identify coral snakes.
Venomous snakes possess venom-producing glands in the upper jaw, behind the eyes (Fig. 139.5). These glands produce the venom that at the time of a bite is passed via ducts to the hollow, needle-like fangs on the anterior maxillae. In pit vipers, these fangs range in length (proportional to the snake’s overall length) from just a few millimeters to more than 2.5 cm.18 The fangs are folded up against the roof of the snake’s mouth when not in use. During a bite, the pit viper opens its mouth widely and swings its fangs into an upright position to drive them into the tissues of its target. Venom is then injected via a set of investing musculature. The speed of a pit viper’s strike has been clocked at 8 feet per second19—faster than a human being can react.
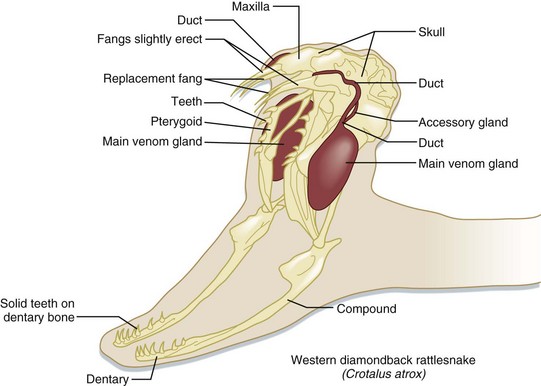
Fig. 139.5 The venom apparatus of pit vipers.
(Adapted from original drawing by Marlin Sawyer. With permission.)
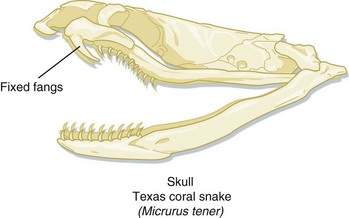
The venom delivery apparatus of a coral snake is less sophisticated than that of a pit viper. Coral snakes have slightly enlarged, anterior, maxillary fangs fixed in an erect position, with which they inject venom (Fig. 139.6). For a coral snake to produce envenomation, it must chew on its victim for a few seconds. Bites by coral snakes frequently involve someone intentionally picking the snake up, often after misidentifying it as a harmless snake.20
Presenting Signs and Symptoms
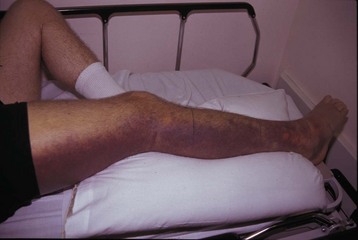
The signs and symptoms of pit viper envenomation are broken down into local and systemic findings. Locally, the victim will have puncture wounds, although the pattern may be misleading and cannot be used to reliably differentiate between a venomous bite and a bite by a harmless snake. Generally, severe, burning pain develops at the bite site within minutes, followed shortly by local swelling that can progress over time to involve the entire bitten extremity and even the trunk. There may be persistent bloody oozing from the fang marks, indicative of coagulopathy. Ecchymosis may be present at the site and, more remotely, as hemorrhagins induce vascular leaks and loss of red blood cells, in tissues (Fig. 139.7). Over several hours to days, blisters and blebs may form on the extremity, particularly at the bite site. These lesions are filled with clear serous fluid or bloody exudate.
In the early stages, hypotension is due to systemic vasodilation. Later, the hypotension is compounded by third spacing of fluids into the bitten extremity and potentially hemolysis.19 Some pit vipers, such as venom A–producing Mohave rattlesnakes, may produce few local signs and symptoms but may cause more systemic toxicity, particularly neurotoxic findings such as ptosis and difficulty swallowing and breathing.
Bites by a coral snake generally cause little in the way of local findings, and their fang marks may be difficult to see.21 Swelling is usually slight and pain is variable (mild to more pronounced). Victims of significant bites by a coral snake may have a delay in the onset of systemic findings, sometimes for many hours.22 Signs of neurotoxicity may then develop, with the earliest findings being altered mental status and cranial nerve dysfunction (e.g., ptosis). Neurotoxicity can progress to frank skeletal muscle paralysis and respiratory failure.
