CHAPTER 208 Vein of Galen Aneurysmal Malformation
Vein of Galen aneurysmal malformations (VGAMs) are arteriovenous (AV) fistulas in the choroid fissure supplied by the choroidal arteries and draining to the dilated median vein of the prosencephalon, which is the precursor of the vein of Galen and the embryonic drainage of the choroid plexus. “VGAMs” were reported to represent less than l% of all arteriovenous malformations (AVMs) in the cooperative study of subarachnoid hemorrhage.1–3 However, its true incidence is difficult to determine because significant diagnostic confusion exists among the various malformations that cause dilation of the vein of Galen or its embryonic precursor. The embryonic nature of the draining veins of VGAMs was first described by Raybaud and colleagues in 1989.4 The concept of this disease was further refined by Lasjaunias and associates, who subclassified VGAMs into choroidal and mural types.5,6 Other vascular lesions that cause dilation of the true vein of Galen are designated as vein of Galen aneurysmal dilation (VGAD) or vein of Galen varix (VGV). VGAD is a group of malformations associated with dilation of the vein of Galen secondary to pial or dural AV shunts draining into the true vein of Galen or its tributary. VGV is a dilated vein of Galen without AV shunts.
Classification
Choroidal Vein of Galen Aneurysmal Malformations
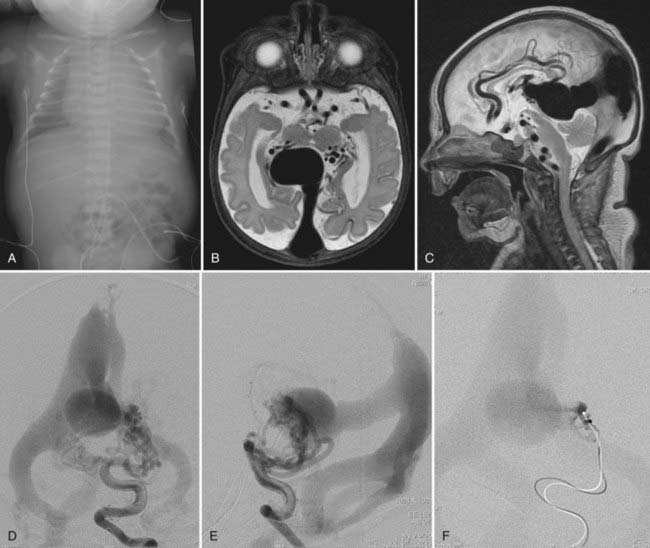
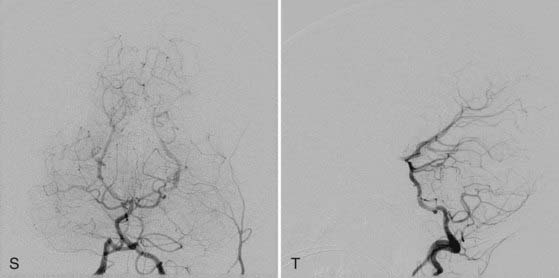
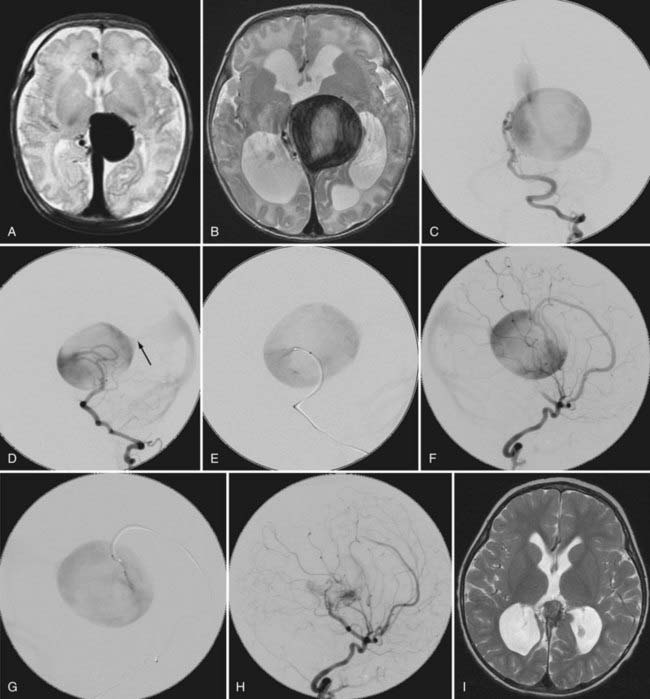
This more primitive type of VGAM consists of multiple fistulas located in the choroid fissure (Figs. 208-1 and 208-2). These multiple fistulas communicate with the anterior aspect of the median vein of the prosencephalon via an arterial network. The arterial feeders are all the choroidal arteries, including the bilateral anterior and posterior choroidal arteries, and the anterior cerebral arteries. There is often additional supply from the quadrigeminal and thalamoperforating arteries.4,7 The choroidal type of VGAM is the most severe expression of the disease and usually causes high-output cardiac failure in the newborn period because of multiple high-flow fistulas with less restriction of outflow than seen with the other type of VGAM. Choroidal VGAM is more challenging to treat because of comorbid conditions such as severe cardiopulmonary failure and the small size of the patient.
