Chapter 54 Varicose Veins
Epidemiology
Varicose veins (VVs) are tortuous, dilated, bulging, superficial veins typically measuring 4 mm or larger.1 Varicose veins are the most common manifestation of chronic venous insufficiency (CVI) and affect up to 25% of women and 15% of men.1,2 In the Framingham Study, which includes men and women between the ages of 30 and 62 from the town of Framingham, Massachusetts, the annual incidence of VVs is 2.6% among women and 1.9% among men.3 Risk factors include female gender, advancing age, family history, pregnancy, prolonged standing, obesity, vascular malformations, and hormone therapy.1,4 Varicose veins are more common in patients of European ancestry compared to Blacks or Asians.3 Approximately 4% of the women presenting with VVs have pelvic vein reflux as the underlying etiology.5 Pregnancy and deep venous reflux are also associated with VV recurrence after treatment.4
Other patterns of venous pathology include reticular veins, which are smaller, 1- to 3-mm diameter, flat, blue-green colored, less tortuous veins.1 Telangiectasias, or spider veins, are 1 mm or less, and blue, black, purple, or reddish in appearance.1 A cross-sectional study of a random sample of 1566 subjects 18 to 64 years of age from the general population in Scotland found that telangiectasias and reticular veins were each present in approximately 80% of men and 85% of women.6
The chronic nature of VVs has a major impact on healthcare resources. It is estimated that venous ulcers cause the loss of approximately 2 million working days annually, generating a cost of more than $3 billion per year in the United States alone.3 Moreover, beyond the purely economical impact of VVs, chronic venous disease is associated with reduced quality of life, with particularly negative impact on pain, physical function, and mobility measures.3 The same is true for patients who develop venous ulcers, with effect on quality of life directly related to severity of disease.3
Anatomy
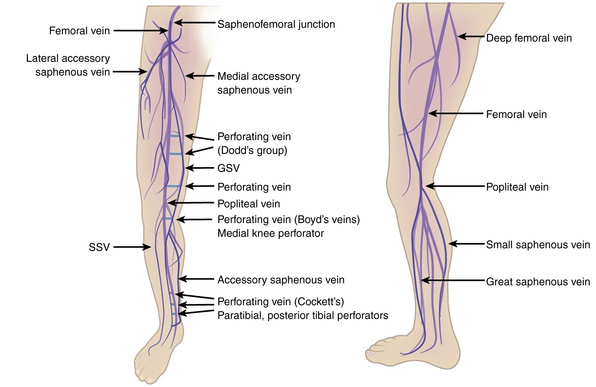
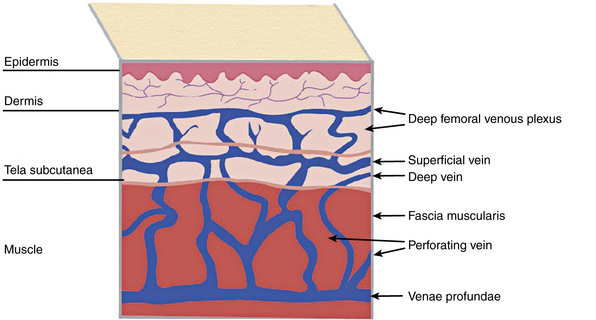
Broadly, the veins of the lower extremity are divided into three systems confluent in a single network, which ultimately drains into the external iliac vein. This venous network includes the superficial veins, deep venous system, and their mutual connections, as well as the perforators (Fig. 54-1). The deep compartment includes the deep venous system and it is bordered by the fascia muscularis. The superficial compartment is externally bordered by the dermis.7 The tissue situated under the dermis is called the tela subcutanea (subcutaneous tissue) and contains the saphenous vein. Within the superficial compartment, a narrow anatomical space called the saphenous compartment can be identified by ultrasound evaluation. Externally bordered by the saphenous fascia, this compartment covers the proper venae saphenae and their beginnings. The term perforating veins or perforators is reserved only for those veins that penetrate the fascia muscularis to connect the superficial system to the deep venous system. Conversely, communicating veins connect veins of the same venous system.
The great saphenous vein (GSV) is the longest vein in the entire human body. It starts at the medial side of the foot and courses proximally along the medial side of the calf as the marginal medial vein together with the saphenous nerve.7 The main tributaries are the posterior accessory GSV and the anterior accessory GSV. The vein continues alone on the medial side of the thigh and crosses through the saphenous hiatus into the common femoral vein. The normal caliber of the GSV is 3 to 4 mm, and it has 10 to 20 valves.7 The GSV is bifid in about 20% of legs, but two venous trunks of the GSV in the same compartment, constituting a true duplication, occurs in only 1% of cases. The small saphenous vein (SSV) is the second largest vein of the lower limb. It begins on the lateral side of the foot dorsum and runs along the lateral margin of the foot as the lateral marginal vein. It penetrates the popliteal fascia into the popliteal vein. In one third of cases, blood flows via various communicating veins to the system of the great saphenous. In one tenth of cases, it flows via the gastrocnemii veins and perforating veins into the deep venous system. The SSV is usually 3 mm wide and contains 7 to 13 valves. It is accompanied by the small saphenous artery, which must not be confused with the vein during sclerotherapy injection.7
Some of the perforating veins are consistently located. The thigh perforators include the medial thigh (formerly Hunter’s perforator), anterior thigh, lateral thigh, and posterior thigh perforating veins, and the pudendal perforating vein. The knee perforators include the medial knee (formerly Boyd’s perforator), suprapatellar, lateral knee, infrapatellar, and popliteal fossa perforating veins. The leg perforators include the paratibial, posterior tibial (formerly Cockett’s perforating vein), anterior leg, lateral leg, and posterior leg (medial and lateral gastrocnemius, intergemellar, para- Achillean) perforating veins. Other groups include the gluteal, ankle, and foot perforating veins. The perforator system does play a key role in calf muscle pump function (Fig. 54-2A).
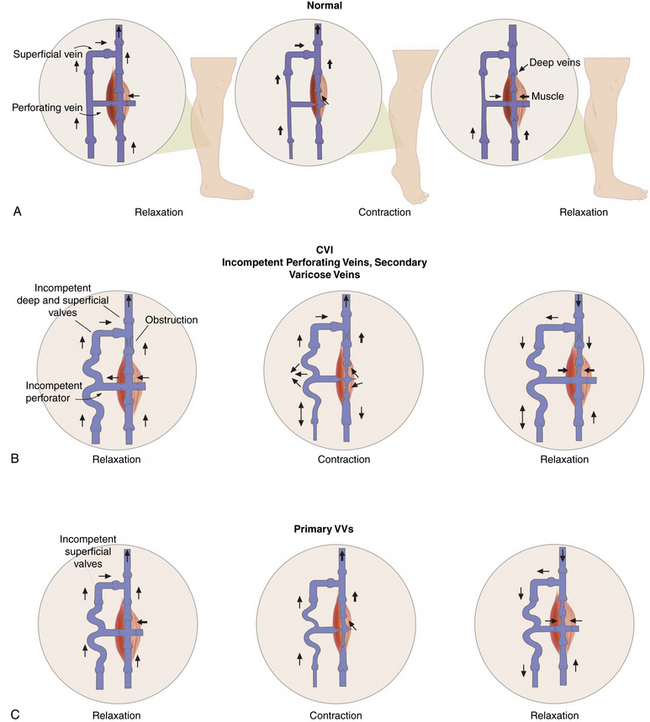
Figure 54-2 Calf “muscle pump” and varicose veins (VVs).
(Adapted from Sumner DS: venous dynamics–varicosities. Clin Obstet Gynecol 24:743–760, 1981.)
A system of subcutaneous veins spreads on the lateral aspect of the thigh and leg as a developmental remnant of the embryonic lateral marginal vein, which fades out and is replaced with the system of the saphenous veins and may be abnormally developed in patients with Klippel-Trénaunay’s or Parkes-Weber’s syndromes. In relation to the surface, there are three levels of venous plexuses: dermal, hypodermal, and deep. The dermal veins involve the superficial subpapillary venous plexus and the deep dermal venous plexus7 (Fig. 54-3).