Diagnostic Testing
In the initial phase of evaluating a victim of a potential pit viper bite, a complete blood count and coagulation studies are repeated every 1 to 2 hours as long as the findings are normal (Table 139.1). If an abnormality appears (e.g., diminished platelets or fibrinogen), antivenom should be administered (see later) and laboratory tests rechecked every 6 hours thereafter until stable. This 6-hour period after antivenom administration is necessary for a healthy liver to replete coagulation factors.
Table 139.1 Diagnostic Testing in Victims of a Potential Venomous Snakebite in North America
| DIAGNOSTIC STUDY | VENOM EFFECTS | COMMENTS |
|---|---|---|
| Potential Pit Viper Bite | ||
| Complete blood count | ||
| White blood cell count | May be elevated without evidence of infection | Caused by stress demargination |
| Hemoglobin/hematocrit | Variable; may be normal, elevated, or decreased | May be elevated if dehydrated, may be low if bleeding or hemolysis is present |
| Platelets | May be low or very low | May see early profound thrombocytopenia |
| Metabolic panel | ||
| Renal function tests | Usually normal | May be abnormal if delayed renal dysfunction occurs |
| Hepatic function tests | Usually normal | May be abnormal with delayed hepatic dysfunction or underlying liver disease (e.g., alcohol abuse) |
| Coagulation studies | ||
| PT, APTT, INR | May be elevated | Abnormalities related to consumptive coagulopathy |
| Fibrinogen | May be reduced | |
| Fibrin degradation products | May be elevated | |
| D-dimer | May be elevated | |
| Type and screen | Venom effects and antivenom can interfere with this process23 | Obtain on first available blood drawn; blood product replacement is rarely necessary, however |
| Blood gases (arterial or venous) | May demonstrate metabolic acidosis or hypoxia, depending on the clinical scenario | Consider in cases of severe envenomation (hypotension, shock, respiratory distress) or if significant underlying comorbidity; use caution in those with coagulopathy |
| Urinalysis | Hematuria, proteinuria | Test each voided sample until the patient is stable; positive blood on bedside testing in the absence of red blood cells on microscopic analysis may indicate myoglobinuria |
| Electrocardiogram | Variable; possible ischemia | Obtain in cases of severe envenomation or in patients with significant underlying comorbidity |
| Chest radiograph | Pulmonary edema, aspiration | Obtain in cases of severe envenomation or in patients with significant underlying comorbidity |
| Computed tomography | Variable | May be indicated if evidence of intracranial, intraabdominal, or retroperitoneal bleeding in patients with consumptive coagulopathy (based on clinical findings) |
| Potential Coral Snake Bite | ||
| Complete blood count | ||
| White blood cell count | May be elevated without evidence of infection | Caused by stress demargination |
| Hemoglobin/hematocrit | Normal | |
| Platelets | Normal | |
| Metabolic panel | ||
| Renal function tests | Normal | |
| Hepatic function tests | Normal | May be abnormal if underlying liver disease (e.g., alcohol abuse) |
| Coagulation studies | All normal | |
| Type and screen | Unnecessary (no need for blood products) | |
| Blood gases (arterial or venous) | May demonstrate hypoxia and/or hypercapnia in the presence of respiratory insufficiency as a result of neurotoxicity | Consider in cases of severe envenomation (hypotension, shock, respiratory distress) or if significant underlying comorbidity is present |
| Urinalysis | Normal | |
| Electrocardiogram | Variable; possible ischemia | Obtain in cases of severe envenomation or in patients with significant underlying comorbidity |
| Chest radiograph | Usually normal; possible aspiration | Obtain in cases of severe envenomation or in patients requiring endotracheal intubation |
| Bedside pulmonary function testing | Variable; may reveal lowered peak flow in early respiratory embarrassment | May use to monitor respiratory status (at first sign of diminished flow, consider early airway control and assisted ventilation) |
APTT, Activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time
Treatment
Prehospital Management
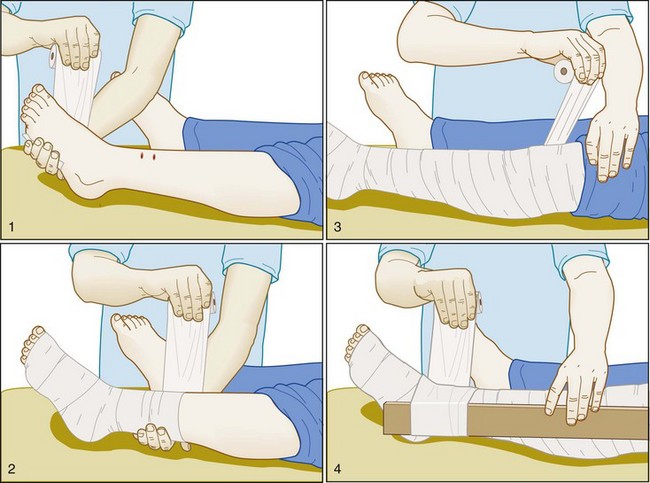
