Chapter 28 Vasculogenic Erectile Dysfunction
Introduction
The first historical descriptions of erectile dysfunction (ED) date back to Egyptian papyrus nearly 4000 years ago. Egyptian scholars described two types of ED: a “natural” form in which the man was incapable of performing the sex act, and a “supernatural” form rooted in evil charms and spells.1 Ancient thinkers such as Hippocrates and Aristotle also theorized on the etiology of ED. However, the first accurate depiction of penile anatomy and rudimentary analysis of erection was not published until 1585, when Ambroise Paré described it in his Ten Books on Surgery and the Book of Reproduction.2 In these texts, Paré portrayed the penis as a tube with concentric coats of nerves, veins, arteries, two “ligaments” composed of the corpora cavernosa, and the urinary tract.
Definition and Classifications
In 1992, the National Institutes of Health convened a Consensus Development Conference on Impotence. The group renamed impotence as male erectile dysfunction and defined it as “the inability to achieve or maintain an erection sufficient for satisfactory sexual performance.”3 Furthermore, they noted that erectile dysfunction represents the most appropriate term, given that sexual desire, orgasm, and ejaculation may be intact despite inability to achieve or maintain erection.
Multiple schema have been proposed to classify the different types of ED. Broadly, ED can be described in terms of organic and psychogenic dysfunction (Box 28-1). The main thrust of this chapter will center on vasculogenic ED, which comprises impaired endothelial function, arterial occlusive disease, veno-occlusive dysfunction, and structural changes to the corpora cavernosa.
Prevalence and Incidence
Erectile dysfunction is quite common, affecting approximately 30 million men in the United States.4 Several population-based studies have been performed to address male sexual function and specifically the prevalence and incidence of ED in the American male population. The 1992 National Health Social and Life Survey (NHSLS) was a national survey of 1410 American men between the ages of 18 and 59. In the study group, the prevalence of ED in men aged 18 to 29 years was 7%, aged 30 to 39 was 9%, aged 40 to 49 was 11%, and aged 50 to 59 was 18%.5 The Massachusetts Male Aging Study (MMAS), a longitudinal population-based study, evaluated 1709 men between the ages of 40 and 70 who returned questionnaires about a broad range of physiological measures, demographic information, and self-reported sexual function. Participants were surveyed between the years 1987 and 1989 and then reevaluated between 1995 and 1997. In this series, the age-adjusted prevalence of significant ED was 39% in men with coronary artery disease (CAD), 25% in men with diabetes mellitus, and 15% in men with hypertension. Incidence of ED on reevaluation was 25.9 cases per 1000 men per year (95% confidence interval [CI], 22.5-29.9).6 Using these data, it was estimated that for Caucasian men, 617,715 new cases of ED would present in the 40 to 69 age group each year.7 Data from European and Brazilian researchers suggest a similar incidence of ED in their respective countries.8,9
Functional Anatomy
Corporal Bodies, Sinusoids, and Glans
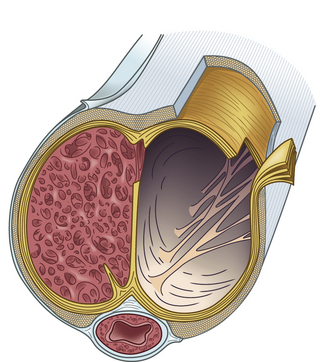
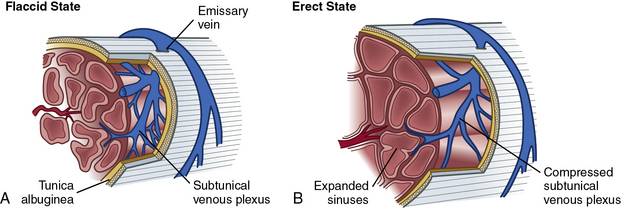
Within the corpora, interconnected sinusoids are enveloped by trabeculae of smooth muscle, collagen, and elastin (Fig. 28-1). The sinusoidal smooth muscle is in intimate association with the cavernous nerves and helicine arteries within the penis. The sinusoids are tonically constricted during the flaccid state. Arterial blood flow diffuses through larger central sinusoids to smaller peripheral sinusoids. In the flaccid state, this slow diffusion of arterial blood results in blood gas values similar to venous blood. During sexual stimulation, release of neurotransmitters causes the smooth muscle around the sinusoids to relax. This results in rapid influx of arterial blood, subsequent entrapment of blood within these expanding sinusoids, and occlusion of veins traversing the tunica albuginea. Subsequent tumescence results in pressure increases of several hundred mmHg and blood gas values approaching arterial levels.10
Tunica Albuginea
The tunica albuginea is composed primarily of tough type I collagen with a minority component of more flexible type III collagen and elastin. It is arranged in a bilayer, with inner circular layers and outer longitudinal layers (see Fig. 28-1). Intervening struts traverse the body of the corpora cavernosa and provide further support.11 The longitudinal layers of the tunica are present from the glans to the proximal crura, where each corporal body inserts into its ischial ramus to form a foundation for support of the erect penis. Emissary veins (Fig. 28-2) pierce the tunica albuginea. During engorgement, these veins become compressed and allow entrapment of blood within the penis.