Pathogenesis
Varicose veins are caused by weakness in the vein wall, and according to their underlying etiology can be divided into primary or secondary. Primary VVs result from idiopathic structural or functional defects in the venous system. Secondary VVs result from underlying venous obstruction, most commonly deep vein thrombosis (DVT) or underlying deep venous insufficiency1 (see Fig. 54-2B-C). Primary valvular incompetence is more frequent. Approximately 8 in 10 individuals with VVs have primary valvular incompetence. Secondary valvular reflux is usually due to trauma or thrombosis. Congenital anomalies only occur in about 2% of cases.3 Secondary chronic venous disease progresses faster than primary.
A key factor in the development of VVs is venous hypertension. Venous pressure is directly proportional to the weight of the column of blood from the right atrium to the foot and is reduced by pressures generated by muscle contractions. When standing, venous pressure is as high as 90 mmHg. It temporarily increases with muscle pumping, but then rapidly decreases as the functioning venous valves guide blood flow toward the heart. A well-functioning calf muscle pumping mechanism decreases the venous pressure to less than 30 mmHg.3 The constant insult of increased venous pressure degenerates in stretching, splitting, tearing, thinning, and adhesion of valves, causing inflammation.3 Adjuvant factors for development of excessive venous hypertension include failure of the calf muscle pump and obesity. Ultimately, prolonged venous hypertension leads to venous valvular incompetence or reflux and venous dilation.1 Venous defects increase venous hypertension and cause weakened venous walls, abnormal distention of the surrounding connective tissue, and separation of valve cusps.
Elevated venous pressure may also generate edema. Prominent swelling is not a usual feature of VVs, but episodic ankle edema is common.8 A small percentage of patients develop complications including dermatitis, superficial thrombophlebitis, or bleeding. Thrombophlebitis may occur spontaneously or result from an injury. Skin changes in chronic venous disease are proportionately related to the severity of venous hypertension. Up to 100% of patients with postexercise venous pressures of more than 90 mmHg develop venous ulcers.3 Patients with CVI and deep vein incompetence are at greatly increased risk of developing ulcers.9 Conversely, frequent dorsiflexion of the ankle and an effective calf muscle pump are protective factors (see Fig. 54-2A). Poor prognostic factors favoring progression include the combination of reflux and obstruction, ipsilateral recurrent DVT, and multisegmental involvement.10
Inflammatory changes also contribute to the genesis of VVs. Blood returning from feet that have been passively dependent for 40 to 60 minutes is depleted of leukocytes, suggesting that leukocytes accumulate and locally participate in the inflammatory cascade.3 Circulating leukocytes and vascular endothelial cells (ECs) express several types of adhesion molecules. Integrin binding promotes firm adhesion of leukocytes, the starting point for their migration out of the vasculature and degranulation. The activated leucocytes shed L-selectin into the plasma and express members of the integrin family.3 This is the backbone of the microvascular leukocyte-trapping hypothesis. Local inflammation associated with intercellular adhesion molecule (ICAM)-1 expression increases monocyte and macrophage adhesion. The valve damage is augmented by disturbed excessive collagen type 1 synthesis, which increases venous rigidity.3 Finally, matrix metalloproteinases (MMPs) and serine proteinases favor the accumulation of extracellular matrix (ECM) material in VVs.3,11
Clinical Manifestations
Clinical manifestations of VV range from cosmetic problems to severe symptoms, including ulceration. Chronic venous insufficiency can be classified by clinical presentation, etiology, anatomy, and pathophysiology (CEAP Classification)8,12 (see Chapter 55). Clinically, however, the patterns may be classified as complicated and uncomplicated varices. Uncomplicated VVs may need only cosmetic treatment or reassurance. Patients with complicated VVs may develop heaviness, fatigue, local pain, spontaneous bleeding, and superficial thrombophlebitis. Varicose veins may cause edema, pain, and skin changes such as stasis dermatitis and ulceration. Venous ulcerations can take more than 9 months to heal, with one study reporting that 66% of ulcers last longer than 5 years.1 All these symptoms impair activities of daily living.1
Multiple questionnaires and instruments measure the effect of venous disease on quality of life. Most are subjective and completed by the patient. The Chronic Venous Insufficiency Questionnaire (CIVIQ) was validated in a sample of 2001 patients and measures the psychological, social, physical, and pain domains. A revised version of the instrument, the CIVIQ 2, equally weighted the categories across 20 questions to provide a global score. This measure is used to follow quality-of-life (QOL) improvement after therapy for chronic venous insufficiency, including VVs. The Aberdeen Varicose Vein Questionnaire (AVVQ) includes 13 questions on physical symptoms and social issues, including pain, ankle edema, ulcers, compression therapy use, and the effect of VVs on daily activities. The disease- specific index is graded from 0 to 100 (extreme venous symptoms).13 This measure has also been validated for patient follow-up after intervention.14 The Venous Insufficiency Epidemiological and Economic Study (VEINES) instrument consists of 35 items in two categories to generate two summary scores. It includes the VEINES-QOL, with 25 QOL questions, and the VEINES-Sym, with 10 symptom questions.14 The focus of this measure is on physical symptoms of venous disease, in particular postthrombotic syndrome. It has been validated in patients with DVT. In 2004, Kahn et al. compared the VEINES and the 36-item Short-Form Health Survey (SF-36) with CEAP classification in 1531 patients from four countries to examine the effect of patient-related QOL reporting on interpreting outcomes in venous studies. Higher CEAP class was directly associated with and predictive of the VEINES-QOL.14 The Charing Cross Venous Ulceration Questionnaire (CXVUQ) was developed for patients with venous ulcers, and its performance is not impaired by the treatment option selected.14 Finally, the Venous Severity Score (VSS) system was derived from the CEAP classification and has three elements: the venous disability score (VDS), venous segmental disease score (VSDS), and venous clinical severity score (VCSS). The VCSS has been recently revised and includes multiple parameters: pain, VVs, inflammation, edema, skin induration, pigmentation, ulcers (size, number, duration), and compression therapy.15 The Venous Clinical Severity Score is useful for following changes with treatment.14,15
Physical Examination
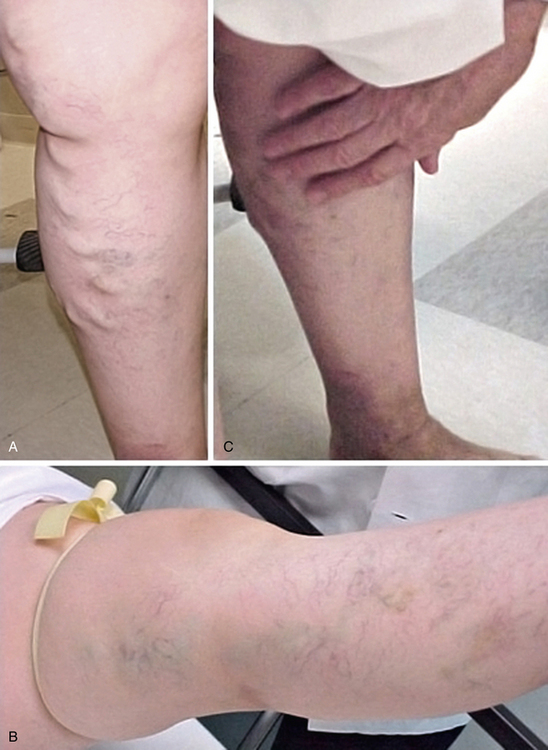
Initial inspection of the leg may reveal edema, prominent VVs, cyanosis, plethora, hyperpigmentation, lipodermatosclerosis, or ulcerations. On inspection, VVs may be observed as tortuous, dilated, bulging, superficial veins measuring 4 mm or larger; the patient is ideally examined in the standing position to allow venous reflux.1Lipodermatosclerosis is a consequence of localized chronic inflammation and fibrosis of the skin and subcutaneous tissue of the lower part of the leg.16 The skin changes will often occur at the “gaiter area” above the medial malleolus. Atrophie blanche is a localized circular, whitish, avascular, atrophic skin area surrounded by capillaries and sometimes hyperpigmentation, consistent with severe chronic venous insufficiency. Similarly, a phlebectatic crown (fan-shaped small intradermal veins on medial or lateral aspects of the foot) may herald severe venous insufficiency.