No first aid measures have ever been proved to be beneficial in victims of pit viper bites. For victims bitten by a coral snake, the Australian pressure-immobilization technique is applied quickly in the field to limit the spread of venom. This involves wrapping the entire bitten extremity snugly in an occlusive wrap followed by the application of a splint (Fig. 139.8). Victims must then be carried from the field for the technique to be effective.24 Rescuers may have difficulty applying the technique in correct fashion in that they tend to underestimate the degree of pressure that needs to be exerted under the wrap.25 The pressure-immobilization technique has been shown to significantly limit the spread of venom after elapid snakebites26 and was effective in one small animal study using eastern coral snake (M. fulvius) venom.27 Although the pressure-immobilization technique may limit the spread of pit viper venom as well, it may actually exacerbate local tissue damage by restricting tissue-destroying venom to the bite site, and it should not be used for such bites.
Hospital Management
If signs of neurotoxicity are presence and any shortness of breath or difficulty speaking or swallowing develops, the airway should be promptly and definitively secured by endotracheal intubation to prevent aspiration. If the victim shows any signs of dehydration or shock, fluid resuscitation is begun with the administration of 1 to 2 L of normal saline (20 to 40 mL/kg for children). If the blood pressure does not respond promptly to crystalloid infusion, albumin is added because it may remain in the leaky vasculature for a longer period. Vasopressors are used only as a last resort after adequate intravascular volume repletion has been achieved.28 Inadequately treated hypotension is a key aspect in many cases of fatal snake envenomation.29,30
Once the ABCs (airway, breathing, and circulation) have been addressed, an attempt is made to identify the offending snake. If the victim has brought the snake to the hospital, extreme caution should be used in examining it, even if it appears to be dead. A severed snake head can have a bite reflex for up to an hour following death and is capable of inflicting a serious bite.31–33
The key to management of significant envenomation is antivenom administration. Initial decisions on antivenom administration are guided by the clinical findings (Box 139.1).
Box 139.1 Indications for Antivenom Administration for Venomous Snakebites in North America
Pit Viper Bite
Progressive evidence of local envenomation (e.g., worsening local swelling)
Any evidence of systemic envenomation—i.e., systemic signs or symptoms (e.g., nausea or vomiting, abnormal taste, numbness or tingling, tachycardia, hypotension)
Laboratory abnormalities (e.g., low platelet count, evidence of coagulopathy)
Pit Viper Antivenom
Antivenom administration is described in detail in Box 139.2 and in the Tips and Tricks box. Antivenom is effective in reversing most systemic and laboratory derangements seen following snake envenomation. However, its ability to reverse thrombocytopenia, rhabdomyolysis, or myokymia is variable.9,35,36 Antivenom’s efficacy in preventing local tissue damage has never been definitively demonstrated. If given very early in the course, it may reduce or limit permanent local damage to some slight degree.
Box 139.2 Administration of Antivenom
Pit Viper Antivenom Administration
Obtain informed consent if possible.
Expand the patient’s intravascular volume with normal saline if safe to do so (e.g., no history of congestive heart failure).
Begin reconstitution of antivenom as soon as its need is evident because this process takes time (see the Tips and Tricks box).
Have epinephrine at the bedside, ready to administer immediately in the event of an acute reaction to the antivenom.
CroFab
Dilute the starting dose in 250 mL of normal saline.
Starting dose (children receive the same dose of antivenom as adults):
Initially administer antivenom intravenously at a slow rate with the emergency physician at the bedside to intervene in the event of an acute (anaphylactoid) adverse reaction.
If tolerated, after 5 to 10 minutes increase the rate of infusion to allow the entire dose to be administered in 1 hour.
