Chapter 20
Venography
David L. Gillespie, Xzabia A. Caliste
Contrast-enhanced venography has been practiced for more than half a century. In 1940, Bauer published details of normal venographic anatomy and the venographic appearance of acute and chronic deep venous thrombosis (DVT).1 Early attempts at venography were marked by complications related to the equipment, contrast agents used at that time, and incomplete understanding of venous hemodynamics. Since 1960, the procedure has become the leading diagnostic test for DVT.2 The technique evolved during many years and became the “gold standard” for the diagnosis of DVT after Rabinov and Paulin published their seminal article on contrast-enhanced venographic technique in 1972.3 Most current techniques are modifications of their approach to venography. Early leg venography required venous cutdown for access to the deep venous system, but this technique was modified by Welch and coworkers, who recommended that contrast material be injected into a superficial vein in the foot with a tourniquet applied above the ankle to prevent filling of the superficial veins, which often obscured the deep system.4
Basic Principles
The two primary techniques of venography that we employ today are ascending and descending venography. Ascending venography is more common and is used to elucidate the presence of DVT in the lower extremity. Descending venography predominantly evaluates incompetent valves in patients with chronic venous insufficiency. This requires direct access to the deep venous system. The techniques of ascending and descending venography are described in the next section on clinical applications.
Clinical Applications
The current indications for venography are detailed in Box 20-1. Despite the availability of less invasive techniques, venography is still considered the gold standard against which all examination modalities are compared to determine the presence of DVT. This is particularly true in instances in which duplex ultrasound is inconclusive or unavailable. Venography also continues to have a higher sensitivity and specificity in detection of infrapopliteal thrombotic disease. Venography is indicated in evaluation of valvular insufficiency and venous malformations, and it has been shown to be helpful in planning adjunctive techniques in the operating room. Relative contraindications to venography are also listed in Box 20-1. Other common modalities used in detection of thrombosis and reflux are compared in Table 20-1.
Table 20-1
Comparison of Various Modalities for the Detection of Venous Disease
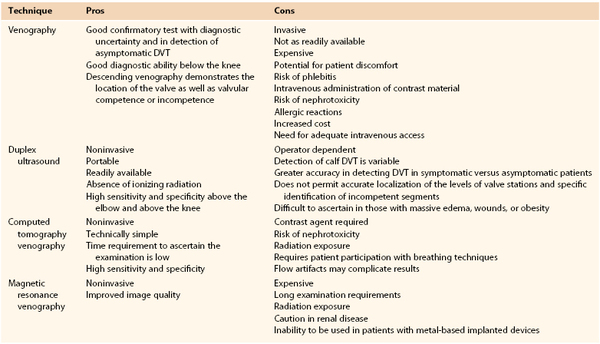
DVT, Deep venous thrombosis.
Ascending Venography
Ascending venography has been used for many indications, including DVT, incompetent perforating veins, and venous aneurysms and malformations.
Deep Venous Thrombosis
DVT cannot be reliably diagnosed by clinical examination. Indications for ascending leg venography include a nondiagnostic ultrasound study and a need for better imaging of calf veins in the setting of high clinical suspicion of DVT.5 Because of its high diagnostic accuracy, a leg venogram is immediately useful for evaluation of the presence of deep venous disease. Another indication is delineation of DVT before endovascular intervention, such as thrombolysis, thrombectomy, or angioplasty.
There are a number of problems with venography, such as inability to cannulate the appropriate vein because of edema, incomplete venous filling, and other technical issues, all of which lead to inadequate studies in as many as 5% to 15% of cases.6,7
Technique.
Ascending venography requires a 22-gauge catheter, three 25-mL syringes, two tourniquets, a tilting fluoroscopic table with footrest, C-arm fluoroscopy, contrast agent, and normal saline. In addition, a 5F sheath should be available for direct access to the venous system through the popliteal vein if needed. It begins with placement of a catheter in a peripheral vein. The access device is usually a 22-gauge intravenous catheter inserted into a superficial vein on the dorsum of the foot. When the affected extremity has significant edema, percutaneous catheterization of peripheral veins becomes difficult. In such cases, we use ultrasound to guide vascular access. It is important that access for ascending venography not be through the saphenous vein adjacent to the medial malleolus at the ankle because contrast material may preferentially fill the superficial system without demonstrating the deep venous systems adequately. The likelihood of this problem can be decreased by placing a tourniquet at the ankle and one at the knee, which will drive the contrast material into the deep venous system. However, these tourniquets should be released just before image acquisition to relieve extrinsic compression and to avoid occlusion of the deep veins.
The patient is placed on a tilting fluoroscopic table and a footrest to enable near-upright positioning. With the patient initially in a reverse Trendelenburg position, the table is tilted to recumbency as the bolus is tracked. This approach enhances visualization of the large-capacity lower extremity venous anatomy as gravity delays the outflow of contrast material. The contralateral leg is supported with a small platform so that no weight is borne on the leg to be examined. Side grip handles can be placed on the table as needed, depending on the patient’s comfort and security in the semiupright position.
The examination is begun with the patient in a 40- to 60-degree semiupright position. As contrast material is injected, fluoroscopic evaluation is performed to ensure that there has been no extravasation of contrast and to optimize deep vein opacification. The bolus is followed by radiographic imaging as the material flows to the central veins. As the contrast agent ascends, additional filming of the deep and superficial femoral veins is performed. At least two projections are needed in the tibial and popliteal locations. About 50 to 100 mL of contrast material is needed to fill the deep venous system adequately from ankle to groin. Additional contrast material can be injected, as needed, to visualize problem areas.
Even with appropriate venous access and the use of tourniquets, adequate opacification of the deep veins of the lower extremities may be difficult because of dilution of the contrast agent and preferential flow from deep to superficial regions, which allows contrast material to escape to the superficial system. In such cases, we place the patient in the prone position, access the popliteal vein under ultrasound guidance, insert a 5F sheath, and inject contrast material into the deep venous system. At completion of the examination, the veins are flushed with 50 mL of normal saline to minimize contact of the contrast agent with the venous endothelium and the chance for development of thrombophlebitis.
Interpretation.
The classic venographic sign of venous thrombosis is a luminal filling defect with a surrounding rim of contrast (Figs. 20-1 to 20-6). This appearance of parallel lines of contrast material around the thrombus is referred to as the “tram-track” sign. Other indicators of thromboembolism are an abrupt termination of intravascular contrast or the formation of a meniscus. Correct and consistent interpretation of lower extremity venography to rule out DVT, however, may be difficult. Intraobserver disagreement about the probable presence or absence of thrombus has been shown to occur in up to 10% of venographic cases.8 The invasive nature of the technique, the exposure to radiation, and the upsurge of ultrasound technology have made venography a secondary technique for the detection of acute and chronic venous thrombosis.
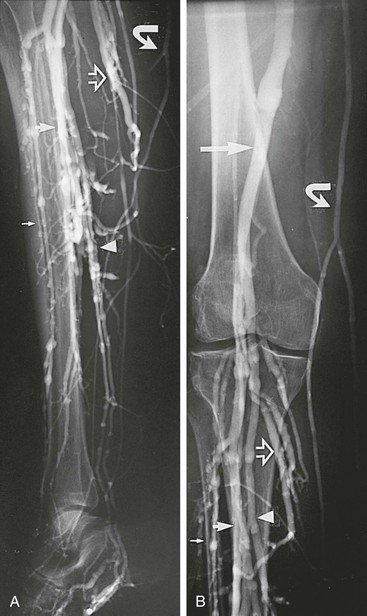
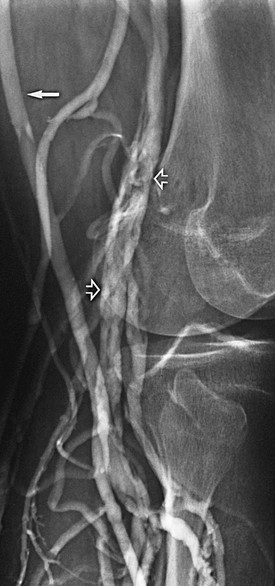
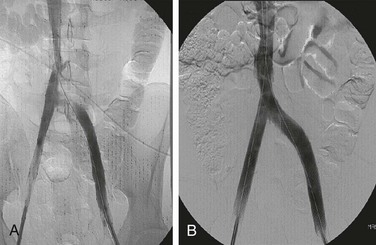
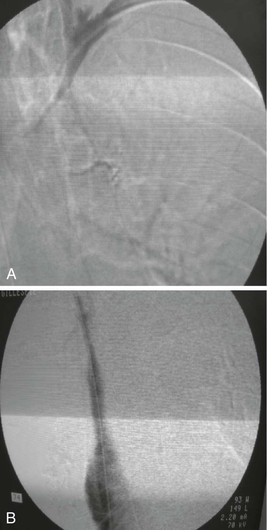
Figure 20-1 Normal right leg venogram. A, Lateral calf projection. B, Anteroposterior projection of the knee. The anterior tibial veins (small arrow), peroneal veins (short arrow), posterior tibial veins (arrowhead), gastrocnemius veins (open arrow), great saphenous vein (curved arrow), and popliteal vein (long arrow) are indicated.