Mural Vein of Galen Aneurysmal Malformations
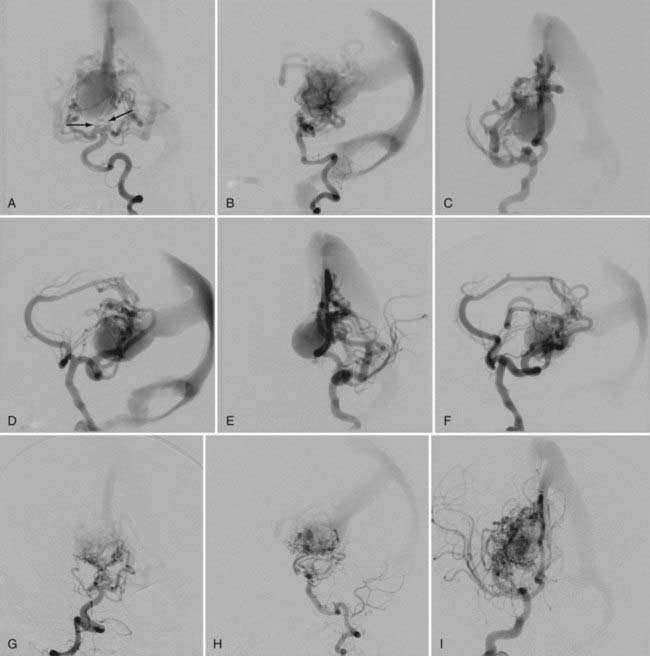
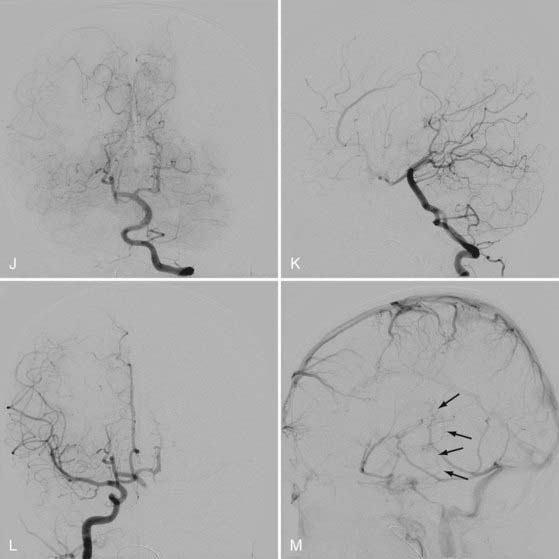
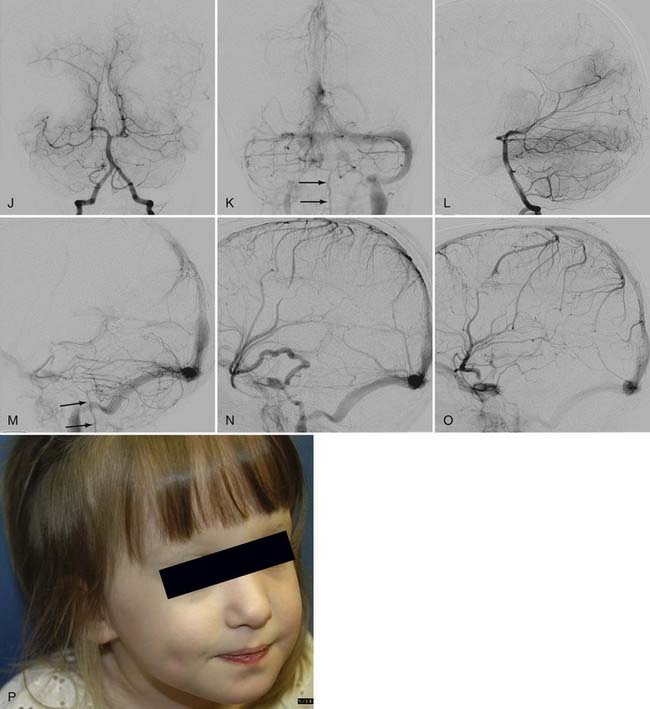
In mural VGAM, the fistulas are single or multiple and located at the wall of the dilated median vein of the prosencephalon, usually at its inferolateral margin (Fig. 208-3). The vessels supplying the shunt are usually the quadrigeminal or the posterior choroidal arteries (or both) and may be unilateral or bilateral. In contrast to choroidal-type VGAM, they have fewer fistulas and typically have more restriction of outflow, which causes more dilation of the median vein of the prosencephalon but protects the heart from high-output failure. The mural type of VGAM is therefore initially manifested later in infancy as macrocephaly, hydrocephalus, or failure to thrive, although it may be associated with mild cardiac failure or asymptomatic cardiomegaly.
Vein of Galen Aneurysmal Dilation
Pial Arteriovenous Malformation with Vein of Galen Aneurysmal Dilation
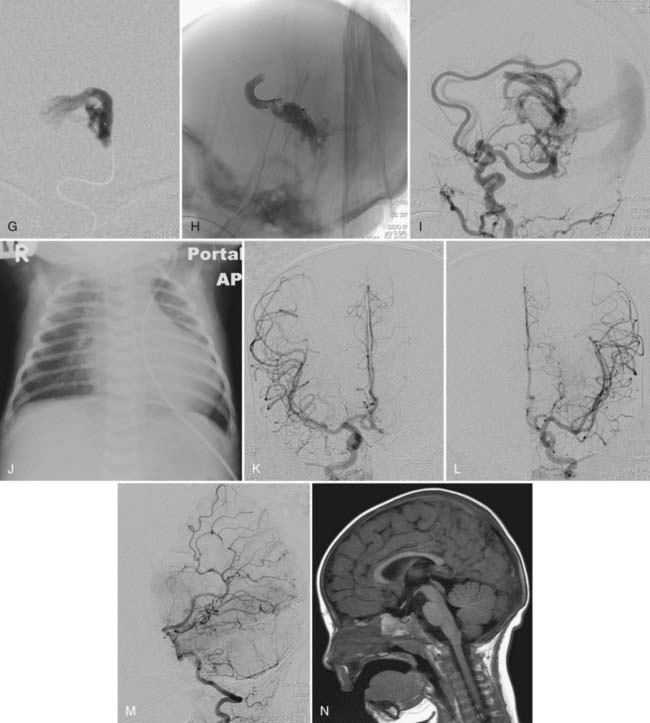
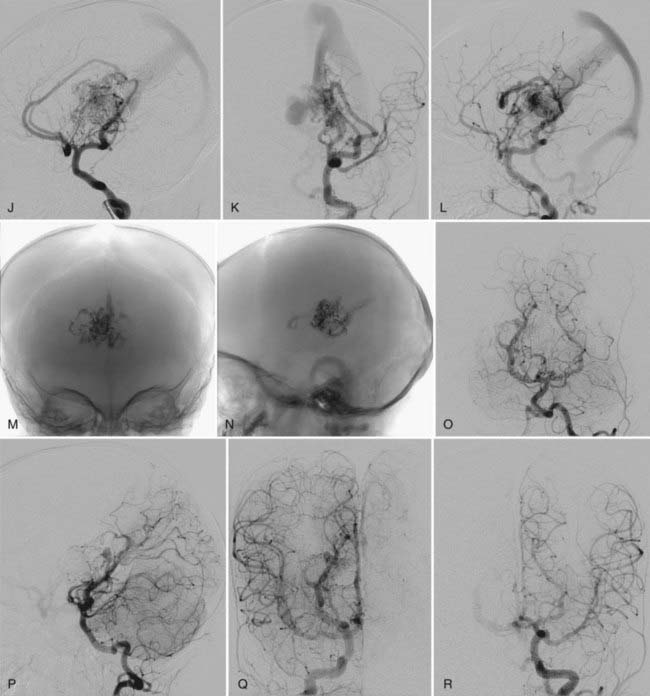
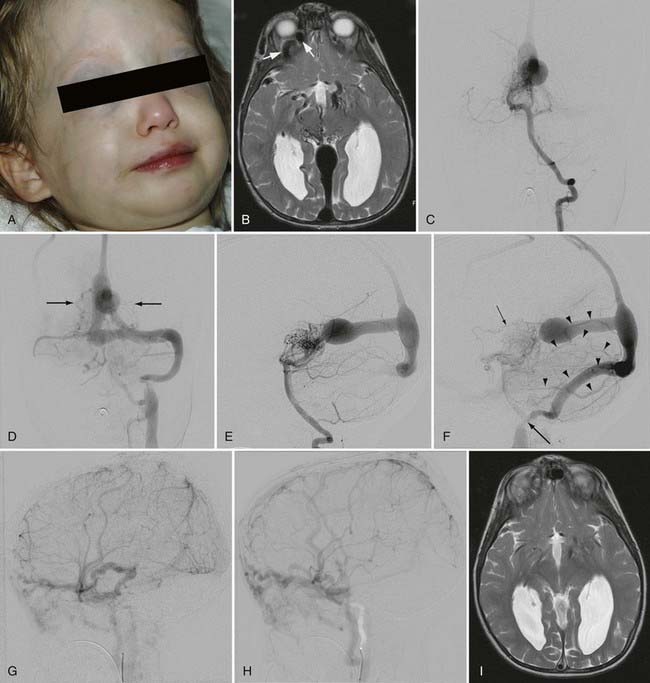
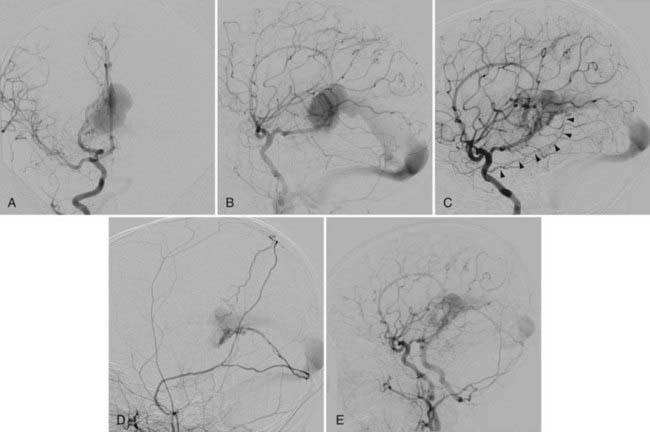
This type of VGAD is a pial or parenchymal AVM that drains into the dilated vein of Galen or its tributary (Figs. 208-4 and 208-5). Dilation of the vein of Galen is secondary to obstruction of outflow. The outflow obstruction can be relative and due to high-flow fistulas or absolute and due to progressive occlusion of the jugular bulb and the sigmoid sinus. Progressive occlusion of the dural sinus is frequently observed with pediatric fistulous malformations of the brain, including VGAM. The etiology of this outflow obstruction is unknown but may be related to underdevelopment of the jugular bulb, abnormal skull base maturation, and high-flow angiopathy of the venous system causing kinking or thrombosis at the tentorial or dural edge of the skull base. Because of this outflow restriction, the vein of Galen dilates and blood flow refluxes into other normal cerebral veins (the internal cerebral, vermian, hippocampal, basal, medial ventricular, parietal, and occipital veins or other normal tributaries of the vein of Galen). Patent embryonic sinuses such as the falcine sinus and the occipital sinus are often seen in both this type of VGAD and VGAM.
This type of VGAD is usually initially manifested in childhood or young adulthood as intracerebral hemorrhage, focal neurological deficit, or seizures. High-output cardiac failure can also occur in young children. Angiographic differentiation between VGAM and VGAD, especially a tectal AVM, can sometimes be difficult. Demonstration of transmesencephalic feeders by magnetic resonance imaging (MRI) or angiography confirms the pial nature of the lesion.8 Transmesencephalic feeders are projected below the P2 segment of the posterior cerebral artery on the lateral view of the vertebral artery angiogram.8 For treatment, transvenous occlusion of the venous dilation in the VGAD is contraindicated because it may produce hemorrhage or venous infarction of the deep cerebral structures as a result of occlusion of the outflow of these veins.
Dural Arteriovenous Malformation with Vein of Galen Aneurysmal Dilation
The dural AVM with VGAD is an acquired lesion in which AV shunts are located in the wall of the vein of Galen itself; it usually develops in the fourth or fifth decade of life. The vein of Galen dilation is due to stenosis or thrombosis of the straight sinus. Reflux is always noted into afferent cerebral veins from the vein of Galen. Typical clinical findings are headaches and progressive dementia secondary to cerebral venous hypertension. The arterial supply is derived predominantly from dural falcotentorial arteries from the internal and external carotid arteries and the vertebral artery, as well as the vasa vasorum to the wall of the vein of Galen from the pial arteries.9 Endovascular treatment of this type of dural AVM had been difficult because the transvenous approach is often not feasible and there are too many feeders from both the carotid and vertebral arteries for transarterial embolization. The recent introduction of Onyx (ev3, Irvine, CA) for embolization of dural AVMs has significantly improved the results of treatment of this type of dural AVM because a large volume of Onyx can be injected through one feeder to occlude an extensive dural vascular network supplied by multiple feeders and shunting to the dilated vein of Galen.
Vein of Galen Varix
VGV indicates dilation of the vein of Galen without the presence of an AV shunt. Two types have been encountered in children. One is transient asymptomatic dilation of the vein of Galen in neonates with cardiac failure from a cause other than VGAM. This dilation is usually noticed on an ultrasound study and disappears in several days after improvement of the cardiac condition. The second type of VGV occurs as an anatomic variation in which venous drainage of the brain converges toward the deep venous system. It is also asymptomatic, but this arrangement of venous drainage may predispose to future venous thrombosis and resultant ischemic symptoms because of the lack of compliance.7