Observe the patient for 1 hour after administration—if findings of envenomation continue to worsen, repeat the starting dose (and continue this sequence until stable; generally no more than two initial doses are required).
Once the patient’s condition is stabilized, admit the patient (to an intensive care unit if any evidence of instability, otherwise to a regular bed) for observation and further management (e.g., wound care; see text).
After stabilization, repeat the dosing of CroFab, 2 vials every 6 hours for 3 additional doses, to prevent recurrence of the effects of the venom.
Coral Snake Antivenom Administration
The basic principles (e.g., informed consent and presence of epinephrine at the bedside) are the same as for pit viper antivenom administration.
Use the manufacturer’s product information to determine proper dosing.
Victims bitten by a coral snake should be admitted to a closely observed setting to allow early detection of any deterioration.
Tips and Tricks
As soon as it is clear that antivenom is indicated, have the pharmacy or nursing staff begin reconstitution of the appropriate number of vials to be given. Reconstitution can be labor-intensive and, if the manufacturer’s recommended procedure is used, can take almost 30 minutes to prepare each vial.
By using 25 mL of sterile water per vial (as opposed to the 10 mL recommended by the manufacturer) and hand mixing via continuous rolling and inverting of the vials, the reconstitution time can be reduced to less than 2 minutes per vial.34
Coral Snake Antivenom
At the time of this writing, production of coral snake antivenom has ceased in the United States, with the current remaining stock set to expire October 31, 2012. Coral snake antivenom is still produced in Mexico (Coralmyn, Instituto Bioclon), but it is not commercially available in the United States. There is reportedly a plan in place for Pfizer to resume production of Micrurus antivenom in the United States.37
Even though the onset of neurotoxic findings following a bite by a coral snake may be delayed, once they start, they may be rapidly progressive and relatively unresponsive to antivenom administration. Management of a victim of a serious bite by a coral snake (definitively identified Micrurus specimen, particularly M. fulvius, and effective penetration of the fangs into the victim’s skin) ideally includes prompt administration of antivenom before evidence of neurotoxicity begins. In the absence of appropriate coral snake antivenom, these victims must be managed conservatively, including securing the airway at the first sign of difficulty swallowing or breathing and providing mechanical ventilation (possibly for many days). If appropriate antivenom is available, it should be administered according to the manufacturer’s guidelines, and all the precautions outlined for administration of pit viper antivenom should be taken (see Box 139.2). Pit viper antivenom is of no benefit to victims of bites by a coral snake.
No antivenom is available for Sonoran coral snake (M. euryxanthus) bites, but this species is quite small and inoffensive and has never caused a known fatality.38 Management is purely supportive.
Other Treatment Considerations
Blood products are rarely needed for victims of venomous snakebites, even with significant coagulopathy noted on laboratory tests.39 Aggressive antivenom administration always precedes the administration of any blood products to avoid feeding further substrate to an ongoing venom-induced consumptive coagulopathy.
Indications for obtaining any consultations that may be needed are listed in Box 139.3.
Box 139.3 When to Consider Consulting a Snakebite Expert*
The treating physician is uncomfortable evaluating or managing snakebite victims.
The envenomation is clearly severe (e.g., victim in respiratory distress, requiring intubation, or in shock).
The victim has significant underlying comorbid conditions (e.g., coronary artery disease, severe pulmonary disease).
No appropriate antivenom is available.
![]() Priority Actions
Priority Actions
Ensure that the airway, breathing, and circulation are adequate.
Begin intravenous crystalloid infusion in patients who are volume depleted or hypotensive.
Assess the severity of the envenomation and remain vigilant for signs of progression.
Measure and record the circumferences of the bitten extremity every 15 minutes during the early stages to assess for progression of local findings.
Send blood for appropriate screening laboratory tests, including blood typing and screening (see Table 139.1).
Begin the process of locating and obtaining appropriate antivenom as soon as possible.
To avoid delays in administration, begin reconstitution of antivenom as soon as it is clear that treatment is needed.
Seek consultation with an expert in snake envenomation as needed (see Box 139.3).