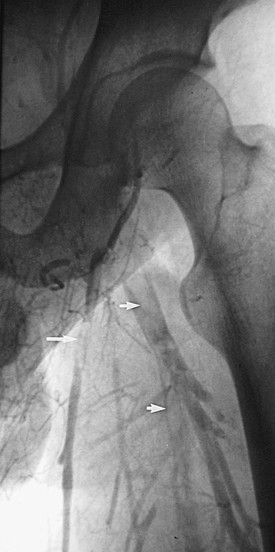
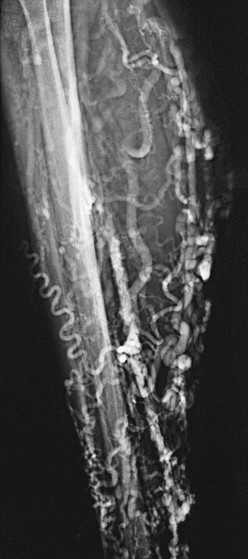
Figure 20-2 Acute thrombus in the deep femoral and great saphenous veins. The deep femoral vein (short arrows) and the great saphenous vein (long arrow) are shown. The femoral vein is occluded.
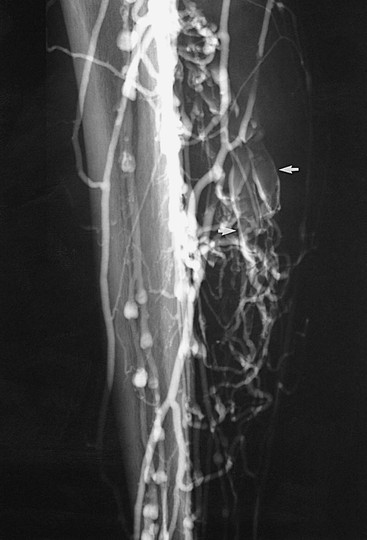
Figure 20-3 Acute thrombosis of the soleal veins. Arrows indicate fresh thrombus filling the soleal veins.
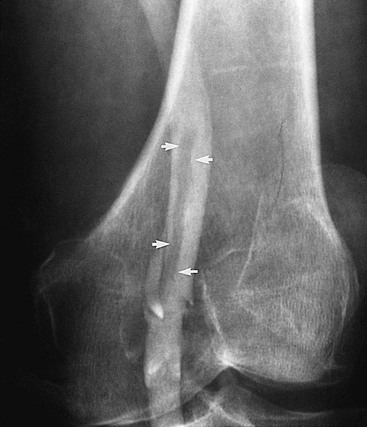
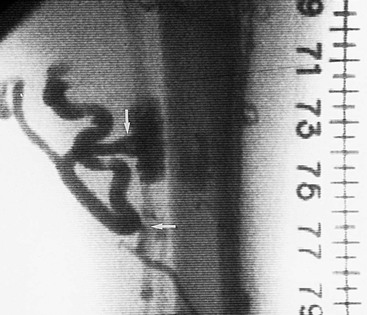
Figure 20-4 Subacute thrombus in the popliteal vein. Arrows denote retracted thrombus, indicative of subacute thrombosis.
Incompetent Perforating Veins
The normal outflow from veins of the lower extremity includes pathways from the superficial system to the deep system through perforating veins. Perforating veins possess unidirectional valves, which, when competent, prevent reflux of blood from the deep into the superficial veins of the leg. Perforating veins with incompetent valves contribute to the development of venous hypertension in the superficial system, which results in the formation of varicose veins and venous ulceration.
Normal perforating veins are small, thin, and smooth. Incompetent veins, however, are dilated, irregular, and valveless. Historically, ascending venography has been a reliable technique for identifying incompetent perforating veins. This invasive technique, although useful, has largely been replaced by color-flow duplex examination of the lower extremity.
Technique.
Ascending venography for incompetent perforating veins involves the injection of contrast material into a foot vein with a tight tourniquet around the ankle to occlude the superficial veins such that reverse flow from the deep veins into the superficial veins through the incompetent perforating veins can be seen. A translucent ruler is then used to measure the location of the incompetent perforating vein from the tip of the medial or lateral malleolus. The table is next moved through the horizontal position and then into Trendelenburg position, and flow of contrast material is followed under fluoroscopy. Lateral views are especially important in the thigh for accurate localization of a midthigh perforating vein. Once a perforating vein is identified, a tourniquet can be used above that level to direct the contrast agent into any incompetent perforating vein.
Interpretation.
Thomas and Bowles compared ascending venography with varicography for identification of incompetent perforating veins.9 Sixty-one legs of 50 patients were examined with both methods. Incompetent perforating veins of the gastrocnemius muscle and midthigh were more accurately shown with varicography than with ascending venography (Fig. 20-7).
Venous Aneurysms and Malformations
The most serious complication associated with lower extremity venous aneurysms is the development of thrombus within the aneurysm and subsequent embolization. Pulmonary embolization has been reported to occur frequently in extremity venous aneurysms, most notably in those involving the popliteal vein (Fig. 20-8). Aldridge and coauthors in 1993 reported 24 cases of popliteal venous aneurysm in which all patients had experienced thromboembolic events.10 Aggressive treatment with immediate anticoagulation and preoperative planning has become the standard of care for this reason. Venography is a useful method for preoperative planning before resection and interposition grafting or lateral suture repair of these aneurysms.
Venous malformations may take the form of isolated venous lakes or diffuse venous aneurysm formation, such as Klippel-Trénaunay syndrome (Fig. 20-9). Patients often become symptomatic from mass effect, reflux, or venous thromboembolism. Operative or interventional management may be indicated in symptomatic patients. Most patients do well without treatment or with elastic compression only. Surgical treatment of the vascular malformation in Klippel-Trénaunay syndrome is rarely needed and continues to be controversial.11 Preoperative venous imaging with ascending venography or direct stick varicography is useful for planning treatment.
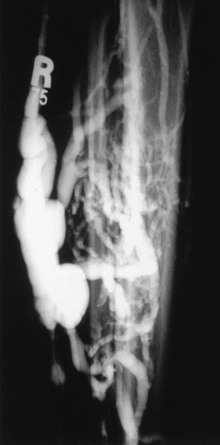
Figure 20-9 Tibial venous malformation in a patient with Klippel-Trénaunay syndrome. (From Gillespie DL, et al: Presentation and management of venous aneurysms. J Vasc Surg 26:845-852, 1997.)
Technique.
The technique used is the same as when ascending venography is performed. Direct stick varicography is most useful for the evaluation of venous malformations (see Figs. 20-8 and 20-9). Ultrasound-guided access provides the ability to perform direct stick venography. In patients being treated for venous malformations, access can be difficult and painful. In such cases, either general or regional anesthesia may be useful.
Interpretation.
Focal dilatation of the deep or superficial venous system is diagnostic of venous aneurysms and malformations (see Figs. 20-8 and 20-9). Because of the small number of venous aneurysms included in most individual series, no single diagnostic method has been reported to be superior to another. Most series, however, do report color-flow duplex examination to be extremely valuable in the diagnosis of popliteal venous aneurysms and to provide information on the presence of mural thrombus.12 Gillespie and associates found in their series that venous aneurysms were correctly diagnosed in 85% of patients with imaging techniques: phlebography (60%), color-flow duplex scanning (27%), and magnetic resonance imaging (10%).13
Passive filling of the vascular tree after compressive exsanguination of an extremity, extrinsic occlusion of its arterial supply, and venous drainage has been shown to be a useful technique for evaluation of venous malformations.14 Closed-system venography was performed in 17 patients, and it correctly identified 11 of 12 surgically confirmed vascular abnormalities. There were no false-positive findings.
Descending Venography
As with ascending venography, lower extremity descending venography is no longer used as a screening study. It has largely been replaced by color-flow duplex study, which has shown good agreement with descending venography in the grading of deep and superficial vein reflux.15 However, duplex examination is limited in that it does not permit accurate localization of the levels of valve stations and specific identification of incompetent segments. For this reason, descending venography remains the definitive test for identifying incompetent valves in patients with chronic venous insufficiency who are candidates for venous valve repair or valve transplantation.
Descending venography is used for evaluation of the anatomy and function of the venous valves in the lower extremities. It shows the location of the valves in the veins and also demonstrates the competence or incompetence of these valves. Kistner16 and Herman17 and colleagues developed a classification system used commonly to categorize the severity of deep venous reflux and the functional integrity of the venous valves (Table 20-2).
Table 20-2
Grades of Venous Reflux
| Grade | Description |
| 0 | Normal valvular function with no reflux |
| 1 | Minimal reflux confined to the upper part of the thigh |
| 2 | More extensive reflux, which may reach the lower part of the thigh; a competent valve is present in the popliteal vein, and there is no reflux to the calf level |
| 3 | Reflux as above but associated with popliteal valvular incompetence and leakage of contrast material into the calf veins |
| 4 | Virtually no valvular competence with immediate and dramatic reflux distally into the calf; this type of reflux often opacifies incompetent calf perforating veins |
From Kistner RL, et al: A method of performing descending venography. J Vasc Surg 4:464-468, 1986; and Herman RJ, et al: Descending venography: a method of evaluating lower extremity venous valvular function. Radiology 137:63-69, 1980.
Technique.