In this chapter, we focus on the diagnosis and treatment of true VGAM.
Embryology
Expansion of the choroid plexus on the roof of the diencephalon induces the development of a midline dorsal vein draining the bilateral choroid plexuses. This vein is the first vein to drain an intracerebral structure and is designated the median vein of the prosencephalon. This vein remains functional during the second month and the first half of the third month of development. Progression of intracerebral vascularization and development of the basal ganglia result in formation of the paired internal cerebral veins, which then annex the venous drainage of the choroid plexus. This leads to regression of the median vein of the prosencephalon except for the most caudal portion, which joins with the internal cerebral veins to form the vein of Galen. Based on analysis of the vascular anatomy of VGAMs and vascular embryology, Raybaud and colleagues estimated that formation of the VGAM probably occurs between the embryonic stage of 21 to 23 mm (6 weeks) and 50 mm (11 weeks).4
Angioarchitecture of Vein of Galen Aneurysmal Malformations
VGAM, including the feeding arteries and the draining vein, exists in the subarachnoid space within the choroid fissure. The choroid fissure consists of the cistern of the velum interpositum and the quadrigeminal cistern. The arteries in the cistern of the velum interpositum are the choroidal arteries, including the anterior and posterior choroidal arteries, and the anterior cerebral arteries. The posterior choroidal arteries can have anastomoses among themselves without the existence of a VGAM. The anterior choroidal artery reaches the foramen of Monro along the choroid plexus. The anterior cerebral artery courses around the splenium of the corpus callosum anteriorly and supplies the choroid plexus at the foramen of Monro. The arteries in the quadrigeminal cistern are quadrigeminal (collicular) arteries coming off the posterior circle of Willis. They usually originate from the crural or ambient segment of the posterior cerebral artery, the superior cerebellar artery, and the medial posterior choroidal artery. They form a dense arterial network in the subarachnoid space above the quadrigeminal plate.4
Other potential contributors to the VGAM include thalamoperforating and subependymal feeders from the posterior circle of Willis. They are secondary feeders because of the “sump” effect of the venous drainage and may regress after occlusion of the primary shunts (see Fig. 208-2). Dural feeders may exist or develop secondarily via the supply to the vasa vasorum at the venodural junction. The choroidal-type VGAM may form fistulas to the choroidal vein at the interventricular foramen and is supplied by perforators from the anterior cerebral artery or Heubner’s artery.7
The dilated draining vein of the VGAM is the embryonic median vein of the prosencephalon, as described in the embryology section. It is located in the midline and receives bilateral supply, although it may be shifted to one side, opposite the major fistula. A choroidal VGAM is more primitive and has multiple fistulas with an interposing arterial network between the feeders and the draining vein. The mural type has single or multiple fistulas on the wall of the dilated draining vein. Multiple feeders can converge into a common vascular channel opening into the dilated vein. A mixed malformation with features of both the choroidal and mural types may also occur.7
The median vein of the prosencephalon has no communication with the drainage system of the normal brain. The choroidal veins embryologically drain into the median vein of the prosencephalon and may remain connected to the median vein with or without the existence of fistulas to the choroidal vein itself. This connection is seen mostly with choroidal-type VGAMs and may mediate communication between the median vein of the prosencephalon and the striate vein, which may be a cause of hemorrhage or venous infarction after transvenous embolization of the VGAM. The straight sinus is absent with most VGAMs, and the dilated median vein of the prosencephalon usually drains to the embryonic falcine sinus, which connects to the posterior third of the superior sagittal sinus.10 Persistence of other embryologic sinuses, such as the occipital and marginal sinuses, is often observed in patients with VGAMs. Persisting arterial anomalies such as a limbic arterial ring involving the anterior and posterior choroidal arteries and pericallosal arteries are also frequently present.