Next Steps: Admission and Discharge
All patients with any evidence of envenomation are admitted to the hospital for further observation and management. If the envenomation is severe, the victim is admitted to an intensive care unit. If it appears that the snake did not inject any venom (i.e., a dry bite—no signs or symptoms of envenomation, simple puncture wounds only), the victim is observed in the emergency department for a minimum of 8 hours. After 8 hours, victims who remain asymptomatic, with normal vital signs and laboratory results, are discharged in the care of a responsible adult and told to return if any evidence of envenomation occurs. Dry bites occur in as many as 20% of pit viper bites, although the precise reason for this remains unclear.6,40 Any child with a possible venomous snakebite is admitted to the hospital regardless of the presence or absence of signs or symptoms of poisoning. Likewise, victims of a possible bite by a coral snake are admitted for observation because of the significant delay that can occur before any evidence of envenomation is apparent.
![]() Documentation
Documentation
Record a careful history in the patient’s own words, including a description of the snake.
Record the results of a thorough screening physical examination.
Have nursing staff measure and record the circumferences of the bitten extremity every 15 minutes until the swelling has stabilized and then every hour for 24 hours.
Note the specifics of any discussion with consultants (e.g., poison control specialists).
Measure and record intracompartmental pressures if concern arises about the development of compartment syndrome.
Document informed consent (for antivenom administration or fasciotomy) if possible.
Complications
Adverse Reactions to Antivenom
Adverse reactions to antivenom include early, acute reactions (which are probably anaphylactoid in nature [also known as nonallergic anaphylaxis]) and delayed serum sickness, an immunoglobulin (IgG and IgM) immune complex–mediated disease. Anaphylactoid reactions may be manifested as hives, wheezing, laryngeal edema, abdominal pain, vomiting, diarrhea, and hypotension. The incidence of anaphylactoid reactions to CroFab is approximately 15%,41 with most reactions being minor and easily treated (Box 139.4).42 Serum sickness occurs in approximately 3% of patients treated with CroFab41 and may be manifested approximately 1 to 2 weeks after administration as fever, urticaria, myalgia, arthralgia, renal dysfunction, or neuropathy (or any combination of these signs). Serum sickness is easily treated with oral steroids (e.g., prednisone, 1 to 2 mg/kg orally per day) administered until the symptoms resolve, followed by a 2-week tapering of the dose. Oral antihistamines may provide additional symptomatic relief. Before discharge from the hospital, patients are warned to watch for signs and symptoms of serum sickness and told to return if they occur.
Box 139.4 Management of Acute Reactions to Antivenom (Allergic or Nonallergic Anaphylaxis)
Immediately stop the infusion.
Treat the signs and symptoms in standard fashion (airway management as needed, epinephrine, crystalloid infusion, antihistamines [H1 and H2 blockers], steroids, and vasopressors as needed).
In most cases the antivenom can be restarted (with the emergency physician at the bedside) once the reaction abates. If the reaction was severe and appears more life-threatening than the envenomation, a decision may be made to withhold further antivenom therapy. It is very helpful to consult an expert on snake envenomation in such cases.
Compartment Syndrome
Some larger rattlesnakes have fangs long enough to penetrate deeply into muscle compartments. Because many significant pit viper bites result in swollen extremities that are discolored and painful on any attempted range of motion, it can be difficult to determine when a compartment syndrome is imminent. This complication is actually rare following pit viper bites, but if there is concern about rising intracompartmental pressure, it is objectively measured with any standard technique. If the pressure is elevated above 30 to 40 mm Hg, the limb is further elevated, more antivenom is administered, and mannitol is given (1.0 g/kg intravenously over a 30-minute period) if the patient is hemodynamically stable.43 If the compartment pressure remains high 1 hour after these measures have been carried out, fasciotomy is considered. Although animal research has suggested an actual increase in myonecrosis following fasciotomy,44 there is still a risk for ischemic neuropathy following unabated rises in compartment pressure. It is important to obtain informed consent, if possible, before proceeding with fasciotomy.
Recurrent Coagulopathy
Patients in whom coagulopathy develops during the initial stages of pit viper envenomation may have delayed recurrence of abnormal coagulation studies for up to 2 weeks following the bite.42,45 This is probably related to continued absorption of venom components from the depot site after all antivenom administered has been cleared from the body, particularly when a low-molecular-weight Fab antivenom (e.g., CroFab) is used. In most cases, delayed coagulopathy is benign, without evidence of clinically significant bleeding, but severe complications, including intracranial bleeding, have been reported.46,47 Patients are warned of this possibility and instructed to avoid any elective surgery, contact sports, and other high-risk activities for a few weeks following the bite. Although administration of antivenom for delayed coagulopathy may be helpful, it is likely to be less effective than in the acute stages of envenomation. Reasonable indications for administering further antivenom in these cases are outlined in Box 139.5.