Descending venography requires a tilt radiographic table, C-arm fluoroscopy, a short 4F or 5F straight catheter with distal side holes, syringes, and contrast agent. It is performed on a tilt radiographic table to allow examination in the 40- to 60-degree upright position. Descending venography always requires direct catheter access into the deep veins. In most cases, the venous access site is the common femoral vein contralateral to the side of interest. The catheter is advanced to the inferior vena cava (IVC) and then to the iliac vein and common femoral vein of the symptomatic lower extremity. A short 4F or 5F straight catheter with distal side holes is positioned at the junction of the external iliac vein and common femoral vein (Fig. 20-10). For unilateral examination, the catheter is placed from the ipsilateral femoral vein, and for bilateral examination, a single femoral vein puncture is used. A transjugular approach may be used to direct the retrograde catheter into both lower extremities from a single access site. The contralateral leg is supported with a small platform, as with ascending venography, so that the leg being examined will be relaxed and bear no weight.
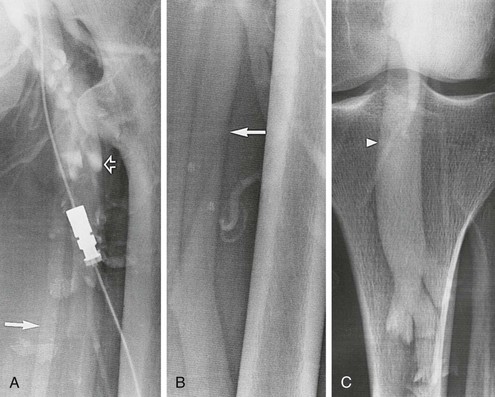
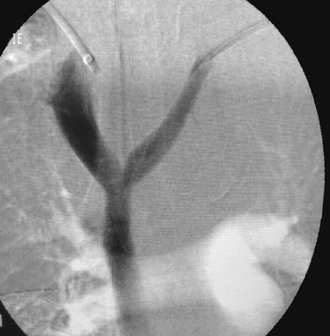
Figure 20-10 A-C, Descending left leg venogram showing grade 4 reflux. Note the lack of valves in the femoral vein (solid arrow) and the large caliber of the popliteal vein (arrowhead). The deep femoral vein (open arrow) is shown.
With the catheter tip in the common femoral vein, the table tilted to a 60-degree semiupright position, and the patient bearing weight on a block placed under the nonexamined leg, contrast material is injected and tracked by fluoroscopy as it flows caudad through the incompetent venous valves. A total of 10 mL of contrast material is slowly injected by hand at a rate of 5 mL/s. As the contrast material is injected, the patient breathes normally, after which the patient is instructed to bear down and perform a Valsalva maneuver to enhance evidence of valvular incompetence. Specific areas of interest can be further studied by sequential injections of contrast material to optimize image capture of valve location and function and the extent of reflux.
Interpretation.
Although superficial varicosities are frequently present, they are rarely a prominent feature of symptomatic advanced chronic venous disease.18 Most patients with symptoms of chronic venous insufficiency who undergo venography have reflux into the deep venous system. The two main abnormalities that cause venous valve reflux are postphlebitic valvular incompetence and primary valvular incompetence, with primary valvular incompetence being responsible more frequently.19 With the use of descending venography, candidates are selected for deep venous valve repair or transplantation, which is offered to patients with grade 3 or grade 4 reflux who have recurrent symptoms of venous insufficiency after treatment of superficial varicosities and perforating vein incompetence (see Fig. 20-10 and Table 20-2).
Iliac Vein Assessment and Inferior Venacavography
IVC anatomy and patency are routinely assessed as part of IVC filter placement. Phlebography allows assessment of caval anatomy and evaluation for the presence of caval thrombus. In addition, there are several anomalies of the IVC, as outlined in previous chapters. Duplicated cavae, megacavae, and left-sided venae cavae are but a few of these anomalies. Although these anomalies are uncommon, it is important to routinely assess the vena cava with venography before placement of IVC filters to avoid complications caused by either filter misplacement or leaving the patient vulnerable to pulmonary embolism through alternative pathways.
Technique
Access to the venous system is achieved through the internal jugular or common femoral vein. Puncture of just a single wall is facilitated by ultrasound guidance, and a micropuncture kit is used to minimize access size. Once access is achieved, a 5F sheath is placed and proper placement confirmed by free flow of venous blood from the sheath.
From the groin, a 0.035-inch J wire is passed into the common iliac vein. A 5F pigtail catheter is advanced over this wire into the common iliac vein, and cavography is performed.
An alternative method of cavography is to proceed with placement of the IVC filter kit, which consists of a sheath, usually 8F. The insertion track is serially dilated from 5F to 8F. The 8F sheath is advanced over the wire to the level of the common iliac vein, and a power-injected cavogram is performed.
From the neck, a 0.035-inch J wire is passed through the right atrium to the common iliac vein. Passing the J wire through the right atrium into the IVC can be somewhat of a challenge, however. If simple manipulation fails, a deflecting wire can be useful. Once the J-wire is in position, an iliocavogram is performed using a protocol of 10 mL/s for a total of 20 mL of nonionic contrast agent.
After completion of the iliocavogram, the pigtail catheter is removed over a wire. All wires are then removed and the sheath is pulled. Hemostasis should be obtained easily with digital compression for 10 to 15 minutes, depending on whether anticoagulation has been administered.
Interpretation
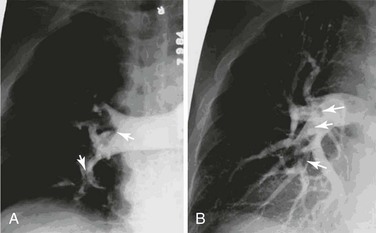
The iliocavogram is reviewed for the diameter of the vena cava, the anatomic level of the bifurcation, the level of the renal veins, and any other anomalies (Figs. 20-11 and 20-12). Typically, the renal veins will not fill with contrast material and instead will be identified by a characteristic flow pattern of the mixing of blood filled with contrast material and unopacified blood. The confluence of the common iliac veins is usually identified by the spilling over of contrast material into the contralateral side.
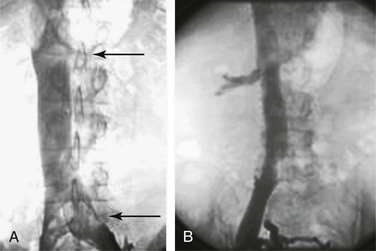
Figure 20-11 A, Inferior venacavogram demonstrating confluence of the common iliac veins and a normal flow pattern formed by mixing of unopacified renal vein blood with contrast material in the inferior vena cava (arrows). B, Inferior venacavogram demonstrating filling of the renal veins.
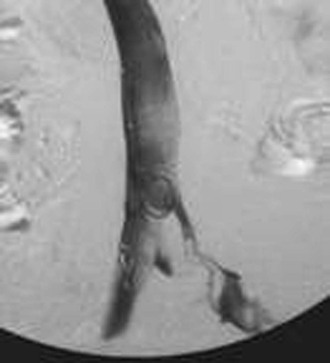
Figure 20-12 Inferior venacavogram demonstrating left common iliac vein deep venous thrombosis (unknown source).
Some centers advocate first-order selective venography instead of or as a supplement to nonselective venography. Danetz and coworkers found that when selective venography was used in conjunction with nonselective venography, 23% of patients had either an abnormal finding or aberrant anatomy, and most of these patients required a major change in vena cava filter position.20
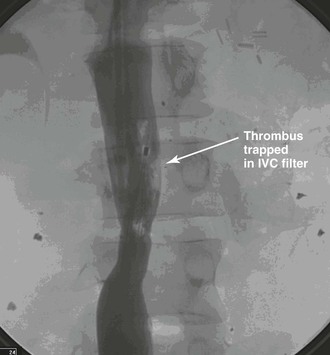
Before removal of the IVC filter, the surgeon should examine whether the filter has retained thrombus, penetrated the IVC, migrated, or angulated (Fig. 20-13). Severely angulated temporary filters with more than 20 degrees of tilt are more likely to make removal unsuccessful.21
Venography During Treatment of Iliocaval Stenosis
Recent advances in angioplasty and stent technology have led to percutaneous treatment of acute and chronic iliocaval occlusion. Many authors have written on the efficacy of this method of treating lower extremity venous outflow obstruction.22–45 Investigation of the safety and efficacy of this treatment has led to refinements of the technique. Neglen and Raju have shown that ensuring adequate inflow into the common femoral vein segment is critical to long-term patency.33–36,46–50
Technique
To facilitate visualization of the confluence of the profunda femoral vein and the superficial femoral vein, it is recommended that access be obtained at the midthigh or popliteal level.51 Ultrasound guidance facilitates placement of a 5F sheath into either of these deep veins. Access to the superficial femoral vein is best achieved in the supine position. To obtain popliteal venous access, however, the patient is placed in the prone position. Once venous access has been achieved, a J wire is advanced to the level of the common femoral vein. A 4F pigtail catheter is then advanced over this wire, and iliocavography is performed in the usual fashion. The surgeon can use either a hand injection technique or preferably power injection with a protocol such as 10 mL/s for a total of 20 mL.
Interpretation
Accurate hemodynamic tests for detecting and grading venous outflow stenosis are currently unavailable. Diagnosis and treatment must be based on morphologic findings. Single-plane transfemoral venography is often used as a standard investigation (Fig. 20-14). These studies may show definite obstruction and the development of collaterals. However, findings are often subtle and only suggestive of an underlying obstruction. Subtle findings of significant venous stenosis include widening of the iliac vein, pancaking, “thinning” of the contrast dye, partial intraluminal defects, septa, and transpelvic collateral formation.34 To increase the overall accuracy of detecting iliac vein stenosis, Neglen and Raju recommend the use of multiplanar venography.52
Diagnostic Venography of Pelvic Congestion Syndrome
Pelvic venous congestion syndrome is characterized by symptoms of dysmenorrhea, dysuria, dyspareunia, vulvar and pelvic varices in women, and varicoceles in men.53 Sources of venous congestion of the pelvic venous plexus include ovarian vein reflux, pelvic varices, and femoral venous insufficiency.