Arterial System
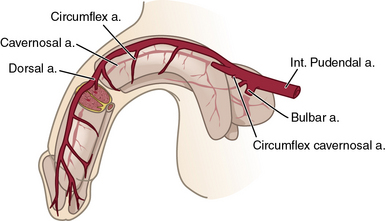
The internal pudendal artery, a branch of the internal iliac artery (IIA), is the principal source of blood flow to the penis. Up to 70% of men may have accessory pudendal branches that originate from the external iliac, obturator, or vesical arteries.12 The internal pudendal artery gives rise to the penile artery, which in turn branches in to the dorsal, bulbourethral, and cavernous arteries (Fig. 28-3). The cavernous artery supplies the corpus cavernosum via helicine arteries, which lie in close approximation to the sinusoidal tissue. During erection, these vessels dilate, resulting in engorgement.
Venous System
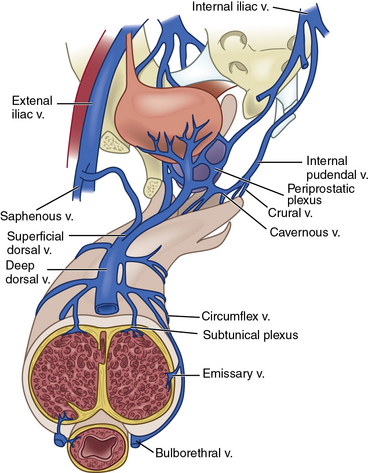
Venous drainage originates from the three corporal bodies. Venules interdigitate through the cavernosal sinusoids and coalesce below the tunica albuginea into a subtunical plexus. The plexi then form emissary veins that penetrate the tunica albuginea. From there, numerous subcutaneous veins course along the shaft of the penis to form the superficial dorsal vein and a deep dorsal venous system, which in turn drain into the saphenous vein and retropubic venous plexus, respectively13 (Fig. 28-4; also see Fig. 28-2).
Nervous System
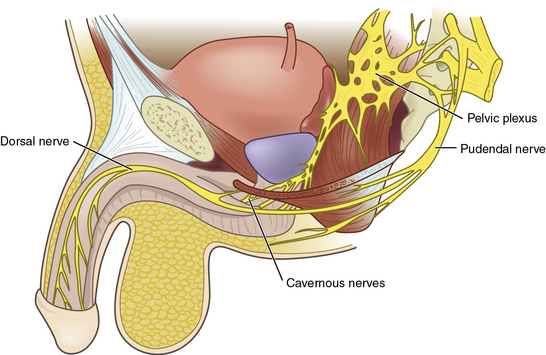
Penile innervation occurs via both autonomic (parasympathetic and sympathetic) and somatic (motor and sensory) pathways. Erection and detumescence are largely regulated via the autonomic system. Sympathetic and parasympathetic nerves coalesce to form the cavernous nerve, which penetrates the corpora cavernosa to exert its effect on erection (Fig. 28-5). Sensation and contraction of penile musculature occurs via the somatic nerves.
Autonomic pathways
Between the T11 and L2 spinal segments, the sympathetic trunk begins. These fibers then form the sympathetic chain ganglia, which continue caudally to the inferior mesenteric and superior hypogastric plexi. Further sympathetic fibers exit to form the hypogastric nerves, and ultimately the sympathetic portions of the pelvic plexus.14
Between the S2-S4 spinal cord segments, the parasympathetic pathway originates. These fibers also continue caudally to the pelvic plexus (see Fig. 28-5), where they join the aforementioned sympathetic nerves. Together, these nerves then join to form a network of nervous tissue that passes along the lateral and posterior aspect of the prostate to create the cavernous nerves.15 Stimulation of the sympathetic trunk via the cavernous nerves results in detumescence. Excitation of the parasympathetic aspects of the pelvic plexus and cavernous nerves is responsible for erection. To avoid iatrogenic ED, clear understanding of the location of these nerves is critical during pelvic surgery such as radical prostatectomy or abdominal perineal resection.