Box 139.5 Indications for Administering Further Antivenom to Patients with Delayed Coagulopathy
Patients found on follow-up to have recurrent coagulopathy following a pit viper bite should receive further antivenom dosing in any of the following situations48:
APTT, Activated partial thromboplastin time; INR, international normalized ratio.
![]() Patient Teaching Tips
Patient Teaching Tips
Never approach, torment, or attempt to catch or kill a venomous or unidentified species of snake.
Do not keep any species of venomous snake as a “pet.”
Never handle a venomous snake that you believe is dead. These snakes may still have a bite reflex and can inflict a serious or fatal bite.
If bitten by a venomous or unknown snake, proceed immediately to the nearest hospital for evaluation. No first aid measures are effective or necessary following bites by pit vipers.
If you have been treated for a venomous snakebite with antivenom, watch for signs of serum sickness (fever, muscle aches, joint aches, hives, or bleeding) in the first 2 weeks after you leave the hospital. If such signs occur, follow up immediately with your physician.
Avoid any elective surgery, contact sports, or high-risk activities for 2 to 3 weeks after a pit viper bite because your blood may not clot normally during this period.
![]() Facts and Formulas
Facts and Formulas
Measurement of Intracompartmental Pressure
If lower than 30 to 40 mm Hg, continue antivenom as indicated along with limb elevation and monitoring.
If higher than 30 to 40 mm Hg, give additional antivenom, keep the limb elevated, administer mannitol (1 g/kg intravenously if the patient’s hemodynamic status allows), observe for 1 hour, and then recheck intracranial pressure.
If the pressure falls below 30 to 40 mm Hg, continue nonoperative treatment. If intracranial pressure remains elevated, consider fasciotomy (with informed patient consent).
![]() Red Flags
Red Flags
Cautions for the Physician
Remain vigilant for worsening severity of venom poisoning; it can progress rapidly and suddenly. Conversely, the onset of clinical findings may be delayed for hours.
Obtain consultation early from a clinician knowledgeable in snake envenomation (e.g., a regional poison control center specialist) whenever needed.
Obtain informed consent whenever possible before giving antivenom.
Give antivenom as soon as possible after its need is identified.
Remain at the patient’s bedside during the initiation of antivenom therapy to intervene if an acute reaction occurs.
Have epinephrine available at the bedside before beginning antivenom infusion.
If a compartment syndrome is suspected, obtain objective measurements of intracompartmental pressure.
If fasciotomy is indicated, obtain and document informed consent.
Cases involving snake envenomation in the United States often end up in medicolegal litigation. Document carefully.
Prognosis
Deaths attributable to bites by endemic snakes are rare in the United States, with only 33 reported to the American Association of Poison Control Centers in the 26-year period from 1983 to 2008.1 This is an underestimate of the total number of deaths because snakebite is not a reportable condition, but it does reflect the limited toll of snakebite in terms of loss of life in the United States. Deaths are even more infrequent in Canada, whereas in Mexico, as many as 150 people die each year of venomous snakebites.43 The case fatality rate following venomous snakebite in the United States when antivenom is used is less than 1%.49 However, long-term complications, such as loss of some degree of function in the bitten extremity, are more likely. Approximately 10% of victims will be left with some functional disability following pit viper bites, and this does not include permanent dysfunction directly related to surgical procedures.43 The incidence of disability may actually be higher if careful, delayed evaluation of extremity function is performed (e.g., using goniometry and precise sensory testing).50
Cannon R, Ruha AM, Kashani J. Acute hypersensitivity reactions associated with administration of Crotalidae polyvalent immune Fab antivenom. Ann Emerg Med. 2008;51:407–411.
Kitchens C, Eskin T. Fatality in a case of envenomation by Crotalus adamanteus initially successfully treated with polyvalent ovine antivenom followed by recurrence of defibrinogenation syndrome. J Med Toxicol. 2008;4:180–183.
Norris RL, Bush SP, Smith J. Bites by venomous reptiles in Canada, the U.S. and Mexico. Auerbach PS, ed. Wilderness medicine, 6th ed, Philadelphia: Saunders, 2012.