The ovarian veins provide drainage of the parametrium, cervix, mesosalpinx, and pampiniform plexus. Two or three trunks form a single vein at L4, with the right ovarian vein draining directly into the IVC and the left into the left renal vein. These veins have an average diameter of 3.1 mm and possess two or three valves.
In addition to primary valvular incompetence of the ovarian vein valves, pelvic congestion syndrome may also result from obstruction of ovarian vein outflow. This obstruction is most often caused by compression of the left renal vein between the superior mesenteric artery (SMA) and the aorta, also known as the nutcracker syndrome. Distal obstruction leads to increased venous pressure and subsequent venodilatation, valvular incompetence, and tortuosity of the ovarian vein. The development of numerous pelvic collateral pathways concurrently allows venous drainage around the point of obstruction. Distal obstruction of ovarian vein outflow leads to the development of an elevated gradient between the left renal vein and the vena cava. Normally, there is minimal or no gradient between the left renal vein and the vena cava, so the presence of an elevated gradient is diagnostic of nutcracker syndrome.54
Vulvar varices may arise from the lower extremity veins, the pelvic veins, or both. Varicose veins of the lower extremities are a frequent source of venous hypertension resulting in pelvic congestion. Almost half of vulvar varices arise from an incompetent great saphenous vein through the superficial external pudendal or posteromedial tributaries. Less often, they arise from a tributary communicating directly with the common or deep femoral vein. The remaining varices arise from the pelvic veins through branches of the internal iliac, including the internal pudendal vein, the obturator vein, and the inferior gluteal vein. The internal pudendal tributaries communicate with the ovarian vein. Rarely, the veins arise from the round ligament.55
Technique
Diagnostic venography of the ovarian vein is achieved with standard catheter-based techniques. Once access to the venous system has been achieved, a 4F angled glide catheter is advanced into the vena cava. The right ovarian vein drains into the IVC just inferior to the renal veins. The left ovarian vein drains into the left renal vein. Selective catheterization of each ovarian vein is then achieved with an angled glide wire and catheter system. Once the catheter is placed in the ovarian vein, the angiographic table is tilted upright. Then, after hand injection, the patient is asked to perform a Valsalva maneuver as images are obtained. Venography can be performed through either the jugular or femoral approach.
The diagnosis of nutcracker syndrome is made by determining the retrograde renocaval gradient. To do so, a glide catheter is placed in the distal left renal vein and the pressure transduced. This pressure is compared with the pressure in the vena cava. An elevated renocaval gradient is one clue in making the diagnosis. In addition, contrast-enhanced visualization of the gonadal system and its pelvic and extrapelvic connections should be performed to look for ovarian vein reflux. In our institution, patients with the clinical and radiologic diagnosis of nutcracker syndrome are studied in both the supine and prone positions. Placing the patient in the prone position theoretically decreases the renocaval gradient and displaces the SMA forward with the intestinal mass.
Interpretation
The venographic criteria for pelvic congestion syndrome were established by Beard and colleagues in 1984.56 The criteria for pelvic congestion syndrome include (1) an ovarian vein diameter of 6 mm or less, (2) retention of contrast material for longer than 20 seconds, (3) congestion of the pelvic venous plexus or opacification of the ipsilateral or contralateral internal iliac vein (or both veins), and (4) filling of vulvovaginal and thigh varicosities (Fig. 20-15). Each variable is assigned a value of 1 to 3, depending on the degree of abnormality, and scores higher than 5 indicate pelvic congestion syndrome.56
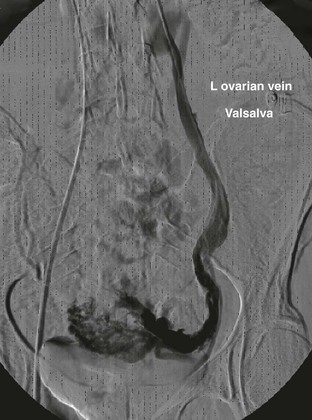
Figure 20-15 Pelvic congestion syndrome resulting from common iliac vein reflux. (Courtesy Mark Meissner, MD.)
Diagnostic criteria for ovarian vein reflux include a left ovarian vein diameter of 8 mm or less and retrograde left ovarian vein flow (Fig. 20-16). Valvular incompetence exists in up to 47% of all women. In a study of preoperative aortography in 273 female kidney donors, Belenky and coworkers found incompetent left ovarian veins in 27 (9.9%).57 Fifty-nine percent of these patients had pelvic symptoms. A secondary cause arising from iliac artery or left renal vein compression was noted in 20% of these patients.
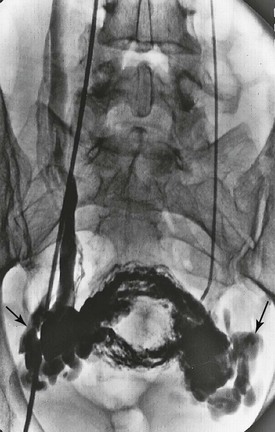
Figure 20-16 Pelvic congestion syndrome resulting from ovarian vein reflux (arrows). (Courtesy Charles Fox, MD.)
Venographic evidence of SMA compression of the renal vein may be seen on an anteroposterior view of midstream venacavography (Fig. 20-17). Further delineation is aided by selective catheterization of the left renal vein and looking for proximal compression or pancaking. Definitive documentation, however, requires measurement of the retrograde renocaval gradient and contrast visualization of the gonadal system as well as of its pelvic and extrapelvic connections. A renocaval gradient greater than 1 mm Hg is consistent with the presence of nutcracker syndrome.58
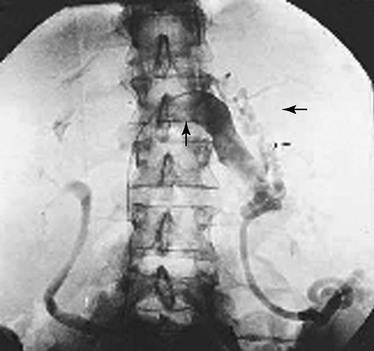
Figure 20-17 Nutcracker syndrome. Retrograde catheterization of the left renal vein shows mesoaortic compression (vertical arrow) of the left renal vein and perirenal varicosities (horizontal arrow). Obstruction of gonadal outflow is evident. Kinking and curls of the gonadal vein are the radiographic manifestations of obstruction. This patient had left flank pain and hematuria. (From Scultetus AH, et al: The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg 34:812-819, 2001.)
Venography for Varicoceles
Abnormal distention of the pampiniform venous plexus, the venous drainage from the testicle, results in varicocele formation. Varicocele can be due to absence of testicular vein valves; however, it is generally due to retrograde flow in or impaired drainage of the testicular vein. Veins of the testes, along with tributaries from the epididymis, converge to form the pampiniform plexus. This plexus begins in the scrotum and extends into the spermatic cord and ascends along the cord in front of the ductus deferens. Below the inguinal ring, the pampiniform plexus unites to form three or four veins that enter the abdomen through the inguinal ring and further coalesce to form two gonadal veins. Intra-abdominally, on the right side, the gonadal vein drains directly into the infrarenal IVC; on the left side, the gonadal vein drains into the left renal vein. The left renal vein then drains into the IVC. Collateral pathways for venous drainage of the testis include perirenal, retroperitoneal, and lumbar veins.
Varicocele predominantly occurs on the left side. It is suspected that this is related to the indirect drainage of the testicular vein to the IVC through the left renal vein. Bilateral and right-sided varicoceles account for about 10% to 15% of varicoceles.59 An isolated right-sided varicocele is less frequent, and its presence should prompt an evaluation for obstructive disease like renal tumors or adenopathy.
Patients presenting with varicocele will often report pain and reduced sperm count. Varicoceles have been associated with a time-dependent decrease in size and function of the testes, and this is assumed to contribute to male infertility. The physical examination is the cornerstone in diagnosis of this entity. The patient should be examined in the standing position after he has been upright for at least 5 minutes. Visual inspection of the scrotum should occur first, and obvious distention of the spermatic cord is noted. Next, the patient is palpated before and after a Valsalva maneuver. Previous descriptions of varicocele have described a “bag of worms” or a thickened cord on palpation. Although the physical examination is key in diagnosis, it is subjective. Therefore, imaging should be obtained to assist in diagnosis. A duplex ultrasound study can be performed to confirm the physical examination findings. On ultrasound, the presence of two prominent tortuous veins in the pampiniform plexus that measure at least 2 mm in diameter and that increase with the Valsalva maneuver is diagnostic. Other potential imaging modalities include computed tomography and magnetic resonance imaging. Venography, however, remains the gold standard in diagnosis of varicocele, and reflux of more than 2 seconds is determined to diagnose it.59
Indications for treatment include an infertile man with a palpable varicocele and an abnormal sperm count, prophylactic repair in adolescent men with a reduction in testicular size, bilateral varicoceles, and impaired spermatogenesis in patients older than 18 years.59
Technique
Access may occur through the femoral or jugular approach. Access through the femoral vein generally requires a sheath or guiding catheter to stabilize the renal vein access and to allow passage of a catheter into the testicular vein. We obtain a venogram from the midpoint of the renal vein to determine renal vein patency and testicular vein incompetence. Venography with a Valsalva maneuver or preferably a tilt table with the patient placed in the reverse Trendelenburg position is performed of the testicular vein, and retrograde flow and collaterals can be visualized. Visualizing the entire anatomy of the testicular vein and pampiniform plexus is necessary to evaluate retrograde and collateral flow as well as aberrant blood supply to the varicocele. At times during instrumentation, the gonadal vein may spasm, and use of nitroglycerin is employed. If reflux is not demonstrated with this technique, renal venography with a Valsalva maneuver may be used, or an internal iliac study with an occlusion balloon may be warranted. This technique, described before, is used to determine a right- or left-sided varicocele.