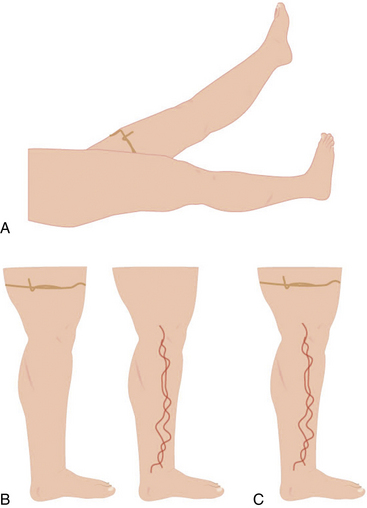
Trendelenburg and Perthes tests may be used during the exam to differentiate superficial from deep venous insufficiency (also see Chapter 55). In the Trendelenburg test, the leg is elevated and a tourniquet applied above the knee. This obstructs the superficial veins, which will promptly fill after standing if the patient has deep vein incompetence (secondary VVs). If after standing, the vein requires more than 20 seconds to refill, but prompt filling follows tourniquet removal, the exam is consistent with primary VVs. In the Perthes test, the leg is elevated and the tourniquet placed at the midthigh or proximal calf. When the patient stands and walks, the VVs will refill owing to incompetent perforators16 (Figs. 54-4 and 55-5). These maneuvers may be complemented with Brodie Trendelenburg percussion: a finger is placed over the distal area of a VV while the proximal segment of the vein is percussed. A transmitted impulse at the lower end suggests incompetence.
Imaging and Physiological Testing
Duplex ultrasonography is useful in the evaluation of VVs (also see Chapter 12). The test is performed with the patient standing or in reverse Trendelenburg position, and is used to detect acute or chronic thrombosis, postthrombotic changes, obstructive flow, and incompetence in the deep veins. Reflux, demonstrated by reversal of flow, is pathological whenever longer than 0.5 seconds.17 Duplex ultrasonography is not reliable for assessment of the iliac and caval veins, but it is sensitive for evaluation of saphenous vein reflux and useful for identification of incompetent perforator veins.
Impedance plethysmography, strain gauge plethysmography, and air plethysmography may be used to detect venous obstruction and reflux in large veins above the knee. Photoplethysmography, most commonly used with and without a tourniquet, can be employed to evaluate superficial venous insufficiency. A venous refilling time less than 20 seconds without a tourniquet that normalizes to over 20 seconds with a tourniquet is compatible with GSV incompetence.17
Venography may provide information regarding pelvic vein obstruction in patients with postthrombotic disease.16 Ascending venography is performed with the patient at 45 degrees, non–weight- bearing, with legs down as contrast is infused. Contrast filling the superficial vein denotes incompetence. Ascending venography is useful to determine vein obstruction, collateralization, and recanalization. Descending venography is more useful to diagnose venous insufficiency. In this scenario, the contrast is injected into the common femoral vein above the saphenofemoral junction. The patient is initially in supine position, and after the contrast dye injection, the table is tilted feet downward. Contrast leakage to the knee or distally is abnormal.17 Venography is usually indicated in the setting of endovenous intervention, but is difficult in the setting of a swollen leg. There is limited experience with magnetic resonance venography (MRV) or computed tomography (CT) to evaluate venous insufficiency and VVs.
Management
Treatment Suitability
Varicose veins treatment may be divided into conservative and invasive modalities. Conservative treatment for VVs and subsequent CVI include lifestyle modifications, compression therapy, and pharmacotherapy. All patients are appropriate for conservative measures. The use of more invasive techniques depends on the size of the vein and the presence of complications. Most commonly, ablation of an incompetent or varicose Great saphenous veins is performed first to decompress more distal varicosities; however, most patients require additional treatments for adequate therapeutic and cosmetic results. Thermal ablation of the GSV using endovenous laser therapy (EVLT) or radiofrequency ablation (RFA) is the most frequently employed technique. GSVs with diameters of 3 to 12 mm are candidates for RFA; EVLT is an option for those larger than 3 mm. A less invasive technique, foam sclerotherapy of the GSV, may also be performed in veins smaller than 1 cm, but has been used to treat larger veins as well.18 When the GSV is larger than 12 mm in diameter, surgery is an option.19 Tortuosity of the vessel is also relevant. For RFA and EVLT, the straight segment of the GSV should extend 15 to 20 cm immediately distal to the saphenofemoral junction.18
In a study of 577 patients with GSV reflux, 55% were suitable for RFA or EVLT, and 57% were suitable for foam sclerotherapy. Stressing the need for careful patient selection, only 41% of the limbs were suitable for all the procedures.18 In one study evaluating patients with recurrent VVs, less than 40% had limbs suitable for RFA or EVLT, while foam sclerotherapy was an option in 58% of the cases.18 Owing to the risk of skin burns, superficial tributary veins are not suitable for catheter-based thermal ablation. Optimal therapeutic results may be achieved with an approach of combined modalities.18
Conservative Management
Diet and lifestyle changes
Prolonged standing or sitting may exacerbate signs and symptoms of VVs. The patient should elevate the legs above the level of the heart as much as possible, lose excess weight, and exercise to minimize swelling and improve calf muscle function.20 Furthermore, moderate-intensity lower-limb exercise training improves microvascular endothelial vasodilator function in postsurgical VV patients.21
Compression
External compression is the cornerstone of therapy for VVs. Compression therapy, including graduated elastic compression stockings and short-stretch bandages, is effective in reducing lower-extremity pain and swelling and preventing progression of VVs and CVI to venous ulceration.20 Among patients with venous ulcerations, improved healing is achieved with multicomponent compression systems. Compression garments should be individualized for maximal patient compliance.20,22
Pharmacotherapy
Low-dose diuretics are often prescribed for patients with significant edema due to VVs, but they are minimally effective in reducing the symptoms of pain and discomfort.20 Patients with stasis dermatitis may be treated with a short course of topical corticosteroids to reduce inflammation. Antibiotics with gram-positive coverage are prescribed to treat cellulitis or infected ulcerations. Antibiotic coverage should be expanded to include gram-negative and anaerobic organisms in diabetic patients.20 Because of the increasing problem of bacterial resistance to antibiotics, current prescribing guidelines recommend that antibacterial preparations should only be used in cases of clinical infection and not for bacterial colonization. At present, there is no evidence to support routine use of systemic antibiotics to promote healing in venous leg ulcers.23
Herbal supplements
Short-term studies have shown the efficacy of horse chestnut seed (Aesculus hippocastanum) extract in reducing edema, ankle and calf circumference, and symptoms of VVs with insufficiency. The horse chestnut is native to southeast Europe, with aescin the active ingredient.20,24 This extract has anti-inflammatory and vasoconstrictive properties that may exert a positive influence on venous tone and increase the flow velocity of venous blood.24 It can be administered orally as a 20- or 50-mg dose. In a meta- analysis by Suter et al., a treated population of 219 adults with CVI stage I/II showed improvement; eight patients reported gastrointestinal upset.24
Micronized purified flavonoid fraction (MPFF) consists of 90% diosmin and 10% flavonoids. MPFF protects the microcirculation from raised ambulatory venous pressure. It decreases the interaction between leucocytes and ECs by inhibiting expression of endothelial ICAM-1 and vascular cell adhesion molecule (VCAM).25 A meta-analysis of randomized prospective studies using MPFF that included 723 patients with venous ulcers suggested improved healing at 6 months among those who used MPFF compared with conventional therapy alone.25
French maritime pine bark extract and rutosides have demonstrated inconsistent results.20 Although herbal products may be beneficial short term, their efficacy and safety have not been proven long term, and these preparations with varying amounts of active and inactive ingredients are not regulated by the U.S. Food and Drug Administration (FDA).
Invasive Therapy
For large VVs, invasive therapy is divided into endovenous procedures, including chemical and thermal ablation, and surgical procedures (Table 54-1).