Somatic pathways
Sensory receptors in the penile skin and glans are unique in the human body.16 They are composed of free nerve endings comprising unmyelinated C fibers and thin myelinated A-delta fibers. These coalesce into the dorsal nerve of the penis, which ultimately forms the pudendal nerve. The pudendal nerve then enters the S2-S4 nerve roots at the spinal cord. Via spinothalamic and spinoreticular pathways, sensations such as touch, pain, and temperature are perceived.17 Interestingly, research by Burnett et al.18 suggests that the dorsal nerve of the penis carries both autonomic and somatic signals, and therefore contributes to penile sensation, erection, and ejaculation.
Pathophysiology of Erectile Dysfunction
Vasculogenic Erectile Dysfunction
As noted in Box 28-1, ED often represents a multifactorial disease state. Although the focus of this chapter is on the vasculogenic determinants of ED, it is worth noting that within an individual patient, neurological, hormonal, or psychological etiologies of ED may be of contributory or even primary importance. With that said, it is clear the vascular system is responsible for providing blood flow to the erectile tissues of the penis, so any dysfunction within the vascular system may affect erectile function.
Arteriogenic erectile dysfunction
Arteriogenic ED can be due to atherosclerotic or traumatic arterial occlusive disease. Michal and Ruzbarsky19 noted impaired penile perfusion is an indicator of generalized atherosclerotic disease, and that the age of onset of ED and CAD is often similar. In fact, ED has been shown to be a bellwether for development of CAD in asymptomatic men,20 and both diseases share the same risk factors—specifically smoking, diabetes, hypercholesterolemia, and hypertension.21
In arteriogenic ED, the corpora cavernosa demonstrate lower oxygen tension,22 which may result in a decreased volume of sinusoidal smooth muscle and subsequent venous leak.23 In an experimental animal model, rabbits with iatrogenic iliac atherosclerotic disease demonstrated alterations in their downstream penile arteries and a reduction in cavernosal smooth muscle content.24 These alterations were associated with decreased nitric oxide synthase (NOS)- and NO-mediated relaxation of corpora cavernosal tissue.25 Erectile dysfunction due to traumatic stenosis of cavernous or pudendal arteries has been noted in young men with pelvic trauma26 and in long-distance cyclists.27
Venogenic erectile dysfunction
Not only can diabetes, hypertension, hypercholesterolemia, and penile injury result in penile arterial disease, these disorders can also result in loss of elastic fibers within the cavernosal venules and sinusoids. This loss of compliance results in diminished venous trapping and subsequent veno-occlusive dysfunction.28 In fact, diminished venous occlusion may represent the most common form of vasculogenic ED.29 Loss of smooth muscle relaxation due to heightened adrenergic tone or decreased NO release may exacerbate already poor compliance in these fibrotic sinusoids.30 Finally, fibrosis leading to increased collagen deposition between cell membranes may abolish critical signaling and intercellular transmission via disrupted gap junctions.31
Drug-Induced (Iatrogenic) Erectile Dysfunction
The data are clear that diabetes, hypercholesterolemia, and hypertension have strong influences on ED. Not surprisingly, some of the medications used to treat these disorders have been implicated as contributing factors in its development. However, it is often difficult to ascertain the direction of causation in medication-induced ED. Of the cardiovascular medications, thiazide diuretics have the strongest association with development of ED. Chang et al.32 demonstrated that men treated with thiazide diuretics showed a significant increase in ED relative to those men prescribed placebo medication. Further evidence of the role of thiazides in ED was noted in the Treatment of Mild Hypertension Study (TOMHS), which demonstrated the prevalence of ED was twofold higher in men taking a thiazide versus placebo or other agent.33 Curiously, after 4 years of treatment in this study, prevalence of ED within the placebo group approached that of those receiving thiazide. The significance of this finding is unclear but may be related to early unmasking of clinically undetected ED in those men receiving thiazides.