Because of lack of connection to the median prosencephalic vein, the deep venous drainage of the brain takes an alternative pathway. The most commonly observed pattern of deep venous drainage is through the thalamic vein connecting to the lateral mesencephalic vein. This drainage system takes an epsilon shape and is seen to connect to the superior petrosal sinus on lateral angiographic views.10,11
Clinical Manifestations of Vein of Galen Aneurysmal Malformations
Cardiac Failure
Thus, intracranial AV shunting causes initially right-sided and then left-sided volume overload in the heart. The neonate responds to the volume overload with tachycardia because of low cardiac reserve. High left ventricular preload results in increased myocardial oxygen requirements. Coronary perfusion occurs mainly during diastole and depends on the systemic–arterial intramyocardial diastolic pressure difference, as well as the duration of diastole. Therefore, a reduction in arterial diastolic pressure because of AV shunts, an increase in end-diastolic pressure because of increased preload, and a reduction in the duration of diastole because of tachycardia are all detrimental to myocardial perfusion and hence aggravate the cardiac failure. The descending aorta shows diastolic flow reversal as a result of decreased flow in the descending aorta and a steal phenomenon from the intracranial high-flow fistulas.12 Low perfusion eventually results in renal, hepatic, and multiple organ failure. Hepatomegaly with hepatic dysfunction, prerenal azotemia followed by oliguria, and metabolic and lactic acidosis are present in neonates with severe cardiac failure and are negative prognostic factors. After a brief initial period of stabilization, in most cases the neonate’s congestive heart failure (CHF) worsens during the first 3 to 5 days of life, followed by stabilization and improvement if the patient responds to appropriate medical management. Prenatal diagnosis of cardiac failure is a poor prognostic sign and is usually associated with major neonatal cerebral ischemia and encephalomalacia.7
The cardiac manifestations of antenatally diagnosed VGAM have been reviewed.13 In Rodesch and colleagues’ series of 18 patients with antenatally diagnosed VGAM, 17 were born with cardiac failure.13 During antenatal ultrasound examination, cardiac enlargement was noted in 4 of the 17 patients. These 4 patients all had a low neonatal score because of systemic failure or encephalomalacia. Treatment was withheld in these 4 patients and they soon died. The others were managed medically and underwent embolization at between 2 and 13 months. Their neurological outcome was reported to be excellent at the 2-year follow-up. An antenatal diagnosis of VGAM is not an absolute indication for emergency embolization at a neonatal age. Macrocrania discovered in utero is not a negative prognostic factor in our experience.
For neonatal high-output cardiac failure, medical treatment is initiated with diuretics and fluid restriction to reduce cardiac preload. Mechanical ventilation may be necessary for severe heart failure. Cardiac output can be improved by increasing cardiac contractility with inotropic agents. The effect of digitalis on the hyperkinetic state secondary to AV fistulas remains controversial, although it is, in general, the main agent used to increase myocardial contractility. There is no clear evidence that a further increase in the contractility of already hyperfunctioning myocardium with digitalis has any clinical benefit. In addition, proper digitalization is difficult in neonates with CHF because it affects liver and kidney function. We recently started digitalizing the fetus through the mother if the diagnosis of VGAM was established in utero. This seems to better help control the digitalis level in the newborn period than if digitalis treatment is started after birth (unpublished data). In neonates with severely compromised cardiac output, dobutamine and dopamine can be used to augment myocardial contractility. Nitric oxide is used for pulmonary hypertension. The goal of medical treatment is to control the cardiac failure so that the baby can tolerate oral feeding and gain weight. If the baby can be discharged home, treatment of the VGAM is scheduled at approximately 5 months of age. If medical treatment is not sufficient to control the cardiac failure, the patient needs emergency embolization in the neonatal period (see Figs. 208-1 and 208-2). The cardiac status of the patient usually improves significantly after embolization, even if complete occlusion of the VGAM cannot be achieved.14
Hydrodynamic Disorder
We use the term hydrodynamic disorder to describe a state of disturbed absorption of cerebrospinal fluid (CSF) and venous hypertension caused by the intracranial AV shunting that occurs in patients with VGAM. It can develop in fetuses, neonates, infants, and young children but usually occurs in infants and young children. The arachnoid granulations are the main mechanism of CSF absorption in the matured brain; they are first recognized around 35 weeks after birth and develop throughout childhood. In neonates and infants, medullary veins are presumed to be the main absorption pathway for CSF. CSF in the ventricle moves freely into the extracellular space of the ependymal cells of the ventricular wall and then into the medullary veins via capillaries. The driving force of this absorption mechanism is a sump effect of the negative pressure in the dural sinuses. Another characteristic feature of the neonatal brain is that the venous drainage of the cerebrum is not connected to the cavernous sinus. Connection of the sylvian vein to the cavernous sinus occurs several months after birth, before which all venous drainage of the cerebrum converges to the torcular. Under these specific anatomic and physiologic circumstances of the brain in neonates and infants, the existence of high-flow AV shunting causes venous hypertension in the superior sagittal sinus, which results in CSF malabsorption. Malabsorption of CSF is also aggravated by pulmonary hypertension, skull base maturation, and premature closure of the cranial sutures.15
The initial manifestations of this disorder are ventriculomegaly (see Fig. 208-3) and macrocranium because of adaptation of the skull to the enlarged CSF space by splaying of the sutures. When it progresses, increased intracranial pressure and developmental delay take place. Thickening of the diploic space of the skull can also occur as a result of the development of collateral circulation to the scalp veins and lymphatic channels.