Treatment
The goal of treatment is to stop the retrograde flow from the gonadal vein or collateral veins to the pampiniform plexus. Occlusion of the testicular vein should extend from just above the inguinal ligament to 5 cm below the convergence of the testicular vein with the left renal vein on the left side. This technique eliminates all collateral and aberrant vessels. Several techniques for occlusion are available: coil embolization, sclerosing agents, and open surgical repair. Coil embolization is commonly employed owing to its painless ease of use. Coils must be sized correctly to at least 10% larger than the diameter of the spermatic vein at the level of deployment. If the coils are undersized, there is a risk of embolization to the pulmonary artery. Other groups, particularly those in Europe, use sclerosants exclusively. In general, if the right side does not evidence reflux, it is not routinely treated. Open surgical options for repair involve retroperitoneal or inguinal access to the testicular vein for ligation. The traditional open approach usually requires general anesthesia and a 2- to 3-day hospitalization.
Complications associated with treatment of varicocele include spasm, dissection, and perforation. Venous puncture–associated complications like false aneurysms or hematomas are generally rare. Phlebothrombosis and phlebitis are the most common complications of embolization specific to embolization of the spermatic vein. Patients may present with fever, pain, and scrotal swelling within 24 to 48 hours of intervention. Patients with this complication should undergo an immediate duplex ultrasound examination followed by a repeated study 6 weeks later for evaluation of signs of testicular necrosis, which rarely occurs. Patients may also be treated with a short course of steroids and nonsteroidal anti-inflammatory drugs. Antibiotics may also be prescribed if there is difficulty in differentiating phlebitis from infection.
Renal Venography
The renal veins may be evaluated for anatomic verification or assessment of congenital anomalies or tumor invasion before resection. Renal venography is also useful for the evaluation of suspected renal vein thrombosis in both native and transplanted kidneys. Patients typically have generalized malaise, flank pain, hematuria, and hypertension. Renal vein thrombosis affects men more than women and most often involves the left renal vein.60 Renal vein thrombosis may arise from four separate causes: (1) thrombus propagating from below to obstruct the IVC and renal veins; (2) caval obstruction or invasion from malignant neoplasms with compromise of the renal veins; (3) primary renal vein thrombosis, which usually occurs in infants; and (4) renal vein thrombosis secondary to nephritis.61 Catheter venography may be especially useful in patients with acute renal vein thrombosis because it may be followed by therapeutic intervention consisting of catheter-directed thrombolysis.
Technique
Midstream venacavography results in characteristic flow voids seen at the L2-3 level as a result of mixing of unopacified renal vein blood with contrast material in the vena cava. Detailed evaluation of the renal veins, however, is best achieved by selective catheterization. Renal vein selection is easily achieved with standard glide wires and angled catheters. Once access to the renal veins has been attained, venography is usually accomplished by hand injections of 5 to 10 mL of dilute contrast material. Improved visualization of the renal veins has been reported with the injection of 10 µg of epinephrine into the renal artery or balloon occlusion of the renal artery (Fig. 20-18).61
Interpretation
Although renal venography is a useful technique for demonstrating renal vein anomalies such as a retroaortic left renal vein or circumaortic venous collar, computed tomography and magnetic resonance imaging are more commonly used today. Venographic diagnosis of renal vein thrombosis depends on the demonstration of a persistent filling defect within the renal vein. Acute renal vein thrombosis may be partial or complete. Detection of intraluminal defects showing partial occlusion and no collateral formation is consistent with acute thrombosis. Chronic renal vein thrombosis would appear as main renal vein obstruction with numerous collaterals around the obstruction. The risk for pulmonary embolism during renal venography and treatment has been reported to be low.60,62
Definitive exclusion of renal vein thrombosis requires selective catheterization of the renal veins if an initial midstream venacavogram is normal. Martin and Gordon found that only 6 of 15 (40%) patients with documented renal vein thrombosis had caval thrombus, whereas the cava was completely normal in 46%. The authors suggest that inferior venacavography lacks specificity in the diagnosis of renal vein thrombosis when it is associated with primary renal disease and nephrotic syndrome.61
Diagnostic Angiography of Mesenteric or Portal Venous Thrombosis
The signs and symptoms of acute mesenteric or portal venous thrombosis are varied and include vague abdominal pain, nausea, vomiting, melena, bloody diarrhea, and ultimately circulatory collapse.63 The diagnosis is usually accomplished with computed tomography or magnetic resonance imaging but can be made with angiography. High clinical suspicion plus early diagnosis may be lifesaving because it allows systemic anticoagulation or thrombolysis (or both) to be performed.64
Technique
Visualization of the superior mesenteric veins may be accomplished either indirectly by review of the venous phase of a superior mesenteric arteriogram (Fig. 20-19) or by direct access (Figs. 20-20 and 20-21). Direct access to the superior mesenteric veins can be accomplished through a transjugular, intrahepatic, or transhepatic approach and allows percutaneous intervention.
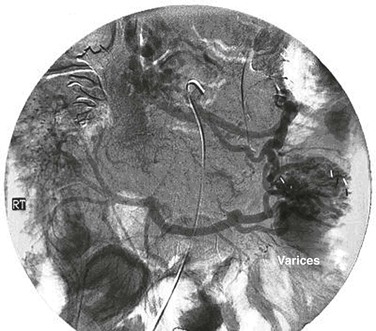
Figure 20-19 Venous phase of a superior mesenteric arteriogram showing occlusion of the superior mesenteric vein. RT, Right. (From Ozkan U, et al: Push enteroscopy for recurrent gastrointestinal hemorrhage due to jejunal anastomotic varices: a case report and review of the literature. Endoscopy 34:735, 2002.)
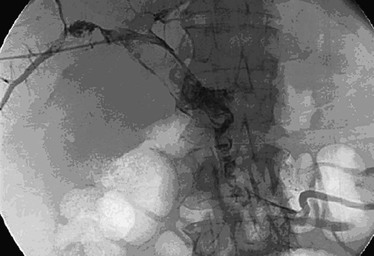
Figure 20-20 Transhepatic venogram showing filling defects attributable to thrombus in the small mesenteric vein, main pulmonary vein, right pulmonary vein, and left pulmonary vein. (From Ozkan U, et al: Percutaneous transhepatic thrombolysis in the treatment of acute portal venous thrombosis. Diagn Interv Radiol 12:105-107, 2006.)
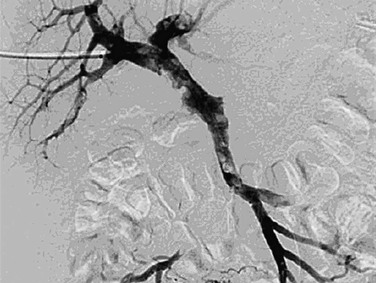
Figure 20-21 Control venography after balloon angioplasty and infusion of recombinant tissue plasminogen activator shows 90% reduction of the thrombus in the superior mesenteric vein and main portal vein, although prominent thrombus is still present in the left pulmonary vein branch. (From Ozkan U, et al: Percutaneous transhepatic thrombolysis in the treatment of acute portal venous thrombosis. Diagn Interv Radiol 12:105-107, 2006.)
The venous phase of an SMA injection provides visualization of superior mesenteric drainage into the portal venous system. After arterial access is achieved, a 5F Simmons 1 catheter is advanced into the aorta. Once the tip is shaped so that it is aimed caudad, the catheter is manipulated into the orifice of the SMA. Hand-injected half-strength contrast dye is helpful in confirming placement of the catheter. Injection of contrast material with a power injector is useful for improving imaging. An injection protocol of 5 mL/s for a total of 10 mL is appropriate. Imaging of the venous phase is accomplished either by delaying acquisition until after injection or by continuing to image after the arterial phase has been completed.
The transcutaneous transhepatic approach is a useful direct technique for access to the portal venous system. Under sonographic guidance, the skin is marked at the point where the right portal vein and the right hepatic vein are aligned in one plane with the portal vein closer to the sonography probe. By use of aseptic technique, the right portal vein is punctured with an 18-gauge needle. After imaging, the superior mesenteric vein has been shown to allow thrombolysis, angioplasty, and stenting in a highly select group of patients.65
The transjugular intrahepatic approach to portal venography was popularized in the 1960s by Rösch, who modified the technique of liver biopsy and developed the technique of transjugular intrahepatic portosystemic shunting.66–69 Transjugular intrahepatic portosystemic shunting is best performed under general anesthesia or heavy intravenous sedation. A 10F introducer sheath is placed through the right internal jugular vein into the IVC. A curved angiographic catheter is used to select a hepatic vein, and the tip of the sheath is then introduced into the hepatic vein. A 16-gauge, 55-cm Colapinto transjugular needle is passed from the hepatic vein through the liver parenchyma into a portal vein branch. A 0.035-inch Amplatz extrastiff guide wire is placed into the superior mesenteric vein and the needle exchanged for a 5F angiography catheter. Portal venography is then performed through a multihole diagnostic catheter placed in the portal vein.70 Postdiagnostic angiographic embolization of the track formed has routinely been performed, although this is changing.