Nonselective β-blockers such as propranolol have been shown to inhibit erection compared to placebo, but this has not been demonstrated in selective β1-antagonists.34 In clinical series, there does not appear to be a deleterious influence of either angiotensin-converting enzyme (ACE) inhibitors or calcium channel blockers on erection.33,35 Interestingly, angiotensin receptor antagonists and some statins, such as atorvastatin, appear to improve erectile function.36,37 Of note, some α-blockers, such as terazosin, have occasionally been implicated in the development of priapism, or pathological erection, likely related to the α-adrenergic blockade of the sympathetic outflow necessary for detumescence.38 Other medications implicated in the development of ED include spironolactone, some antipsychotics, selective serotonin reuptake inhibitors, opiates, and antiandrogens.39
Evaluation of Erectile Dysfunction
Evaluation and treatment of a man presenting with ED has changed considerably over the last 30 years. This shift is largely due to the influence of oral therapies for treatment and a transition to a more patient-centered and evidence-based treatment plan. Evaluation should begin with an in-person and detailed medical, sexual, and psychosocial history (Fig. 28-6). Additionally, the use of a quantifiable questionnaire such as the International Index of Erectile Function (IIEF)40 may be useful to establish baseline sexual function and to assess future treatment efficacy.
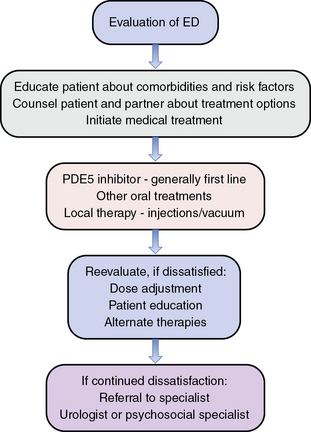
Figure 28-6 Algorithm for evaluation of erectile dysfunction (ED).
(Adapted from Lue TF, Giuliano F, Montorsi F, et al: Summary of the Recommendations on sexual dysfunctions in men, J Sex Med 1:6–23, 2004, and Lue, TF, Broderick, GA: Evaluation and nonsurgical management of erectile dysfunction and premature ejaculation. In Wein AJ, Kavoussi LR, Nocik AC, et al, editors: Campbell-Walsh Urology, ed 9, Philadelphia, 2007, Saunders, pp 751–752.)
History
The history should begin with a thorough discussion of the patient’s medical history, with particular attention to medical comorbidities. As previously mentioned, ED is an early marker for systemic atherosclerotic disease, and all patients should be questioned about their cardiovascular health.41 In particular, a detailed discussion regarding any chest pain, palpitations, dyspnea, and limb pain may reveal occult CAD, congestive heart failure, or peripheral artery disease. Querying the patient about his medication use may elucidate agents that contribute to ED or may be contraindications to oral PDE therapy (e.g., nitrates). A sexual history should focus on timing, duration, and severity of the patient’s ED, as well as the occurrence of other associated problems, such as premature ejaculation, desire, or anorgasmia. To complete the sexual history, a psychosocial evaluation may be relevant; sexual dysfunction may affect self-esteem and coping. Both depression and treatment for depression may be associated with ED.
Laboratory Assessments
Laboratory testing for men with sexual dysfunction may include fasting glucose, lipid levels, sex hormone values (including morning free and total testosterone levels), and other endocrine tests, such as thyroid function tests and prolactin levels. Again, this testing may reveal comorbid conditions such as diabetes, hyperlipidemia, or hypogonadism that may contribute to ED. Prostate-specific antigen (PSA) testing may be considered in men over 40, men with a strong family history of prostate cancer, or men in whom testosterone replacement is being considered, although this is a point of debate in some circles.42 The optimal laboratory test panel for the evaluation of ED has yet to be determined.
Education and Referral
Although rarely performed in 2010 and usually only after an empirical trial of oral PDE5 inhibitors, first-line urological evaluation of penile blood flow consists of a combined intracavernous injection of a single or combination of vasodilators and some form of penile stimulation. This testing allows the urologist to evaluate the specific mechanics of the erectile response and avoid the confounding influence of neurological or hormonal factors. Second-line urological evaluation includes intracavernous injection of a vasodilator and blood flow measurement with duplex ultrasonography, possibly Doppler waveform analysis, and peak systolic velocity calculations. Third-line evaluations may include calculations of cavernous arterial systolic occlusion pressures (CASOP), pharmacological arteriography, pharmacological cavernosometry, or cavernosography.43 These increasingly invasive procedures are often reserved for young men with traumatic pelvic or penile arterial injuries who may be candidates for arterial revascularization.