Chemical sclerotherapy
Liquid sclerosants have been a treatment for VVs for almost a century. The introduction of endovenous foam sclerotherapy (EFS) in 1944, with standardization of the method by Cabrera in the early 1990s, improved the nonsurgical obliteration of VVs. Endovenous foam sclerotherapy is especially effective when administered using ultrasound guidance.20
Procedure
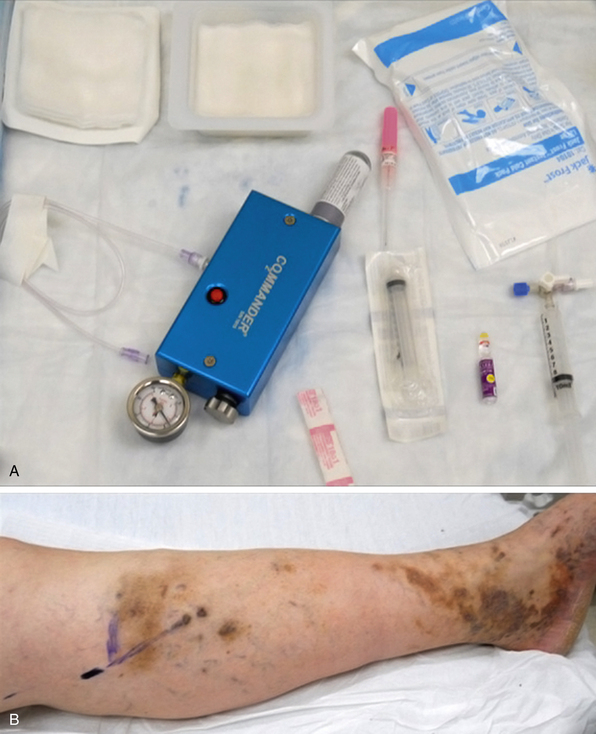
A chemical sclerosant (e.g., polidocanol, sodium morrhuate, sodium tetradecyl sulfate) is combined with carbon dioxide/oxygen (CO2/O2) or room air to produce foam (concentration 1%-3%; volume 6-10 mL) using the two-syringe, or Tessari, method26 (Fig. 54-6). Because it is more soluble in blood and water than the nitrogen in room air, CO2/O2 may reduce the risk of microbubble embolization. The Tessari method generates foam by pumping the contents of two disposable syringes, one containing the liquid sclerosant and the other containing air, backward and forward through a two-way stopcock.26 Ultrasound is performed to localize the most superficially accessible segment of the varicosity or GSV proper into which a catheter can be easily inserted. The foam is prepared immediately before the procedure and injected into the GSV or its tributaries under ultrasound guidance.26 The leg is elevated 45 degrees during injection, and the foam is massaged distally to fill the tributaries. Subsequently, a compression garment is applied to the treated leg.26,27
The foam displaces the blood in the vein, resulting in local inflammation, sclerosis, and obliteration of the VV over 1 to 2 weeks.26,28 The effectiveness of foam sclerotherapy lies in the utilization of detergent sclerosants that work by denaturation of proteins. By forming a lipid bilayer, the endothelial surface is disrupted in the absence of essential proteins, which produces a delayed cell death.27
A systematic review of EFS by van den Bos et al.29 that selected 64 studies and assessed 12,320 limbs followed for an average of 32 months determined an overall obliteration rate of 82.1% at 3 months (95% confidence interval [CI], 72.5-88.9), 80.9% at 1 year (95% CI, 71.8-87.6), and 73.5% at 5 years (95% CI, 62.8-82.1) (see Table 54-1). Contrary to catheter thermal-based techniques, increasing age does not impact sclerotherapy suitability.18 Endovenous foam sclerotherapy is also effective in treating venous stasis ulcers and congenital vascular malformations.1 In a study by Barrett et al. that analyzed a total of 100 randomly chosen legs with VVs treated with ultrasound-guided EFS, patient satisfaction was rated highly, with a 90% improvement in QOL 2 years after treatment with EFS1 (see Table 54-1). In the van den Bos review, after adjusting for follow-up, foam therapy and RFA were as effective as surgical stripping.29 In the absence of large comparative randomized clinical trials between multiple techniques, the minimally invasive techniques appear to be at least as effective as surgery in the treatment of lower-extremity VVs. Endovenous foam sclerotherapy is often used in conjunction with thermal ablation for sclerosis of tributary VVs.
In a study of 1931 treatment sessions that included 852 patients treated with ultrasound-guided EFS, the risk of deep venous occlusion was lower when treating veins less than 5 mm in diameter, and when restricting the volume of foam injected to less than 10 mL.30 The most common complications include mild to moderate pain and hyperpigmentation (up to 30%).1,13 Hyperpigmentation typically resolves within 6 to 12 months.1 Less common adverse events include superficial thrombophlebitis, DVT and pulmonary embolism (PE), trapped coagulum, hematoma, skin necrosis, transient neurological events (migraines, visual disturbance), and pulmonary symptoms (cough). Trapped coagulum resulting in superficial thrombophlebitis occurs in less than 5% of treated veins. Deep vein thrombosis results from propagation of foam into the deep venous system and typically involves the popliteal and calf veins. The incidence of DVT/PE is less than 1%.1,12 Transient neurological events may occur with the use of large amounts of foam in patients with a patent foramen ovale. To date, there have been three reported cases of major posttreatment neurological events (transient ischemic attack [TIA] and cerebrovascular accident) suspected to be associated with EFS. In all three cases, symptoms were associated with the presence of patent foramen ovale and resolved within 2 weeks.1,12,31
ClariVein, recently approved by the FDA, is a novel sclerotherapy approach for large VVs that uses a mechanical rotating dispersion wire to mix and disperse a sclerosant on the vessel wall. Potential advantages are that it does not require tumescence anesthesia, can be used near nerve bundles, is fully disposable, and does not require a generator.32 However, to date, no large-scale comparative studies have been published.
Endovenous laser therapy
Endovenous laser therapy was introduced in 1999 for obliteration of the GSV and its tributaries. The direct action of the laser on the vein wall and heating of the venous blood result in damage to the vein wall and, over weeks to months, obliteration of the varicosity.20 The pattern of injury is eccentrically distributed, with maximum injury occurring along the path of laser contact. Temperatures during EVLT may reach 1000 °C at the fiber tip and 300 °C in the firing zone. There is also steam generated during the photothermolytic process, but this accounts only for 2% of applied energy dose.33 The occlusion and complication rate after EVLT are proportional to the total energy (joules) per centimeter of vein (J/cm). The laser energy can be applied in continuous or pulsed mode, the continuous mode being more effective.
Procedure
Endovenous laser therapy is performed using tumescent anesthesia. A 5-mL syringe with a 25-gauge needle may be used to subcutaneously infiltrate 2 mL of tumescent anesthetic solution (420 mL of normal saline, 60 mL of 1% lidocaine with epinephrine, and 20 mL of sodium bicarbonate) over the access site. This solution is delivered manually or with an infusion pump under ultrasound guidance, aiming to surround the vein segment to be treated.20,34 A laser-tipped catheter is inserted into the GSV at the level of the knee and advanced just distal to the saphenofemoral junction with ultrasound guidance. The laser is continuously pulled distally in the GSV as continuous energy is applied to the vein.