The existence of high-flow AV shunts inhibits maturation of the jugular bulb, which usually occurs over a period of several months after birth, and causes persistent patent embryonic sinuses such as the occipital sinus and the marginal sinus. Persistent flow to these patent embryonic sinuses from the torcular, in turn, prevents maturation of the sigmoid sinus. When the embryonic sinuses eventually close, the immature sigmoid sinus and jugular bulb may also be occluded. High-flow angiopathy of the dural sinuses as a result of high-flow AV shunts and abnormal maturation of the skull base as a result of macrocrania are suspected causes of sinus occlusion, but the mechanisms are unknown. If the alternative pathway of venous drainage of the brain from cortical veins to the cavernous sinus is established at the time of sinus occlusion, the symptoms of venous hypertension are relatively mild because the brain can drain to the tributaries of the cavernous sinus. Drainage pathways from the cavernous sinus include the ophthalmic vein to the facial vein, the pterygoid plexus, and the inferior petrosal sinus to the internal jugular vein. Increased flow to the ophthalmic vein as a result of venous drainage of the brain, as well as AV shunting, can cause exophthalmos and engorgement of the facial veins (see Fig. 208-4). If the alternative pathway of venous drainage of the brain is not established at the time of sinus occlusion, significant symptoms of venous hypertension, such as seizures, hemorrhages, and neurological deficits, can develop as a result of pial cortical venous reflux.16
There are two pathways of venous drainage of the posterior fossa in neonates and infants. One is anterior drainage to the cavernous sinus through the petrosal vein and the superior petrosal sinus, and the other is inferior drainage to the jugular bulb and the spinal cord veins. When occlusion of the transverse/sigmoid sinuses or the jugular bulb/vein occurs, venous congestion of the cerebellum develops. This venous congestion is severe if the cavernous sinus is underdeveloped and leads to tonsillar prolapse. The prolapse is not related to global increased intracranial pressure and is not an indication for emergency ventricular shunting but rather an indication for endovascular embolization to decrease the venous hypertension. The tonsillar prolapse is reversible if the venous congestion of the cerebellum is alleviated early enough by endovascular embolization of the VGAM. Hydrocephalus results from impaired reabsorption of CSF secondary to venous hypertension. Although compression of the aqueduct of Sylvius by the VGAM may be present, obstructive hydrocephalus is rare. It is also possible for the ventriculomegaly to progress without increased intracranial pressure because of subependymal atrophy. Chronic venous ischemia of the brain induces subcortical white matter calcification. It is clinically manifested as irritability initially and then as psychomotor developmental delay. In the advanced stage, seizures, hemorrhages, and focal neurological deficits can develop. Ventricular shunting interferes with water balance between the extracellular and intravascular compartments. It reverses the normal pressure gradient from the ventricle to the brain surface. Such reversal causes brain edema, further enlargement of the dilated draining vein of the VGAM, calcification of the white matter opposite the side of shunt placement, and subependymal atrophy. Furthermore, acute changes in the pressure gradient may give rise to subdural hematoma and slit ventricles. If the venous hypertension is reduced early enough by endovascular embolization, hydrocephalus is prevented or treated without placement of a ventricular shunt. If endovascular treatment is delayed until after the development of hydrocephalus, ventricular shunt placement may be necessary even if endovascular embolization succeeds in significantly reducing the AV shunts. Even then, the complication of ventricular shunting is reduced if the VGAM has been well treated by endovascular embolization. Third ventriculostomy may be sufficient and more preferable than ventricular shunting for the treatment of hydrocephalus if it is combined with embolization of the VGAM.15
Melting Brain Syndrome
We use the term melting brain syndrome to describe an advanced stage of the hydrodynamic disorder. In this situation, cerebral blood flow is decreased because of venous hypertension, and the brain parenchyma is acutely and progressively destroyed, mainly in the white matter. The ventricular system is enlarged, but intracranial pressure is not elevated. Although it occurs bilaterally and symmetrically, brain atrophy around a pial AVM can be regarded as a focal expression of the same phenomenon. Melting brain syndrome can occur in fetuses, neonates, and infants, but it is not observed in adults. It can be associated with all types of AV fistulas, including VGAMs, pial AVMs, and dural sinus malformations.15 In general, emergency treatment is indicated when the early stage of this phenomenon is detected because it is inclined to progress rapidly and irreversibly once it starts. In VGAMs, melting brain syndrome tends to occur in fetuses and neonates and is indicative of a poor prognosis.
Treatment
A major breakthrough in the treatment of VGAM began in the early 1980s with endovascular embolization.17–21 Before the introduction of endovascular embolization for the treatment of VGAMs, surgery had been performed with uniformly poor results.22 Mickle and Quisling introduced the neurosurgical transtorcular venous approach to control severe CHF in 1986.23 The first complete transarterial occlusion plus cure was reported by Lasjaunias and coworkers in 1989.24–26 If treatment is properly timed and performed by a well-trained group in pediatric interventional neuroradiology/endovascular neurosurgery, embolization of the VGAM is the present treatment of choice and can lead to good clinical outcomes. Medical, surgical, or radiosurgical treatment (or any combination of the three) is also used as an adjunct to endovascular therapy.
Indications for and Goal of Treatment
The final goal of treatment of a VGAM is complete obliteration of the lesion and normal development of the patient without neurological deficits. The immediate treatment goal, however, depends on the age and the symptoms of the patient. The primary goal of treatment in neonates is to alleviate the CHF so that they can tolerate oral feeding and gain weight. Once this goal is achieved, the patient is discharged with oral cardiac medication and brought back in several months for further treatment. Complete occlusion of the lesion is not the objective at this age because of the increased risk for complications and limitation in the use of contrast material. The extent of angiographic evaluation before treatment should be limited in neonates because they usually have compromised cardiac and renal function and cannot tolerate a significant volume and load of contrast media. Treatment is much safer and easier if the patient is clinically stable and weighs several kilograms more than the birth weight. If significant brain damage is noted on computed tomography (CT), ultrasonography, or MRI, treatment is not indicated because the clinical outcome is poor even if the lesion is anatomically cured.27–29
For infants and children, the immediate goal is to restore the normal hydrovenous equilibrium to permit normal development of the patient. Our special interest in patients at this age is to avoid ventricular shunting by performing timely endovascular treatment. If performed before the full development of hydrocephalus and its clinical symptoms, transarterial embolization is effective in decreasing venous pressure, improving the clinical symptoms of the hydrodynamic disorder, and avoiding placement of a ventricular shunt. Therefore, urgent embolization should be performed if the head circumference increases rapidly, regression in clinical milestones or a significant developmental delay occurs, or increased CSF spaces, subcortical calcifications, or other evidence of increased intracranial pressure is detected by CT or MRI (see Fig. 208-3). If endovascular treatment is performed after the full development of hydrocephalus, the effect of embolization is usually insufficient, and third ventriculostomy or a ventricular shunt should then be considered. Of note, embolization should be avoided for at least a few days after placement of a ventricular shunt to avoid the risk for upward cerebellar herniation secondary to a rapid decrease in supratentorial pressure. For a mural-type VGAM, complete obliteration of the lesion can usually be achieved in one or two sessions of endovascular treatment. For a choroidal-type VGAM, several sessions of staged embolization may be necessary over a period of several years to achieve complete obliteration. In such a case, the treatment interval is determined according to the response of the patient to the treatment. Complete obliteration of the VGAM fistulas is not necessary to achieve hydrovenous equilibrium and clinical stabilization. If a patient is clinically stable, the next treatment is planned for 3 to 6 months after the previous treatment until either complete obliteration is accomplished or technical limitations prevent further safe and effective embolization, in which case technical advancements in the future may be awaited for further treatment.