Treatment
As noted previously, the initial step in penile tumescence is arousal and subsequent release of NO into vascular and cavernous smooth muscle cells (SMCs). This causes stimulation of guanylyl cyclase, with a concomitant rise in cyclic guanosine monophosphate (cGMP) and resultant reduction of cytoplasmic calcium. This leads to smooth muscle relaxation, increased arterial inflow, venous trapping, and subsequent erection.44 With the discovery that PDE inhibitors prevent breakdown of cGMP,45 and ensuing FDA approval of sildenafil in 1998, a new era in ED treatment was born.
Alprostadil (Prostaglandin E1)
Prostaglandin E1 (PGE1) mediates relaxation of corporal cavernosal tissue by activating prostaglandin (PG) receptors and subsequently increasing cyclic adenosine monophosphate (cAMP) levels in the corporal smooth muscle. Elevated cAMP results in reduction of cytosolic calcium and relaxation of smooth muscle. Alprostadil is formulated for both intraurethral placement and intracavernosal injection. Intraurethral administration involves insertion of a pellet approximately 3 mm in size, 2 to 3 cm within the distal urethra. Alprostadil is absorbed via the urethral mucosa, passes through the corpus spongiosum, and then via the emissary veins. The medication passes into the corpora cavernosa to exert its vasodilatory effects. Efficacy of intraurethral alprostadil is about 66% in office placement and about 50% in home placement.46 Penile pain is often a significant side effect of alprostadil treatment.
Intracavernous injection of alprostadil works by the same mechanism as intraurethral placement. Researchers have noted higher efficacy with intracavernosal placement. However, alprostadil is still limited by pain during erections in 16.8% of patients, penile fibrosis in 2%, hematoma at the injection site in 1.5%, and priapism in 1.3% of patients.47 Alprostadil is formulated in a powder and must be reconstituted and refrigerated.
Papaverine and Phentolamine
Other injectable agents include papaverine and phentolamine. Papaverine exerts its effect on penile erectile tissue by an inhibitory action on PDE, resulting in increased cAMP and cGMP as well as inhibition of voltage-gated calcium channels. These mechanisms each result in cavernosal smooth muscle relaxation and subsequent erection. Papaverine’s efficacy is approximately 50%, though it has not been expressly approved by the FDA for use in ED therapy. The incidence of priapism may be as high as 33% in patients receiving solitary papaverine treatment, and the incidence of penile fibrosis with this form of treatment is high.43
Phentolamine is also used for injection therapy but works by a different mechanism; it functions as a competitive α-adrenergic antagonist. It is postulated to induce erection by releasing sympathetic tone and thereby increasing corporal blood flow. Systemic hypotension, reflex tachycardia, and nasal congestion are its principal side effects.48
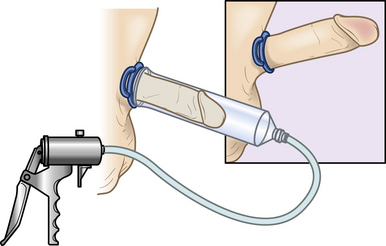
Vacuum Erection Devices
In 1917, Otto Lederer was awarded the first patent for a surgical device to induce and maintain erection.49 Since then, the vacuum erection device (VED) has been modified and perfected, yet the principle remains the same. The VED consists of a cylinder and suction pump that induces erection by negative pressure and subsequent increased corporal flow. A compression band is then placed at the base of the penis to trap engorged blood (Fig. 28-7). The erection is different than a physiological erection in that girth is increased, the penis is cooler, and it is less rigid than a natural erection. However, success rates are good, and patient and partner satisfaction are high. Cookson et al. noted a 90% chance of achieving a good-quality erection with satisfaction rates over 80%.50 For patients failing the treatments discussed, surgery is typically reserved as a final treatment option.