Seifert SA, Boyer LV, Benson BE, et al. AAPCC database characterization of native U.S. venomous snake exposures, 2001-2005. Clin Toxicol (Phila). 2009;47:327–335.
1 Campbell JA, Lamar WM. The venomous reptiles of the western hemisphere. Ithaca, NY: Comstock; 2004.
2 Norris RL, Bush SP, Smith J. Bites by venomous reptiles in Canada, the U.S. and Mexico. Auerbach PS, ed. Wilderness medicine, 6th ed, Philadelphia: Saunders, 2012.
3 Russell FE. AIDS, cancer, and snakebite—what do these three have in common? West J Med. 1988;148:84–85.
4 Dart RC, Gold BS. Crotaline snakebite. In: Dart RC, Caravati EM, McGuigan MA, et al. Medical toxicology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:1559–1565.
5 Kamiguti AS. Platelets as targets of snake venom metalloproteinases. Toxicon. 2005;45:1041–1049.
6 Russell FE. Snake venom poisoning. New York: Scholium International; 1983.
7 Gutierrez JM, Rucavadoa R, Escalantea T, et al. Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005;45:997–1011.
8 White J. Snake venoms and coagulopathy. Toxicon. 2005;45:951–967.
9 Clark RF, Williams SR, Nordt SP, et al. Successful treatment of crotalid-induced neurotoxicity with a new polyspecific crotalid Fab antivenom. Ann Emerg Med. 1997;30:54–57.
10 Jansen PW, Perkin RM, Van Stralen D. Mojave rattlesnake envenomation: prolonged neurotoxicity and rhabdomyolysis. Ann Emerg Med. 1992;21:322–325.
11 Glenn JL, Straight RC. Venom characteristics as an indicator of hybridization between Crotalus viridis viridis and Crotalus scutalatus scutulatus in New Mexico. Toxicon. 1990;28:857–862.
12 Glenn JL, Straight RC, Wolt TB. Regional variation in the presence of canebrake toxin in Crotalus horridus venom. Comp Biochem Physiol. 1994;107:337–346.
13 Hendon RA, Bieber AL. Presynaptic toxins from rattlesnake venoms. In: Tu AT, ed. Rattlesnake venoms: their actions and treatment. New York: Marcel Dekker, 1982.
14 Weinstein SA, Minton SA, Wilde CE. The distribution among ophidian venoms of a toxin isolated from the venom of the Mojave rattlesnake (Crotalus scutalatus scutulatus). Toxicon. 1985;23:825–844.
15 French WJ, Hayes WK, Bush SP, et al. Mojave toxin in venom of Crotalus helleri (southern Pacific rattlesnake): molecular and geographic characterization. Toxicon. 2004;44:781–791.
16 Chang CC. The action of snake venoms on nerve and muscle. In: Lee CY, ed. Snake venoms. New York: Springer-Verlag, 1979.
17 Van Mierop LHS. Poisonous snakebite: a review—snakes and their venom. J Fla Med Assoc. 1976;63:191–200.
18 Klauber LM. Rattlesnakes: their habits, life histories, and influence on mankind, 2nd ed. Berkeley, CA: University of California Press; 1997.
19 Wingert WA, Wainschel J. Diagnosis and management of envenomation by poisonous snakes. South Med J. 1975;68:1015–1026.
20 Pettigrew LC, Glass JP. Neurologic complications of a coral snake bite. Neurology. 1985;35:589–592.
21 Norris RL, Dart RC. Apparent coral snake envenomation in a patient without fang marks. Am J Emerg Med. 1989;7:402–405.
22 Kitchens CS, Van Mierop LHS. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615–1618.
23 Van Mierop LH, Kitchens CS. Defibrination syndrome following bites by the eastern diamondback rattlesnake. J Fla Med Assoc. 1980;67:21–27.
24 Howarth DM, Southee AE, Whyte M. Lymphatic flow rates and first-aid in simulated peripheral snake or spider envenomation. Med J Aust. 1994;161:695–700.
25 Norris RL, Ngo J, Nolan K, et al. Physicians and lay people are unable to apply pressure immobilization properly in a simulated snakebite scenario. Wilderness Environ Med. 2005;16:16–21.