The systematic review by van den Bos et al. (see Table 54-1) reported an overall obliteration rate of 92.9% at 3 months (95% CI, 90.2-94.8), 93.3% at 1 year (95% CI, 91.1-95.0), and 95.4% at 5 years (95% CI, 79.7-99.1).29 Several studies have reported that EVLT is more effective than venous stripping and other endovenous procedures in terms of obliteration and recurrence rates.1 Endovenous laser therapy recurrence of reflux in the treated vein occurs secondary to new incompetent perforators in the thigh and calf, and new saphenofemoral junction incompetence accounted for the progression of new vein disease.35 In a study of 3000 treated limbs by Ravi et al., overall patient satisfaction, as assessed by symptom relief and absence of VVs after ablation, was 86%.36 There are no absolute contraindications for EVLT.37 Relative contraindications include uncorrectable coagulopathy, liver dysfunction limiting local anesthetic use, immobility, pregnancy, and breastfeeding.37
Higher energy results in increased occlusion rates but is associated with higher complication rates, including paresthesia and thermal injuries. Other complications include pain, edema, erythema, ecchymosis, hematoma, vesiculation, hypo- or hyperpigmentation, superficial thrombophlebitis, and DVT.1,38 In a meta-analysis of 29 studies, Luebke and Brunkwall described more than a 50% incidence of ecchymosis in 12 of the studies where this complication was reported.13 In the same meta-analysis, the incidence of paresthesias was 1.7%. Moderate pain along the treated vein and superficial thrombophlebitis occurs in up to 50% and 12% of the limbs, respectively.39 In the same meta-analysis, seven studies with a total of 9317 patients reported only 27 cases of incident DVT (0.3%). In other studies, the incidence of DVT has been reported to be as much as 7%.1,13 Pulmonary embolism has not been a reported complication with EVLT.13 Because EVLT is usually performed in the outpatient setting, it may be more cost-effective than surgical treatment for VVs.1
Radiofrequency ablation
Radiofrequency ablation, introduced in the United States in 1999, results in obliteration of the GSV and its tributaries by delivering controlled heat using radiofrequency energy passed through an endovenous electrode.1
The theoretical advantage of segmental RFA is greater consistency in the vein treatment and increased speed of ablation; each 7-cm segment can be treated in 20 seconds.40
Procedure
Radiofrequency ablation is performed under general or local anesthesia. Tumescent anesthesia is required, and a dilute mixture of lidocaine in normal saline may be used (50 mL of 1% lidocaine with 1:200,000 epinephrine in 500 mL 0.9% saline for unilateral procedures, or in 1000 mL 0.9% saline for bilateral procedures). The volumes of tumescence are commonly between 75 and 100 mL per 10 cm of vein.40 Ultrasound is used for catheter placement and guidance during the procedure (Fig. 54-7). The GSV is cannulated at the knee, and the catheter is advanced to the saphenofemoral junction.
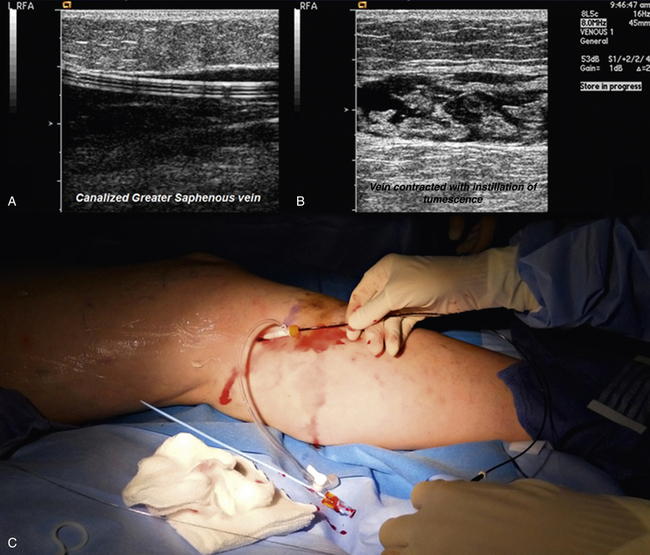
Figure 54-7 Insertion of radiofrequency catheter, with ultrasound visualization of inserted catheter and tumescence.
Radiofrequency ablation’s mechanism of action is based on resistive (or ohmic) heating caused by current. The heat generated in the vein wall (not in the catheter tip) is then dissipated and causes controlled collagen contraction or total thermocoagulation of the vein. The outcome is controlled tissue destruction that ultimately seals the lumen with minimal thrombus or coagulum.41 The thermal effect is proportional to the temperature and treatment time. For instance, with a temperature of 85 °C to 90 °C and a pullback speed of 3 to 4 cm/min, the thermal effect is sufficient to cause collagen contraction and occlude the lumen.41
A compression garment is applied for several days after the procedure. Recovery time is 3 to 5 days. Complications include paresthesia, hematoma, skin burns, infection, bruising, and thrombophlebitis/thromboembolism. Transient paresthesia is reported in up to in 15%, hematoma in 5%, skin burns in 2.1%, superficial thrombophlebitis in 2.1%, DVT in 16%, and nonfatal PE in 1% of 286 treated limbs.1 Venous size of less than 2 mm or more than 15 mm, previous superficial thrombophlebitis with residual obstruction, and GSV tortuosity are relative contraindications for RFA.37
In a systematic review of more than 12,000 limbs of patients who received stripping, foam sclerotherapy, or thermal ablation, the overall occlusion rate for RFA was 88.8% at 3 months (95% CI, 83.6-92.5), 87.7% at 1 year (95% CI, 83.1-91.2), and 79.9% at 5 years (95% CI, 59.5-91.5).29 Symptomatic improvement was reported in 85% to 94% of limbs with anatomical success and 70% to 80% of limbs with anatomical failures.1 In a meta-analysis by Luebke et al., the QOL at 72 hours and 1 week was significantly better with RFA than with surgery.19
Ultrasound examination is recommended within 72 hours to 1 week after the procedure to evaluate for DVT. Although no studies exist validating efficacy and safety of VTE prevention, patients with high thromboembolic risk can be considered for medical thromboprophylaxis after the procedure.1 Radiofrequency ablation requires less hospitalization and recovery time than surgical procedures. In a study of 458 patients treated with RFA followed at 3 months and 1 year, the main predictors of long-term occlusion were pullback speed (odds ratio [OR] 3.7; 95% CI, 1.1-12.4) and CEAP classification (OR 3.1; 95% CI, 1.7-5.6).42
Surgical procedures
Surgical interventions were traditionally the alternative treatment for VVs when conservative management had been unsuccessful. These are now rarely performed. Surgical treatments of VVs include saphenous vein stripping, ligation of the saphenofemoral junction, and ambulatory phlebectomy.1 The hemodynamic effectiveness of surgical procedures is supported by air plethysmography studies. In a study of 2120 limbs by Park et al., hemodynamic parameters including venous volume, venous filling index, residual volume function, and ejection fraction were significantly improved after surgery.43
Saphenous Vein Stripping
First reported in 1844, this procedure is performed in patients with incompetent GSVs or SSVs, reflux through the saphenofemoral or saphenopopliteal junctions, or superficial thrombophlebitis identified by duplex ultrasounds.1 Additional chemical sclerotherapy is often needed for residual tributary VVs.44
Saphenous vein stripping is performed under general anesthesia and involves making an incision in the groin, along with ligation of the GSV and its major branches. A stiff but flexible wire is inserted into the free end of the GSV and advanced along its length and out through a second incision at the upper calf. The vein is then tied to the wire in the groin and retrieved through the second incision at the upper calf, stripping the entire GSV. The incisions are then closed and compression bandages applied.1
Of note, venous stripping from ankle to groin is not always necessary. Ligation of the vein at the saphenofemoral junction in conjunction with removal of the thigh portion of the vein can also reduce venous reflux. Venous stripping may be performed in conjunction with ligation of the saphenofemoral junction, phlebectomy, or chemical sclerotherapy. Saphenous vein stripping has a higher initial cost due to hospitalization and results in more time lost from work compared with endovenous procedures.1 Recovery time varies from 2 to 3 weeks. The procedure is contraindicated in patients with a history of DVT, Klippel-Trénaunay’s syndrome, or the presence of severe peripheral artery disease (PAD) or neuropathy, which may impede wound healing and increase the risk of infection.