Surgery
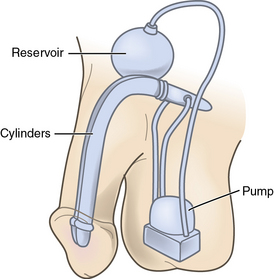
In 1936, a Russian surgeon named Bogoraz was the first person to create a functional autologous penile implant. He used rib cartilage in an attempt to correct ED. Although innovative, his success with this treatment was largely limited by resorption of the cartilage.51 In 1973, Scott ushered in the modern era of penile implantation with development of the three-piece inflatable penile prosthesis.52 Penile prostheses are typically reserved for men with organic ED who have failed or rejected treatments such as oral medications, vacuum erection devices, intraurethral alprostadil, or injection therapy. Three classes of penile implants exist: malleable, semirigid, and inflatable. Malleable and semirigid prostheses are typically placed via a distal penile approach. The inflatable prosthesis comes either as a two-piece or three-piece model that is composed of inflatable cylinders, tubing, a pump mechanism, and a reservoir (Fig. 28-8). Typically these are placed under general anesthetic, via a penoscrotal or infraumbilical incision. Infection rates vary from 3% to 8%.53 Patient and partner satisfaction is over 90%, and freedom from mechanical failure ranges between 80% and 95% at 5 years.54
Longitudinal Psychological Outcomes
There is significant interplay between psychological health and ED, but the direction of that relationship is not always clear. Few studies have evaluated the psychological benefit from ED treatment or baseline psychological characteristics of men with ED. In 2006, we evaluated 153 men in an observational ED registry and collected clinical and psychosocial data at baseline and during follow-up. Of those patients who responded to treatment, these men reported significant improvements in sexual self-efficacy, while nonresponders reported small decrements. Surprisingly, nearly 42% of the patients describing ED were not offered treatment by their physicians.55 Given the benefits in psychological health suggested by this study, and the high incidence of occult cardiovascular disease noted in previous studies, this should be a call to all healthcare providers to actively diagnose and treat ED in their patients.
Guidelines
In 2005, the American Urologic Association convened a consensus group of experts within the discipline of erectile dysfunction.56 The committee created a set of guidelines to help clarify the standard of care, recommended treatments, and offered expert opinions on treatment of ED. The primary findings of these guidelines are presented in Box 28-2 and form the basis of current standard practice.
![]() Box 28-2 American Urological Association Management Guidelines for Erectile Dysfunction
Box 28-2 American Urological Association Management Guidelines for Erectile Dysfunction
Standards of Care
Oral phosphodiesterase type 5 (PDE5) inhibitors, unless contraindicated, should be offered as first-line therapy for erectile dysfunction (ED).
PDE5 inhibitors are contraindicated in patients taking organic nitrates.
The initial trial dose of alprostadil intraurethral suppositories should be administered under healthcare provider supervision, owing to the risk of syncope.
The initial trial dose of intracavernous injection therapy should be administered under healthcare provider supervision.
Physicians who prescribe intracavernous injection therapy should (1) inform patients of the potential occurrence of prolonged erections, (2) have a plan for urgent treatment of prolonged erections, and (3) inform the patient of the plan.
The patient considering prosthesis implantation and, when possible, his partner should be informed of the following: types of prostheses available; possibility and consequences of infection and erosion, mechanical failure, and resulting reoperation; differences from the normal flaccid and erect penis, including penile shortening; and potential reduction of effectiveness of other therapies if the device is subsequently removed.
Prosthetic surgery should not be performed in the presence of systemic, cutaneous, or urinary tract infection.
Antibiotics providing gram-negative and gram-positive coverage should be administered preoperatively.
Recommendations
Monitoring of patients receiving continuing PDE5 inhibitor therapy should include a periodic follow-up of efficacy, side effects, and any significant change in health status, including medications.
Prior to proceeding to other therapies, patients reporting failure of PDE5 inhibitor therapy should be evaluated to determine whether the trial of PDE5 inhibition was adequate.
Patients who have failed a trial with PDE5 inhibitor therapy should be informed of the benefits and risks of other therapies, including use of a different PDE5 inhibitor, alprostadil intraurethral suppositories, intracavernous drug injection, vacuum constriction devices, and penile prostheses.
Only vacuum constriction devices containing a vacuum limiter should be used, whether purchased over the counter or procured with a prescription.
Use of trazodone in the treatment of ED is not recommended.
Testosterone therapy is not indicated for treatment of ED in the patient with a normal serum testosterone level.
Yohimbine is not recommended for treatment of ED.
Herbal therapies are not recommended for treatment of ED.