Pretherapeutic Evaluation
Pretherapeutic evaluation of a patient with a VGAM should assess the following information: (1) physical parameters, including weight and head circumference and their changes in time; (2) information on the lesion obtained by MRI, magnetic resonance angiography (MRA), and magnetic resonance venography (MRV), including feeders, venous dilation, and sinus occlusion; (3) information about the brain, such as congestion, encephalomalacia, atrophy, calcification, and the size of the ventricles, obtained by transfontanelle ultrasound, CT, and MRI; (4) cardiac status, including associated cardiac anomalies, determined by clinical assessment and cardiac echocardiography; and (5) renal and liver function and coagulation profile. Angiography in neonates and infants should be performed only when embolization is being considered at the same setting. In view of the limited arterial access, diagnostic angiography alone is not indicated.30
The indications for and timing of treatment should be decided after careful assessment of the patient’s neurological symptoms, growth and development, cardiac and other systemic manifestations, and imaging studies of the VGAM and the brain parenchyma. If the patient is clinically stable with or without cardiac medication, it is preferable to delay the treatment until 5 to 6 months of the age. Lasjaunias and associates created a neonatal scoring system that includes cardiac, cerebral, respiratory, hepatic, and renal function.15 According to these authors, treatment is not indicated in those with a score of less than 8 out of 21, a score between 8 and 12 is an indication for emergency endovascular treatment, and a score of more than 12 out of 21 is an indication for medical management until the child is 5 months of age. In their experience, 42 of 140 neonates with VGAM were not treated because of a low neonatal score, and only 23 patients were treated during the neonatal period.7 We use less strict exclusion criteria and more individualized inclusion criteria for treatment.
Endovascular Treatment
Endovascular treatment of VGAMs is performed either by occluding the fistula sites via a transarterial approach or by occluding the ectatic vein via a transvenous approach. Transfemoral transarterial embolization is our first and predominant choice of treatment. A transumbilical artery approach is possible for newborn patients and sometimes preferable because of the small size of the femoral artery.31 The risk for immediate or delayed hemorrhagic complications is significantly less with transarterial embolization. Many centers use a combination of transarterial and transvenous embolizations, usually in multiple stages. If the venous route is being considered, one must be absolutely certain that the dilated vein is not connected to normal cerebral veins.
A 4 French sheath is used for the transfemoral access. Doppler ultrasound is a useful aid for femoral artery puncture in a neonate. Surgical exposure of the femoral artery is usually no longer necessary. Pretherapeutic angiography is performed with a 4 French catheter and low-osmolarity, nonionic contrast material. In neonates with CHF, we proceed to the initial transarterial embolization immediately after the first angiographic injection without completing a full angiographic assessment because of the limited toleration of contrast material by these patients (see Fig. 208-1). The first angiographic injection, therefore, should be for the vessel harboring the largest fistula, which is the first target for embolization. This vessel should be determined on the basis of MRI or MRA but is usually the left or right vertebral artery. In most neonates, up to 8 mL/kg body weight of contrast material is well tolerated. The total amount of contrast material that can be tolerated by a patient depends on the duration of the procedure and urinary output. In older patients, full angiography can generally be performed before initiation of the endovascular treatment.
Transarterial embolization is performed with a flow-guided microcatheter through the 4 French guiding catheter. We almost always use N-butyl-2-cyanoacrylate (NBCA) as a transarterial embolic agent because we believe that it is the best agent for closing the fistula site itself, which is the key for successful treatment. To close a high-flow fistula, we use a high concentration of NBCA of greater than 70% mixed with Ethiodol and tantalum powder for radiopacity. The high-concentration NBCA mixture is injected under systemic hypotension to avoid migration of the mixture to the lung.30 Coils tend to result in proximal occlusion of the feeder, which often induces collateralization without occluding the fistula site and makes further treatment difficult. Complete closure of the malformation can be accomplished in one session, particularly in patients with a mural-type VGAM. We have not experienced perfusion pressure breakthrough phenomena in our series. After total or nearly total occlusion of the fistulas, progressive thrombosis and shrinkage of the ectatic vein is observed on CT or MRI over a period of several weeks. In patients with more complex arterial supply, especially with choroidal VGAMs, embolization should be staged. The clinical symptoms usually improve even after partial embolization.
Transvenous embolization of a VGAM may be accomplished from either a percutaneous transfemoral or a transtorcular approach. Transtorcular treatment can be performed by either surgical exposure of the torcular or ultrasound-guided percutaneous penetration of the overlying dura with a needle.32 Reduction of AV shunting is achieved by packing the venous pouch with a variety of materials, including coils,32,33 nylon,33 and balloons.32 Several sessions of treatment may be required to achieve a satisfactory response.23,34,35 The extent of the embolization may be monitored during the procedure by injection of contrast material transarterially or directly into the pouch or, alternatively, by measurement of intra-aneurysmal pressure.36 One may also consider angioplasty and stenting of the dural sinus for progressive sigmoid/jugular occlusion and severe intracranial venous hypertension when endovascular embolization does not improve the symptoms.37 The long-term durability of dural sinus stenting is unknown, however.
The venous approach is technically easier but is associated with a higher rate of postprocedure hemorrhage than is the case with transarterial embolization because of the sudden increase in venous backpressure with a patent fistula. The venous approach is contraindicated when the venous pouch is connected to subependymal veins via the choroidal veins because of an even higher rate of postprocedure hemorrhage. The long-term neurological outcome after venous embolization is less clear, and it is contraindicated for VGAD associated with a pial AVM.38 A combined transvenous and transarterial embolization may be beneficial for certain patients in whom complete occlusion of the fistulas cannot be achieved by the transarterial approach alone. In such cases, transvenous embolization is performed at the end to close the small residual fistulas for complete obliteration of the malformation.
Surgical Treatment
Surgery is no longer indicated as the primary treatment of VGAM because the surgical results are uniformly poor. Johnston and associates’ review in 1987 showed 38% to 91% mortality in the overall group and 33% to 77% mortality in the operated group.22 Mickle and coauthors reported 26% overall mortality with 78% of children having normal neurological status on follow-up after transarterial embolization of more than 120 VGAMs.23,39 Surgical craniotomy to allow transtorcular embolization may be considered when an arterial or venous transfemoral approach is not possible, although we have not needed this approach for the past 14 years.