Interpretation
The superior mesenteric vein joins with the splenic vein to form the portal vein. In general, noninvasive methods of imaging are used as the initial diagnostic modality in the case of portal or mesenteric vein disease. Indirect visualization of the portomesenteric system can be achieved during mesenteric arteriography by prolonged imaging of the venous phase. This method, however, does not provide great detail or access for treatment. Advances in percutaneous technology have led to increased use of the transjugular and transhepatic approaches.64,65,71 These methods carry increased risk for subcapsular or intra-abdominal bleeding complications, especially if they are combined with thrombolytic therapy.
Diagnostic Venography of Budd-Chiari Syndrome
Normal hepatic venous outflow occurs from the right and left hepatic veins into the retrohepatic vena cava and then into the right atrium (Fig. 20-22). Budd-Chiari syndrome results from thrombosis of hepatic venous outflow, which gives rise to portal hypertension and its sequelae. The liver becomes congested and enlarged, and as the hepatic congestion progresses, hepatic function deteriorates and failure results. For unclear reasons, some patients have mild initial symptoms, whereas others exhibit fulminant hepatic failure and massive hepatic necrosis.72 Budd-Chiari syndrome can result from various causes, including membranous obstruction of the IVC, pregnancy, oral contraceptive use, tumors, infections, and blood dyscrasias, or it can be idiopathic. The most useful initial test is a color-flow duplex scan of the portal vein, which will establish the diagnosis. This scan should then be followed by venacavography and portal vein venography with portal venous and vena cava pressure measurements, as well as liver biopsy, to help determine further management.73
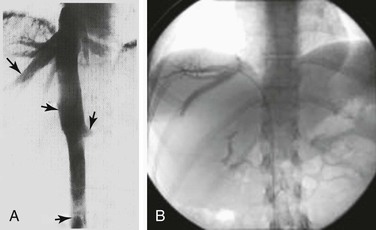
Figure 20-22 A and B, Normal hepatic venous outflow (arrows) demonstrated by cavography and selective catheterization of the hepatic veins. (B, Courtesy Charles Fox, MD.)
Technique
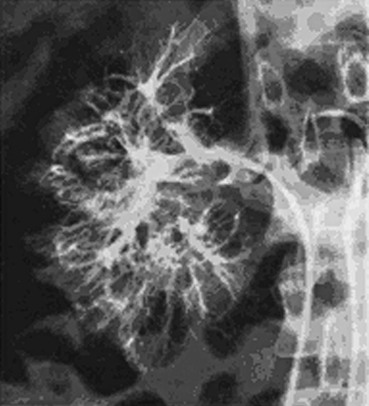
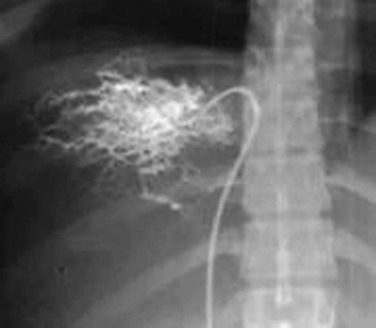
The diagnosis of Budd-Chiari syndrome may be confirmed by demonstrating obstruction of hepatic venous outflow, most often by selective catheterization of the right or left hepatic vein. This can easily be accomplished through a transfemoral or transjugular approach. From the groin, a curved catheter, such as a Simmons 1, is manipulated into the suprahepatic cava and then used to select the hepatic vein. From the jugular vein, a straight or angled catheter is easily manipulated into the hepatic outflow tract. Hand injection of 5 to 10 mL of contrast material should reveal a characteristic spider-like appearance of the occluded hepatic venous system.
In some cases, such as membranous obstruction of the vena cava, imaging is best accomplished with venacavography. A pigtail catheter is placed at the level of the distal IVC. Power injection at 15 mL/s for a total volume of 30 mL would be used. The surgeon should look for filling defects in the distal IVC.
Interpretation
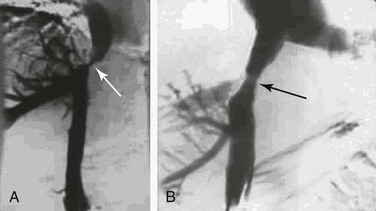
Thrombosis of the hepatic venous outflow tract may be detected by direct catheterization of the hepatic veins. A characteristic spider-like appearance of the occluded or recanalized hepatic veins is seen (see Fig. 20-22). Distal obstruction of the IVC may take the appearance of a weblike band or stenosis (Figs. 20-23 and 20-24).
Upper Extremity Venography
Upper extremity venography is the procedure of choice for most clinicians in the evaluation of central thoracic veins and for investigation of upper extremity edema. Upper extremity edema can be caused by acute or chronic venous thrombosis, external venous compression by a tumor of the lymph nodes, venous obstruction secondary to fibrosis after radiation therapy or surgery, superior vena cava (SVC) obstruction, and lymphatic obstruction. Although primary axillary-subclavian vein thrombosis is relatively uncommon (<2% of all cases of DVT), the incidence of central venous occlusion is still rising because of the increasing long-term use of central venous catheters and transvenous cardiac pacers (Fig. 20-25).74,75
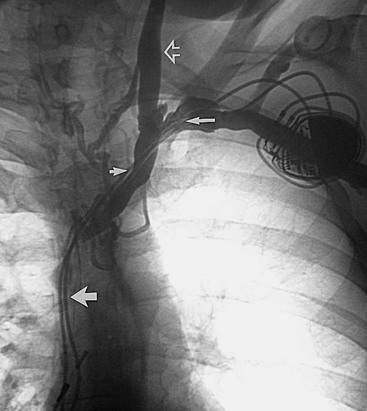
Figure 20-25 Chronic thrombosis of the superior vena cava, the innominate veins, and the subclavian veins caused by cardiac pacer wires. Chronic subclavian venous thrombosis (long arrow), the innominate vein (short arrow), and the expected location of the superior vena cava (wide arrow) are shown. Note the reflux of contrast material up the left internal jugular vein (open arrow).
Upper extremity venography is most commonly used in the investigation of arm edema in patients with primary axillary-subclavian venous thrombosis who are candidates for thrombolytic therapy. The examination is performed to show the extent of thrombosis and to guide placement of infusion catheters for the administration of lytic agents. Other common indications are to evaluate patients with suspected central venous occlusion who are not candidates for magnetic resonance angiography (e.g., because of a cardiac pacemaker or claustrophobia) and to rule out venous injuries from trauma.
Technique
Arm venography techniques were initially described in 1971 by Thomas and Andress.76 The most common method involved accessing the median antecubital vein or a peripheral hand vein and positioning the upper extremity by the side of the patient in 5 to 10 degrees of abduction without the use of tourniquets (Fig. 20-26). Natu and associates modified this technique by abducting the arm 90 degrees, introducing the catheter into a hand vein or forearm vein, and injecting 50 mL of contrast material (Renografin-60) in 5 seconds.77 Patients are asked to hold their breath, and the arm is elevated 3 seconds after the contrast agent is infused to approximately 60 degrees from the horizontal. Andrews and colleagues further modified this technique with the use of digital subtraction.78 Venous access was obtained in similar fashion, and after 10 mL of contrast material was injected, the patient was asked to breath-hold and imaging was started. The flow of contrast material was followed, and injection was terminated once adequate images were obtained. The use of digital subtraction techniques allows the examination to be performed with a lower volume of contrast medium, the immediate availability of images, and the ability to perform the study with use of small peripheral veins.
Interpretation
Significant findings in subclavian venous thrombosis include nonopacification of the vein with filling of collaterals and visualization of the thrombus within the vein. Stenosis or occlusion of the subclavian vein associated with thoracic outlet syndrome typically occurs as the vein enters the chest at the sternoclavicular junction. This point of impingement is often due to hypertrophy of the subclavius muscle. Large collateral vein formation is seen and allows venous outflow from the arm around the point of obstruction. In patients with pacemaker wires, subclavian vein stenosis will typically occur more distally. Patients with central venous catheters will have stenosis or occlusion from the midclavicle, centrally.
Central Thoracic Venography
Contrast-enhanced venography is considered the diagnostic method of choice for evaluation of SVC obstruction.79 SVC evaluation is necessary in patients with clinical suspicion of SVC or axillary-subclavian vein obstruction (or both) caused by thoracic outlet compression.80,81
Technique
Sharma and colleagues described their technique for evaluation of the SVC, which involves access in a peripheral vein of each upper extremity and injection of 20 mL of contrast material at a rate of 5 mL/s and a pressure of 150 psi.82 We perform central thoracic venography similarly by injecting through an intravenous site in the antecubital fossa or forearm with the arm in a neutral position at the patient’s side. For each projection needed, 20 to 30 mL of contrast material is injected by hand. Digital images of the mediastinum are obtained at one frame per second until contrast material is seen within the veins, and the frame rate is then increased to three per second. The imaged field should include the upper mediastinum and shoulders for careful evaluation of the site of venous obstruction, collateral channels, and reconstitution of the SVC distal to the obstruction.