In a large systematic review, there was persistent GSV and SSV obliteration of 80.4% (95% CI, 72.3-86.5) at 3 months, 79.7% (95% CI, 71.8-85.8) at 1 year, and 75.7% (95% CI, 67.9-82.1) at 5 years after saphenous vein stripping.1,29 In a study of 245 extremities in 210 patients operated on for either GSV or SSV incompetence, there was a recurrence rate of 30%, as determined by ultrasound examination 14 years after the procedure, with only 6.9% having clinically significant recurrence of their VVs.11 A multicenter study evaluated predictors of persistence or redevelopment of reflux in 1638 limbs. After adjustment for follow-up, independent predictors were found to be: groin mapping by ultrasound (OR, 0.28; 95% CI, 0.20-0.40), less than 3-cm groin incisions at or below groin crease (OR, 0.50; 95% CI, 0.32-0.78), prior parity (OR, 2.69; 95% CI, 1.45-4.97), body mass index (BMI) over 29 (OR, 1.65; 95% CI, 1.12-2.43), less than 3-cm suprainguinal incisions (OR, 3.71; 95% CI, 1.70-5.88), stripping to the ankle (OR, 2.43; 95% CI, 1.71-3.46), and interim pregnancy (OR, 4.74; 95% CI, 2.47-9.12).45 Perforating vein incompetence and postthrombotic deep vein incompetence are also relevant considerations for postoperative VV recurrence.46 The exact causes of VV recurrence are speculative, but may include neovascularization, presence of superficial and deep venous insufficiency, presence of incompetent perforator veins, or surgical failure.4,8
Although saphenous vein stripping improves the QOL for patients with symptomatic VVs, in a multicenter retrospective analysis of 376 limbs of 296 patients treated for primary VVs due to GSV insufficiency, the patient satisfaction rate decreased from 86% at 1 year to 74% at 5 years.4 Complications of venous stripping include pain, bleeding (24%), infection (2%-15%), nerve injury (25%), superficial thrombophlebitis, and venous thromboembolism (<2%).47
Ligation of the Saphenofemoral Junction
Ligation of the saphenofemoral junction can be performed in patients with saphenofemoral reflux. However, owing to the high VV recurrence rate, it is typically performed in conjunction with venous stripping, phlebectomy, or EFS.1
Ligation is performed under local anesthesia through an incision made parallel to the inguinal ligament at the site of the saphenofemoral junction. Saphenous vein tributaries are identified and ligated until reaching the saphenofemoral junction. The GSV is then ligated at its junction with the common femoral vein. The incision is then closed, and compression garments are applied.1 Ligation has been used with endovenous ablation techniques to improve efficacy and safety. In one study of 210 legs in 182 patients with primary saphenofemoral junction incompetence, the recurrence rate for saphenofemoral junction ligation was 5.4% at 1 year and 35.5% at 4 years. The relative risk of recurrence after ligation of the saphenofemoral junction alone is 2.4 times greater than that of venous stripping.48 However, the percentage of patients with symptomatic recurrence of their varicosities is low.1 In a study with long-term follow-up of 10 years of 245 extremities in 210 patients operated on for either GSV or SSV incompetence, only 7% of the limbs had recurrence (>3 mm diameter), with neovascularization as the main cause.49 Complications of saphenofemoral ligation include pain, bleeding and hematoma (<30%), infection (2%-15%), nerve injury (4%-25%), thrombophlebitis, and DVT/PE (<2%).47 Contraindications are similar to saphenous vein stripping.1
Stab or Transilluminated Phlebectomy
Transilluminated phlebectomy (TIPP) was proposed in 2000 as a more reliable and less invasive outpatient alternative to saphenous vein stripping.1 The use of TIPP is associated with fewer incisions compared with conventional stab phlebectomy, but with a potentially higher cost, longer operating time, and greater complication rate.
For incision phlebectomy, small incisions are made along the GSV and its tributaries, which are retrieved with the use of a phlebectomy hook and subsequently avulsed.1 Transilluminated phlebectomy is performed with a fiberoptic light channel inserted into the GSV. A mixture of saline and local anesthetic is infused into the subcutaneous tissue to produce tumescence and transilluminate the vein. With an endoscopic dissector, provided with a rotating blade and suction channel, the GSV and its tributaries are resected and aspirated.50 Contraindications are similar to those for saphenous vein stripping.
A randomized prospective trial on 188 limbs comparing stab phlebectomy with TIPP reported a significant difference in the number of incisions for phlebectomy between the two groups (29 ± 1.28 vs. 5 ± 0.17; P <0.001). However, there was a higher recurrence rate at 52 weeks with TIPP (21.2% vs. 6.2%), with no significant difference in complication rates.51 Complications include pain, hyperpigmentation (<2%), cellulitis (<3%), hematoma (5%-12%), and nerve injury (up to 25%).1 The rate of calf hematoma is higher for TIPP than for stab phlebectomy (25% vs. 2.5%).1 Phlebectomy is useful for larger truncal veins, in which higher venous flow limits the use of endovenous procedures, and in younger patients with thicker vein walls.1 This procedure is often performed in conjunction with traditional surgical ligation of the saphenofemoral junction.1 Both stab phlebectomy and TIPP prolong the return to work and resumption of activities of daily living. However, most patients recover fully by 6 weeks post surgery.52
Microphlebectomy
Dr. Robert Muller, a Swiss dermatologist from Neuchâtel, Switzerland, rediscovered this technique in 1956.53 Ambulatory phlebectomy is a minimally invasive technique that can be performed in an office-based practice. This technique may be suitable for GSV, SSV, and pudendal veins in the groin, but more commonly is used to treat reticular varices in the popliteal fold or lateral part of the thigh.53
The VVs are carefully identified with a marking pen while the patient is standing. After tumescent anesthesia, with the patient in Trendelenburg position, cutaneous incisions are made with a #11 scalpel blade or 18-gauge needle, vertically oriented along the thigh and lower leg following the skin lines at the knee or the ankle.53 The distance between the incisions is determined by experience, anatomy, and history of phlebitis. The vein is then dissected with the phlebectomy hook and mosquito forceps. Incompetent perforators can be dissected and eliminated with gentle traction or torsion, but this is more difficult. Venous ligation is not necessary, since hemostasis may be achieved with local compression during and after surgery. No skin closure is needed with small incisions of 1 to 3 mm. Postoperative bandaging is essential.54 Dressings are removed after 24 or 48 hours. Ongoing compression therapy with elastic bandages or compression stockings is recommended for up to 3 weeks. Complementary chemical sclerotherapy may be used several weeks after the initial procedure.
Complications are minor and benign and usually resolve spontaneously. Periprocedurally, patients should avoid early sun exposure because hyperpigmentation may result at the puncture or incision sites.53 Complications include edema, bleeding, hematoma formation, scarring, trauma-induced telangiectatic matting, neotelangiectasia, occasional nerve injury with sensory disturbances, and blisters due to wound dressings.54 Very rarely, skin necrosis due to the high pH caused by adding excess bicarbonate to the anesthetic solution may occur. Deep venous thrombosis has not been reported.54 Contraindications to ambulatory phlebectomy include reflux at the saphenofemoral or saphenopopliteal junctions. These junctions may be treated by thermal ablation.53 Small studies and case series have reported a high rate of success with this procedure.
CHIVA cure
This is a relatively new outpatient method described by Franceschi in 1988. “Cure Conservatrice et Hémodynamique de l’Insuffisance Veineuse en Ambulatoire” (CHIVA), or “Ambulatory Conservative Hemodynamic Management of Varicose Veins,” aims for preservation of the superficial venous system and its cutaneous and subcutaneous drainage. The CHIVA method consists of breaking up the hydrostatic pressure column by disconnecting venous shunts. This is achieved by using venous ligatures guided by hemodynamic and duplex ultrasonography data derived from the deep and superficial venous system.55 A variation of the CHIVA technique may be done using sclerotherapy.56 Recurrence at 5 years of follow-up were 44.3% cured, 24.6% improved, and 31.1% failure in a study by Pares et al.55 There were no occurrences of DVT, pulmonary thromboembolism, saphenous vein neuralgia, or deaths. Potential complications include bruises (47.5%), wound infection (2.5%), and phlebitis (1.3%).55 Although the technique was invented in 1988, it is not yet widely available in United States. The practice guidelines of the American Venous Forum and the Society for Vascular Surgery do not currently endorse widespread use of the CHIVA technique.37
Management of Incompetent Perforator Veins
Poor deep venous function caused by venous reflux, obstruction, or calf muscle pump failure will ultimately lead to an increase in ambulatory venous pressure and recurrence of VV through incompetent perforators, resulting in chronic venous insufficiency. Incompetent perforator veins in patients with venous ulcerations were previously treated with ligation using the open Linton procedure, and now occasionally with subfascial endoscopic perforator surgery (SEPS).1 More commonly, endovenous thermal ablation or sclerotherapy is the treatment of choice for patients with venous ulcers who have failed conservative compression therapy and require ablation of incompetent perforator veins. The current Society for Vascular Surgery and American Venous Forum guidelines do suggest treatment of so-called pathological perforating veins, defined as those with outward flow of 500-ms duration, diameter of 3.5 mm, and located beneath a healed or open venous ulcer.37
Surgical Treatments of Incompetent Perforator Veins
Patients who undergo ligation of perforator veins typically have severe resistant CVI complicated by venous ulcerations. The Linton procedure was introduced in the 1950s for treating perforator veins and has largely been replaced by SEPS. About 45% of incompetent perforator veins are located in an area 10 to 15 cm above the medial malleolus, the typical Cockett 2/3 area, but the anatomy of the subfascial compartments makes only 32% of Cockett 2, 4% of Cockett 3, and 40% of Cockett 4 perforators available in the superficial posterior compartment for interruption via a SEPS procedure.57
Linton or subfascial endoscopic perforator surgery procedure
The Linton procedure involves making a long incision across the calf including the diseased tissue, forming a skin/soft tissue/fascial flap, and ligating the perforator veins under direct visualization.1 SEPS can be performed in the ambulatory care setting, with less time away from work required. The SEPS procedure involves making two incisions below the knee and inserting ports into the subfascial space.1 The subfascial plane is kept open with infusion of CO2 for visualization of the structures. The perforator veins are identified and ligated.