Surgeries performed with the intent to limit the venous outflow of the penis are not recommended.
Modified from http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm.?sub=ed. Accessed December 2010.
1 Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Campbell-Walsh urology, ed 9,. 2007.
2 Brenot P. Male impotence—a historical perspective. L’ Esprit du Temps. 1994.
3 Impotence. NIH Consens Statement. 1992;10:1.
4 Sun P, Cameron A, Seftel A, et al. Erectile dysfunction—an observable marker of diabetes mellitus? A large national epidemiological study. J Urol. 2006;176:1081–1085.
5 Laumann E, Paik A, Rosen R. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544.
6 Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–463.
7 Lewis RW, Hatzchistou D, Laumann E, et al. Epidemiology and natural history of erectile dysfunction: risk factors including iatrogenic and aging. In: Proceedings of the First International Consultation on Erectile Dysfunction. Health Publication; 2000:21–51.
8 Schouten BW, Bosch JL, Bernsen RM, et al. Incidence rates of erectile dysfunction in the Dutch general population. Effects of definition, clinical relevance, and duration of follow-up in the Krimpen study. Int J Impot Res. 2005;17:58–62.
9 Moreira ED, Lbo CR, Diament A, et al. Incidence of erectile dysfunction in men 40-69 years old: results from a population based cohort in Brazil. Urology. 2003;61:431–436.
10 Sattar AA, Salpigidis G, Schulman CC, et al. Relationship between intrapenile O2 lever and quantity of intracavernous smooth muscle fibers: current physiopathological concept. Acta Urol Belg. 1995;63:53–59.
11 Hsu GL, Brock G, Martinez-Pineiro L, et al. The three dimensional structure of the human tunica albuginea: anatomical and ultrastructural levels. Int J Impot Res. 1992;4:117–129.
12 Droupy S, Benoit G, Giuliano F, et al. Penile arteries in humans. Surg Radiol Anat. 1997;19:161–167.
13 Hsu GL, Hsieh CH, Wen HS, et al. Penile venous anatomy: an additional description and its clinical implication. J Androl. 2003;24:921–927.
14 de Groat WC, Booth A. Neural control of penile erection. In: Maggi CA, ed. The autonomic nervous system. London: Harwood; 1993:465–513.
15 Walsh PC, Brendler CB, Chang T, et al. Preservation of sexual function in men during radical pelvic surgery. Md Med J. 1990;39:389–393.
16 Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res. 1986;371:205–230.
17 McKenna KE. Central control of penile erection. Int J Impot Res. 1998;10(Suppl):S25–S34.
18 Burnett AL, Tillman SL, Chang TS, et al. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J Urol. 1993;150:73–76.
19 Michal V, Ruzbarsky V. Histological changes in the penile arterial bed with aging and diabetes. In: Zorgniotti AW, Rossi G. Vasculogenic impotence: proceedings of the First International Conference on Corpus Cavernosum Revascularization. Ill: Springfield; 1980:113–119.
20 Vlachopoulos C, Rokkas K, Ioakeimidis N, et al. Prevalence of asymptomatic coronary artery disease in men with vasculogenic erectile dysfunction: a prospective angiographic study. Eur Urol. 2005;48:996–1003.
21 Gratzke C, Angulo J, Chitaley K, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–475.
22 Tarhan F, Kuyumcuoglu U, Kolsuz A, et al. Cavernous oxygen tension in the patients with erectile dysfunction. Int J Impot Res. 1997;9:149–153.
23 Nehra A, Azadoi KM, Moreland RB, et al. Cavernosal expandability is an erectile tissue mechanical property which predicts trabecular histology in an animal model of vasculogenic erectile dysfunction. J Urol. 1998;159:2229–2236.
24 Azadzoi KM, Park K, Andry C, et al. Relationship between cavernosal ischemia and corporal veno-occlusive dysfunction in an animal model. J Urol. 1997;157:1011–1017.
25 Azadzoi KM, Krane RJ, Saenz de Tejada I, et al. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992;147:220–225.
26 Levine FJ, Greenfield AJ, Goldstein I. Arteriographically determined occlusive disease within the hypogastric-cavernous bed in impotent patients following blunt perineal and pelvic trauma. J Urol. 1990;144:11147–11153.
27 Richiuti VS, Haas CA, Seftel AD, et al. Pudendal nerve injury associated with avid bicycling. J Urol. 1999;162:2099–2100.