In general, contrast material is injected unilaterally for suspected subclavian vein obstruction and bilaterally for suspected SVC obstruction (Fig. 20-27). This helps compensate for washout of contrast material in the SVC by unopacified blood from the opposite innominate vein. Images are acquired at one frame per second, and the duration of injection and acquisition is determined by opacification of the SVC, brachiocephalic veins, and medial third of the subclavian veins. These structures are commonly obscured within the mediastinal shadows on conventional venography.
Interpretation
Obstruction of the central veins may be associated with either benign or malignant processes. Benign processes are most often related to indwelling catheters for hemodialysis or other infusions. Malignant tumors of the chest may also cause SVC syndrome through external compression or tumor invasion. The existence of large collateral veins arising from the innominate veins and reconstituting in the SVC is indicative of significant obstruction. One may also see lesser degrees of obstruction from extraluminal compression or intraluminal thrombus. Options for treatment of central venous obstruction include operative reconstruction with spiral vein grafts, angioplasty and stenting in select patients, or both (Fig. 20-28).
Pulmonary Arteriography
Pulmonary arteriography is most commonly performed in situations in which computed tomographic angiography or magnetic resonance angiography is nondiagnostic in a patient with strong clinical suspicion of pulmonary embolism. Pulmonary arteriography is superior to computed tomographic angiography or magnetic resonance angiography for identification of small or subtle peripheral or chronic emboli and has the advantage of being able to measure pulmonary arterial pressure.83
Technique
Selective catheter pulmonary arteriography is performed by advancing a pigtail catheter through the right atrium and ventricle. Angulated pigtail catheters such as the Grollman catheter are preshaped to facilitate advancement from the right ventricle into the pulmonary outflow tract. Caution should be exercised when the catheter is advanced through the right ventricle because this maneuver can produce transient right bundle branch block. In patients with left bundle branch block, it may cause complete heart block. One should consider the use of a temporary pacemaker in this subset of patients when pulmonary angiography is performed. Once the target artery has been selectively catheterized, baseline pulmonary arterial pressure measurements are obtained. The right and left pulmonary artery trunks are normally evaluated separately. Contrast material is injected at 15 to 25 mL/s for a volume of 30 to 50 mL per injection. Fluoroscopy should be set at high frame rates (4 to 15 frames per second), and patients should be holding their breath during image acquisition. For complete evaluation, frontal and oblique views of each pulmonary artery should be obtained.
In patients with pulmonary hypertension, injection of large volumes of contrast material into the pulmonary artery is contraindicated. However, patients with chronic compensated pulmonary hypertension can tolerate pulmonary arteriography without difficulty. A right ventricular end-diastolic pressure of greater than 20 mm Hg is considered a relative contraindication to high-volume injection of contrast material, and only small amounts should be injected by hand in such cases.
Interpretation
The classic angiographic sign of acute pulmonary embolism is an intraluminal defect with diminished distal contrast enhancement (Fig. 20-29). Main and segmental pulmonary artery emboli should be easily detectable. Smaller subsegmental pulmonary emboli may be missed with this technique unless digital spot films are obtained. This is often time-consuming and less successful. Chronic pulmonary emboli are more difficult to detect than acute occlusion and are typically identified as stenosis, webs, or mural thickening consistent with organized thrombus.
Limitations and Risks
Although venography is still considered the gold standard in diagnosis of DVT and reflux disease, several limitations and risk accompany its use.
Limitations include the invasive nature of the technique, radiation exposure, differences in intraobserver interpretation of the presence or absence of thrombus, and inability to cannulate the appropriate vein in the setting of extremity edema. Several technical issues, like incomplete filling of the vein, may lead to 5% to 15% inadequacy of this study. In instances in which the external or common iliac veins are not well visualized, access through the common femoral or iliac vein for venography may need to be accomplished.
Risks of venography can range from minor to major and include nausea or vomiting, pain at the site of injection, local infection at the injection site, extravasation of contrast material, contrast-induced allergic reaction, thrombophlebitis, intra-abdominal bleeding complications with the transhepatic approach to evaluate mesenteric or portal vein thrombosis, DVT, and pulmonary embolism. Risks specifically associated with pulmonary angiography include transient right bundle branch block. In those with an already present left bundle branch block, complete heart block may ensue.
Future Advances
It is difficult to ascertain what future role venography will continue to play in the diagnosis of thrombosis or reflux disease. Vascular surgery is a rapidly progressive field, and improvements in less invasive methodologies for diagnosis of disease are evolving. Currently, venography is still the reference by which imaging modalities are compared, and its use is preferred in particular instances described earlier in the chapter. As the demand for less invasive technology continues, the use of more invasive techniques like venography may become obsolete. However, knowledge of this technique and its performance is important for the vascular surgeon to maintain in his or her armamentarium.
Acknowledgment
We would like to acknowledge the previous contributions of Gilbert Aidinian to this work.
Selected Key References
Baldt MM, Böhler K, Zontsich T, Bankier AA, Breitenseher M, Schneider B, Mostbeck GH. Preoperative imaging of lower extremity varicose veins: color coded duplex sonography or venography. J Ultrasound Med. 1996;15:143–154.
Lower extremity descending venography is no longer used as a screening study. It has largely been replaced by color duplex sonography, which has shown good agreement with descending venography in the grading of deep and superficial vein reflux.
Beard RW, Highman JH, Pearce S, Reginald PW. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2:946–949.
Established the venographic criteria for diagnosis of pelvic congestion syndrome.
Danetz JS, McLafferty RB, Ayerdi J, Gruneiro LA, Ramsey DE, Hodgson KJ. Selective venography versus nonselective venography before vena cava filter placement: evidence for more, not less. J Vasc Surg. 2003;38:928–934.
Found that when selective venography was used in conjunction with nonselective venography, 23% of patients had either an abnormal finding or aberrant anatomy, and most of these required a major change in vena cava filter position.
Gillespie DL, Villavicencio JL, Gallagher C, Chang A, Hamelink JK, Fiala LA, O’Donnell SD, Jackson MR, Pikoulis E, Rich NM. Presentation and management of venous aneurysms. J Vasc Surg. 1997;26:845–852.
Showed that venous aneurysms were correctly diagnosed in 85% of patients by imaging techniques: phlebography (60%), color-flow duplex (27%), and magnetic resonance imaging (10%).
Kistner RL, Ferris EB, Randhawa G, Kamida C. A method of performing descending venography. J Vasc Surg. 1986;4:464–468.
Developed a classification system used commonly to categorize the severity of deep venous reflux and functional integrity of the venous valves.
McLachlan MS, Thomson JG, Taylor DW, Kelly ME, Sakett DL. Observer variation in the interpretation of lower limb venograms. AJR Am J Roentgenol. 1979;132:227–229.
Showed intraobserver disagreement about the probable presence or absence of thrombus occurring in up to 10% of venographic cases.
Neglen P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694–700.
Showed that subtle findings of significant venous stenosis include widening of the iliac vein, pancaking, “thinning” of the contrast dye, partial intraluminal defects, septa, and transpelvic collateral formation. To increase the overall accuracy of detecting iliac vein stenosis, the use of multiplanar venography is recommended.
Rösch J, Antonovic R, Dotter CT. Transjugular approach to the liver, biliary system, and portal circulation. Am J Roentgenol Radium Ther Nucl Med. 1975;125:602–608.
Popularized the transjugular intrahepatic approach to portal venography.
Thomas ML, Bowles JN. Incompetent perforating veins: comparison of varicography and ascending phlebography. Radiology. 1985;154:619–623.
Showed that incompetent perforating veins of the gastrocnemius muscle and midthigh were more accurately demonstrated with varicography than with ascending venography.
Welch CE, Faxon HH, McGahey CE. The application of phlebography to the therapy of thrombosis and embolism. Surgery. 1942;12:162.
First to recommended injection of contrast material into a superficial vein in the foot with a tourniquet applied above the ankle to prevent filling of the superficial veins, which often obscured the deep system.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Bauer G. A venographic study of thromboembolic problems. Acta Chir Scand. 1940;84(Suppl 61):1–75.
2. Hager K. Problems of acute deep venous thrombosis: I. The interpretation of signs and symptoms. Angiology. 1969;20:219–223.
3. Rabinov K, et al. Roentgen diagnosis of venous thrombosis of the leg. Arch Surg. 1972;104:134–144.
4. Welch CE, et al. The application of phlebography to the therapy of thrombosis and embolism. Surgery. 1942;12:162.
5. Wheeler HB, et al. Diagnostic tests for deep vein thrombosis: clinical usefulness depends on probability of disease. Arch Intern Med. 1994;154:1921–1928.
6. Huisman MV, et al. Serial impedance plethysmography for suspected deep venous thrombosis in outpatients. The Amsterdam General Practitioner Study. N Engl J Med. 1986;314:823–828.
7. Lea Thomas M. Techniques of phlebography: a review. Eur J Radiol. 1990;11:125–130.
8. McLachlan MS, et al. Observer variation in the interpretation of lower limb venograms. AJR Am J Roentgenol. 1979;132:227–229.
9. Thomas ML, et al. Incompetent perforating veins: comparison of varicography and ascending phlebography. Radiology. 1985;154:619–623.
10. Aldridge SC, et al. Popliteal venous aneurysm: report of two cases and review of the world literature. J Vasc Surg. 1993;18:708–715.