As noted, the Linton procedure has largely been replaced by SEPS because of higher complication rates, including wound infections (40%-50%), nerve injury (11%), and DVT (4%). Complications associated with SEPS are less frequent and include wound infection (5%-7%), nerve injury (6%), superficial thrombophlebitis (3%), and cellulitis (2.5%).18,20,51 Presence of peripheral artery disease, which carries the risk of poor wound healing, is a relative contraindication to these procedures. Similarly, performance of these procedures in patients with deep vein occlusion is associated with poor outcomes.58
Lower-extremity activity is limited for 5 to 7 days.1 SEPS may be performed in conjunction with other surgical and endovenous procedures that ablate an incompetent GSV.
Sclerotherapy
Ultrasound-guided sclerotherapy uses a relatively small catheter to gain access to the incompetent perforator or its tributary. Masuda et al. treated 80 limbs in 68 patients by sclerotherapy using liquid sodium morrhuate. The initial incompetent perforator closure rate was 90%, but fell to 70% at a mean follow-up of 20 months.57 After treatment, the VCSS was improved from a median of 8 to 2. Skin necrosis is a reported adverse effect.
Endovenous ablation
Percutaneous endovenous RFA of incompetent perforator veins can be carried out with a small incision or puncture site in the calf. However, because this entry site is usually made in compromised skin directly over the perforator, there may be risk of infection or exacerbation of the wound. In a meta-analysis of 1573 incompetent perforator veins treated by RFA, the occlusion rate varied from 64% to 99% during a short follow-up.57 In a study of 37 patients who underwent Doppler ultrasound surveillance 5 years after incompetent perforator ablation, of 125 incompetent perforators analyzed, 101 were closed (81%).59 Although this is a promising procedure, data regarding ulcer healing rates and long-term efficacy are limited.57
Management of Telangiectasia/Reticular Veins
Surface Transcutaneous Laser Therapy
Procedure
The laser is applied to the surface of the skin and targets a wavelength of light to the Hb within the vessel, resulting in heating and obliteration of the vessel. Small (<1 mm) superficial vessels with higher oxygenated Hb content are treated with shorter wavelengths (580-1064 nm), shorter pulse durations (15-30 ms), higher fluences (350-600 J/cm2), and smaller spot sizes (<2 mm).40 Larger, deeper vessels with lower oxygenated Hb content are treated with longer wavelengths (800-1064 nm), longer pulse durations (30-50 ms), moderate fluences (100-350 J/cm2), and larger spot sizes (2-8 mm).1 Pulsed light therapy delivers a high-intensity spectrum of light to the vessel, resulting in its obliteration. Pulsed light therapy generally is used for longer vessels. Typically, one to three laser treatments are scheduled at 6- to 12-week intervals.1
Reports of effectiveness are based on case series reporting small numbers of patients. In a study of 40 female patients 24 to 58 years old, the leg veins were treated with synchronized micropulses from a long-pulsed 1064-nm Nd:YAG laser, 6-mm diameter spot size, and 130 and 140 J/cm.2 After one to three laser treatment sessions, there was a 50% to 75% disappearance of veins in approximately 60% of the limbs at 4 weeks, and in more than 80% of the limbs at 12 weeks. The patient subjective satisfaction index, measuring cosmetics, increased from 42.5% at 6 months to 75% at 12 months. Objective improvement in cosmetic appearance, measured with computer-assessed medical photography, increased from 57% at 6 months to 82.5% at 12 months.60
Postprocedure pain is a common side effect of laser and light therapy. Other complications include edema, erythema, bruising, vesiculation, hypo/hyperpigmentation, transient hemosiderin staining, telangiectatic matting, and scarring. This procedure is contraindicated during pregnancy and in those with tanned or dark skin, history of photosensitivity disorder, or keloidal scarring. Patients are advised to avoid tanning before the procedure to avoid absorption of shorter wavelengths from the laser by sun-induced melanin, resulting in blistering and hyperpigmentation.3 Sunscreen is advised after treatment with laser.
Chemical Sclerotherapy
Sclerotherapy for treating telangiectasias and reticular veins is generally performed using liquid sclerosant (glycerin, hypertonic saline, polidocanol, and sodium tetradecyl sulfate) rather than foam, although foam can also be used in lower volumes. To decrease telangiectatic matting and postsclerosis hyperpigmentation, a reduced amount of foam per injection (0.5 mL) and per treatment session is recommended for telangiectasias and reticular veins. Telangiectatic matting describes a network of tiny vessels less than 0.2 mm in diameter that may appear after sclerotherapy treatment.61 In a large retrospective analysis of 2120 patients, the overall incidence of telangiectatic matting was 16%.61
Follow-Up and Prognosis
A relevant disclosure before treating the patient with VVs is that current methodologies continue to require long-term follow-up and retreatment. The possibility of recurrence at 5 years is 5% to 30%, depending on the treatment administered and ongoing risk factors. Obesity, multiple pregnancies, incompetent perforators, and saphenofemoral junction incompetence are some of the often-mentioned risk factors for recurrence. Regular compression stocking use will minimize the signs and symptoms associated with recurrent VVs and progression of chronic venous insufficiency. However, patients will commonly return for repeated treatments over their lifetime.45,46
1 Nael R., Rathbun S. Treatment of varicose veins. Curr Treat Options Cardiovasc Med. 2009;11(2):91–103.
2 Callam M.J. Epidemiology of varicose veins. Br J Surg. 1994;81(2):167–173.
3 Bergan J.J., et al. Chronic venous disease. N Engl J Med. 2006;355(5):488–498.
4 Miyazaki K., et al. Long-term results of treatments for varicose veins due to greater saphenous vein insufficiency. Int Angiol. 2005;24(3):282–286.
5 Marsh P., et al. Pelvic vein reflux in female patients with varicose veins: comparison of incidence between a specialist private vein clinic and the vascular department of a National Health Service District General Hospital. Phlebology. 2009;24(3):108–113.
6 Evans C.J., et al. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53(3):149–153.
7 Kachlik D., Pechacek V., Baca V., et al. The superficial venous system of the lower extremity: new nomenclature. Phlebology. 2010;25:113–123.
8 Raju S., Neglen P. Clinical practice. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360(22):2319–2327.
9 Robertson L., et al. Risk factors for chronic ulceration in patients with varicose veins: a case control study. J Vasc Surg. 2009;49(6):1490–1498.
10 Labropoulos N., et al. Secondary chronic venous disease progresses faster than primary. J Vasc Surg. 2009;49(3):704–710.
11 Raffetto J.D., Khalil R.A. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol. 2008;6(3):158–172.
12 Rutherford R.B., et al. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31(6):1307–1312.
13 Luebke T., Brunkwall J. Systematic review and meta-analysis of endovenous radiofrequency obliteration, endovenous laser therapy, and foam sclerotherapy for primary varicosis. J Cardiovasc Surg (Torino). 2008;49(2):213–233.