26 Sutherland SK, Coulter AR, Harris RD. Rationalisation of first-aid measures for elapid snakebite. Lancet. 1979;1:183–185.
27 German BT, Hack JB, Brewer K, et al. Pressure-immobilization bandages delay toxicity in a porcine model of eastern coral snake (Micrurus fulvius fulvius) envenomation. Ann Emerg Med. 2005;45:603–608.
28 Schaeffer RC, Carlson RW, Puri VK, et al. The effects of colloidal and crystalloidal fluids on rattlesnake venom shock in the rat. J Pharmacol Exp Ther. 1978;206:687–695.
29 Dart RC, McNally JT, Spaite DW. The sequelae of pit viper poisoning in the United States. In: Campbell JA, Brodie ED. Biology of the pitvipers. Tyler, Tex: Selva, 1992.
30 Hardy DL, Bush SP. Pressure/immobilization as first aid for venomous snakebite in the United States. Herpetol Rev. 1998;29:204–208.
31 Carroll RR, Hall EL, Kitchens CS. Canebrake rattlesnake envenomation. Ann Emerg Med. 1997;30:45–48.
32 Kitchens CS, Hunter S, Van Mierop LHS. Severe myonecrosis in a fatal case of envenomation by the canebrake rattlesnake (Crotalus horridus atricaudatus). Toxicon. 1987;25:455–458.
33 Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead [letter]. N Engl J Med. 1999;340:1929.
34 Quan AN, Quan D, Curry SC. Improving Crotalidae polyvalent immune Fab reconstitution times. Am J Emerg Med. 2010;28:593–595.
35 Bond RG, Burkhart KK. Thrombocytopenia following timber rattlesnake envenomation. Ann Emerg Med. 1997;30:40–44.
36 Bush SP, Wu VH, Corbett SW. Rattlesnake venom–induced thrombocytopenia response to antivenom (Crotalidae) polyvalent. Acad Emerg Med. 2000;7:181–185.
37 Jones A. Pfizer is building two new facilities in Richland Township. Kalamazoo Gazette. Available at http://www.mlive.com/business/west-michigan/index.ssf/2010/11/pfizer_building_two_new_facili.html Accessed December 2, 2010
38 Davidson TM, Eisner J. United States coral snakes. Wilderness Environ Med. 1996;1:38–45.
39 Burgess JL, Dart RC. Snake venom coagulopathy: use and abuse of blood products in the treatment of pit viper envenomation. Ann Emerg Med. 1991;10:795–801.
40 Parrish HM, Goldner JC, Silberg SL. Poisonous snakebites causing no venenation. Postgrad Med. 1966;39:265–269.
41 Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347–356.
42 CroFab full prescribing information. Brentwood, Tenn: Protherics, Inc., 2010. Available at http://www.crofab.com/pdf/CroFab_PI.pdf Accessed November 28, 2010
43 Gomez HF, Dart RC. Clinical toxicology of snakebite in North America. In: Meier J, White J. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, Fla: CRC Press, 1995.
44 Tanen DA, Danish DC, Grice GA, et al. Fasciotomy worsens the amount of myonecrosis in a porcine model of crotaline envenomation. Ann Emerg Med. 2004;44:99–104.
45 Bogdan GM, Dart RC, Falbo SC, et al. Recurrent coagulopathy after antivenom treatment of crotalid snakebite. South Med J. 2000;93:562–566.
46 Kitchens C, Eskin T. Fatality in a case of envenomation by Crotalus adamanteus initially successfully treated with polyvalent ovine antivenom followed by recurrence of defibrinogenation syndrome. J Med Toxicol. 2008;4:180–183.
47 O’Brien NF, DeMott MD, Suchard JR, et al. Recurrent coagulopathy with delayed significant bleeding after crotaline envenomation. Pediatr Emerg Care. 2009;25:457–459.
48 Boyer LV, Seifert SA, Cain JS. Recurrence phenomena after immunoglobulin therapy for snake envenomations: part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann Emerg Med. 2001;37:196–201.
49 Dart RC, McNally J. Efficacy, safety, and use of snake antivenoms in the United States. Ann Emerg Med. 2001;37:181–188.
50 Simon TL, Grace TG. Envenomation coagulopathy from snake bites. N Engl J Med. 1981;305:1347–1348.