11. Gloviczki P, et al. Klippel-Trénaunay syndrome: the risks and benefits of vascular interventions. Surgery. 1991;110:469–479.
12. Chahlaoui J, et al. Popliteal venous aneurysm: a source of pulmonary embolism. AJR Am J Roentgenol. 1981;136:415–416.
13. Gillespie DL, et al. Presentation and management of venous aneurysms. J Vasc Surg. 1997;26:845–852.
14. Braun SD, et al. Closed-system venography in the evaluation of angiodysplastic lesions of the extremities. AJR Am J Roentgenol. 1983;141:1307–1310.
15. Baldt MM, et al. Preoperative imaging of lower extremity varicose veins: color coded duplex sonography or venography. J Ultrasound Med. 1996;15:143–154.
16. Kistner RL, et al. A method of performing descending venography. J Vasc Surg. 1986;4:464–468.
17. Herman RJ, et al. Descending venography: a method of evaluating lower extremity venous valvular function. Radiology. 1980;137:63–69.
18. Morano JU, et al. Chronic venous insufficiency: assessment with descending venography. Radiology. 1990;174:441–444.
19. Raju S, et al. Valve reconstruction procedures for nonobstructive venous insufficiency: rationale, techniques, and results in 107 procedures with two- to eight-year follow-up. J Vasc Surg. 1988;7:301–310.
20. Danetz JS, et al. Selective venography versus nonselective venography before vena cava filter placement: evidence for more, not less. J Vasc Surg. 2003;38:928–934.
21. Rosenthal D, et al. Retrievability of the Gunther Tulip vena cava filter after dwell times longer than 180 days in patients with multiple trauma. J Endovasc Ther. 2007;14:406–410.
22. Broholm R, et al. [Endovascular treatment of chronic iliac vein obstruction in patients with venous claudication.]. Ugeskr Laeger. 2007;169:1564–1568.
23. Delis KT, et al. Successful iliac vein and inferior vena cava stenting ameliorates venous claudication and improves venous outflow, calf muscle pump function, and clinical status in post-thrombotic syndrome. Ann Surg. 2007;245:130–139.
24. Elson JD, et al. Vena caval and central venous stenoses: management with Palmaz balloon-expandable intraluminal stents. J Vasc Interv Radiol. 1991;2:215–223.
25. Hartung O, et al. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138–1144.
26. Heniford BT, et al. May-Thurner syndrome: management by endovascular surgical techniques. Ann Vasc Surg. 1998;12:482–486.
27. Hurst DR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
28. Husmann MJ, et al. Stenting of common iliac vein obstructions combined with regional thrombolysis and thrombectomy in acute deep vein thrombosis. Eur J Vasc Endovasc Surg. 2007;34:87–91.
29. Knipp BS, et al. Factors associated with outcome after interventional treatment of symptomatic iliac vein compression syndrome. J Vasc Surg. 2007;46:743–749.
30. Kwak HS, et al. Stents in common iliac vein obstruction with acute ipsilateral deep venous thrombosis: early and late results. J Vasc Interv Radiol. 2005;16:815–822.
31. Mickley V, et al. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg. 1998;28:492–497.
32. Mussa FF, et al. Iliac vein stenting for chronic venous insufficiency. Tex Heart Inst J. 2007;34:60–66.
33. Neglen P, et al. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7:79–91.
34. Neglen P, et al. Proximal lower extremity chronic venous outflow obstruction: recognition and treatment. Semin Vasc Surg. 2002;15:57–64.
35. Neglen P, et al. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38:879–885.
36. Neglen P, et al. In-stent recurrent stenosis in stents placed in the lower extremity venous outflow tract. J Vasc Surg. 2004;39:181–187.
37. Neglen P, et al. Combined saphenous ablation and iliac stent placement for complex severe chronic venous disease. J Vasc Surg. 2006;44:828–833.
38. Neglen P, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990.
39. O’Sullivan GJ, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
40. Raju S, et al. Reversal of abnormal lymphoscintigraphy after placement of venous stents for correction of associated venous obstruction. J Vasc Surg. 2001;34:779–784.
41. Raju S, et al. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35:8–15.
42. Raju S, et al. Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg. 2006;44:820–827.
43. Raju S. Endovenous treatment of patients with iliac-caval venous obstruction. J Cardiovasc Surg (Torino). 2008;49:27–33.
44. Sawada S, et al. Application of expandable metallic stents to the venous system. Acta Radiol. 1992;33:156–159.
45. Tacke J, et al. [The palliative treatment of venous stenoses in tumor patients with self-expanding vascular prostheses.]. Rofo. 1994;160:433–440.
46. Raju S, et al. Recanalization of totally occluded iliac and adjacent venous segments. J Vasc Surg. 2002;36:903–911.
47. Neglen P, et al. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7:79–91.
48. Neglen P, et al. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583–589.
49. Ing FF, et al. Reconstruction of stenotic or occluded iliofemoral veins and inferior vena cava using intravascular stents: re-establishing access for future cardiac catheterization and cardiac surgery. J Am Coll Cardiol. 2001;37:251–257.
50. Chang TC, et al. Treatment of inferior vena cava obstruction in hemodialysis patients using Wallstents: early and intermediate results. AJR Am J Roentgenol. 1998;171:125–128.
51. Neglen P, et al. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583–589.
52. Neglen P, et al. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694–700.
53. Zerhouni EA, et al. Elevated pressure in the left renal vein in patients with varicocele: preliminary observations. J Urol. 1980;123:512–513.
54. Beinart C, et al. Left renal to inferior vena cava pressure relationship in humans. J Urol. 1982;127:1070–1071.
55. Hobbs JT. The pelvic congestion syndrome. Br J Hosp Med. 1990;43:200–206.
56. Beard RW, et al. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2:946–949.
57. Belenky A, et al. Ovarian varices in healthy female kidney donors: incidence, morbidity, and clinical outcome. AJR Am J Roentgenol. 2002;179:625–627.
58. Scultetus AH, et al. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001;34:812–819.
59. Bittles MA, et al. Gonadal vein embolization: treatment of varicocele and pelvic congestion syndrome. Semin Intervent Radiol. 2008;25:261–270.
60. O’Dea MJ, et al. Renal vein thrombosis. J Urol. 1976;116:410–414.
61. Martin EC, et al. Selective renal venography in patients with primary renal disease and the nephrotic syndrome. Cardiovasc Intervent Radiol. 1980;3:175–179.
62. Beckmann CF, et al. Renal venography: anatomy, technique, applications, analysis of 132 venograms, and a review of the literature. Cardiovasc Intervent Radiol. 1980;3:45–70.
63. Gertsch P, et al. Acute thrombosis of the splanchnic veins. Arch Surg. 1993;128:341–345.
64. Ozkan U, et al. Percutaneous transhepatic thrombolysis in the treatment of acute portal venous thrombosis. Diagn Interv Radiol. 2006;12:105–107.
65. Rosen MP, et al. Transhepatic mechanical thrombectomy followed by infusion of TPA into the superior mesenteric artery to treat acute mesenteric vein thrombosis. J Vasc Interv Radiol. 2000;11:195–198.
66. Rösch J, et al. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112–1114.
67. Rösch J, et al. Transjugular intrahepatic portacaval shunt. An experimental work. Am J Surg. 1971;121:588–592.
68. Rösch J, et al. Transjugular approach to the liver, biliary system, and portal circulation. Am J Roentgenol Radium Ther Nucl Med. 1975;125:602–608.
69. Rösch J, et al. Coaxial catheter-needle system for transjugular portal vein entrance. J Vasc Interv Radiol. 1993;4:145–147.
70. Hebbard GS, et al. Transjugular intrahepatic portal-systemic shunts (TIPS)—initial experience and clinical outcome. Aust N Z J Med. 1994;24:141–148.
71. Ferro C, et al. Transjugular intrahepatic portosystemic shunt, mechanical aspiration thrombectomy, and direct thrombolysis in the treatment of acute portal and superior mesenteric vein thrombosis. Cardiovasc Intervent Radiol. 2007;30:1070–1074.
72. Dilawari JB, et al. Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore). 1994;73:21–36.
73. Rosa P, et al. Budd-Chiari syndrome: case report and review of the literature. Vasc Surg. 2000;34:591–595.
74. Phibbs B, et al. Complications of permanent transvenous pacing. N Engl J Med. 1985;312:1428–1432.
75. Warden GD, et al. Central venous thrombosis: a hazard of medical progress. J Trauma. 1973;13:620–626.
76. Thomas ML, et al. Axillary phlebography. Am J Roentgenol Radium Ther Nucl Med. 1971;113:713–721.
77. Natu SS, et al. An improved technique for axillary phlebography. Radiology. 1982;142:529–530.
78. Andrews JC, et al. Digital subtraction venography of the upper extremity. Clin Radiol. 1987;38:423–424.
79. Shimm DS, et al. Evaluating the superior vena cava syndrome. JAMA. 1981;245:951–953.
80. Hudson GW. Venography in superior vena caval obstruction. Radiology. 1957;68:499–506.
81. Rutherford RB, et al. Primary subclavian-axillary vein thrombosis: consensus and commentary. Cardiovasc Surg. 1996;4:420–423.
82. Sharma RP, et al. Superior vena cava obstruction: evaluation with digital subtraction angiography. Radiology. 1986;160:845.
83. Ruiz Y, et al. Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement. Eur Radiol. 2003;13:823–829.