Treatment Results
Review of the literature shows that the outcome of both conservative management and surgical treatment has been poor, particularly for newborns and infants. In Johnston and coworkers’ review of 245 cases, overall mortality was 91.4% in the neonatal group, with the majority of patients dying within 1 week of diagnosis or treatment.22 Mortality was 100% (12 patients) without treatment, 95% (38/40) with medical treatment, and 82% (14/17) with surgical treatment. In patients 1 to 12 months of age, the overall mortality was 48%, and 50% of the survivors were neurologically impaired. Mortality was 73% (8/11) without treatment, 33% (2/6) with treatment only for CHF, 64% (7/11) with shunt placement only, and 32% (13/41) with direct surgery, although they most likely included a significant number of pial AVMs with VGAD, which are poor surgical candidates.
Lasjaunias and coauthors reported the results of endovascular treatment in the largest number of patients.7 Their technical strategies for embolization are similar to ours, and they predominantly perform transarterial embolization with NBCA. In their series of 216 patients treated with embolization, there were 23 neonates, 153 infants, and 40 children. The mortality after embolization was 10.6% overall, including 52% in neonates, 7.2% in infants, and 0% in children. Of the 193 patients who survived after embolization, 74% were neurologically normal, 15.6% were moderately retarded, and 10.4% were severely retarded. In these 193 patients, 55% had 90% to 100% occlusion, 38.5% had 50% to 90% occlusion, and 6.2% had less than 50% occlusion of the lesion with embolization. These results are significantly better than those of surgical or conservative management.
Spontaneous thrombosis of the venous pouch or its outlets (or both) has rarely been reported.40 It appears to occur more frequently in mural-type than in choroidal-type VGAMs. It may be associated with increased intracranial pressure, venous ischemic episodes, or both.
Follow-Up
After complete or nearly complete obliteration of the fistulas by embolization, we perform follow-up angiography in 6 to 12 months. Complete obliteration has thus far been reconfirmed on follow-up angiography in all cases. Many cases of nearly complete obliteration progressed to complete obliteration on follow-up angiography, especially in those with mural-type VGAMs. In these cases, the dilated draining vein and the falcine sinus were completely thrombosed and shrunk, with deep venous drainage of the brain being maintained through the collateral pathway. After angiographic confirmation of complete occlusion of the fistulas, follow-up MRI is performed every 1 to 3 years. After partial occlusion of the fistulas, the timing of the next embolization is determined by clinical assessment of the symptoms and the complexity of the residual lesion. If clinically stable, we usually bring back the patient for next treatment in 6 to 12 months. We perform MRI under the same anesthesia before each treatment to evaluate the brain. We sometimes observe the development of dural fistulas either at the venodural junction of the dilated vein or near the torcular after partial embolization of the VGAM or VGAD (see Fig. 208-5).
Andeweg J. Intracranial venous pressures, hydrocephalus and effects of cerebrospinal fluid shunts. Childs Nerv Syst. 1989;5:318-323.
Berenstein A, Masters LT, Nelson PK, et al. Transumbilical catheterization of cerebral arteries. Neurosurgery. 1997;41:846-850.
Berenstein A, Niimi Y, Song J, et al. Vein of Galen aneurysmal malformation. In: Albright A, Pollack I, Adelson P, editors. Principles and Practice of Pediatric Neurosurgery. 2nd ed. New York: Thieme; 2008:1014-1028.
Casasco A, Lylyk P, Hodes JE, et al. Percutaneous transvenous catheterization and embolization of vein of Galen aneurysms. Neurosurgery. 1991;28:260-266.
Fournier D, Rodesch G, Terbrugge K, et al. Acquired mural (dural) arteriovenous shunts of the vein of Galen. Report of 4 cases. Neuroradiology. 1991;33:52-55.
Johnston IH, Whittle IR, Besser M, et al. Vein of Galen malformation: diagnosis and management. Neurosurgery. 1987;20:747-758.
Lasjaunias P, Garcia-Monaco R, Rodesch G, et al. Vein of Galen malformation. Endovascular management of 43 cases. Childs Nerv Syst. 1991;7:360-367.
Lasjaunias P, Rodesch G, Terbrugge K, et al. Vein of Galen aneurysmal malformations. Report of 36 cases managed between 1982 and 1988. Acta Neurochir (Wien). 1989;99:26-37.
Lasjaunias P, Ter Brugge K, Berenstein A. Vein of Galen aneurysmal malformation. In Surgical Neuroangiography 3. Clinical and Interventional Aspects in Children, 2nd ed, Berlin: Springer; 2006:105-226.
Lasjaunias P, Ter Brugge K, Berenstein A. Introduction and general comments regarding pediatric intracranial arteriovenous shunts. In Surgical Neuroangiography 3. Clinical and Interventional Aspects in Children, 2nd ed, Berlin: Springer; 2006:27-104.
Lasjaunias P, Terbrugge K, Choi IS. Trans-mesencephalic arteries and veins. Angiographic aspects in tectal vascular lesions. Acta Neurochir (Wien). 1988;92:138-143.
Lasjaunias P, Terbrugge K, Piske R, et al. [Dilatation of the vein of Galen. Anatomoclinical forms and endovascular treatment apropos of 14 cases explored and/or treated between 1983 and 1986.]. Neurochirurgie. 1987;33:315-333.
Lylyk P, Vinuela F, Dion JE, et al. Therapeutic alternatives for vein of Galen vascular malformations. J Neurosurg. 1993;78:438-445.
Mickle JP, Quisling RG. The transtorcular embolization of vein of Galen aneurysms. J Neurosurg. 1986;64:731-735.
Mickle JP, Quisling RG. Balloon embolization of high-flow traumatic arteriovenous fistulae to the brain. J Fla Med Assoc. 1982;69:767-774.
Mickle JP, Quisling R, Ryan P. Transtorcular approach to vein of Galen aneurysms. 1985. Pediatr Neurosurg. 1994;20:163-168.
Patton D, Fouron J. Cerebral arteriovenous malformation: prenatal and postnatal cerebral blood flow dynamics. Pediatr Cardiol. 1995;16:141-144.
Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology. 1989;31:109-128.
Rodesch G, Hui F, Alvarez H, et al. Prognosis of antenatally diagnosed vein of Galen aneurysmal malformations. Childs Nerv Syst. 1994;10:79-83.
Rodesch G, Lasjaunias P, Terbrugge K, et al. [Intracranial arteriovenous vascular lesions in children. Role of endovascular technics apropos of 44 cases.]. Neurochirurgie. 1988;34:293-303.
1 Sahs AL, Perret G, Locksley HB, et al. Preliminary remarks on subarachnoid hemorrhage. J Neurosurg. 1966;24:782-788.
2 Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1966;25:321-368.
3 Locksley HB, Sahs AL, Knowler L. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section II. General survey of cases in the central registry and characteristics of the sample population. J Neurosurg. 1966;24:922-932.