14 Vasquez M.A., Munschauer C.E. Venous Clinical Severity Score and quality-of-life assessment tools: application to vein practice. Phlebology. 2008;23(6):259–275.
15 Vasquez M.A., et al. Revision of the Venous Clinical Severity Score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg.. 2010;52:1387–1396.
16 Rathbun S. Chronic venous disease and lymphatic disease. In: Rooke T., ed. Vascular medicine and endvascular interventions. Oxford, UK: Blackwell; 2009:44–58.
17 Rumwell C., McPharlin M. Vascular technology, ed 4. Pasadena, CA: Davies; 2009.
18 Goode S.D., et al. Suitability of varicose veins for endovenous treatments. Cardiovasc Intervent Radiol. 2009;32(5):988–991.
19 Luebke T., et al. Meta-analysis of endovenous radiofrequency obliteration of the great saphenous vein in primary varicosis. J Endovasc Ther. 2008;15(2):213–223.
20 Rathbun S.W., Kirkpatrick A.C. Treatment of chronic venous insufficiency. Curr Treat Options Cardiovasc Med. 2007;9(2):115–126.
21 Klonizakis M., et al. Exercise training improves cutaneous microvascular endothelial function in post-surgical varicose vein patients. Microvasc Res. 2009;78(1):67–70.
22 Milic D.J., et al. The influence of different sub-bandage pressure values on venous leg ulcers healing when treated with compression therapy. J Vasc Surg. 2010;51(3):655–661.
23 O’Meara S., et al. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. (1):2010. CD003557
24 Suter A., Bommer S., Rechner J. Treatment of patients with venous insufficiency with fresh plant horse chestnut seed extract: a review of 5 clinical studies. Adv Ther. 2006;23(1):179–190.
25 Coleridge-Smith P., Lok C., Ramelet A.A. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30(2):198–208.
26 Breu F.X., Guggenbichler S. European Consensus Meeting on Foam Sclerotherapy, April, 4-6, 2003, Tegernsee, Germany. Dermatol Surg. 2004;30(5):709–717. discussion 717
27 Bergan J., Cheng V. Foam sclerotherapy for the treatment of varicose veins. Vascular. 2007;15(5):269–272.
28 Belcaro G., et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology. 2003;54(3):307–315.
29 van den Bos R., et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49(1):230–239.
30 Myers K.A., Jolley D. Factors affecting the risk of deep venous occlusion after ultrasound-guided sclerotherapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;36(5):602–605.
31 Morrison N., Neuhardt D.L. Foam sclerotherapy: cardiac and cerebral monitoring. Phlebology. 2009;24(6):252–259.
32 PRNewswire. ClariVein®: New method for vein ablation introduced in Europe. 2010. [cited 2010 October 3, 2010]; Available from: http://www.vasculardiseasemanagement.com/content/clarivein%C2%AE-new-method-vein-ablation-introduced-europe
33 Fan C.M., Rox-Anderson R. Endovenous laser ablation: mechanism of action. Phlebology. 2008;23(5):206–213.
34 Perkowski P., et al. Endovenous laser ablation of the saphenous vein for treatment of venous insufficiency and varicose veins: early results from a large single-center experience. J Endovasc Ther. 2004;11(2):132–138.
35 King J. Progression and recurrence of vein disease in patients treated with endovenous laser ablation: one year experience. The 11th Annual Scientific Meeting & Workshops. 2007. Sydney
36 Ravi R., et al. Endovenous thermal ablation of superficial venous insufficiency of the lower extremity: single-center experience with 3000 limbs treated in a 7-year period. J Endovasc Ther. 2009;16(4):500–505.
37 Gloviczki P, et al: The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum, J Vasc Surg 53(5 Suppl):2S–48S.
38 Nwaejike N., Srodon P.D., Kyriakides C. 5-years of endovenous laser ablation (EVLA) for the treatment of varicose veins – a prospective study. Int J Surg. 2009;7(4):347–349.
39 Pannier-Fischer F., Rabe E. Endovenous laser therapy and radiofrequency ablation of saphenous varicose veins. J Cardiovasc Surg (Torino). 2006;47(1):3–8.
40 Gohel M.S., Davies A.H. Radiofrequency ablation for uncomplicated varicose veins. Phlebology. 2009;24(Suppl 1):42–49.
41 Roth S.M. Endovenous radiofrequency ablation of superficial and perforator veins. Surg Clin North Am. 2007;87(5):1267–1284. xii
42 Boon R, Akkersdijk GJ, Nio D: Percutaneous treatment of varicose veins with bipolar radiofrequency ablation, Eur J Radiol 75(1):43–47.
43 Park U.J., et al. Analysis of the postoperative hemodynamic changes in varicose vein surgery using air plethysmography. J Vasc Surg. 2010;51(3):634–638.
44 Nishibe T., et al. Fate of varicose veins after great saphenous vein stripping alone. Int Angiol. 2009;28(4):311–314.
45 Fischer R., et al. Patient characteristics and physician-determined variables affecting saphenofemoral reflux recurrence after ligation and stripping of the great saphenous vein. J Vasc Surg. 2006;43(1):81–87.
46 Allegra C., Antignani P.L., Carlizza A. Recurrent varicose veins following surgical treatment: our experience with five years follow-up. Eur J Vasc Endovasc Surg. 2007;33(6):751–756.
47 Beale R.J., Gough M.J. Treatment options for primary varicose veins–a review. Eur J Vasc Endovasc Surg. 2005;30(1):83–95.
48 Winterborn R.J., et al. Randomised trial of flush saphenofemoral ligation for primary great saphenous varicose veins. Eur J Vasc Endovasc Surg. 2008;36(4):477–484.
49 Hartmann K., et al. Recurrent varicose veins: sonography-based re-examination of 210 patients 14 years after ligation and saphenous vein stripping. Vasa J Vasc Dis. 2006;35(1):21–26.
50 Beale R.J., Gough M.J. Treatment options for primary varicose veins – a review. Eur J Vasc Endovasc Surg. 2005;30(1):83–95.
51 Aremu M.A., et al. Prospective randomized controlled trial: conventional versus powered phlebectomy. J Vasc Surg. 2004;39(1):88–94.
52 Chetter I.C., et al. Randomized clinical trial comparing multiple stab incision phlebectomy and transilluminated powered phlebectomy for varicose veins. Br J Surg. 2006;93(2):169–174.
53 Ramelet A.A. Phlebectomy. Technique, indications and complications. Int Angiol. 2002;21(2 Suppl 1):46–51.
54 Weiss R.A., Dover J.S. Leg vein management: sclerotherapy, ambulatory phlebectomy, and laser surgery. Semin Cutan Med Surg. 2002;21(1):76–103.
55 Pares J.O., et al. Varicose vein surgery: stripping versus the CHIVA method: a randomized controlled trial. Ann Surg. 2010;251(4):624–631.
56 Bernardini E., et al. Echo-sclerosis hemodynamic conservative: a new technique for varicose vein treatment. Ann Vasc Surg. 2007;21(4):535–543.
57 Donnell T.F. The role of perforators in chronic venous insufficiency. Phlebology. 2010;25(1):3–10.
58 Gloviczki P., et al. Mid-term results of endoscopic perforator vein interruption for chronic venous insufficiency: lessons learned from the North American subfascial endoscopic perforator surgery registry. The North American Study Group. J Vasc Surg. 1999;29(3):489–502.
59 Bacon J.L., et al. Five-year results of incompetent perforator vein closure using trans-luminal occlusion of perforator. Phlebology. 2009;24(2):74–78.
60 Trelles M.A., et al. Long pulse Nd:YAG laser for treatment of leg veins in 40 patients with assessments at 6 and 12 months. Lasers Surg Med. 2004;35(1):68–76.
61 Davis L.T., Duffy D.M. Determination of incidence and risk factors for postsclerotherapy telangiectatic matting of the lower extremity: a retrospective analysis. J Dermatol Surg Oncol. 1990;16(4):327–330.