28 Sattar AA, Wespes E, Schulman CC. Computerized measurement of penile elastic fibers in potent and impotent men. Eur Urol. 1994;25:142–144.
29 Rajfer J, Rosciszewski A, Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol. 1988;140:69–71.
30 Christ GJ, Maayani S, Valcic M, et al. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoreceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101:375–381.
31 Christ GJ, Moreno AP, Parker ME, et al. Intercellular communication through gap junctions: a potential role in pharmacomechanical coupling and syncytial tissue contraction in vascular smooth muscle isolated from the human corpus cavernosum. Life Sci. 1991;49:PL195–PL200.
32 Chang SW, Fine R, Siegel D, et al. The impact of diuretic therapy on reported sexual function. Arch Intern Med. 1991;151:2402–2408.
33 Grimm RHJr, Grandits GA, Prineas RJ, et al. Long term effects on sexual function of five anti-hypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of Mild Hypertension Study (TOMHS). Hypertension. 1997;29:8–14.
34 Franzen D, Metha A, Seifert N, et al. Effects of beta-blockers on sexual performance in men with coronary artery disease. A prospective, randomized, and double blinded study. Int J Impot Res. 2001;13:348–351.
35 Fogari R, Zoppi A, Corradi L, et al. Sexual function in hypertensive males treated with lisinopril or atenolol: a crossover study. Am J Hypertens. 1998;11:1244–1247.
36 Llisteri JL, Lozano JV, Aznar VJ, et al. Sexual dysfunction in hypertensive patients treated with losartan. Am J Med Sci. 2001;321:336–341.
37 Salzman EA, Guay AT, Jacobson J. Improvement in erectile function in men with organic erectile dysfunction by correction of elevated cholesterol levels: a clinical observation. J Urol. 2004;172:255–258.
38 Sadeghi-Nejah H, Jackson I. New onset priapism associated with ingestion of terazosin in an otherwise healthy man. J Sex Med. 2007;6:1766–1768.
39 Gratzke C, Angulo J, Chitaley K, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–475.
40 Rosen RC, Riley A, Wagner G, et al. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830.
41 Schwartz BG, Kloner RA. How to save a life during a clinic visit for erectile dysfunction by modifying cardiovascular risk factors. Int J Impot Res. 2009;21:327–335.
42 Green KL, Albertsen PC, Babaian RL, et al. AUA prostate specific antigen best practice guideline: 2009 update. J Urol. 2009;182:2232–2241.
43 Lue TF, Broderick GA. Evaluation and nonsurgical management of erectile dysfunction and premature ejaculation. Wein AJ, Kavoussi LR, Novick AC. Campbell-Walsh Urology, ed 9,. 2007.
44 Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29.
45 Francis SH, Lincoln TM, Corbin JD. Guanosine 3′-5′ cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci U S A. 1976;73:2559–2563.
46 Hellastrom WJ, Bennett AH, Gesundheit N, et al. A double blind, placebo controlled evaluation of the erectile response to transurethral alprostadil. Urology. 1996;48:851–856.
47 Linet OL, Neff LL. Intracavernous prostaglandin E1 in erectile dysfunction. Clin Invest. 1994;72:139–149.
48 Padma-Nathan J, Christ G, Adaikan G, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2004;1:128–140.
49 Earle CM, Seah M, Coulden SE, et al. The use of the vacuum erection device in the management of erectile impotence. Int J Impot Res. 1996;8:237–240.
50 Cookson MS, Nadig PW. Long term results with vacuum constriction device. J Urol. 1993;149:290–294.
51 Mulcahy JJ, Austoni E, Barada JH, et al. The penile implant for erectile dysfunction. J Sex Med. 2004;1:98–109.
52 Subrini L. Subrini penile implants: surgical, sexual, and psychological results. Eur Urol. 1982;8:222–226.
53 Hellstrom JG, Montague DK, Moncada I, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7:501–523.
54 Montorsi F, Rigatti P, Carmignani G, et al. AMS three-piece inflatable implants for erectile dysfunction: a long term multi-institutional study in 200 consecutive patients. Eur Urol. 2000;37:50–55.
55 Latini DM, Penson DF, Wallace KL, et al. Longitudinal differences in psychological outcomes for men with erectile dysfunction: Results from ExCEED. J Sex Med. 2006;3:1068–1076.
56 http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ed. Accessed December 2010