4 Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology. 1989;31:109-128.
5 Berenstein A, Lasjaunias P. Arteriovenous fistulas of the brain. In: Surgical Neuroangiography 4. Endovascular Treatment of Cerebral Lesions. Berlin: Springer-Verlag; 1992:267-317.
6 Garcia-Monaco R, Lasjaunias P, Berenstein A. Therapeutic management of vein of Galen aneurysmal malformations. In: Vinuela F, Halbach V, Dion J, editors. Interventional Neuroradiology: Endovascular Therapy of the Central Nervous System. New York: Raven Press; 1992:113-127.
7 Lasjaunias P, Ter Brugge K, Berenstein A. Vein of Galen aneurysmal malformation. In Surgical Neuroangiography 3. Clinical and Interventional Aspects in Children, 2nd ed, Berlin: Springer; 2006:105-226.
8 Lasjaunias P, Terbrugge K, Choi IS. Trans-mesencephalic arteries and veins. Angiographic aspects in tectal vascular lesions. Acta Neurochir (Wien). 1988;92:138-143.
9 Fournier D, Rodesch G, Terbrugge K, et al. Acquired mural (dural) arteriovenous shunts of the vein of Galen. Report of 4 cases. Neuroradiology. 1991;33:52-55.
10 Lasjaunias P, Garcia-Monaco R, Rodesch G, et al. Vein of Galen malformation. Endovascular management of 43 cases. Childs Nerv Syst. 1991;7:360-367.
11 Lasjaunias P, Garcia-Monaco R, Rodesch G, et al. Deep venous drainage in great cerebral vein (vein of Galen) absence and malformations. Neuroradiology. 1991;33:234-238.
12 Patton D, Fouron J. Cerebral arteriovenous malformation: prenatal and postnatal cerebral blood flow dynamics. Pediatr Cardiol. 1995;16:141-144.
13 Rodesch G, Hui F, Alvarez H, et al. Prognosis of antenatally diagnosed vein of Galen aneurysmal malformations. Childs Nerv Syst. 1994;10:79-83.
14 Garcia-Monaco R, De Victor D, Mann C, et al. Congestive cardiac manifestations from cerebrocranial arteriovenous shunts. Endovascular management in 30 children. Childs Nerv Syst. 1991;7:48-52.
15 Lasjaunias P, Ter Brugge K, Berenstein A. Introduction and general comments regarding pediatric intracranial arteriovenous shunts. In Surgical Neuroangiography 3. Clinical and Interventional Aspects in Children, 2nd ed, Berlin: Springer; 2006:27-104.
16 Andeweg J. Intracranial venous pressures, hydrocephalus and effects of cerebrospinal fluid shunts. Childs Nerv Syst. 1989;5:318-323.
17 Berenstein A, Krischeff II. Catheter and material selection for transarterial embolization: technical considerations. I. Catheters. Radiology. 1979;132:619-630.
18 Berenstein A, Kricheff II. Microembolization techniques of vascular occlusion: radiologic, pathologic, and clinical correlation. AJNR Am J Neuroradiol. 1981;2:261-267.
19 Berenstein A, Kricheff II. Catheter and material selection for transarterial embolization: technical considerations. II. Materials. Radiology. 1979;132:631-639.
20 Berenstein A, Kricheff II. Therapeutic vascular occlusion. J Dermatol Surg Oncol. 1978;4:874-880.
21 Berenstein A, Kricheff II. A new balloon catheter for coaxial embolization. Neuroradiology. 1979;18:239-241.
22 Johnston IH, Whittle IR, Besser M, et al. Vein of Galen malformation: diagnosis and management. Neurosurgery. 1987;20:747-758.
23 Mickle JP, Quisling RG. The transtorcular embolization of vein of Galen aneurysms. J Neurosurg. 1986;64:731-735.
24 Lasjaunias P. Vein of Galen malformations. Neurosurgery. 1989;25:666-667.
25 Lasjaunias P, Rodesch G, Pruvost P, et al. Treatment of vein of Galen aneurysmal malformation. J Neurosurg. 1989;70:746-750.
26 Lasjaunias P, Rodesch G, Terbrugge K, et al. Vein of Galen aneurysmal malformations. Report of 36 cases managed between 1982 and 1988. Acta Neurochir (Wien). 1989;99:26-37.
27 Friedman DM, Madrid M, Berenstein A, et al. Neonatal vein of Galen malformations: experience in developing a multidisciplinary approach using an embolization treatment protocol. Clin Pediatr (Phila). 1991;30:621-629.
28 Friedman DM, Verma R, Madrid M, et al. Recent improvement in outcome using transcatheter embolization techniques for neonatal aneurysmal malformations of the vein of Galen. Pediatrics. 1993;91:583-586.
29 Nass R, Kramer E, Molofsky W, et al. Perfusion brain scintigraphy studies in infants and children with malformations of the vein of Galen. Childs Nerv Syst. 2001;17:519-523.
30 Berenstein A, Niimi Y, Song J, et al. Vein of Galen aneurysmal malformation. In: Albright A, Pollack I, Adelson P, editors. Principles and Practice of Pediatric Neurosurgery. 2nd ed. New York: Thieme; 2008:1014-1028.
31 Berenstein A, Masters LT, Nelson PK, et al. Transumbilical catheterization of cerebral arteries. Neurosurgery. 1997;41:846-850.
32 Lylyk P, Vinuela F, Dion JE, et al. Therapeutic alternatives for vein of Galen vascular malformations. J Neurosurg. 1993;78:438-445.
33 Borthne A, Carteret M, Baraton J, et al. Vein of Galen vascular malformations in infants: clinical, radiological and therapeutic aspect. Eur Radiol. 1997;7:1252-1258.
34 Mickle JP, Quisling RG. Balloon embolization of high-flow traumatic arteriovenous fistulae to the brain. J Fla Med Assoc. 1982;69:767-774.
35 Mickle JP, Quisling RG. Vein of Galen fistulas. Neurosurg Clin N Am. 1994;5:529-540.
36 Casasco A, Lylyk P, Hodes JE, et al. Percutaneous transvenous catheterization and embolization of vein of galen aneurysms. Neurosurgery. 1991;28:260-266.
37 Brew S, Taylor W, Reddington A. Stenting of a venous stenosis in vein of Galen aneurysmal malformation. Intervent Neuroradiol. 2001;7:237-240.
38 Lasjaunias P, Terbrugge K, Piske R, et al. [Dilatation of the vein of Galen. Anatomoclinical forms and endovascular treatment apropos of 14 cases explored and/or treated between 1983 and 1986.]. Neurochirurgie. 1987;33:315-333.
39 Mickle JP, Quisling R, Ryan P. Transtorcular approach to vein of Galen aneurysms. 1985. Pediatr Neurosurg. 1994;20:163-168.
40 Rodesch G, Lasjaunias P, Terbrugge K, et al. [Intracranial arteriovenous vascular lesions in children. Role of endovascular technics apropos of 44 cases.]. Neurochirurgie. 1988;34:293-303.